Abstract
Bacille-Calmette-Guerin (BCG) has variable efficacy as an adult tuberculosis (TB) vaccine but can reduce the incidence and severity of TB infection in humans. We have engineered modified vaccinia Ankara (MVA) strain vaccine constructs to express the secreted mycobacterial proteins Ag85A and ESAT-6 (MVA-AE) and evaluated their immunogenicity and protective efficacy as mucosal booster vaccines for BCG given subcutaneously in early life. Intranasal delivery of MVA-AE to young adult mice induced CD4+ and CD8+ T cell responses to both Ag85A and ESAT-6 in lung mucosae. These responses were markedly enhanced in mice that had been primed neonatally with BCG prior to intranasal MVA-AE immunization (BCG/MVA-AE), as evidenced by numbers of pulmonary Ag85A-, ESAT-6-, and PPD-specific CD4+ and CD8+ T cells and by their capacity to secrete multiple antimicrobial factors, including IFNγ, IL-2 and IL-17. Moreover, MVA-AE boosting generated multifunctional lung CD4+ T cells responding to ESAT-6, which were not, as expected, detected in control mice given BCG, and elevated Ag85A-specific circulating antibody responses. After aerosol challenge with M. tuberculosis H37Rv (Mtb), the BCG/MVA-AE group had significantly reduced mycobacterial burden in the lungs, compared with either BCG primed mice boosted with control MVA or mice given only BCG. These data indicate that intranasal delivery of MVA-AE can boost BCG-induced Th1 and Th17-based immunity locally in the lungs and improve the protective efficacy of neonatally-administered BCG against M. tuberculosis infection.
Keywords: Neonates, TB vaccination, BCG, recombinant MVA, pulmonary immunity, protection
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb) infection, remains one of the deadliest diseases globally, resulting in an estimated 1.45 million deaths in 2018 alone [1]. The emergence of multi-drug-resistant Mtb strains has rendered the search for effective vaccination strategies even more acute. Bacille-Calmette-Guérin (BCG), the only currently licensed vaccine against TB, fails to provide long-term protection in adults [2], but has shown efficacy in reducing the childhood incidence of pulmonary TB and severe non-pulmonary TB [2, 3] and childhood mortality among both TB-exposed and unexposed children [4]. Since up to 80% of the global population is vaccinated with BCG as neonates or in childhood, any new TB vaccination strategies should take this into account. It should also be noted that the protective efficacy of BCG administered in neonatal life appears to decline over time. While re-vaccination with BCG did not appear to enhance protection against TB in a large-scale clinical study in Brazil [5], partial prevention of Mtb infection, determined indirectly by IFNγ release assay, was recently reported in individuals given BCG in infancy and revaccinated with BCG as adolescents [6].
Several studies in animal models and in humans have shown that priming with BCG followed by boosting with protective antigens, including antigen 85A (Ag85A), which is present in all BCG strains and is highly conserved among circulating Mtb strains, results in increased numbers of antigen-specific CD4+ and CD8+ T cells and enhanced protection against Mtb challenge [7–9]. However, a phase 2 clinical vaccine trial involving a modified vaccinia Ankara strain (MVA) vaccine construct expressing Ag85A given intradermally to infants previously vaccinated with BCG failed to provide protection beyond the limited efficacy of BCG alone [10], despite evidence that the MVA85A vaccine enhanced durable circulating Ag85A-specific T cell responses [11, 12]. Interestingly, MVA85A is in now clinical trials as an aerosol vaccine [13, 14], after earlier indications of its efficacy via this route in animal models of Mtb infection [15, 16].
We have previously shown that DNA or viral vector-based heterologous prime-boost immunization strategies generate sustained immune responses to encoded Mtb antigens in both mucosal tissues and in the spleen, particularly when the booster dose is given mucosally, via the respiratory route [17–20]. Given the renewed interest in respiratory delivery of TB immunogens [13, 14, 21], we have developed a heterologous prime-boost vaccination strategy in a mouse model of acute Mtb infection using neonatal BCG priming via the subcutaneous route followed by intranasal boosting with MVA expressing the secreted M. tuberculosis antigens Ag85A and ESAT-6 (MVA-AE) several weeks later. Our data show that local delivery of MVA-AE can boost both pulmonary and circulating Mtb-specific immunity and improve the protective efficacy of BCG, given shortly after birth, against Mtb infection.
Materials and Methods
Recombinant MVA construction and testing
MVA constructs encoding Ag85A and ESAT-6 were generated as follows using standard molecular cloning techniques for recombinant MVA [22]. A gene fusion encoding Mtb Ag85A (GenBank Acc. No. AY207395.1) upstream of ESAT-6 (GenBank Acc. No. FJ014499.1) was codon-optimized using the Java Codon Optimization Tool (http://www.jcat.de) and manufactured as a synthetic gene by GenScript (Piscataway, New Jersey). The fusion was cloned into the pLW44 transfer vector (provided by L. Wyatt, National Institutes of Health), bringing it under the control of the vaccinia virus (VV) modified H5 early/late promoter and adjacent to the gene encoding GFP regulated by the VV P11 late promoter. Recombinant MVA-Ag85A-ESAT-6 was generated by transfecting pLW44-Ag85A-ESAT-6 into primary chick embryo fibroblast cells infected with MVA (provided by B. Moss, National Institutes of Health). Fluorescent plaques were selected through five rounds of plaque isolation, and seed stock was purified by sucrose gradient centrifugation [22]. The presence of the Mtb gene inserts was confirmed by PCR, and by Western Blot, as described elsewhere [19], using BHK-21 cells (CCL-10, ATCC, Manassas, VA), rabbit anti-mouse anti-Ag85 complex polyclonal antibody, NR-13800 and rabbit anti-mouse anti-ESAT-6 polyclonal antibody, NR-13803 (BEI Resources, Manassas, VA) at 1: 5000 dilution. Recombinant Ag85A, NR-49427 and recombinant ESAT-6, NR-49424 (BEI Resources) were included as positive controls. Control MVA (MVA-c) was generated and tested as above using pLW44 that had not been engineered to encode the Mtb gene fusion.
Mice
All procedures in these studies were conducted under the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Louisiana State University Health Sciences Center (LSUHSC) in accordance with the National Institutes of Health guidelines for the care and use of Laboratory animals. Mice were housed in the AAALAC-accredited LSUHSC Animal Care Facility. Pregnant BALB/c mice were purchased from Charles River (Wilmington, MA) and housed individually. Neonates were weaned and separated according to gender at 3 weeks of age. All Mtb-challenged mice were housed in a Biosafety Level 3 (BSL3) Laboratory operated in accordance with safety precautions recommended by the Centers for Disease Control and Prevention and monitored by the LSUHSC Institutional Biosafety Committee. Invasive procedures were performed under anesthesia with a mixture of KetaThesia (ketamine HCl 100 mg/mL, Butler Animal Health Supply, Dublin, OH) and xylazine (10 mg/mL, Henry Schein, Mandeville, LA) diluted eight-fold in phosphate buffered saline (PBS, Gibco, Invitrogen, Carlsbad, CA).
Immunization
Neonatal BALB/c mice at 4 days of age were immunized subcutaneously with 1 × 106 colony forming units (CFU) of M. bovis BCG, obtained from ATCC (Rockville, MD, catalog #35734). BCG was given in 50μL PBS, while controls were sham immunized with PBS only. Six weeks later, the mice were boosted via the intranasal route with 5 × 107 PFU of MVA vector vaccine resuspended in 20 μL PBS (10 μL given into each nostril). At 12 wk after intranasal MVA boosting, mice were euthanized and spleens and tracheobronchial lymph nodes harvested by gross dissection. Harvested organs were processed into single-cell suspension in complete medium for use in immune assays as described previously [17–19].
Peptides and antigens
Synthetic peptide oligomers representing H-2d -restricted CD4+ T cell epitopes in Ag85A (P1: TFLTSELPGWLQANRHVKPT) [23], and ESAT-6 (eP1: MTEQQWNFAGIEAAASAIQG) [24], or a CD8+ T cell epitope in Ag85A (MPVGGQSSF) [25], were used for restimulation of mononuclear cells in immune assays. All peptides were synthesized by Genscript (Piscataway, NJ). Purified protein derivative (PPD) of Mycobacterium bovis was obtained from the Statens Serum Institut (SSI), Copenhagen, Denmark. Peptides and PPD were used at a final assay concentration of 5 μg/ml.
IFN-γ ELISpot assays
Assays for CD4+ and CD8+ T cell IFN-γ responses were performed as described elsewhere [17, 18]. Data are presented as spot-forming cells (SFCs) per million cells ± SEM.
Intracellular cytokine staining (ICS) assays and tetramer staining
Expression of IL-2, IFNγ and IL-17 by mononuclear cells in response to restimulation by Ag85A and ESAT-6 peptides was assayed by ICS, and data were analyzed using a hierarchical gating strategy, as outlined elsewhere [17, 19]. Fluorochrome antibodies used for staining included CD3-APC-eFluor780 (Invitrogen), CD4-APC, CD8-PE-Cy7, IFNγ-PerCP Cy5.5, IL-17A-PE/Dazzle 594 (all from Biolegend) and IL-2-PE (BD Pharmingen). For tetramer staining, single cell suspensions of 1–2 × 106 cells per well were stained with the fluorescent antibodies CD3e-Pacific Blue and CD8-FITC (BD Pharmingen) and the PE-labeled MVPGGQSSF/H-2d tetramer (MHC Tetramer Production Facility, Baylor College of Medicine, Houston, Texas) for 45 minutes at room temperature. Cells were then washed twice with PBS, fixed, and 200,000 events were acquired on the FACS Canto II (Beckman Coulter).
ELISA
Serum antibody responses to Ag85A and ESAT-6 were measure by ELISA as described elsewhere [19], except that plates were coated with recombinant Ag85A, NR-49427, or recombinant ESAT-6, NR-49424 (BEI Resources). IgG titers were established using a calibration curve generated using known concentrations of mouse IgG.
Aerosolized M. tuberculosis challenge and determination of bacterial load
At 12 weeks after boosting with MVA vectors, mice were challenged with 50–100 CFU of aerosolized M. tuberculosis H3Rv strain (ATCC No. 27294, Rockville, MD) in a BSL-3 laboratory using a GlasCol aerosol infection system (Terre Haute, IN), as described elsewhere [17, 19]. Six weeks later, the mice were euthanized for harvest of lungs and spleens. To determine bacterial loads, harvested organs were homogenized, serially diluted, and plated on Middlebrook 7H10 agar plates containing 10% oleic-acid albumin-dextrose-catalase (OADC – BD, Sparks, MD) and 2 μg/mL of 2-thiophenecarboxylic acid hydrazide (Sigma-Aldrich, St. Louis, MO) to selectively inhibit the growth of residual BCG. The plates were incubated at 37°C for 21 days before colonies were counted.
Statistical analysis
One-way ANOVA with Tukey’s correction for multiple comparisons was used to analyze statistical differences using GraphPad Prism software 9.0. Data are presented as mean values ± SEM. P values ≤ 0.05 were considered statistically significant.
Results
MVA-AE expresses both ESAT-6 and MVA-AE.
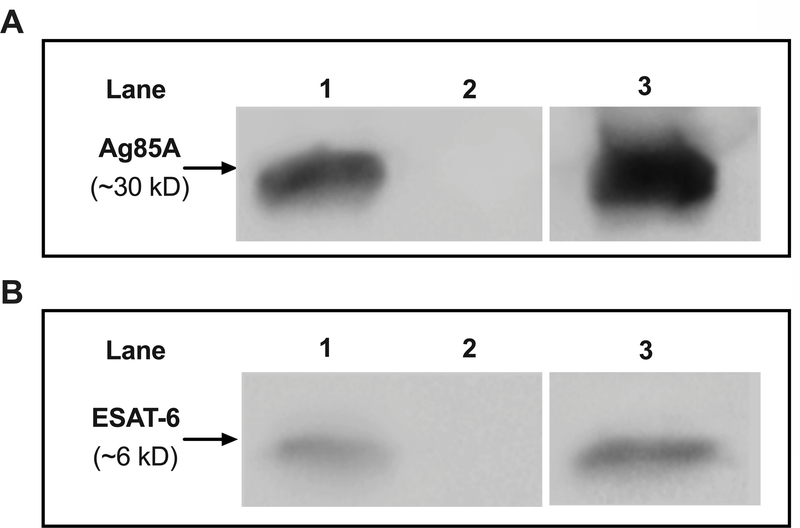
Expression of both Ag85A and ESAT-6 by MVA-AE, but not by MVA-c, was confirmed by Western blot (Fig. 1).
Fig. 1. Expression of Ag85A and ESAT-6 by MVA-AE.
Expression of Mtb proteins encoded in MVA was tested by Western blot as described in Materials and Methods. Ag85A expression (A) is shown as follows: MVA-AE in lane 1; MVA-c in lane 2; recombinant Ag85A in lane 3. ESAT-6 expression (B) is shown as follows: MVA-AE in lane 1; MVA-c in lane 2; recombinant ESAT-6 in lane 3.
Intranasal boosting with MVA enhanced pulmonary and splenic T cell responses and circulating antibody responses in mice primed with BCG as neonates.
Initially, we evaluated the immunogenicity of MVA expressing Ag85A and ESAT-6 proteins of Mtb when given as a pulmonary booster vaccination to mice immunized with BCG in early life. Thus, in each of the experiments described below, mice given BCG subcutaneously at 4 days of age were boosted intranasally with MVA 6 wk later, and 12 wk thereafter, lymphoid cells were harvested from tracheobronchial lymph nodes and spleens for assay.
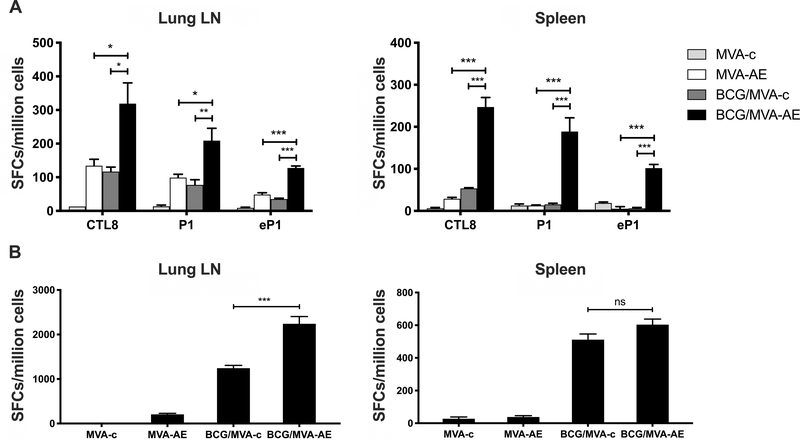
Intranasal MVA-AE boosting of mice primed with BCG as neonates resulted in a significant increase in numbers of Ag85A-specific CD4+ and CD8+ T cell subsets expressing IFN-γ in pulmonary lymphoid tissues and in spleens compared to animals primed with BCG and boosted with control MVA, or given MVA-AE intranasally without neonatal BCG priming (Fig. 2A). MVA-AE delivery also induced local and splenic subsets of CD4+ T cells that were specific for ESAT-6, a protein that is not expressed by BCG. The use of purified protein derivative (PPD) of Mtb for in vitro restimulation confirmed significantly higher pulmonary anti-mycobacterial T cell responses in BCG-primed neonates boosted with MVA-AE (Fig. 2B).
Fig. 2. Mtb-specific pulmonary and splenic T cell responses generated following neonatal BCG prime /MVA boost vaccination.
Neonates at 4 days of age were immunized subcutaneously with BCG or with PBS vehicle only and were boosted IN 6 wk later with MVA-AE or MVA-c, as described in Materials and Methods. Ag85A-specific (P1) and ESAT-6-specific (eP1) CD4+ T cell responses, and Ag85A-specific (CTL8) CD8+ T cells were evaluated in lung LN and spleen by IFN-γ ELISPOT assay at 12 wk after boosting (A), while PPD-specific T cell responses in lung LN and spleen were evaluated by IFN-γ ELISPOT assay at 12 wk after boosting (B), as described in Materials and Methods. Data are expressed as mean numbers of spot-forming cells (SFC) per million cells ± SEM for five mice per group and are representative of two independent experiments. Statistical significance of differences between vaccine groups was determined using one-way ANOVA with Tukey’s correction for multiple comparisons and are denoted as *** (P < 0.001), ** (P < 0.01), or * (P < 0.05).
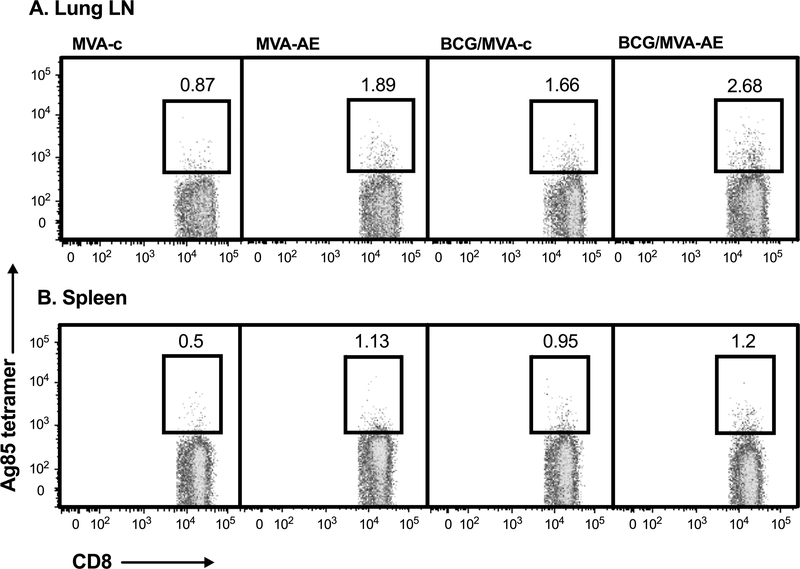
Ag85A tetramers were used to further evaluate specific CD8+ T cell responses that were readily detectable in lung mucosae and spleens at 12 wk after MVA-AE boosting (Fig. 3). Splenic Ag85A-specific CD8+ T cell numbers were similar in all vaccine groups at around 2-fold higher than in mice given only the control vector MVA-c. However, the BCG/ MVA-AE group had pulmonary mucosal Ag85A-specific CD8+ T cell numbers three-fold greater than this control group, with more moderate increases seen in mice given MVA-AE alone or BCG followed by boosting with MVA-control vaccine.
Fig. 3. Tetramer analysis of Mtb-specific pulmonary and splenic T cell responses generated following neonatal BCG prime/intranasal MVA boost vaccination.
Mice were immunized as described in Fig. 2 and Ag85A-specific CD8+ T cell responses in lung LN (A) and spleens (B) at 12 wk after boosting were evaluated by tetramer analysis as described in Materials and Methods. Data shown are frequencies of Ag85A-specific tetramer-positive cells within the total CD3+CD8+ parent cell population within each vaccine group. Representative data from one of two independent experiments are shown.
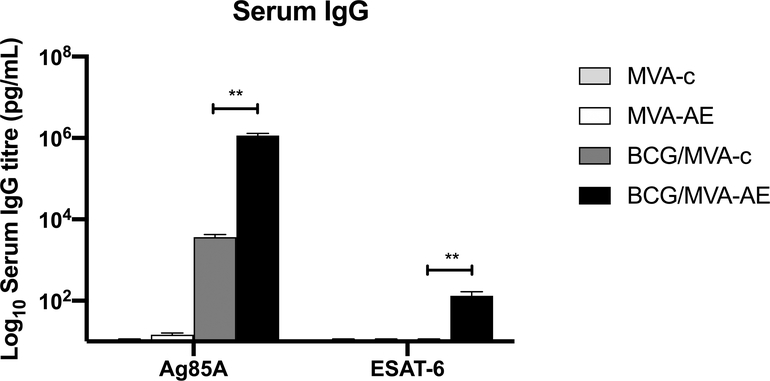
IgG antibody responses against Ag85A were detected in sera at levels over 100-fold greater in mice boosted IN with MVA-AE at 12 wk after priming with BCG as neonates than in mice boosted with MVA-c (Fig. 4). |IgG responses against ESAT-6 were detected at lower levels after the IN MVAE-booster, as expected, since ESAT-6 is not present in the BCG priming vaccine.
Fig. 4. IgG antibody responses generated following neonatal BCG prime /MVA boost vaccination.
Sera from mice immunized as described in Fig. 2 were tested for Mtb-specific IgG responses by ELISA as described in Materials and Methods. Data shown are Ag85A-specific and ESAT-6-specific IgG responses generated from five mice per group and are representative of two independent experiments. Statistical significance of differences between BCG/MVA-AE and BCG/MVA-c vaccine groups was determined using one-way ANOVA with Tukey’s correction for multiple comparisons and are denoted as ** (P < 0.01).
Taken together, these data indicate that intranasal boosting with MVA expressing Ag85A and ESAT-6 enhances both pulmonary and splenic Ag85A-specific T cell responses and circulating antibody responses generated in mice primed neonatally with BCG, with de novo generation of CD4+ T cells responses to ESAT-6 protein expressed only in the booster vaccine.
Intranasal MVA boosting of mice primed neonatally with BCG generates local T cell populations secreting multiple antimicrobial cytokines.
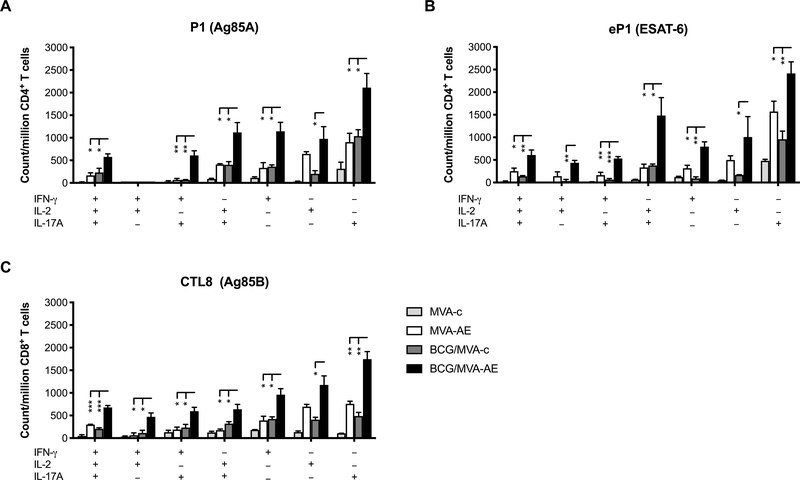
To further evaluate vaccine-induced T cell responses, we used intracellular cytokine staining to characterize the capacity of single cells to express multiple antimicrobial cytokines. It was clear that intranasal MVA-AE boosting of young adult mice that had been primed with BCG as neonates generated significantly higher numbers of Ag85A-specific CD4+ and CD8+ T cells in pulmonary mucosal tissues that were capable of concomitantly expressing IFN-γ, IL-2, and IL-17, compared to mice boosted with control MVA or given only MVA-AE as young adults (Fig. 5). This was also the case for T cell subsets producing IFN-γ/IL-2, IFN-γ/IL-17, or IL-2/IL-17. In addition, gating for CD4+ and CD8+ T cell subsets expressing only one of these factors revealed uniformly greater pulmonary expression of IFN-γ, IL-2 or IL-17 in the BCG/MVA-AE group.
Fig. 5. Mtb-specific production of multiple antimicrobial cytokines by pulmonary T cells generated following neonatal BCG prime/intranasal MVA boost vaccination.
Cells from lung-associated lymph nodes of mice immunized as described in Fig. 2 were analyzed for concomitant secretion of IFNγ, IL-2 and IL-17 by ICS as described in Materials and Methods. Ag85A-specific (A) and ESAT-6-specific (B) CD4+ T cell, and Ag85A-specific CD8+ T cell (C) cytokine secretion profiles for triple- (3+), dual- (2+) and single-cytokine (1+) secretion, and comparisons between vaccination groups, are shown. Data represent mean counts of Mtb peptide-responsive cells per million CD4+ T cells ± SEM from five mice per group and are representative of two independent experiments. Statistical significance of differences between vaccine groups was determined using one-way ANOVA with Tukey’s correction for multiple comparisons and are denoted as *** (P < 0.001), ** (P < 0.01), or * (P < 0.05).
Interestingly, significant increases in ESAT-6-specific pulmonary CD4+ T cells with the same multifunctional characteristics were confirmed in mice primed with BCG and boosted with MVA-AE (Fig. 5). It is possible that the effects of neonatal priming with BCG (lacking ESAT-6) non-specifically enhanced local pulmonary T cell responses to subsequent intranasal immunization with MVA-AE encoding this protein.
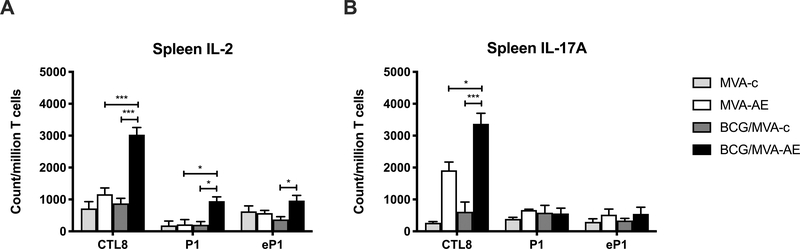
While there was no evidence for multifunctional T cell populations in the spleens of immunized mice, significant numbers of Mtb-specific CD4+ and CD8+ T cells expressing IL-2 and CD8+ T cells expressing IL-17 were seen in the BCG/ MVA-AE vaccine group (Fig. 6).
Fig. 6. Mtb-specific splenic CD4+ and CD8+ T cell IL-2 and IL-17 responses generated following neonatal BCG prime /MVA boost vaccination.
Spleen cells from mice immunized as described in Fig. 2 were analyzed for cytokine secretion by ICS as described in Materials and Methods. Ag85A-specific and ESAT-6-specific CD4+ T cell, and Ag85A-specific CD8+ T cell IL-2 and IL-17A secretion profiles are shown. Data represent mean counts of Mtb peptide-responsive cells per million T cells ± SEM from five mice per group and are representative of two independent experiments. The statistical significance of differences between vaccine groups was determined using one-way ANOVA with Tukey’s correction for multiple comparisons and are denoted as *** (P < 0.001), ** (P < 0.01), or * (P < 0.05).
Intranasal MVA boost enhances the protective efficacy of neonatal BCG immunization against pulmonary challenge with virulent M. tuberculosis.
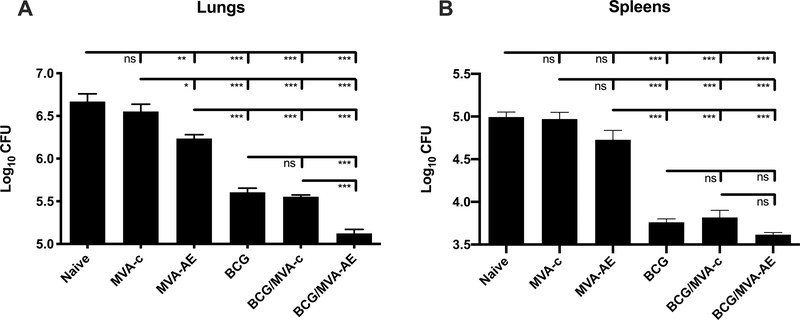
To evaluate the protective efficacy of our vaccine approach, neonates primed subcutaneously with BCG and boosted intranasally with MVA as young adults were challenged with pathogenic Mtb H37Rv in an aerosol chamber at 12 wk after boosting. Bacterial loads in lungs and spleens were determined at 6 wk thereafter (Fig. 7). Neonatal priming with BCG alone reduced bacillary loads in the lungs by 11-fold after aerosol Mtb challenge compared to vehicle control (naïve) mice. Notably, these values were reduced 3-fold further following boosting with MVA-AE (p<0.001), but not with control MVA. While intranasal MVA-AE alone was marginally protective compared to vehicle control mice (p<0.05), as expected neither this construct, nor control MVA, when given alone were effective compared to neonatal BCG/intranasal MVA-AE vaccination. While there was a trend toward reductions in bacterial growth in the spleens of mice given BCG/MVA-AE compared to those given BCG/MVA-control, these differences were not statistically significant.
Fig. 7. Bacillary loads in vaccinated mice following pulmonary challenge with Mtb.
Mice were immunized with BCG and/or MVA, subsequently challenged with aerosolized virulent Mtb H37Rv, and lungs (A) and spleens (B) were examined for bacillary loads at 6 weeks after challenge, as described in Materials and Methods. Data represent mean colony-forming units ± SEM for ten mice per group and are representative of two independent experiments.
Statistical analyses were performed using one-way ANOVA with Tukey’s correction for multiple comparisons. The significance of differences between vaccine groups are denoted as *** (P < 0.001), ** (p < 0.01) or * (P < 0.05).
Discussion
Given increased recent interest in respiratory delivery of TB immunogens [13, 14, 21], we have developed a heterologous prime-boost vaccination strategy in a mouse model of acute Mtb infection using intranasal boosting with MVA vaccine vectors engineered to express the secreted M. tuberculosis antigens Ag85A and ESAT-6 (MVA-AE) in mice primed neonatally with BCG. Our data show that delivery of MVA-AE to the respiratory tract can improve the protective efficacy of BCG given shortly after birth against M. tuberculosis infection, while boosting circulating antibody responses, and Mtb-specific Th1 and Th17-based immunity and CD8+ cytotoxic T cell responses, particularly in the lung mucosae.
Although the neonatal immune system has been characterized as immature, having antigen-presenting cells, T cells and marginal zone B cells in low numbers and/or of reduced functionality, and with a tendency to mount Th2-biased immune responses [26–28], it is clear that neonates are able to mount strong responses to a variety of immunogens including oral polio vaccine [29], hepatitis B vaccine [30], and acellular pertussis vaccine [31]. In addition, while the efficacy of BCG vaccine for protection against Mtb has varied between trials, neonatal BCG vaccination has consistently been associated with a reduced incidence of TB [32] and the generation of strong Th1-type immune responses [33, 34]. Our current data underline the capacity of the neonatal immune system for priming by BCG for strong Th1 and Th17-type anti-mycobacterial immunity in the spleen and lung mucosae and circulating antibody responses, despite its relative immaturity.
A further, widely reported, feature of BCG immunization in early life is the decreased incidence of neonatal and early childhood mortality due to unrelated and antigenically diverse pathogens, termed ‘pathogen agnostic’ protection [4, 30, 35, 36]. In the present study, the construction of MVA-AE, in addition to facilitating vaccination with two immunogenic proteins that are highly expressed by Mtb, also provided the opportunity to assess de novo development of immune responses against ESAT-6. It was notable that neonatal priming with BCG non-specifically enhanced ESAT-6-specific T cell responses to subsequent intranasal immunization with MVA-AE encoding this protein. BCG, which does not express ESAT-6, has been shown to activate non-specific antimicrobial activity of myeloid cells, a phenomenon recently termed ‘trained innate immunity’ [37, 38], and to promote heterologous lymphocyte activation [30, 36, 39], with T cell responses against unrelated bacterial and fungal antigens also enhanced and sustained in adults given BCG [40]. In our study, the marked enhancement of IFN-γ, IL-2 and IL-17 expression by ESAT-6-specific pulmonary CD4+ T cells in mice given BCG/MVA-AE may be reflective of these phenomena and should be beneficial in a vaccine setting, although further studies will be required to evaluate the contribution of such responses to the increased level of protection seen in this vaccine group.
Interleukin-17 appears to play a role in regulating cell-mediated adaptive immunity in TB infection. In humans, mutations in the transcription factor RORγT, crucial for development of IL-17A-producing cells, markedly decreased Mtb-specific IFNγ production by CD4+ CCR6+ αβ T cells and γδ T cells and increased susceptibility to Mtb infection [41]. In mice, adoptive transfer studies showed that Th17 cells could mediate protection against Mtb infection [42], while Th17 cells promote CD4+ T cell recruitment to pulmonary sites of infection, favor granuloma formation, and accelerate pathogen clearance in BCG-immunized mice challenged with Mtb [43, 44], and this process was enhanced by boosting with mycobacterial proteins [45]. IL-17 expression is apparently regulated by IL-23 in driving Th1 cell responses in this system [43, 46], while Mtb infection has been shown to dysregulate STAT3 signaling, selectively impairing production of IL-23 required for the differentiation of IL-23 receptor-expressing Th17 cells [43]. In the present study, it was notable that intranasal boosting of mice given BCG as neonates with MVA-AE significantly enhanced numbers of Mtb-specific CD4+ and CD8+ T cells producing IL-17 in the lung mucosae, including populations that concomitantly expressed combinations of IL-17, IL-2 and/or IFNγ, while markedly reducing Mtb titers recovered from pulmonary tissues after challenge. While CD8+ T cells expressing IL-17 were also numerous in the spleens of these mice, there was no evidence for CD4+ T cells expressing IL-17 or multifunctional CD4+ populations in the spleen, and no significant reduction in splenic Mtb growth, albeit occurring at much lower levels compared to the lungs.
Prime-boost strategies have been widely used in vaccine development, including for TB [21, 47]. While no clear correlates of protection have yet been defined for BCG, there is considerable interest in the development of effective booster vaccines for those previously vaccinated with BCG, given its widespread administration to neonates and children in regions where TB is endemic [4]. Several studies in small animal models of Mtb infection and in humans have shown that priming with BCG followed by boosting with recombinant viral vaccine vectors expressing TB proteins, or with mycobacterial proteins, generated strong CD4+ and CD8+ T cell immunity and enhanced protection against Mtb challenge [7–9, 15, 16, 45]. Data from recent studies in non-human primates have been mixed in this respect. Boosting BCG-primed animals with protein-based, or adenovirus or cytomegalovirus vector-based, Mtb immunogens failed to enhance BCG-mediated protection against Mtb challenge [48, 49], while a recent macaque study clearly demonstrated enhanced protective efficacy following intravenous BCG administration. These animals had markedly elevated levels of pulmonary T cell responses (mostly of the Th1-type but also some contribution from Th17 cells), including evidence for localized tissue-resident memory T cells (TRM), and higher antibody levels in bronchial fluids and plasma, compared to animals vaccinated intradermally or via aerosol [50]. In clinical studies, re-vaccination with BCG failed to enhance protection against TB in a large-scale study in Brazil [5], although a reduced rate of QuantiFERON-TB-Gold assay conversion was seen in subjects in a high-transmission South African setting given BCG in infancy and revaccinated as adolescents with either BCG or H4:IC31, an immunogenic subunit vaccine [6]. An earlier phase 2 clinical vaccine trial involving recombinant MVA vaccine expressing Ag85A given intradermally to infants previously vaccinated with BCG failed to demonstrate protection beyond that mediated by BCG alone [10], despite enhancement of circulating Ag85A-specific T cell responses that were detected for several years [11, 12], while MVA85A is now in clinical trials as an aerosol vaccine [13, 14].
In summary, we have shown that delivery of recombinant MVA vaccine encoding Ag85A and ESAT-6 to the respiratory tract significantly improves the protective efficacy of neonatally-administered BCG against Mtb infection, while boosting BCG-induced Th1 and Th17-based immunity particularly in lung mucosae, and circulating antibody responses. Studies are underway in this system to further characterize both innate and adaptive immune responses, particularly in terms of local pulmonary innate lymphoid cell responses, lung tissue-resident memory T cell (TRM) responses, and local pulmonary antibody responses, and also to assess the protective efficacy of mucosal MVA boosting with other Mtb antigens in this system.
Highlights.
A recombinant poxvirus vaccine encoding Ag85A and ESAT-6 of M. tuberculosis (MVA-AE) was generated.
Respiratory boosting with MVA-AE enhanced protection against TB in mice given BCG as neonates.
Elevated Th1 and Th17 immunity was seen after MVA-AE boosting in lungs of mice given BCG as neonates.
MVA-AE boosting also generated lung CD4+ T cells against ESAT-6, not seen in mice given only BCG.
Acknowledgments
We thank Olga Nichols, Elizabeth Porretta and Robert Kutner for valuable technical assistance, and Drs. L. Wyatt and B. Moss (National Institutes of Health) for providing pLW44 and MVA. We also acknowledge the MHC Tetramer Production Facility, Baylor College of Medicine, Houston, Texas for generating PE-labeled Ag85A tetramers. The following reagents were obtained through BEI Resources, NIAID, NIH: Polyclonal Anti-Mycobacterium tuberculosis Antigen 85 Complex (FbpA/FbpB/FbpC; Genes Rv3804c, Rv1886c, Rv0129c) (antiserum, Rabbit), NR-13800; Polyclonal Anti-Mycobacterium tuberculosis ESAT6 (Gene Rv3875) (antiserum, Rabbit), NR-13803; Ag85A, Recombinant Protein Reference Standard, NR-49427; ESAT-6, Recombinant Protein Reference Standard, NR-49424.
Funding: This work was supported by NIH grants R01 AI058810 (AJR) and 5PO1 HL076100 (AJR), and by the Louisiana Vaccine Center funded by the Louisiana Board of Regents (PKSFI-PRS-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO,Global Tuberculosis Report. 2019, World Health Organization, Geneva. [Google Scholar]
- 2.Colditz GA, et al. , Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Jama, 1994. 271(9): p. 698–702. [PubMed] [Google Scholar]
- 3.Trunz BB, Fine P, and Dye C, Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet, 2006. 367(9517): p. 1173–80. [DOI] [PubMed] [Google Scholar]
- 4.Thysen SM, et al. , Neonatal BCG vaccination and child survival in TB-exposed and TB-unexposed children: a prospective cohort study. BMJ Open, 2020. 10(2): p. e035595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues LC, et al. , Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet, 2005. 366(9493): p. 1290–5. [DOI] [PubMed] [Google Scholar]
- 6.Nemes E, et al. , Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med, 2018. 379(2): p. 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathan AA, et al. , Boosting BCG with recombinant modified vaccinia ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One, 2007. 2(10): p. e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McShane H, et al. , Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med, 2004. 10(11): p. 1240–4. [DOI] [PubMed] [Google Scholar]
- 9.Williams A, et al. , Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun, 2005. 73(6): p. 3814–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tameris MD, et al. , Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. The Lancet, 2013. 381(9871): p. 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scriba TJ, et al. , Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis, 2011. 203(12): p. 1832–43. [DOI] [PubMed] [Google Scholar]
- 12.Tameris M, et al. , The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS One, 2014. 9(2): p. e87340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satti I, et al. , Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis, 2014. 14(10): p. 939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manjaly Thomas ZR, et al. , Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: A phase I randomised controlled trial. PLoS Med, 2019. 16(4): p. e1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goonetilleke NP, et al. , Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol, 2003. 171(3): p. 1602–9. [DOI] [PubMed] [Google Scholar]
- 16.Stylianou E, et al. , Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine, 2015. 33(48): p. 6800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalmia N, et al. , DNA-Launched Alphavirus Replicons Encoding a Fusion of Mycobacterial Antigens Acr and Ag85B Are Immunogenic and Protective in a Murine Model of TB Infection. PLoS One, 2015. 10(8): p. e0136635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auten MW, et al. , CD40 Ligand enhances immunogenicity of vector-based vaccines in immunocompetent and CD4+ T cell deficient individuals. Vaccine, 2012. 30(17): p. 2768–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rady HF, et al. , Flagellin Encoded in Gene-Based Vector Vaccines Is a Route-Dependent Immune Adjuvant. PLoS One, 2016. 11(2): p. e0148701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai G, et al. , Gene-based neonatal immune priming potentiates a mucosal adenoviral vaccine encoding mycobacterial Ag85B. Vaccine, 2016. 34(50): p. 6267–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Counoupas C, Triccas JA, and Britton WJ, Deciphering protective immunity against tuberculosis: implications for vaccine development. Expert Rev Vaccines, 2019. 18(4): p. 353–364. [DOI] [PubMed] [Google Scholar]
- 22.Wyatt LS, Earl PL, and Moss B, Generation of Recombinant Vaccinia Viruses. Curr Protoc Protein Sci, 2017. 89: p. 5.13.1–5.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Souza S, et al. , Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect Immun, 2003. 71(1): p. 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt L, et al. , Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol, 1996. 157(8): p. 3527–33. [PubMed] [Google Scholar]
- 25.Radosevic K, et al. , Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun, 2007. 75(8): p. 4105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dakic A, et al. , Development of the dendritic cell system during mouse ontogeny. J Immunol, 2004. 172(2): p. 1018–27. [DOI] [PubMed] [Google Scholar]
- 27.Dadaglio G, et al. , Efficient in vivo priming of specific cytotoxic T cell responses by neonatal dendritic cells. J Immunol, 2002. 168(5): p. 2219–24. [DOI] [PubMed] [Google Scholar]
- 28.Chen N and Field EH, Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation, 1995. 59(7): p. 933–41. [DOI] [PubMed] [Google Scholar]
- 29.Mateen FJ, Shinohara RT, and Sutter RW, Oral and inactivated poliovirus vaccines in the newborn: a review. Vaccine, 2013. 31(21): p. 2517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ota MO, et al. , Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine, 2004. 22(3–4): p. 511–9. [DOI] [PubMed] [Google Scholar]
- 31.Wood N, et al. , Immunogenicity and Safety of Monovalent Acellular Pertussis Vaccine at Birth: A Randomized Clinical Trial. JAMA Pediatr, 2018. 172(11): p. 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangtani P, et al. , Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis, 2014. 58(4): p. 470–80. [DOI] [PubMed] [Google Scholar]
- 33.Vekemans J, et al. , Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol, 2001. 31(5): p. 1531–5. [DOI] [PubMed] [Google Scholar]
- 34.Hussey GD, et al. , Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology, 2002. 105(3): p. 314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lund N, et al. , The Effect of Oral Polio Vaccine at Birth on Infant Mortality: A Randomized Trial. Clin Infect Dis, 2015. 61(10): p. 1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodridge HS, et al. , Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol, 2016. 16(6): p. 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Netea MG, Quintin J, and van der Meer JW, Trained immunity: a memory for innate host defense. Cell Host Microbe, 2011. 9(5): p. 355–61. [DOI] [PubMed] [Google Scholar]
- 38.Joosten SA, et al. , Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest, 2018. 128(5): p. 1837–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brook B, et al. , BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med, 2020. 12(542). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinnijenhuis J, et al. , Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun, 2014. 6(2): p. 152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada S, et al. , Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science, 2015. 349(6248): p. 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wozniak TM, et al. , Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun, 2010. 78(10): p. 4187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khader SA, et al. , IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol, 2007. 8(4): p. 369–77. [DOI] [PubMed] [Google Scholar]
- 44.Umemura M, et al. , IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol, 2007. 178(6): p. 3786–96. [DOI] [PubMed] [Google Scholar]
- 45.Choi HG, et al. , Antigen-Specific IFN-γ/IL -17-Co-Producing CD4(+) T-Cells Are the Determinants for Protective Efficacy of Tuberculosis Subunit Vaccine. Vaccines (Basel), 2020. 8(2): p. 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopal R, et al. , IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol, 2012. 42(2): p. 364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalmia N and Ramsay AJ, Prime-boost approaches to tuberculosis vaccine development. Expert Rev Vaccines, 2012. 11(10): p. 1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen SG, et al. , Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med, 2018. 24(2): p. 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darrah PA, et al. , Boosting BCG with proteins or rAd5 does not enhance protection against tuberculosis in rhesus macaques. NPJ Vaccines, 2019. 4: p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darrah PA, et al. , Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature, 2020. 577(7788): p. 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]