Abstract
Temporally unpredictable stimuli influence behavior across species, as previously demonstrated for sequences of simple threats and rewards with fixed or variable onset. Neuroimaging studies have identified a specific frontolimbic circuit that may become engaged during the anticipation of temporally unpredictable threat (U-threat). However, the neural mechanisms underlying processing of temporally unpredictable reward (U-reward) are incompletely understood. It is also unclear whether these processes are mediated by overlapping or distinct neural systems. These knowledge gaps are noteworthy given that disruptions within these neural systems may lead to maladaptive response to uncertainty. Here, using functional magnetic resonance imaging data from a sample of 159 young adults, we showed that anticipation of both U-threat and U-reward elicited activation in the right anterior insula, right ventral anterior nucleus of the thalamus and right inferior frontal gyrus. U-threat also activated the right posterior insula and dorsal anterior cingulate cortex, relative to U-reward. In contrast, U-reward elicited activation in the right fusiform and left middle occipital gyrus, relative to U-threat. Although there is some overlap in the neural circuitry underlying anticipation of U-threat and U-reward, these processes appear to be largely mediated by distinct circuits. Future studies are needed to corroborate and extend these preliminary findings.
Keywords: unpredictable threat, unpredictable reward, NPU, fMRI
Introduction
The adaptive ability to predict and effectively prepare for the possible (that is uncertain) future outcomes, both positive and negative in valence, is essential for the well-being and self-preservation of organisms. Empirical research to date has shown that under uncertain conditions, both animals and humans exhibit sustained vigilance and apprehensive responding, particularly when there is a potential for an aversive outcome (Prokasy, 1956; Cantor and LoLordo, 1970; Seligman and Meyer, 1970; Imada and Nageishi, 1982; Herry et al., 2007; Bar-Anan et al., 2009; Grupe and Nitschke, 2011; Davies and Craske, 2015; Dieterich et al., 2016; Ran et al., 2016; Anselme and Güntürkün, 2018). These anticipatory responses to uncertain events and situations with valenced outcomes (threatening or rewarding) are notably aberrant in clinical populations with anxiety, depressive, obsessive-compulsive and other disorders (Greco and Roger, 2003; Dugas et al., 2004; Gentes and Ruscio, 2011; Olino et al., 2011; Carleton et al., 2012; Baskin-Sommers and Foti, 2015; Carleton, 2016; Nelson et al., 2016; Shihata et al., 2016). Increasing our understanding of the neural mechanisms underlying response to uncertainty may therefore shed light on pathophysiology of multiple psychopathologies.
Most of the research regarding uncertainty has been focused on examining reactivity to unpredictable threats, which come in many forms including uncertain timing, intensity, frequency and/or duration (Shankman et al., 2011; Schmitz and Grillon, 2012; Bradford et al., 2013; Daldrup et al., 2015; Davies and Craske, 2015; Quelhas Martins et al., 2015; Bennett et al., 2018). In particular, uncertainty associated with not knowing when an aversive event may occur (temporal unpredictability) is a potent elicitor of sustained anxiety and hypervigilance across species (Sudha and Pradhan, 1993; Grillon et al., 2004, 2006; Herry et al., 2007; Seidenbecher et al., 2016).
One of the most common paradigms used to measure response to temporally unpredictable threat in humans is the NPU task, which compares the anticipatory responses to three within-subject conditions—no threat (N; participants are safe from threat), predictable threat (P; threat is signaled by a predictable warning cue) and unpredictable threat (U; threat is unsignaled; Schmitz and Grillon, 2012; Grupe and Nitschke, 2013; Ferry and Nelson, 2020). NPU studies of healthy individuals have found that unpredictable negative events (e.g. shocks, aversive tones or images) elicit stronger psychophysiological responses than predictable ones, as evidenced by an increased startle eyeblink potentiation, a somatic marker of aversive responding (Grillon et al., 2004, 2006; Schmitz et al., 2011; Bach et al., 2015; Nelson et al., 2015; Schroijen et al., 2016).
In order to elucidate the neural circuitry underlying heightened reactivity to unpredictable threat, studies have begun to employ variants of the NPU task during functional magnetic resonance imaging (fMRI). To date, studies have identified a specific frontolimbic circuit that may become engaged during the processing of aversive stimuli (Bach and Dolan, 2012; Grupe and Nitschke, 2013; Fox and Shackman, 2019; Hur et al., 2020). This circuit is comprised of regions such as the amygdala, insula, bed nucleus of the stria terminalis, orbitofrontal cortex (OFC), anterior cingulate cortex (ACC) and the dorsolateral, ventrolateral and ventromedial prefrontal cortices (Herry et al., 2007; Grupe and Nitschke, 2013; Shankman et al., 2014; Tovote et al., 2015; Goode et al., 2019). Of these regions, insula appears to be particularly involved in responding to uncertainty, with the anterior agranular region of the insular cortex (anterior insula, AIC) playing a critical role in the anticipation of unpredictable aversiveness (Clark et al., 2008; Craig, 2009; Khalsa et al., 2009; Sarinopoulos et al., 2010; Gu et al., 2013; Shankman et al., 2014). Evidence indicates that the AIC integrates information about the internal and external states to produce interoceptive awareness and generate anticipatory emotional responses to future events (Craig, 2011).
Interestingly, the AIC has recently also been implicated in processing of uncertain or unpredictable rewards. For example, using single-unit recordings, Mizuhiki et al. (2012) examined neural activity during stochastic reward delivery and found that dynamics of neuronal population activity in the AIC was modulated as a function of reward outcome uncertainty (i.e. whether a trial was rewarded or not). A separate study done in rodents found that magnitude and temporal dynamics of neuronal activity in the AIC encoded reward probability (i.e. the likelihood that a trial would be rewarded; Jo and Jung, 2016). In addition, a recent human fMRI study from our lab found increased activation in the bilateral AIC during the anticipation of unpredictable monetary rewards of varying magnitudes (Gorka et al., 2016). Therefore, the activity in the AIC appears to be modulated by unpredictable rewards as well.
The AIC is also preferentially interconnected with the OFC, ACC and the ventral striatum (Mesulam and Mufson, 1982; Augustine, 1996; Chikama et al., 1997), brain regions implicated in processing of information about uncertain outcomes (Critchley et al., 2001; O’Doherty et al., 2001; Dreher et al., 2006; Yu et al., 2011; Li et al., 2016; Monosov, 2017; O’Neill and Schultz, 2018). Of these regions, the ventral striatum has emerged as a key node involved in reward anticipation (Berridge and Robinson, 1998, 2003; Diekhof et al., 2012; Bartra et al., 2013; Oldham et al., 2018), particularly when the rewards occurred unexpectedly or were uncertain (Mirenowicz and Schultz, 1994; Apicella et al., 1997; Schultz, 1998). Related to this, accumulating evidence suggests that activity of the striatal dopamine system reflects the anticipated reward magnitude, probability or delay within various behavioral contexts ranging from classic Pavlovian conditioning paradigms to widely used instrumental paradigms such as the monetary incentive delay (MID) task (Knutson et al., 2000, 2001; Satoh et al., 2003; Morris et al., 2004; Tobler et al., 2005; Dreher et al., 2006; Preuschoff et al., 2006; Kobayashi and Schultz, 2008; Hart et al., 2015).
It is worth noting, however, that the experimental methodologies used to test neural response to reward uncertainty in humans, such as the MID task, often conflated explicit manipulations of multiple parameters of uncertain reward (e.g. probability and magnitude) with the implicit temporal unpredictability of the reward (i.e. when the reward may be delivered). Such approach made it difficult to examine the neural correlates of temporally unpredictable reward well separated from the coding of other reward parameters. This gap in the literature is noteworthy, given that temporal unpredictability influences behavior not only during the anticipation of aversive stimuli (as previously described), but also during the anticipation of appetitive stimuli (Mirenowicz and Schultz, 1994; Galtress et al., 2012; Bermudez and Schultz, 2014). In this regard, it is critical to examine whether these two processes are mediated by overlapping or distinct neural systems, given that disruptions within these systems may lead to maladaptive anticipatory response to uncertainty (as a broadly defined construct), which is central to many clinical disorders.
To our knowledge, no study has run a direct, within-subject comparison of the neural circuitry underlying response to temporally unpredictable threat and reward. In addition, the paradigmatic differences between the tasks used to examine neural response to unpredictable threat (e.g. NPU) and reward (e.g. MID) represent a major confound when comparing the neural systems underlying these respective processes (e.g. blocked vs event-related design; passive vs button press). In order to fully assess the integrity of neural systems that signal temporally unpredictable threats and rewards, it is critical to employ experimental paradigms with little or no learning component, decision-making or active participation needed. The goal of the present study was to therefore examine the shared and unique neural correlates of temporally unpredictable threat and reward processing in a sample of young adults using fMRI variants of the well-validated NPU paradigm, in which temporal unpredictability of threat (i.e. mild electric shock) and reward (i.e. monetary incentive) was manipulated. The two tasks were specifically designed for the purposes of this study and were therefore analogous.
Methods
Participants
Participants (total N = 159) were taken from two samples recruited from the community as part of larger investigations on abnormal reactivity to uncertain stimuli (threat and reward) in relation to psychopathology. Participants were recruited via advertisements posted in the Chicago community, local psychiatric clinics and nearby college campuses. Demographic characteristics of the individual and pooled samples are listed in Table 1. General exclusion criteria included any major medical or neurological illness, psychosis, active suicidal ideation, deafness, traumatic brain injury, psychotropic medication use within the past four months, contraindications for fMRI, pregnancy, positive urine drug screen for illicit substances (including tetrahydrocannabinol, cocaine, amphetamine, morphine, phencyclidine, barbiturates, benzodiazepines, MDMA, oxycodone and buprenorphine) or breathalyzer test. Psychopathology was assessed via the Structured Clinical Interview for DSM-5 Disorders (First et al., 2015), in person, by trained assessors and supervised by a clinical psychologist.
Table 1.
Participant demographics and baseline clinical characteristics
| Sample 1 (N = 88) | Sample 2 (N = 71) | Pooled (N = 159) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 18.5 (0.7)a | 24.2 (2.9)b | 21.0 (3.5) |
| Sex (% female) | 67.0%a | 49.3%b | 59.1% |
| Ethnicity (% Hispanic) | 35.2%a | 31.0%a | 33.3% |
| Education level (years) | 12.9 (1.3)a | 16.1 (1.9)b | 14.3 (2.3) |
| Race | |||
| White | 60.2%a | 57.7%a | 59.1% |
| Black | 8.0%a | 4.2%a | 6.3% |
| Asian | 8.0%a | 14.1%a | 10.7% |
| American Indian or Alaskan Native | 4.5%a | 1.4%a | 3.1% |
| Biracial, other or unknown | 19.4%a | 22.5%a | 20.8% |
| SCID diagnoses | |||
| Current major depressive disorder | 5.7%a | 6.9%a | 6.3% |
| Current generalized anxiety disorder | 5.7%a | 9.7%a | 7.5% |
| Current social anxiety disorder | 12.6%a | 1.4%b | 7.5% |
| Current panic disorder | 2.3%a | 0.0%b | 1.3% |
| Current specific phobia | 1.1%a | 1.4%a | 1.3% |
| Current post-traumatic stress disorder | 3.4%a | 1.4%a | 2.5% |
| Current eating disorder | 0.0%a | 2.6%b | 1.3% |
| Current alcohol use disorder | 0.0%a | 39.1%b | 19.5% |
| Lifetime major depressive disorder | 34.5%a | 26.4%b | 30.8% |
| Lifetime generalized anxiety disorder | 9.2%a | 15.3%a | 11.9% |
| Lifetime social anxiety disorder | 16.1%a | 2.8%b | 10.1% |
| Lifetime panic disorder | 5.7%a | 6.9%a | 6.3% |
| Lifetime-specific phobia | 2.3%a | 1.4%a | 1.9% |
| Lifetime post-traumatic stress disorder | 10.3%a | 2.8%b | 6.9% |
| Lifetime eating disorder | 0.0%a | 5.6%b | 2.5% |
| Lifetime alcohol use disorder | 0.0%a | 56.6%b | 28.3% |
| Substance use | |||
| No. of drinks per week in the past month | 0.7 (2.0)a | 8.1 (6.7)b | 4.1 (6.0) |
| No. of binges in the past month | 0.1 (0.3)a | 2.1 (2.7)b | 1.0 (2.1) |
| Daily cigarette smoker (yes/no) | 0.0%a | 0.0%a | 0.0% |
| No. of cigarettes smoked in the past month | 1.3 (5.6)a | 0.2 (1.7)b | 0.8 (4.3) |
| Used cannabis in the past month (yes/no) | 20.7%a | 11.1%b | 16.4% |
| No. of times used cannabis in the past month | 0.7 (3.4)a | 0.2 (0.6)b | 0.5 (2.5) |
| Used other illicit drugsa in the past month (yes/no) | 2.7%a | 4.6%b | 3.8% |
| No. of times used other illicit drugs in the past month | 0.07 (0.08)a | 0.04 (0.27)b | 0.03 (0.02) |
| Clinical variables | |||
| AUDIT | 3.0 (3.1)a | 7.0 (4.7)b | 4.8 (4.3) |
| IDAS-II General Depression | 41.0 (11.9)a | 35.0 (11.0)b | 38.3 (11.8) |
| IDAS-II Anxiety | 8.6 (2.6)a | 6.9 (1.5)b | 7.8 (2.3) |
Note: Means (and s.d.) or percentages with different subscripts across rows were significantly different in pairwise comparisons (P < 0.05, chi-square test for categorical variables and Tukey’s honestly significant test for continuous variables).
AUDIT, Alcohol Use Disorders Identification Test; IDAS-II, Inventory for Depression and Anxiety Symptoms-II; SCID, Structured Clinical Interview for DSM-5.
Other illicit drugs refers to any illicit drug other than cannabis (e.g. cocaine, heroin and nonmedical prescription medications).
Sample 1.
Due to the aims of the larger study (not yet published), young adults were required to have had minimal alcohol exposure (i.e. self-reported consuming >1 but <100 standard alcoholic drinks in their lifetime), but be at risk for the onset of alcohol abuse by virtue of affiliating with risky peers and having access to alcohol. Participants were also required to be between the ages of 17 and 19. Study-specific exclusion criteria included lifetime history of alcohol or substance use disorder (SUD). A total of 109 individuals met inclusionary criteria; however, 18 were excluded due to missing/poor-quality fMRI data and 3 participants were excluded due to difficulty maintaining wakefulness, thus yielding a final sample of 88 individuals.
Sample 2.
As part of the aims of the larger study (Gorka et al., 2019), participants were required to either have no personal or family history of alcohol use disorder (AUD) or meet the criteria for AUD within the past two years. Both groups were otherwise matched on the rates of other internalizing disorders. Participants were also required to be between 21 and 30 years old. Study-specific exclusion criteria included lifetime moderate or severe SUD (other than alcohol and nicotine). A total of 82 individuals met inclusionary criteria; however, 11 were excluded due to missing/poor quality fMRI data, thus yielding a final sample of 71 individuals.
Of note, poor fMRI data quality was defined in terms of excessive motion (i.e. >3 mm displacement in any direction) and/or presence of scanning artifacts.
Both studies took place at the University of Illinois at Chicago and were approved by the University Institutional Review Board. All participants provided written informed consent after review of the respective study protocols and were monetarily compensated for their time.
Study procedure and fMRI tasks
Participants completed an initial screening and orientation visit during which they provided written informed consent and completed a clinical interview and battery of self-report questionnaires. During a separate fMRI visit, participants completed the following two complementary fMRI tasks designed to assess separate anticipatory processes (i.e. anticipating threat and reward). The tasks were presented in a counterbalanced order. For both studies, individuals were instructed to abstain from drugs and alcohol at least 24 h prior to the lab assessments, which was verified via breath alcohol and urine screens.
NPU threat task.
The fMRI threat task and laboratory procedures described here have been used previously by our group (Lieberman et al., 2017; Gorka et al., 2019). Briefly, each participant had two shock electrodes placed on their left foot in order to minimize movement and potential scan artifacts. Next, a shock work-up procedure was completed to determine the level of shock intensity that participants described as ‘highly annoying but not painful’ (between 1 and 5 mA). Ideographic shock levels were used to ensure equality in perceived shock aversiveness (Rollman and Harris, 1987). The shock stimuli lasted 400 ms and were delivered using a Biopac MP150 with an STM100C module (Biopac Systems, Inc., Goleta, CA) connected to a 200 V maximum stimulus isolation unit (STMISOC, Biopac System, Inc., Goleta, CA). Task stimuli were administered using Presentation software package (Neurobehavioral Systems, Inc., Albany, CA).
To examine the neural correlates of temporally unpredictable threat, we used a modified version of the original NPU-threat task developed by Grillon and colleagues (Figure 1A; Schmitz and Grillon, 2012). The task included three, within-subject conditions: no shock (N), predictable shock (P) and unpredictable shock (U). During each condition, participants viewed a numeric countdown that ranged between 3 and 8 s, jittered (M = 5 s). Text at the bottom of the computer monitor informed participants of the current condition. During N trials, no shocks were delivered and the text read ‘No Shock’. During P trials, participants received a shock only when the countdown reached ‘1’ and the text read ‘Shock at 1’. During U trials, participants received a shock at random, regardless of the number on the screen and the text read ‘Shock at Anytime’. Following each countdown, individuals saw a fixation cross for 5–7 s, jittered (M = 6 s). N, P and U countdowns were presented in blocks of 6, and each condition/block was administered in a randomized order (counterbalanced) 6 times over the course of two runs. Participants received 10 electric shocks during P and 10 electric shocks during U, during each run. The rate of ‘Shock at 1’ during the P condition was 60%, consistent with the NPU version used by Grillon and colleagues (Schmitz and Grillon, 2012).
Fig. 1.
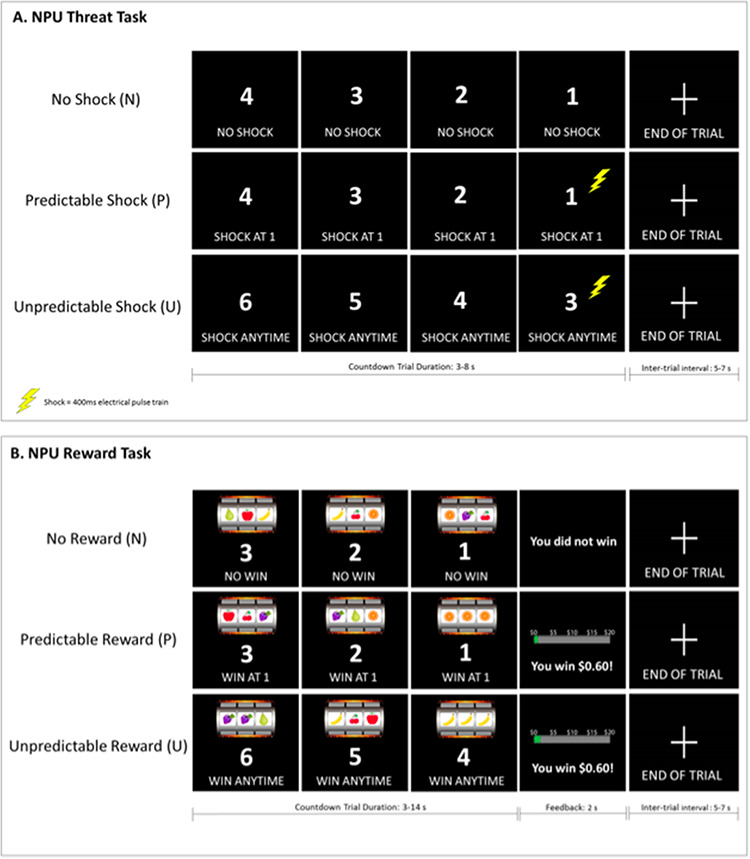
Illustrations of the NPU (A) threat and (B) reward tasks administered during scan.
NPU reward task.
In order to examine the neural correlates of temporally unpredictable reward, our lab developed an analogous NPU reward task similar in its design and timing to the NPU threat task. Before the task, participants were told that they would be playing a slot machine game, similar to the one they would see at a casino, and that they had the chance to win up to $20 in cash. The task itself was a computerized, passive slot machine paradigm with three within-subject conditions: no reward (N), predictable reward (P) and unpredictable reward (U). The reward was a monetary prize of $0.60. During each condition, participants viewed a numeric countdown that ranged between 3 and 14 s, jittered (M = 8 s), and three reels of fruit, which ‘spun’ simultaneously for the duration of the countdown and then ‘landed’ on a result at the same time (Figure 1B). Text at the bottom of the computer monitor informed participants of the current condition. During N trials, no reward was delivered (i.e. the reels landed on three different fruits) and the text read ‘No Win’. During P trials, participants received a reward only when the countdown reached ‘1’ (i.e. the reels landed on three identical fruits) and the text read ‘Win at 1’. During U trials, participants received a reward at random (i.e. when the reels landed on three identical fruits), regardless of the number on the screen and the text read ‘Win at Anytime’. A feedback screen notified participants whether they won money or not during that trial and indicated their cumulative total winnings at that point. Following the feedback screen, individuals saw a fixation cross for 5–7 s, jittered (M = 6 s). N, P and U countdowns were presented in blocks of 4, and each condition/block was administered in a randomized order (counterbalanced) 6 times over the course of two runs. Participants were told before the game that the reward probabilities were random. Unbeknownst to the participants, the game was rigged to ensure that, consistent with the NPU threat task, 60% of the P and U trials resulted in a win, per each run.
fMRI data collection and processing
fMRI was performed on a 3.0 Tesla GE MR 750 scanner (General Electric Healthcare; Waukesha, WI) using an 8-channel phased-array radio frequency head coil. A standard T2-sensitive gradient-echo echoplanar imaging sequence was used (2 s repetition time (TR); 22.2 ms echo time (TE); 90° flip; 64 × 64 matrix; 22 cm FOV; 44 axial slices; 3.44 × 3.44 × 3.0 mm voxels; 308 volumes per run). Structural scans were obtained with a 3D BRAVO pulse sequences with the following parameters: flip angle 13°, inversion time 450 ms, field of view 22 × 22 cm, matrix size 256 × 256, slice thickness 1 mm and 182 axial slices of the whole brain.
Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuroscience, London, UK) was used to perform conventional preprocessing steps. Images were spatially realigned to correct for head motion, slice-time corrected (44 slices, TR = 2, TA = 2, slice order: ascending interleaved, reference slice 21), spatially normalized to Montreal Neurological Institute (MNI) space using the participants’ T1 structural image (default settings), resampled to 2 mm3 voxels and smoothed with an 8 mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128 s high-pass filter. Condition effects for U, P and N anticipation were separately estimated at each voxel for each subject. For each condition, only the countdowns prior to the shock (or reward), or prior to trial termination in instances where there was no shock (or no reward), were modeled. Importantly, number of data points (i.e. TRs/repetition times) was the same across the three conditions (N, P and U). Movement parameters obtained during realignment were included in the model as regressors-of-no-interest to account for motion-related effects on blood-oxygen-level-dependent (BOLD). In line with our study aims, we created individual contrast maps for unpredictable threat (U-threat) > No-threat and unpredictable reward (U-reward) > No-reward for each person during first-level analysis.
These contrast maps were then first entered into a second-level one-way repeated measure analysis of variance (ANOVA) conducted using flexible factorial design in SPM, in order to examine the main effects of U-threat and U-reward across all participants (i.e. both samples). Next, to identify areas where significant activity was elicited by both U-threat and U-reward stimuli, a conjunction analysis (Nichols et al., 2005) was performed within the framework of SPM using the two family-wise error (FWE) thresholded statistical maps identified in the above analysis that showed significant main effects of U-threat and U-reward (i.e. [U-threat > No-threat] AND [U-reward > No-reward]) in order to create an intersection map that revealed voxels with significant common activation. Finally, to identify areas where activity in the two tasks differed significantly, we performed a paired t-test comparing the main effects of U-threat and U-reward for each participant (i.e. [U-threat > U-reward], [U-reward > U-threat]). Regions were identified using built-in Talairach Atlas Labels in xjView (v.9.7; Human Neuroimaging Laboratory; Houston, TX) in conjunction with the Allen Brain Atlas (Allen Institute for Brain Science, Seattle, WA). Given the characteristics of our study sample, age (mean centered), gender, current or lifetime AUD and major depressive disorder (MDD) diagnoses were entered as covariates of no interest in all analyses to account for potentially confounding effects. In all second-level analyses, we considered activations that survived FWE whole-brain cluster extent correction at P < 0.05, with a cluster size greater than 20 contiguous voxels (volume > 160 mm3; Lieberman and Cunningham, 2009; Han and Glenn, 2018), as significant. These results were subsequently verified with permutations tests. Based on simulations (10 000 iterations) performed using 3dFWHMx and 3dClustSim with the autocorrelation function, correction at α < 0.05 is achieved with a voxel threshold of P < 0.001 and cluster size of at least 106 contiguous voxels for U-threat (volume > 848 mm3) and 121 contiguous voxels for U-reward (volume > 968 mm3).
Results
Main effects of U-threat and U-reward
Detailed neural activation elicited by U-threat and U-reward is presented in Figure 2 and Table 2. U-threat significantly activated the right insula (anterior and posterior), right supplementary motor area, left precuneus, left cerebellum, left dorsal anterior cingulate cortex (dACC) and left precentral gyrus. For U-reward, the whole-brain results yielded significant activations in regions previously associated with reward processing, including the right AIC, right ventral anterior nucleus of the thalamus (VA) and bilateral inferior frontal gyrus (IFG). Additional activations were found in bilateral fusiform gyrus.
Fig. 2.
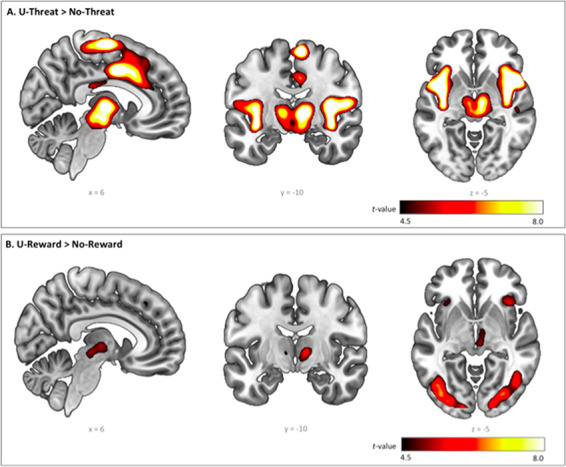
Whole-brain voxel-wise statistical t maps overlaid on a canonical brain, displaying significant activations at P < 0.05, family-wise error corrected (FWE), with a cluster size of 20 or more contiguous voxels, during (A) unpredictable threat > no threat and (B) unpredictable reward > no reward, across all participants. Color bars represent statistical t-scores.
Table 2.
Main effects of the U-threat and U-reward task conditions
| Region | MNI coordinates | Cluster (voxels) | Volume (mm3) | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| U-threat ≥ No-threat | ||||||
| R insula (anterior and posterior) | 36 | −16 | 12 | 20 726 | 165 808 | >8 |
| R supplementary motor area | 8 | −10 | 66 | 6068 | 48 544 | >8 |
| L precuneus | −16 | −46 | 66 | 81 | 648 | 6.16 |
| L cerebellum | −34 | −56 | −34 | 228 | 1824 | 6.10 |
| L anterior cingulate cortex (dorsal) | −14 | −22 | 36 | 57 | 456 | 5.62 |
| L precentral gyrus | −36 | 0 | 46 | 34 | 272 | 4.80 |
| U-reward ≥ No-reward | ||||||
| L fusiform gyrus | −32 | −78 | −16 | 2264 | 18 112 | >8 |
| R fusiform gyrus | 34 | −64 | −14 | 1907 | 15 256 | 7.72 |
| R inferior frontal gyrus | 46 | 8 | 26 | 491 | 3928 | 6.04 |
| R thalamus (ventral anterior nucleus) | 10 | −10 | 2 | 29 | 232 | 4.68 |
| L inferior frontal gyrus | −38 | 8 | 28 | 36 | 288 | 4.56 |
| R insula (anterior) | 36 | 24 | −2 | 20 | 160 | 4.45 |
Note: Reporting of all significant peak voxels at P < 0.05, family-wise error corrected (FWE), with a cluster size of >20 contiguous voxels.
L, left; MNI, Montreal Neurologic Institute; R, right; U, unpredictable.
Common and differing activation for U-threat and U-reward
The conjunction analysis revealed clusters of common activation in several brain regions, including the right AIC, right VA and right IFG. Significant differences between the two tasks were also identified; notably, activity in the right insula (anterior and posterior) and dACC was higher during the threat task (U-threat > No-threat) relative to the reward task (U-reward > No-reward). The opposite contrast revealed significantly higher activity primarily in the right fusiform and left middle occipital gyrus for reward relative to threat task. Figure 3 illustrates significant findings (see also Table 3). Of note, adjusting our analyses for covariates of no interest (i.e. age, gender, AUD and MDD diagnoses) did not change the results.
Fig. 3.
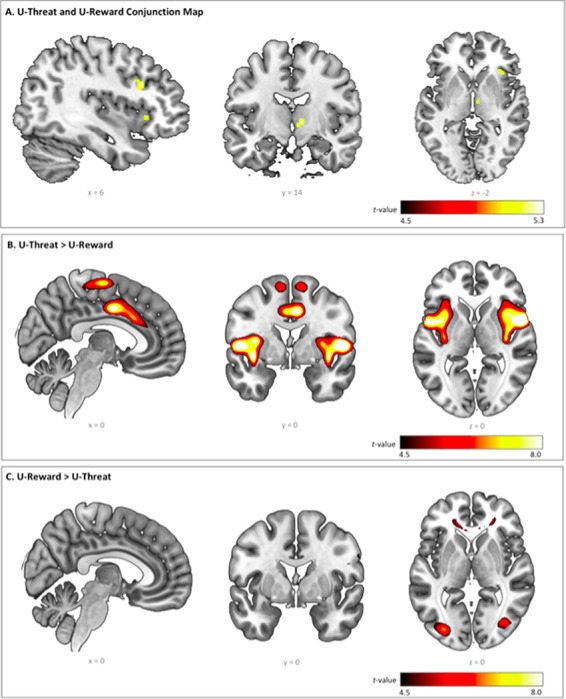
Whole-brain voxel-wise statistical t maps overlaid on a canonical brain, displaying significant activations at P < 0.05, family-wise error corrected (FWE), with a cluster size of 20 or more contiguous voxels, during (A) conjunction of unpredictable threat and unpredictable reward, (B) unpredictable threat > unpredictable reward and (C) unpredictable reward > unpredictable threat, across all participants. Color bars represent statistical t-scores.
Table 3.
Common (conjunction) and differing activation between U-threat and U-reward task conditions
| Region | MNI coordinates | Cluster (voxels) | Volume (mm3) | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Conjunction analysis | ||||||
| R inferior frontal gyrus | 46 | 16 | 22 | 240 | 1920 | 5.26 |
| R thalamus (ventral anterior nucleus) | 10 | −10 | 2 | 29 | 232 | 4.68 |
| R insula (anterior) | 36 | 24 | −2 | 20 | 160 | 4.45 |
| Paired t-test (U-threat ≥ U-reward) | ||||||
| R insula (anterior and posterior) | 50 | −26 | 22 | 5362 | 42 896 | >8 |
| L supramarginal gyrus | −56 | −28 | 20 | 4578 | 36 624 | >8 |
| R postcentral gyrus | 18 | −42 | 64 | 1116 | 8928 | >8 |
| R anterior cingulate cortex (dorsal) | 6 | −2 | 40 | 1648 | 13 184 | >8 |
| L supplementary motor area | −10 | −2 | 66 | 185 | 1480 | 5.93 |
| L precuneus | −16 | −46 | 66 | 39 | 312 | 5.18 |
| R thalamus | 16 | −12 | 10 | 110 | 880 | 4.77 |
| Paired t-test (U-reward ≥ U-threat) | ||||||
| R fusiform gyrus | 30 | −60 | −12 | 3658 | 29 264 | 7.46 |
| L middle occipital gyrus | −32 | −84 | 6 | 2930 | 23 440 | 7.38 |
| R precentral gyrus | 42 | −14 | 62 | 49 | 392 | 5.23 |
| R postcentral gyrus | 60 | −4 | 32 | 119 | 952 | 5.04 |
| L postcentral gyrus | −60 | −6 | 30 | 76 | 608 | 4.69 |
Note: Reporting of all significant peak voxels at P < 0.05, family-wise error corrected (FWE), with a cluster size of >20 contiguous voxels.
L, left; MNI, Montreal Neurologic Institute; R, right; U, unpredictable.
Discussion
Temporally unpredictable stimuli influence behavior across species, as previously demonstrated for sequences of simple threats and rewards with fixed or variable onset (e.g. Bermudez and Schultz, 2014; Nelson et al., 2015). Here, we showed that both temporally unpredictable threat and reward elicited activation in several common brain regions. Specifically, within the frontolimbic circuit, both processes engaged the right AIC. This finding is consistent with previous neuroimaging studies, which showed that the AIC is involved in processing of unpredictable threats (e.g. Shankman et al., 2014), and more recently, unpredictable rewards as well (e.g. Gorka et al., 2016). Thus, the AIC may be an important neural substrate involved in processing physiological and subjective responses to uncertainty (as a broadly defined construct).
Evidence indicates that the AIC integrates information about internal bodily states and salient environmental stimuli to produce interoceptive awareness and facilitate the generation of anticipatory emotional responses to positive or negative future outcomes (Craig, 2009). Fundamentally, during times of uncertainty, the AIC creates a subjective response to the question, ‘How is this going to feel?’. Related to this, the AIC uses information about interoceptive states to also perceive the passage of time, which is important given that we specifically manipulated the temporal predictability of uncertain stimuli (i.e. not knowing when the stimulus would occur). In the anticipation of uncertain stimuli, the AIC engages adaptive preparatory cognitive and behavioral resources that help an individual, avoid, minimize and cope with possible negative consequences. Dysfunction of the AIC may therefore lead to both (i) negatively biased perception of unpredictable threat, regardless of its true potential to confer harm (Paulus and Stein, 2006), and (ii) faulty appraisal of unpredictable reward (i.e. winning money) as distressing or over-arousing, which may diminish the hedonic and approach-eliciting aspects of reward (Nelson et al., 2014). Therefore, chronic abnormal AIC activation may repeatedly impair appraisal of appetitive and aversive stimuli under uncertain conditions.
In addition to the AIC, both unpredictable threat and reward elicited activation in the right VA and right IFG, the two important auxiliary brain regions to the AIC that may play a role in responding to uncertainty. Studies have indicated that the VA has anatomical and functional connections with the rest of the thalamic nuclei and regions within the frontolimbic circuit, namely the basal ganglia and prefrontal cortices (McFarland and Haber, 2002; Grodd et al., 2020), and is thus thought to be an important center for executive and motor functioning as well as reward and emotion processing (Xiao and Barbas, 2002, 2004; Child and Benarroch, 2013; Asami et al., 2018; Wolff and Vann, 2019). Furthermore, in conjunction with other thalamic nuclei, the VA plays a role in both downstream and upstream pathways that carry viscerosensory information to be conveyed to the insula, cingulate cortex and prefrontal cortices. Increased VA activation during the anticipation of unpredictable threat and reward may therefore be related to its proposed role in processing of salient information to redirect attention and behavior (Cho et al., 2013).
Research has also shown that IFG, a subregion of the lateral prefrontal cortex, may be another important node within the frontolimbic circuitry that may be implicated in emotion regulation (Cha et al., 2016), as this region shows abnormal function in disorders with hyperarousal (anxiety) or hypoarousal (depression). More specifically, the IFG is important for the maintenance of the biological homeostasis, and its role is to effectively respond to salient emotional stimuli (appetitive or aversive) and efficiently return the neural system to baseline, and thus protect it from harm. Given the roles of the VA and IFG in maintenance of bodily homeostasis during the processing of uncertain stimuli, their dysfunction may lead to exacerbated aversive responding to uncertainty. However, this is speculative and remains to be further tested.
Although there are similarities in the neural circuitries underlying unpredictable threat and reward processing, there are also some notable differences. Relative to unpredictable reward, unpredictable threat recruited both the anterior and posterior clusters of the insular cortex, whereas unpredictable reward elicited activation only within the AIC cluster. Unpredictable threat also elicited greater activation (both in cluster size and in signal intensity) in the AIC compared with unpredictable reward (although see the ‘Limitations’ section below). The AIC is typically considered a key node involved in interoceptive awareness. However, posterior insula may also act as an integrative hub for information on subjective evaluation of internal and external states (Stephani et al., 2011). Based on the current findings, however, posterior insula activation may be specific to the processing of unpredictable threat.
In addition to anterior and posterior insula, unpredictable threat, but not unpredictable reward, activated the dACC, which is also thought to contribute to the appraisal and expression of negative emotion and has a regulatory role with respect to limbic regions involved in generating emotional responses (Khalsa et al., 2009; Etkin et al., 2011). The connections between the dACC and the insula invite the hypothesis that the dACC plays a complementary role in generating a warning signal toward upcoming threat in order to encourage avoidance behavior (Klumpp et al., 2012). The dACC is also a primary target of the mesocortical dopamine neurons (Paus, 2001), and therefore one might expect increased dACC response to rewarding or appetitive stimuli. However, we did not observe dACC activation during unpredictable reward. This may be due, in part, to the design of the NPU reward task. Most notably, unlike the MID task, this slot-machine task did not include a punishment condition in which participants lose money. This design distinction is important considering prior reports that showed greater dACC activation following trials that resulted in a loss relative to those that resulted in a gain (Gehring and Willoughby, 2002; Holroyd et al., 2004).
Related to this, we also did not observe activation in the ventral striatum during the anticipation of unpredictable reward. This may be surprising given that previous studies often reported striatal activation during reward anticipation (Liu et al., 2011). Nevertheless, prior work has also shown that anticipatory striatal activation may be contingent on an instrumental response (button press) and not just on imminent, potential reward delivery itself (Tricomi et al., 2004; Bjork and Hommer, 2007). Thus, the lack of striatal response in the present study may be in part due to the task design being entirely passive (i.e. there were no behavioral performance component and no decision-making aspect).
On the other hand, unpredictable reward relative to unpredictable threat elicited more activation particularly in the visual cortex (i.e. right fusiform and left middle occipital gyrus), which could mean that unpredictable reward was perhaps more engaging.
The present study had several strengths including a relatively large sample size and the use of fMRI variants of the well-established NPU paradigm in order to independently examine the shared and unique neural correlates of temporally unpredictable threat and reward for the first time within the same sample of young adults. However, the present findings should be interpreted in light of several limitations. First, it is difficult to subjectively match the two reinforcers (i.e. mild electric shock and monetary reward) on emotional engagement. For each person, it is therefore possible that the observed differences in the neural response to unpredictable threat and reward may have been due to the difference in emotional intensity of the aversive and appetitive stimuli. The range and mean of the ITI (and the jitter) across the tasks were also different. One way to circumvent these problems in the future, and better equate the two tasks, may be to use reinforcers that are similar in nature (e.g. pairing primary reward [food, liquid] with primary threat [shock, aversive tone] or secondary reward [pleasant images, monetary gains] with secondary threat [negative images, monetary losses]) and tightly control the timing across paradigms. Related to this, future studies may also consider measuring additional indices of motivational engagement during the anticipatory periods in both shock and reward contexts (i.e. stimuli ratings, skin conductance) to control for in subsequent fMRI analyses and to ensure that the tasks worked as designed. Third, the present study examined neural response to temporally unpredictable threat and reward, but there are many ways one can manipulate uncertainty. Future studies should consider tasks that allow for a comparison of neural reactivity during the anticipation of unpredictable relative to predictable threats and rewards (such as NPU), which could involve manipulations of probability and/or magnitude. Doing so would ultimately allow for a better assessment of the neural circuitry underlying aversion/preference for uncertainty and its impact on behavior. Finally, adjusting our analyses for potential confounds (i.e. age, gender and AUD and MDD diagnoses) did not change the results. However, given our sample selection and characteristics, there still may be some other unmeasured confounding variable(s) that could not be accounted for in our analyses. Future studies are therefore needed to replicate and expand the present findings.
In conclusion, this study compared the neural response to temporally unpredictable threat and reward in a sample of young adults and found overlapping activation in the right AIC, right VA and right IFG. We also found preliminary evidence to suggest that some regions may be threat and reward specific. For instance, unpredictable threat may also recruit the posterior insula and dACC, while unpredictable reward may elicit increased activation in the visual cortex (i.e. fusiform and occipital gyrus). Taken together, the present findings suggest that although there is overlap in the neural circuitry underlying anticipation of temporally unpredictable threat and reward, these processes appear to be largely mediated by distinct circuits. However, more research is needed to corroborate these results using tasks that are better matched by design. Finally, future studies should also examine the generalizability of these findings to clinical populations and investigate how disruption of the neural activity within the aforementioned brain regions may contribute to psychopathology.
Contributor Information
Milena Radoman, Department of Psychiatry, University of Illinois-Chicago, Chicago, IL 60612, USA; Graduate Program in Neuroscience, University of Illinois-Chicago, Chicago, IL 60612, USA.
Lynne Lieberman, Road Home Program, Rush University Medical Center, Chicago, IL 60612, USA.
Jagan Jimmy, Department of Psychiatry, University of Illinois-Chicago, Chicago, IL 60612, USA.
Stephanie M Gorka, Department of Psychiatry and Behavioral Health, Ohio State University, Columbus, OH 43205, USA.
Funding
Research reported in this paper was supported by the National Institute of Alcohol Abuse and Alcoholism (NIAAA) under awards P50AA022538 and K23AA025111. The study was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR002003.
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
S.M.G. was the principal investigator of the study, S.M.G. contributed to the conceptual design, made important contributions to the editing of the manuscript and assisted in data interpretation. M.R. conducted the imaging analyses, interpreted the data and wrote the initial draft of the manuscript. L.L. and J.J. assisted in data preprocessing and manuscript preparation.
References
- Anselme, P, Güntürkün, O. (2018). How foraging works: uncertainty magnifies food-seeking motivation. Behavioral and Brain Sciences, 8, 1–106. [DOI] [PubMed] [Google Scholar]
- Apicella, P., Legallet, E., Trouche, E. (1997). Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Experimental Brain Research, 116, 456–66. [DOI] [PubMed] [Google Scholar]
- Asami, T., Yoshida, H., Takaishi, M., et al. (2018). Thalamic shape and volume abnormalities in female patients with panic disorder. PLoS One, 13, e0208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine, J.R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews, 22, 229–44. [DOI] [PubMed] [Google Scholar]
- Bach, D.R., Dolan, R.J. (2012). Knowing how much you don’t know: a neural organization of uncertainty estimates. Nature Reviews Neuroscience, 13, 572–86. [DOI] [PubMed] [Google Scholar]
- Bach, D.R., Seifritz, E., Dolan, R.J. (2015). Temporally unpredictable sounds exert a context-dependent influence on evaluation of unrelated images. PLoS One, 10, e0131065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Anan, Y., Wilson, T.D., Gilbert, D.T. (2009). The feeling of uncertainty intensifies affective reactions. Emotion, 9, 123–7. [DOI] [PubMed] [Google Scholar]
- Bartra, O., McGuire, J.T., Kable, J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers, A.R., Foti, D. (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98, 227–39. [DOI] [PubMed] [Google Scholar]
- Bennett, K.P., Dickmann, J.S., Larson, C.L. (2018). If or when? Uncertainty’s role in anxious anticipation. Psychophysiology, 55, e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez, M.A., Schultz, W. (2014). Timing in reward and decision processes. Philosophical Transactions of the Royal Society B: Biological Sciences, 369, 20120468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, K.C., Robinson, T.E. (2003). Parsing reward. Trends in Neurosciences, 26, 507–13. [DOI] [PubMed] [Google Scholar]
- Berridge, K.C., Robinson, T.E. (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28, 309–69. [DOI] [PubMed] [Google Scholar]
- Bjork, J.M., Hommer, D.W. (2007). Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behavioural Brain Research, 177, 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, D.E., Shapiro, B.L., Curtin, J.J. (2013). How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science, 24, 2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor, M.B., LoLordo, V.M. (1970). Rats prefer signaled reinforcing brain stimulation to unsignaled ESB. Journal of Comparative and Physiological Psychology, 71, 183–91. [DOI] [PubMed] [Google Scholar]
- Carleton, R.N. (2016). Into the unknown: a review and synthesis of contemporary models involving uncertainty. Journal of Anxiety Disorders, 39, 30–43. [DOI] [PubMed] [Google Scholar]
- Carleton, R.N., Mulvogue, M.K., Thibodeau, M.A., et al. (2012). Increasingly certain about uncertainty: intolerance of uncertainty across anxiety and depression. Journal of Anxiety Disorders, 26, 468–79. [DOI] [PubMed] [Google Scholar]
- Cha, J., DeDora, D., Nedic, S., et al. (2016). Clinically anxious individuals show disrupted feedback between inferior frontal gyrus and prefrontal-limbic control circuit. Journal of Neuroscience, 36, 4708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama, M., McFarland, N.R., Amaral, D.G., et al. (1997). Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. Journal of Neuroscience, 17, 9686–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child, N.D., Benarroch, E.E. (2013). Anterior nucleus of the thalamus: functional organization and clinical implications. Neurology, 81, 1869–76. [DOI] [PubMed] [Google Scholar]
- Cho, Y.T., Fromm, S., Guyer, A.E., et al. (2013). Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage, 66, 508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, L., Bechara, A., Damasio, H., et al. (2008). Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain: A Journal of Neurology, 131, 1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A.D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Craig, A.D.B. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82. [DOI] [PubMed] [Google Scholar]
- Critchley, H.D., Mathias, C.J., Dolan, R.J. (2001). Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron, 29, 537–45. [DOI] [PubMed] [Google Scholar]
- Daldrup, T., Remmes, J., Lesting, J., et al. (2015). Expression of freezing and fear-potentiated startle during sustained fear in mice. Genes, Brain, and Behavior, 14, 281–91. [DOI] [PubMed] [Google Scholar]
- Davies, C.D., Craske, M.G. (2015). Psychophysiological responses to unpredictable threat: effects of cue and temporal unpredictability. Emotion, 15, 195–200. [DOI] [PubMed] [Google Scholar]
- Diekhof, E.K., Kaps, L., Falkai, P., et al. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50, 1252–66. [DOI] [PubMed] [Google Scholar]
- Dieterich, R., Endrass, T., Kathmann, N. (2016). Uncertainty is associated with increased selective attention and sustained stimulus processing. Cognitive, Affective and Behavioral Neuroscience, 16, 447–56. [DOI] [PubMed] [Google Scholar]
- Dreher, J.-C., Kohn, P., Berman, K.F. (2006). Neural coding of distinct statistical properties of reward information in humans. Cerebral Cortex (New York, NY: 1991), 16, 561–73. [DOI] [PubMed] [Google Scholar]
- Dugas, M.J., Schwartz, A., Francis, K. (2004). Brief report: intolerance of uncertainty, worry, and depression. Cognitive Therapy and Research, 28, 835–42. [Google Scholar]
- Etkin, A., Egner, T., Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry, R.A., Nelson, B.D. (2020). Differential impact of threat type on defensive motivation and attention during the NPU-threat task. Motivation and Emotion, 1, 3. [Google Scholar]
- First, M.B., Williams, J.B.W., Karg, R.S., Spitzer, R. (2015). Structured Clinical Interview for DSM-5-Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Fox, A.S., Shackman, A.J. (2019). The central extended amygdala in fear and anxiety: closing the gap between mechanistic and neuroimaging research. Neuroscience Letters, 693, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtress, T., Marshall, A.T., Kirkpatrick, K. (2012). Motivation and timing: clues for modeling the reward system. Behavioural Processes, 90, 142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring, W.J., Willoughby, A.R. (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science, 295, 2279–82. [DOI] [PubMed] [Google Scholar]
- Gentes, E.L., Ruscio, A.M. (2011). A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive–compulsive disorder. Clinical Psychology Review, 31, 923–33. [DOI] [PubMed] [Google Scholar]
- Goode, T.D., Ressler, R.L., Acca, G.M., et al. (2019). Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. eLife, 8, e46525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka, S.M., Kreutzer, K.A., Petrey, K.M., et al. (2019). Behavioral and neural sensitivity to uncertain threat in individuals with alcohol use disorder: associations with drinking behaviors and motives. Addiction Biology, 25, e12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka, S.M., Nelson, B.D., Phan, K.L., et al. (2016). Intolerance of uncertainty and insula activation during uncertain reward. Cognitive, Affective and Behavioral Neuroscience, 16, 929–39. [DOI] [PubMed] [Google Scholar]
- Greco, V., Roger, D. (2003). Uncertainty, stress, and health. Personality and Individual Differences, 34, 1057–68. [Google Scholar]
- Grillon, C., Baas, J.M.P., Cornwell, B., et al. (2006). Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biological Psychiatry, 60, 752–9. [DOI] [PubMed] [Google Scholar]
- Grillon, C., Baas, J.P., Lissek, S., et al. (2004). Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience, 118, 916–24. [DOI] [PubMed] [Google Scholar]
- Grodd, W., Kumar, V.J., Schüz, A., et al. (2020). The anterior and medial thalamic nuclei and the human limbic system: tracing the structural connectivity using diffusion-weighted imaging. Scientific Reports, 10, 10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe, D.W., Nitschke, J.B. (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14, 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe, D.W., Nitschke, J.B. (2011). Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion, 11, 413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, X., Hof, P.R., Friston, K.J., et al. (2013). Anterior insular cortex and emotional awareness. The Journal of Comparative Neurology, 521, 3371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H., Glenn, A.L. (2018). Evaluating methods of correcting for multiple comparisons implemented in SPM12 in social neuroscience fMRI studies: an example from moral psychology. Social Neuroscience, 13, 257–67. [DOI] [PubMed] [Google Scholar]
- Hart, A.S., Clark, J.J., Phillips, P.E.M. (2015). Dynamic shaping of dopamine signals during probabilistic Pavlovian conditioning. Neurobiology of Learning and Memory, 117, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry, C., Bach, D.R., Esposito, F., et al. (2007). Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience, 27, 5958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd, C.B., Nieuwenhuis, S., Yeung, N., et al. (2004). Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience, 7, 497–8. [DOI] [PubMed] [Google Scholar]
- Hur, J., Smith, J.F., DeYoung, K.A., et al. (2020). Anxiety and the neurobiology of temporally uncertain threat anticipation. Journal of Neuroscience, 40, 7949–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada, H., Nageishi, Y. (1982). The concept of uncertainty in animal experiments using aversive stimulation. Psychological Bulletin, 91, 573–88. [Google Scholar]
- Jo, S., Jung, M.W. (2016). Differential coding of uncertain reward in rat insular and orbitofrontal cortex. Scientific Reports, 6, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa, S.S., Rudrauf, D., Feinstein, J.S., et al. (2009). The pathways of interoceptive awareness. Nature Neuroscience, 12, 1494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp, H., Angstadt, M.,Phan, K.L. (2012). Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological psychology, 89, 273–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B., Adams, C.M., Fong, G.W., et al. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21, RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B., Westdorp, A., Kaiser, E., et al. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12, 20–7. [DOI] [PubMed] [Google Scholar]
- Kobayashi, S., Schultz, W. (2008). Influence of reward delays on responses of dopamine neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28, 7837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Vanni-Mercier, G., Isnard, J., et al. (2016). The neural dynamics of reward value and risk coding in the human orbitofrontal cortex. Brain, 139, 1295–309. [DOI] [PubMed] [Google Scholar]
- Lieberman, L., Gorka, S.M., Shankman, S.A., et al. (2017). Impact of panic on psychophysiological and neural reactivity to unpredictable threat in depression and anxiety. Clinical Psychological Science, 5, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, M.D., Cunningham, W.A. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience, 4, 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Hairston, J., Schrier, M., et al. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35, 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland, N.R., Haber, S.N. (2002). Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22, 8117–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam, M.M., Mufson, E.J. (1982). Insula of the old world monkey. III: efferent cortical output and comments on function. Journal of Comparative Neurology, 212, 38–52. [DOI] [PubMed] [Google Scholar]
- Mirenowicz, J., Schultz, W. (1994). Importance of unpredictability for reward responses in primate dopamine neurons. Journal of Neurophysiology, 72, 1024–7. [DOI] [PubMed] [Google Scholar]
- Mizuhiki, T., Richmond, B.J., Shidara, M. (2012). Encoding of reward expectation by monkey anterior insular neurons. Journal of Neurophysiology, 107, 2996–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov, I.E. (2017). Anterior cingulate is a source of valence-specific information about value and uncertainty. Nature Communications, 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, G., Arkadir, D., Nevet, A., et al. (2004). Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron, 43, 133–43. [DOI] [PubMed] [Google Scholar]
- Nelson, B.D., Hodges, A., Hajcak, G., et al. (2015). Anxiety sensitivity and the anticipation of predictable and unpredictable threat: evidence from the startle response and event-related potentials. Journal of Anxiety Disorders, 33, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, B.D., Kessel, E.M., Jackson, F., et al. (2016). The impact of an unpredictable context and intolerance of uncertainty on the electrocortical response to monetary gains and losses. Cognitive, Affective and Behavioral Neuroscience, 16, 153–63. [DOI] [PubMed] [Google Scholar]
- Nelson, B.D., Shankman, S.A., Proudfit, G.H. (2014). Intolerance of uncertainty mediates reduced reward anticipation in major depressive disorder. Journal of Affective Disorders, 158, 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T., Brett, M., Andersson, J., et al. (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25, 653–60. [DOI] [PubMed] [Google Scholar]
- O’Doherty, J., Kringelbach, M.L., Rolls, E.T., et al. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4, 95–102. [DOI] [PubMed] [Google Scholar]
- O’Neill, M., Schultz, W. (2018). Predictive coding of the statistical parameters of uncertain rewards by orbitofrontal neurons. Behavioural Brain Research, 355, 90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham, S., Murawski, C., Fornito, A., et al. (2018). The anticipation and outcome phases of reward and loss processing: a neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping, 39, 3398–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino, T.M., McMakin, D.L., Dahl, R.E., et al. (2011). ‘I won, but I’m not getting my hopes up’: depression moderates the relationship of outcomes and reward anticipation. Psychiatry Research Neuroimaging, 194, 393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus, M.P., Stein, M.B. (2006). An insular view of anxiety. Biological Psychiatry, 60, 383–7. [DOI] [PubMed] [Google Scholar]
- Paus, T. (2001). Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews Neuroscience, 2, 417–24. [DOI] [PubMed] [Google Scholar]
- Preuschoff, K., Bossaerts, P., Quartz, S.R. (2006). Neural differentiation of expected reward and risk in human subcortical structures. Neuron, 51, 381–90. [DOI] [PubMed] [Google Scholar]
- Prokasy, W.F. (1956). The acquisition of observing responses in the absence of differential external reinforcement. Journal of Comparative and Physiological Psychology, 49, 131–4. [DOI] [PubMed] [Google Scholar]
- Quelhas Martins, A., Mcintyre, D., Ring, C. (2015). Aversive event unpredictability causes stress-induced hypoalgesia. Psychophysiology, 52, 1066–70. [DOI] [PubMed] [Google Scholar]
- Ran, G., Chen, X., Zhang, Q., et al. (2016). Attention modulates neural responses to unpredictable emotional faces in dorsolateral prefrontal cortex. Frontiers in Human Neuroscience, 10, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollman, G.B., Harris, G. (1987). The detectability, discriminability, and perceived magnitude of painful electrical shock. Perception and Psychophysics, 42, 257–68. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos, I., Grupe, D.W., Mackiewicz, K.L., et al. (2010). Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex (New York, NY: 1991), 20, 929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, T., Nakai, S., Sato, T., et al. (2003). Correlated coding of motivation and outcome of decision by dopamine neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 9913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, A., Grillon, C. (2012). Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protocols, 7, 527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, A., Merikangas, K., Swendsen, H., et al. (2011). Measuring anxious responses to predictable and unpredictable threat in children and adolescents. Journal of Experimental Child Psychology, 110, 159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroijen, M., Fantoni, S., Rivera, C., et al. (2016). Defensive activation to (un)predictable interoceptive threat: the NPU respiratory threat test (NPUr). Psychophysiology, 53, 905–13. [DOI] [PubMed] [Google Scholar]
- Schultz, W. (1998). Predictive reward signal of dopamine neurons. Journal of Neurophysiology, 80, 1–27. [DOI] [PubMed] [Google Scholar]
- Seidenbecher, T., Remmes, J., Daldrup, T., et al. (2016). Distinct state anxiety after predictable and unpredictable fear training in mice. Behavioural Brain Research, 304, 20–3. [DOI] [PubMed] [Google Scholar]
- Seligman, M.E., Meyer, B. (1970). Chronic fear and ulcers in rats as a function of the unpredictability of safety. Journal of Comparative and Physiological Psychology, 73, 202–7. [DOI] [PubMed] [Google Scholar]
- Shankman, S.A., Gorka, S.M., Nelson, B.D., et al. (2014). Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport, 25, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman, S.A., Robison-Andrew, E.J., Nelson, B.D., et al. (2011). Effects of predictability of shock timing and intensity on aversive responses. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 80, 112–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihata, S., McEvoy, P.M., Mullan, B.A., et al. (2016). Intolerance of uncertainty in emotional disorders: what uncertainties remain? Journal of Anxiety Disorders, 41, 115–24. [DOI] [PubMed] [Google Scholar]
- Stephani, C., Fernandez-Baca Vaca, G., Maciunas, R., et al. (2011). Functional neuroanatomy of the insular lobe. Brain Structure and Function, 216, 137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha, S., Pradhan, N. (1993). Behavioral consequences of predictable vs. unpredictable shocks in rats. Physiology and Behavior, 54, 243–7. [DOI] [PubMed] [Google Scholar]
- Tobler, P.N., Fiorillo, C.D., Schultz, W. (2005). Adaptive coding of reward value by dopamine neurons. Science (New York, NY), 307, 1642–5. [DOI] [PubMed] [Google Scholar]
- Tovote, P., Fadok, J.P., Lüthi, A. (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 16, 317–31. [DOI] [PubMed] [Google Scholar]
- Tricomi, E.M., Delgado, M.R., Fiez, J.A. (2004). Modulation of caudate activity by action contingency. Neuron, 41, 281–92. [DOI] [PubMed] [Google Scholar]
- Wolff, M., Vann, S.D. (2019). The cognitive thalamus as a gateway to mental representations. Journal of Neuroscience, 39, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, D., Barbas, H. (2004). Circuits through prefrontal cortex, basal ganglia, and ventral anterior nucleus map pathways beyond motor control. Thalamus and Related Systems, 2, 325–43. [Google Scholar]
- Xiao, D., Barbas, H. (2002). Pathways for emotions and memory II. Afferent input to the anterior thalamic nuclei from prefrontal, temporal, hypothalamic in the rhesus monkey. Thalamus and Related Systems, 2, 33–48. [Google Scholar]
- Yu, R., Zhou, W., Zhou, X. (2011). Rapid processing of both reward probability and reward uncertainty in the human anterior cingulate cortex. PLoS One, 6, e29633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


