ABSTRACT
Background
Cervical cancer caused by human papillomavirus (HPV) infections is one of the most common cancers affecting women worldwide. Current preventative HPV vaccines on the market are composed of HPV L1 protein produced either in the yeast such as Gardasil or in the insect cells such as Cervarix. The duration of efficacy and cross-protection remain highly desirable for the improvement of current prophylactic HPV vaccine. Given that HPV carries out infection and replicates in mammalian cells, L2 protein, which is not included in the current licensed vaccines, is included in the third generation of HPV vaccine in pursuing of providing broader prevention. We hypothesize that a virus-like particle (VLP) consisting of HPV L1 plus L2 proteins generated in mammalian cells will present conformations more closely to native HPV, thus it will provide more durable and broader efficacy of prevention.
Methods
We took advantage of 293TT cells to produce VLP containing L1 and L2 proteins of HPV16 and HPV18, respectively.
Results
VLP particles of uniformed size and morphology were observed, and potent and broadly neutralizing antibodies were induced in mice and rabbits. In addition, compared to bivalent HPV vaccine of Cervarix, our HPV L1-L2 VLPs elicited higher titer of anti-sera, and the anti-sera also presented comparable neutralization potency against HPV16 and HPV18 infections even a much less potent adjuvant was used in our case.
Conclusion
Our VLPs were capable of eliciting stronger and more broadly neutralizing activities against various HPV subtypes and were potential candidate HPV vaccines.
Keywords: HPV, cervical cancer, vaccine, mammalian cells, neutralizing antibodies
Statement of Significance
VLP containing L1 and L2 proteins were generated in mammalian cells. Potent and broadly neutralizing antibodies against HPV were induced by our VLPs. Compared to bivalent HPV vaccine of Cervarix, our HPV VLPs induced higher titer of anti-sera.
INTRODUCTION
Cervical cancer is one of the most common cancers in woman with an estimated 530 000 new cases worldwide every year [1]. Cervical cancer is caused by persistent cervical infection of high-risk (human papillomaviruses) HPVs [2–4]. Though HPV vaccine has provided high degrees of protection, the duration of efficacy and cross-protection remains highly desirable for the improvement of current prophylactic HPV vaccines [5].
HPVs are small, nonenveloped viruses containing 2 structural proteins, L1 and L2, forming the viral capsid with 50–55 nm in diameter. Each viral particle contains 360 copies of the major capsid protein L1 and 72 copies of the minor capsid protein L2 [6, 7]. L1 and L2 together form a viral ectodomain glycoprotein that mediates viral entry and thus are the targets for prophylactic vaccine development [8].
Vaccines have demonstrated high degrees of efficacy in the prevention of HPV transmission. The first-generation licensed vaccines, Gardasil and Cervarix, were developed by Merck and GlaxoSmithKline (GSK), respectively [5]. Gardasil is constituted of virus-like particles (VLPs) containing L1 proteins of HPV6, HPV11, HPV16 and HPV18 and could provide protection against the infection of HPV6/11/16/18. HPV16/18 infection causes approximately 70% of cervical cancer. HPV6 and HPV11 account for approximately 90% of external genital warts [9–11]. Cervarix was based on VLPs containing L1 proteins of HPV16 and HPV18, which could prevent HPV16/18 infection [12, 13]. L1 protein of Gardasil and Cervarix was produced in the yeast and insect cells, respectively. Gardasil uses alum as adjuvant in its formulation, while Cervarix uses AS04, a proprietary adjuvant composed of alum plus monophosphoryl lipid A (a detoxified form of lipopolysaccharide). Head-to-head trials of Gardasil vs Cervarix in women suggested that significantly higher antibody titer was induced for Cervarix than Gardasil [14]. Gardasil protection against HPV16/18 infections could last at least 5 years while Cervarix protected against HPV16/18 infections for more than 10 years. In addition, Cervarix could provide cross-protection against high-risk HPV31/45 infection as compared to Gardasil that does not [15]. The application of both first-generation HPV vaccines significantly reduced HPV16/18 infection. The two HPV vaccines were highly immunogenic, had an excellent safety profile and had conferred complete type-specific protection against persistent infection and associated lesions in fully vaccinated women [16].
The second-generation vaccine, Gardasil 9 developed by Merck, prevent nine types of HPV infection including HPV6/11/16/18/31/33/45/52/58 [17]. Long-term follow-up studies are ongoing to evaluate immunogenicity, safety and effectiveness of 9vHPV [18]. Immunization with L2 peptides or proteins elicited cross-reactive, neutralizing antibodies, making L2-based immunogen as a promising candidate for the third-generation HPV vaccine [5, 8, 19, 20]. In order to improve the duration and the cross-protective of neutralizing antibodies induced by HPV vaccine, we hypothesized that HPV L1 plus L2 proteins generated by mammalian cells will present conformation more closely to native HPV virus, thus it will provide more durable and broader prevention than the currently licensed HPV vaccines from other expression systems. In this study, we exploited 293TT cells to produce VLP containing L1 plus L2 proteins (L1-L2 VLPs) of HPV16 and L1-L2 VLPs of HPV18. L2 protein was introduced into VLP, expectedly to form L1/L2 VLP with more stable and size consistent VLPs and to induce cross-neutralizing antibodies. The consistent VLP morphology was observed under electron microscopy and the immunogenicity was evaluated in mice and rabbits, in which potent and broadly neutralizing antibodies were induced. Because head-to-head trials of Cervarix vs Gardasil demonstrated that Cervarix was superior to Gardasil with the regard of geometric mean titers (GMT) and seropositivity retention [14], Cervarix was chosen for the head-to-head comparison of Cervarix vs our HPV16/18 L1-L2 VLPs. Compared to Cervarix, our HPV L1-L2 VLPs elicited higher titer of anti-sera, and the anti-sera also present comparable neutralization potency against HPV16 and HPV18 infections, indicating the potentially longer duration of protection; in addition, it also inhibited HPV31 and HPV45 infection while Cervarix showed little inhibitory activity. Taken together, our study suggests that our HPV16/18 L1-L2 VLPs expressed in mammalian cells is a potential HPV vaccine candidate, probably with longer duration and more broadly neutralizing activity.
MATERIALS AND METHODS
HPV VLP preparation
HPV VLP was prepared as previously described [21] with the following modifications. In brief, L1-L2 expression plasmid (Addgene, Watertown, MA, USA) mixed with TurboFect Transfection Reagent (R0531, Thermo Fisher Scientific, Waltham, MA, USA) was transfected into 293 TT cells (Supplementary Fig. 1). Producer cells were collected by trypsinization about 48 hours after transfection. After centrifugation, the cell pellet was suspended at approximately 100 million cells per ml in lysis buffer. Cell lysate was incubated at 37oC for at least 16 hours and then chilled on ice for 5 min. The matured lysate on ice was incubated for 15 min with the final concentration of 850 mM NaCl. After centrifugation, the supernatant was transferred for OptiPrep (D1156, Sigma-Aldrich, St.Louis, Missouri, USA) gradient purification at 40000 rpm/min for 4.75 hours. Different fractions were collected for verification.
Ultrastructural negative staining
The purified VLPs were prepared for electron microscopy negative staining analysis according to a standard two-step negative staining method using phosphotungstic acid (Macklin P829844-5g) as contrasting agent. Briefly, a drop of the sample was added on a nickel grid (400 mesh) with carbon-coated formvar film (BZ1102, Electron Microscopy China, Beijing, China) for 5 min at room temperature to allow adsorption of the sample. Excess solution was removed, and the sample was left to air dry for less than 2 min. The grid was briefly (less than 30 sec) placed on a drop of distilled water to remove salts and was then transferred onto a drop of stain consisting of 2% phosphotungstic acid (w/v) for 4 min. After the grid was blotted dry, it was left to dry completely (more than 10 hours) and examined under electron microscopy of JEM-2100. Representative fields were imaged at standard 50, 100 and 200 K original magnifications.
Animal immunization
The immunization was conducted according to our previous protocols with the following modifications [22]. Briefly, 6–8 week female mice or 2–2.5 kg New Zealand rabbits received immunizations with purified VLPs prepared in complete Freund's adjuvant (Sigma-Aldrich), followed by 4 boots 3, 6, 9 and 12 weeks later in incomplete Freund's adjuvant (Sigma-Aldrich). Blood were collected 1 week after every boost immunization. Alternatively, in the head-to-head comparison of Cervarix vs our HPV VLPs, 20 μg HPV16 VLP and 20 μg 18 VLP were mixed with 0.5 mg aluminum hydroxide (Sigma-Aldrich) in our HPV VLPs. Rabbits were given the same dose of our HPV VLPs and Cervarix with the adjuvant of AS04 (purchased from GSK, Middlesex, United Kingdom) according to the manufacturer's protocols of GSK. Rabbits were administered with antigen at 0, 4 and 10 weeks. Sera were collected 1 week after every boost immunization.
ELISA for characterizing sera titer
Anti-sera titer was assessed by ELISA as previously described with the following modifications [23]. In brief, HPV L1 protein (Abcam, Cambridge, MA, USA) was coated in high-affinity, protein-binding ELISA plates (Corning, Corning, New York, USA) at 4oC overnight or 37oC for 2 hours. After washing, blocking buffer with 5% non-fat milk in PBS was added for 1 hour at 37oC. Serially diluted sera were added, followed by secondary antibodies anti-mouse IgG or anti-rabbit IgG with HRP (Sigma-Aldrich). The color reaction was developed with addition of 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich) substrate at 37oC for 30 min and stopped by 10 μl 0.2 M H2SO4. Optical densities were measured at 450 nm using Infinite 200 (Tecan, Ramsey, MN, USA). Serum antibody titers were defined as the highest dilution where the diluted sample produced at least 2.1-folds greater optical density readout than that of the control serum sample at the same dilution.
SDS-PAGE and western blotting
Purified protein was separated by electrophoresis in a 7.5–12% polyacrylamide gel. The separated proteins were revealed by either Coomassie blue or transferred to PVDF membrane. The membrane was first blocked and then incubated at 4oC overnight or 37oC for 1 hour with the diluted plasma or antibody (anti-HPV16 L1 antibody; ab69, Abcam; anti-HPV18 L1 antibody; ab31492, Abcam), followed by a secondary antibody of either anti-mouse IgG or anti-rabbit IgG with an IRDye 800CW (catalog 926-32232, Rockland, Li-COR, Lincoln, NE, USA). Protein bands were visualized using Odyssey Image System (Li-COR, Lincoln, NE, USA).
HPV neutralization assay
HPV neutralization was conducted as previously described with the following modifications [21]. Briefly, 30 000 293TT cells were seeded in a 96-well cell culture plate. HPV pseudovirus mixed with various serial dilutions of antibodies or anti-sera was added in triplicate. After 72 hours incubation at 37oC, 50 μl cell supernatant was transferred to test the activity of Secreted Alkaline Phosphatase (SEAP). The inhibitory dose of 50% (ID50) or inhibitory concentration of 50% (IC50) titers was calculated based on the standard algorithm published previously [24].
Statistics
Graphs were generated with GraphPad Prism 5.01 software (GraphPad Software, San Diego, CA, USA). Two-tailed Student's t-tests were used for group comparisons. P < 0.05 was considered statistically significant with necessary mean ± Standard Error of Mean (SEM) or mean ± Standard Deviation (SD).
Ethics
The study and the protocol for this research were approved by the Center for Public Health Research, Medical School, Nanjing University. All the authors declare their compliance with publishing ethics.
RESULTS
Production and analysis of purified HPV16/18 L1-L2 protein
L1-L2 proteins of HPV16 and HPV18 were produced by transfecting plasmids encoding HPV16/18 L1-L2 into 293TT cells, respectively. In order to evaluate L1-L2 protein, SDS-PAGE and western blotting were used to characterize the proteins of three batches. L1 (55 kDa) and L2 (64–78 kDa) bands were identified as predicted molecular weights, and proteins from each batch were consistent, indicative of the purity of the L1 and L2 proteins greater than 90% (as estimated by Coomassie brilliant blue staining; Fig. 1A and B). These bands were confirmed by western blotting (Fig. 1C and D). These results indicate that the purified L1-L2 protein of HPV16 and HPV18 were generated in mammalian cell of 293TT with high purity. Very similar SDS-PAGE and western blot patterns were observed for all batches suggesting that the process consistently produced highly pure L1 and L2 proteins of HPV16/18.
Figure 1.
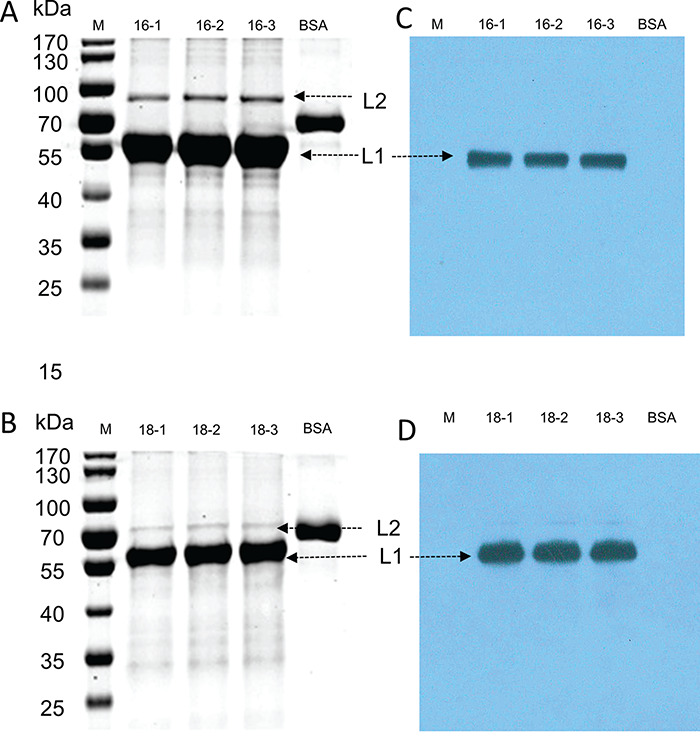
The characterization of L1-L2 proteins by SDS-PAGE and western blotting. (A and B) Purified L1 and L2 proteins were separated by SDS-PAGE and stained with Coomassie blue R-250. (C and D) After SDS-PAGE, proteins were transferred onto blotting membranes and probed with respective antibodies specific for HPV16 (Anti-HPV16 L1 antibody) and HPV18 (Anti-HPV18 L1 antibody).
Structural characterization of HPV16/18 L1-L2 VLPs
The morphology and homogeneity of VLPs were the important indications of the efficacy for HPV vaccine for inducing neutralizing antibodies against conformational epitope [25, 26]. The structure of mammalian cell-produced HPV16/18 L1-L2 proteins was analyzed by negative staining electron microscopy. L1-L2 proteins of HPV16 and HPV18 were assembled as VLPs at the end of the multi-step purification process. For both types, the great majority of VLPs appeared spherical with a diameter of around 50 nm (Fig. 2) and displayed typical HPV VLP morphology as previously published [5, 27]. The mammalian cell-produced L1-L2 VLPs exhibited high homogeneity, indicating potentially improved immunogenicity.
Figure 2.
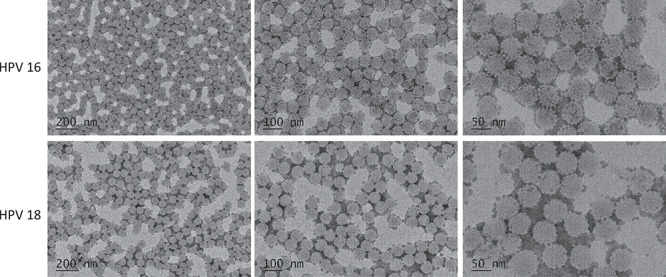
Electron microscopy negative staining image showing HPV-16 VLPs and HPV-18 VLPs from L1-L2 VLP proteins at different scales as indicated. Representative fields were imaged at standard 50, 100 and 200 K original magnifications.
Immunogenicity analysis in rabbits and mice
In order to analyze the immunogenicity of the mammalian cell-produced HPV16/18 VLPs, mice and rabbits were immunized as the regimen indicated in Fig. 3A. Anti-sera were collected and analyzed (Fig. 3A). The mean titers of murine anti-sera against HPV16 L1 and HPV18 L1 proteins were 243 000 and 6 561 000, respectively (Fig. 3B and C). The mean titers of rabbit anti-sera against HPV16 and HPV18 L1 proteins were 2 187 000 and 19 683 000, respectively (Fig. 3D and E). These results indicate that high titers of anti-sera against L1 protein of HPV16/18 were induced by our HPV16/18 L1-L2 VLPs in both mice and rabbits after five immunizations.
Figure 3.
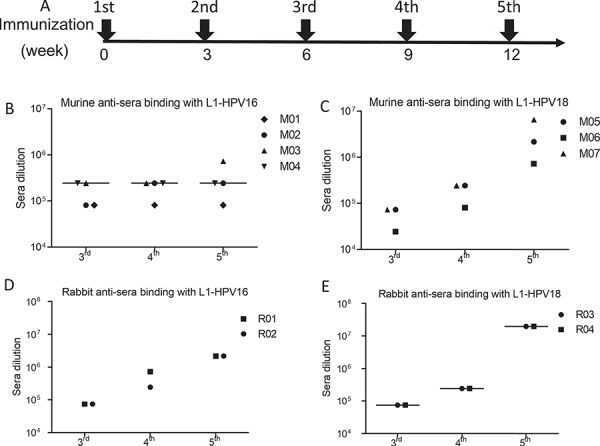
Immunogenicity of HPV16 and HPV18 VLPs. L1 was coated for ELISA detection.(A) The schedule of immunization; the titer of the anti-sera induced by VLP-HPV16 (B) and VLP-HPV18 (C) in mice receiving 50 μg HPV L1-L2 VLPs. X-axis represented the immunization times; Y-axis was the anti-sera dilution fold. The titer of anti-sera induced by VLP-HPV16 (D) and VLP-HPV18 (E) in rabbits receiving 100 μg HPV L1-L2 VLPs immunization.
Neutralizing activities of anti-sera induced by VLPs of HPV
HPV vaccines developed by GSK Biologicals and Merck all indicated that the in vitro titers of neutralization antibody were highly correlated with protection [5, 15]. Thus, neutralization assay was conducted to evaluate the rabbit anti-sera induced by our HPV16/18 L1-L2 VLPs. HPV16 L1-L2 VLPs induced high titers of neutralizing anti-sera against HPV16 infection in rabbits (ID50, >1.0 × 106 dilution and ID90, >1.0 × 105 dilution); in addition, one rabbit anti-serum induced by HPV16 L1-L2 VLP could inhibit HPV45 infection at the ID50 of 250 sera dilution; however, it failed to neutralize HPV18 and HPV31 infections, suggesting the induction of highly type-specific neutralizing activity (Fig. 4). With the similar results of anti-sera induced by HPV16 L1-L2 VLP, the anti-sera induced by HPV18 L1-L2 VLP also exhibited robust inhibitory activity against autologous HPV18 infection (ID50, >1.0 × 106 dilution and ID90, >1.0 × 106 dilution; Fig. 4). Interestingly, anti-sera from both rabbits also prevented HPV31 infection (ID50 = 2.76 × 105 and ID50 = 2.99 × 103; Fig. 4). In order to confirm the anti-sera neutralization result, purified antibody from these anti-sera was used to further evaluate the neutralization against HPV infection. The results of purified antibody were consistent with the results of anti-sera inhibition of HPV infection (Supplementary Fig. 2). Interestingly, the IC50 of heparin (H4784, Sigma-Aldrich), one of the drugs used in the treatment of HPV infection serving as the positive control, in inhibiting HPV16 infection was 25200 ng/ml, much higher than the IC50 of both HPV16 L1-L2 VLP-induced antibodies at 2.774 and 5.06 ng/ml. The IC50 of heparin at 24890 ng/ml against HPV18 infection was also much higher than the IC50 of 5.25 and 8.148 ng/ml of both antibodies. In another word, compared to the heparin control, the HPV16/18 L1-L2 VLP-induced antibodies are at least three orders of magnitude more potent in neutralizing the viral infection (Supplementary Fig. 2). In conclusion, robust neutralizing anti-sera and antibodies were elicited by our HPV16/18 L1-L2 VLPs (Supplementary Fig. 2). It is interesting that HPV16 is genetically much more close to HPV31 and HPV18 much more close to HPV45; they did not show any preferential neutralization against these two strains.
Figure 4.
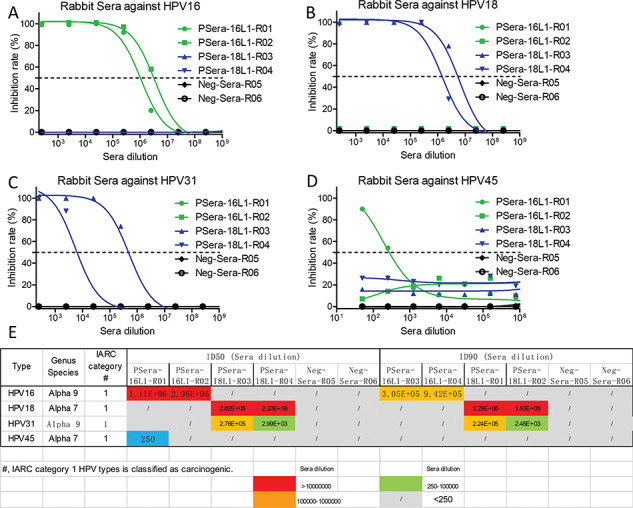
The neutralization end-point of the anti-sera. Green line was labeled as rabbits receiving HPV16 L1-L2 VLPs immunization; blue line was labeled as rabbits receiving HPV18 L1-L2 VLPs immunization; and black line was labeled as rabbits receiving PBS immunization. The end-point of the anti-sera from different groups inhibited the infection of HPV16 (A), HPV18 (B), HPV31 (C) and HPV45 (D), respectively. (E) Summary of the neutralization titers (IC50 and/or IC90) of the anti-sera against different subtypes of HPV.
Head-to-head comparison of Cervarix vs HPV16/18 VLPs
Head-to-head trials of Cervarix vs Gardasil demonstrated that Cervarix was superior to Gardasil with the regard of GMT and seropositivity retention [14]. Thus, Cervarix was chosen for the head-to-head comparison of bivalent HPV vaccine Cervarix vs our HPV16/18 VLPs in rabbits. In order to compare the HPV16/18 VLPs with Cervarix based on insect cell-produced HPV16/18 L1 VLP by GSK, the same dosage and immunization regimen were conducted (Fig. 5A). Though the adjuvant of AS04 in Cervarix was better than alum adjuvant in our VLPs [28], the titers of the anti-sera induced by VLPs were higher or at least comparable at the end of the second immunization and the third immunization (Fig. 5B–E). In addition, the second anti-sera induced by VLPs still exhibited comparable potency of neutralizing activity against HPV16 and HPV18 infections as compared with that of Cervarix (Fig. 6A and B). In addition, anti-sera induced by the VLPs showed significantly higher potency of inhibition against HPV31 and HPV45 infections than the anti-sera induced by Cervarix (Fig. 6C and D). The third anti-sera showed the similar neutralization potency as the second anti-sera (Supplementary Fig. 3). These data indicate that at least comparable neutralizing activities were induced by mammalian cell-produced VLPs and more broadly neutralizing anti-sera was induced as compared with Cervarix even though a much less potent adjuvant was used in our VLPs.
Figure 5.
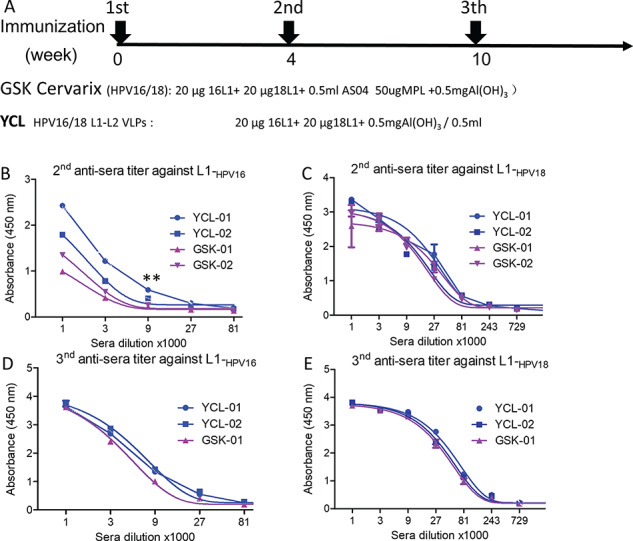
Head-to-head comparison of GSK-Cervarix vs YCL-HPV16/18. The blue line (YCL-01 and YCL02) indicated the animal number receiving our YCL-HPV16/18 L1-L2 VLPs immunization; the pink line (GSK-01 and GSK-02) indicated the animal number receiving the immunization of the Cervarix of GSK. (A) The schedule of immunization; the characterization of the second anti-sera against HPV16 L1 (B) and HPV18 L1 (C), respectively; the characterization of the third anti-sera against HPV16 L1 (D) and HPV18 L1 (E), respectively. Data represent mean ± SD; two-tailed, unpaired, Student’s t-tests were performed. **P < 0.01.
Figure 6.
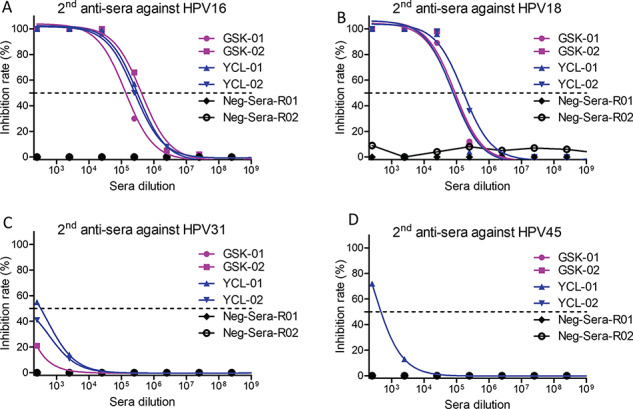
The neutralization of the second anti-sera. Line was labeled as Fig. 5; the second anti-sera from different animal neutralized HPV16 (A) HPV18 (B), HPV31 (C) and HPV45 (D), respectively.
DISCUSSION
In this study, plasmid encoding L1 and L2 proteins was transfected into 293TT cells to produce VLPs containing L1-L2 proteins of HPV16 and HPV18. Our L1-L2 VLPs formed nanoparticles of about 50 nm in diameter, which was the similar size as the native HPV virus [7]. The morphology of the VLPs was agreement with published results [5, 29] and the VLP preparations contained highly homogeneous particles as revealed by electron microscopy. The immunogenicity of the VLPs was investigated in mice and rabbits, in which potent and broadly neutralizing antibodies were induced.
Head-to-head comparison of bivalent HPV vaccine of Cervarix vs our HPV16/18 L1-L2 protein was conducted. Compared to VLPs of Cervarix, the size homogeneity of our VLP particles was better [29]. One possible reason lies that our VLPs were generated in mammalian cells system in which HPV virus infects and replicates; thus, the proteins may have proper glycosylation and conformation, while Cervarix was produced using baculovirus expression system; another possible reason is that the introduction of L2 protein may help to stabilize VLPs by forming a structurally native 5xL1-1xL2 pentamer [30]. The consistent size of VLP was required for, or surrogate of, the immunogenicity of prophylactic HPV vaccine [25, 26]. Moreover, head-to-head comparisons of bivalent HPV vaccine of Cervarix vs our HPV16/18 L1-L2 VLPs were conducted and showed that our HPV16/18 L1-L2 VLP presented higher titers of anti-sera and elicited comparable neutralization activity against HPV16 and HPV18 infections though Alum adjuvant in our HPV L1-L2 VLPs formulation was much inferior to AS04 in Cervarix, indicating potential longer duration of protection by L1-L2 VLPs. AS04 formulation was previously shown to elicit over 5-fold higher titers of HPV16/18 L1 VLP-specific antibodies than aluminum salt formulation did [28]. In addition, anti-sera induced by our VLPs showed significantly higher activity in the prevention of HPV31 and HPV45 infections than the anti-sera induced by Cervarix. Compared to Cervarix, the improved immunogenicity of our HPV16/18 L1-L2 VLPs may result from the consistent morphology, and the sizes of VLPs in that VLPs were produced in mammalian cells with proper glycosylation and conformation and the introduction of L2 protein, which may help to stabilize VLPs. L2 protein functions in both the infection process and virion assembly, and it could induce cross-neutralizing antibodies for its highly conserved sequence among various HPVs [19]. While the major challenge for developing L2 protein as a vaccine is its poor immunogenicity as compared to L1. L2 is concealed inside the L1 pentamer [19]. Natural infection or immunization against HPV L1/L2 VLPs produced predominantly L1 antibodies with little or no L2 antibodies. Thus, it is highly plausible that L2 protein stabilizes VLPs and, therefore, contributes to the improved immunogenicity of the VLP [5, 19, 20]. Since there was a lack of suitable animal model for mimicking HPV infection, in vitro antibody titers and the potency of neutralization were taken as the indication of efficacy of our HPV16/18 L1-L2 VLPs.
In order to further confirm whether mammalian cell system could be applied to produce other types of VLPs HPV6, HPV11, HPV31, HPV52 and HPV58 were generated in our mammalian cell system. VLPs were formed at the end of multi-purification process and were observed by negative staining electron microscopy. All VLPs from different subtypes showed consistent morphology and uniformed sizes, which indicates that such mammalian cell system could be widely applied to produce VLPs of various HPV subtypes (Supplementary Fig. 4). Given that Chinese Hamster Ovary (CHO) cells now dominate the domain of mass production of recombinant protein products because of their capacity for single-cell suspension growth, VLPs produced in this system will enjoy the benefits of scale-up processes and regulatory advantages [31]. In the future, we are going to generate CHO stable cell line to produce L1-L2 VLPs and are going to test the efficacy of other HPV types generated by mammalian cell system. It is desirable to demonstrate that such a potential candidate HPV VLP vaccine based on L1-L2 proteins produced in mammalian cell system will provide with longer duration and more broadly protection against HPV infection.
AUTHORS' CONTRIBUTIONS
XW performed most experiments, analyzed the data and wrote the manuscript. XM, YL, YX, NZ, SX, provided technical assistance and did animal experiments. ZW designed the study, monitored and financially supported the study and revised the manuscript. All authors critically reviewed the drafts of the manuscript and approved the final version.
Supplementary Material
FUNDING
This work was supported by National Science Foundation of China (NSFC) (81803414), Jiangsu Province Natural Science Foundation for Young Scholar (BK20170653), The National Key Research and Development Program of China from the Ministry of Sciences and Technologies (2016YFC1201000), Nanjing University–Ninxia University Collaborative Project (2017BN04), Jiangsu Province “Innovative and Entrepreneurial talent” and Six Talent Peaks Project in Jiangsu Province of China.
Conflicts of Interest Statement. None declared.
References
- 1. Small, W Jr, Bacon, MA, Bajaj, A et al. Cervical cancer: a global health crisis. Cancer 2017; 123: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 2. Roden, RBS, Stern, PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer 2018; 18: 240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brianti, P, De Flammineis, E, Mercuri, SR. Review of HPV-related diseases and cancers. New Microbiol 2017; 40: 80–85. [PubMed] [Google Scholar]
- 4. Doorbar, J, Quint, W, Banks, L et al. The biology and life-cycle of human papillomaviruses. Vaccine 2012; 30: F55–F70. [DOI] [PubMed] [Google Scholar]
- 5. Castle, PE, Maza, M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect 2016; 144: 449–468. [DOI] [PubMed] [Google Scholar]
- 6. Araldi, RP, Assaf, SMR, Carvalho, RF et al. Papillomaviruses: a systematic review. Genet Mol Biol 2017; 40: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doorbar, J, Egawa, N, Griffin, H et al. Human papillomavirus molecular biology and disease association. Rev Med Virol 2015; 25: 2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schellenbacher, C, Roden, R, Kirnbauer, R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol 2009; 83: 10085–10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villa, LL, Costa, RL, Petta, CA et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6: 271–278. [DOI] [PubMed] [Google Scholar]
- 10. Lowy, DR, Schiller, JT. Prophylactic human papillomavirus vaccines. J Clin Invest 2006; 116: 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FUTURE II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356: 1915–1927. [DOI] [PubMed] [Google Scholar]
- 12. Paavonen, J, Jenkins, D, Bosch, FX et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161–2170. [DOI] [PubMed] [Google Scholar]
- 13. Skinner, SR, Szarewski, A, Romanowski, B et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet 2014; 384: 2213–2227. [DOI] [PubMed] [Google Scholar]
- 14. Einstein, MH, Baron, M, Levin, MJ et al. Comparison of the immunogenicity and safety of Cervarix (TM) and Gardasil (R) human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin 2009; 5: 705–719. [DOI] [PubMed] [Google Scholar]
- 15. Harper, DM, DeMars, LR. HPV vaccines—a review of the first decade. Gynecol Oncol 2017; 146: 196–204. [DOI] [PubMed] [Google Scholar]
- 16. Kash, N, Lee, MA, Kollipara, R et al. Safety and efficacy data on vaccines and immunization to human papillomavirus. J Clin Med 2015; 4: 614–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huh, WK, Joura, EA, Giuliano, AR et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet 2017; 390: 2143–2159. [DOI] [PubMed] [Google Scholar]
- 18. Zhai, L, Tumban, E. Gardasil-9: a global survey of projected efficacy. Antiviral Res 2016; 130: 101–109. [DOI] [PubMed] [Google Scholar]
- 19. Wang, D, Li, Z, Xiao, J et al. Identification of broad-genotype HPV L2 neutralization site for pan-HPV vaccine development by a cross-neutralizing antibody. PLoS One 2015; 10: e0123944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jagu, S, Karanam, B, Gambhira, R et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst 2009; 101: 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buck, CB, Pastrana, DV, Lowy, DR et al. Generation of HPV pseudovirions using transfection and their use in neutralization assays. In: Davy C., Doorbar J. (eds). Methods in Molecular Medicine, NJ, USA: Humana Press, 2005, 445–462 [DOI] [PubMed] [Google Scholar]
- 22. Zhou, J, Cheung, AK, Tan, Z et al. PD1-based DNA vaccine amplifies HIV-1 GAG-specific CD8+ T cells in mice. J Clin Invest 2013; 123: 2629–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu, X, Guo, J, Niu, M et al. Tandem bispecific neutralizing antibody eliminates HIV-1 infection in humanized mice. J Clin Invest 2018; 128: 2239–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leigh Brown, AJ, Frost, SD, Mathews, WC et al. Transmission fitness of drug-resistant human immunodeficiency virus and the prevalence of resistance in the antiretroviral-treated population. J Infect Dis 2003; 187: 683–686. [DOI] [PubMed] [Google Scholar]
- 25. Zhao, Q, Allen, MJ, Wang, Y et al. Disassembly and reassembly improves morphology and thermal stability of human papillomavirus type 16 virus-like particles. Nanomedicine 2012; 8: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 26. Lowy, DR. HPV vaccination to prevent cervical cancer and other HPV-associated disease: from basic science to effective interventions. J Clin Invest 2016; 126: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen, XS, Garcea, RL, Goldberg, I et al. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell 2000; 5: 557–567. [DOI] [PubMed] [Google Scholar]
- 28. Giannini, SL, Hanon, E, Moris, P et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24: 5937–5949. [DOI] [PubMed] [Google Scholar]
- 29. Deschuyteneer, M, Elouahabi, A, Plainchamp, D et al. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in CervarixTM, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum Vaccin 2010; 6: 407–419. [DOI] [PubMed] [Google Scholar]
- 30. Sapp, M, Fligge, C, Petzak, I et al. Papillomavirus assembly requires trimerization of the major capsid protein by disulfides between two highly conserved cysteines. J Virol 1998; 72: 6186–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wurm, FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 2004; 22: 1393–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


