Abstract
Objective
In our study, it was aimed to determine whether there were differences in genial tubercle dimensions depending on age and gender.
Methods
In this study, 220 cone beam computed tomography (CBCT) images of patients (110 female and 110 male) between the ages of 20–80 years were obtained from the archive of İzmir Katip Çelebi University Faculty of Dentistry. All patients were divided into decade groups according to their age, and each decade group was divided into two subgroups according to gender. The genial tubercle was defined radiologically using axial, coronal and sagittal sections as well as 3D reconstruction image with NNT software program. Sagittal, vertical and horizontal dimensions of the genial tubercle were measured and statistically analyzed.
Results
There was a weak negative correlation between age groups and vertical values (r=−0.142; p=0.036) whereas the correlation coefficients between age groups and sagittal and horizontal values were not statistically significant (r=−0.043; p=0.530 and r=−0.039; p=0.563). There was a strong positive correlation between vertical and sagittal values in men (r=0.705, p<0.001) and women (r=0.714, p<0.001) in the whole group. There was a weak positive correlation between horizontal and sagittal, horizontal and vertical values in men (r=0.362, p<0.001; r=0.231, p<0.001) and women (r=0.304, p<0.001; r=0.257, p=0.007) in the whole group.
Conclusion
The vertical and horizontal dimensions of genial tubercle of men were higher than that of women. As the age of the patients increased, a decrease in the vertical values of the genial tubercle was observed.
Keywords: Age, chin, forensic dentistry, gender
INTRODUCTION
During the growth process, the mandible grows downward and forward as a whole (1). The corpus region of the mandible, which contains the genial tubercle, grows forward with the effect of the ramus and the alveolar bone of the upper jaw (2).
The aging process, which begins after the development is completed, causes morphological changes in the bone structure of the face and bones are subject to remodeling (3). Microstructural changes are observed in the bone matrix as a result of the change of mineral components during the aging process. The aging process has a significant impact on bone mineral density and calcium concentration. In order to reveal age-related changes in skull structure, studies have been carried out on skulls of young and old individuals and Computed Tomography (CT) data (4). Aging of the craniofacial structure is not only due to bone atrophy, but also due to bone expansion and changes in dynamics due to bone loss (5).
Facial bones, which loose their density and thickness due to age, cause some changes in face shape. Bone quantity loss belonging to age groups are as follows (6):
20–30 age group: A small percentage of bone loss is seen in this age group.
30–40 age group: The base of the nose gets bigger. Some changes are observed in the chin area.
40–50 age group: The chin area continues to expand. Eye sockets begin to widen.
50–60 age group: Menopause effects in women. There is a decrease in bone density due to the combination of low growth hormone and estrogen levels.
The genial tubercle is located on the midline on the lingual face of the mandible and slightly above the lower margin as four bone ledge called spina mentalis; The two upper protrusions are called spina mentalis superior, and the lower two protrusions are called spina mentalis inferior. The genioglossus muscle attaches to the upper protrusions, and the geniohyoideus muscle to the lower protrusions (7).
There are studies reporting that defining the morphology of the genial tubercles is valuable for different dental applications, and the morphology, position and dimensions of the genial tubercle are important in some cases (8–11). Some studies have reported that genial tubercle dimensions are important in complete denture stability in the mandible (12, 13). Genial tubercles can also be used as a reference in the assessment of mandibular asymmetry (11). It can also be a guide in determining a safe area in the mental foramina region in the mandible before implant surgery. Besides, it is reported that genial tubercles are an anatomical hard tissue parameter that can be used in the surgical treatment of Obstructive Sleep Apnea (OSA) (14).
The muscles attached to the genial tubercle are closely related to the function and support of the tongue and its associated soft tissues (15). The first of these is the genioglossus muscle, which is the main muscle that protrudes the tongue, and its contraction expands and stabilizes the area of the upper airway most prone to collapse. The other is the geniohyoideus muscle; It is a narrow muscle located superior to the medial border of the mylohyoid muscle and originates from the spina mentalis inferior, behind the mandibular symphysis, and moves back and slightly downward, inserting into the anterior part of the body of the hyoid bone. It is in contact with its fellow of the other side. The geniohyoid muscle, which belongs to the group of suprahyoid muscles, pulls the hyoid bone forward and upward (16). And by expanding the upper airway, it helps breathing (17). In the first stage of swallowing, as the food mass moves from the mouth to the pharynx, the hyoid bone and accompanying tongue is pulled up and forward by the anterior bellies of the digastric muscle, the mylohyoid muscle, and geniohyoid muscle. It also helps to lower the mandible (18). The tongue prevents posterior collapse by contracting the genioglossus muscle during sleep (19). Especially during the REM period of sleep, relaxation of the genioglossus and the geniohyoid muscles has been reported to play a role in OSA (18).
In order to better understand its morphology, it is useful to use CBCT to measure the height and width of the tubercle in individuals of different ages and genders. Studies in the literature also show that CBCT is an accurate method for evaluating the morphology, dimensions and position of the genial tubercle (19). Studies have shown that it provides acceptable precision in linear measurements on alveolar bone and mandible (20).
Another area of use where the awareness of the importance of knowing the location of the morphology of genial tubercles is increasing in forensic dentistry. As in forensic medicine, x-ray records are used in many parameters (age and gender) related to identification in forensic dentistry and radiology science is used. X-ray records are one of the important evaluation criteria used by forensic medicine and forensic dentistry, as they shed light on many issues both in determining the current situation and in comparisons with the past (21). Forensic dentistry is not only concerned with the identification of the dead and the finding of the disappeared, but also deals with the forensic cases in the living. In this case, the forensic odontologist can assist in the investigation by narrowing the age group in which the deceased may be included, in the light of the data obtained (22).
In this study, we aimed to examine the genial tubercles of patients by making 3D measurements on the CBCT images in the archive of Izmir Katip Çelebi University Faculty of Dentistry. We aimed to investigate whether there are age- and gender-related differences in the genial tubercle dimensions.
METHODS
In our cross-sectional study, tomography data which was previously taken and kept ready at our university in order to observe the changes in the bones, teeth, soft tissues and airways of the patients were used. In the study, 220 CBCT images (110 female and 110 male) were obtained from the archive of İzmir Katip Çelebi University Faculty of Dentistry Department of Oral and Maxillofacial Radiology. All patients were divided into ten-year groups according to their age, and each ten-year group was divided into two subgroups according to gender. Patients who did not show asymmetry were selected considering the criteria defined by Wang et al. (23)
The study was approved by the “İzmir Katip Çelebi Üniversity Non-Interventional Clinical Research Ethics Committee” (Date:04.09.2020, Approval Number: 2020-GOKAE-0175). CBCT images included in this retrospective study were determined according to the following criteria:
Inclusion criteria:
Being twenty years old or older,
No dental anomaly or tooth loss in the anterior mandible,
No craniofacial deformity (i.e. any bone related disorders) and/or facial asymmetry observed.
380 individuals who met the general criteria determined by measuring cephalometric radiographs of 1100 individuals who had previously registered for diagnosis and treatment purposes in the archives of X University Faculty of Dentistry Department of Oral and Maxillofacial Radiology were selected and their CBCT images were screened. Among the individuals whose CBCT images were screened, CBCT images of 220 persons who comply with the inclusion criteria of the study were included in the study. The study did not have a control group. In our study, there are a total of 18 groups; 6 main groups and 12 subgroups.
All CBCT images were obtained with NewTom 5G (QR, Verona, Italy) flat panel CBCT device using 110 kVp and 1–20 mA parameters, 15×12 FOV (Field Of View) field and 0.2 mm voxel size. Images were analyzed using the NNT (QR-NNT V9.1, Verona, Italy) software program. The head orientation is standardized so that the Frankfurt horizontal plane is perpendicular to the ground and the Porion plane (the line passing through the right and left Porion points) is parallel to the ground, because CBCT device in the Radiology Department of the hospital takes images in the supine position.
The genial tubercle was defined radiologically using axial, coronal and sagittal sections as well as 3D reconstruction image (Figure 1); and it has been identified as the center of the four genial tubercles of the mandible. Genial tubercle dimensions were calculated in axial and sagittal sections. In the axial section, the most concave points were identified on the lingual surface of the anterior segment of the mandible near the genial tubercle. A line was obtained by combining these two points and this line was measured in millimeters. The horizontal width of the genial tubercle was calculated using this line. In the sagittal section, first the most concave points on both sides of the genial tubercle were marked and a line was formed between these two points. Through this line, the vertical width of the genial tubercle was calculated in millimeters. Then, a point was marked at the top of the genial tubercle and a second line was drawn perpendicular to the vertical width line from this point and terminating on this line to measure the height of the genial tubercle in millimeters. (Figure 2).
Figure 1.
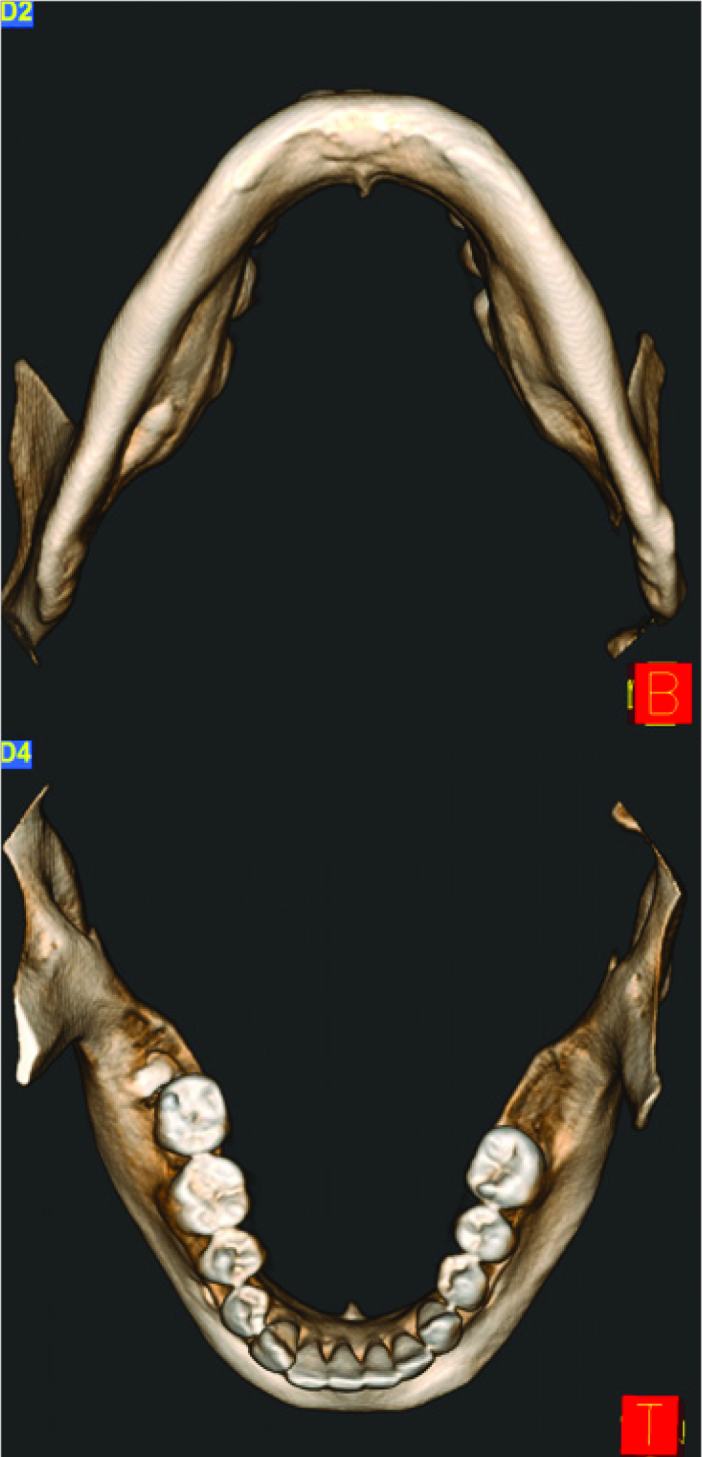
The images of the genial tubercle on 3D reconstruction
Figure 2.
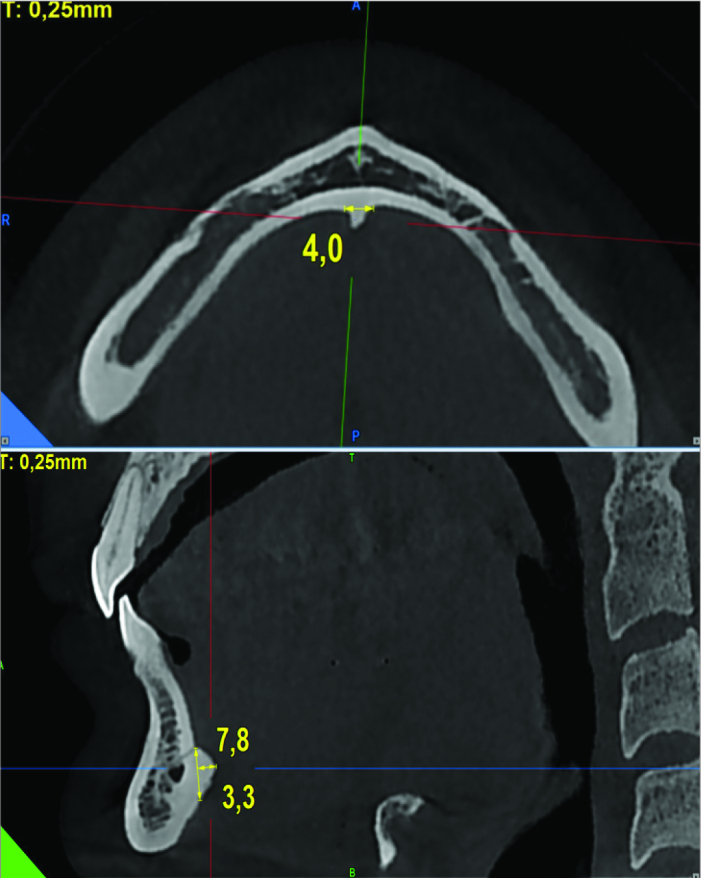
Measurement of the genial tubercle in the axial, horizontal and vertical direction in the sections
Statical Analysis
The data were evaluated by The Statistical Package for Social Sciences version 25.0 software (IBM Corp.; Armonk, NY, USA). The normal distribution of the data was analyzed using the Shapiro Wilk test. Homogeneity of variances was evaluated by Levene’s test. Since the data for sagittal, vertical and horizontal values had a skewed distribution, logarithmic transformation of the data was performed. Descriptive statistics were given as geometric mean (GM) and 95% confidence limits of the real values, since the data were transformed logarithmically. Independent samples t-test was used to compare sagittal, vertical and horizontal values according to gender, and one-way analysis of variance was used for comparison of age groups. Comparison of sagittal, vertical and horizontal values in age groups by gender was made by two-factor analysis of variance from general linear models. The Sidak test was used as a multiple comparison test in the two-factor analysis of variance. The relationship between sagittal, vertical and horizontal values was evaluated by Pearson correlation analysis. p value of <0.05 was considered statistically significant.
RESULTS
Sagittal, vertical and horizontal values of men and women in all age groups with logarithmic transformations showed normal distribution. Sagittal values did not differ according to gender (p=0.171). Vertical values of men were statistically higher than women (p=0.015). Horizontal values of men were statistically higher than women (p<0.001) (Table 1).
Table 1.
Comparison of sagittal, vertical and horizontal values by gender groups
| Gender | Geometric Mean | 95% Confidence Limits | Test Statistics | |||
|---|---|---|---|---|---|---|
|
| ||||||
| t | p | |||||
| Sagittal | Male | 1.77 | 1.62 | 1.93 | 1.374 | 0.171 |
| Female | 1.61 | 1.45 | 1.78 | |||
| Vertical | Male | 5.58 | 5.22 | 5.97 | 2.46 | 0.015 |
| Female | 5 | 4.72 | 5.3 | |||
| Horizontal | Male | 8.16 | 7.70 | 8.64 | 5.094 | <0.001 |
| Female | 6.54 | 6.13 | 6.97 | |||
Sagittal, vertical and horizontal values were compared according to age groups. Sagittal, vertical and horizontal values were not statistically different according to age groups (p<0.05) (Table 2).
Table 2.
Comparison of sagittal, vertical and horizontal values by age groups
| Age Groups | Geometric Mean | 95% Confidence Limits | Test Statistics | |||
|---|---|---|---|---|---|---|
|
| ||||||
| f | p | |||||
| Sagittal | 20–30 | 1.84 | 1.61 | 2.09 | 1.256 | 0.284 |
| 30–40 | 1.65 | 1.41 | 1.93 | |||
| 40–50 | 1.71 | 1.44 | 2.04 | |||
| 50–60 | 1.57 | 1.33 | 1.84 | |||
| 60–70 | 1.53 | 1.29 | 1.81 | |||
| 70–80 | 2.03 | 1.57 | 2.63 | |||
| Vertical | 20–30 | 5.81 | 5.21 | 6.48 | 1.686 | 0.139 |
| 30–40 | 5.4 | 4.75 | 6.14 | |||
| 40–50 | 5.42 | 4.95 | 5.93 | |||
| 50–60 | 4.87 | 4.41 | 5.37 | |||
| 60–70 | 4.88 | 4.35 | 5.47 | |||
| 70–80 | 5.48 | 4.84 | 6.2 | |||
| Horizontal | 20–30 | 7.5 | 6.67 | 8.44 | 0.771 | 0.572 |
| 30–40 | 7.37 | 6.61 | 8.24 | |||
| 40–50 | 6.93 | 6.18 | 7.77 | |||
| 50–60 | 7.31 | 6.54 | 8.17 | |||
| 60–70 | 7.01 | 6.39 | 7.69 | |||
| 70–80 | 8.14 | 7.07 | 9.37 | |||
When the model statistics were examined, sagittal values were similar according to age and gender groups. Considering the average values, the sagittal values of female patients in the 60–70 age group were higher than that of men; and also, in other age groups, although the sagittal values of men were higher than that of women, the differences were not statistically significant (Table 3). According to the model and test statistics, the vertical values of male patients in the 40–50 age group were statistically higher than women. The differences between vertical values between male and female patients in other age groups were not statistically significant (Table 3). According to the model and test statistics, the horizontal values of male patients in the 20–30, 40–50 and 60–70 age groups were statistically higher than that of women. In the 30–40 and 70–80 age groups, the values of men were high and the difference with women is close to statistical significance (Table 3).
Table 3.
Comparison of sagittal, vertical and horizontal values according to group and age variables
| Direction | Age Groups | Gender | Geometric Mean | 95% Confidence Limits | Test Statistics | Model Statistics | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| f | p | |||||||
| SAGITTAL | 20–30 | Male | 2.01 | 1.66 | 2.43 | 1.294 | 0.257 | Age groups effect: f= 1.250; p= 0.287; Gender effect: f=2.360; p= 0.126; Age groups* gender effect F= 0.646; p= 0.665 |
| Female | 1.68 | 1.38 | 2.04 | |||||
| 30–40 | Male | 1.69 | 1.31 | 2.17 | 0.067 | 0.795 | ||
| Female | 1.62 | 1.31 | 2 | |||||
| 40–50 | Male | 1.81 | 1.51 | 2.15 | 0.403 | 0.526 | ||
| Female | 1.63 | 1.19 | 2.24 | |||||
| 50–60 | Male | 1.72 | 1.37 | 2.16 | 1.285 | 0.258 | ||
| Female | 1.44 | 1.13 | 1.83 | |||||
| 60–70 | Male | 1.44 | 1.19 | 1.75 | 0.631 | 0.428 | ||
| Female | 1.63 | 1.23 | 2.18 | |||||
| 70–80 | Male | 2.33 | 1.65 | 3.28 | 1.433 | 0.233 | ||
| Female | 1.78 | 1.15 | 2.76 | |||||
| VERTICAL | 20–30 | Male | 6.26 | 5.27 | 7.44 | 2.025 | 0.156 | Age groups effect: f= 1.715; p=0.132; Gender effect: f=5.941; p=0.016; Age groups* gender effect: f=0.726; p=0.605 |
| Female | 5.39 | 4.68 | 6.21 | |||||
| 30–40 | Male | 5.58 | 4.42 | 7.03 | 0.352 | 0.554 | ||
| Female | 5.24 | 4.57 | 6 | |||||
| 40–50 | Male | 6.01 | 5.3 | 6.83 | 3.890 | 0.049 | ||
| Female | 4.89 | 4.32 | 5.53 | |||||
| 50–60 | Male | 5.29 | 4.66 | 6.01 | 2.64 | 0.118 | ||
| Female | 4.49 | 3.85 | 5.22 | |||||
| 60–70 | Male | 4.78 | 4.02 | 5.68 | 0.168 | 0.682 | ||
| Female | 4.99 | 4.22 | 5.9 | |||||
| 70–80 | Male | 5.87 | 5.16 | 6.69 | 0.846 | 0.359 | ||
| Female | 5.12 | 4.07 | 6.46 | |||||
| HORIZONTAL | 20–30 | Male | 8.52 | 7.39 | 9.82 | 6.125 | 0.014 | Age groups effect: f= 0.851; p= 0.515; Gender effect: f= 24.315; p<0.001; Age groups* gender effect: f= 0.570; p= 0.723 |
| Female | 6.61 | 5.5 | 7.95 | |||||
| 30–40 | Male | 8.04 | 6.89 | 9.39 | 2.821 | 0.095 | ||
| Female | 6.77 | 5.75 | 7.97 | |||||
| 40–50 | Male | 8.23 | 7 | 9.67 | 11.266 | 0.001 | ||
| Female | 5.84 | 5.12 | 6.66 | |||||
| 50–60 | Male | 7.75 | 6.7 | 8.96 | 1.284 | 0.258 | ||
| Female | 6.9 | 5.77 | 8.25 | |||||
| 60–70 | Male | 7.78 | 6.97 | 8.7 | 4.176 | 0.042 | ||
| Female | 6.32 | 5.48 | 7.29 | |||||
| 70–80 | Male | 9.19 | 7.55 | 11.18 | 2.781 | 0.097 | ||
| Female | 7.22 | 5.87 | 8.88 | |||||
When the relationships between age groups and sagittal, vertical and horizontal values were evaluated, there was a weak negative correlation between age groups and vertical values (r=−0.142; p=0.036). As the age of the patients increased, a decrease in the vertical values was observed. The correlation coefficients between age groups and sagittal and horizontal values were not statistically significant (r=−0.043; p=0.530 and r=−0.039; p=0.563).
According to correlation coefficients between age groups and sagittal, vertical and horizontal values were not statistically significant (r=−0.037; p=0.701; r=−0.109; P=0.257 and r=−0.023; p=0.809). In males, there was a weak negative correlation between age groups and vertical values (r=−0.211; p=0.027). As the age of male patients increased, a decrease in vertical values was observed. Correlation coefficients between age groups and sagittal and horizontal values in males were not statistically significant (r=−0.049; p=0.611 and r=−0.083; p=0.386).
In the whole group, there was a weak positive correlation between horizontal and sagittal, horizontal and vertical values in men and women (Table 4; Figure 3, 4). In the whole group, there was a strong positive correlation between vertical and sagittal values in men and women (Figure 5).
Table 4.
Correlations between sagittal, vertical and horizontal values for the whole group and gender
| All Groups | Male | Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Sagittal | Vertical | Sagittal | Vertical | Sagittal | Vertical | |||||||
|
|
|
|
|
|
|
|||||||
| r | p | r | P | R | p | r | p | r | p | r | p | |
| Vertical | 0.704 | <0.001 | - | - | 0.705 | <0.001 | - | - | 0.714 | <0.001 | - | - |
| Horizontal | 0.34 | <0.001 | 0.279 | <0.001 | 0.362 | <0.001 | 0.231 | 0.015 | 0.304 | <0.001 | 0.257 | 0.007 |
r: Pearson’s correlation coefficient
Figure 3.
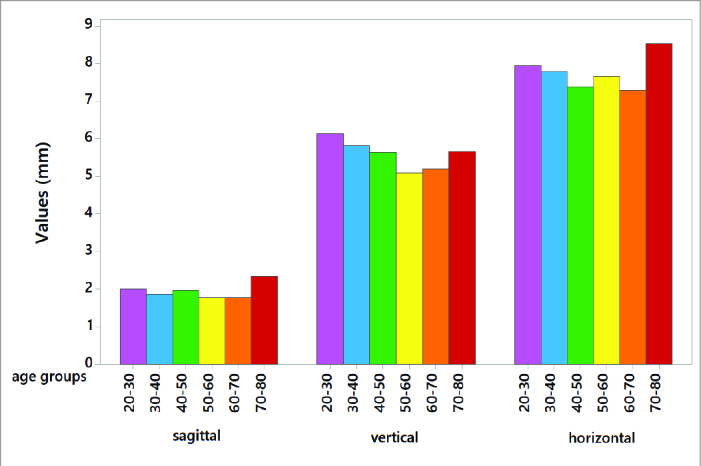
Graphical illustration of the sagittal, vertical and horizontal values by different age groups.
Figure 4.
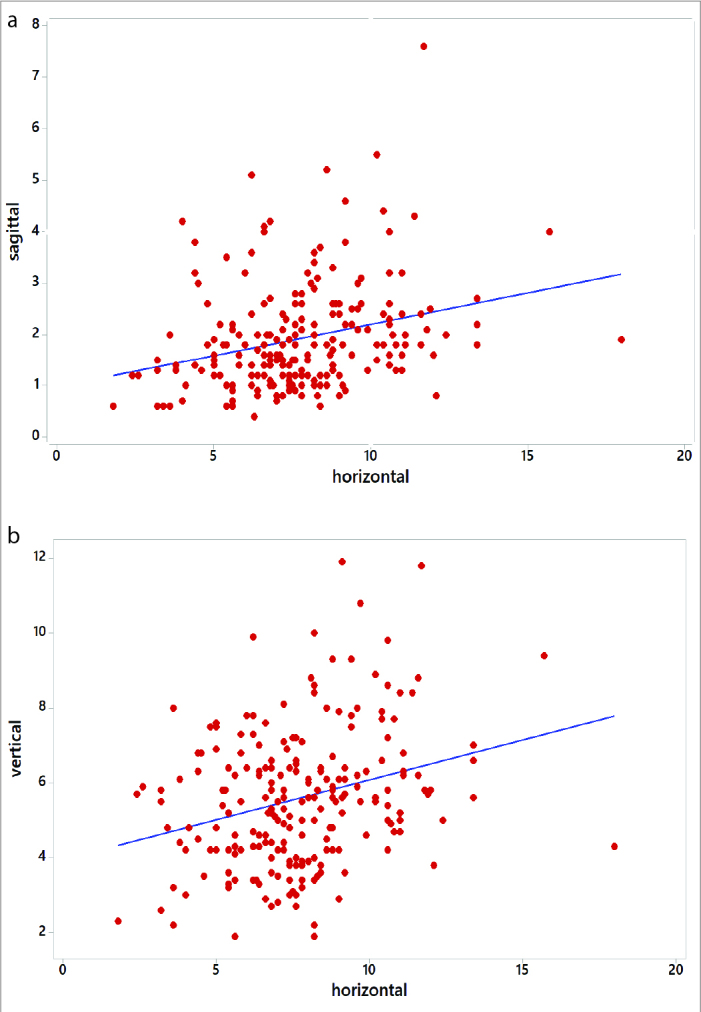
Graphs showing positive correlation between (a) sagittal and horizontal and (b) vertical and horizontal values in the pooled sample (men and women).
Figure 5.
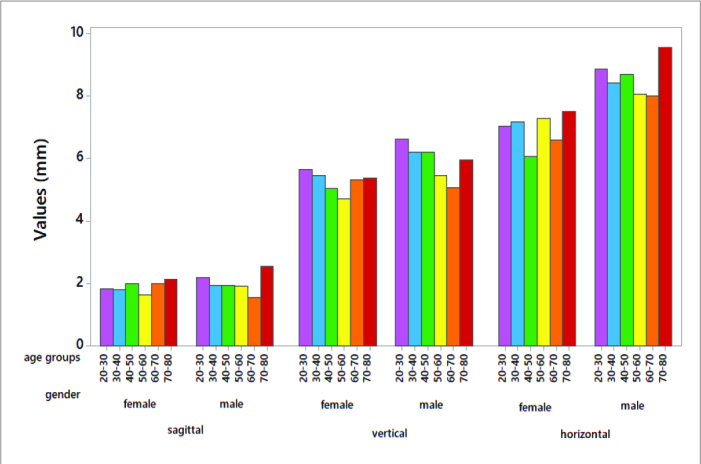
Graphical illustration of the sagittal, vertical and horizontal values by different age groups.
DISCUSSION
Although the genial tubercles are defined as four tubercles bilaterally surrounding the lingual foramina in pairs on the lingual side of the mandible, on the right and left sides of the midline, it is stated that the morphology and dimensions of these anatomical structures are still controversial (24).
Many studies have shown that the anatomical linear measurements of the alveolar bone and mandible with CBCT are acceptable (21). However, cadaver studies have also been conducted to define the genial tubercle region more clearly (25). Results of a study conducted by Hueman et al. (26) showed that CBCT is a reliable method for determining the anatomical location of genial tubercles. Compared to conventional CT, CBCT has the advantages of relatively less radiation dose, low cost, accessibility, and multiplanar reconstruction images (24). Therefore, in our study, CBCT images were preferred for anatomical measurements of genial tubercles.
Studies based on cadaver dissections and radiographic imaging have found some differences among the patient population, as in many anatomical structures (25). In genioglossus advancement surgery, various studies have been conducted in various populations examining the morphology, position and dimensions of the genial tubercle to contribute to pre-surgical evaluation and planning (15, 27). Kolsuz et al. (28) examined the location and morphology of the genial tubercle using CBCT in a Turkish population. Only a few of the studies in the literature divided the total study group into groups by gender (15, 26). Nejaim et al. (15) found that the height of the genial tubercle was significantly higher in men compared to women; on the other hand, they did not find a significant difference in width. However, in the study of Kai Yin et al. (27), no statistically significant difference related to genial tubercle height between genders was found. In our study, vertical and horizontal measurements were compared both between genders and age groups.
After the development is completed and the aging process begins, there is no study that specifically compares the morphology and dimensions of the genial tubercle between different age groups. Some researchers draw attention to genial tubercle fractures observed especially in older ages, and it is not known whether there is a significant difference in genial tubercle morphology and dimensions between age groups (10, 29).
It has been reported that CBCT, which has been increasingly used in clinical dentistry practice, is especially useful in age and gender determination studies in the field of forensic dentistry and orthodontics (21, 30). Today, archive records are created with the presence of CBCT devices in imaging centers and dentistry faculties, and these records can be consulted in forensic cases when needed for the identification of individuals, age and gender determination (30). Some authors have reported that the evaluation of anatomical structures with the help of CBCT can be used in determining age and gender in forensic dentistry (21, 30). In our study, although not statistically significant, the genial tubercle values of men in the 30–40 and 70–80 age groups were found to be high, and the difference with women is close to statistical significance. In addition, according to the model and test statistics made in our study, it was observed that the vertical values of male patients in the 40–50 age group were statistically higher than women. However, according to the model and test statistics, the horizontal values of male patients in the 20–30, 40–50 and 60–70 age groups were found to be statistically higher than women. It is thought that these findings will provide important information in determining the age range and gender, especially in forensic dentistry.
According to the findings we obtained in our study, the genial tubercle vertical values of men are statistically higher than that of women. Similarly, the mean horizontal values of the genial tubercle were found to be statistically significantly higher in men than in women. These differences in the vertical and horizontal dimensions of the genial tubercles between genders can be attributed to the fact that the anatomical structures of men are physically larger than that of women. This finding is again an important finding for forensic dentistry.
Various surgical approaches have been reported to advance the mandible forward to increase the hypopharyngeal airway space in patients with mandibular retrognathie. These approaches are to position the tongue muscles forward to reduce airway resistance. However, the anatomy and morphology of these muscles are not clearly defined in the literature (31). Therefore, preoperative planning requires an important anatomical hard tissue such as genial tubercles, and the localization, morphology and dimensions of these structures gain importance. There are not many studies in the literature that associate the localization, morphology and dimensions of genial tubercles with orthodontics. In a retrospective study of 101 women and 100 men, with 201 CBCT images, Kolsuz et al. (28) concluded that the genial tubercle was different in terms of its localization, morphology, and dimensions in the study group. Although they used different measurement methods from our study, they found that the dimensions of the genial tubercle were larger in male patients compared to female patients, similar to our findings. In addition, a high positive correlation was found between vertical and sagittal values in the whole group, for men and women. In the whole group, there was a weak positive correlation between horizontal and sagittal, horizontal and vertical values in men and women. When the relationships between age groups and sagittal, vertical and horizontal values were evaluated, it was observed that there was a weak negative correlation between age groups and vertical values. These findings show that the vertical values of the genial tubercles decrease as the patients’ age increases. When these current findings are evaluated together, it is thought that the age-and gender-related genial tubercle size differences obtained in our study will give more insight into the medicine; and more comprehensive studies should be conducted on this subject.
When all age-and gender-related findings are considered together, it is seen that the evaluation of the dimensions of the genial tubercles with the help of CBCT is an important anatomical parameter that can be used in determination of age-gender in forensic dentistry.
CONCLUSION
The vertical and horizontal dimensions of genial tubercle of men were higher than that of women.
There was a negative correlation between vertical dimensions of the genial tubercle and age.
Our study suggests that the morphological analysis of the genial tubercles can be a valuable and effective tool in determining the morphological differences in these structures according to age and gender factors; It also shows that it can have important contributions in the preoperative planning of maxillofacial surgery and in forensic dentistry.
Main points.
The anatomy of the genial tubercle differs between genders and ages.
Men have larger genial tubercles compared to women, regarding vertical and horizontal dimensions.
As the age of the patients increases, the vertical dimensions of the genial tubercle decrease.
Three-dimensional evaluation of the genial tubercle can be used in forensic dentistry for age-gender determination and preoperative planning of mandibular osteotomy.
Acknowledgement
We would like to thank to Prof. Dr. Sema Aka, who established the hypothesis of this study and contributed to the material and method.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee of İzmir Katip Celebi University, (Approval No: 2020-GOKAE-0175).
Informed Consent: Verbal/Written informed consent was obtained from the patients who agreed to take part in the study. (This was a retrospective study)
Peer-review: Externally peer-reviewed.
Author Contributions: Supervision – B.K.Ü., Design – M.İ.K.; Conception – M.İ.K., İ.H.H., Materials – E.A.; Data Collection and/or Processing – B.K., Ö.B., Analysis and/or Interpretation – F.E.; Literature Search – N.Ö..; Writing Manuscript –E.A., B.K., Ö.B.; Critical Review – B.K., M.İ.K.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Bishara SE. Textbook of Orthodontics. Philadelphia: WB Saunders Company; 2001. pp. 43–8. [Google Scholar]
- 2.Enlow DH, Hans MG. Essentials of facial growth. Philadelphia: WB Saunders Company; 1996. pp. 1–110. [Google Scholar]
- 3.Creutz U. Architecture of the human skullcap in the region of the parts bregmatica suturae sagittalis- I. Age dependence. Gegenbaurs Morphol Jahrab. 1977;123:666–88. [PubMed] [Google Scholar]
- 4.Skrzat J, Brzegowy P, Walocha J, Wojciechowski W. Age dependent changes of the diploe in the human skull. Folia Morphologica. 2004;63:67–70. [PubMed] [Google Scholar]
- 5.Bartlett SP, Grossman R, Whitaker LA. Age-Related Changes of the Craniofacial Skeleton. Plast Reconstr Surg. 1992;90:592–600. doi: 10.1097/00006534-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Treacy P. The Science of Facial Aging. Global Press Release Distribution. 2009;1(1 screens) ( www.prlog.org) Available From: https://www.prlog.org/10299776-the-science-of-facial-aging.html. [Google Scholar]
- 7.Nejaim Y, Moreira DD, Fernandes ABN, de Souza MMG, Groppo FC, Neto FH. Evaluation of the morphology of the genial tubercle using cone-beam computed tomography. Br J Oral Maxillofac Surg. 2018;56:155–6. doi: 10.1016/j.bjoms.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Moss ML. Twenty Years of Functional Cranial Analysis. Am J Orthod Dentofac. 1972;61:479–85. doi: 10.1016/0002-9416(72)90152-2. [DOI] [PubMed] [Google Scholar]
- 9.Enlow DH. Postnatal craniofacial growth and development. J Plastic Surgery. 1990;4:2496–514. [Google Scholar]
- 10.Santos-Oller JM, Junquera Gutierrez LM, De Vicente Rodriguez JC, Llorente Pendas S. Spontaneous fracture of hypertrophied genial tubercles. Oral Surg Oral Med Oral Pathol. 1992;74:28–9. doi: 10.1016/0030-4220(92)90210-H. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Choi DS, Jang I, Song GS, Cha BK. The genial tubercle: A prospective novel landmark for the diagnosis of mandibular asymmetry. Korean J Orthod. 2017;47:50–8. doi: 10.4041/kjod.2017.47.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Păuna M, Babiuc I, Farcasiu T. Prosthodontic management of an extreme atrophy of the mandible correlated with a prominent genial tubercle-A clinical report. Revue roumaine de morphologie et embryologie. 2015;1:867–70. [PubMed] [Google Scholar]
- 13.Solomon EG. A critical analysis of complete denture impression procedures: contribution of early prosthodontists in India-part I. J Indian Prosthodont Soc. 2011;11:172–82. doi: 10.1007/s13191-011-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voon YS, Patil PG. Safe zone in anterior mandible related to the genial tubercle for implant osteotomy in a Chinese-Malaysian population: A CBCT study. J Prosthet Dent. 2018;119:568–73. doi: 10.1016/j.prosdent.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein K. Genioglossus muscle attachments: an anatomic analysis and the implications for genioglossus advancement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:686–8. doi: 10.1067/moe.2000.111187. [DOI] [PubMed] [Google Scholar]
- 16.Singh IB. Essentials of anatomy. 2nd ed. New Delhi: Jaypee Bros; 2009. p. 346. [DOI] [Google Scholar]
- 17.Takahashi S. Breathing modes, body positions, and suprahyoid muscle activity. J Orthod. 2002;29:307–13. doi: 10.1093/ortho/29.4.307. [DOI] [PubMed] [Google Scholar]
- 18.Herder C, Schmeck J, Appelboom DJK, de Vries N. Risks of general anaesthesia in people with obstructive sleep apnoea. BMJ (Clinical Research ed) 2004;329:955–9. doi: 10.1136/bmj.329.7472.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hueman EM, Noujeim ME, Langlais RP, Prihoda TJ, Miller FR. Accuracy of cone beam computed tomography in determining the location of the genial tubercle. Otolaryngol Head Neck Surg. 2007;137:115–8. doi: 10.1016/j.otohns.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Ludlow JB, Laster WS, See M, Bailey LJ, Hershey HG. Accuracy of measurements of mandibular anatomy in cone beam computed tomography images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:534–42. doi: 10.1016/j.tripleo.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canger EM, Arslan S. Adli diş hekimliğinde radyolojinin kullanımı. [The use of radiology in forensic dentistry]. Atatürk Üniv Diş Hek Fak Derg. 2013;23:252–60. [Google Scholar]
- 22.Harorlı A. Adli Dişhekimliği [Forensic Dentistry]. 1. baskı. Erzurum: Atatürk Üniversitesi Yayınları; 2006. pp. 5pp. 25–6.pp. 53–68. [Google Scholar]
- 23.Wang TT, Wessels L, Hussain G, Merten S. Discriminative thresholds in facial asymmetry: A review of the literature. Aesthetic Surgery Journal. 2017;37:375–85. doi: 10.1093/asj/sjw271. [DOI] [PubMed] [Google Scholar]
- 24.Araby YA, Alhirabi AA, Santawy AH. Genial tubercles: Morphological study of the controversial anatomical landmark using cone beam computed tomography. World J Radiol. 2019;11:94. doi: 10.4329/wjr.v11.i7.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennessee J, Miller FR. Anatomic analysis of the genial bone advancement trephine system’s effectiveness at capturing the genial tubercle and its muscular attachments. Otolaryngol Head Neck Surg. 2005;133:229–33. doi: 10.1016/j.otohns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Hendler BH, Costello BJ, Silverstein K, Yen D, Goldberg A. A protocol for uvulopalatopharyngoplasty, mortised genioplasty, and maxillomandibular advancement in patients with obstructive sleep apnea: an analysis of 40 cases. J Oral Maxillofac Surg. 2001;59:892–7. doi: 10.1053/joms.2001.25275. [DOI] [PubMed] [Google Scholar]
- 27.Kai YS, Liang YH, Ying LW, Guan J, Min WH, Yu CZ, et al. Anatomic and spiral computed tomographic study of the genial tubercles for genioglossus advancement. Otolaryngol Head Neck Surg. 2007;136:632–7. doi: 10.1016/j.otohns.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Kolsuz ME, Orhan K, Bilecenoglu B, Sakul BU, Ozturk A. Evaluation of genial tubercle anatomy using cone beam computed tomography. J Oral Sci. 2015;57:151–6. doi: 10.2334/josnusd.57.151. [DOI] [PubMed] [Google Scholar]
- 29.Reifman S. Genial tubercle fracture. Report of a case. Oral Surg Oral Med Oral Pathol. 1969;27:595–7. doi: 10.1016/0030-4220(69)90089-9. [DOI] [PubMed] [Google Scholar]
- 30.MÖ Özemre, Köseoğlu, Seçgin C, Gülşahı A. Adli diş hekimliği ve konik ışınlı bilgisayarlı tomografi. Kamburoğlu K, editör. Dentomaksillofasiyal Konik Işınlı Bilgisayarlı Tomografi: Temel Prensipler, Teknikler ve Klinik Uygulamalar. 1. Baskı. Ankara: Türkiye Klinikleri;; 2019. pp. 162–5. [Google Scholar]
- 31.Barbosa D, Bezerra T, Silva P, Pimenta A, Kurita L, Costa F. Clinical-Imaginological and Therapeutic Aspects of Genial Tubercles Fractures: A Systematic Review. J Oral Maxillofac Surg. 2019;77:1674. doi: 10.1016/j.joms.2019.03.030. [DOI] [PubMed] [Google Scholar]


