Abstract
Obesity represents the single greatest ongoing roadblock to improving cardiovascular health. Prolonged obesity is associated with fundamental changes in the integrative control of energy balance, including the development of selective leptin resistance, which is thought to contribute to obesity-associated hypertension, and adaptation of resting metabolic rate (RMR) when excess weight is reduced. Leptin and the melanocortin system within the hypothalamus contribute to the control of both energy balance and blood pressure. While the development of drugs to stimulate RMR and thereby reverse obesity through activation of the melanocortin system has been pursued, most of the resulting compounds simultaneously cause hypertension. Evidence supports the concept that although feeding behaviors, RMR, and blood pressure are controlled through mechanisms that utilize similar molecular mediators, these mechanisms exist in anatomically dissociable networks. New evidence supports a major change in molecular signaling within Agouti-related peptide (AgRP) neurons of the arcuate nucleus of the hypothalamus during prolonged obesity, and the existence of multiple distinct subtypes of AgRP neurons that individually contribute to control of feeding, RMR, or BP. Finally, ongoing work by our lab and others support a unique role for angiotensin II signaling through its type 1 receptor (AT1) within one specific subtype of AgRP neuron for the control of RMR. We propose that understanding the unique biology of the AT1-expressing, RMR-controlling subtype of AgRP neurons will help to resolve the selective dysfunctions in RMR control that develop during prolonged obesity, and potentially point toward novel druggable anti-obesity targets that will not simultaneously cause hypertension.
Keywords: Obesity, Angiotensin, Leptin, Hypertension, Agouti-related peptide
Graphical Abstract
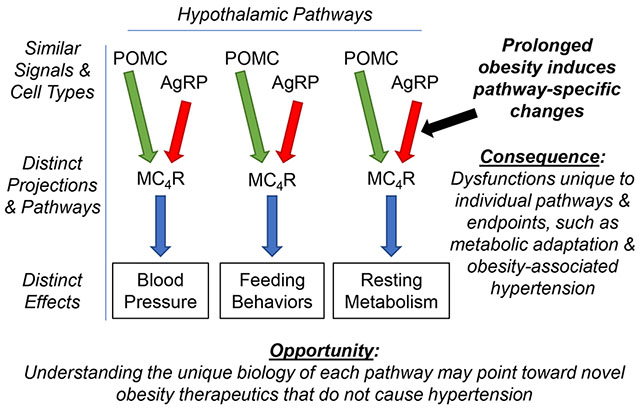
Introduction
Obesity is caused by a complex interaction of genes, behavior, and environmental factors. Increased body weight is associated with increased blood pressure (BP), while weight loss is associated with reduced BP.1–3 Obesity is an independent risk factor for the development of hypertension, and the Framingham Heart Study suggests that roughly 70% of primary hypertension is linked to obesity.2
Although the American Heart Association (AHA) made great strides toward its ambitious 2020 Impact Goal “to improve the cardiovascular health of all Americans by 20% while reducing deaths from CVDs and stroke by 20% by 2020”, we failed to achieve this goal primarily due to metabolic diseases, including obesity. In the AHA 2030 Impact Goal, Angell et al.4 state:
“Over the past decade (NHANES 2007–2016), there has been significant progress in the United States toward the 2020 Impact Goal, with less smoking in adults, increased physical activity in adults, improved dietary habits in both adults and youth, lower total cholesterol in adults and youth, and lower blood glucose in adults. However, these areas of progress have been offset by adverse forces: increases in high blood pressure and body mass index among adults and youth, higher blood glucose in youth, and lower physical activity in youth. The continued worsening of overweight and obesity rates among adults and youth throughout the decade is especially concerning and largely accounts for worsening blood pressure in adults and youth and fasting blood glucose among youth.”
Thus, it is increasingly clear that cardiovascular disease and metabolic disease are inextricably intertwined, and that future progress in hypertension and cardiovascular health is dependent upon improvements in our understanding and treatment of metabolic disease, especially obesity.
Obesity: Not ‘just’ overeating?
In 1985, the NIH Consensus Development Conference recognized obesity as a disease with high morbidity and mortality.5 Unfortunately, it took almost 30 years for obesity to be formally appreciated by major medical associations as a ‘disease’ and a global public health burden rather than ‘simply’ a consequence of overeating.6 It is well-recognized that obesogenic environmental factors abound in Western societies,7 yet obesity is the result of long-term caloric imbalance which may be caused by overfeeding, reduced energy expenditure, or a combination of both.
Energy balance is achieved through a complex interplay among systems controlling input versus output. Ingestive behaviors and the efficiency of the digestive tract to extract calories from consumed food both modulate caloric input into the system. In contrast, resting metabolic rate (RMR) and activity-based energy expenditure both contribute to caloric output. Dysfunctions in any of these systems can mediate the development and/or maintenance of obesity and targeting any of these systems may be utilized to promote weight loss, at least in the short-term.
One of the critical challenges to correcting obesity is that, after obesity is established, the body appears to defend this increased mass against targeted efforts to lose weight. Indeed, individuals enrolled in structured behavioral interventions for weight loss often regain roughly 80% of lost weight within 5 years.8 Similarly, although reductions in digestive efficiency and subsequent weight loss can be achieved with orlistat, weight relapse is common within a few years.9
Increasing evidence supports the concept that weight loss triggers an adaptative but dysfunctional RMR response, such that RMR is suppressed relative to body mass and composition (TABLE 1), which hinders long-term reduced weight maintenance.10–12 Scattered evidence also supports the concept that this adaptation of RMR may predict subsequent weight regain.12, 13 Stimulating RMR via mitochondrial ionophores works to combat obesity in humans and animal models, however, because such compounds are highly toxic their use in humans is strongly prohibited.14–19 Such compounds, however, provide critical proof-of-concept data regarding the potential utility of targeting RMR to treat obesity, while highlighting the ongoing need for a better understanding of the biology of RMR control (during both health and disease) to support the development of safe drugs to stimulate RMR.
Table 1:
Clinical studies reporting on metabolic adaptation after weight loss
| Author | Metabolic adaptation, kcal/day (mean±SD) | n | Baseline BMI, kg/m2 (mean ± SD or range) | Sex | Max weight loss | Follow-up (months) | Daily calorie intake or calorie restriction | Duration of dietary intervention | Procedure |
|---|---|---|---|---|---|---|---|---|---|
| LIFESTYLE CHANGES | |||||||||
| Short term studies (≤ 6 months) | |||||||||
| Leibel88 | -137±305 | 9 | n/r | n/r | 10% | 2.5? | 800 kcal/day | until weight goal achieved | - |
| Leibel88 | -79±294 | 10 | n/r | n/r | 20% | n/r | 800 kcal/day | until weight goal achieved | - |
| Doucet89 | M ~ -112 F ~ -143 |
35 | 34.2±0.6 36.8±0.9 |
15 M 20 F |
10% 9% |
4.5 | -700 kcal/day | 15 weeks | - |
| Knuth90 | -419±13 | 35 | 46.7±9.5 | 7M 9F |
35% | 7 | >70% caloric needs | 28 weeks | - |
| Rosenbaum91 | NS | 10 | 35.3±3.8 | 5 M 5 F |
10% | 3.75 | 800 kcal/day | until weight goal achieved | - |
| Bosy-Westphal92 | -55±165 | 45 | 35.9±4.2 | F | 9% | 3 | ~900 kcal/day | 13 weeks | - |
| Müller93 | -80±97 | 32 | 20.7–29.3 | M | 7.5% | 0.75 | ~1400 kcal/day# | 3 weeks | - |
| Nymo10 | -111±165 | 31 | 36.7±4.5 | 18 M 13 F |
10% | 2 | ~600 kcal/day | 8 weeks | - |
| Martins94 | −49±128 | 71 | 34.6±3.4 | 33 M 38 F |
13% | 3.25 |
1000 kcal/day | 8 weeks | - |
| Long term studies (> 6 months) | |||||||||
| Rosenbaum95 | -150±50 | 7 | n/a | 2 M 5 F |
≥10% | >12 (R) | n/r | n/r | - |
| Fothergill12 | -500±150 | 14 | 34.9±10.3 | M, F | 39%* | 72 | >70% caloric needs | 28 weeks | - |
| Ostendorf96 | NS | 34 | 33.0±4.6 | 8 M 26 F |
29% | 72 (R) | n/r | n/r | - |
| Martins94 | NS | 45 | 34.6±3.4 | 14%** | 12 | n/r | n/r | - | |
| Thom97 | -150±162 | 15 | 39.4±4.3 | F | 13.5% | 6 | ~850 kcal/day | 12–20 weeks | - |
| BARIATRIC SURGERY | |||||||||
| Short term studies (≤ 6 months) | |||||||||
| Carrasco98 | -85±124 | 38 | 44.4±4.8 | 4 M 34 F |
29% | 6 | n/r | n/r | RYGB |
| Browning99 | NS | 8 | 47.1±6.8 | 2 M 6 F |
28% | 6 | n/r | n/r | RYGB |
| Knuth90 | -201±182 | 13 | 47±7.6 | 4 M 9 F |
25% | 6 | ~1000 kcal/day | 24 weeks | RYGB |
| Golzarand100, 101 | -230±234 -308±210 |
22 21 |
42.6±5.4 | 1 M, 21 F 21 F |
23% | 6 | n/r | n/r | RYGB SG |
| Long term studies (> 6 months) | |||||||||
| Das102 | NS | 30 | 50.1±9.3 | 4 M 26 F |
38% | 14 | n/r | n/r | RYBG |
| Knuth90 | NS | 13 | 47±7.6 | 4 M 9 F |
30% | 12 | ~1000 kcal/day | 52 weeks | RYGB |
| Tam103 | -134±28 -255±122 |
5 9 |
45.1±2.5 49.9±3.4 |
5 F 2 M, 7 F |
35% | 12 | 800 | 8 weeks | RYGB SG |
Metabolic adaptation calculated as measured RMR – predicted RMR; RYGB: Roux en Y gastric bypass; SG: sleeve gastrectomy; (R): retrospective study; n/r: not reported; NS: not significant;
50% energy requirements;
regained 70% of weight lost by end of follow-up (final weight loss 12%);
regained 30% of weight lost by end of follow-up (final weight loss 10%)
Leptin & Melanocortin Systems
Leptin serves as an afferent signal to communicate fat stores to the brain, thereby influencing the integrative control of energy stores.20–22 Leptin modulates the activity of several types of cells, including Proopiomelanocortin (POMC) and Agouti-related peptide (AgRP) neurons, to influence feeding behavior, RMR, and BP.23–27 POMC neurons stimulate the melanocortin receptor 4 receptor (MC4R) via α-melanocyte stimulating hormone (αMSH), and also release γ-aminobutyric acid (GABA), adrenocorticotropic hormone, β-endorphin, and the cocaine and amphetamine regulated transcript. AgRP neurons utilize a combination of AgRP, Neuropeptide Y (NPY) and GABA to influence postsynaptic neurons. Importantly, AgRP acts as an inverse agonist at MC4R.28, 29 Furthermore, MC4R exhibits an unusually strong constitutive intrinsic (ie, ligand-independent) activity, highlighting the complex biochemistry of this signaling pathway and the importance of changes in AgRP neurotransmission even in the absence of αMSH signaling.30 The MC4R is expressed in multiple hypothalamic and extra-hypothalamic regions that are variably implicated in cardiovascular and metabolic control.30, 31 Activation of MC4R by αMSH increases cardiovascular sympathetic activity, BP and heart rate in mice, whereas pharmacological inhibition of MC4R or Mc4r deletion cause increased body weight, without affecting BP.32
Because MC4R is implicated in energy balance and BP control, it represents an obvious target for manipulating cardiometabolic functions.33, 34 Unfortunately, because MC4R is involved in multiple pre-autonomic networks that contribute to cardiovascular versus metabolic control, such stimulation of sympathetic drive is typically non-selective, leading to simultaneous stimulation of both RMR and BP. For example, first-generation synthetic MC4R agonists were abandoned as potential anti-obesity treatments because although they caused weight loss in rodents, monkeys, and humans, they also caused significant increases in BP.35 Next-generation MC4R agonists, represented by Setmelanotide,36 reduce food intake and body weight in several species (rodents, dogs, and non-human primates), without affecting BP.37, 38 Setmelanotide increases resting energy expenditure in C57BL/6 mice supplied a high fat diet (HFD),38 and decreases food intake and increases energy expenditure without causing hypertension in Rhesus monkeys fed obesogenic diets.37 In a phase 1 human clinical trial (NCT01867437), it increased RMR (~111 kcal/d), with no effect on BP or heart rate.39 Similarly, in subjects carrying MC4R mutations, Setmelanotide induced weight loss (~0.6 kg/week) without any effects on cardiovascular parameters (NCT02431442).36 It also reduced hyperphagia and body weight in obese individuals with POMC and/or leptin receptor (LEPR) mutations without hemodynamic effects (NCT02507492).40, 41 As such, Setmelanotide received FDA approval for chronic weight management in individuals with selected mutations in the POMC, PCSK1, or LEPR genes on November 27, 2020. Future work to explore the utility of this compound for treating obesity that results from other mutations and causes (ie, beyond dysfunctions primarily attributed to the POMC neuron) is clearly warranted.
The mechanisms by which Setmelanotide induces weight loss without influencing BP are unclear. It may act as a biased ligand for MC4R, differentially stimulating Gαs/cAMP versus Gαq second messengers.36, 41 Additionally, it may exhibit selective distribution/compartmentalization, bringing the compound into contact with cardiovascular versus metabolic control regions at different effective concentrations. Based on in silico docking and crystallization studies, it may alter binding kinetics of endogenous ligands.42 Regardless of mechanism, this differential action provides exciting proof-of-concept support for the idea that metabolic and cardiovascular control networks are dissociable, even if they utilize the same molecular mediators.
Selective Leptin Resistance
Although obesity is associated with increased adiposity and circulating leptin, metabolic responses to leptin are attenuated while cardiovascular responses to leptin are maintained. This phenomenon, termed Selective Leptin Resistance (SLR), is proposed to contribute to obesity-associated hypertension.43 Studies of C57BL/6J mice fed HFD for 10 weeks to cause diet-induced obesity (DIO) demonstrate that SLR involves changes in hypothalamic leptin signaling. Indeed, cardiovascular (ie, renal) sympathetic responses to acute ICV leptin injection remain intact in DIO mice, while metabolic (ie, brown adipose) sympathetic responses are blunted.44
To explore changes in hypothalamic leptin signaling during obesity, we performed single nucleus RNA-sequencing (snRNAseq) on arcuate nucleus of the hypothalamus (ARC) from mice after 10 weeks of HFD feeding.45 Mice displayed expected body and fat mass gains, increased plasma leptin concentrations, and changes in food intake and energy expenditure metrics. Interestingly, the gene expression signature for leptin signaling was only significantly altered in 5 of the 23 unique clusters of cell types, including astrocytes, oligodendrocytes, and AgRP neurons – but notably and iconoclastically, not POMC neurons. In addition, the canonical signature for leptin signaling in AgRP neurons was awarded a negative Z-score, indicating that the canonical leptin-sensitive genes responded opposite to leptin’s typical effects. Thus, molecular evidence of SLR is observable within the ARC, and SLR is most obviously occurring within AgRP neurons.
Multiple groups have demonstrated in various rodent models that, while short-term HFD feeding suppresses expression of Agrp within the ARC, 46–54 prolonged HFD leads to attenuation of this effect or even stimulation of Agrp expression.48, 55–57 Similarly, in our study, Agrp expression was significantly increased within AgRP neurons after DIO despite large increases in circulating leptin.45 Further, within AgRP neurons we identified derangements of multiple signaling pathways implicated in transcriptional control of Agrp, including CREB and MAPK/ERK cascades.
Although it is classically considered a ‘hunger’ hormone, evidence suggests that AgRP is also important for the control of energy expenditure. Egan et al. previously demonstrated that genetic deletion of the leptin receptor from cells expressing Agrp resulted in increased body mass without effects on feeding behaviors, implying a suppression of energy expenditure.58 Similarly, Makimura et al. demonstrated that RNAi-mediated knockdown of Agrp in the mouse hypothalamus increased heat production,59 Small et al. reported that ICV injection of recombinant AgRP protein suppressed oxygen consumption in Wistar rats,60 and Krashes et al. showed reduced oxygen consumption in mice after DREADD-mediated stimulation of AgRP neurons.61
Collectively, these findings support the concepts that SLR involves fundamental changes in the behavior of AgRP neurons, and that AgRP neurons contribute to the integrative control of energy homeostasis. Thus, understanding the unique biology of these cells in both health and disease may help to explain the pathogenesis of SLR and weight loss-associated adaptation of RMR.
The ARC renin-angiotensin system
Local paracrine versions of the RAS have been identified in the brain and other tissues, which operate independently of the circulating RAS .62–65 We and others have implicated the brain RAS in the control of energy homeostasis and ingestive behaviors, including roles of the RAS within the ARC, the paraventricular nucleus, the nucleus tractus solitarius, the lateral parabrachial nucleus, and other regions.66–70 Pharmacological activation of the brain RAS by angiotensin II (ANG) infusion71, 72 or deoxycorticosterone acetate (DOCA)-salt treatment,73 or transgenic activation of the RAS within the brain74 all stimulate RMR with mechanisms dependent upon angiotensin II type 1 receptors (AT1). Pharmacological blockade of the brain RAS or genetic disruption of AT1A (Agtr1a) receptors attenuate thermogenic brown adipose sympathetic nerve activity (BAT SNA) responses to leptin.75 We demonstrated that leptin-mediated activation of BAT SNA and heat production responses to an array of stimuli are all dependent upon AT1A specifically on AgRP neurons.31, 76, 77 Interestingly, however, disruption of the Agtr1a gene in AgRP neurons had no major effect on feeding behaviors or BP control,76 whereas others have demonstrated that deletion of Agtr1a in other brain regions does modulate feeding behavior.78 This observation suggests the existence of multiple distinct subtypes of AgRP neurons within the ARC; some of which contribute to feeding, some to BP control, and others to RMR control. Indeed, in silico reanalysis of single-cell RNA sequencing datasets and RNAscope-based in situ hybridization experiments support the concept that Agtr1a is only expressed in a specific subset of AgRP neurons within the ARC.31, 76, 77 These findings lead us to hypothesize the distinct involvement of Agtr1a-expressing AgRP neurons in the control of RMR, but not feeding or BP (FIGURE 1).
Figure 1. Working model: labelled-line encoding of feeding, BP and RMR control in the ARC.
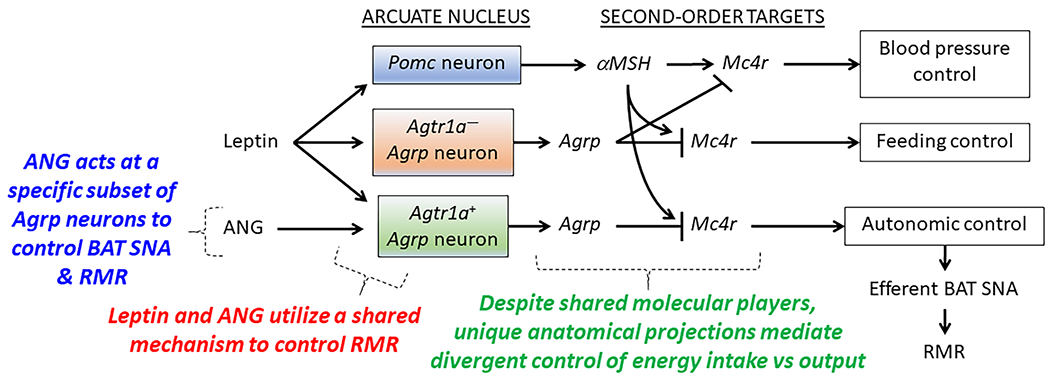
Evidence supports the working hypothesis that a specific subset of AgRP neurons express the AT1A receptor (Agtr1a), and that these receptors simultaneously (i) identify the subset of AgRP neurons involved in RMR control, and (ii) serve a critical molecular role in the integrative control of RMR. We hypothesize that molecular dysfunctions specifically within the Agtr1a-expressing subset of AgRP neurons contributes to the development of SLR and weight loss-associated adaptations in RMR control. Pomc, proopiomelanocortin; αMSH, α-melanocyte stimulating hormone; Mc4r, melanocortin type 4 receptor; Agrp, Agouti-related peptide; ANG, angiotensin II; BAT SNA, brown adipose tissue sympathetic nerve activity; RMR, resting metabolic rate.
Ongoing questions
Together, these findings prompt many ongoing questions:
How do changes in the local ARC RAS contribute to the pathogenesis of SLR and obesity-associated adaptations in RMR control?
How do anatomically distinct neural pathways projecting from the ARC differentially contribute to feeding, RMR, and BP control, and how do these pathways functionally or structurally change with prolonged obesity? How can these pathways be targeted individually for therapeutic applications?
What unique molecular pathways exist and differentially contribute to cellular functions in each of the dissociable neural pathways controlling feeding, RMR, and BP? What druggable targets can be identified to selectively stimulate each individual pathway?
It is known that there are sex differences in the biology of the ARC and AgRP function.58, 79–84 How do sex differences in AgRP function contribute to sex differences in RMR and BP control?
Ghrelin, glucagon-like peptide-1, cholecystokinin, and other circulating peptides also act at the ARC in parallel or in opposition to leptin to control energy balance, and are also implicated in BP control.85, 86 How do alterations in ARC ANG signaling impact these other systems?
Do these results translate to human physiology? Circulating AgRP levels appear to correlate with hypothalamic Agrp expression, which may identify AgRP as a useful biomarker for cardiometabolic dysfunctions.87 Is circulating AgRP a valid marker of hypothalamic melanocortin activity in humans and does it correlate with RMR control? Can it be used to identify subjects at risk of metabolic adaptation?
Conclusions
Obesity represents the primary hurdle for continued improvement in cardiovascular health. The local melanocortin and renin-angiotensin systems within the hypothalamus contribute to both cardiovascular and metabolic control, and ongoing work to dissect the anatomical and molecular mechanisms by which these systems contribute to normal and pathological control of energy homeostasis promises to identify novel therapeutic targets for both obesity and cardiovascular disease. These findings also highlight the need for greater integration of cardiovascular and metabolic endpoints in preclinical research, as the development of next-generation therapeutics for obesity versus hypertension in isolation is often derailed by failure to appreciate the simultaneous ‘off target’ consequences of manipulations of one system upon the other.
Acknowledgments
The authors wish to thank the Council on Hypertension for this opportunity.
Sources of Funding
Authors were supported by the NIH (HL134850, HL084207), the American Heart Association (18EIA33890055), the MCW Clinical & Translational Science Institute “Obesity” Ensemble (UL1TR001436), and the Advancing a Healthier Wisconsin Endowment to MCW.
Footnotes
Declarations of Interest: None
Disclosures
None.
Literature Cited
- 1.Jones DW, Miller ME, Wofford MR, Anderson DC Jr., Cameron ME, Willoughby DL, Adair CT, King NS. The effect of weight loss intervention on antihypertensive medication requirements in the hypertension optimal treatment (hot) study. Am J Hypertens. 1999;12:1175–1180 [DOI] [PubMed] [Google Scholar]
- 2.Garrison RJ, Kannel WB, Stokes J 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: The framingham offspring study. Prev Med. 1987;16:235–251 [DOI] [PubMed] [Google Scholar]
- 3.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention, phase ii. Ann Intern Med. 2001;134:1–11 [DOI] [PubMed] [Google Scholar]
- 4.Angell SY, McConnell MV, Anderson CAM, Bibbins-Domingo K, Boyle DS, Capewell S, Ezzati M, de Ferranti S, Gaskin DJ, Goetzel RZ, et al. The american heart association 2030 impact goal: A presidential advisory from the american heart association. Circulation. 2020;141:e120–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Health implications of obesity. National institutes of health consensus development conference. 11–13 february 1985. Ann Intern Med. 1985;103:977–1077 [PubMed] [Google Scholar]
- 6.Coulter AA, Rebello CJ, Greenway FL. Centrally acting agents for obesity: Past, present, and future. Drugs. 2018;78:1113–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin JP, Rodríguez-Durán LF, Guzmán-Ramos K, Perez-Cruz C, Ferreira G, Diaz-Cintra S, Pacheco-López G. Palatable hyper-caloric foods impact on neuronal plasticity. Front Behav Neurosci. 2017;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102:183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362 [DOI] [PubMed] [Google Scholar]
- 10.Nymo S, Coutinho SR, Torgersen LH, Bomo OJ, Haugvaldstad I, Truby H, Kulseng B, Martins C. Timeline of changes in adaptive physiological responses, at the level of energy expenditure, with progressive weight loss. Br J Nutr. 2018;120:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tremblay A, Chaput JP. Adaptive reduction in thermogenesis and resistance to lose fat in obese men. Br J Nutr. 2009;102:488–492 [DOI] [PubMed] [Google Scholar]
- 12.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD. Persistent metabolic adaptation 6 years after “the biggest loser” competition. Obesity (Silver Spring). 2016;24:1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravussin E, Bogardus C. Energy expenditure in the obese: Is there a thrifty gene? Infusionstherapie. 1990;17:108–112 [DOI] [PubMed] [Google Scholar]
- 14.Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol. 2015;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ost M, Keipert S, Klaus S. Targeted mitochondrial uncoupling beyond ucp1 - the fine line between death and metabolic health. Biochimie. 2017;134:77–85 [DOI] [PubMed] [Google Scholar]
- 16.Colman E. Dinitrophenol and obesity: An early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol. 2007;48:115–117 [DOI] [PubMed] [Google Scholar]
- 17.Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (dnp): A weight loss agent with significant acute toxicity and risk of death. J Med Toxicol. 2011;7:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa D, Carmo H, Roque Bravo R, Carvalho F, Bastos ML, Guedes de Pinho P, Dias da Silva D. Diet aid or aid to die: An update on 2,4-dinitrophenol (2,4-dnp) use as a weight-loss product. Arch Toxicol. 2020;94:1071–1083 [DOI] [PubMed] [Google Scholar]
- 19.Tainter ML. Dinitrophenol in the treatment of obesity. Journal of the American Medical Association. 1935;105:0332–0337 [Google Scholar]
- 20.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 21.Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1:754–764 [DOI] [PubMed] [Google Scholar]
- 22.Farooqi IS, O’Rahilly S. Leptin: A pivotal regulator of human energy homeostasis. The American journal of clinical nutrition. 2009;89:980S–984S [DOI] [PubMed] [Google Scholar]
- 23.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat Rev Nephrol. 2019;15:367–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seoane-Collazo P, Ferno J, Gonzalez F, Dieguez C, Leis R, Nogueiras R, Lopez M. Hypothalamic-autonomic control of energy homeostasis. Endocrine. 2015;50:276–291 [DOI] [PubMed] [Google Scholar]
- 25.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandit R, Beerens S, Adan RAH. Role of leptin in energy expenditure: The hypothalamic perspective. American journal of physiology. Regulatory, integrative and comparative physiology. 2017;312:R938–r947 [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Morgan DA, Cui H, Rahmouni K. Activation of hypothalamic agrp and pomc neurons evokes disparate sympathetic and cardiovascular responses. American journal of physiology. Heart and circulatory physiology. 2020;319:H1069–h1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138 [DOI] [PubMed] [Google Scholar]
- 29.Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL. Hypothalamic expression of art, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602 [DOI] [PubMed] [Google Scholar]
- 30.Tao YX. Constitutive activity in melanocortin-4 receptor: Biased signaling of inverse agonists. Adv Pharmacol. 2014;70:135–154 [DOI] [PubMed] [Google Scholar]
- 31.Morselli LL, Claflin KE, Cui H, Grobe JL. Control of energy expenditure by agrp neurons of the arcuate nucleus: Neurocircuitry, signaling pathways, and angiotensin. Current hypertension reports. 2018;20:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154 [DOI] [PubMed] [Google Scholar]
- 33.da Silva AA, do Carmo JM, Wang Z, Hall JE. Melanocortin-4 receptors and sympathetic nervous system activation in hypertension. Current hypertension reports. 2019;21:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52 [DOI] [PubMed] [Google Scholar]
- 36.Collet TH, Dubern B, Mokrosinski J, Connors H, Keogh JM, Mendes de Oliveira E, Henning E, Poitou-Bernert C, Oppert JM, Tounian P, et al. Evaluation of a melanocortin-4 receptor (mc4r) agonist (setmelanotide) in mc4r deficiency. Mol Metab. 2017;6:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemmensen C, Finan B, Fischer K, Tom RZ, Legutko B, Sehrer L, Heine D, Grassl N, Meyer CW, Henderson B, Hofmann SM, Tschöp MH, Van der Ploeg LH, Müller TD. Dual melanocortin-4 receptor and glp-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice. EMBO Mol Med. 2015;7:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, Zhao X, Ring M, Psota TL, Cone RD, Panaro BL, Gottesdiener KM, Van der Ploeg LH, Reitman ML, Skarulis MC. Rm-493, a melanocortin-4 receptor (mc4r) agonist, increases resting energy expenditure in obese individuals. J Clin Endocrinol Metab. 2015;100:1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kühnen P, Clément K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, Mai K, Blume-Peytavi U, Grüters A, Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375:240–246 [DOI] [PubMed] [Google Scholar]
- 41.Clément K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C, Puder L, Fiedorek F, Gottesdiener K, Kleinau G, et al. Mc4r agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018;24:551–555 [DOI] [PubMed] [Google Scholar]
- 42.Falls BA, Zhang Y. Insights into the allosteric mechanism of setmelanotide (rm-493) as a potent and first-in-class melanocortin-4 receptor (mc4r) agonist to treat rare genetic disorders of obesity through an in silico approach. ACS Chem Neurosci. 2019;10:1055–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark AL. Selective leptin resistance revisited. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R566–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018 [DOI] [PubMed] [Google Scholar]
- 45.Deng G, Morselli LL, Wagner VA, Balapattabi K, Sapouckey SA, Knudtson KL, Rahmouni K, Cui H, Sigmund CD, Kwitek AE, Grobe JL. Single-nucleus rna sequencing of the hypothalamic arcuate nucleus of c57bl/6j mice after prolonged diet-induced obesity. Hypertension. 2020;76:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barson JR, Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Effect of dietary fatty acid composition on food intake, triglycerides, and hypothalamic peptides. Regulatory peptides. 2012;173:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Storlien LH, Huang XF. Effects of dietary fat types on body fatness, leptin, and arc leptin receptor, npy, and agrp mrna expression. American journal of physiology. Endocrinology and metabolism. 2002;282:E1352–1359 [DOI] [PubMed] [Google Scholar]
- 48.Densmore VS, Morton NM, Mullins JJ, Seckl JR. 11 beta-hydroxysteroid dehydrogenase type 1 induction in the arcuate nucleus by high-fat feeding: A novel constraint to hyperphagia? Endocrinology. 2006;147:4486–4495 [DOI] [PubMed] [Google Scholar]
- 49.Archer ZA, Rayner DV, Mercer JG. Hypothalamic gene expression is altered in underweight but obese juvenile male sprague-dawley rats fed a high-energy diet. The Journal of nutrition. 2004;134:1369–1374 [DOI] [PubMed] [Google Scholar]
- 50.Stofkova A, Skurlova M, Kiss A, Zelezna B, Zorad S, Jurcovicova J. Activation of hypothalamic npy, agrp, mc4r, and il-6 mrna levels in young lewis rats with early-life diet-induced obesity. Endocrine regulations. 2009;43:99–106 [PubMed] [Google Scholar]
- 51.Dalvi PS, Chalmers JA, Luo V, Han DY, Wellhauser L, Liu Y, Tran DQ, Castel J, Luquet S, Wheeler MB, Belsham DD. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and tnf-α on appetite-regulating npy neurons. International journal of obesity (2005). 2017;41:149–158 [DOI] [PubMed] [Google Scholar]
- 52.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate npy/agrp neurons. Endocrinology. 2010;151:4745–4755 [DOI] [PubMed] [Google Scholar]
- 53.Shibata M, Banno R, Sugiyama M, Tominaga T, Onoue T, Tsunekawa T, Azuma Y, Hagiwara D, Lu W, Ito Y, Goto M, Suga H, Sugimura Y, Oiso Y, Arima H. Agrp neuron-specific deletion of glucocorticoid receptor leads to increased energy expenditure and decreased body weight in female mice on a high-fat diet. Endocrinology. 2016;157:1457–1466 [DOI] [PubMed] [Google Scholar]
- 54.Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased arc npy/agrp, but no alteration in pomc/cart, mrna expression in diet-induced obese mice. Behavioural brain research. 2009;205:50–56 [DOI] [PubMed] [Google Scholar]
- 55.Patterson CM, Villanueva EC, Greenwald-Yarnell M, Rajala M, Gonzalez IE, Saini N, Jones J, Myers MG, Jr. Leptin action via lepr-b tyr1077 contributes to the control of energy balance and female reproduction. Molecular metabolism. 2012;1:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell metabolism. 2007;5:181–194 [DOI] [PubMed] [Google Scholar]
- 57.Fraser M, Dhaliwal CK, Vickers MH, Krechowec SO, Breier BH. Diet-induced obesity and prenatal undernutrition lead to differential neuroendocrine gene expression in the hypothalamic arcuate nuclei. Endocrine. 2016;53:839–847 [DOI] [PubMed] [Google Scholar]
- 58.Egan OK, Inglis MA, Anderson GM. Leptin signaling in agrp neurons modulates puberty onset and adult fertility in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017;37:3875–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makimura H, Mizuno TM, Mastaitis JW, Agami R, Mobbs CV. Reducing hypothalamic agrp by rna interference increases metabolic rate and decreases body weight without influencing food intake. BMC neuroscience. 2002;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Small CJ, Liu YL, Stanley SA, Connoley IP, Kennedy A, Stock MJ, Bloom SR. Chronic cns administration of agouti-related protein (agrp) reduces energy expenditure. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27:530–533 [DOI] [PubMed] [Google Scholar]
- 61.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of agrp neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sigmund CD, Grobe JL. A colorful view of the brain renin-angiotensin system. Hypertens Res. 2020;43:357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pahlavani M, Kalupahana NS, Ramalingam L, Moustaid-Moussa N. Regulation and functions of the renin-angiotensin system in white and brown adipose tissue. Compr Physiol. 2017;7:1137–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nehme A, Zouein FA, Zayeri ZD, Zibara K. An update on the tissue renin angiotensin system and its role in physiology and pathology. J Cardiovasc Dev Dis. 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin ii receptor isoforms in the brain. J Clin Invest. 2000;106:103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka M, Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Current hypertension reports. 2019;21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roncari CF, David RB, Johnson RF, De Paula PM, Colombari DS, De Luca LA Jr., Johnson AK, Colombari E, Menani JV. Angiotensinergic and cholinergic receptors of the subfornical organ mediate sodium intake induced by gabaergic activation of the lateral parabrachial nucleus. Neuroscience. 2014;262:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Speretta GF, Ruchaya PJ, Delbin MA, Melo MR, Li H, Menani JV, Sumners C, Colombari E, Bassi M, Colombari DSA. Importance of at1 and at2 receptors in the nucleus of the solitary tract in cardiovascular responses induced by a high-fat diet. Hypertension research : official journal of the Japanese Society of Hypertension. 2019;42:439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sá JM, Barbosa RM, Menani JV, De Luca LA Jr., Colombari E, Almeida Colombari DS. Cardiovascular and hidroelectrolytic changes in rats fed with high-fat diet. Behavioural brain research. 2019;373:112075. [DOI] [PubMed] [Google Scholar]
- 70.Gasparini S, Melo MR, Nascimento PA, Andrade-Franzé GMF, Antunes-Rodrigues J, Yosten GLC, Menani JV, Samson WK, Colombari E. Interaction of central angiotensin ii and aldosterone on sodium intake and blood pressure. Brain research. 2019;1720:146299. [DOI] [PubMed] [Google Scholar]
- 71.Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin ii on body weight and food intake in adult rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2004;287:R422–428 [DOI] [PubMed] [Google Scholar]
- 72.Porter JP, Anderson JM, Robison RJ, Phillips AC. Effect of central angiotensin ii on body weight gain in young rats. Brain research. 2003;959:20–28 [DOI] [PubMed] [Google Scholar]
- 73.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (doca)-salt in c57 mice. Hypertension. 2011;57:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell metabolism. 2010;12:431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. American journal of physiology. Heart and circulatory physiology. 2012;303:H197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin at1a receptors on leptin receptor-expressing cells control resting metabolism. The Journal of clinical investigation. 2017;127:1414–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sapouckey SA, Deng G, Sigmund CD, Grobe JL. Potential mechanisms of hypothalamic renin-angiotensin system activation by leptin and doca-salt for the control of resting metabolism. Physiological genomics. 2017;49:722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4825–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lensing CJ, Adank DN, Doering SR, Wilber SL, Andreasen A, Schaub JW, Xiang Z, Haskell-Luevano C. Ac-trp-dphe(p-i)-arg-trp-nh2, a 250-fold selective melanocortin-4 receptor (mc4r) antagonist over the melanocortin-3 receptor (mc3r), affects energy homeostasis in male and female mice differently. ACS chemical neuroscience. 2016;7:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamada C, Saegusa Y, Nahata M, Sadakane C, Hattori T, Takeda H. Influence of aging and gender differences on feeding behavior and ghrelin-related factors during social isolation in mice. PloS one. 2015;10:e0140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asarian L, Geary N. Sex differences in the physiology of eating. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R1215–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheffer-Babila S, Sun Y, Israel DD, Liu SM, Neal-Perry G, Chua SC Jr. Agouti-related peptide plays a critical role in leptin’s effects on female puberty and reproduction. American journal of physiology. Endocrinology and metabolism. 2013;305:E1512–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of gaba by agrp neurons is required for normal regulation of energy balance. Nature neuroscience. 2008;11:998–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goodin SZ, Kiechler AR, Smith M, Wendt D, Strader AD. Effect of gonadectomy on agrp-induced weight gain in rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;295:R1747–1753 [DOI] [PubMed] [Google Scholar]
- 85.Mikulášková B, Maletínská L, Zicha J, Kuneš J. The role of food intake regulating peptides in cardiovascular regulation. Molecular and cellular endocrinology. 2016;436:78–92 [DOI] [PubMed] [Google Scholar]
- 86.Matsumura K, Tsuchihashi T, Fujii K, Iida M. Neural regulation of blood pressure by leptin and the related peptides. Regulatory peptides. 2003;114:79–86 [DOI] [PubMed] [Google Scholar]
- 87.Page-Wilson G, Peters JB, Panigrahi SK, Jacobs TP, Korner J, Otten M, Bruce JN, Wardlaw SL. Plasma agouti-related protein and cortisol levels in cushing disease: Evidence for the regulation of agouti-related protein by glucocorticoids in humans. The Journal of clinical endocrinology and metabolism. 2019;104:961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England journal of medicine. 1995;332:621–628 [DOI] [PubMed] [Google Scholar]
- 89.Doucet E, St-Pierre S, Alméras N, Després JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. The British journal of nutrition. 2001;85:715–723 [DOI] [PubMed] [Google Scholar]
- 90.Knuth ND, Johannsen DL, Tamboli RA, Marks-Shulman PA, Huizenga R, Chen KY, Abumrad NN, Ravussin E, Hall KD. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring, Md.). 2014;22:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. The Journal of clinical investigation. 2005;115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bosy-Westphal A, Kossel E, Goele K, Later W, Hitze B, Settler U, Heller M, Glüer CC, Heymsfield SB, Müller MJ. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. The American journal of clinical nutrition. 2009;90:993–1001 [DOI] [PubMed] [Google Scholar]
- 93.Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Glüer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A. Metabolic adaptation to caloric restriction and subsequent refeeding: The minnesota starvation experiment revisited. The American journal of clinical nutrition. 2015;102:807–819 [DOI] [PubMed] [Google Scholar]
- 94.Martins C, Roekenes J, Salamati S, Gower BA, Hunter GR. Metabolic adaptation is an illusion, only present when participants are in negative energy balance. The American journal of clinical nutrition. 2020;112:1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. The American journal of clinical nutrition. 2008;88:906–912 [DOI] [PubMed] [Google Scholar]
- 96.Ostendorf DM, Melanson EL, Caldwell AE, Creasy SA, Pan Z, MacLean PS, Wyatt HR, Hill JO, Catenacci VA. No consistent evidence of a disproportionately low resting energy expenditure in long-term successful weight-loss maintainers. The American journal of clinical nutrition. 2018;108:658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thom G, Dombrowski SU, Brosnahan N, Algindan YY, Rosario Lopez-Gonzalez M, Roditi G, Lean MEJ, Malkova D. The role of appetite-related hormones, adaptive thermogenesis, perceived hunger and stress in long-term weight-loss maintenance: A mixed-methods study. European journal of clinical nutrition. 2020;74:622–632 [DOI] [PubMed] [Google Scholar]
- 98.Carrasco F, Papapietro K, Csendes A, Salazar G, Echenique C, Lisboa C, Díaz E, Rojas J. Changes in resting energy expenditure and body composition after weight loss following roux-en-y gastric bypass. Obesity surgery. 2007;17:608–616 [DOI] [PubMed] [Google Scholar]
- 99.Browning MG, Rabl C, Campos GM. Blunting of adaptive thermogenesis as a potential additional mechanism to promote weight loss after gastric bypass. Surg Obes Relat Dis. 2017;13:669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Golzarand M, Toolabi K, Hedayati M, Azam K, Douraghi M, Djafarian K. Comparative study of resting metabolic rate and plasma amino acid profile in patients who underwent laparoscopic roux-en-y gastric bypass and laparoscopic sleeve gastrectomy: 6-month follow-up study. Obesity surgery. 2019;29:3125–3132 [DOI] [PubMed] [Google Scholar]
- 101.Golzarand M, Toolabi K, Djafarian K. Changes in body composition, dietary intake, and substrate oxidation in patients underwent laparoscopic roux-en-y gastric bypass and laparoscopic sleeve gastrectomy: A comparative prospective study. Obesity surgery. 2019;29:406–413 [DOI] [PubMed] [Google Scholar]
- 102.Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. The American journal of clinical nutrition. 2003;78:22–30 [DOI] [PubMed] [Google Scholar]
- 103.Tam CS, Redman LM, Greenway F, LeBlanc KA, Haussmann MG, Ravussin E. Energy metabolic adaptation and cardiometabolic improvements one year after gastric bypass, sleeve gastrectomy, and gastric band. The Journal of clinical endocrinology and metabolism. 2016;101:3755–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]


