Abstract
Intestinal inflammation and microbiota are two important components of colorectal cancer (CRC) etiology. However, it is not clear how tuning inflammation using clinically relevant anti-inflammatory treatment impacts microbiota or whether this consequently influences CRC outcome. Here, using chemically induced (DSS/Apcmin/+) and spontaneous (Apcmin/+;Il10−/−) mouse CRC models colonized by colibactin-producing Escherichia coli, we established the role of microbiota in mediating the antitumorigenic effect of anti–tumor necrosis factor (TNF) therapy. We found that TNF blockade attenuated colitis and CRC development. Microbiota community structure and gene activities significantly changed with disease development, which was prevented by TNF blockade. Several microbiota functional pathways underwent similar changes in patients following anti-TNF therapy. Under cohousing condition, TNF blockade failed to prevent colitis, cancer development and disease-associated microbiota structural changes. Finally, microbiota transplantation showed reduced carcinogenic activity of microbiota from anti-TNF-treated mice. Together, our data demonstrate the plasticity of microbiota, which could be reverted to noncarcinogenic status by targeting inflammation.
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, affects over 0.3% of the Western population and shows accelerating incidence in newly industrialized countries1. Patients with IBD, especially those with colonic inflammation, are at increased risk for developing CRC2. While inflammation per se contributes to all stages of tumorigenesis3, intestinal inflammation is associated with broad perturbations of gut microbiota composition and activities4, which can further promote inflammation and cancer development5.
The pro-tumorigenic interplay between inflammation and bacteria is exemplified by colibactin-producing (clb+) E. coli. Colibactin alkylates DNA and induces DNA double-stranded breaks6-8. clb+ E. coli are commonly present in the microbiota of healthy individuals, but a higher prevalence of mucosa-associated clb+ E. coli was observed in patients with IBD or CRC compared with nondisease individuals9-12. In addition, clb genes are enriched in the fecal metagenome of patients with CRC13,14. Recently, a unique colibactin mutational signature has been detected in human CRC genomes and driver mutations15. clb+ E. coli enhance CRC development via colibactin activity in various mouse models9,16-20. Intestinal inflammation appears essential for clb+ E. coli-driven carcinogenesis, as clb+ E. coli failed to induce CRC in inflammation-free mice9,12,18,20. Mechanistically, inflammation could influence clb+ E. coli carcinogenic activity by at least two means: by upregulating virulence genes such as clb genes18 and by facilitating E. coli colonization to the mucosa12, both presumably leading to increased colibactin-induced DNA damage in colonic epithelial cells.
Accordingly, targeting the colibactin synthesis machinery or E. coli colonization could protect against clb+ E. coli-driven CRC. For example, small boron-based compounds that inhibit the activity of ClbP, a peptidase necessary for colibactin prodrug activation21, reduced clb+ E. coli genotoxic and pro-tumorigenic effects22. A bacteriophage cocktail selectively targeting the clb+ E. coli strain NC101 reduced colonization levels of the bacterium and CRC development23. E. coli could obtain a growth advantage in the inflamed gut by molybdoenzyme-dependent nitrate respiration24 and formate oxidation25. Tungstate, by inactivating molybdoenzymes, prevented inflammation-associated luminal E. coli expansion26 and NC101-mediated CRC development20. These E. coli-directed treatments, while showing promising results in mouse models, require further validation before moving into the human clinical setting.
Given the impact of inflammation on CRC development, anti-inflammatory agents such as those used to treat IBD could be chemopreventive2. These clinically relevant agents could be particularly advantageous in protecting against inflammation-dependent carcinogenesis, for which clb+ E. coli-driven CRC represents an ideal model. However, such efficacy has not been demonstrated. Inflammation status could affect not only clb+ E. coli but also other members of the microbiota as well as microbial interactions within the gut ecosystem27. It is not clear how anti-inflammatory treatments would change clb+ E. coli and overall microbiota activities. More importantly, does the treatment-induced microbiota change functionally impact CRC pathogenesis? To address these questions, we evaluated the effect of anti-TNF therapy, a clinical treatment for moderate and severe IBD, on two mouse CRC models that were mainly driven by clb+ E. coli. We showed that TNF blockade altered microbiota composition and activities, a process subject to environmental influence and critical for preventing colorectal carcinogenesis.
Results
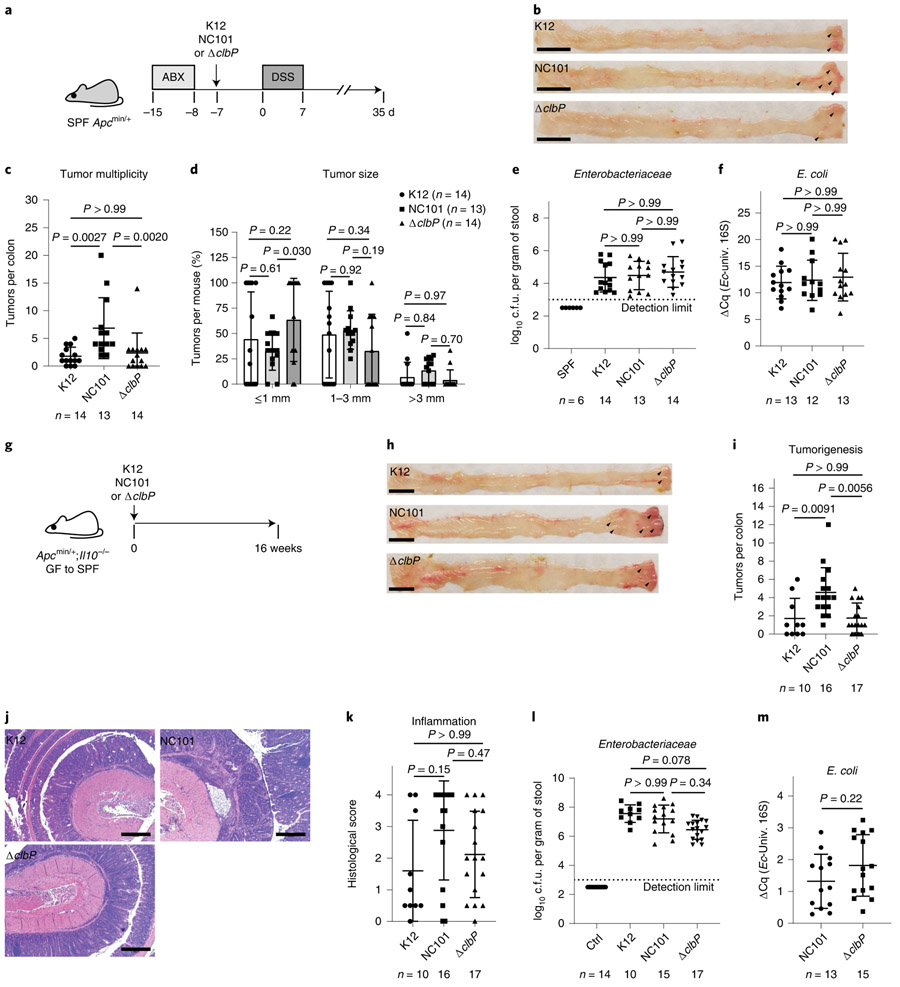
clb+ E. coli promotes CRC development via colibactin activity in mice harboring a complex microbiota.
The clb+ E. coli stain NC101 was shown to promote CRC development via colibactin activity in mono-associated mouse models9,18,19. Consistently, deletion of the clb genomic island attenuated NC101-driven CRC in the azoxymethane/dextran sulfate sodium (AOM/DSS) mouse model in specific-pathogen-free (SPF) environment20. However, clb genes could be involved in bacterial functions besides colibactin synthesis; for example, clbA contributes to siderophore production and therefore iron acquisition28. To determine colibactin contribution to NC101 carcinogenic activity in the presence of a complex microbiota, we colonized SPF Apcmin/+ mice with NC101, NC101 isogenic mutant ΔclbP (ref. 19) or the commensal strain K12 (control), and treated mice with DSS to induce colonic tumorigenesis (Fig. 1a). The temporary low-dose DSS treatment led to comparable self-resolving inflammation in Apcmin/+ mice (data not shown). Notably, NC101-colonized mice developed significantly more colon tumors than K12- and ΔclbP-colonized mice (P = 0.0027 and 0.0020, respectively), with no difference observed between the latter two groups (Fig. 1b,c). Tumor size, fecal Enterobacteriaceae loads and E. coli relative abundance were comparable among mice colonized with different E. coli strains (Fig. 1d-f).
Fig. 1 ∣. E. coli NC101 promotes CRC development via colibactin activity in mice harboring a complex microbiota.
a, Timeline for the DSS/Apcmin/+ model. b, Representative pictures of mouse colon (tumors marked by arrowheads). Scale bars, 1 cm. c, Macroscopic tumor counts with the number of mice indicated in each group. d, Tumor size. e, Endpoint fecal Enterobacteriaceae loads. SPF mice were shown as comparison. f, E. coli relative abundance in DSS/Apcmin/+ mice associated with various E. coli strains. Quantification cycle differences (ΔCq) are shown between E. coli (Ec) 16S and universal bacterial (univ.) 16S qPCR. g, Timeline for the Apcmin/+;Il10−/− model. h, Representative pictures of mouse colon. Scale bars, 1 cm. i, Macroscopic tumor counts with the number of mice indicated in each group. j, H&E-stained colon Swiss roll sections (distal). Scale bars, 500 μm. k, Distal colon histological scores. l, Endpoint fecal Enterobacteriaceae loads. Conventionalized (Ctrl) mice without E. coli inoculation were shown for comparison. m, E. coli relative abundance in Apcmin/+;Il10−/− mice. Due to difficulty of collecting stools from certain mice, which did not correlate with tumor or inflammation status, the number of samples for Enterobacteriaceae and E. coli quantification does not always match the number of mice used for tumor characterization. Data are mean±s.d. One-way ANOVA followed by Dunn’s multiple comparisons test was performed for multi-group comparisons, two-way ANOVA followed by Tukey’s multiple comparisons test was used for tumor size comparison and two-tailed Mann–Whitney U-test was used for paired comparisons. Experiments comparing effects of different E. coli strains on colitis and tumorigenesis in DSS/Apcmin/+ and Apcmin/+;Il10−/− models were done once, with mice staggered due to limited mouse availability per experimental setup. The number of animals (n) used for each graph was indicated. GF, germ-free.
As an independent model, we transferred germ-free Apcmin/+;Il10−/− mice to SPF environment and inoculated mice with the various E. coli strains (Fig. 1g). Apcmin/+;Il10−/− mice develop microbiota-dependent aggravating colitis and CRC19. Similar to the DSS/Apcmin/+ model, Apcmin/+;Il10−/− mice colonized with NC101 developed significantly more colon tumors than those colonized with K12 or ΔclbP (P = 0.0091 and 0.0056, respectively; Fig. 1h,i). Colonic inflammation was comparable among Apcmin/+;Il10−/− mice colonized by different E. coli strains, as shown by histological scores (Fig. 1j,k) and messenger RNA levels of key inflammatory cytokines (that is, Tnfa, Il1b, Ifng, Il17a, Il6, Il18, Il22, Cxcl1 and Cxcl2; data not shown) in the distal colon. Fecal Enterobacteriaceae loads were not significantly different among Apcmin/+;Il10−/− mice colonized with different E. coli strains (Fig. 1l), and E. coli relative abundance was not affected by colibactin production (Fig. 1m). Together, results from the two mouse models, chemically induced and spontaneous, demonstrate that colibactin production promotes colorectal carcinogenesis, particularly tumor initiation, in mice harboring a complex microbiota, without affecting E. coli colitogenic activity or luminal colonization. It is noteworthy that SPF mice in our facility have very low levels of Enterobacteriaceae (Fig. 1e,l) and E. coli (undetectable in stools by E. coli 16S gene quantitative PCR (qPCR)).
TNF blockade attenuates colitis and CRC development.
Given that inflammation is essential for clb+ E. coli-induced carcinogenesis9,12,18,20, we reasoned that anti-inflammatory treatments such as TNF blockade could prevent CRC development in our mouse models. To test this hypothesis, we treated NC101-colonized Apcmin/+ mice with PBS (vehicle control) or a TNF-neutralizing antibody after inflammation induction. At day 14 (after four antibody injections), anti-TNF-treated mice displayed lower histological scores of colon inflammation compared with PBS-treated control (Extended Data Fig. 1a-c), confirming the anti-inflammatory effect of TNF blockade. Interestingly, anti-TNF treatment was associated with a reduced incidence of DNA damage response in regenerating colon crypts as shown by γH2AX immunohistochemistry (Extended Data Fig. 1d,e), suggesting that TNF blockade protects against DNA damage. At day 35, anti-TNF-treated mice developed significantly fewer colon tumors than PBS-treated mice (P = 0.0020), although tumor size was comparable between the two treatment groups (Fig. 2a-d). This effect was not due to impaired NC101 colonization, as TNF blockade did not significantly affect fecal Enterobacteriaceae loads, fecal NC101 relative abundance or tissue-associated NC101 levels along disease development (Fig. 2e-g and Extended Data Fig. 1f).
Fig. 2 ∣. TNF blockade attenuates NC101-mediated CRC development.
a, Timeline for TNF blockade in the DSS/Apcmin/+ model. b, Representative pictures of mouse colon. Scale bars, 1 cm. c, Macroscopic tumor counts with number of mice in each group. d, Tumor size. e, Fecal Enterobacteriaceae loads at indicated time points. f, Fecal NC101 relative abundance. Due to difficulty of collecting stools from certain mice, which did not correlate with tumor or inflammation status, the number of samples for Enterobacteriaceae and E. coli quantification do not always match the number of mice used for tumor characterization. g, Colon tissue-associated NC101 levels at the endpoint in DSS/Apcmin/+ mice. Cq, quantification cycles from PCR. h, Timeline for TNF blockade in the Apcmin/+;Il10−/− model. i, Macroscopic tumor counts with number of mice in each group. j, Representative pictures of H&E-stained colon Swiss roll sections (distal). Scale bars, 500 μm. k, Distal colon histological scores. l, Endpoint fecal Enterobacteriaceae loads. m, Fecal NC101 relative abundance. n, Colon tissue-associated NC101 levels in Apcmin/+;Il10−/− mice. Data are mean±s.d. Two-tailed Mann–Whitney U-test was performed for paired comparisons, and two-way ANOVA followed by Sidak’s multiple comparisons test was used for tumor size comparison. Anti-TNF treatments in DSS/Apcmin/+ and Apcmin/+;Il10−/− models were performed twice, with similar results. Data show results from one representative experiment. The number of animals (n) used for each graph was indicated.
We next examined the effect of TNF blockade on NC101-colonized Apcmin/+;Il10−/− mice. Similarly, compared with vehicle-treated control, anti-TNF-treated Apcmin/+;Il10−/− mice developed significantly fewer colon tumors (P = 0.0038) and had lower colon inflammation scores (Fig. 2h-k). These responses correlated with lower levels of Il1b and Ifng mRNA but higher Il18 in the distal colon of anti-TNF-treated mice (Extended Data Fig. 1g), consistent with the divergent roles of these cytokines in CRC pathogenesis29. In the Apcmin/+;Il10−/− model, TNF blockade also reduced incidence of colon crypt DNA damage response (P = 0.0092, Fisher’s exact test), without significantly affecting fecal or tissue-associated Enterobacteriaceae and NC101 levels (Fig. 2l-n). Together, data from the two models showed that the inflammation-targeting anti-TNF treatment prevented CRC development.
TNF blockade alters microbiota composition and activities.
Anti-TNF therapy was reported to induce microbiota compositional changes in patients with IBD30. To determine microbiota community structure changes associated with disease development and anti-TNF treatment in our preclinical models, we performed 16S ribosomal RNA gene sequencing on longitudinal stool samples and endpoint distal colon snips. In the DSS/Apcmin/+ model, longitudinal fecal microbiota profiling revealed significant time-associated alterations of microbiota alpha and beta diversities in both control and anti-TNF-treated mice (Extended Data Fig. 2a,b). This suggests that microbiota dynamically evolved with CRC development. From day 0 to day 35, the relative abundances of Erysipelotrichaceae and two genera of Lachnospiraceae (that is, Hespellia and Marvinbryantia) increased in the control (false discovery rate (FDR) P=6.8 X 10−11, 0.0074 and 0.00024, respectively) but not in the anti-TNF-treated mice, suggesting their association with tumorigenesis. Between treatment groups, significant differences were observed in beta but not alpha diversity of fecal and colon tissue-associated microbiota (Fig. 3). Only Erysipelotrichaceae was significantly impacted by TNF blockade, with abundance reduced in endpoint stools from anti-TNF-treated mice compared with control (FDR P = 0.018). No significant change in Enterobacteriaceae relative abundance was observed longitudinally or between control and anti-TNF-treated mice. Similarly, in the Apcmin/+;Il10−/− model, fecal microbiota composition significantly changed over time, and microbiota beta but not alpha diversity was significantly different in endpoint stools and colon tissues between PBS- and anti-TNF-treated mice (Extended Data Fig. 3). Only the genus 02d06 of Clostridiaceae was significantly different between treatment groups, that is, higher in endpoint stools from anti-TNF-treated mice (FDR P=0.00039). Although not significantly affected by TNF blockade, endpoint fecal Erysipelotrichaceae abundance positively correlated with tumor numbers in Apcmin/+;Il10−/− mice (Pearson’s r=0.61, FDR P=0.041). Overall, the impact of TNF blockade on microbiota composition was reproducible in the two mouse models.
Fig. 3 ∣. TNF blockade alters microbiota composition in DSS/Apcmin/+ mice.
a, Comparison of microbiota alpha diversity (Chao1) in fecal samples collected at indicated time points and colon tissue snips at the endpoint between PBS- and anti-TNF-treated mice. The box horizontal lines are the 25th (lower) and 75th (upper) percentiles. The middle line is the 50th percentile (the median). The lower whisker is the 25th percentile–1.5 times the interquartile range and the upper whisker is the 75th percentile+ 1.5 times the interquartile range. b, PCoA comparison of microbiota community structure in fecal samples collected at indicated time points and colon tissue snips at the endpoint between PBS- and anti-TNF-treated mice. NS, not significant. Sequencing data analysis was described in detail in the Methods. The number of animals (n) used for each graph was indicated.
We next assessed microbiota activities by performing microbial RNA sequencing (RNA-seq) on day-14 and -35 stools collected from PBS- and anti-TNF-treated DSS/Apcmin/+ mice. To specifically evaluate changes due to microbial gene expression, we normalized metatranscriptomes with metagenomes. We found that microbiota gene activities were significantly different between time points as well as treatment groups (Fig. 4a,b). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed decreased microbial porphyrin and chlorophyll metabolism (ko00860) along CRC development in control mice (Fig. 4c). This pathway, along with nine other pathways such as aminoacyl-tRNA biosynthesis (ko00970), cell cycle—Caulobacter (ko04112), biosynthesis of amino acids (ko01230), biosynthesis of antibiotics (ko01130) and ribosome (ko03010), was significantly downregulated in anti-TNF-treated mice compared with control mice at day 14 (Fig. 4d), during early tumorigenesis when inflammation was higher in control mice (Extended Data Fig. 1c). Only two pathways were significantly upregulated at day 14 in anti-TNF-treated mice, that is, degradation of aromatic compounds (ko01220) and phenylalanine metabolism (ko00360) (Fig. 4d). ko004112, ko00970, ko01230 and ko03010 remained at lower activities in anti-TNF-treated mice at day 35, with three additional pathways including amino sugar and nucleotide sugar metabolism (ko00520), peroxisome (ko04146) and phenylalanine, tyrosine and tryptophan biosynthesis (ko00400) also being repressed (Fig. 4e).
Fig. 4 ∣. TNF blockade alters microbiota activity in DSS/Apcmin/+ mice.
a, PCA comparison of fecal microbiota metatranscriptomes normalized by metagenomes between day 14 and day 35 in PBS- and anti-TNF-treated mice. b, PCA comparison of fecal microbiota metatranscriptomes normalized by metagenomes between PBS- and anti-TNF-treated mice at days 14 and 35. c, Differentially expressed KEGG pathways in day-14 and -35 stools from PBS-treated mice. d, Differentially expressed KEGG pathways in PBS- and anti-TNF-treated mice at day 14. e, Differentially expressed KEGG pathways in PBS- and anti-TNF-treated mice at day 35. Pathways similarly affected by anti-TNF therapy in patients are highlighted (magenta). Mice were randomly selected from each group, and sequencing data analysis was described in detail in the Methods. The number of animals (n) used for each graph was indicated. PC1, first principal component; PC2, second principal component.
To see whether similar changes in microbiota activity occur in humans, we compared longitudinal fecal metatranscriptomes from patients with IBD before and after anti-TNF therapy in the IBD Multi’omics Database (IBDMDB) 31. After metagenome normalization, we found that five KEGG pathways were significantly higher pre-therapy and four were higher post-therapy (Supplementary Table 1). Notably, several pathways affected by TNF blockade in our mouse models underwent similar changes in patients with IBD receiving anti-TNF therapy, for example, downregulation of ko01230, ko01130 and ko03010, and upregulation of ko01220 and ko00360 (highlighted in Fig. 4d,e).
As for the differentially expressed genes (Supplementary Table 2), the largest number (n = 1,139) was found at day 14 between PBS- and anti-TNF-treated mice, whereas the smallest (n = 241) was observed between day 14 and day 35 in PBS-treated mice. This suggests stronger impact of treatment, particularly during early tumorigenesis, than time on microbiota gene activities. Since the reference mouse gut metagenome database32 does not contain clb genes, we aligned RNA-seq reads to NC101 clb gene sequences18 to assess activity of the clb island. Our metagenome sequencing data suggest that NC101 abundance did not change over time or by treatment (Extended Data Fig. 2c). Nonetheless, after metagenome normalization, although no significant difference in individual clb gene expression was observed between PBS- and anti-TNF-treated mice, overall expression of the clb island was significantly higher in PBS- than anti-TNF-treated mice at both day 14 and day 35 (Extended Data Fig. 2d). This suggests increased NC101 clb activity concurrent with higher inflammation in control mice during early tumorigenesis and maintained throughout tumor progression.
Microbiota mediates the CRC-preventive effect of TNF blockade.
To determine the impact of microbiota on CRC prevention by TNF blockade, we cohoused DSS/Apcmin/+ mice receiving PBS or the TNF-neutralizing antibody (Fig. 5a). 16S rRNA gene sequencing of longitudinal stool samples and endpoint distal colon tissues revealed similar microbiota structure in cohoused PBS- and anti-TNF-treated mice throughout the experiment, suggesting successful normalization of microbiota (Fig. 5b). Consistently, fecal NC101 abundance was comparable between cohoused control and anti-TNF-treated mice (Fig. 5c). Interestingly, enrichment of Erysipelotrichaceae and Marvinbryantia, as aforementioned occurring in separately housed PBS- but not anti-TNF-treated mice, was observed in cohoused anti-TNF-treated mice (Supplementary Table 3). Overall, bacterial taxa that underwent significant abundance changes and the direction of changes along disease development in cohoused anti-TNF-treated mice mirrored those of PBS-treated mice. This suggests that the impact of TNF neutralization on microbiota composition was nullified under cohousing condition, and that microbiota in PBS-treated mice played a dominant role in shaping the community in cohoused anti-TNF-treated mice. Importantly, under cohousing condition TNF blockade failed to attenuate colon inflammation, as shown by fecal lipocalin-2 levels at day 14 (Fig. 5d), or reduce colorectal carcinogenesis (Fig. 5e-g). Similarly, cohousing abrogated the protective effect of TNF blockade on inflammation and tumorigenesis in the Apcmin/+;Il10−/− mouse model (Extended Data Fig. 4). Together, these data suggest that the CRC-preventive effect of TNF blockade relies on microbiota shifting towards a nondisease state, a process that could be overridden by continuous exposure to disease-associated microbiota.
Fig. 5 ∣. Cohousing abrogates TNF blockade effect on microbiota, inflammation and CRC.
a, Timeline for TNF blockade in DSS/Apcmin/+ mice under cohousing condition. b, PCoA comparison of microbiota structure in longitudinal stool samples and endpoint colon tissue snips between cohoused PBS- and anti-TNF-treated mice. Sequencing data analysis was described in detail in the Methods. c, Endpoint fecal NC101 relative abundance. d, Fecal lipocalin-2 (LCN2) levels. Randomly selected mice at day 0 (before DSS treatment) were used as uninflamed reference. e, Representative pictures of mouse colon. Scale bars, 1 cm. f, Macroscopic tumor counts. g, Tumor size from cohoused PBS- and anti-TNF treated DSS/Apcmin/+ mice. Data are mean±s.d. Two-tailed Mann–Whitney U-test was performed for paired comparisons, one-way ANOVA followed by Sidak’s multiple comparisons test was performed for multi-group comparisons and two-way ANOVA followed by Sidak’s multiple comparisons test was used for tumor size comparison. The cohousing experiment was done once. The number of animals (n) used for each graph was indicated. ABX, antibiotics.
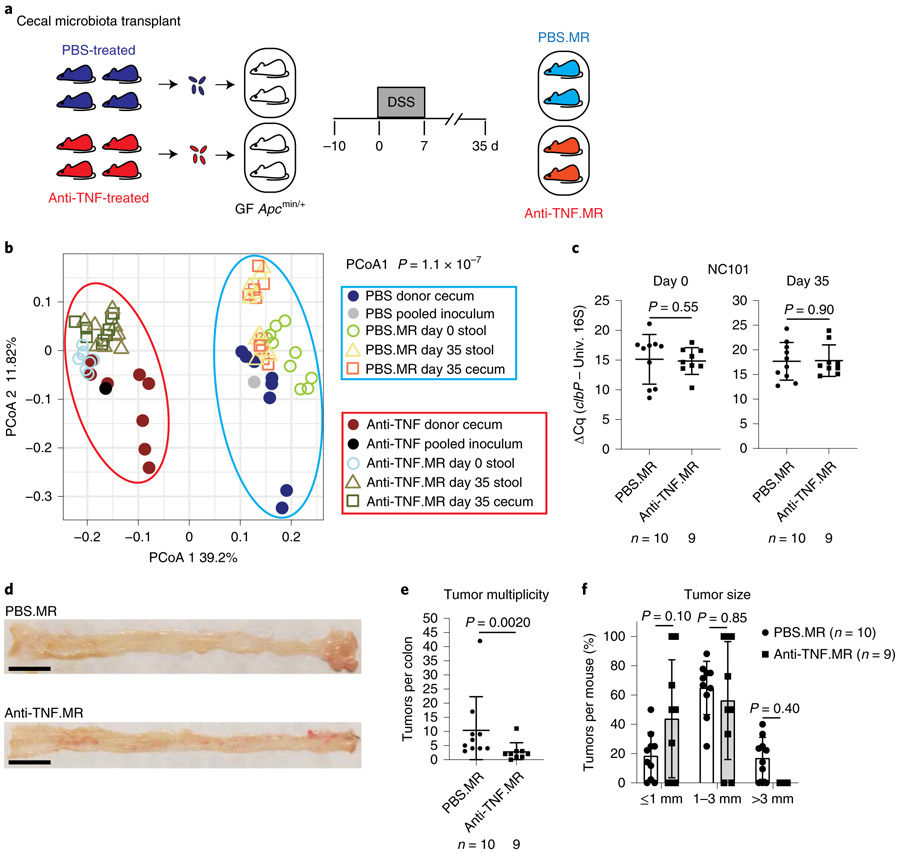
To determine whether treatment-induced microbiota alteration is sufficient to influence CRC development, we performed microbiota transplantation by inoculating germ-free Apcmin/+ mice with pooled cecal contents from separately housed PBS- or anti-TNF-treated mice, and then treated mice with DSS to induce CRC (Fig. 6a). Microbiota profiling by 16S rRNA gene sequencing showed clustering of donors and matched recipients, and clear separation between PBS- and anti-TNF-related samples (Fig. 6b). Specifically, PBS-treated microbiota donors had significantly higher Erysipelotrichaceae and lower Clostridiaceae/Clostridium abundance compared with anti-TNF-treated microbiota donors (FDR P=0.0027, 0.0016 and 0.0016, respectively). These differences were transferred to the recipient cohort and maintained along disease development (FDR P=0.012, 9.0×10−05 and 0.00031, respectively, in recipients’ endpoint stools). No difference in NC101 relative abundance was observed between PBS and anti-TNF microbiota recipients (Fig. 6c). Fecal abundance of the Turicibacter genus and the Turicibacteraceae and Erysipelotrichaceae families significantly increased with CRC pathogenesis in PBS microbiota recipients (FDR P=3.1 × 10−06, 1.3×10−06 and 0.018, respectively) but not anti-TNF microbiota recipients. Notably, PBS microbiota recipients developed significantly more tumors than anti-TNF microbiota recipients (P=0.0020), although tumor size was comparable (Fig. 6d-f). Thus, alteration of microbiota by TNF blockade functionally attenuates CRC development.
Fig. 6 ∣. TNF blockade-associated microbiota show reduced carcinogenic activity.
a, Schematic diagram of cecal microbiota transplantation using gnotobiotic DSS/Apcmin/+ mice. Pooled cecal contents from PBS- or anti-TNF-treated mice (separately caged as described in Fig. 2a) were used to inoculate germ-free Apcmin/+ mice to generate PBS microbiota recipient (PBS.MR) and anti-TNF microbiota recipient (anti-TNF.MR) mice. b, PCoA comparison of microbiota composition in donor and recipient cohorts shows separate clustering by treatment (PBS or anti-TNF). Sequencing data analysis was described in detail in the Methods. c, Fecal NC101 relative abundance at indicated time points. d, Representative pictures of mouse colon. Scale bars, 1 cm. e, Macroscopic tumor counts. f, Tumor size in PBS.MR and anti-TNF.MR mice. Data are mean±s.d. Two-tailed Mann–Whitney U-test was performed for paired comparisons, and two-way ANOVA followed by Sidak’s multiple comparisons test was used for tumor size comparison. Microbiota transplantation was performed once due to limited materials for inoculation. The number of animals (n) used for each graph was indicated.
Discussion
Inflammation is a hallmark of cancer and directly involved in every stage of tumorigenesis3, and as a result anti-inflammatory drugs could prevent cancer development. Indeed, use of nonsteroidal anti-inflammatory drugs (NSAIDs), especially COX inhibitors such as aspirin, has been linked to a reduced risk for various types of cancer33. IBD is associated with increased risk for CRC, and hence anti-inflammatory drugs used for IBD have been investigated for impact on CRC development2. For example, epidemiological surveys suggest that anti-TNF therapy is associated with reduced frequency of CRC34,35, but the association is not always statistically significant36. Similarly, in preclinical animal models, TNF blockade attenuated CRC development in several studies37-40 but failed to elicit significant effects in others41,42. The discrepancy reconciles with the clinical observation that up to 40% of patients do not respond to initial anti-TNF therapy 43. The events dictating responsiveness to TNF blockade are currently unknown. Our study suggests that microbiota variations among individuals and institutes could be accountable. Since our study provides proof of concept that targeting inflammation attenuates CRC development through a microbiota-dependent manner, it would be important to evaluate the impact of microbiota on chemopreventive agents such as aspirin and other NSAIDS. Due to the potential adverse effects of chronic NSAID usage on the gastrointestinal tract44, it would be necessary to determine whether intermittent intake of NSAIDs is sufficient to convert the microbiota into a ‘noncarcinogenic’ form in humans.
Our analyses of mouse microbiota revealed features that were also associated with CRC in humans. For example, Erysipelotrichaceae abundance positively correlated with tumorigenesis and was reduced by TNF blockade in our mouse models. Enrichment of Erysipelotrichaceae (associated with tumorigenesis in our mouse models) or members of the family (for example, Solobacterium moorei) was reported in CRC across multiple patient cohorts 14,45-48. Regarding microbiota activities, ribosome and biosynthesis of amino acids and antibiotics were repressed, whereas degradation of aromatic compounds and phenylalanine metabolism was enhanced by TNF blockade in mice. Similar changes in these microbiota functional pathways were observed in patients with IBD following anti-TNF therapy, although these associations need to be verified by larger patient cohorts. To what extent these bacteria and microbiota functions influence CRC development is worth further investigation.
CRC was primarily driven by NC101 colibactin activity in our mouse models, as ΔclbP-colonized mice had very few tumors. Intestinal inflammation could confer a growth benefit to E. coli, which are able to use nitrate24 and formate25 generated in the inflamed environment. And inflammation-associated mucus barrier impairment could enhance E. coli attachment to the mucosa12. However, in our mouse models, neither fecal nor tissue-associated NC101 abundance significantly changed with inflammation status (along disease progression or following TNF blockade). Instead, our normalized microbial RNA-seq data support the notion that inflammation modulates NC101 carcinogenic activities by regulating bacterial gene expression. We found that clb island expression was significantly higher in control mice compared with anti-TNF-treated mice, starting at early tumorigenesis when inflammation was higher in the former (day 14). This is concordant with the previous observation that inflammation enhanced clb gene activities in mono-associated AOM/Il10−/− mouse colitis-associated CRC model18. The lower clb island transcription could account for the reduced DNA damage response in the colon epithelium of anti-TNF-treated mice, although we cannot rule out the contribution of attenuated inflammation-associated genotoxic oxidative stress or other microbiota activities.
In summary, we show that TNF blockade alters microbiota composition and activities, thereby attenuating colorectal carcinogenesis. Our study reveals a previously unappreciated role of microbiota in mediating the antitumorigenic effect of TNF blockade and suggests that microbiota are amenable by targeting host immune signaling. In light of our findings, it would be important to determine the tumorigenic potential of pre- and post-therapy microbiota from patients with IBD using gnotobiotic animal models. The reciprocal influence between disease status and microbiota, which is also under environmental pressure, underlines the multifactorial nature of IBD and CRC etiology. For disease prevention and therapy, a holistic approach devised to address the host–microbiota–environment tripartite interaction would likely be most effective.
Methods
Mice.
All 129/SvE Apcmin/+ and Apcmin/+;Il10−/− mice were bred and all experiments were conducted at the University of Florida. Germ-free mouse colonies were maintained in isolators, supplied with sterile water and food. For all experiments, mice were randomized into treatment groups and matched to the best for age and sex. At least three cages, each harboring at least two mice, were used in each group for all experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Florida (protocol no. 201609606).
Bacteria and culture conditions.
E. coli NC101, NC101ΔclbP or K12 were cultured from glycerol stocks in Luria-Bertani broth at 37 °C overnight, then diluted 1:10 into fresh Luria-Bertani medium and cultured for 3 h. Bacteria were then collected and resuspended in sterile PBS for gavage.
Mouse models.
SPF Apcmin/+ mice (12–18 weeks old, males and females) were pretreated with an antibiotic cocktail in the drinking water (2 g l−1 streptomycin, 1 g l−1 bacitracin, 0.5 g l−1 gentamycin and 0.125 g l−1 ciprofloxacin) for a week, with water replaced once on day 4. After switching to regular drinking water for 24 h, mice were inoculated with 108 colony-forming units (c.f.u.) of E. coli via single oral gavage. At 1 week after E. coli gavage, mice received 1.5% DSS (molecular weight 40 kDa, Alfa Aesar, J63606) in drinking water for 7 d. After switching to regular drinking water for 4 weeks, mice were euthanized and examined. Germ-free Apcmin/+;Il10−/− mice (12–15 weeks old, males and females) were transferred from breeding isolators to an SPF room, and immediately inoculated with 108 c.f.u. of E. coli via single oral gavage. Mice were euthanized 16 weeks after gavage and examined.
For TNF neutralization, Apcmin/+ and Apcmin/+;Il10−/− mice were inoculated with NC101 as described in the previous paragraph. An anti-mouse TNF monoclonal antibody with increased affinity for mouse TNF (Janssen)49 was intraperitoneally injected into Apcmin/+ mice (10 mg kg−1) every other day for up to six times starting immediately after DSS retrieval, or into Apcmin/+;Il10−/− mice twice weekly starting 8 weeks after E. coli gavage till the endpoint. PBS (vehicle) injection was used as control. Anti-TNF- and PBS-treated mice were separately housed or cohoused as indicated starting at the beginning of experiments.
Stools at indicated time points and cecal contents at the endpoint were collected, flash-frozen in liquid nitrogen and stored at −80 °C until analyzed. Mouse colon was dissected at the experimental endpoint, cut open longitudinally using sterile blades and gently rinsed twice with sterile PBS on the lumenal side. Macroscopic tumors were counted and sizes (diameter) were measured. Colon tissue was then cut in half longitudinally. One half was flash-frozen in liquid nitrogen and stored at −80 °C until analyzed. The other half was Swiss rolled and fixed in 10% neutral buffered formalin solution (Fisher Scientific, SF100-4). Swiss rolls were processed, paraffin-embedded, sectioned and stained with hematoxylin and eosin (H&E) by the Molecular Pathology Core at the University of Florida. Histological scoring of the distal colon was performed blindly by at least two investigators, as described previously9,50.
Microbiota transplantation.
Approximately 50 mg of cecal contents from each mouse was pooled from NC101-colonized DSS/Apcmin/+ mice treated with PBS or anti-TNF antibody in a type B vinyl anaerobic chamber (Coy Laboratory, 032714), resuspended in 10 ml of reduced sterile PBS and kept in sealed tubes on ice until gavage. Germ-free Apcmin/+ mice (12–18 weeks old) were transferred from breeding isolators to individually ventilated cages on the IsoCage P Bioexclusion System (Techniplast) and supplied with autoclaved water and food. After 1-week acclimation, mice were inoculated with 200 μl of freshly prepared cecal content slurry by single oral gavage. At 10 d after gavage, mice received 1.5% DSS (molecular weight 40 kDa, Alfa Aesar, J63606; filter-sterilized) for 1 week. Mice were then kept on autoclaved regular drinking water for 4 weeks before euthanasia. Stools and cecal contents were collected using sterile tools at indicated time points.
Enterobacteriaceae and NC101 quantification.
Enterobacteriaceae load was determined by quantitative plating of stool samples on MacConkey agar plates. DNA was extracted from 20–100 mg of fecal material using phenol/chloroform separation followed by DNeasy Blood & Tissue Kit (QIAGEN, 69506). DNA concentration and quality were examined using a NanoDrop 2000. qPCR was performed on 20 ng of DNA on the CFX384 Touch Real-Time PCR Detection System (Bio-Rad, 1855485) using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, 1725274). The relative abundances of E. coli and NC101 was determined using specific primers targeting E. coli 16S and clbP gene, respectively, normalized to universal bacterial 16S. Primer sequences are shown in Supplementary Table 4.
Cytokine qPCR.
First, ~0.5-cm-long snips were cut from the distal end of frozen colon tissues. RNA was extracted using TRIzol reagent followed by phenol/chloroform separation. DNA was removed using the Turbo DNA-free Kit (Thermo Fisher Scientific, AM1907). Then, 500 ng of RNA, quantified by NanoDrop 2000, was used for complementary DNA synthesis using the iScript cDNA Synthesis Kit (Bio-Rad, 1708891). PCR was performed on the CFX384 Touch Real-Time PCR Detection System (Bio-Rad, 1855485) using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, 1725274). 36B4 and GusB were used as references. Primer sequences are shown in Supplementary Table 4.
γH2AX immunohistochemistry.
γH2AX was detected using the phospho-Histone H2A.X (Ser139) (20E3) rabbit monoclonal antibody (Cell Signaling, 9718), followed by Vectastain Elite ABC Rabbit IgG Kit (Vector Labs, PK-6101) according to manufacturer’s instructions. Slides were examined using a Leica DM5500 B bright-field microscope.
16S rRNA gene sequencing.
DNA was extracted using phenol/chloroform separation followed by DNeasy Blood & Tissue Kit (QIAGEN, 69506), from 20–100 mg of fecal materials, cecal or pooled cecal contents, or 0.5-cm distal colon snips. DNA concentration and quality were evaluated using NanoDrop 2000. The V1-V3 hypervariable region of the 16S rRNA gene was amplified using the primer pair 8F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 534R (5’-ATTACCGCGGCTGCTGG-3’). Both the forward and the reverse primer contained universal Illumina paired-end adapter sequences, as well as unique individual 4–6-nucleotide barcodes between the PCR primer sequence and the Illumina adapter sequence to allow multiplex sequencing. PCR products were visualized on an agarose gel before and after sample purification using the Agencourt AMPure XP kit (Beckman Coulter, A63880), and quantified by qPCR using the KAPA Library Quantification Kit (KAPA Biosystems, KR4824). Equimolar amounts of samples were then pooled and sequenced using paired-ends (read length=300 bases) on an Illumina MiSeq. Each experiment was sequenced separately.
16S rRNA gene sequencing analysis.
Sequencing reads were preprocessed using Quantitative Insights into Microbial Ecology (QIIME) 51 v.1.9.1, including trimming and filtering at quality score (Q) 20. The final set of reads was fed to QIIME’s pick_open_reference_otus.py to pick operational taxonomic units (OTUs) at 97% similarity level using the Greengenes 97% reference dataset (release 13_8). Chimeric sequences were detected and removed using QIIME. OTUs that had ≤0.005% of the total number of sequences were excluded according to Bokulich and colleagues52. Taxonomic assignment was done using the RDP (Ribosomal Database Project) classifier53 through QIIME with confidence set to 50%. For the first experiment (Fig. 3 and Extended Data Figs. 2 and 3), this resulted in an average of 52,204 reads per sample (minimum = 2,724 reads; maximum = 107,872 reads) for the distal colon samples and an average of 74,971 reads per sample (minimum = 55,371 reads; maximum = 119,395 reads) for the stool samples. For the cohousing experiment (Fig. 5), this resulted in an average of 15,576 reads per sample (minimum = 8,763 reads; maximum = 23,951 reads) for the distal colon samples and an average of 29,754 reads per sample (minimum = 12,829 reads; maximum = 39,609 reads) for the stool samples. For the transplant experiment (Fig. 6), this resulted in an average of 55,244 reads per sample (minimum = 47,455 reads; maximum = 85,560 reads) for the cecal samples and an average of 50,678 reads per sample (minimum = 45,766 reads; maximum = 55,591 reads) for the inoculum samples.
For each dataset, we generated a Principal Coordinate Analysis (PCoA) using R (v.3.5.1) phyloseq package54 (v.1.26) from the Bray–Curtis dissimilarity matrix, using the log10-normalized counts55 according to the following formula:
where RC is the read count for a particular OTU in a particular sample, n is the total number of reads in that sample, the sum of x is the total number of reads in all samples and N is the total number of samples.
Alpha diversity (Chao1 diversity index) was calculated using the phyloseq R package from the rarefied counts (set to the minimum read count in each comparison) and differences were tested using the models described in the next paragraph.
Difference in the microbial community composition, alpha diversity and taxa abundances were tested as described previously 18,55. Briefly, differences were detected using a linear mixed-effects model (lme function) in the R nlme package (v.3.1–140), with the restricted maximum likelihood (REML) method to fit a generalized mixed linear model of the following form: variable ≈ treatment + 1∣cage + ε, where variable indicates the PCoA axis, alpha diversity index or taxa abundance; treatment indicates the treatment (PBS or anti-TNF); 1∣cage indicates that we used the cage as a random effect to account for cohousing effects; and ε is the random error. For comparisons that involved time with multiple measures from the same animal, we used the following model: variable ≈ time + ~1∣cage/mouse + ε, where variable is as defined above and the random effect is set to ~1∣cage/mouse to account for both cage and mice-within-cage55. For comparisons that involved the Apcmin/+;Il10−/− model, we detected significant effect for sex and therefore sex was also included in the models above.
Then, analysis of variance (ANOVA) on the model was performed to generate P values. Whenever multiple comparisons were performed, the P values were FDR corrected (denoted as FDR P value) using R’s p.adjust function employing the method of Benjamini and Hochberg56.
Metagenome shotgun sequencing.
At least one mouse was randomly selected from each cage for microbial RNA-seq, except one cage in the PBS treatment group where remaining fecal material was too little for sequencing. DNA was extracted using phenol/chloroform separation followed by DNeasy Blood & Tissue Kit (QIAGEN, 69506), from 20–100 mg of fecal materials. Libraries for metagenome shotgun sequencing were constructed at the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida. Briefly, DNA samples (0.5–1.0 μg) were fragmented in 50-μl microTUBES-50 (AFA Fiber Screw-Cap) on the Covaris S220 sonicator to generate ~300-base pair (bp) fragments. Fragmentation was followed by AMPure clean-up (0.9:1.0 beads/sample ratio). Equal amounts of the fragmented material (125 ng) were used as input for library construction. Sequencing libraries were constructed using the NEBNext Ultra II DNA Library Prep Kit (NEB, E7645S), following the manufacturer’s protocol with a few modifications. First, 10–20 ng of DNA was used to barcode and generate full adapter-ligated libraries through PCR. PCR was done for only four cycles of amplification to minimize duplicate reads. Barcoding was done using the indexing reagents provided in the NEBNext Unique Dual Index Oligos kit (NEB, E6440S). The final libraries were quantified by fluorescence (QUBIT, ThermoFisher) and sized on the Agilent TapeStation (DNA5000 Screen Tape). A yield of 50–60 ng of library was obtained, with an average size range of 460–600 bp. Libraries were normalized and pooled equimolarly. This was followed by treatment with the ‘Free Adapter Blocking Reagent’ protocol (FAB, 20024145) to minimize index-hopping rates. The pooled library was diluted to 2.5 nM and sequenced (one S4 lane, 2×150 cycles) according to the XP (lane splitting) Illumina NovaSeq6000 sequencing protocol, using 200-pM loading concentration and 1% PhiX spike-in control. A total of 3.03 billion paired-end reads were obtained from a single S4 lane run (Q30% ≥ 84%; cluster passing filter (PF)=80.5%).
Metagenome shotgun sequencing analysis.
Reads were quality trimmed and filtered (at Q20) to remove mouse (using iGenome Mus musculus Ensembl GRCm38 reference genome) rRNA and transfer RNA (using a collection of National Center for Biotechnology Information rRNA and tRNA in addition to SILVA database sequences) by employing Trimmomatic57 and KneadData (http://huttenhower.sph.harvard.edu/kneaddata). The remaining reads were aligned to the mouse gut microbiome integrated gene catalog32 using Bowtie2 (ref. 58) (v.2.3.5) followed by quantification using featureCounts59 from the subread package (v.1.5.3). For clb island gene expression analysis, we aligned the trimmed and filtered reads to the E. coli NC101 genome assembly18 using Bowtie2 then quantified clb gene expression using featureCounts59.
For Extended Data Fig. 2c, we used COSMOSID (https://www.cosmosid.com/) platform to perform strain-level identification of our metagenome shotgun sequencing samples. Strain counts obtained from COSMOSID were normalized and log10-transformed according to the formula shown in the 16S rRNA gene sequencing analysis section. E. coli NC101 normalized and log10-transformed abundances were then used for the analysis shown in Extended Data Fig. 2c.
Microbial RNA-seq.
The same stools used for shotgun metagenome sequencing were selected for microbial RNA-seq. Total RNA was extracted from ~50 mg of fecal material using mirVana miRNA Isolation Kit with phenol (Thermo Fisher Scientific, AM1560) according to the manufacturer’s instructions, with the addition of a tissue disruption and lysis step using a Precellys 24 (Bertin Instruments, EQ03119-200-RD000.0) bead beater with an approximately 1:1 mix of 1-mm acid-washed glass beads and 0.1-mm zirconia beads. DNA was removed using Turbo DNA-free Kit (Thermo Fisher Scientific, AM1907). Quality control, ribosomal RNA depletion and cDNA library preparation were performed by the Gene Expression and Genotyping Core at the University of Florida ICBR, using the Agilent 2100 Bioanalyzer (Agilent Genomics, G2939BA), the MICROBExpress Bacterial mRNA Enrichment Kit (Thermo Fisher Scientific, AM1905) and the ScriptSeq RNASeq Library Preparation Kit (Illumina, SSV21124), starting with 1 μg of total RNA. Samples were sequenced on an Illumina HiSeq 3000 (2×100 run) by the University of Florida ICBR NextGen DNA Sequencing Core, multiplexed into two lanes to avoid lane effect.
Microbial RNA-seq analysis.
Reads were quality trimmed, filtered, aligned and quantified as described above in the ‘Metagenome shotgun sequencing analysis’ section. Metagenomic normalization of microbial RNA-seq data was done using HUMAnN2’s humann2_rna_dna_norm script60. Metagenomic normalized counts were then imported to edgeR (v.3.26) to normalize for library sizes using the weighted trimmed mean of M values (TMM) method, and those normalized counts were then used for both principal component analysis (PCA) and differential expression analysis. A gene was considered for differential expression analysis only if it was present in at least 30% of the samples. We considered a transcript differentially expressed if its edgeR FDR adjusted P value was <0.05. Pathway analysis was conducted through GAGE61 (v.2.34) using KEGG reference pathways. We considered a pathway significant if its GAGE Q value was less than 0.05.
For clb island gene expression analysis, we aligned the trimmed and filtered RNA-seq reads to E. coli NC101 genome assembly using Bowtie2, quantified gene expression using featureCounts and performed metagenomic normalization of microbial RNA-seq data using HUMAnN2’s humann2_rna_dna_norm script, then normalized for library sizes using the edgeR TMM method.
IBDMDB metatranscriptome pathway analysis.
Samples were pulled from the hmp2_IBDMDB metadata (https://ibdmdb.org/) with available information on anti-TNF therapy (Remicade (Infliximab), Humira (Adalimumab) and Cimzia (Certlizumab)). In the original metadata, the treatment status was recorded in ‘host_genome’, ‘methylome’ and ‘serology’ but not ‘metagenomics’ or ‘metatranscriptomics’ datasets. By matching unique patient IDs, we generated a list of 88 patients with information on anti-TNF therapy. Out of these patients, those with samples showing before (‘never taken’) and after (‘current’) anti-TNF treatment were selected. This led to a list of 21 patients. In these patients, ‘metatranscriptomics’ samples were checked for ‘week_num’, and pre- or post-anti-TNF-treatment status was inferred based on ‘week_num’ and ‘duration’ of treatment provided in ‘host_genome’, ‘methylome’ and ‘serology’ datasets. Only samples with ≥1,000,000 reads60 and matching metagenome shotgun sequencing available were kept, and then only patients with both pre- and post-treatment samples were included for analyses. This led to a total of six patients (one Cincinnati, three Massachusetts General Hospital (MGH) and two MGH pediatrics) with pre- (n=36) and post- (n=21) treatment samples. The metadata for samples included in our analysis are shown in Supplementary Table 5.
The raw sequence files for both the IBDMDB metatranscriptomes and their matching metagenome shotgun sequences62 were downloaded from https://ibdmdb.org/tunnel/public/HMP2/MTX/1750/rawfiles and https://ibdmdb.org/tunnel/public/HMP2/WGS/1818/rawfiles, respectively, and processed as described above in the ‘Metagenome shotgun sequencing analysis’ section except for the use of the human reference genome (iGenome Homo sapiens GRCh38) instead of the mouse genome. The trimmed and filtered reads were then aligned to a local copy of KEGG bacterial orthologs using Diamond (blastx) aligner (v.0.9.24)63, and metagenomic normalization of metatranscriptomic data was done using HUMAnN2’s humann2_rna_dna_norm script then normalized for library sizes using edgeR TMM method and log10-transformed. Pathway analysis was conducted using GAGE as described in the ‘Microbial RNA-seq analysis’ section.
Statistics and reproducibility.
Statistics for 16S rRNA gene sequencing, shotgun metagenomics sequencing and microbial RNA-seq are described above. Other data were graphed and analyzed using GraphPad Prism 8, and specific statistical methods used are indicated in the text or figure legends. Briefly, two-tailed Mann–Whitney U-test was used for comparison between two groups. For multi-group comparisons, one-way or two-way ANOVA followed by Dunn’s, Tukey’s, Sidak’s or Holm–Sidak’s multiple comparisons test was performed. Fisher’s exact test was used to determine association.
No statistical method was used to predetermine sample size. No data were excluded from the analyses. Mice were randomly assigned to experimental groups and matched to the best for age and sex. The investigators were not blinded to group allocation for mouse stool collection and euthanasia to avoid sample cross-contamination. Otherwise, the investigators were blinded to allocation during experiments and outcome assessment. Experiments comparing effects of different E. coli strains on colitis and tumorigenesis in DSS/Apcmin/+ and Apcmin/+;Il10−/− models were done once, with mice staggered due to limited mouse availability per experimental setup. TNF blockade was performed twice in DSS/Apcmin/+ and Apcmin/+;Il10−/− models, with reproducible results. Cohousing was performed once in DSS/Apcmin/+ and Apcmin/+;Il10−/− models. Cecal microbiota transplantation was performed once in DSS/Apcmin/+ model.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The sequencing data generated in this study were deposited in the National Center for Biotechnology Information Sequence Read Archive under the following accessions: PRJNA564272 (transplant 16S rDNA sequences), PRJNA564144 (cohousing 16S rDNA sequences), PRJNA564137 (main experiment 16S rDNA sequences), PRJNA564115 (microbial RNA-seq) and PRJNA610017 (metagenome shotgun sequences). The human metatranscriptome and metagenome data were derived from the IBDMDB (https://ibdmdb.org/), and the unique ‘External ID’ for individual samples included in our analysis was listed in Supplementary Table 5. Data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
The Leica Application Suite X (https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/) was used for histology and immunohistochemistry visualization and imaging. The following open-source code was used in the analyses performed in this research: QIIME v.1.9.1 (ref. 51), Trimmomatic v.0.36 (ref. 57), KneadData v.0.6.1 (http://huttenhower.sph.harvard.edu/kneaddata), Bowtie2 v.2.3.5 (ref. 58), featureCounts v.1.5.3 (ref. 59), HUMAnN2 v.2.8.1 (ref. 60), edgeR v.3.26, GAGE v.2.34 (ref. 61), Diamond v.0.9.24 (ref. 63), R statistical framework v.3.5.1, R phyloseq package v.1.26, R nlme package v.3.1–140 and R ggplot2 package v.3.2. In addition, the following commercial code was used for data analyses: COSMOSID (https://www.cosmosid.com/) and GraphPad Prism 8 (https://www.graphpad.com/scientific-software/prism/). Preprocessing steps were done using in-house scripts that are available upon request.
Extended Data
Extended Data Fig. 1 ∣. TNF blockade attenuates colonic inflammation and DNA damage response.
a, Timeline for short-term TNF blockade in NC101-colonized DSS/Apcmin/+ mice. Experiment was done once. b, Representative pictures of H&E stained colon Swiss roll section (distal). Scale bars = 500 μm. c, Distal colon histological scores. d, Representative pictures of colon Swiss roll sections stained for γH2AX. Scale bars = 100 μm. e, Association between treatment and incidence of γH2AX+ regenerating crypts. Fisher’s exact test was used to determine association. f, Fecal NC101 relative abundance in PBS- and anti-TNF-treated mice at day 14. g, Cytokine expression in distal colon snips of NC101-colonized Apcmin/+;Il10−/− mice treated with PBS or anti-TNF antibody (related to Fig. 2h). Data are mean ± s.d. Two-tailed Mann-Whitney U test was performed for paired comparisons. The number of animals (n) used for each graph was indicated.
Extended Data Fig. 2 ∣. Fecal microbiota and NC101 changes in DSS/Apcmin/+ mice treated with PBS or anti-TNF antibody.
a, Alpha diversity (Chao1) and beta diversity (PCoA) comparison of microbiota in PBS-treated mice between indicated time points. The box horizontal lines are the 25th (lower) and 75th (upper) percentile. The middle line is the 50th percentile (the median). The lower whisker is 25th percentile - 1.5 times the interquartile range and the upper whisker is 75th percentile + 1.5 times the interquartile range. b, Alpha diversity (Chao1) and beta diversity (PCoA) comparison of microbiota in anti-TNF-treated mice between indicated time points. Sequencing data analysis was described in details in Methods. The box horizontal lines are the 25th (lower) and 75th (upper) percentile. The middle line is the 50th percentile (the median). The lower whisker is 25th percentile - 1.5 times the interquartile range and the upper whisker is 75th percentile + 1.5 times the interquartile range. c, NC101 abundance by metagenome analysis. One-way ANOVA followed by Holm-Sidak’s multiple comparisons test was performed for multi-group comparisons. d, Normalized counts of transcripts aligned to clb genes. Two-tailed Mann-Whitney U test was performed for paired comparisons. Data are mean ± s.d. The number of animals (n) used for each graph was indicated.
Extended Data Fig. 3 ∣. Microbiota community structure changes in NC101-colonized Apcmin/+;Il10−/− mice treated with PBS or anti-TNF antibody.
a, Alpha diversity (Chao1) and beta diversity (PCoA) comparison of microbiota in PBS-treated mice between indicated time points. The box horizontal lines are the 25th (lower) and 75th (upper) percentile. The middle line is the 50th percentile (the median). The lower whisker is 25th percentile - 1.5 times the interquartile range and the upper whisker is 75th percentile + 1.5 times the interquartile range. b, Alpha diversity (Chao1) and beta diversity (PCoA) comparison of microbiota in anti-TNF-treated mice between indicated time points. The box horizontal lines are the 25th (lower) and 75th (upper) percentile. The middle line is the 50th percentile (the median). The lower whisker is 25th percentile - 1.5 times the interquartile range and the upper whisker is 75th percentile + 1.5 times the interquartile range. c, Alpha diversity (Chao1) and beta diversity (PCoA) comparison between PBS- and anti-TNF-treated mice in longitudinal stool samples and endpoint colon tissues. The box horizontal lines are the 25th (lower) and 75th (upper) percentile. The middle line is the 50th percentile (the median). The lower whisker is 25th percentile - 1.5 times the interquartile range and the upper whisker is 75th percentile + 1.5 times the interquartile range. ns, not significant. Sequencing data analysis was described in details in Methods. The number of animals (n) used for each graph was indicated.
Extended Data Fig. 4 ∣. Cohousing abolishes CRC-preventive effect of TNF blockade in NC101-colonized Apcmin/+;Il10−/− mice.
a, Schematic diagram for TNF blockade under cohousing condition. b, Macroscopic colon tumor counts. c, Distal colon histological scores. d, Fecal NC101 relative abundance in cohoused PBS- and anti-TNF-treated mice. Data are mean ± s.d. Two-tailed Mann-Whitney U test was performed for paired comparisons. Experiment was done once. The number of animals (n) used for each graph was indicated.
Supplementary Material
Acknowledgements
This research was supported by NIH grant R01DK073338, the University of Florida Health Cancer Center Funds and the University of Florida Department of Medicine Gatorade Fund (all to C.J.). Y.Y. was supported by the Crohn’s & Colitis Foundation of America research fellowship award (ref. no. 409472). R.Z.G. was supported by UF Health Cancer Center Funds. R.C.N. was supported by an NIH TL1 training grant (TL1TR001428). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We are grateful to the Germ-Free Services division of UF Animal Care Services for assistance with gnotobiotic experiments, the UF Molecular Pathology Core for assistance with tissue processing and staining, the UF Interdisciplinary Center for Biotechnology Research Core for assistance with shotgun metagenome and microbial RNA sequencing and J. Gauthier for assistance with 16S rRNA gene sequencing.
Footnotes
Extended data is available for this paper at https://doi.org/10.1038/s43018-020-0078-7.
Supplementary information is available for this paper at https://doi.org/10.1038/s43018-020-0078-7.
Competing interests
The authors declare no competing interests.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ng SC et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Beaugerie L & Itzkowitz SH Cancers complicating inflammatory bowel disease. N. Engl. J. Med 372, 1441–1452 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Greten FR & Grivennikov SI Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schirmer M, Garner A, Vlamakis H & Xavier RJ Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol 17, 497–511 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang M & Martin A Microbiome and colorectal cancer: unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin. Immunol 32, 3–13 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Nougayrède J-P et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Wilson MR et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science 363, eaar7785 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue M et al. Structure elucidation of colibactin and its DNA cross-links. Science 365, eaax2685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur JC et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buc E et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 8, e56964 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prorok-Hamon M et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 63, 761–770 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dejea CM et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eklöf V et al. Cancer-associated fecal microbial markers in colorectal cancer detection. Int. J. Cancer 141, 2528–2536 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wirbel J et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med 25, 679–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pleguezuelos-Manzano C et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnet M et al. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res 20, 859–867 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Cougnoux A et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63, 1932–1942 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Arthur JC et al. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun 5, 4724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomkovich S et al. Locoregional effects of microbiota in a preclinical model of colon carcinogenesis. Cancer Res. 77, 2620–2632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W et al. Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J. Exp. Med 216, 2378–2393 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brotherton CA & Balskus EP A prodrug resistance mechanism is involved in colibactin biosynthesis and cytotoxicity. J. Am. Chem. Soc 135, 3359–3362 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Cougnoux A et al. Small-molecule inhibitors prevent the genotoxic and protumoural effects induced by colibactin-producing bacteria. Gut 65, 278–285 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Gogokhia L et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 25, 285–299.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter SE et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes ER et al. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 21, 208–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu W et al. Precision editing of the gut microbiota ameliorates colitis. Nature 553, 208–211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aden K et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology 157, 1279–1292.e11 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Martin P et al. Interplay between siderophores and colibactin genotoxin biosynthetic pathways in Escherichia coli. PLoS Pathog. 9, e1003437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karki R & Kanneganti T-D Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer 19, 197–214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estevinho MM et al. Features of fecal and colon microbiomes associate with responses to biologic therapies for inflammatory bowel diseases: a systematic review. Clin. Gastroenterol. Hepatol 18, 1054–1069 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Lloyd-Price J et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao L et al. A catalog of the mouse gut metagenome. Nat. Biotechnol 33, 1103–1108 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Chen F & Shang L Advances in antitumor effects of NSAIDs. Cancer Manag. Res 10, 4631–4640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biancone L et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: does blocking TNFɑ reduce colitis-associated colorectal carcinogenesis? Gut 58, 1703 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Baars JE et al. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case–control study. Am. J. Gastroenterol 106, 319–328 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Nyboe Andersen N et al. Association between tumor necrosis factor-α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA 311, 2406–2413 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Rao VP et al. Proinflammatory CD4+CD45RBhi lymphocytes promote mammary and intestinal carcinogenesis in ApcMin/+ mice. Cancer Res. 66, 57–61 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Popivanova BK et al. Blocking TNF-ɑ in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest 118, 560–570 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onizawa M et al. Signaling pathway via TNF-ɑ/NF-κB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol 296, G850–G859 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Kim YJ, Hong KS, Chung JW, Kim JH & Hahm KB Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev. Res. (Phila.) 3, 1314–1333 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Craven B, Zaric V, Martin A, Mureau C & Egan LJ Effect of genetic deletion or pharmacological antagonism of tumor necrosis factor alpha on colitis-associated carcinogenesis in mice. Inflamm. Bowel Dis 21, 485–495 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Lopetuso LR et al. Infliximab does not increase colonic cancer risk associated to murine chronic colitis. World J. Gastroenterol 22, 9727–9733 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roda G, Jharap B, Neeraj N & Colombel J-F Loss of response to anti-TNFs: definition, epidemiology, and management. Clin. Transl. Gastroenterol 7, e135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjarnason I et al. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology 154, 500–514 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Thomas AM et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med 25, 667–678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Liu F, Ling Z, Tong X & Xiang C Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 7, e39743 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchesi JR et al. Towards the human colorectal cancer microbiome. PLoS ONE 6, e20447 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yachida S et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med 25, 968–976 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Heaton WL et al. Autocrine Tnf signaling favors malignant cells in myelofibrosis in a Tnfr2-dependent fashion. Leukemia 32, 2399–2411 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Z et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 68, 289–300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caporaso JG et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bokulich NA et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q, Garrity GM, Tiedje JM & Cole JR Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol 73, 5261–5267 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMurdie PJ & Holmes S phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCafferty J et al. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J. 7, 2116–2125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benjamini Y & Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol 57, 289–300 (1995). [Google Scholar]
- 57.Bolger AM, Lohse M & Usadel B Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langmead B & Salzberg SL Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao Y, Smyth GK & Shi W featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Franzosa EA et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 15, 962–968 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo W, Friedman MS, Shedden K, Hankenson KD & Woolf PJ GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinf. 10, 161 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature 569, 641–648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buchfink B, Xie C & Huson DH Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data generated in this study were deposited in the National Center for Biotechnology Information Sequence Read Archive under the following accessions: PRJNA564272 (transplant 16S rDNA sequences), PRJNA564144 (cohousing 16S rDNA sequences), PRJNA564137 (main experiment 16S rDNA sequences), PRJNA564115 (microbial RNA-seq) and PRJNA610017 (metagenome shotgun sequences). The human metatranscriptome and metagenome data were derived from the IBDMDB (https://ibdmdb.org/), and the unique ‘External ID’ for individual samples included in our analysis was listed in Supplementary Table 5. Data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.