Mussel-inspired adhesion chemistry provides a useful alternative to the use of fibrin glues.
Abstract
Since the first report of underwater adhesive proteins of marine mussels in 1981, numerous studies have reported mussel-inspired synthetic adhesive polymers. However, none of them have developed up to human-level translational studies. Here, we report a sticky polysaccharide that effectively promotes hemostasis from animal bleeding models to first-in-human hepatectomy. We found that the hemostatic material instantly generates a barrier layer that seals hemorrhaging sites. The barrier is created within a few seconds by in situ interactions with abundant plasma proteins. Therefore, as long as patient blood contains proper levels of plasma proteins, hemostasis should always occur even in coagulopathic conditions. To date, insufficient tools have been developed to arrest coagulopathic bleedings originated from genetic disorders, chronic diseases, or surgical settings such as organ transplantations. Mussel-inspired adhesion chemistry described here provides a useful alternative to the use of fibrin glues up to a human-level biomedical application.
INTRODUCTION
Bleeding is a leading cause of intraoperative and postoperative morbidity and mortality (1). Although various hemostatic agents have been developed, arresting perioperative bleeding, especially in patients with coagulopathy, remains a challenge. The first choice of hemostatic material in most clinical settings is fibrin-containing materials. However, fibrin-containing materials are not useful for coagulopathic patients. For example, ineffective hemostatic abilities for thrombocytopenia and thrombotic thrombocytopenic purpura have been reported (2–6).
Coagulopathy, also called a bleeding disorder, is a condition in which the blood’s ability to form clots is impaired (7). Coagulopathy occurs for a variety of reasons: genetic disorders (e.g., hemophilia and von Willebrand’s disease), chronic diseases (e.g., cancer, chronic liver, or renal disease), medications (e.g., heparin, warfarin, and aspirin) or disseminated intravascular coagulation (DIC), etc. (7–10). Chronic liver diseases frequently result in impaired ability to maintain necessary levels of coagulation factors and platelets (7). Coagulopathy is also observed in renal failure because of decreased erythropoietin biosynthesis and the accumulation of uremic toxins, which result in anemia and platelet dysfunctions (7). The administration of anticoagulants also affects hemostasis. The down-regulation of vitamin K by warfarin, anti-platelet function by aspirin, or antithrombin activation by heparin causes delayed blood clotting (7–9). Another case is DIC, also known as consumptive coagulopathy, in which hemostasis is severely impaired because blood clotting factors are quickly consumed by the formation of blood clots throughout the body. DIC can be caused by sepsis, infectious disease, obstetrical complications, massive tissue injury, and so on (7, 10).
Therefore, effective hemostasis “independent of blood conditions” is required to reduce the occurrence of postoperative complications related to coagulopathic bleeding. Recent studies are in the line of developing hemostatic materials aiming at blood condition–independent coagulations. The intravenously injected polymers conjugated with fibrin-binding peptides selectively identify vascular injury sites and induce hemostasis, forming fibrin matrixes (11). In addition, thrombin-loaded, gas-generating microparticles locomotive against hemorrhage streams show effective hemostasis (12). Being different from these studies, the fundamental premise in developing hemostatic materials is that biopolymer itself, without actions of thrombin and fibrinogen, can induce active blood coagulation upon the aforementioned coagulopathic conditions. Topical on organs and external applications of hemostatic materials are primary targets in this study, which can be extended to diffuse injuries later. We hypothesized that achieving blood condition–independent hemostasis essentially requires the formation of adhesive barriers at hemorrhaging sites in situ. In the hemostatic context, the term “adhesion” is a molecular-level phenomenon in which blood plasma proteins are rapidly glued by extrinsically provided adhesive components to form a barrier membrane against hemorrhage. To incorporate an adhesive property to achieve hemostasis for both normal and coagulopathic bleeding, we used the wet-resistant mussel adhesion principle (13–16). Mussel-inspired adhesive hemostatic materials such as poly(organophosphazene)-catechol (17), poly(ethylene glycol)–citric acid-catechol (18), gelatin-catechol (19), and catechol-functionalized chitosan/Pluronic F127 (20) have been reported to stop bleeding. However, none of them showed a hemostatic effect under coagulopathy. Furthermore, the demonstrated bleeding models have been small mouse models without a clear discussion of hemostasis mechanisms.
Here, we demonstrate that mussel-inspired catechol chemistry plays a pivotal role in hemostasis not only in healthy blood but also in coagulopathic blood. Furthermore, its hemostatic ability was shown from preclinical animal models to human clinical trials. By optimizing catechol derivative chemistry, we found that the synergy of catechol and cationic charges instantly (<1 s) triggers the formation of a barrier membrane for hemostasis by intermolecular complexation with plasma proteins. In this study, catechol-conjugated chitosan (CHI-C) was used as a hemostatic material to demonstrate the catechol-cation synergy. The opposite type of synergies such as catechol and anion did not trigger such membrane formation. As long as a certain level of plasma protein exists in the blood, independent of blood conditions, catechol and cationic synergy generate adhesive membranes for hemostasis. We demonstrated the hemostatic capability of CHI-C in blood with considerably low levels of platelets from human patients who underwent liver transplantation. Compared to the previous study using CHI-C coated on needle surfaces with an extremely small dose (<50 μg per needle injection) (21), this study demonstrates that CHI-C is a standalone hemostatic material with a large dose (~60 mg/kg for a porcine model and ~8 mg/kg for a human clinical study), which affects the mode of actions, toxicity, and degradation broadly compared to the previous study. This previously unidentified hemostatic mechanism shown by CHI-C has enormous potential for controlling the bleeding of surgical patients with unresolved medical coagulation problems.
RESULTS
Blood protein barrier formation by CHI-C in blood and plasma
We fabricated porous CHI-C hemostatic sponges using a mold and freeze-drying method (Fig. 1A). The lyophilized CHI-C sponges were a bright white color, indicating no occurrence of catechol oxidation (22). When the oxidation occurs, generating amine-catechol or catechol-to-catechol adducts via catecholquinone, absorption at 300 nm (and longer wavelength) should be observed. However, no catechol oxidation was observed (Fig. 1B, red arrow). The degree of conjugation (DOC) of catechol was determined using an ultraviolet-visible (UV-vis) spectrophotometer (Fig. 1B) and a proton nuclear magnetic resonance (1H-NMR) spectrometer (Fig. 1C). The DOC was about 10%, and its conjugation degree was consistent from different reaction batches. For example, the DOC values calculated using the absorbance at 280 nm of the catechol group was 10.2% for CHI-C batch #1, 10.5% for batch #2, and 9.5% for batch #3. 3,4-Dihydroxyhydrocinnamic acid (HCA) was used as a standard compound. 1H-NMR spectra were also used to confirm the DOC values in which the area under the curve of catechol protons at 6.67 parts per million (ppm) was compared to that of ─CH3 protons in the N-acetyl group along a chitosan backbone (1.95 ppm). The 1H-NMR characterization resulted in similar DOC values: 10.0% for batch #1, 10.4% for batch #2, and 10.4% for batch #3. These comparative results indicate that the two methods are compatible with each other without any instrument-dependent bias. Thus, any convenient method can be used to determine the DOC value.
Fig. 1. Preparation and characterization of CHI-C sponge and observation of CHI-C/blood (and plasma) membrane formation named the blood protein barrier.
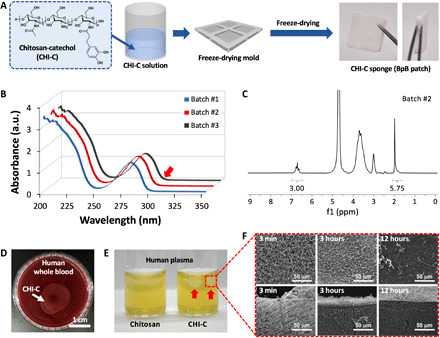
(A) Chemical structure of CHI-C and schematic illustration of the preparation of the CHI-C sponge. Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (B) UV-vis and (C) 1H-NMR spectra of CHI-C. (D) A photographic image of the blood protein barrier (BpB) membrane formed by CHI-C in human whole blood. Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (E) A photographic image of BpB membranes (right vial, red arrows) formed by CHI-C in human plasma. However, no BpB membrane was formed by chemically unmodified chitosan (left vial). Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (F) Scanning electron microscope (SEM) images of the surface (top) and cross section (bottom) of the BpB membrane at predetermined immersion times (3 min, 3 hours, and 12 hours). a.u., absorbance unit.
We unexpectedly found that the CHI-C solution spontaneously formed an immediate interfacial membrane upon contact with blood and plasma (Fig. 1, D and E). The formation of a large-scale CHI-C membrane was observed at the interface between the CHI-C solution [2 weight % (wt %), 500 μl] and human whole blood (Fig. 1D), indicating that membrane formation was enhanced more in whole blood than in plasma. In addition, we dropped CHI-C solution (2 wt %) into human blood plasma (Fig. 1E), and opaque white beads were formed in a few seconds (Fig. 1E, right vial). In contrast, for chemically unmodified chitosan (control), no beads were generated (Fig. 1E, left vial). Chitosan and CHI-C showed different interfacial behaviors when in contact with human blood plasma, proving that catechol plays an important role in this phenomenon. Moreover, as the contact time increased, a mechanically rigid and visually distinct membrane was formed. Considering that CHI-C remains soluble up to 60 mg/ml in phosphate-buffered saline (PBS) at pH 7 (23), the insolubility of the polymer in blood plasma was an unexpected result. The formation of the interfacial membrane indicates that plasma components (mostly proteins) were continuously bound/complexed to the dropped CHI-C solutions. To monitor the morphological changes in the membranes, we prepared them at predetermined time intervals of 3 min, 3 hours, and 12 hours. As shown in Fig. 1F, the membrane formed at the initial time (3 min) showed microporous structures both on the surface (top) and in the bulk cross section (bottom). However, after 3 hours, the surface porosity of the membrane decreased (top middle) and completely disappeared after 12 hours (top right). In contrast, as the contact time increased from 3 min to 12 hours, the bulk porosity was maintained (bottom). The film-like morphology of the surface gradually became obvious, indicating that complex formation between plasma components and the CHI-C solution continued for several hours. This membrane, formed by CHI-C in the blood, was called the blood protein barrier (BpB).
Identification of plasma proteins that reacted with CHI-C in the BpB membrane
The major components of blood plasma are mostly proteins such as albumins, globulins, fibrinogen, glycoproteins, and lipoproteins. In general, blood plasma contains proteins (approximately 60 to 80 mg/ml) and other minor components, such as vitamins, lipids, hormones, and salts (24). Thus, we hypothesized that the BpB membrane might be formed by rapid complexation between CHI-C and macromolecules (e.g., proteins) in plasma. Other molecules, such as ionic salts, were determined not to participate in BpB membrane formation because CHI-C is soluble in buffer solutions such as PBS, as mentioned previously (23). Because many studies of polymer/protein interactions have been reported (25–28), we hypothesized CHI-C/blood protein interaction as a plausible mechanism. To test this hypothesis, we performed two-dimensional (2D) gel electrophoresis to identify the main components of plasma that reacted with CHI-C (Fig. 2A). We dissociated the unknown macromolecules from the BpB aggregates by treatment with SDS. The proteins that appeared in the 2D gel were albumin, fibrinogen (α, β, and γ chains), immunoglobulin G (IgG) light chains (λ and κ), apolipoproteins (apo A-I and J), haptoglobin (α2 and β chains), various glycoproteins (α2 HS, α1 acid glycoprotein, α1B glycoprotein, and hemopexin), and other proteins (α1 antichymotrypsin, antithrombin III, α1 antitrypsin, Gc-globulin, and prealbumin). The proteins detected in the 2D gel spots were assigned by the previous studies (24, 29, 30). In particular, albumin, which is the most abundant plasma protein (~55% of the total plasma proteins) (31), appeared abundantly in the 2D gel. Fibrinogens (α, β, and γ chains, ~7%), which are essential glycoproteins for blood clot formation, also appeared to react with CHI-C. Thus, albumin and fibrinogen were the two major protein factors responsible for the rapid formation of BpB membranes. The other proteins detected showed that CHI-C nonspecifically bound to virtually all plasma proteins to form BpB membranes. Previous studies have shown that catecholamine polymers such as polydopamine and poly(norepinephrine) exhibit material-independent and water-resistant adhesive properties (15, 32, 33). Similarly, CHI-C is also a kind of catecholamine polymer due to the coexistence of catechol and amine groups. Thus, CHI-C exhibited excellent adhesiveness at the molecular level, which made CHI-C and plasma proteins rapidly bind to form BpB membranes. We observed that the BpB membrane could be formed in single protein solutions of albumin (first photo), γ-globulin (second photo), fibrinogen (third photo), and IgG (fourth photo) (Fig. 2B). Thus, the rapid formation of the BpB membrane by interpolymer complexation between CHI-C and plasma proteins suggests that CHI-C can stop bleeding by forming an effective physical barrier at the bleeding site.
Fig. 2. Characterization of the BpB membrane.
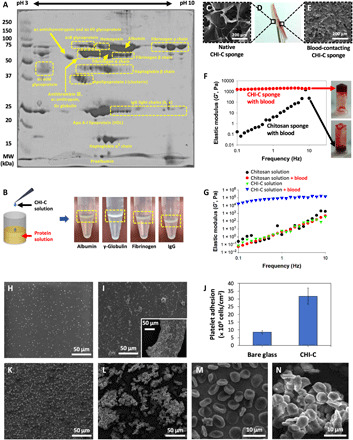
(A) A photographic image of 2D acrylamide gel (12%) showing the plasma proteins that interacted with CHI-C to form BpB membranes. (B) Left: A schematic illustration of the experiment to confirm BpB membrane formation by CHI-C in each identified plasma protein solution. Right: Photographic images of BpB membranes formed by CHI-C in each protein (albumin, ɣ-globulin, fibrinogen, and IgG) solution. Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (C to E) Photographic images of the CHI-C sponge after contact with heparin-treated human blood (D) and SEM images of the native (photo credit: Ji Hyun Ryu, Wonkwang University) (C) and blood-contacting (E) surfaces of the CHI-C sponge. (F) Elastic modulus (G′) values of the CHI-C sponge (red circles) and chemically unmodified chitosan sponge (black circles) after mixing with heparin-treated human blood. Photographic images of test-tube inverting test results of CHI-C sponge (top photo) and chemically unmodified chitosan sponge (bottom photo) after mixing with heparin-treated human blood. Photo credit: Ji Hyun Ryu, Wonkwang University. (G) Changes in the mechanical properties (elastic modulus, G′) of CHI-C and chemically unmodified chitosan solutions after mixing with heparin-treated human blood. (H to J) SEM images of platelets adhered to bare glass surface (H) and CHI-C film (I) (large panel, mild and small panel, severe). (J) Quantification of platelet adhesion on the glass substrates and CHI-C films. (K to N) SEM images of red blood cells (RBCs) that adhere onto bare glass (K and M) and CHI-C film (L and N).
3D structure formation of the BpB membrane
To confirm BpB membrane formation on the CHI-C sponge, we contacted one side of the CHI-C sponge with heparin-treated human blood (Fig. 2D) and observed the surface changes using a scanning electron microscope (SEM). The blood-contacting surface showed that the micropores were closed entirely (Fig. 2E), whereas the other side (non–blood-contacting surface) maintained the original micropores (Fig. 2C). In addition, a rheological analysis was performed to reveal BpB membrane formation at the molecular level. CHI-C and chitosan (control) (2 wt %) solutions were mixed with heparin-treated human blood (1:1, v/v), respectively. For CHI-C solution with blood, the elastic modulus values (~1.5 kPa) did not substantially change with increasing frequency, indicating cross-linked, hydrogel-like behavior (Fig. 2F, red circles). The slight increase (to 2 kPa) in the elastic moduli indicated that the intermolecular binding in the CHI-C/blood complex (i.e., BpB membrane) was in progress. In contrast, for the chemically unmodified chitosan solution with blood, the elastic modulus values increased substantially, indicating a viscous solution, not a cross-linked 3D structure, demonstrating that chitosan alone cannot form a BpB membrane (Fig. 2F, black circles). In the test-tube inversion test, the mixture of CHI-C with heparin-treated blood exhibited gel-like behavior (Fig. 2F, top photo), but the mixture of chitosan with blood flowed down (Fig. 2F, bottom photo). The mechanical properties of the CHI-C sponge were also confirmed to be improved by adding blood. The elastic modulus value of the CHI-C sponge (solid state) after the addition of heparin-treated human blood (9.4 × 104 Pa) at a 1-Hz frequency was much higher than that of the chitosan sponge with blood (6.2 Pa) (Fig. 2G). It was found that CHI-C and blood react to form a 3D structure via various interactions, such as charge interactions, ionic complexes, hydrophobic interactions, and covalent bonds (34–37).
In addition to the rapid interactions with plasma proteins, cellular components in blood also bind with CHI-C because of the presence of cellular receptor proteins. This largely contributes to CHI-C–mediated blood coagulation. CHI-C–coated glass surfaces were prepared, to which platelet-rich plasma (PRP) solution was applied and then incubated at 37°C for 2 hours. The SEM image showed that the number of platelet adhesion was significantly increased from 8.5 × 109 for unmodified glass to 3.2 × 1010 for the CHI-C–coated one (Fig. 2, H to J). Most platelets adhered onto the unmodified glass are round shapes, indicating an inactivated cellular state. However, the numerous pseudopods were observed for the platelet attached to CHI-C surfaces, indicating activated states of the platelets. In addition, red blood cells (RBCs) were rapidly aggregated upon CHI-C interactions. Purified human RBCs were incubated with CHI-C films at 37°C for 2 hours. Remarkable degrees of RBC aggregations similar to the platelet result were observed (Fig. 2, K and L). Unexpectedly, the normal biconcave cellular morphology was changed to polyhedral shapes upon CHI-C interactions (Fig. 2, M and N). The RBC morphological change during clotting was previously reported (38). Thus, the unprecedented hemostatic ability of CHI-C is attributed to two types of interactions: One is blood plasma proteins, and the other is blood cells such as platelets, RBCs, and possibly others.
Preclinical study I: The hemostatic ability of a CHI-C sponge in a heparinized rabbit model of femoral artery bleeding
From the above results, we hypothesized that the CHI-C sponge could be used as a hemostatic agent to stop bleeding by forming an effective physical barrier (BpB) and cellular aggregations and activations at the bleeding site. To confirm the hemostatic ability of the CHI-C sponge, we performed a preclinical study in a heparinized rabbit model of femoral artery bleeding. The coagulopathic rabbit model was induced by injecting heparin (200 IU/kg) via a marginal ear vein. Figure 3A shows massive bleeding (50 to 60 cm high) after puncture with a 19-gauge needle in the femoral artery. The CHI-C sponge was applied to the bleeding site with manual compression, and complete hemostasis was observed after 1 min and 30 s, as shown in Fig. 3B. The hemostasis time of the CHI-C sponge was 90 ± 0 s, which was significantly shorter than that of gauze (390 ± 60 s; P < 0.05), as shown in Fig. 3C. When studying the hemostasis ability of dressings, manual pressure is an important factor to affect the results. We would like to emphasize that gentle manual pressure was applied because of the strong eruption of the bloodstream from the artery. However, a manual press was not used for all other subsequent animal models including human studies to decouple pressure-induced hemostasis from hemostatic materials.
Fig. 3. Preclinical studies on the hemostatic ability of the CHI-C sponge for coagulopathic bleeding in rabbits and pigs.
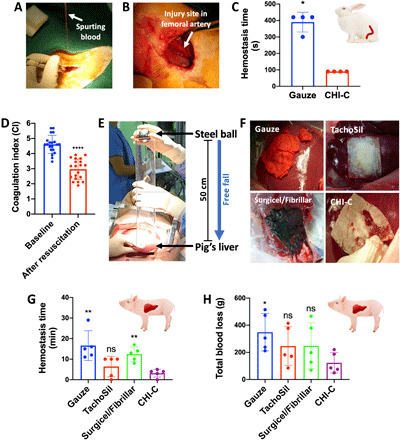
(A) Photographic image showing spurting arterial bleeding at the puncture site of the femoral artery in a heparinized rabbit. Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (B) Photographic image showing the femoral artery in a heparinized rabbit after applying the CHI-C sponge for 1 min and 30 s. The CHI-C sponge completely stopped the massive arterial bleeding. Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (C) Hemostasis time (in seconds) of the CHI-C sponge and gauze (control) in heparinized rabbits with arterial bleeding (n = 4 per group). The data are presented as means ± SD, and comparisons were performed using the Mann-Whitney U test (*P < 0.05). (D) Coagulation index (CI) values before (baseline) and after resuscitation to cause coagulopathy in pigs (n = 20). The data are presented as means ± SD, and normality was evaluated by the Shapiro-Wilk test, in which confirmed a normal distribution (P > 0.05). The comparison was performed using the parametric paired two-sample t test (****P < 0.0001). (E) A photographic image showing the causation of traumatic blunt liver injury (grade IV) in a pig. A stainless steel ball (d = 5 cm, 512 g) was freely dropped at a height of 50 cm from the liver surface. Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (F) Photographic images showing the materials after application to the coagulopathic liver bleeding caused in pigs. Photo credit: Keumyeon Kim, KAIST and InnoTherapy Inc. (G to H) The hemostatic ability of the CHI-C sponge was determined by measuring the hemostasis time (in minutes) (G) and total blood loss (in grams) (H) in a pig model of traumatic liver bleeding with coagulopathy (n = 5 per group). The data are presented as means ± SD, and comparisons were performed using the Mann-Whitney U test (ns, not significant; *P < 0.05 and **P < 0.01).
Preclinical study II: The hemostatic ability of a CHI-C sponge in a pig model of traumatic blunt liver injury with hemodilutional and hypothermic coagulopathy
After confirming the excellent hemostatic effect of the CHI-C sponge in heparinized arterial bleeding in rabbits, we were convinced that the CHI-C sponge could serve as a hemostatic agent. Before clinical trials, we performed an additional preclinical experiment to compare CHI-C sponge with conventional hemostatic agents in a pig model of traumatic coagulopathic bleeding. The coagulopathic condition was induced by hemodilution and hypothermia and confirmed by the coagulation index (CI) of thromboelastography (TEG), which has been widely used to analyze blood coagulation by quantitatively measuring the elastic moduli (i.e., solid properties) of blood (39, 40). As shown in fig. S1, there are four major TEG parameters for blood coagulation interpretation: (i) R-time (reaction time), which is the time of coagulation initiation; (ii) α angle, which represents the speed of blood clot formation; (iii) maximum amplitude (MA), which represents the maximum mechanical strength of the final clots; and (iv) K-time (the time required for moderate clot formation), which is determined from the end of R-time to the time to reach MA = 20 mm. The CI, which represents the overall blood coagulation status, is calculated with these four parameters of TEG (39, 40). After the hemodilution and hypothermia procedures, the CI value significantly decreased from 4.61 ± 0.60 (for baseline) to 2.97 ± 0.67 (P < 0.0001), showing that coagulopathy was successfully produced (Fig. 3D). A stainless steel ball (512 g) was dropped at the height of 50 cm from the liver to mimic traumatic blunt liver injury (Fig. 3E). Grade IV liver injury (41) and traumatic bleeding with coagulopathy occurred. As shown in Fig. 3F, the CHI-C sponge and TachoSil (Baxter) adhered firmly to the liver surface, resulting in effective hemostasis. However, for gauze and Surgicel/Fibrillar (Johnson & Johnson), blood leaked through not only the entire test material because of the excess blood absorption capacity but also the gap between the liver tissue and the test material because of weak tissue adhesion. Hemostatic efficacy was determined by quantitatively measuring the hemostasis time and blood loss. As shown in Fig. 3G, complete hemostasis of the CHI-C sponge was achieved at 3.2 ± 1.9 min, which was significantly shorter than the time required for the gauze (16.6 ± 7.2 min) and the Surgicel/Fibrillar (12.4 ± 3.5 min) (P < 0.01). The mean hemostasis time of the TachoSil sponge was 6.4 ± 5.0 min, which was not significantly different but longer and more variable than that of the CHI-C sponge. Moreover, the total blood loss during hemostasis was the lowest for the CHI-C sponge (123.4 ± 75.1 ml) among the test groups: 348.6 ± 137.6 ml for gauze (P < 0.05), 246.1 ± 142.5 ml for TachoSil (P > 0.05), and 248.1 ± 168.0 ml for Surgicel/Fibrillar (P > 0.05), as shown in Fig. 3H. These results demonstrate that the CHI-C sponge is effective in a traumatic blunt liver bleeding pig model, even with coagulopathy.
Ex vivo hemostatic activity of CHI-C in blood from liver transplant recipients with coagulopathy
We designed an ex vivo hemostasis experiment on the blood of liver transplant recipients with coagulopathy. We selected three patients with coagulopathy exhibiting PT/INR > 2.0 (prothrombin time/international normalized ratio) and platelet count < 50,000 μl−1 and one patient without coagulopathy. To investigate the coagulation capacity of CHI-C in the blood of a patient with coagulation, a TEG analysis, which was already mentioned in the section describing the pig model and fig. S1, was performed. In Fig. 4A, black represents a thromboelastogram of normal whole blood without coagulopathy. The R-time (20.4 min), K-time (6.1 min), α angle (38.8°), and MA (59.3 mm) values were all in the normal range. After the addition of 30 μl of CHI-C solution (0.5 wt %) to the normal blood, the blood entered a hypercoagulable state, as depicted by the four hemostasis TEG parameters (20.4 min ➔ 5.4 min for R-time, 6.1 min ➔ 4.8 min for K-time, 38.8° ➔ 58° for α angle, and 59.3 mm ➔ 63 mm for MA) (Fig. 4A, red lines). In addition, we treated coagulopathic blood from three patients (PT/INR > 2.0) who considerably lacked hemostatic capability with CHI-C. The blood of patient no. 1 (Fig. 4B, black lines) exhibited poor coagulation, namely, delayed coagulation initiation, slow coagulation progress, and weak clots, which were all remarkably improved by CHI-C (Fig. 4B, red lines): 26.6 min ➔ 14.9 min for R-time, 19.4 min ➔ 7.35 min for K-time, 13.8° ➔ 33.6° for α angle, and 30.9 mm ➔ 33.8 mm for MA. For patient no. 2 (Fig. 4C), CHI-C resulted in an R-time decrease from 6.25 to 4.5 min, a K-time decrease from 6.15 to 0.85 min, an α angle increase from 37.9° to 79.1°, and an MA increase from 36.6 to 41 mm. Patient no. 3 (Fig. 4D, black lines) showed extremely poor blood coagulation: delayed coagulation initiation (R-time, 33.25 min), low speed (α angle, 6.4°), and weak blood clots (MA, 9.1 mm). After the addition of CHI-C, all parameters improved (Fig. 4D, red lines): 21.5 min for R-time, 8.7° for α angle, and 18.7 mm for MA. Regardless of coagulopathy, the addition of CHI-C hemostatic materials improved blood coagulation. These positive hemostatic results by CHI-C can be attributed to the results shown in Figs. 1 and 2, in which BpB membranes were formed by rapid intermolecular complexation between CHI-C and plasma proteins. As long as proteins are present in plasma, CHI-C–induced coagulation is expected to be independent of the presence or absence of coagulation factors. In addition, cellular components of platelets and RBCs are also aggregated by CHI-C, which is another important contribution to exhibit the improved TEG parameters such as R-time reduction even in coagulopathic conditions.
Fig. 4. The hemostatic activity of CHI-C in the blood of liver transplant patients with coagulopathy.
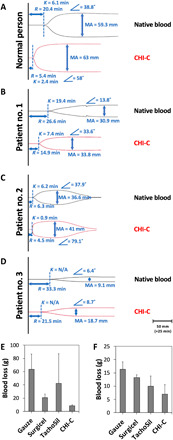
TEG tracings with measured quantitative parameters showing the hemostatic activity of CHI-C solution in the blood of a patient exhibiting a normal coagulation state (A) and patients exhibiting various coagulopathic states (B to D). (E and F) The hemostatic ability of CHI-C sponges for liver bleeding caused by a liver resection in anticoagulated rabbit models [(E) warfarin treated and (F) clopidogrel treated].
Preclinical study III: The hemostatic ability of a CHI-C sponge in an anticoagulant-treated rabbit model of liver resection bleeding
Furthermore, the blood treated with anticoagulants such as warfarin or clopidogrel is not easily coagulated even in the presence of thrombin/fibrinogen containing hemostatic materials because intrinsic coagulation cascade is largely impaired. However, CHI-C sponge showed a fairly good level of blood coagulation for warfarin-treated (Fig. 4E) or clopidogrel-treated blood (Fig. 4F) because of the presence of considerable amounts of plasma proteins, which are rapidly complexed with CHI-C forming BpB. As shown in Fig. 4E (warfarin-treated rabbit liver resection model), blood loss amount for a negative control (gauze treatment) was about 64 ± 23 g. However, effective hemostasis was observed for CHI-C, resulting in blood loss of 9 ± 2 g. Blood loss of 21 ± 5 g for Surgicel and 42 ± 45 g for TachoSil was observed. To confirm the effective hemostatic ability of CHI-C in anticoagulant-treated blood, another model was prepared, which was the clopidogrel-treated rabbit liver resection model. Similar to the warfarin hemostasis result, CHI-C showed the least bleeding (7 ± 4 g) compared to other groups: 16 ± 3 g for gauze (negative control), 13 ± 1 g for Surgicel, and 10 ± 4 g for TachoSil (Fig. 4F).
Clinical study on the hemostatic ability of CHI-C sponge in patients with hepatectomy
We conducted a single-center, controlled, single-blind, and exploratory clinical study on the early hemostatic efficacy and safety of CHI-C. To approve clinical trials, all necessary toxicity tests including ISO-10993 (ISO 10993-5, cytotoxicity; ISO 10993-6, histopathological test; ISO 10993-10, intracutaneous reactivity and skin sensitization; and ISO 10993-11, acute toxicity and subchronic toxicity) were passed. Patients who voluntarily signed consent forms and underwent hepatectomy for hepatocellular carcinoma, intrahepatic cholangiocarcinoma, intrahepatic cholelithiasis, and metastatic liver cancer according to the standard practice of the institution (Pusan National University Hospital, Busan, Republic of Korea) were selected. The CHI-C sponge or the comparators (conventional and commercially available drugs, in this study, TachoSil and Surgicel/Fibrillar) were applied to local bleeding sites that were continuously oozing from the transection after hepatectomy despite first-line hemostasis, such as suture, ligation, vascular clips, argon beam coagulation (ABC), and electrocautery (Fig. 5A). All 15 patients completed the study without exclusion (five subjects per group). The demographic data including gender, age, body weight, and height as well as drinking and smoking history of patients were summarized in table S1. In addition, the complete blood cell counts (table S2), blood coagulation (table S3), liver enzyme levels (table S4), and C-reactive protein contents (table S5) after treatment of CHI-C were described in the Supplementary Materials, respectively. As shown in Fig. 5B, the mean hemostasis time of the CHI-C sponge group was 168.0 ± 65.7 s, which was shorter than 180.0 ± 112.2 s for the TachoSil group and 288.0 ± 155.3 s for the Surgicel/Fibrillar group. However, the differences between the test group and each comparator group were not statistically significant (P > 0.05). There were no rebleeding or adverse effects leading to withdrawal or death during the 1-month follow-up. The CHI-C sponge group showed excellent hemostatic effects compared with the TachoSil and Surgicel/Fibrillar groups. These results demonstrate that the CHI-C sponge can effectively stop oozing hemorrhage that cannot be controlled by primary hemostasis during hepatectomy and suggest the potential of the CHI-C sponge as a medical hemostatic agent.
Fig. 5. Clinical study on the hemostatic effect of CHI-C sponge in patients undergoing hepatectomy and a proposed hemostatic mechanism of CHI-C.
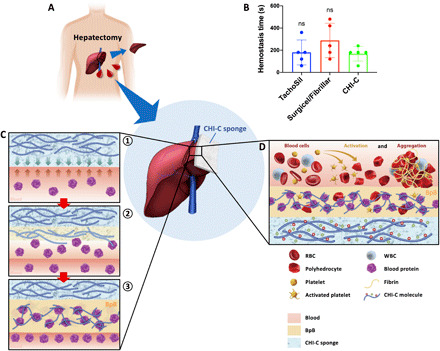
(A) A schematic illustration of the clinical trial design. A CHI-C sponge was applied for persistent local bleeding despite the first-line hemostatic procedures. (B) Hemostasis time (in seconds) results of the CHI-C sponge compared with the conventional hemostatic agents (TachoSil and Surgicel/Fibrillar). The data are presented as means ± SD, and comparisons were performed using the Mann-Whitney U test. (C) Schematic illustrations of the primary hemostatic mechanism of CHI-C, which forms physical barriers (BpB) by interacting with blood proteins: ① contact and dissolution of CHI-C at bleeding site, ② interaction of the dissolved CHI-C with blood proteins, and ③ rapid BpB (physical barrier) formation. (D) A schematic illustration of the secondary hemostatic mechanism of CHI-C, which activates and aggregates blood cells. WBC, white blood cell.
Proposal of the hemostatic mechanism of CHI-C sponge
Considering the overall results, we propose a hemostatic mechanism for the CHI-C sponge, as shown in Fig. 5C. First, when a CHI-C sponge comes into contact with blood, in contrast to chemically unmodified chitosan, CHI-C dissolves at the interface between the incoming blood and the CHI-C sponge because of the increased solubility resulting from the catechol moiety (Fig. 5C, ①). Subsequently, the dissolved CHI-C interacts with blood plasma proteins (Fig. 5C, ②), resulting in rapid BpB membrane formation within a few seconds (Figs. 1, D to F, and 5C, ③) via ionic/covalent/hydrogen bonds and reversible hydrophobic interactions (34–37). Through this process, the CHI-C sponge forms robust physical membranes (i.e., BpB) at the bleeding site, leading to successful hemostasis regardless of blood status (blood status–independent hemostasis). In addition to plasma proteins, the cellular components of platelets and RBCs are also activated and aggregated by CHI-C, making another important contribution to achieving successful hemostasis (Fig. 5D).
DISCUSSION
The physical properties of CHI-C and chemically unmodified chitosan differ markedly. Chitosan has been used as a hemostatic agent because it causes platelet and erythrocyte aggregations (42); nevertheless, its intrinsic insolubility in neutral conditions has been problematic and led to insufficient chitosan/blood contact issues. However, CHI-C (>100 kDa) is highly water soluble under neutral conditions (~60 mg/ml) (23). Figures 1 and 2 show instant complexation and association of CHI-C with virtually all plasma proteins. The identified proteins complexed with CHI-C by 2D electrophoresis are albumin, fibrinogen, globulins, and others. The mentioned three proteins constitute ~95% of the total blood proteins (29–31). Thus, rapid BpB formation was observed because CHI-C was able to bind with abundant plasma proteins. We also performed the same BpB formation experiments in human plasma for other catechol-conjugated natural polymers, such as hyaluronic acid–catechol and gelatin-catechol (fig. S2). However, they did not form BpB membranes at all. We concluded that BpB formation by CHI-C in the blood is a unique phenomenon because the BpB membrane is formed only when synergy between chitosan and catechol exists. This is different from the well-known protein-binding anionic macromolecule heparin, which binds to cationic domains of proteins/peptides that exist in tiny amounts in the blood. For example, apoE is a heparin-binding protein, and it was found that the microenvironments of lysine residues in apoE contribute to heparin binding (43). Conclusively, the cationic water-soluble polysaccharide, CHI-C, binds to plasma proteins abundantly present in the blood, whereas heparin, the anionic water-soluble polysaccharide, binds to minority yet essential proteins/peptides that have strong positive surface charges.
Although catechol-conjugated polymers such as hyaluronic acid–catechol, alginate-catechol, poly(ethylene glycol)–catechol, gelatin-catechol, and others have been published, most of them so far have been developed to a benchtop level. Thus, the use of CHI-C is the first report among various mussel-inspired, catechol-conjugated polymers aiming for a human trial (approval no. 650), which exhibited excellent hemostatic efficacy and reasonable safety. The remaining tasks include investigations for an excretion route of CHI-C. We prepared Cy3-labeled CHI-C (Cy3–CHI-C) to monitor its excretion pathway. Urine samples obtained after intraperitoneal injection of CHI-C (60 mg/kg) exhibited significant levels of Cy3 fluorescence within 1 day, which was gradually decreased after 3 days. We found that weak Cy3 signals were detected 10 weeks after implantation (fig. S3). However, at the same time, the Cy3 fluorescence in the blood was a nondetectable level for the same time scale up to 10 weeks. Nonetheless, detailed studies are required in the future. In addition to monitoring degradation in small-sized animal models, we also performed degradation experiments in a large-sized porcine model (n = 3). The majority (80 to 90%) of the CHI-C was degraded with 10 to 20% residuals 4 weeks after implantations (fig. S4). Thus, degradation speed in a small animal model was observed rather slow (3 months), but the speed turned out to be fast in a large animal porcine model (80 to 90% degradation in 4 weeks).
Coagulopathy is a broad-term disease that originated from a variety of plasma protein defects such as coagulation factors or fibrinogen. In addition, low levels and/or dysfunctions of platelets are often the cause of coagulopathy. Thus, the development of a material exhibiting effective hemostasis for all types of coagulopathic conditions remains to be an ultimate goal. First, the ex vivo TEG experiments (Fig. 4, A to D) using human blood from liver transplantation patients are closely related to “platelet deficiency” coagulopathic conditions. All patient blood exhibited serious problems of coagulation due to dysfunction of the liver, which is the organ for producing platelets. This experiment was performed only when platelet levels are ≤50,000/μl (normal platelet range, 140,000 to 450,000/μl) (similar to the number dysfunction like thrombocytopenia) and PT/INR > 2 (normal range, 0.8 to 1.1). In addition, PT/INR > 2 indicates that some coagulation factors such as factors I (which is fibrinogen), II (which is prothrombin), V, VII, and others are lacking. The results shown in Fig. 4 (A to D) demonstrated that CHI-C is an effective hemostasis ability for the coagulopathic states of platelet and some factor defect human blood. Second, preventing blood loss from heparin- or warfarin-treated animal models (Figs. 3, A to C, and 4E) by CHI-C applications demonstrates that it is an effective hemostat against coagulation factor defects. Last, the hypothermic hemodilution model by removing 40% (v/v) of total blood volume followed by supplying a cold (4°C) isotonic solution instead was prepared (Fig. 3, D to H). The coagulopathic conditions for this porcine model present low levels of all coagulation factors and platelets. In addition, the low temperature of blood decreases the overall activities of coagulation factors. CHI-C also showed effective hemostatic abilities for the hemodilution-induced coagulopathic blood by forming adhesive barriers with existing low-level plasma proteins. This indicates that CHI-C is a nearly coagulopathy-independent hemostatic material. However, further studies using heparin-induced thrombocytopenia models and other animal models are necessary to supplement demonstrations of CHI-C’s ability of coagulopathy state–independent hemostasis.
Typically, the topical/external hemostats including fibrin/thrombin-based materials, mechanical hemostats (collagen, gelatin, and polysaccharides), and CHI-C have a limitation in noncompressible hemorrhage. As shown in the successful clinical results of human liver hemorrhages in which one did not apply a compressive force, a noncompressible organ such as pancreas will be a good target for the next hemostasis design. This discussion indicates the practical advantages of CHI-C over existing hemostatic materials.
MATERIALS AND METHODS
Materials
Chitosan (viscosity, 100 mPa·s; 70% deacetylated) was purchased from Heppe Medical Chitosan GmbH [Halle (Saale), Germany]. HCA was purchased from Sigma-Aldrich (Milwaukee, WI, USA). 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) was purchased from Tokyo Chemical Industry Co., Ltd. (TCI) (Tokyo, Japan). All other chemicals were of analytical grade.
Synthesis of CHI-C
CHI-C was synthesized using standard EDC chemistry. Briefly, chitosan (3 g, 19.48 mmol) was dissolved in HCl solutions (pH 5; 100 ml). HCA (2.37 g, 15.58 mmol) and EDC (2.02 g, 10.54 mmol) were dissolved separately in deionized and distilled water (DDW; 25 ml). Both HCA and EDC solutions were added slowly to the chitosan solution. The pH of the solution was maintained at 5.0 to prevent the oxidation of catechol groups. Ethanol (50 ml) was added to the EDC/HCA mixture solution (50 ml) as a cosolvent to avoid possible precipitation during the EDC coupling reaction (total, 100 ml). After 12 hours, the product was purified by membrane dialysis [molecular weight cut-off (MWCO), 12,000 to 14,000; SpectraPor, USA] against pH 4.0 NaCl solutions for 2 days and against DDW for 4 hours and then freeze-dried.
Characterization of CHI-C
The synthesis of CHI-C was confirmed using either a UV-vis spectrophotometer (Hewlett Packard 8453, Agilent) or a 1H-NMR spectrometer (Bruker AVANCE III). The DOC of catechol moiety was determined using the absorbance at 280 nm by a UV-vis spectrophotometer. A standard curve of catechol concentration was generated by measuring the absorbance at 280 nm of HCA standard solutions, and the catechol content was quantified. In addition, 1H-NMR spectroscopy (D2O, 400 MHz) was also used to confirm the DOC of the catechol group, which was calculated by dividing the relative integral value of the catechol proton peaks by those of the acetyl group protons multiplied by five because 20% acetylation was used.
Preparation of chitosan and CHI-C sponges
CHI-C and chemically unmodified chitosan sponges were prepared by a freeze-drying method. Briefly, CHI-C or unmodified chitosan (1 wt %) was dissolved in DDW and poured into rectangular-shaped polyethylene terephthalate molds. The CHI-C or unmodified chitosan solutions in the molds were frozen in a refrigerator (−20°C) for 6 hours, and then, the solutions were freeze-dried for 3 days.
BpB membrane formation in human blood and plasma
All protocols related to human blood in this study were approved by the Institutional Review Board (IRB) of the Korea Advanced Institute of Science and Technology (KAIST) (approval no. KH2015-44). Two solutions were prepared for plasma interactions as follows. First, CHI-C (2 wt %) was dissolved in DDW. Second, chemically unmodified chitosan (2 wt %) was dissolved in DDW adjusted to pH 2 by 5 M HCl solution. Human whole blood was drawn into a Vacutainer (containing 0.109 M sodium citrate; BD, USA), and the plasma was prepared by centrifugation at 2000g for 10 min. To observe the interaction between CHI-C and citrate-treated whole blood, we transferred 5 ml of citrate-treated whole blood into a petri dish, and 500 μl of the CHI-C solution was dropped into the blood. To observe the interaction between CHI-C or unmodified chitosan and blood plasma, 1-ml aliquots of plasma were transferred into glass vials, and one or two drops of the CHI-C or unmodified chitosan solutions (approximately 10 μl) were dropped into the plasma. Kinetic analysis for morphological changes in the BpB membranes produced by CHI-C was performed by scanning electron microscopy (Inspect F50, FEI) after lyophilization.
2D gel electrophoresis analysis
To analyze the specific proteins bound to the BpB membranes, we used 2D gel electrophoresis. CHI-C (0.5 wt %) was dissolved in DDW, and the CHI-C solution and plasma were mixed at a volume ratio of 3:1 (e.g., 3 ml for CHI-C and 1 ml for plasma) for 15 min of vortexing. BpB aggregates were collected by centrifugation (13,000 rpm). The collected BpB aggregates were washed in DDW at least five times using a vortex and centrifuge. SDS solution (1%) was added to the aggregates, and the mixture of washed aggregates and SDS solution was vortexed and centrifuged. The supernatant was precipitated twice using ice-cold acetone, and the precipitated proteins were obtained using a Centrifan PE evaporator (KD Scientific, USA). For 2D gel electrophoresis, the proteins were dissolved in IEF sample buffer (final volume, 200 μl) containing 8 M urea, 2 M thiourea, 2% CHAPS, 30 mM tris, 100 mM dithiothreitol (DTT), 0.8% ampholyte, and bromophenol blue (Bio-Rad, Hercules, CA, USA). The samples were diluted to adjust their concentrations in the range of 10 to 100 μg/ml, and 125 μl of the diluted sample solution was loaded onto immobilized pH gradient (IPG) strips (pH 3 to 10, 7 cm; Bio-Rad) for passive rehydration. Isoelectric focusing was carried out at 250 V for 15 min, followed by a linear increase to 4000 V for 2 hours before being held constant at 20,000 V · hour at 4000 V using the PROTEAN IEF Cell System (Bio-Rad). After focusing, the IPG strips were incubated with 2% (w/v) DTT in equilibration buffer [50 mM tris-HCl, (pH 8.8), 6 M urea, 20% glycerol, 2% SDS, and 2% DTT] for 10 min and then with 2.5% (w/v) iodoacetamide in equilibration buffer for 10 min. The equilibrated IPG strip was placed on a 12% SDS–polyacrylamide gel with Precision Plus Protein Standards (dual color; Bio-Rad), and a 0.5% agarose overlay in cathode buffer [192 mM glycine, 15 mM tris, and 0.1% SDS, (pH 8.3)] containing bromophenol blue was applied. The second dimension was performed at a constant of 200 V until the dye front reached the lower end of the gel. The gels were stained using Coomassie Brilliant Blue R-250 (Bio-Rad).
Confirmation of BpB membrane formation in individual plasma proteins
To demonstrate the interactions of CHI-C and individual plasma proteins, each plasma protein (albumin, γ-globulin, fibrinogen, and IgG; Sigma-Aldrich, USA) was dissolved in PBS (pH 7.4) at a concentration of 100 mg/ml. Then, 10 to 20 μl of CHI-C (2 wt %) solution was dropped into each plasma protein solution (300 μl).
Rheological analysis
First, for solid-state analysis, 3 mg of CHI-C sponge or chemically unmodified chitosan sponge (control) was placed between rheometer plates (Bohlin Advanced Rheometer, Malvern Instruments, UK), and 150 μl of heparin-treated human blood was applied. We monitored the elastic modulus (G′) and viscous modulus (G″) as functions of frequency. To confirm the gel-like behavior of the CHI-C sponge after blood mixing, a test-tube inverting method was also performed under the same conditions. Second, for liquid-state analysis, CHI-C (2 wt %) sponge or chitosan was dissolved in DDW, and heparin-treated human blood was added to the solution at a 1:1 (v/v) ratio. For frequency sweep measurements, the frequency was varied from 0.1 to 10 Hz, and the stress applied to the mixture was fixed to 100 Pa. All samples were measured in triplicate.
SEM analysis of the interaction of CHI-C with platelets/RBCs
Preparation of CHI-C–coated substrates
Glass substrates were cut into squares (1 × 1 cm2) and cleaned by ultrasonication in ethanol. CHI-C solution was prepared by dissolving CHI-C (0.5 wt %) in 1× PBS solution (pH 8.5). The cleaned glass substrates were immersed in the CHI-C solution (0.5 wt %) overnight. The CHI-C–coated glass substrates were prepared by taking them out after overnight immersion and rinsing with water gently.
Preparation of platelet solution
A volume of 40 ml of blood was collected into blood collection tubes (containing acid citrate dextrose solution; Vacutainer, BD). PRP was obtained by centrifugation at 100g for 20 min at room temperature. The PRP supernatant was transferred into a new plastic tube, and further centrifugation to remove RBCs was performed at 200g for 20 min at room temperature. The RBC-removed PRP supernatant was transferred into another new plastic tube, and the tube was centrifuged again at 800g for 20 min to obtain the platelet pellet. The platelet pellet was washed very carefully (without resuspension to avoid unnecessary platelet activation) three times with 1× PBS solution. The resuspended platelet solution was obtained by adding 100 ml of 1× PBS solution (pH 7.4) containing human serum albumin (3 mg/ml) to the platelet pellet and resuspending slowly. The resuspended platelets were stabilized by incubating in a 37°C water bath for 30 min before use.
Preparation of RBC solution
A volume of 6 ml of blood was collected into blood collection tubes (containing lithium heparin; Vacutainer, BD). RBCs were obtained by centrifugation at 200g for 10 min at room temperature, and the supernatant was removed. The RBCs were washed three times with 1× PBS solution and resuspended in 100 ml of 1× PBS solution (pH 7.4) containing human serum albumin (3 mg/ml).
Interactions of CHI-C with platelets/RBCs
The CHI-C–coated glass substrates and bare glass substrates were immersed into the resuspended platelet or RBC solution and incubated at 37°C for 2 hours (n = 3). The substrates were taken out and rinsed with 1× PBS solution and primarily fixed in a 2.5% electron microscopy–grade glutaraldehyde in a sodium cacodylate buffer overnight. The substrates were rinsed the next morning with DDW three times for 10 min each. The subsequent fixation was performed with a 1% aqueous solution of osmium tetroxide for 1 hour. Then, the substrates were rinsed again three times with DDW, dehydrated in 50, 70, 80, and 90% ethanol for 10 min each, and dehydrated in 100% ethanol twice for 10 min each. Scanning electron microscopy (Hitachi S-4800, Japan) was performed to analyze the platelet/RBC adhesion and activation onto the substrates.
Preclinical study: A femoral artery bleeding model in heparinized rabbits
This animal protocol was approved by the IRB and Institutional Animal Care and Use Committee of the Seoul National University Hospital. All animal care and experiments were conducted by the Guidelines for the Care and Use of Laboratory Animals of Seoul National University Hospital. Twelve New Zealand white rabbits (male, 3 to 4 kg) were used to evaluate the hemostatic efficacy of the CHI-C sponge compared with gauze (control; n = 4 for each group). Briefly, anesthesia was induced by intramuscular injection of Zoletil (33 mg/kg; Virbac, France) and Rompun (7 mg/kg; Bayer, Germany). An approximate 5-cm skin incision was made over the groin area, and approximately 3 to 4 cm of the femoral artery was dissected from surrounding tissues. To induce coagulopathic conditions, heparin sodium (200 IU/kg; Green Cross, Korea) was intravenously administered via the marginal ear vein. After 5 min of stabilization, the artery was punctured using a 23-gauge needle. A CHI-C sponge or gauze (3 × 2.5 cm2) was applied to the bleeding site with manual compression. Visual hemostasis observations were performed at predetermined time intervals (1 min and 30 s, 2 min and 30 s, 3 min and 30 s, 5 min, and 7 min). The same procedure was also performed using regular gauze only (3 × 3 cm2) as a negative control group.
Preclinical study: A traumatic liver bleeding model in hemodilutional coagulopathic pigs
This animal protocol was approved by the IRB and Institutional Animal Care and Use Committee of the Pusan National University Hospital. All animal care and experiments were conducted by the Guidelines for the Care and Use of Laboratory Animals of Pusan National University Hospital. Twenty pigs weighing approximately 60 kg were used. The pigs were sedated by intramuscular injection of ketamine (Huons, Korea) and Rompun. General anesthesia was maintained using 2% isoflurane, and a rectal temperature probe was placed. Peripheral catheterization into the ear vein was performed to provide maintenance fluid, and warm normal saline (NS) solution was infused at 4 ml/kg per hour. A central venous catheter (8 Fr) was advanced into the left external jugular vein via a cutdown technique for central venous pressure (CVP) monitoring, fluid resuscitation, and blood withdrawal. The right femoral artery was also catheterized for mean arterial pressure (MAP) monitoring, blood sampling, and blood withdrawal. Measured parameters included heart rate, respiratory rate, MAP, CVP, oxygen saturation, end-tidal oxygen concentration, body temperature, and urine output. In addition, TEG was performed to investigate the blood coagulation status. A laparotomy was performed to expose the liver, and a splenectomy was then performed to limit fluid compensation. The animals were hemodiluted by exchanging 40% of the estimated total blood volume with a cold NS solution (4°C) of three times the amount of the removed blood and spleen. The body temperature was lowered to approximately 32°C and maintained during the experiment. After resuscitation, the animals were stabilized for 30 min. Coagulopathy status was determined by the CI values of TEG. A liver lobe over 3 cm thick was placed on an acryl plate, and a plastic bag was placed under the liver to collect bleeding. As shown in Fig. 3E, a reproducible and severe liver injury (grade IV) was induced by freely dropping a stainless steel ball (512 g, d = 5 cm) at the height of 50 cm from the liver surface through a transparent acryl pipe. The amount of bleeding for 3 min after the liver injury was measured. The CHI-C sponge, gauze, TachoSil, or Surgicel/Fibrillar (5 × 3 cm2) was cut into several pieces (1 × 1 cm2) and placed on the bleeding site without manual compression. Subsequently, the whole test material (5 × 3 cm2) was lastly applied to the bleeding surface. Active bleeding was visually examined, and blood loss was measured by collection with gauze and plastic bags.
TEG analysis
This study was approved by the IRB of Seoul National University Hospital. Coagulopathy was defined as a platelet count less than 50,000 cells/μl and a PT/INR greater than 2. These values require correction by the infusion of blood in general operations. All patients who donated their blood were informed by a verbal explanation and signed consent forms. Fresh native whole blood (2 to 3 ml) was drawn from each patient, and 660 μl of fresh native blood was gently mixed with 60 μl of CHI-C solution (0.5 wt %). The TEG analysis was performed at 37°C in a TEG 5000 (Haemonetics, USA) channel using 360 μl of the CHI-C/blood mixture. As a control, TEG analysis for healthy blood was also performed under the same conditions.
Preclinical study: A liver resection model in anticoagulant-treated rabbits
This animal protocol was approved by the IRB of the Osong Medical Innovation Foundation Laboratory Animal Center (approval no. KBIO-IACUC-2017-051). Twenty-two New Zealand white rabbits (male, 2.7 to 3.6 kg) were used to evaluate the hemostatic efficacy of the CHI-C sponge compared with gauze (Daehan Medical, Korea), Surgicel/Fibrillar (Ethicon, USA), TachoSil (Baxter, USA) in warfarin- or clopidogrel-treated rabbits. Warfarin (warfarin sodium tablet; Jeil Pharmaceutical, Korea) was administered orally at a dose of 2 mg/kg per day for 5 days before bleeding induction. PT/INR values were measured before and after the warfarin treatment to confirm the action of warfarin. Clopidogrel (clopidogrel bisulfate tablet; Sinil Pharmaceutical, Korea) was administered orally at a dose of 20 mg/kg per day for 3 days before bleeding induction. PFA-100 test was performed before and after the clopidogrel administration to confirm the action of clopidogrel. Anesthesia was induced by intramuscular injection of Zoletil (5 mg/kg; Virbac, France) and Rompun (15 mg/kg; Bayer, Germany). A laparotomy was performed to expose the liver, and approximately 3 g of the left medial lobe was resected. The test material (3 × 5 cm2) was applied to the resected liver surface. The bleeding leaking out of the test material was absorbed using preweighed gauzes, and the change in the weight of the gauzes was measured to evaluate the blood loss.
The exploratory clinical study
This study protocol was approved by the IRB (H-1604-007-040) of Pusan National University Hospital and the Ministry of Food and Drug Safety of the Republic of Korea according to local regulations. Patients who were diagnosed with hepatocellular carcinoma, intrahepatic cholangiocarcinoma, intrahepatic cholelithiasis, and metastatic liver cancer at Pusan National University Hospital in outpatient settings and required hepatectomy were enrolled in the study. The patients voluntarily signed the informed consent form and met all of the inclusion/exclusion criteria through screening. Dates were set for hospital admission and surgery. A potential subject underwent hepatectomy as scheduled according to the standard practice of the institution. Patients with persistent oozing local bleeding from the transection after hepatectomy despite first-line hemostasis (suture, ligation, vascular clips, ABC, electrocautery, etc.) were lastly enrolled in this study and assigned a subject enrollment number. The CHI-C sponge (5.8 mg/kg) or the absorbable hemostatic comparator (TachoSil and Surgicel/Fibrillar) was applied to the bleeding site for hemostasis only without manual compression. For efficacy assessment, the duration of time from the application of the CHI-C sponge or the comparator and follow-up for adverse events was conducted for 30 days. Chitosan-based hemostatic materials known as ChitoSorb and ChitoFlex cannot be used as control devices because they must be removed after dermatological and operative uses. They are not absorbable materials for internal uses shown in this study.
Statistical analysis
All results are expressed as means ± SD. All comparisons (except the CI value results in a pig model) were performed by Mann-Whitney U test using Prism 8.0 software (GraphPad Software Inc., USA). For comparison of CI values in a pig model, first, normality was evaluated by the Shapiro-Wilk test, and the comparison was performed using the parametric paired two-sample t test. Statistical significance was defined at P < 0.05.
Acknowledgments
We would like to thank J. S. Lee and J.-H. Kim for assistance on the pig experiment. Funding: This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant no. HI16C0820), and the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (grant no. HA16C0016). This study is also supported by Center for Multiscale Chiral Architecture from NRF of South Korea. Author contributions: K.K., J.H.R., and M.-Y.K. designed and performed the experiments, analyzed the data, and wrote the manuscript. S.P.Y. and H.-I.S. designed and conducted the exploratory clinical trial. M.-Y.K. and S.K. assisted with the heparinized rabbit experiment. J.P.P. performed 2D gel electrophoresis experiments and helped with the manuscript writing and figure designing. C.-W.J. provided expertise in TEG experiments and designed and analyzed TEG experimental data. M.S.L. helped with data interpretation and writing the manuscript. H.-I.S. and J.H.K. supervised all of the experiments including the hypothesis and analyzed the experimental data. H.L. supervised all of the experiments including the hypothesis, analyzed the experimental data, and wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/13/eabc9992/DC1
REFERENCES AND NOTES
- 1.Burks S., Spotnitz W., Safety and usability of hemostats, sealants, and adhesives. AORN J. 100, 160–176 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Radosevich M., Goubran H. A., Burnouf T., Fibrin sealant: Scientific rationale, production methods, properties, and current clinical use. Vox Sang. 72, 133–143 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Figueras J., Llado L., Miro M., Ramos E., Torras J., Fabregat J., Serrano T., Application of fibrin glue sealant after hepatectomy does not seem justified: Results of a randomized study in 300 patients. Ann. Surg. 245, 536–542 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonstra E. A., Molenaar I. Q., Porte R. J., De Boer M. T., Topical haemostatic agents in liver surgery: Do we need them? HPB 11, 306–310 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer M. T., Klaase J. M., Verhoef C., van Dam R. M., van Gulik T. M., Molenaar I. Q., Bosscha K., Dejong C. H. C., Van der Jagt E. J., Porte R. J., Fibrin sealant for prevention of resection surface-related complications after liver resection: A randomized controlled trial. Ann. Surg. 256, 229–234 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Duarte A. P., Coelho J. F., Bordado J. C., Cidade M. T., Gil M. H., Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 37, 1031–1050 (2012). [Google Scholar]
- 7.Hunt B. J., Bleeding and coagulopathies in critical care. N. Engl. J. Med. 370, 847–859 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Mannucci P. M., Levi M., Prevention and treatment of major blood loss. N. Engl. J. Med. 356, 2301–2311 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Harter K., Levine M., Henderson S. O., Anticoagulation drug therapy: A review. West. J. Emerg. Med. 16, 11–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levi M., ten Cate H., Disseminated intravascular coagulation. N. Engl. J. Med. 341, 586–592 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Chan L. W., Wang X., Wei H., Pozzo L. D., White N. J., Pun S. H., A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci. Transl. Med. 277, 277ra29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baylis J. R., Yeon J. H., Thomson M. H., Kazerooni A., Wang X., John A. E. St., Lim E. B., Chien D., Lee A., Zhang J. Q., Piret J. M., Machan L. S., Burke T. F., White N. J., Kastrup C. J., Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Sci. Adv. 1, e1500379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waite J. H., Tanzer M. L., Polyphenolic substance of Mytilus edulis: Novel adhesive containing L-dopa and hydroxyproline. Science 212, 1038–1040 (1981). [DOI] [PubMed] [Google Scholar]
- 14.Lee H., Scherer N. F., Messersmith P. B., Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. U.S.A. 103, 12999–13003 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H., Dellatore S. M., Miller W. M., Messersmith P. B., Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier G. P., Rapp M. V., Waite J. H., Israelachvili J. N., Butler A., Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 349, 628–632 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Kim Y.-M., Kim C.-H., Park M.-R., Song S.-C., Development of an injectable dopamine-conjugated poly(organophophazene) hydrogel for hemostasis. Bull. Korean Chem. Soc. 37, 372–377 (2016). [Google Scholar]
- 18.Mehdizadeh M., Weng H., Gyawali D., Tang L., Yang J., Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 33, 7972–7983 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan C., Fu J., Zhu W., Wang D.-A., A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 33, 51–63 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Ryu J. H., Lee Y., Kong W. H., Kim T. G., Park T. G., Lee H., Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 12, 2653–2659 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Shin M., Park S.-G., Oh B.-C., Kim K., Jo S., Lee M. S., Oh S. S., Hong S.-H., Shin E.-C., Kim K.-S., Kang S.-W., Lee H., Complete prevention of blood loss with self-sealing haemostatic needles. Nat. Mater. 16, 147–152 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Ryu J. H., Hong S., Lee H., Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 27, 101–115 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Kim K., Ryu J. H., Lee D. Y., Lee H., Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 1, 783–790 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Herosimczyk A., Dejeans N., Sayd T., Ozgo M., Skrzypczak W. F., Mazur M., Plasma proteome analysis: 2D gels and chips. J. Physiol. Pharmacol. 57, 81–93 (2006). [PubMed] [Google Scholar]
- 25.Knoll D., Hermans J., Polymer-protein interactions. Comparison of experiment and excluded volume theory. J. Biol. Chem. 258, 5710–5715 (1983). [PubMed] [Google Scholar]
- 26.Ye A., Complexation between milk proteins and polysaccharides via electrostatic interaction: Principles and applications—A review. Int. J. Food Sci. Tech. 43, 406–415 (2008). [Google Scholar]
- 27.Turgeon S. L., Schmitt C., Sanchez C., Protein-polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 12, 166–178 (2007). [Google Scholar]
- 28.Zhao Y., Li F., Carvajal M. T., Harris M. T., Interactions between bovine serum albumin and alginate: An evaluation of alginate as protein carrier. J. Colloid Interface Sci. 332, 345–353 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Anderson L., Anderson N. G., High resolution two-dimensional electrophoresis of human plasma proteins. Proc. Natl. Acad. Sci. U.S.A. 74, 5421–5425 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson N. L., Anderson N. G., A two-dimensional gel database of human plasma proteins. Electrophoresis 12, 883–906 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Anderson N. L., Anderson N. G., The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteomics 1, 845–867 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Kang S. M., Rho J., Choi I. S., Messersmith P. B., Lee H., Norepinephrine: Material-independent, multifunctional surface modification reagent. J. Am. Chem. Soc. 131, 13224–13225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong S., Kim J., Na Y. S., Park J., Kim S., Singha K., Im G.-I., Han D.-K., Kim W. J., Lee H., Poly(norepinephrine): Ultrasmooth material-independent surface chemistry and nanodepot for nitric oxide. Angew. Chem. Int. Ed. 52, 9187–9191 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Faure E., Falentin-Daudré C., Jérôme C., Lyskawa J., Fournier D., Woisel P., Detrembleur C., Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 38, 236–270 (2013). [Google Scholar]
- 35.Dreyer D. R., Miller D. J., Freeman B. D., Paul D. R., Bielawski C. W., Elucidating the structure of poly(dopamine). Langmuir 28, 6428–6435 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Hong S., Na Y. S., Choi S., Song I. T., Kim W. Y., Lee H., Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 22, 4711–4717 (2012). [Google Scholar]
- 37.Yang J., Stuart M. A. C., Kamperman M., Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 43, 8271–8298 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Cines D. B., Lebedeva T., Nagaswami C., Hayes V., Massefski W., Litvinov R. I., Rauova L., Lowery T. J., Weisel J. W., Clot contraction: Compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 123, 1596–1603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donahue S. M., Otto C. M., Thromboelastography: A tool for measuring hypercoagulability, hypocoagulability, and fibrinolysis. J. Vet. Emerg. Crit. Care 15, 9–16 (2005). [Google Scholar]
- 40.Thakur M., Ahmed A. B., A review of thromboelastography. Int. J. Periop. Ultrasound Appl. Technol. 1, 25–29 (2012). [Google Scholar]
- 41.Moore E. E., Cogbill C. T. H., Jurkovich G. J., Shackford S. R., Malangoni M. A., Champion H. R., Organ injury scaling: Spleen and liver (1994 revision). J. Trauma Inj. Infect. Crit. Care 38, 323–324 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Wedmore I., McManus J. G., Pusateri A. E., Holcomb J. B., A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma Inj. Infect. Crit. Care 60, 655–658 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Saito H., Dhanasekaran P., Nguyen D., Baldwin F., Weisgraber K. H., Wehrli S., Philips M. C., Lund-Katz S., Characterization of the heparin binding sites in human apolipoprotein E. J. Biol. Chem. 278, 14782–14787 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/13/eabc9992/DC1


