Abstract
Recent advances in oxygen tolerant controlled/living radical polymer chemistry now enable efficient synthesis of diverse and combinatorial polymer libraries. While library synthesis has been dramatically simplified, equally efficient purification strategies for removal of small molecule impurities are not yet established in high throughput settings. It is shown that gel filtration columns for chromatography (GFC) frequently used in the protein science community are well suited for high throughput polymer purification. Using either single-use columns or gel filtration plates, we demonstrate the purification of 32 diverse polymers in a library with >95% removal of small molecule impurities and >85% polymer retention in a single purification step. Doing so replaces the typical procedure of polymer precipitation which requires solvent optimization for each polymer in a complex library. Overall, this work raises awareness in the polymer science community that gel filtration is amenable to purification of large polymer libraries and can speed up the progress of combinatorial polymer chemistry.
Keywords: polymer purification, low-volume combinatorial polymer libraries, PET-RAFT, gel filtration columns
Graphical Abstract
Combinatorial polymer chemistry enables rapid and efficient testing of design parameters to obtain desired material functionality. This high throughput approach has found success in many applications including drug delivery and biomaterial sciences.[1–3] Controlled/living radical polymerization (CLRP) was previously difficult to implement in a combinatorial setting because of the requirement for deoxygenated conditions. This would often necessitate the use of gloveboxes or polymer synthesizer workstations to provide inert atmosphere and limited throughput.[4–7] Recently, multiple oxygen tolerant CLRP techniques have been developed such as photoinduced electron/energy transfer-reversible addition-fragmentation chain-transfer (PET-RAFT),[8, 9] enzyme-assisted RAFT (Enz-RAFT),[10, 11] and open air initiators for continuous activator regeneration atom-transfer radical polymerization (ICAR ATRP).[12] Because of this, we are now able to complete this chemistry in labware open to the air including well plates for combinatorial polymer chemistry.[4, 13]
While oxygen tolerant CLRP has advanced synthetic throughput capability, challenges remain in the purification of diverse polymer libraries. In order to remove small molecule impurities associated with synthesis or post-polymerization modification the polymer science community typically uses precipitation or phase extraction[13–15] or avoids purification entirely using a one-pot approach.[16] While precipitation of polymers is an effective strategy, it is not practical for a diverse polymer library where solvent/polymer preferences can vary widely. Usually, purification by precipitation of a new polymer requires multiple experiments to determine which solvent leads to efficient precipitation. Another purification method involves preparative HPLC with multiple columns in parallel to automatically purify about 1000 polymers/day.[5, 6, 14, 17, 18] Unfortunately, this HPLC-based approach requires substantial equipment investment and maintenance as well as dilution of polymer into other solvents (e.g. H2O and acetonitrile). This necessitates downstream processing in the form of rotary evaporation and lyophilization which are time consuming and low throughput.
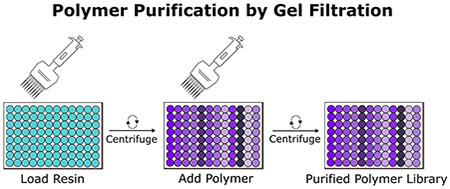
In the protein science community, this problem of high throughput purification has been solved by gel filtration chromatography (GFC) in the form of disposable columns. These devices are offered as individual columns or as 96-well filter plates to enable high throughput purification. They are sold as pre-packed devices or they can be manually packed with resins that have different solvent compatibilities and molecular weight cutoffs (MWCOs) by centrifugation or vacuum.[19] GFC columns are highly versatile and have been used for purifying or buffer exchanging proteins[19–22] and viruses[22] for many years. While GFC columns are used for polymer purification as well, this technique is less common than precipitation for practical reasons when purifying large amounts of material (>100 mg). However, in the situation where one wishes to purify large and diverse polymer libraries, we find GFC columns are ideally suited (Figure 1).
Figure 1.
Experimental schemes for traditional polymer purification by precipitation versus polymer purification by gel filtration chromatography (GFC). For precipitation purifications, each polymer must be individually precipitated (in poor solvent which can vary polymer by polymer in a diverse library). For gel filtration, multiple rounds of centrifugation are required to load the resin, wash with desired solvent, and purify the polymer directly into 96-well plates. Vacuum manifold filtration can also be done rather than centrifugation. Purification by traditional precipitation takes ≈45 min for 1 polymer, while purification by gel filtration takes ≈10 min for 96 polymers.

Here, we demonstrate the utility of GFC columns to purify diverse polymers. In our experiments, we have chosen to purify a common PET-RAFT initiator, zinc tetraphenylporphyrin (ZnTPP), and post-polymerization click chemistry tool, dibenzocyclooctyne-amine (DBCO-NH2), from commercially available poly(ethylene glycol) (PEG) and PET-RAFT synthesized mPEG acrylate. We also show the potential of different resins (pre-packed resin along with Sephadex G-10, G-25 superfine, and LH20), of various purification devices (0.5, 2, 5, and 10 mL columns and 96-well filter plate), and the ability to purify a diverse library of 32 polymers (Supporting Information Table S1 and Figure S1). Our objective is to determine the removal efficiency of small molecule impurities and retention of polymer for low-volume purification applications. We also saught to compare our approach to purification by precipitation. We believe that this work will further enable synthesis of combinatorial libraries and more productive exploration of polymer designs.
First, polymers were prepared and purified to test the efficiency of GFC when removing ZnTPP, the most common PET-RAFT initiator. Experimental conditions are displayed in Supporting Information Table S2. Small molecule removal efficiency is based on UV-Vis standard absorbance plots (Supporting Information Figure S2). Table 1 contains results for PEG +ZnTPP and mPEG acrylate +ZnTPP polymers that were purified using pre-packed 96-well filter plates and 0.5 mL desalting columns. Removal efficiency represents the ability of the resin to purify out ZnTPP, while the wash sum is the percentage of the amount of recovered material from all five wash steps relative to the amount of material determined to be retained in the column after the first purification. Purification for synthesized mPEG acrylate polymers using GFC columns and plates was compared against precipitation purification (3 rounds of precipitation by addition to ethyl acetate). Concentrations used to derive removal efficiency and wash sum were determined by interpolating experimental intensities from the standard curve for ZnTPP. Polymer retention was quantified by dividing area under the curve (AUC) of the purified polymers by AUC of the crude polymers by gel permeation chromatography (GPC). All volume retentions were determined by pipette measurement.
Table 1.
Removal efficiency, reusability, and volume retention associated with PEG +ZnTPP and mPEG acrylate +ZnTPP
| Condition | Removal efficiency | Wash sum | Polymer retention | Volume retention |
|---|---|---|---|---|
| 1st Purificationa | 83.9% | 98.3% | 100% | 99% |
| 2nd Purificationa | 84.1% | – | – | 100% |
| 3rd Purificationa | 95.6% | – | 70.0% | 98% |
| 1st Purificationb | 90.1% | 103% | 80.3% | 103% |
| 2nd Purificationb | 97.5% | – | – | 102% |
| 3rd Purificationb | 105% | – | 60.2% | 97% |
| 1st Purificationc | 96.9% | 101% | 70.6% | 105% |
| 2nd Purificationc | 97.5% | – | – | 103% |
| 3rd Purificationc | 97.9% | – | 60.2% | 100% |
| Precipitation | 101% | – | 47.0% | – |
| 1st Purificationd | 101% | 105% | 69.9% | 94% |
| 2nd Purificationd | 101% | – | – | 99% |
| 3rd Purificationd | 103% | – | 50.3% | 98% |
| Precipitation | 101% | – | 47.0% | – |
96-well late PEG +ZnTPP
96-well mPEG acrylate +ZnTPP
0.5 mL column PEG +ZnTPP
0.5 mL column mPEG acrylate +ZnTPP. PEG solutions were prepared at 20 mg mL−1 and mPEG acrylate (DP 400) was polymerized by PET-RAFT. Polymer retention is based on comparing AUC of purified polymers to crude by GPC.
While removal efficiency is important for these purification systems, it is also necessary for polymers of interest to be retained. From the Supporting Information, Figure S3 includes representative GPC plots which display the approximate concentration of crude polymer, 1st purification, and 3rd purification. A blank (DMF with LiBr) is also included. Figure S3A–B contains GPC plots for 10 mg/mL PEG +ZnTPP for the 96-well plate and 0.5 mL pre-packed columns (MWCO 7 kDa), respectively. Figure S3C–D contains the GPC plots for PET-RAFT synthesized mPEG acrylate for purification using 96-well plate and 0.5 mL pre-packed columns, respectively. Included in Figure S3C–D is a GPC trace of mPEG acrylate after three precipitations in ethyl acetate. The concentration displayed is slightly lower than that of a polymer purified three times by 96-well and 0.5 mL column purification.
After the purification was validated for removing ZnTPP using pre-packed columns, we aimed to test the efficacy of manually-packed columns in removing DBCO-NH2, a click chemistry molecule relevant to bioconjugate chemistry.[8] We selected Sephadex G-25 superfine as an optimal resin for these specific removal processes by conducting a head-to-head comparison against Sephadex LH20 and G-10, found in Supporting Information Figure S4–S6 and Table S3–S4. Table 2 details the removal efficiency of manually packed 96-well filter plates with Sephadex G-25 (MWCO 5 kDa) in removing DBCO-NH2 and mPEG acrylate +DBCO-NH2. Concentrations needed to calculate removal efficiency and wash sum were determined from the standard curve of DBCO-NH2 at 295 nm, while the volume retention was estimated by pipette measurement. Also included is average removal efficiency, wash sum, polymer retention, and volume retention associated with the polymer library (#1-32). Additional data for these 32 polymers can be found in the Supporting Information, which includes removal of ZnTPP by GPC in Figure S7–S12, polymer retention by GPC in Figure S13–S18, and individual quantities for purification efficiency, wash sum, polymer retention, and volume retention in Table S5.
Table 2.
Removal efficiency, reusability, and volume retention associated with mPEG acrylate +DBCO-NH2 and polymers #1-32 +DBCO purified using manually packed 96-well filter plates
| Condition | Removal efficiency | Wash sum | Polymer retention | Volume retention |
|---|---|---|---|---|
| 1st Purificationa | 100% | 110% | 95.2% | 99% |
| 2nd Purificationa | 96.7% | – | – | 99% |
| 3rd Purificationa | 100% | – | 66.2% | 102% |
| 1st Purificationb | 99.5% | 70.3%d | 89.4% | 96% |
| 2nd Purificationb | 99.8% | – | – | 96% |
| 3rd Purificationb | 100% | – | 66.6% | 98% |
| 1st Purificationc | 97.2% ± 1.7% | 95.4% ± 0.9% | 87.2% ± 13.8% | 98% ± 2.7% |
| 3rd Purificationc | 98.0% ± 0.9% | – | 72.6% ± 11.5% | – |
DBCO-NH2 purified using 96-well filter plates manually packed with Sephadex G-25
PEG acrylate +DBCO purified using 96-well filter plates manually packed with Sephadex G-25
Polymer library (polymers #1-32) purified using 96-well filter plates manually packed with Sephadex G-25
Wash sum for mPEG acrylate +DBCO was 70.3% after five washes but column was removed of DBCO-NH2 with three additional washes.
Similarly, polymer retention was determined for mPEG acrylate +DBCO purified using 96-well filter plates manually packed with Sephadex G-25 and removal efficiency was further visualized by GPC (Figure 2). These plots display the UV and RI traces associated with polymer crude, polymer purified once, polymer purified three times, and a blank injection. In order to show removal of ZnTPP and DBCO-NH2, Figure 2A contains the UV trace for mPEG acrylate at the elution time of ZnTPP while Figure 2B contains the UV trace for mPEG acrylate +DBCO at the elution time of ZnTPP and DBCO-NH2. Meanwhile, to demonstrate polymer retention, Figure 2C displays the RI trace associated with just mPEG acrylate purification at the elution time associated with the polymer while Figure 2D displays the RI trace for mPEG acrylate +DBCO purification. From these results, we can conclude that a significant amount of polymer is not lost due to purification.
Figure 2.
GPC UV and RI traces for purification of mPEG acrylate and mPEG acrylate +DBCO. A) UV trace of mPEG acrylate at the elution time for ZnTPP, showing removal of PET-RAFT initiator. B) UV trace of mPEG acrylate +DBCO at elution time for ZnTPP and DBCO-NH2, showing their removal. RI traces for C) mPEG acrylate and D) mPEG acrylate +DBCO, demonstrating the level of polymer retention.

The goal of this work was to demonstrate the utility of GFC columns for the purification of polymer libraries. For these diverse libraries with low volume of polymer, 96-well and 0.5 mL devices would be most useful. However, we have also deemed that 2, 5, and 10 mL columns have value in purifying large quantities of polymer. We chose to purify commercially available PEG spiked with ZnTPP as a model polymer as well as mPEG acrylate synthesized by PET-RAFT. We chose to characterize ZnTPP removal, an initiator for PET-RAFT, and DBCO-NH2, a tool for click chemistry, to reveal the applicability of this process for purification of polymers and modified polymers.
First, we compared the purification ability of 96-well and 0.5 mL GFC columns by purifying commercially available PEG +ZnTPP and synthesized mPEG acrylate in Table 1. We found that removal efficiency was high for all purifications (>80% for all and >95% for most conditions) as determined by UV-Vis spectroscopy. By completing five column washes with DMSO post-purification, we determined that roughly 100% of the ZnTPP was collected by the column and eluted by later washes. This not only shows the potential to reuse gel filtration media but also verifies our measured removal efficiency. Note that any removal efficiencies >100% are due to minor experimental variability. We also found that the polymer retention was greater than 70% for the first purification and decreased by the third purification. This indicates that some polymer is being retained in the column, particularly because we observed that volume retention due to sample addition was close to 100% for all purifications. Further, we did a head-to-head comparison of GFC purification to precipitation of PET-RAFT synthesized mPEG acrylate. Doing so proved that this technique is comparable to precipitation in terms of removal efficiency. However, not only can polymer retention be sacrificed due to multiple precipitation and transfer steps but also time required to purify each polymer as precipitation is much more labor-intensive. Polymer retention is further visualized by GPC RI traces displayed in Figure S3, which represents ZnTPP removal for PEG +ZnTPP in 96-well and 0.5 mL columns (Figure S3A–B) and mPEG acrylate purification in 96-well and 0.5 mL columns (Figure S3C–D). Thus, completing three purification steps would be valuable in ensuring close to 100% small molecule impurities are removed, but the main drawback is that additional polymer is retained in the column.
Next we studied the ability of gel filtration to remove both ZnTPP and DBCO-NH2 from mPEG acrylate synthesized by PET-RAFT in 96-well purification devices that were manually packed with Sephadex G-25 superfine resin. We decided to use this specific resin by completing a comparison between Sephadex G-10, G-25 superfine, and LH20. Table 2 contains removal efficiency of DBCO-NH2, wash sum, polymer retention, and volume retention for mPEG acrylate and mPEG acrylate +DBCO. For both cases (polymer and polymer +DBCO), removal efficiency was close to 100% for the first purification and polymer retention was greater than 89%. We believe that the spiking of DBCO slightly increases the solution viscosity which would explain why retention is lower and the wash sum is lower after five post-purification washes. With respect to removing DBCO-NH2, we have found that three purification steps would be excessive. These results are further supported by Figure 2 which contains GPC UV traces for mPEG acrylate and mPEG acrylate +DBCO (Figure 2A–B), showing removal of ZnTPP (Figure 2A) along with ZnTPP and DBCO-NH2 (Figure 2B). Polymer retention of mPEG acrylate and mPEG acrylate +DBCO is represented by GPC RI traces in Figure 2C–D, respectively. Lastly, we confirmed the ability of GFC columns and filter plates to remove small molecule impurities associated with 32 diverse polymers spiked with DBCO-NH2 (Table S5 from Supporting Information). On average, from just one purification step, this technique enabled removal of >95% free-DBCO and retention of >87% polymer (Table 2). Any variations in removal efficiency and polymer retention between the 32 polymers listed in Table S5 and the individual PEG-based polymers in Table 1–2 are likely due to differences in polymer viscosities and molecular weight of the impurities (ZnTPP or DBCO-NH2).
As previously described, the current purification strategies used in the polymer community include precipitation and two-phase extraction, which require polymer-specific optimization and are therefore not practical methods for purifying libraries.[13–15] An additional consideration is needed while recognizing that the majority of PET-RAFT polymerizations proceed in DMSO. While DMSO is a favorable solvent for synthesis, it is a poor solvent to precipitate from. Therefore, GFC represents a better approach for purifying libraries prepared by PET-RAFT.[9] Similarly, HPLC-based purification only seems to increase the complexity and even adds additional dialysis or lyophilization steps.[17] GFC columns and filter plates have been used for a wide variety of applications[19–23] and should be used more frequently by the synthetic polymers community.
CONCLUSION
In conclusion, we found that 96-well filter plates are well suited to purify polymer combinatorial libraries. With this work, we demonstrated that gel filtration can be easily incorporated into a routine for preparing large, diverse, and low-volume combinatorial libraries. By increasing the awareness of these accessible and affordable tools, the barrier for creating libraries of polymers is greatly reduced. Since current polymer purification strategies of precipitation and preparative HPLC are practically insufficient for high throughput applications, this approach will enable more efficient protocols for library preparation and improve the overall research productivity.
Experimental Section
Preparation of Polymers and PET-RAFT Polymerization:
Preliminary purification experiments were completed using 35 kDa PEG purchased from Sigma Aldrich, which was diluted to 20 mg/mL in DMSO and spiked with 50 mM ZnTPP in order to determine purification efficiency. Meanwhile, the remaining polymers were purified by PET-RAFT with targeted degree of polymerization (DP) = 400.[8] These polymers include mPEG acrylate along with 32 random heteropolymer combinations detailed in Table S1. Additional experimental details for PET-RAFT are contained in the Supporting Information.
Polymer Purification:
Polymer purification was completed in both pre-packed and manually packed desalting columns and plates. The various Sephadex resins were freshly prepared by addition to DMSO and allowed to swell about two hours. All parameters used for each purification device are listed in Table S2, including resin volume, DMSO equilibration volume (repeated three times), equilibration spin rate and spin time, sample volume, and sample spin rate and spin time. This table provides a starting point for others, however, further optimization may be required. These parameters did not differ between pre-packed and manually packed devices. Following sample purification, five washes were completed using the same sample volumes and spin conditions to demonstrate that the small molecule was in fact removed and that columns can be re-used. For all sample recovery steps, volume retention was determined using calibrated pipettes to ensure minimal volume fluctuations. After the five wash steps, the purified sample was re-purified for a second and third time.
Polymer Precipitation:
To compare the GFC purification to traditional precipitation purification, the same mPEG acrylate polymer was precipitated by addition to 10-fold excess ethyl acetate in a 1:9 v/v ratio (polymer:ethyl acetate). Vortexing was used to ensure full precipitation followed by centrifugation at 3,300 g for 10 minutes. The organic phase was decanted and the polymer was re-dissolved in methanol for subsequent precipitation. After three cycles of precipitation, the final polymer solution (dissolved in methanol) was rotary evaporated and diluted accordingly for GPC and UV-Vis analysis.
Supplementary Material
Acknowledgements
RU acknowledges the support of the National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008339. AJG acknowledges financial support from the New Jersey Health Foundation.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- [1].Celasun S, Remmler D, Schwaar T, Weller MG, Du Prez F, Börner HG, Angew. Chem., Int. Ed. Engl 2019, 58, 1960–1964. [DOI] [PubMed] [Google Scholar]
- [2].Chun YW, Balikov DA, Feaster TK, Williams CH, Sheng CC, Lee JB, Boire TC, Neely MD, Bellan LM, Ess KC, Bowman AB, Sung HJ, Hong CC, Biomaterials 2015, 67, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mishra B, Wilson DR, Sripathi SR, Suprenant MP, Rui Y, Wahlin KJ, Berlinicke CA, Green JJ, Zack DJ, Reg Eng Transl Med 2019, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yeow J, Chapman R, Gormley AJ, Boyer C, Chem. Soc. Rev 2018, 47, 4357–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schmatloch S, Meier MAR, Schubert US, Macromol. Rapid Commun 2003, 24, 33–46. [Google Scholar]
- [6].Chighine A, Sechi G, Bradley M, Drug Discov. Today 2007, 12, 459–464. [DOI] [PubMed] [Google Scholar]
- [7].Zhang H, Hoogenboom R, Meier MAR, Schubert US, Meas. Sci. Technol 2005, 16, 203–211. [Google Scholar]
- [8].Gormley AJ, Yeow J, Ng G, Conway O, Boyer C, Chapman R, Angew. Chem., Int. Ed. Engl 2018, 57, 1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu J, Jung K, Atme A, Shanmugam S, Boyer C, J. Am. Chem. Soc 2014, 136, 5508–5519. [DOI] [PubMed] [Google Scholar]
- [10].Chapman R, Gormley AJ, Stenzel MH, Stevens MM, Angew. Chem., Int. Ed. Engl 2016, 128, 4576–4579. [DOI] [PubMed] [Google Scholar]
- [11].Chapman R, Gormley AJ, Herpoldt K, Stevens MM, Macromolecules 2014, 47, 8541–8547. [Google Scholar]
- [12].Enciso AE, Fu L, Russell AJ, Matyjaszewski K, Angew. Chem., Int. Ed. Engl 2018, 57, 933–936. [DOI] [PubMed] [Google Scholar]
- [13].Oliver S, Zhao L, Gormley AJ, Chapman R, Boyer C, Macromolecules 2019, 52, 3–23. [Google Scholar]
- [14].Hughes I, Hunter D, Curr. Opin. Chem. Biol 2001, 5, 243–247. [DOI] [PubMed] [Google Scholar]
- [15].Fagan JA, Khripin CY, Silvera Batista CA, Simpson JR, Hároz EH, Walker ARH, Zheng M, Adv. Mater 2014, 26, 2800–2804. [DOI] [PubMed] [Google Scholar]
- [16].Wang S, Fu C, Wei Y, Tao L, Macromol. Chem. Phys 2014, 215, 486–492. [Google Scholar]
- [17].Hughes I, SLAS Technol. 2000, 5. [Google Scholar]
- [18].Hoogenboom R, Schubert US, J. Polym. Sci., Part A: Polym. Chem 2003, 41, 2425–2434. [Google Scholar]
- [19].Howden AJM, Geoghegan V, Katsch K, Efstathiou G, Bhushan B, Boutureira O, Thomas B, Trudgian DC, Kessler BM, Dieterich DC, Davis BG, Acuto O, Nat. Methods 2013, 10, 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chavez JD, Wu J, Bisson W, Maier CS, J Proteomics 2011, 74, 2417–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wu L, Murat P, Matak-Vinkovic D, Murrell A, Balasubramanian S, Biochemsitry 2013, 52, 9519–9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reitinger S, Petriv OI, Mehr K, Hansen CL, Withers SG, J. Virol. Methods 2012, 185, 171–174. [DOI] [PubMed] [Google Scholar]
- [23].Chandak MS, Nakamura T, Takenaka T, Chaudhuri TK, Yagi-Utsumi M, Chen J, Kato K, Kuwajima K, Protein Sci. 2013, 22, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


