Abstract
Background:
Phosphatidylinositol-binding clathrin assembly protein (PICALM) is a validated genetic risk factor for late-onset Alzheimer’s disease (AD) and is associated with other neurodegenerative diseases. However, PICALM expression in the blood of neurodegenerative diseases remains elusive.
Objective:
This study aimed to assess the usefulness of PICALM expression levels in the blood of patients with AD, Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and geriatric major depressive disorder (MDD) as a diagnostic biomarker.
Methods:
In total, 45, 20, 21, and 19 patients with AD, PD, DLB, and geriatric MDD, respectively, and 54 healthy controls (HCs) were enrolled in the study. Expression data from Gene Expression Omnibus database (GSE97760), (GSE133347) and (GSE98793), (GSE48350), and (GSE144459) were used to validate the ability of biomarkers in the blood of patients with AD, PD, geriatric MDD, and a postmortem human AD brain and animal model of AD (3xTg-AD mouse), respectively.
Results:
PICALM mRNA expression in human blood was significantly increased in patients with AD compared with that in HCs. PICALM mRNA expression and age were negatively correlated only in patients with AD. PICALM mRNA expression in human blood was significantly lower in patients with PD than in HCs. No changes in PICALM mRNA expression were found in patients with DLB and geriatric MDD.
Conclusion:
PICALM mRNA expression in blood was higher in patients with AD, but lower in patients with PD, which suggests that PICALM mRNA expression in human blood may be a useful biomarker for differentiating neurodegenerative diseases and geriatric MDD.
Keywords: Alzheimer’s disease, blood, gene expression, major depressive disorder, phosphatidylinositol-binding clathrin assembly protein
INTRODUCTION
About 50 million people worldwide are living with dementia, and this number is predicted to increase to 152 million by 2050; therefore, dementia prevention, precise interventions, and person-centered care are expected to receive increasing attention in the years to come [1]. Most cases of Alzheimer’s disease (AD) (>95%) are sporadic and have a late onset (80–90 years of age) [2]. Many genetic risk factors for sporadic AD have been consistently identified in large-scale genome-wide association studies, and pathway analysis has implicated immunity, lipid metabolism, tau-binding proteins, and amyloid-β protein precursor metabolism [3]. Growing evidence suggests an overlap between AD and Parkinson’s disease (PD) pathophysiology in a subset of patients. A recent study demonstrated common genetic variants in genome-wide association with AD as predictors of concomitant AD pathology in the brains of people with a primary clinicopathological diagnosis of PD or dementia with Lewy bodies (DLB) [4]. Furthermore, the shared genetic etiology underlying AD and major depressive disorder (MDD) showed common pathways related to immune response and the regulation of endocytosis [5]. These neurodegenerative diseases and geriatric MDD may have overlapping signatures underpinning common phenotypic manifestations such as cognitive impairment and depressed mood.
Phosphatidylinositol-binding clathrin assembly protein (PICALM) is a highly validated genetic risk factor for late-onset AD [3]. PICALM is involved in trans-vascular Aβ clearance through the process of PICALM/clathrin-dependent endocytosis [6], and PICALM reductions in the brain endothelium of patients with AD correlate with AD neuropathology and cognitive impairment [7]. In addition to Aβ pathology, PICALM is associated with other neurodegenerative diseases with tau-mediated neuropathology, such as frontotemporal dementia and progressive supranuclear palsy [8, 9]. Although the relationship between PICALM expression and the reduction of endocytosis in the brain has been reported in several studies [8, 9], PICALM expression in the blood of neurodegenerative diseases remains elusive. Moreover, recent studies have suggested that both Aβ and tau pathologies may be associated with the pathophysiology of geriatric MDD [10–12], which is not only frequently comorbid, but also necessary to differentiate from AD [13], PD [14], and DLB [15].
In addition to the presence of amyloid plaques in the brain parenchyma and intraneuronal neurofibrillary tangles, recent evidence suggests additional AD pathophysiological pathways, such as innate immune responses, neuroinflammation, and vascular and cell membrane dysregulation [16–19]. Our previous studies suggest that the detection of transcriptome biomarkers related to cell stress and inflammation in the blood has significant potential as a minimally invasive and inexpensive diagnostic tool for the diagnosis and early detection of developing AD [20–28].
Therefore, the purpose of this study was to assess the usefulness of PICALM expression levels in the blood of patients with AD and other neurodegenerative diseases (i.e., PD and DLB), as well as geriatric MDD, as a diagnostic biomarker.
MATERIALS AND METHODS
Participants
We enrolled 45 patients with AD, 20 with PD, 21 with DLB, 19 with geriatric MDD, and 54 healthy controls (HCs). All patients met the diagnostic criteria for AD, PD, DLB, and MDD according to the Aging/Alzheimer’s Association [29], UK Parkinson’s Disease Society Brain Bank [30], 2017 DLB clinical diagnostic criteria [31], and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [32], respectively. All patients with MDD were age >60 years. HCs had no current cognitive impairment, psychiatric symptoms, or history of mental disorders. The demographic characteristics of the patients and HCs are shown in Table 1. Cognitive function was evaluated using the Mini-Mental State Examination (MMSE) and psychiatric symptoms in patients with MDD were evaluated using the 21-item Hamilton Rating Scale for Depression (HAM-D21) (Table 1). All participants were unrelated and of Japanese origin and provided written informed consent using forms approved by the institutional ethics committees of Ehime University (Approval Number: 31-K8 and R2–4). This study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Characteristics of each group
| HCs | AD | PD | DLB | MDD | p | |
| N | 54 | 45 | 20 | 21 | 19 | |
| Age (y) | 77.0±5.6 | 77.3±4.4 | 75.8±4.2 | 77.8±7.5 | 70.7±7.6* | p < 0.001a |
| Sex (male/female) | 18/36 | 15/30 | 6/14 | 7/14 | 7/12 | p = 0.995b |
| MMSE | 18.2±5.5 | 27.2±3.3 | ||||
| Duration (y) | 4.2±4.1 | |||||
| NPI | 13.8±16.5 | |||||
| MADRS | 5.9±4.9 | |||||
| ADAS | 19.5±9.0 | |||||
| CDR | 1.5±0.6 | |||||
| L-dopa (mg/day) | 415.4±116.2 | |||||
| UPDRS | 27.9±11.3 | |||||
| HAM-D21 | 22.8±10.1 |
Data are shown as mean±standard deviation. HCs, healthy controls; AD, Alzheimer’s disease; PD, Parkinson’s disease; DLB, dementia with Lewy bodies; MDD, major depressive disorder; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; MADRS, Montgomery–Åsberg Depression Rating Scale; ADAS, Alzheimer’s Disease Assessment Scale; CDR, Clinical Dementia Rating; L-dopa, L-3, 4-dihydroxyphenylalanine; UPDRS, Unified Parkinson’s Disease Rating Scale; HAM-D21, 21-item Hamilton Depression Rating Scale. *Dunnett’s test, p < 0.001 (HCs versus MDD). aOne-way analysis of variance. bχ2 test for independence.
Blood collection, RNA isolation, and cDNA synthesis
Total RNA was extracted from whole peripheral blood samples using PAXgene Blood RNA Tubes and the PAXgene Blood RNA kit (Qiagen, Tokyo, Japan) according to the manufacturer’s protocol. RNA concentration and purity were determined by spectrophotometric analysis using a spectrophotometer (Nano Drop-1000; Thermo Fisher Scientific, Yokohama, Japan). A high-capacity cDNA reverse transcription kit (Applied Biosystems, Yokohama, Japan) was used for reverse transcription. RNA (1.0μg) was reverse transcribed in a total reaction volume of 40μL. We examined the RNA integrity of all samples using the Bioanalyzer DNA kit (Agilent Technologies Japan, Ltd., Tokyo, Japan). The mean (±standard deviation [SD]) RNA integrity numbers in each group were over 7.6 (HCs: 7.62±0.52, AD: 7.78±0.68, PD: 7.78±0.61, DLB: 7.74±0.63, MDD: 7.76±0.68), and no significant differences were found (analysis of variance [ANOVA] p = 0.84). These results indicated that the RNA qualities were appropriate for real-time PCR experiments.
mRNA expression
We performed real-time qPCR to examine PICALM and GAPDH mRNA expression using the TaqMan method (Applied Biosystems). Specific TaqMan probes were Hs00200318_m1 for PICALM and Hs02758991_g1 for GAPDH, which was used as an internal standard. Because preceding studies, including ours, identified GAPDH as the most suitable reference gene for blood gene expression analysis using the PAXgene blood RNA system [33, 34], we have presented the results of gene expression analyses using GAPDH for future validation. Because some reports have indicated that GAPDH gene expression decreases with age [35, 36], we examined the relationship between age and GAPDH levels (threshold cycle value) and found no significant correlation between them (Pearson correlation test: r = –0.095, p = 0.233). Additionally, we selected 18S rRNA as the second reference gene for validation. The specific TaqMan probe for 18S rRNA was Hs99999901_s1. RT-qPCR was performed with 2.0μL cDNA in a reaction mixture containing TaqMan Gene Expression Master Mix (Applied Biosystems). The total volume of each well was 20μL. RT-qPCR was run in 96-well reaction plates using a StepOnePlus real-time PCR system (Applied Biosystems). mRNA expression was determined in duplicate. Thermal cycling conditions included one cycle at 50°C for 2 min and one cycle at 95°C for 10 min, followed by 50 cycles of amplification at 95°C for 15 s and 60°C for 1 min. Relative mRNA levels were calculated via the ΔΔCt method using StepOne software (Applied Biosystems).
Validation analyses using public functional genomics data
To validate the ability of biomarkers in the blood of patients with AD, PD, geriatric MDD, and a postmortem human AD brain and animal model of AD (3xTg-AD mouse), we used expression data from Gene Expression Omnibus database (GSE97760) [37], (GSE133347) and (GSE98793) [38], (GSE48350) [39], and (GSE144459), [40] respectively. From human blood AD data (GSE97760), old female HCs (N = 10, mean age±SD: 72.1±13.1 years) and female patients with advanced AD (N = 9, mean age±SD: 79.3±12.3 years) were examined. From human blood PD data (GSE133347), HCs (N = 5, mean age±SD: 59.2±3.5 years) and patients with PD (N = 5, mean age±SD: 61.8±3.3 years) were examined. From human blood MDD data (GSE98793), old HCs (N = 19, M/F = 2/17, mean age±SD: 65.8±3.8 years) and patients with geriatric MDD (N = 38, M/F = 4/34, mean age±SD: 66.0±4.1 years) were examined. From human hippocampal data (GSE48350), old HCs (N = 21, M/F = 10/11, mean age±SD: 84.7±8.4 years) and patients with AD (N = 19, M/F = 9/10, mean age±SD: 83.1±8.5 years) were examined. From animal model blood and hippocampal data (GSE144459), wild-type male mice (n = 8 each, age 12 and 52 weeks) (named as C12bld, C52bld, C12 hip, and C52 hip) and 3xTg-AD male mice (n = 8 each, age 12 and 52 weeks) (named as AD12bld, AD52bld, AD12 hip, and AD52 hip) were examined.
Statistical analysis
SPSS software (version 22.0; IBM Japan, Tokyo, Japan) was used for the statistical analysis. After the normal distribution of age and mRNA expression data was analyzed using the Shapiro–Wilk test, expression data were examined using one-way ANOVA to compare age and PICALM mRNA expression between patients with AD, PD, DLB, geriatric MDD, and HCs. Sex differences were analyzed using Pearson’s chi-squared test. The Spearman correlation test was used to determine correlations with clinical parameters. Comparisons of the expression data from the Gene Expression Omnibus database were analyzed using a t-test or one-way ANOVA with Tukey’s honestly significant difference (HSD) test. Values of p < 0.05 were considered statistically significant.
RESULTS
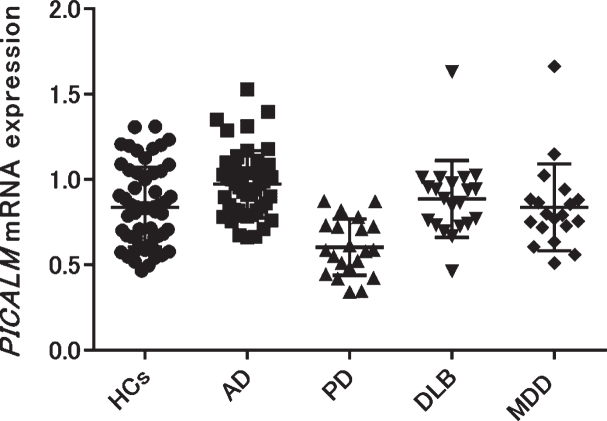
We examined PICALM mRNA expression in the blood of patients with AD, PD, DLB, geriatric MDD, and HCs. Each group except geriatric MDD showed no significant differences in age or sex (Table 1). The geriatric MDD group was significantly younger than the other groups (one-way ANOVA p < 0.001, Tukey’s HSD p < 0.001 [HCs versus MDD]). The mean±SD PICALM mRNA expression in patients with AD was significantly higher than that in HCs (AD: 0.98±0.19; HCs: 0.84±0.23, Tukey’s HSD p < 0.01), while that in patients with PD was significantly lower than that in HCs (PD: 0.60±0.17, HCs: 0.84±0.23, Tukey’s HSD p < 0.001). No significant differences were found between patients with DLB, geriatric MDD, and HCs (DLB: 0.89±0.22; geriatric MDD: 0.84±0.25, HCs: 0.84±0.23) (Fig. 1). Validation experiments using 18S rRNA as the second reference gene showed similar expressional changes (Supplementary Figure 2).
Fig. 1.
PICALM mRNA expression was compared among patients with several neuropsychiatric diseases (AD, PD, DLB, and geriatric MDD) and HCs. PICALM mRNA expression was significantly higher in patients with AD, but significantly lower in patients with PD compared with HCs (AD versus HCs: p = 0.004, PD versus HCs: p < 0.001). AD, Alzheimer’s disease; PD, Parkinson’s disease; DLB, dementia with Lewy bodies; MDD, major depressive disorder; HCs, healthy controls.
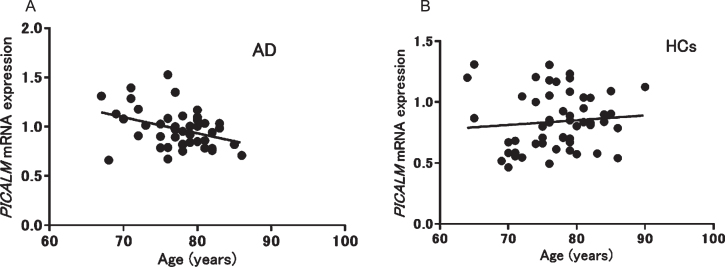
We also analyzed the correlations between PICALM mRNA expression and clinical parameters. A significant negative correlation between PICALM mRNA expression and age was observed only in patients with AD (p = 0.013, r = –0.37) (Fig. 2), whereas no significant correlations were found with other clinical parameters in patients with AD (MMSE): p = 0.29, r = 0.16, duration: p = 0.24, r = 0.19, Neuropsychiatric Inventory: p = 0.49, r = 0.11, Montgomery–Åsberg Depression Rating Scale: p = 0.14, r = –0.27, Alzheimer’s Disease Assessment Scale: p = 0.61, r = 0.09, Clinical Dementia Rating: p = 0.19, r = –0.21). In patients with PD, no significant correlation was found between mRNA expression and age. In addition, no correlation was seen between PICALM mRNA expression and other clinical parameters in patients with PD (L-3,4-dihydroxyphenylalanine: p = 0.91, r = –0.038, MMSE: p = 0.98, r = –0.009, Unified Parkinson’s Disease Rating Scale: p = 0.3, r = 0.31) or geriatric MDD (HAM-D21): p = 0.08, r = 0.42).
Fig. 2.
Correlation between PICALM mRNA expression and age in patients with Alzheimer’s disease (AD; A: r = –0.37, p = 0.013) and healthy controls (HCs; B: r = 0.10, p = 0.48).
Validation analyses using public functional genomics data showed significantly higher PICALM mRNA expression levels in the blood of patients with AD (p < 0.001 logFC: 1.46) (Supplementary Figure 1A), in that of 3xTg-AD mice (one-way ANOVA p < 0.001, Tukey’s HSD p < 0.001 [C12 bld versus AD12 bld and C12 bld versus AD52 bld]) (Supplementary Figure 1E), and in the hippocampus of 3xTg-AD mice (one-way ANOVA p < 0.001, Tukey’s HSD p < 0.001 [C12 hip versus AD52 hip]) (Supplementary Figure 1F). No changes were seen in PICALM mRNA expression in the blood of patients with PD or geriatric MDD (Supplementary Figure 1B, C) or in the hippocampus of patients with AD (Supplementary Figure 1D).
DISCUSSION
This study had four major findings. First, PICALM mRNA expression in blood was significantly increased in patients with AD compared with that in HCs. Inconsistent with our results, a previous study using postmortem human brain and AD model mice showed reduced expression of PICALM in AD endothelium correlated with amyloid-β (Aβ) pathology and cognitive impairment [7]. This inconsistency may be due to tissue differences. Another study reported a decrease in PICALM gene methylation in the blood of patients with AD [41]. As DNA methylation levels were generally inversely correlated with RNA expression levels, this result may be consistent with our result. Although PICALM gene methylation in the same study was positively associated with MMSE scores, PICALM mRNA expression in our study was not associated with MMSE scores, probably because of the small sample size. Interestingly, validation analyses using public functional genomics data in the blood of patients with AD (Supplementary Figure 1A) and in the blood and hippocampus of 3xTg-AD mice (Supplementary Figure 1E, F) could replicate the increased PICALM mRNA expression in the blood of patients with AD. Although public functional genomics data in the hippocampus of patients with AD (Supplementary Figure 1D) did not show a significant change, PICALM mRNA expression in the hippocampus of 3xTg-AD mice showed a significant increase (Supplementary Figure 1F). Further analyses of PICALM mRNA expression in the human postmortem brain are therefore warranted.
Second, we observed a negative correlation between PICALM mRNA expression and age only in patients with AD (Fig. 2); no association was seen in HCs. In a previous study, PICALM was identified as having gene-based genome-wide significance with larger effects at a younger age in AD [42]. Therefore, the negative correlation observed between expression and age in patients with AD may suggest that the risk of PICALM for AD is modified by age.
Third, PICALM mRNA expression in human blood was significantly lower in patients with PD compared with that in HCs. In a previous study, an association was reported between PICALM rs3851179 and cognitive impairment in patients with PD [43]. Regarding the relationship between PICALM polymorphisms and PD, significant differences have been reported in the genotypes and allele frequencies of an SNP PICALM rs3851179 A allele among patients with AD, PD, and HCs [44]; however, it has also been reported that PICALM rs3851179 polymorphism is not related to PD [45, 46]. Although we could not confirm the same result in our validation analysis (Supplementary Figure 1B), PICALM may also be involved in the pathophysiology of PD.
Fourth, we did not find any changes in PICALM mRNA expression in patients with DLB and geriatric MDD. Public functional genomics data also showed that PICALM mRNA expression in the blood of patients with geriatric MDD had not changed (Supplementary Figure 1C). Because geriatric MDD is frequently comorbid with neurodegenerative diseases such as AD, PICALM mRNA expression may be useful as a differential biomarker for treating geriatric diseases that show psychiatric symptoms.
This study had several limitations. As few studies have reported finding relationships between PICALM and neurodegenerative diseases, patients should be recruited in various stages and the effects of medication should be considered in future studies. Moreover, this study had a small sample size. Due to the limited power, the negative findings in this study cannot be interpreted as showing no associations.
CONCLUSIONS
We demonstrated that PICALM mRNA expression in blood was higher in patients with AD, but lower in patients with PD. Therefore, PICALM mRNA expression in human blood may be a useful biomarker for differentiating neurodegenerative diseases and geriatric MDD. Because PICALM mRNA expression using another reference gene (18S rRNA) was not changed in PD and decreased in geriatric MDD, these results need further validations.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank Ms. Chiemi Onishi for her technical assistance and all participants in this study. This research was supported by AMED under Grant No. JP20dk0307076 and Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, JSPS KAKENHI (Grant Nos. 18K07564, 18H02752, 19K17113, 20K07971, and 20K16628).
Authors’ disclosures available online (http://www.j-alz.com/manuscript-disclosures/20-1046r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-201046.
REFERENCES
- [1]. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimaki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1, 15056. [DOI] [PubMed] [Google Scholar]
- [3]. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, Amlie-Wolf A, et al. (2019) Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet 51, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Dai DL, Tropea TF, Robinson JL, Suh E, Hurtig H, Weintraub D, Van Deerlin V, Lee EB, Trojanowski JQ, Chen-Plotkin AS (2020) ADNC-RS, a clinical-genetic risk score, predicts Alzheimer’s pathology in autopsy-confirmed Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol 140, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Lutz MW, Sprague D, Barrera J, Chiba-Falek O (2020) Shared genetic etiology underlying Alzheimer’s disease and major depressive disorder. Transl Psychiatry 10, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK, Zlokovic BV (2015) Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat Neurosci 18, 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Ando K, De Decker R, Vergara C, Yilmaz Z, Mansour S, Suain V, Sleegers K, de Fisenne MA, Houben S, Potier MC, Duyckaerts C, Watanabe T, Buee L, Leroy K, Brion JP (2020) Picalm reduction exacerbates tau pathology in a murine tauopathy model. Acta Neuropathol 139, 773–789. [DOI] [PubMed] [Google Scholar]
- [9]. Ando K, Tomimura K, Sazdovitch V, Suain V, Yilmaz Z, Authelet M, Ndjim M, Vergara C, Belkouch M, Potier MC, Duyckaerts C, Brion JP (2016) Level of PICALM, a key component of clathrin-mediated endocytosis, is correlated with levels of phosphotau and autophagy-related proteins and is associated with tau inclusions in AD, PSP and Pick disease. Neurobiol Dis 94, 32–43. [DOI] [PubMed] [Google Scholar]
- [10]. Gatchel JR, Rabin JS, Buckley RF, Locascio JJ, Quiroz YT, Yang HS, Vannini P, Amariglio RE, Rentz DM, Properzi M, Donovan NJ, Blacker D, Johnson KA, Sperling RA, Marshall GA, Harvard Aging Brain Study (2019) Longitudinal association of depression symptoms with cognition and cortical amyloid among community-dwelling older adults. JAMA Netw Open 2, e198964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Gatchel JR, Donovan NJ, Locascio JJ, Schultz AP, Becker JA, Chhatwal J, Papp KV, Amariglio RE, Rentz DM, Blacker D, Sperling RA, Johnson KA, Marshall GA (2017) Depressive symptoms and tau accumulation in the inferior temporal lobe and entorhinal cortex in cognitively normal older adults: A pilot study. J Alzheimers Dis 59, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Chung JK, Plitman E, Nakajima S, Chow TW, Chakravarty MM, Caravaggio F, Gerretsen P, Brown EE, Iwata Y, Mulsant BH, Graff-Guerrero A, Alzheimer’s Disease Neuroimaging Initiative (2015) Lifetime history of depression predicts increased amyloid-beta accumulation in patients with mild cognitive impairment. J Alzheimers Dis 45, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Hu M, Shu X, Wu X, Chen F, Hu H, Zhang J, Yan P, Feng H (2020) Neuropsychiatric symptoms as prognostic makers for the elderly with mild cognitive impairment: A meta-analysis. J Affect Disord 271, 185–192. [DOI] [PubMed] [Google Scholar]
- [14]. Goodarzi Z, Mrklas KJ, Roberts DJ, Jette N, Pringsheim T, Holroyd-Leduc J (2016) Detecting depression in Parkinson disease: A systematic review and meta-analysis. Neurology 87, 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Yamane Y, Sakai K, Maeda K (2011) Dementia with Lewy bodies is associated with higher scores on the Geriatric Depression Scale than is Alzheimer’s disease. Psychogeriatrics 11, 157–165. [DOI] [PubMed] [Google Scholar]
- [16]. Yulug B, Hanoglu L, Ozansoy M, Isik D, Kilic U, Kilic E, Schabitz WR (2018) Therapeutic role of rifampicin in Alzheimer’s disease. Psychiatry Clin Neurosci 72, 152–159. [DOI] [PubMed] [Google Scholar]
- [17]. Delvaux E, Mastroeni D, Nolz J, Chow N, Sabbagh M, Caselli RJ, Reiman EM, Marshall FJ, Coleman PD (2017) Multivariate analyses of peripheral blood leukocyte transcripts distinguish Alzheimer’s, Parkinson’s, control, and those at risk for developing Alzheimer’s. Neurobiol Aging 58, 225–237. [DOI] [PubMed] [Google Scholar]
- [18]. Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC, Alzheimer’s Disease Neuroimaging Initiative (2016) Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 7, 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Mise A, Yoshino Y, Yamazaki K, Ozaki Y, Sao T, Yoshida T, Mori T, Mori Y, Ochi S, Iga JI, Ueno SI (2017) TOMM40 and APOE gene expression and cognitive decline in Japanese Alzheimer’s disease subjects. J Alzheimers Dis 60, 1107–1117. [DOI] [PubMed] [Google Scholar]
- [21]. Mori Y, Yoshino Y, Ochi S, Yamazaki K, Kawabe K, Abe M, Kitano T, Ozaki Y, Yoshida T, Numata S, Mori T, Iga J, Kuroda N, Ohmori T, Ueno S (2015) TREM2 mRNA expression in leukocytes is increased in Alzheimer’s disease and schizophrenia. PLoS One 10, e0136835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Ozaki Y, Yoshino Y, Yamazaki K, Sao T, Mori Y, Ochi S, Yoshida T, Mori T, Iga JI, Ueno SI (2017) DNA methylation changes at TREM2 intron 1 and TREM2 mRNA expression in patients with Alzheimer’s disease. J Psychiatr Res 92, 74–80. [DOI] [PubMed] [Google Scholar]
- [23]. Sao T, Yoshino Y, Yamazaki K, Ozaki Y, Mori Y, Ochi S, Yoshida T, Mori T, Iga JI, Ueno SI (2018) TREM1 mRNA expression in leukocytes and cognitive function in Japanese patients with Alzheimer’s disease. J Alzheimers Dis 64, 1275–1284. [DOI] [PubMed] [Google Scholar]
- [24]. Sao T, Yoshino Y, Yamazaki K, Ozaki Y, Mori Y, Ochi S, Yoshida T, Mori T, Iga JI, Ueno SI (2018) MEF2C mRNA expression and cognitive function in Japanese patients with Alzheimer’s disease. Psychiatry Clin Neurosci 72, 160–167. [DOI] [PubMed] [Google Scholar]
- [25]. Yamazaki K, Yoshino Y, Mori T, Okita M, Yoshida T, Mori Y, Ozaki Y, Sao T, Iga J, Ueno S (2016) Association study and meta-analysis of polymorphisms, methylation profiles, and peripheral mRNA expression of the serotonin transporter gene in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 41, 334–347. [DOI] [PubMed] [Google Scholar]
- [26]. Yamazaki K, Yoshino Y, Mori T, Yoshida T, Ozaki Y, Sao T, Mori Y, Ochi S, Iga JI, Ueno SI (2017) Gene expression and methylation analysis of ABCA7 in patients with Alzheimer’s disease. J Alzheimers Dis 57, 171–181. [DOI] [PubMed] [Google Scholar]
- [27]. Yoshino Y, Funahashi Y, Nakata S, Ozaki Y, Yamazaki K, Yoshida T, Mori T, Mori Y, Ochi S, Iga JI, Ueno SI (2018) Ghrelin cascade changes in the peripheral blood of Japanese patients with Alzheimer’s disease. J Psychiatr Res 107, 79–85. [DOI] [PubMed] [Google Scholar]
- [28]. Yoshino Y, Yamazaki K, Ozaki Y, Sao T, Yoshida T, Mori T, Mori Y, Ochi S, Iga JI, Ueno SI (2017) INPP5D mRNA expression and cognitive decline in Japanese Alzheimer’s disease subjects. J Alzheimers Dis 58, 687–694. [DOI] [PubMed] [Google Scholar]
- [29]. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51, 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Association AP (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).
- [33]. Watanabe SY, Iga J, Ishii K, Numata S, Shimodera S, Fujita H, Ohmori T (2015) Biological tests for major depressive disorder that involve leukocyte gene expression assays. J Psychiatr Res 66-67, 1–6. [DOI] [PubMed] [Google Scholar]
- [34]. Carrol ED, Salway F, Pepper SD, Saunders E, Mankhambo LA, Ollier WE, Hart CA, Day P (2007) Successful downstream application of the Paxgene Blood RNA system from small blood samples in paediatric patients for quantitative PCR analysis. BMC Immunol 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Vigelso A, Dybboe R, Hansen CN, Dela F, Helge JW, Guadalupe Grau A (2015) GAPDH and beta-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J Appl Physiol (1985) 118, 386–394. [DOI] [PubMed] [Google Scholar]
- [36]. El Kadmiri N, Cuardos R, El Moutawakil B, Slassi I, Avila J, Nadifi S, Hachem A, Soukri A (2014) A proteomic approach for the involvement of the GAPDH in Alzheimer disease in the blood of Moroccan FAD cases. J Mol Neurosci 54, 774–779. [DOI] [PubMed] [Google Scholar]
- [37]. Naughton BJ, Duncan FJ, Murrey DA, Meadows AS, Newsom DE, Stoicea N, White P, Scharre DW, McCarty DM, Fu H (2015) Blood genome-wide transcriptional profiles reflect broad molecular impairments and strong blood-brain links in Alzheimer’s disease. J Alzheimers Dis 43, 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S, Henderson R, Freeman TC, Pariante CM, Harrison NA, MRC Immunopsychiatry Consortium, Perry VH, Drevets WC, Wittenberg GM, Bullmore ET (2018) Replicable and coupled changes in innate and adaptive immune gene expression in two case-control studies of blood microarrays in major depressive disorder. Biol Psychiatry 83, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, Zielke HR, Cotman CW (2008) Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A 105, 15605–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Ochi S, Iga JI, Funahashi Y, Yoshino Y, Yamazaki K, Kumon H, Mori H, Ozaki Y, Mori T, Ueno SI (2020) Identifying blood transcriptome biomarkers of Alzheimer’s disease using transgenic mice. Mol Neurobiol 57, 4941–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Mercorio R, Pergoli L, Galimberti D, Favero C, Carugno M, Dalla Valle E, Barretta F, Cortini F, Scarpini E, Valentina VB, Pesatori AC (2018) PICALM gene methylation in blood of Alzheimer’s disease patients is associated with cognitive decline. J Alzheimers Dis 65, 283–292. [DOI] [PubMed] [Google Scholar]
- [42]. Lo MT, Kauppi K, Fan CC, Sanyal N, Reas ET, Sundar VS, Lee WC, Desikan RS, McEvoy LK, Chen CH, Alzheimer’s Disease Genetics Consortium (2019) Identification of genetic heterogeneity of Alzheimer’s disease across age. Neurobiol Aging 84, 243.e241–243.e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Barrett MJ, Koeppel AF, Flanigan JL, Turner SD, Worrall BB (2016) Investigation of genetic variants associated with Alzheimer disease in Parkinson disease cognition. J Parkinsons Dis 6, 119–124. [DOI] [PubMed] [Google Scholar]
- [44]. Santos-Reboucas CB, Goncalves AP, Dos Santos JM, Abdala BB, Motta LB, Laks J, de Borges MB, de Rosso ALZ, Pereira JS, Nicaretta DH, Pimentel MMG (2017) rs3851179 Polymorphism at 5’ to the PICALM gene is associated with Alzheimer and Parkinson diseases in Brazilian population. Neuromolecular Med 19, 293–299. [DOI] [PubMed] [Google Scholar]
- [45]. Kalinderi K, Bostantjopoulou S, Katsarou Z, Clarimon J, Fidani L (2012) Lack of association of the PICALM rs3851179 polymorphism with Parkinson’s disease in the Greek population. Int J Neurosci 122, 502–605. [DOI] [PubMed] [Google Scholar]
- [46]. Gao J, Huang X, Park Y, Hollenbeck A, Chen H (2011) An exploratory study on CLU, CR1 and PICALM and Parkinson disease. PLoS One 6, e24211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.