Abstract
Background:
The frontal variant of Alzheimer’s disease (fAD) is poorly understood and poorly defined. The diagnosis remains challenging. The main differential diagnosis is the behavioral variant of frontotemporal degeneration (bvFTD). For fAD, there is some dissociation between the clinical frontal presentation and imaging and neuropathological studies, which do not always find a specific involvement of the frontal lobes. DAPHNE is a behavioral scale, which demonstrated excellent performance to distinguish between bvFTD and AD.
Objective:
The aim of the present study was to assess the reliability of this new tool to improve the clinical diagnosis of fAD.
Methods:
Twenty fAD patients and their caregivers were prospectively included and were compared with 36 bvFTD and 22 AD patients.
Results:
The three main behavioral disorders in the fAD patients were apathy, loss of empathy, and disinhibition. Three disorders were discriminant because they were less frequent and less severe in the fAD patients than in the bvFTD patients, namely hyperorality, neglect, and perseverations. This specific pattern of behavioral disorders was corroborated by SPECT or 18FDG PET-CT scan that showed that patients with fAD could have a medial frontal hypoperfusion, whereas in bvFTD patients the orbitofrontal cortex was the main involved region, with more diffuse hypoperfusion.
Conclusion:
We demonstrated that DAPHNE had good sensitivity and good specificity to discriminate between the three groups and in particular between fAD and bvFTD patients. DAPHNE is a quick tool that could help clinicians in memory clinics not only to differentiate bvFTD from typical AD but also from fAD.
Keywords: Alzheimer’s disease, behavioral disorders, frontotemporal dementia, scale
INTRODUCTION
With the increase in life expectancy and the aging of the population, the number of patients suffering from neurocognitive disorders due to Alzheimer’s disease (AD) keeps growing. The classical clinical presentation is an episodic memory deficit that becomes progressively established. It is accompanied through its evolution by a more global alteration of cognitive and behavioral functions, which compromises autonomy. Described more recently, progressive focal cortical presentations represent an unusual expression of AD. In 1982, Mesulam described isolated progressive language disorders, associated with atrophy of the left perisylvian regions [1]. In 1988, Benson et al. reported several cases of patients presenting difficulties in associative visual functions, associated with an atrophy of the posterior regions of the brain [2]. In 2011, the diagnostic criteria for AD were reviewed, and for the first time these two non-amnestic variants of AD were included. On this occasion, a third, lesser known and non-consensual entity, called frontal, behavioral, or dysexecutive variant of AD, was individualized and brought closer to language and neurovisual variants due to the focal progressive dysfunction dimension [3].
The concept of frontal AD (fAD) was introduced by Johnson et al. in 1999, through the study of three cases of patients presenting executive dysfunctions in whom the subsequent pathological examination revealed neurofibrillary tangles predominantly in the frontal lobes [4]. Since this initial article, numerous studies on fAD have contributed to a more accurate definition of the clinical, radiological, neuropsychological and anatomopathological characteristics of this as yet little-known variant [5–9].
The diagnosis of atypical forms of AD is often delayed, especially for the frontal variant. Early diagnosis is, however, an important issue in order to avoid misdiagnosis and provide early, specific, and adapted management and consider possible inclusion in a therapeutic trial.
According to data from recent studies, fAD generally appears to affect younger patients than the amnestic variant and is characterized by:
1. A specific clinical presentation, characterized by an insidious beginning and a progressive worsening, with behavioral and/or dysexecutive deficits.
The behavioral presentation is the more frequent and results in a clinical phenotype very close to that observed in the behavioral/frontal variant of frontotemporal degeneration (bvFTD), which represents the most frequent differential diagnosis.
The dysexecutive form of AD is characterized by a predominant executive dysfunction, although the most frequent reason for consulting is memory loss complaints [8].
2. The demonstration of a dysfunction in the anterior cerebral regions through imaging. However, the results of functional or morphological imaging are not always consistent. Through morphological imaging by MRI with post-processing techniques (voxel-to-voxel comparison or voxel-based morphometry), the cerebral atrophy of fAD patients is rather temporo-parietal, and therefore very close to that observed in the classical amnestic variant [8]. Nevertheless, one study in an fAD group showed the morphological damage to be slightly stronger in the orbito-frontal and frontal regions than in typical AD but less than in bvFTD [9].
3. The etiological proof of the AD process, which can be established through an anatomopathological examination but also, and more commonly, in vivo. The current means of diagnosis is the study of CSF biomarkers (the typical CSF profile of AD associates a decrease in Aβ42 peptide levels with an increase in total and hyperphosphorylated tau protein levels) and/or increased tracer retention on amyloid PET. IWG-2 criteria for atypical AD include these markers [10].
The “gold standard” to confirm the diagnosis of AD still remains pathology. But the anatomopathological studies in fAD are contradictory. Indeed, in the initial description by Johnson et al. [4], there seemed to be a predominance of neurofibrillary tangles in the frontal regions, but for Blennerhaset et al. [5], the predominance of the frontal expression would be due not to neurofibrillary tangles but to a larger amount of amyloid plaques in the frontal regions. More recently, Larner’s study did not show any anatomopathological pattern that could correlate with the clinical phenotype [11].
In clinical practice, the main issue is to distinguish between fAD and bvFTD. Paraclinical explorations are usually required to diagnose these diseases but it would be interesting to explore deeper the clinical differences, including the neuropsychological assessment, between these two groups of patients.
Some scales have been specifically built to assist in the diagnosis of specific pathologies. This is the case, for example, with the Frontal Behavioral Inventory (FBI) proposed by Kertesz et al. [12], or the Frontotemporal behavioral scale (FBS) proposed by Lebert et al. [13], for the exploration of behavioral disorders in bvFTD. The lack of a simple tool for the behavioral rating of bvFTD recently led us to propose and validate a scale named DAPHNE (Disinhibition, Apathy, Perseveration, Hyperorality, early Negligence, and Empathy loss). DAPHNE explores six domains: three with positive items (disinhibition, perseveration, and hyperorality) and three with negative items (apathy, negligence, and empathy loss). Domains were selected from the core diagnostic features of Rascovsky’s criteria. A ‘personal neglect’ domain was added in accordance with Kertesz’s FBI, Lebert et al.’s FBS and based on our own experience.
The DAPHNE scale allows the screening and diagnosis of bvFTD versus typical amnestic AD with a sensitivity and a specificity of 92% [14].
In order to better and more readily identify fAD, we studied the contribution of DAPHNE to the differential diagnosis and characterization of behavioral forms of AD.
The primary aim of this prospective multicenter study (‘DAPHNE 2’) was to test the DAPHNE scale as a tool for differential diagnosis between bvFTD and fAD. The secondary aim, bearing in mind the absence of consensual criteria, was to better prospectively characterize fAD at a neurological, neuropsychological and paraclinical level.
METHODS
Study design
DAPHNE 2 was a prospective multicenter cohort study. Patients and caregivers were enrolled from five expert memory centers in France: Nantes, Lyon, Dijon, Bordeaux/Dax and Angers. All these centers have extensive experience in neurodegenerative diseases and especially in bvFTD and AD. The patients and their caregivers were seen for evaluation at their respective investigation center.
Subjects
For inclusion in the study, the following criteria had to be met: 1) the patient had a caregiver and both agreed to participate in the study; 2) the patient was referred to one of the five expert memory centers; 3) the diagnosis was the frontal variant of AD (fAD).
bvFTD patients and AD patients were included in the previous DAPHNE study [14]. Inclusion of healthy subjects did not seem relevant since the aim was to investigate a new behavioral disturbance scale among different groups of ill patients.
The following clinical features were required. To avoid inclusion of patients with a very advanced form of the disease, a Mini-Mental State Examination (MMSE) score >16 was required. fAD patients had to fulfill the McKhann criteria and IGW 2 criteria, with clinically prominent behavioral dysfunctions as well as evidence of amyloid evaluated by biomarkers. For bvFTD patients, the revised Rascovsky criteria had to be met for “possible bvFTD” [15]. AD patients had to fulfill the modified McKhann criteria [3, 10].
Procedures
fAD patients were followed in their expert memory center in the course of caregiving for their cognitive and/or behavioral complaint. They were recruited following a routine care consultation.
At the inclusion visit, which lasted half a day, the following clinical parameters were collected: age at the beginning of the disease, duration and symptoms of the beginning of the disease, significant personal and family background, educational level, history of head trauma and any psychotropic treatments. Moreover, the investigator (a neurologist and/or a geriatrician) performed a full neurological and general examination to rule out differential diagnoses based on atypical clinical signs.
All patients underwent a cerebral MRI and a lumbar puncture with CSF biomarkers for AD (amyloid, tau and p-tau). Most of the patients had a PET scan or SPECT.
Behavioral and neuropsychological assessments
The behavioral evaluation and the neuropsychological assessment were carried out by a neuropsychologist with experience in neurodegenerative diseases.
The DAPHNE scale explores the following six domains (with a combined total of 10 items): disinhibition (4 items), apathy (1 item), empathy loss (1 item), perseveration (1 item), hyperorality (2 items), and neglect (1 item). The scale was designed as a series of semi-structured propositions to be asked of caregivers. It was composed of items with five possible answer categories with description and examples. Items were scored on a five-point scale (none = 0, very mild = 1, mild = 2, moderate = 3, and severe = 4) according to their severity and/or frequency of the behavioral disorder. As in the earlier validation study, two scores were computed. (1) DAPHNE-6 (screening) was computed from 6 synthetic binary domains. For a given domain, we scored one point if at least one symptom was present, regardless of the number of items present in the domain and irrespective of the severity. The maximum score was 6. (2) DAPHNE-40 (diagnosis) was computed as the sum of the 10 items. The maximum score was 40.
In addition, patients underwent a full neuropsychological evaluation. Global efficiency was assessed using the MMSE. Episodic memory was tested using the Free and Cued Selective Reminding Test (FCSRT) [16] as well as recall of the Rey figure [17]. Attentional capacities and executive functions were rated using the Frontal Assessment Battery [18], the Trail Making Test B, verbal fluency [19], the Zoo test (from the Behavioural Assessment of Dysexecutive Syndrome battery) [20], and the Wisconsin Card Sorting Test [21]. Denomination and language were evaluated using the DO 80 (oral denomination of images test) [22] and visuo-constructive praxis was evaluated using the copy of the Rey figure. Social cognition and affective processes were assessed using several specific tests: the Faux Pas Recognition Test [23], Reading mind in the eyes Test [24], and Ekman’s global study of faces for joy, surprise, fear, disgust, sadness, anger, and neutrality [25].
Finally, caregiver burden was evaluated using the 22-item Zarit Burden Inventory to assess disease impact on the caregiver’s quality of life [26].
MRI
MRI scans were made on different 1.5 Tesla scanners in order to contribute to the diagnostic process by excluding other neurological diseases. Since imaging was performed as part of clinical routine, MRI acquisition parameters were not homogenized. Radiologist and neurologist analyzed visually cortical atrophy blind to clinical data. Frontal atrophy, temporal atrophy and global atrophy scores for each patient were dichotomized as normal (without atrophy) or abnormal (atrophy).
Functional imaging
Cerebral 99 mTc-HMPAO SPECT or 18F-FDG PET were performed for routine indication under usual conditions. In both PET and SPECT, patients were injected in quiet surroundings with minimal distractions. PET and SPECT scans were both oriented to the orbitomeatal line to obtain transverse, sagittal and coronal section. Visual analysis and description of SPECT or PET images were retrospectively performed by two operators blind to neuropsychological, MRI and all follow-up clinical data. Only SPECT and PET performed at Nantes Hospital center were included in the study. Reduced regional uptake was evaluated on SPECT and PET images according to the color scale in percent and describe as no reduced (uptake > 80%), mild reduced uptake (uptake > 65 and ≤80%), moderate reduced uptake (uptake > 50 and ≤65%) and severe reduced uptake (uptake ≤50%). Visual grading was performed on each side of hemispheres, considered frontal superior, frontal dorsolateral, orbitofrontal, frontal mesial, temporal anterior, temporal lateral and parietal regions. The same grading was performed on the SPECT images of 20 bvFTD patients and 13 AD patients who had participated in the previous DAPHNE 1 study [14].
Ethical considerations
The study protocol was approved by the Committee on Ethics and Human Research (Comité de Protection des Personnes Ouest VI) and the study was performed in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients and caregivers gave written informed consent prior to their participation in the study.
Gathering of data
All the information required under the study protocol was gathered by an investigator from the clinical team of each participating investigating center. This confidential information was compiled in the patient observation notebook, which also included all the data needed to confirm compliance with the protocol, perform the statistical analysis and allow any major deviations from the protocol to be detected.
Statistical analysis
Given the low prevalence of fAD, we planned to include 15 to 20 patients. Descriptive data for the three groups (AD, fAD, and bvFTD) were reported as median and interquartile range. Patient characteristics were compared between the three groups according to a non-parametric Kruskal-Wallis analysis. A pairwise comparison of groups was performed using the Dunn’s multiple comparisons test. Cohen’s d were presented when two groups were compared only if the difference reached significance. The diagnostic performances of DAPHNE-6 and DAPHNE-40 were compared using receiver operating characteristic (ROC) curves. The different neuropsychological tests were also compared for all three groups and then in pairs. Here, non-parametric tests were also used (again Kruskal-Wallis test and Dunn’s post-hoc test).
Fischer’s exact test was performed for MRI analysis. For functional imaging analysis, Kruskal-Wallis tests and Dunn’s post-hoc tests were performed for comparison for the three groups then between fAD and bvFTD and between fAD and AD.
For statistical analysis the level of significance was set at 5% (p = 0.05).
RESULTS
Twenty fAD patients and their caregivers were prospectively included between June 2016 and June 2017 within the five recruitment centers: 8 in Nantes, 6 in Lyon, 3 in Dijon, 2 in Bordeaux/Dax, and 1 in Angers. All patients were diagnosed with fAD defined by a suggestive neuropsychological profile and by amyloid biomarkers (amyloid, tau, and p-tau in the CSF for 19 patients and positive amyloid PET for 1 patient).
Thirty-six bvFTD patients and 22 AD patients were included in the previous DAPHNE study [14]. The patients’ demographic characteristics for the three groups are shown in Table 1.
Table 1.
Clinical characteristics for the three groups of patients
| AD (n = 22) | fAD (n = 20) | bvFTD (n = 36) | |
| Age (y) | 66.5 [59–70] | 71.5 [66–76] | 67 [60–73] |
| Female/Male (number) | 10/12 | 7/13 | 15/21 |
| Education (y) | 9 [9–12] | 9 [8–13] | 9 [5–12] |
| Disease duration (y) | 48 [36–57] | 60 [36–78] | 48 [36–66] |
| MMSE / 30 | 24 [21–26] | 25 [21–26] | 24 [20–26] |
AD, Alzheimer’s disease; fAD, frontal Alzheimer’s disease; FTD, behavioral variant frontotemporal dementia; MMSE, Mini-Mental State Examination. Data are expressed as median [IQR].
There was no statistical difference between the three groups except for the age. For the later, Kruskal-Wallis test revealed a difference (p = 0.039) between the three groups. Dunn’s post-hoc analysis shown only a significant between AD and fAD patients (p = 0.047); fAD being slightly older.
Behavioral evaluation: DAPHNE as a clinical tool to distinguish patients? (3 groups)
The results of the behavioral evaluation in terms of the DAPHNE-6 and DAPHNE-40 scores for the AD, fAD, and bvFTD groups are shown in Table 2. In terms of global scores, there was a significant difference for DAPHNE-6 and DAPHNE-40 between the three groups (p < 10-3). Moreover, the post-hoc analysis showed that the difference was significant whatever the comparison between any two groups.
Table 2.
DAPHNE-6 and DAPHNE-40 scores for the three groups of patients
| AD group (n = 22) | fAD group (n = 20) | bvFTD group (n = 36) | |
| DAPHNE-6 | 1 [1–2] | 3 [3–4] | 5 [4–6] |
| DAPHNE-40 | 2 [1–4] | 8 [5–14] | 16 [10–20] |
AD, Alzheimer’s disease; fAD, frontal Alzheimer’s disease; FTD, behavioral variant frontotemporal dementia. Data are expressed as median [IQR].
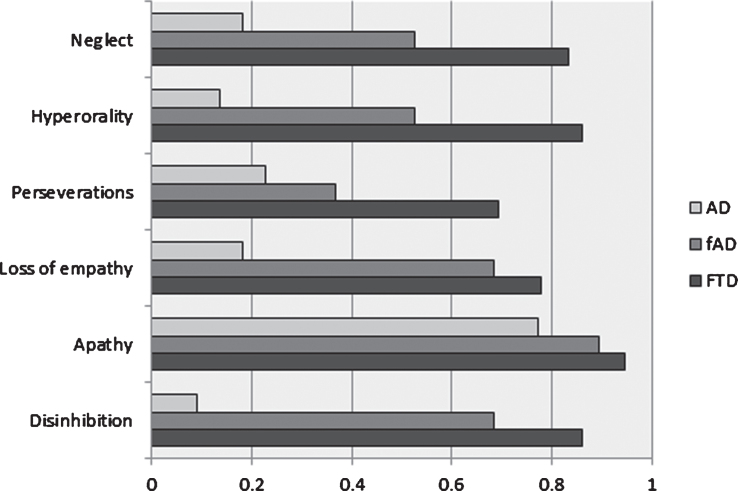
Concerning each out of the six domains of DAPHNE-6 (Fig. 1), whatever the domain, the score was higher for fAD patients than for AD patients and higher again for bvFTD patients. These differences were significant between the three groups, except for apathy.
Fig.1.
Mean score for the 6 domains of DAPHNE-6 scale for the 3 groups. AD, Alzheimer’s disease; fAD, frontal Alzheimer’s disease; FTD, behavioral variant frontotemporal dementia. The x-axis represents a maximum value of 1 for each of the six domains of DAPHNE-6. For each domain, the score was significantly different between the three groups (Kruskal-Wallis; all p values < 10-3) except for apathy.
Since DAPHNE-40 is a more precise behavioral assessment, unsurprisingly, the 10 items of DAPHNE-40 revealed results close to those of DAPHNE-6. The score were statistically higher between the three groups, except for the items “apathy” and “sexual disinhibition”. Again, scores were higher for bvFTD patients (bvFTD >fAD>AD).
DAPHNE as a specific tool for clinical diagnosis of fAD versus bvFTD?
When we focused on the comparison between fAD and bvFTD of the six domains of DAPHNE-6 we showed that three disorders were discriminants to differentiate between the two groups of patients: hyperorality, neglect, and perseverations (for all p < 0.05). These symptoms were more frequent and more severe in bvFTD. The three main behavioral disorders of fAD were apathy, loss of empathy, and disinhibition.
The comparison of quantitative data for the 10 items of DAPHNE-40 between fAD and bvFTD showed a slight significant difference for spending (p = 0.04), but this difference was also clear for loss of initiative (p = 0.01), eating disorders (p = 0.007), bulimia, etc. (p = 0.01), and personal neglect (p = 0.013) (Fig. 2). Whatever the item, the mean score was always higher in the bvFTD group.
Fig.2.
Mean scores for the ten items of DAPHNE for fAD and bvFTD patients. fAD, frontal Alzheimer’s disease; FTD, behavioral variant frontotemporal dementia. The y-axis (theoretical maximum value of 4) was censored, considering the highest mean observed. * indicates a significant statistical difference with a p-value<0.05. ** indicates a significant statistical difference with a p-value<0.01. Cohen’s d for spending, loss of initiative, eating disorders, bulimia, and personal neglect are respectively 0.56, 0.67, 0.75, 0.87, and 0.91.
The ROC curves for DAPHNE-6 and DAPHNE-40 highlighted discriminatory values between the two groups (fAD and bvFTD patients). A cut-off on DAPHNE-6 of 4/6 allowed to distinguish fAD (score <4) from bvFTD (score ≥4), with a sensitivity of 92%. A cut-off on DAPHNE-40 of 16/40 allowed to distinguish fAD (score <16) from bvFTD (score ≥ 16), with a specificity of 85%. We obtained a high positive likelihood ratio at 6.1 with the score of DAPHNE ‘combined’. In contrast, in this cohort, Rascovsky’ criteria demonstrated a lower diagnostic performance. Fifteen fAD patients fulfilled Rascovsky’s criteria for possible bvFTD, having been assessed with three or more clinical criteria. These results are summarized in Table 3.
Table 3.
Diagnostic accuracy of DAPHNE and of the Rascovsky’s revised clinical criteria to differentiate FTD and fAD patients
| Threshold | Sensitivity | Specificity | Positive likelihood ratio | |
| Rascovsky’s clinical criteria | ≥3 | 100% | 25% | 1.3 |
| DAPHNE-6 | ≥4 | 92% | 55% | 2.0 |
| DAPHNE-40 | ≥16 | 47% | 85% | 3.1 |
| DAPHNE ‘combined’ | - | 92% | 85% | 6.1 |
fAD, frontal Alzheimer’s disease; FTD, behavioral variant frontotemporal dementia. The positive likelihood ratio is assumed to demonstrate the interest of a diagnostic tool when >5. Thus, the value > 5 is shown in bold.
The second part of the Results section explore the characteristics of the fAD patients in this cohort, in terms of neuropsychological evaluation, but also regarding caregiver burden, MRI atrophy and functional imaging.
Neuropsychological evaluation
The results are reported in Table 4. We observed that neuropsychological evaluation would not allow an easy distinction between the three groups. The Kruskal-Wallis test revealed a significant difference only for total recall on the FCSRT, recognition and intrusions in this FCSRT, speed of execution on the Zoo test and verbal fluency (P letter).
Table 4.
Neuropsychological assessment in the three groups of patients
| AD | fAD | FTD | P AD/fAD/FTD | ||
| FCSRT | Total recall /48 | 27 [22–32] | 38 [30–45] | 41 [33–46] | 0.005 |
| Free total recall /48 | 13 [4–17] | 14 [7–20] | 13 [8–20] | NS | |
| Recognition /16 | 14 [13–15] | 16 [15–16] | 15 [15–16] | 0.028 | |
| Intrusions | 4 [2–7] | 8 [3–10] | 2 [0–5] | 0.022 | |
| Delayed total recall | 10 [6–14] | 14 [10–15] | 15 [10–16] | NS | |
| Oral denomination | /80 | 77 [72–78] | 77 [76–78] | 77 [73–78] | NS |
| Verbal fluency 2’ | Animals | 14 [12–20] | 14 [11–17] | 12 [9–20] | NS |
| P letter | 13 [9–20] | 14 [10–17] | 8 [5–16] | 0.022 | |
| Rey figure | Copy | 31 [21–33] | 31 [21–34] | 31 [23–34] | NS |
| Memory | 7 [3–9] | 12 [6–15] | 8 [6–16] | NS | |
| FAB | /18 | 15 [12–16] | 13 [8–15] | 12 [10–16] | NS |
| TMT A | Time in sec | 69 [50–90] | 72 [46–119] | 70 [50–102] | NS |
| TMT B | Time in sec | 164 [97–262] | 180 [133–248] | 164 [107–300] | NS |
| Wisconsin Card Sorting Test | Criteria /6 | 2 [1–5] | 3 [2–4] | 2 [2–5] | NS |
| Errors | 12 [9–17] | 16 [13–19] | 15 [11–22] | NS | |
| Perseverations | 5 [2–8] | 8 [5–9] | 7 [4–12] | NS | |
| Zoo test | Latency time | 129 [101–302] | 50 [29–83] | 39 [7–90] | 0.001 |
| Execution time | 163 [98–253] | 209 [149–315] | 97 [79–152] | 0.006 | |
| “Faux pas” test | Detection /10 | 9 [7–10] | 10 [6–10] | 9 [7–10] | NS |
| Explanation /100 | 66 [51–74] | 70 [30–80] | 45 [29–90] | NS | |
| No intentionality /100 | 25 [10–39] | 50 [25–70] | 30 [11–50] | NS | |
| Attrition score /100 | 40 [30–75] | 38 [20–53] | 30 [0–58] | NS | |
| Global score /60 | 35 [29–45] | 46 [28–50] | 30 [19–39] | NS | |
| Reading mind in the eyes | Global score /36 | 20 [14–22] | 19 [17–22] | 17 [14–21] | NS |
| Ekman faces | Global score /35 | 27 [24–28] | 24 [22–28] | 21 [16–26] | 0.017 |
| Happiness/5 | 5 [5–5] | 5 [5–5] | 5 [5–5] | NS | |
| Surprise /5 | 5 [4–5] | 4 [3–4] | 3 [2–4] | 0.003 | |
| Fear /5 | 1 [1–2] | 1 [1–3] | 1 [1–3] | NS | |
| Disgust /5 | 4 [3–5] | 4 [3–5] | 3 [2–4] | 0.057 | |
| Sadness /5 | 4 [3–5] | 4 [2–4] | 3 [2–4] | 0.052 | |
| Anger /5 | 3 [3–4] | 3 [2–4] | 3 [2–4] | NS | |
| Neutral /5 | 5 [4–5] | 5 [4–5] | 3 [2–5] | 0.056 |
AD, Alzheimer’s disease; fAD, frontal Alzheimer’s disease; FTD, behavioral variant frontotemporal dementia; FCSRT, Free and Cued Selective Reminding Test; FAB, Frontal assessment battery; NS, not significant. The three groups were compared using a Kruskal-Wallis test. Data are expressed as median [IQR]. p-values < 0.06 are detailed. p-values < 0.05 were considered significant (bold).
For social cognition, we found statistical differences for Ekman faces recognition for the global score and for “surprise” items.
When we compared fAD and bvFTD (Dunn’s post-hoc multiple comparisons test), we observed significant differences for intrusions in FCSRT (p < 0.05), and execution time of Zoo test (p < 0.01). Always considering fAD versus bvFTD patients, there was a trend for a better verbal fluency for letter P and a trend for a higher score for the recognition of “disgust” on the Ekman test for the fAD patients but these differences did not reach significance.
Evaluation of caregiver burden
Caregiver burden was studied using the Zarit Burden Inventory. The median score [IQR] was 20 [7–31] for the AD group but higher for fAD or bvFTD caregivers, respectively 45 [32–54] and 52 [28–59]. Considering the three groups, these differences were highly significant (p < 10-3) but they did not significant when considering only the two groups fAD versus bvFTD.
MRI atrophy
All the fAD patients underwent an MRI, which in most cases (n = 18) showed global cerebral atrophy. MRI morphological imaging data were compared based on nominal qualitative variables by a neurologist and a radiologist. Comparing the three groups, there was no significant difference concerning global cerebral atrophy, or frontal atrophy considered separately. However, there were significant differences in temporal atrophy (p < 10-3).
When we compared pairs of groups, we found a significant difference between the FTD and AD groups. FTD patients presented more temporal atrophy (p < 10-3) and frontal atrophy (p < 10-3). We also found a significant difference between the fAD and AD groups with more frontal atrophy (p < 10-2) for fAD patients.
In contrast, there were no significant differences between the fAD and bvFTD groups.
Functional imaging
Twelve fAD patients (63.2%) had a SPECT or an 18FDG PET-CT scan and one patient had an amyloid PET-CT scan. SPECT or 18FDG PET-CT scans were analyzed for the eight fAD patients at Nantes; six had SPECT scans and two had 18FDG PET-CT scans. Four out of 8 fAD patients had moderate parietal reduced uptake; bilaterally (n = 1) or predominant on the left (n = 3). We observed frontal mesial reduced uptake in all fAD patients (8/8 patients, severe for 3 and moderate for 5), while mesial reduced uptake was present in 58.9% of AD patients (10/17 patients, severe for 2, moderate for 2, mild for 6) and in 77.3% of bvFTD patients (17/22 patients, severe for 5, moderate for 8, mild for 4). There was reduced regional uptake in frontal superior regions in 7/8 fAD patients (severe for 2 on the left, moderate for 5 predominantly on the left) and in dorsolateral region in 6/8 fAD patients (severe on the left for one, moderate for 5 with more intensity on the left). Five of the 8 fAD patients had mild or normal orbitofrontal uptake. There was no significant differences in regional uptake between fAD patients and bvFDT or AD groups, but we noted however a trend towards a more severe frontal mesial reduced uptake in fAD patients than AD patients and a trend towards a more preserved uptake in temporal anterior regions in fAD patients when compared to bvFTD patients.
DISCUSSION
The results of this study support the usefulness of the DAPHNE scale for the clinical differential diagnosis between fAD and bvFTD. We demonstrated that DAPHNE has good sensitivity and good specificity to discriminate between these diseases. History-taking with caregivers is therefore crucial to support the diagnosis and it appears necessary to use behavioral scales in order to proceed to an exhaustive and precise evaluation of these disorders.
Behavior
DAPHNE allowed us to distinguish these populations from each other, as demonstrated by a high positive likelihood ratio. In our study, bvFTD patients obviously fulfilled Rascovsky criteria but most of the fAD patients did so too (15/20). This again shows the low specificity of the diagnostic criteria and the need for more specific diagnostic tools. Furthermore, DAPHNE enables behavioral symptoms to be quantified.
The three main behavioral disorders of fAD in our study were apathy, loss of empathy, and disinhibition. These results are in line with those obtained in the cohort of Ossenkoppele et al. [8]. The presence of apathy did not differentiate between the groups, as this symptom is not specific.
On the other hand, we showed that three disorders were discriminants to differentiate patients, namely hyperorality, neglect, and perseverations, as these were more frequent and more severe in bvFTD. Indeed, in our study more than 80% of bvFTD patients presented early personal neglect. This symptom, not referenced in Rascovsky’s criteria, is very frequent in bvFTD [27], and is detected by the DAPHNE scale. The FBI and the FBS also include this symptom. Our results highlight that this symptom is relevant for the differential diagnosis of FTD versus fAD, as already demonstrated in comparison with amnestic AD, bipolar disorder, or progressive supranuclear palsy patients [14].
It is now well-known that caregiver burden is strongly linked to behavior and is heavier in relatives of FTD patients than in relatives of typical AD patients [28]. Our findings confirm the link between heavy caregiver burden and patients’ behavior.
Neuropsychological assessment
We observed that neuropsychological assessment would not enable fAD and bvFTD patients to be readily differentiated. The core discriminatory result for executive functions was the Zoo Test latency time, which was very low in bvFTD and fAD patients. bvFTD and fAD patients rushed to begin the test without adequate reflection, a tendency linked to behavioral disturbances and demonstrating impulsivity in both groups, in comparison with patients with typical AD.
Concerning memory, it was recently established that memory testing has limited value in differentiating AD from FTD [29]. Our bvFTD and fAD groups both had a similar memory deficit, which was less pronounced than amnestic syndrome of the AD type. The two frontal groups had a better total score on the FCSRT. The criterion of relative preservation of memory, proposed by Rascovsky et al. for distinguishing FTD, needs to be considered with caution.
Our study confirms that traditional neuropsychological tests have a low diagnostic accuracy in differentiating between behavioral diseases. In the literature, the ‘theory of mind’ was assumed to be more relevant [30, 31]. When we compared bvFTD and fAD patients, the only difference we found on the Ekman faces recognition test was for the ‘disgust’ item. Neither the Faux pas test nor Reading mind in the eyes was contributive. Thus, social cognition does not appear to be very discriminant.
Imagery
The analysis of functional imaging showed frontotemporal abnormalities and especially medial prefrontal cortex hypoperfusion in fAD patients, while FTD patients have a main involvement of the orbitofrontal cortex with more diffuse hypoperfusion. Medial prefrontal cortex could be linked to apathy and emotional blunting, whereas the orbitofrontal cortex could be more linked to disinhibition, personal neglect, or perseveration.
Thus, this could explain a specific pattern of behavioral disorders. Imaging was performed as part of clinical routine, so these results are very preliminary.
In summary, we obtained three distinct groups, with a progression of intensity of behavior disorders: typical AD with few behavioral disorders, fAD with moderate behavioral disorders, and bvFTD with severe behavioral disorders.
This demonstrates that although behavioral disorders are a core characteristic of fAD patients, the pattern of behavioral disorders is different from that of bvFTD patients and could be linked to the cortical regions involved.
The question that arises is the origin of a frontal presentation affecting the mesial prefrontal cortex at an early stage. What factors may influence this? Are there events that affect the frontal cognitive reserve? One could hypothesize that a history of head trauma will favor a frontal presentation and it is found in 10% to 20% of the cases of fAD [32]. Likewise, the premorbid personality, such as a very sthenic underlying personality, could influence the clinical presentation of AD at the beginning stage.
Other authors have advanced the hypothesis of genetics with some case studies highlighting presenilin mutation [33]. APOE is also more often E4 type in patients with fAD [8]. With regard to early autosomal genetic mutations, given the later age of onset of AD, this criterion cannot of course explain all the cases.
The strength of this study is the prospective methodology. Clinical data were collected after obtaining the patients’ consent and with a standardized method. It will be very interesting to conduct a longitudinal study to acquire a better knowledge of the course of the disease in patients with a frontal presentation of AD. Do the behavioral disorders give way to a more typical profile of AD when cognitive disorders increase?
The limitations are related to the small size of our groups of patients. We focused our study on the most frequent form of frontal AD, the behavioral variant, and therefore excluded the isolated dysexecutive variant. These differences could possibly be explained by the fact that in our study, 75% of fAD patients fulfilled Rascovsky’s criteria for bvFTD. The low prevalence of fAD compelled us to include patients at five different sites. The patients with typical AD were relatively young as they were age matched to patients with behavioral AD or FTD. This is because patients with fAD are generally younger than patients with the amnestic variant. Consequently, they may differ in profile from late onset AD patients. Furthermore, our diagnoses were not confirmed by autopsy. However, we used CSF amyloid and tau biomarkers to support the diagnosis of AD pathology.
There are currently no consensus clinical criteria for fAD. By analogy with criteria of bvFTD [15], they could be defined as: 1) diagnosis of possible fAD based on behavior and cognitive features after use clinical diagnostic tools (behavioral scales, neuropsychological testing), 2) diagnosis of probable fAD based on imaging findings and also biological biomarkers. It seems therefore relevant to propose if available, CSF biomarker analysis or amyloid PET to confirm AD as the causative etiology in patients with a behavioral clinical presentation, 3) diagnosis of definite fAD based on the presence of a known pathogenic mutation or histopathological evidence of AD (on biopsy or autopsy).
With this aim in mind, behavioral inventory appears to be a very good criterion to help in the differential clinical diagnosis of fAD and bvFTD, compared to executive assessment or social cognition for possible fAD. Definition of the behavioral clinical syndrome is important for routine screening, as well as for optimal management of patients and their families. DAPHNE, with a scoring system on a 5-point scale, has the advantage of swiftness and efficiency.
ACKNOWLEDGMENTS
This work is the end-of-study dissertation of Elsa Lehingue, Julien Gueniat and Sandra Jourdaa for the DIU MA2 course. The DIU MA2 course (Diplôme InterUniversitaire de diagnostic et de prise en charge des Maladies d’Alzheimer et Apparentées) is the French transdisciplinary course on diagnosis and care of Alzheimer’s Disease and related disorders (www.diu-ma2.fr). The course is supported by the Fondation Alzheimer and the Fondation Vaincre Alzheimer.
The first study on DAPHNE (DAPHNE 1) was supported by the Association France Alzheimer.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1088r2).
REFERENCES
- [1]. Mesulam M-M (1982) Slowly progressive aphasia without generalized dementia. Ann Neurol 11, 592–598. [DOI] [PubMed] [Google Scholar]
- [2]. Benson DF, Davis RJ, Snyder BD (1988) Posterior cortical atrophy. Arch Neurol 45, 789–793. [DOI] [PubMed] [Google Scholar]
- [3]. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Johnson JK, Head E, Kim R, Starr A, Cotman CW (1999) Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol 56, 1233–1239. [DOI] [PubMed] [Google Scholar]
- [5]. Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ (2014) Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis 39, 63–70. [DOI] [PubMed] [Google Scholar]
- [6]. Li P, Zhou Y-Y, Lu D, Wang Y, Zhang H-H (2016) Correlated patterns of neuropsychological and behavioral symptoms in frontal variant of Alzheimer disease and behavioral variant frontotemporal dementia: A comparative case study. Neurol Sci 37, 797–803. [DOI] [PubMed] [Google Scholar]
- [7]. Dickerson BC, Wolk DA, Alzheimer’s Disease Neuroimaging Initiative (2011) Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry 82, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Ossenkoppele R, Pijnenburg YAL, Perry DC, Cohn-Sheehy BI, Scheltens NME, Vogel JW, Kramer JH, van der Vlies AE, La Joie R, Rosen HJ, van der Flier WM, Grinberg LT, Rozemuller AJ, Huang EJ, van Berckel BNM, Miller BL, Barkhof F, Jagust WJ, Scheltens P, Seeley WW, Rabinovici GD (2015) The behavioural/dysexecutive variant of Alzheimer’s disease: Clinical, neuroimaging and pathological features. Brain 138, 2732–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Woodward MC, Rowe CC, Jones G, Villemagne VL, Varos TA (2015) Differentiating the frontal presentation of Alzheimer’s disease with FDG-PET. J Alzheimers Dis 44, 233–242. [DOI] [PubMed] [Google Scholar]
- [10]. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert M-O, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [11]. Larner AJ (2006) “Frontal variant Alzheimer’s disease”: A reappraisal. Clin Neurol Neurosurg 108, 705–708. [DOI] [PubMed] [Google Scholar]
- [12]. Kertesz A, Davidson W, Fox H (1997) Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 24, 29–36. [DOI] [PubMed] [Google Scholar]
- [13]. Lebert F, Pasquier F, Souliez L, Petit H (1998) Frontotemporal behavioral scale. Alzheimer Dis Assoc Disord 12, 335–339. [DOI] [PubMed] [Google Scholar]
- [14]. Boutoleau-Bretonnière C, Evrard C, Hardouin JB, Rocher L, Charriau T, Etcharry-Bouyx F, Auriacombe S, Richard-Mornas A, Lebert F, Pasquier F, Sauvaget A, Bulteau S, Vercelletto M, Derkinderen P, Bretonnière C, Thomas-Antérion C (2015) DAPHNE: A new tool for the assessment of the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord Extra 5, 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini M-L, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Grober E, Buschke H, Crystal H, Bang S, Dresner R (1988) Screening for dementia by memory testing. Neurology 38, 900–903. [DOI] [PubMed] [Google Scholar]
- [17]. Osterrieth PA (1944) Le test de copie d’une figure complexe: Contribution à l’étude de la perception et de la mémoire. Arch Psychol 30, 286–356. [Google Scholar]
- [18]. Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: A Frontal Assessment Battery at bedside. Neurology 55, 1621–1626. [DOI] [PubMed] [Google Scholar]
- [19]. Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y (1990) [Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level]. Acta Neurol Belg 90, 207–217. [PubMed] [Google Scholar]
- [20]. Allain P, Roy A Allain, P., Roy, A., et al. (2004) Fonctions exécutives et traumatisme crânien sévère: évaluation à l’aide de ‘Behavioural Assessment of the Dysexecutive Syndrome’. Rev Neuropsychol 14, 285–323. [Google Scholar]
- [21]. Nelson HE (1976) A modified card sorting test sensitive to frontal lobe defects. Cortex 12, 313–324. [DOI] [PubMed] [Google Scholar]
- [22]. Deloche G, Hannequin D (1997) DO 80 Epreuve de dénomination orale d’images. Paris. [Google Scholar]
- [23]. Stone VE, Baron-Cohen S, Knight RT (1998) Frontal lobe contributions to theory of mind. J Cogn Neurosci 10, 640–656. [DOI] [PubMed] [Google Scholar]
- [24]. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I (2001) The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 42, 241–251. [PubMed] [Google Scholar]
- [25]. Ekman P, Friesen WV (1976) Pictures of facial affect. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- [26]. Zarit SH, Reever KE (1980) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20, 649–655. [DOI] [PubMed] [Google Scholar]
- [27]. Lebert F (2005) Diogene syndrome, a clinical presentation of fronto-temporal dementia or not? Int J Geriatr Psychiatry 20, 1203–1204. [DOI] [PubMed] [Google Scholar]
- [28]. Boutoleau-Bretonnière C, Vercelletto M, Volteau C, Renou P, Lamy E (2008) Zarit burden inventory and activities of daily living in the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord 25, 272–277. [DOI] [PubMed] [Google Scholar]
- [29]. Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR (2010) How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology 74, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, Hodges JR (2002) Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain 125, 752–764. [DOI] [PubMed] [Google Scholar]
- [31]. Funkiewiez A, Bertoux M, de Souza LC, Lévy R, Dubois B (2012) The SEA (Social cognition and Emotional Assessment): A clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology 26, 81–90. [DOI] [PubMed] [Google Scholar]
- [32]. Corrigan JD, Selassie AW, Orman JAL (2010) The epidemiology of traumatic brain injury. J Head Trauma Rehabil 25, 72–80. [DOI] [PubMed] [Google Scholar]
- [33]. Larner AJ (2013) Presenilin-1 mutations in Alzheimer’s disease: An update on genotype-phenotype relationships. J Alzheimers Dis 37, 653–659. [DOI] [PubMed] [Google Scholar]