Dear Editor,
We read with great interest the paper by Sotirchos et al. (2020)1 in the June issue of Annals of Neurology describing retinal atrophy in multiple sclerosis (MS). Although we found their results generally consistent with data from our deeply-phenotyped observational cohort (UCSF EPIC),2 their described pattern of inner nuclear layer (INL) change differs significantly from our own findings. Sotirchos reported that increasing age is associated with increasingly rapid INL thinning, a finding most prominent in progressive MS patients. This suggests that at every age patients are losing INL thickness, which therefore prompts their interpretation of INL as reflecting neurodegeneration from the earliest stage of disease. This does not comport with our observations. We performed the same analysis in 517 patients, 466 RMS (97 CIS, 369 RR), and 51 PMS (30 PP, 21 SP), excluding eyes with a history of optic neuritis, to similarly assess for interactions3 between age at first visit and disease course on INL thickness changes using a multilevel linear model. Our RMS patients (70% female) had a low EDSS score [median (inter-quartile range):1.5(1)], age [mean ± SD:44.4±10.8)], disease duration [median(IQR) 9(12) years] and follow-up time [median (IQR) 4.34 [3.1] years], while progressive MS patients (57% females) had higher EDSS [median (IQR): 4(2.5)], age [mean±SD:54.2±9.8], disease duration [median(IQR) 11(19) years] and similar follow up time [median(IQR) 4.1(2.6)] at baseline.
For both our younger relapsing and progressive subjects, we observed evidence of prominent INL thickening during the first phase of the disease – a phenomenon which evolves to the loss described by Sotirchos only at older ages(fig 1). Earlier reports noted cystic changes and increased volume in the INL,4,5 and we propose an integrated model that synthesizes all available data including the observation that swelling might be most prominent during early disease when inflammation is most active. Later in the disease course the rate of thickening declines until such a time that atrophy begins to supersede the swelling. This means that rather than solely reflecting the progressive degenerative nature of MS, INL may evidence early inflammation followed by later neurodegeneration. In contrast to Sotirchos et al.(fig 1.b), our data may capture the early thickening of INL due to the characteristics of our cohort, including 97 MS patients in the few years after an initial demyelinating event. This means that taken together our data may help expand our understanding of what is happening in the INL across the entire disease course.
Figure 1.
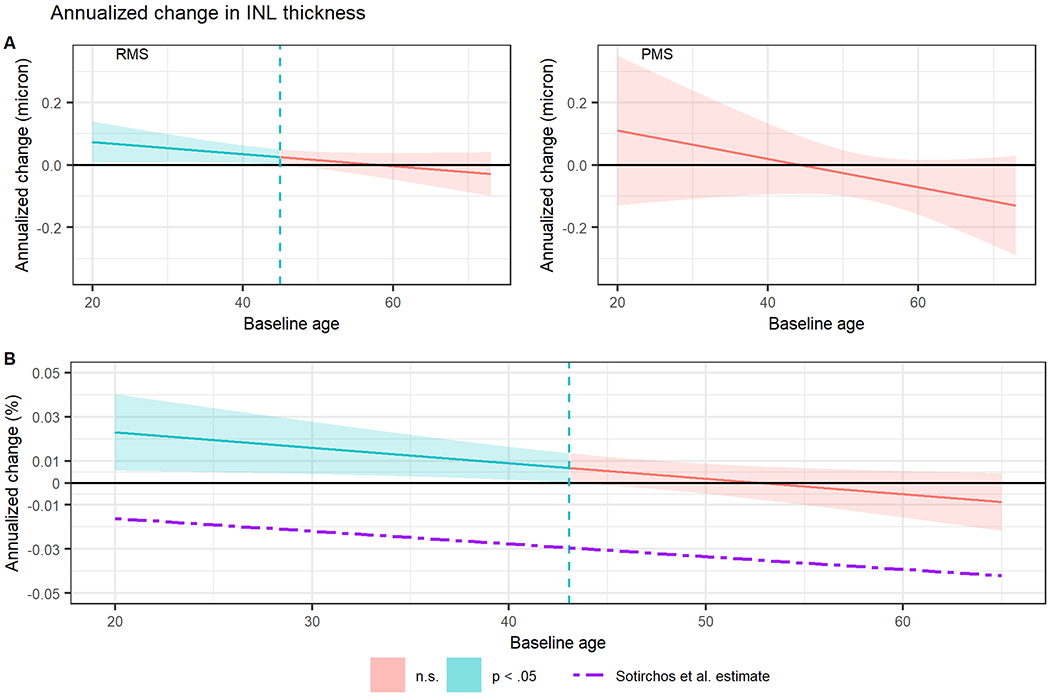
Simple slopes analysis showing interaction between age at first visit and change in inner nuclear layer (INL) thickness over time in (A) patients with remitting multiple sclerosis (RMS) and progressive multiple sclerosis (PMS) and in (B) the whole cohort, comparing our results to the Sotirchos et al model. Shaded regions represent 95% confidence intervals for annualized change in INL thickness and the dashed vertical lines represent upper bounds of baseline age where the annualized change is statistically significant (Johnson–Neyman interval). n.s., not significant.
Footnotes
Potential Conflicts of Interest:
Nothing to report.
References
- 1.Sotirchos ES, Gonzalez Caldito N, Filippatou A, et al. Progressive Multiple Sclerosis Is Associated with Faster and Specific Retinal Layer Atrophy. Ann Neurol. 2020;87(6):885–896. doi: 10.1002/ana.25738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.University of California, San Francisco MS-EPIC Team, Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–666. doi: 10.1002/ana.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long JA (2019). interactions: Comprehensive, User-Friendly Toolkit for Probing Interactions. R package version 1.1.0, https://cran.r-project.org/package=interactions. [Google Scholar]
- 4.Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135(Pt 6):1786–1793. doi: 10.1093/brain/aws098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 2012;11:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]


