Abstract
Background
Although several COVID-19 vaccines have been developed so far, they will not be sufficient to meet the global demand. Development of a wider range of vaccines, with different mechanisms of action, could help control the spread of SARS-CoV-2 globally. We developed a protein subunit vaccine against COVID-19 using a dimeric form of the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein as the antigen. We aimed to assess the safety and immunogenicity of this vaccine, ZF2001, and determine the appropriate dose and schedule for an efficacy study.
Methods
We did two randomised, double-blind, placebo-controlled, phase 1 and phase 2 trials. Phase 1 was done at two university hospitals in Chongqing and Beijing, China, and phase 2 was done at the Hunan Provincial Center for Disease Control and Prevention in Xiangtan, China. Healthy adults aged 18–59 years, without a history of SARS-CoV or SARS-CoV-2 infection, an RT-PCR-positive test result for SARS-CoV-2, a history of contact with confirmed or suspected COVID-19 cases, and severe allergies to any component of the vaccine were eligible for enrolment. In phase 1, participants were randomly assigned (2:2:1) to receive three doses of the vaccine (25 μg or 50 μg) or placebo intramuscularly, 30 days apart. In phase 2, participants were randomly assigned (1:1:1:1:1:1) to receive the vaccine (25 μg or 50 μg) or placebo intramuscularly, 30 days apart, in either a two-dose schedule or a three-dose schedule. Investigators, participants, and the laboratory team were masked to group allocation. For phase 1, the primary outcome was safety, measured by the occurrence of adverse events and serious adverse events. For phase 2, the primary outcome was safety and immunogenicity (the seroconversion rate and the magnitude, in geometric mean titres [GMTs], of SARS-CoV-2-neutralising antibodies). Analyses were done on an intention-to-treat and per-protocol basis. These trials are registered with ClinicalTrials.gov (NCT04445194 and NCT04466085) and participant follow-up is ongoing.
Findings
Between June 22 and July 3, 2020, 50 participants were enrolled into the phase 1 trial and randomly assigned to receive three doses of placebo (n=10), the 25 μg vaccine (n=20), or the 50 μg vaccine (n=20). The mean age of participants was 32·6 (SD 9·4) years. Between July 12 and July 17, 2020, 900 participants were enrolled into the phase 2 trial and randomly assigned to receive two doses of placebo (n=150), 25 μg vaccine (n=150), or 50 μg vaccine (n=150), or three doses of placebo (n=150), 25 μg vaccine (n=150), or 50 μg vaccine (n=150). The mean age of participants was 43·5 (SD 9·2) years. In both phase 1 and phase 2, adverse events reported within 30 days after vaccination were mild or moderate (grade 1 or 2) in most cases (phase 1: six [60%] of ten participants in the placebo group, 14 [70%] of 20 in the 25 μg group, and 18 [90%] of 20 in the 50 μg group; phase 2: 37 [25%] of 150 in the two-dose placebo group, 43 [29%] of 150 in the two-dose 25 μg group, 50 [33%] of 150 in the two-dose 50 μg group, 47 [31%] of 150 in the three-dose placebo group, 72 [48%] of 150 in the three-dose 25 μg group, and 65 [43%] of 150 in the three-dose 50 μg group). In phase 1, two (10%) grade 3 or worse adverse events were reported in the 50 μg group. In phase 2, grade 3 or worse adverse events were reported by 18 participants (four [3%] in the two-dose 25 μg vaccine group, two [1%] in the two-dose 50 μg vaccine group, two [1%] in the three-dose placebo group, four [3%] in the three-dose 25 μg vaccine group, and six [4%] in the three-dose 50 μg vaccine group), and 11 were considered vaccine related (two [1%] in the two-dose 25 μg vaccine group, one [1%] in the two-dose 50 μg vaccine group, one [1%] in the three-dose placebo group, two [1%] in the three-dose 25 μg vaccine group, and five [3%] in the three-dose 50 μg vaccine group); seven participants reported serious adverse events (one [1%] in the two-dose 25 μg vaccine group, one [1%] in the two-dose 50 μg vaccine group, two [1%] in the three-dose placebo group, one [1%] in the three-dose 25 μg vaccine group, and two [1%] in the three-dose 50 μg vaccine group), but none was considered vaccine related. In phase 2, on the two-dose schedule, seroconversion rates of neutralising antibodies 14 days after the second dose were 76% (114 of 150 participants) in the 25 μg group and 72% (108 of 150) in the 50 μg group; on the three-dose schedule, seroconversion rates of neutralising antibodies 14 days after the third dose were 97% (143 of 148 participants) in the 25 μg group and 93% (138 of 148) in the 50 μg group. In the two-dose groups in phase 2, the SARS-CoV-2-neutralising GMTs 14 days after the second dose were 17·7 (95% CI 13·6–23·1) in the 25 μg group and 14·1 (10·8–18·3) in the 50 μg group. In the three-dose groups in phase 2, the SARS-CoV-2-neutralising GMTs 14 days after the third dose were 102·5 (95% CI 81·8–128·5) in the 25 μg group and 69·1 (53·0–90·0) in the 50 μg group.
Interpretation
The protein subunit vaccine ZF2001 appears to be well tolerated and immunogenic. The safety and immunogenicity data from the phase 1 and 2 trials support the use of the 25 μg dose in a three-dose schedule in an ongoing phase 3 trial for large-scale evaluation of ZF2001's safety and efficacy.
Funding
National Program on Key Research Project of China, National Science and Technology Major Projects of Drug Discovery, Strategic Priority Research Program of the Chinese Academy of Sciences, and Anhui Zhifei Longcom Biopharmaceutical.
Translation
For the Chinese translation of the abstract see Supplementary Materials section.
Introduction
The COVID-19 pandemic caused by SARS-CoV-2 is still ongoing worldwide.1, 2, 3 As of March 23, 2021, more than 123 million people have been diagnosed with COVID-19 in 223 countries and regions, with 2·7 million deaths worldwide, according to the Johns Hopkins Coronavirus Resource Center.
Safe and effective vaccines are urgently needed to control the spread of COVID-19 globally. After 1 year of global efforts, substantial progress has been made in the development of vaccines for COVID-19,4 and several vaccine candidates based on mRNA technology or a virus-vector platform have been developed and showed protective efficacy against SARS-CoV-2 in interim analyses (70·4% for AZD1222, 94·1% for mRNA-1273, 95% for BNT1622, and 91·6% for Sputnik V).5, 6, 7, 8 To date, nine vaccines have received conditional marketing authorisation: two mRNA vaccines (developed by BioNTech–Pfizer and Moderna) first approved in the USA; four adenovirus vaccines (developed by Oxford–AstraZeneca, Gamaleya, Janssen, and Cansino–Bejing Institute of Biotechnology) approved in the UK, Russia, the USA, and China; and three inactivated vaccines (developed by Sinopharm–China National Institute for Communicable Disease Control and Prevention, Sinopharm–Chinese Academy of Sciences, and Sinovac–China National Institute for Communicable Disease Control and Prevention) first approved in China. However, the vaccines developed so far cannot meet global vaccination requirements because of production and transport constraints in many remote areas. Additionally, the long-term efficacy of these vaccines and related safety concerns are under investigation.9 An ideal COVID-19 vaccine should have high protective efficacy and be safe and deployable for billions of potential vaccinees. To achieve this goal, an optimal vaccine target, platform, and dose regimen are important.4 Therefore, development of a range of COVID-19 vaccines with different mechanisms of action would be beneficial for diversifying the global vaccine pipeline. More than 80 COVID-19 vaccines in development, as documented by WHO, are based on the protein subunit platform, indicating that this technology is an effective platform for the development of COVID-19 vaccines. Two protein-subunit-based vaccine candidates targeting the SARS-CoV-2 spike (S) protein have entered phase 3 (NVX-CoV2373; Novavax) and phase 2–3 trials (SCB-2019; Clover), with their preliminary phase 1 or phase 1–2 results reported.10, 11 Both vaccines showed high immunogenicity in recipients.
Research in context.
Evidence before this study
We searched PubMed up to Feb 8, 2021, with the search terms “COVID-19”, “vaccine”, and “trial”. No language restrictions were applied. We identified 26 clinical trials for 15 COVID-19 vaccines, with five phase 3 studies and 21 studies described as phase 1 or phase 2, or both. These 15 vaccines include five inactivated vaccines, four adenovirus-vectored vaccines, three mRNA-based vaccines, two protein subunit vaccines, and one DNA vaccine. These vaccines mainly target either the whole virus or the spike protein. One vaccine targeting the receptor-binding domain (RBD) of SARS-CoV-2, BNT162b1, manufactured by BioNTech and Pfizer, comprises mRNA expressing the RBD trimer. Two studies of a protein subunit vaccine against COVID-19 have been published: NVX-CoV2373 (Novavax) and SCB-2019 (Clover). Both vaccines have used the full-length SARS-CoV-2 spike protein as the antigen, and are formulated with adjuvants (Matrix-M1 for NVX-CoV2373; AS03 and CpG/Alum for SCB2019). Various RBD-based protein subunit vaccines are under clinical and preclinical development, but no clinical studies have been published.
Added value of this study
ZF2001 is one of two protein-subunit-based COVID-19 vaccine candidates that has advanced into phase 3 clinical trials. This study is, to the best of our knowledge, the first to report phase 1 and phase 2 clinical data for an RBD-based protein subunit vaccine against COVID-19 comprising a conventional alum adjuvant. We evaluated the safety profile, immunogenicity, dose effect, and vaccination schedule for ZF2001. ZF2001 was found to be well tolerated, without causing any vaccine-related serious adverse events. Three immunisations at day 0, 30, and 60 achieved 93–100% seroconversion of neutralising antibodies, with the geometric mean titres exceeding the magnitude of convalescent serum samples obtained from patients admitted to hospital with RT-PCR-confirmed COVID-19. Additionally, the vaccine elicited moderate cellular immune responses, as demonstrated by the balanced production of cytokines associated with T-helper 1 and T-helper 2 cells.
Implications of all the available evidence
These findings indicate that the RBD-based protein subunit vaccine ZF2001 is safe and immunogenic. Phase 3 clinical trials are ongoing to further investigate its safety and protective efficacy.
In response to the COVID-19 pandemic, we launched a vaccine development programme against SARS-CoV-2. The resulting vaccine candidate, ZF2001, is a protein subunit vaccine that has advanced into phase 3 development (NCT04646590). Compared with other vaccine candidates in clinical trials targeting mainly the whole virus or the S protein, ZF2001 targets the receptor-binding domain (RBD) of the SARS-CoV-2 S protein. The RBD is responsible for engagement of its cellular receptor, angiotensin converting enzyme 2 (ACE2), and is an attractive vaccine target to induce immune responses focusing on blocking receptor binding.12, 13, 14 We rationally designed a dimeric form of the SARS-CoV-2 RBD with high yields (at levels of g/L) and with substantially enhanced immunogenicity compared with the conventional monomeric form of RBD in a mouse model.15 ZF2001 is made with an RBD-dimer protein produced in Chinese hamster ovary (CHO) cells adjuvanted with aluminium hydroxide.16
The phase 1 trial for ZF2001 began on June 22, 2020, in China, and the phase 2 trial began on July 12, 2020, in China. Here, we report the results of both trials, in which we aimed to assess vaccine safety and immunogenicity.
Methods
Study design and participants
We did two randomised, double-blind, placebo-controlled phase 1 and phase 2 trials. The phase 1 trial was done at two hospitals in China: The Second Affiliated Hospital of Chongqing Medical University (Chongqing) and the Beijing Chao-Yang Hospital of Capital Medical University (Beijing). The phase 2 trial was done at the Hunan Provincial Center for Disease Control and Prevention (Xiangtan, China). Eligible participants were healthy men and non-pregnant women aged 18–59 years. Health status, assessed during the screening period, was based on medical history and clinical laboratory findings, vital signs, and physical examination. Individuals with a history of SARS-CoV infection or COVID-19, who tested positive for SARS-CoV-2 exposure (by real-time PCR assay or ELISA), or who had contact with a confirmed or suspected COVID-19 case were excluded. Exclusion criteria also included a history of seizures or mental illness; allergy to any ingredient in the vaccine; acute febrile disease in the preceding 24 h before enrolment and gastrointestinal symptoms in the preceding 7 days before enrolment; congenital or acquired immune diseases; serious chronic disease; abnormal chest CT image (in the phase 1 trial); a positive test for hepatitis B virus, hepatitis C virus, HIV, or syphilis; a history of a tumour or cancer; receipt of any blood products in the past 3 months; receipt of any investigational medicines or vaccines in the past 3 months; and an inability to comply with the study schedule. Full details of the eligibility criteria are summarised in the trial protocols provided in appendix 2 (pp 26–238).
Participants were recruited through community recruitment advertisements. All participants provided written informed consent before enrolment. The trial protocol was approved by the institutional review board of The Second Affiliated Hospital of Chongqing Medical University, Beijing Chao-Yang Hospital of Capital Medical University, Hunan Provincial Center for Disease Control and Prevention, and the National Medical Products Administration of China, and was done in accordance with the Declaration of Helsinki and Good Clinical Practice. Safety oversight for specific vaccination pause rules and for advancement was done by an independent safety monitoring committee.
Randomisation and masking
Participants in phase 1 were randomly assigned (2:2:1) into three groups to receive three doses of the vaccine (25 μg or 50 μg dose) or placebo on day 0, 30, and 60. Participants in phase 2 were randomly assigned (1:1:1:1:1:1) into six groups, with three groups receiving two doses of the vaccine (25 μg or 50 μg dose) or placebo, 30 days apart, and three groups receiving three doses of the vaccine (25 μg or 50 μg dose) or placebo, 30 days apart.
The study statisticians used SAS statistical software (version 9.4) to generate a random table of participants in each dose group. These statisticians were not allowed to participate in other aspects of these clinical trials and were not allowed to disclose the masking code to any personnel participating in the clinical trials. Participants were randomly assigned to the vaccine group or the placebo group according to the block randomisation method, with a block of five and block size of five, in phase 1, and a block of 75 and block size of 12 in phase 2. Investigators at the trial site assigned random numbers according to the order of the screening sequence of eligible participants, and experimental vaccines were obtained and administered according to these random numbers. Trial participants, field investigators, and the laboratory team were masked to group allocation during the trial.
Procedures
The vaccine was jointly developed by the Institute of Microbiology, Chinese Academy of Sciences, and Anhui Zhifei Longcom Biopharmaceutical.15 The vaccine was manufactured according to good manufacturing practice guidelines by Anhui Zhifei Longcom Biopharmaceutical. The recombined vaccine encodes the SARS-CoV-2 RBD antigen (residues 319–537, accession number YP_009724390), with two copies in tandem repeat dimeric form, and was manufactured in the CHOZN CHO K1 cell line (Sigma-Aldrich Trading; Shanghai, China) as a liquid formulation containing 25 μg or 50 μg per 0·5 mL in a vial, with aluminium hydroxide as the adjuvant. The placebo contained only aluminium hydroxide in buffer. Vaccines were stored at 2–8°C before use. The vaccine or placebo was administrated intramuscularly in the arm of each participant.
For both trials, participants were observed in the observation room for 30–60 min after each dose of vaccination for emergent adverse events. During the first 7 days after each vaccination, any adverse events were self-reported by participants daily on the diary cards, and verified by investigator visits and confirmation. Adverse events occurring 8–30 days after each vaccination were reported by participants through contact cards. Any serious adverse events occurring within 1 year after the first dose of vaccination will be monitored, and follow-up is ongoing. Solicited local adverse events at the injection site within 7 days after vaccination included pain, swelling, induration, redness, rash, and pruritus; and solicited systemic adverse events within 7 days after vaccination included fever, cough, dyspnoea, diarrhoea, anorexia, nausea, vomiting, muscle pain (not at the injection site), arthritis, joint pain, headache, fatigue, acute allergic reaction, irritation, and mental disorders (including anxiety, depression, mania, and insanity). Laboratory safety tests including routine blood and serum chemistry and routine urine testing were done to assess any toxicity after vaccination. Adverse events and abnormal changes in laboratory tests were graded according to the latest scale issued by the National Medical Products Administration of China (2019 version).
Serum samples were collected to evaluate binding responses against the RBD antigen and neutralising activity against live SARS-CoV-2. Peripheral blood mononuclear cells (PBMCs) were collected to assess specific T-cell responses. Blood samples were taken from participants at the scheduled site visits before vaccination, and on day 14, 30, 37, 60, 67, and 90 after the first vaccination in phase 1, and on day 14, 30, 44, 60, 74, and 90 in phase 2. We used ELISA kits (Wantai BioPharm, Beijing, China) to evaluate binding antibody responses against RBD proteins according to the manufacturer's protocol. We also assessed neutralising activities against live SARS-CoV-2 by microcytopathogenic effect assays (appendix 2 p 23). PBMCs from whole blood were isolated and stored in liquid nitrogen in advance, then thawed before testing. An enzyme-linked immunospot (ELISpot) assay (BD Biosciences, San Diego, CA, USA) was used to quantify the specific T-cell responses according to a previously reported method (appendix 2 p 24). PBMCs were stimulated with overlapping peptide pools covering RBD protein for around 20–24 h (interferon-γ [IFNγ] and IL-2) and 40–48 h (IL-4 and IL-5) before detection, and the number of spot-forming cells per 300 000 cells was calculated. We measured the secretion of IFNγ, IL-2, IL-4, and IL-5 with BD ELISpot.
Convalescent serum samples (appendix 2 pp 20–21) were obtained by health-care workers from patients admitted to hospital (Beijing Ditan hospital, Capital Medical University, Beijing, China) with RT-PCR-confirmed SARS-CoV-2 infection between Jan 22 and Aug 1, 2020. Written consent from these patients was obtained by the hospital to use these samples for this study. The neutralising activities of these serum samples against live SARS-CoV-2 were measured by a microcytopathogenic effect assay.
Outcomes
For the phase 1 trial, the primary outcome was the safety of the COVID-19 vaccine. The secondary outcome was immunogenicity. For the phase 2 trial, primary outcomes were safety and immunogenicity.
Safety outcomes were the occurrence of adverse events between the first injection to 30 days after the final injection, including all adverse events, adverse events related to vaccination, adverse events at grade 3 and worse, and adverse events leading to withdrawal of participants. Serious adverse events will be monitored for up to 1 year. All adverse events and serious adverse events related to vaccination in all groups and dose regimens were analysed.
Outcomes for immunogenicity were the seroconversion rate and magnitude, in geometric mean titres (GMTs), of the RBD-binding antibody, SARS-CoV-2 neutralising antibody, and T-cell cytokine production. Seroconversion was defined as the highest reciprocal dilution of serum greater than 11 for binding antibodies, or higher than four for neutralising antibodies. Serum samples from the vaccine groups and convalescents were compared to assess the immunogenicity of the vaccine.
Statistical analysis
The safety analysis was done in all participants who received the first dose after enrolment. The immunogenicity analyses were done on both the intention-to-treat and per-protocol sets at various timepoints, as defined in the study protocol. The intention-to-treat population comprised all participants who completed randomisation and received at least one dose of vaccine or placebo, and had a valid pre-immunisation immunogenicity result. The per-protocol sets refer to participants who conformed to the inclusion and exclusion criteria and the trial protocol, who completed the course of vaccination and had valid immunogenicity results both before immunisation and the indicated days after vaccination as designated in the protocol. For phase 2 the sample size calculation was based on the assumption that the positive rate of the neutralising antibody would reach 80% in the vaccine group and 30% in the placebo group, and the test level would be a unilateral α=0·025. We calculated that, with a sample size of 900 participants in phase 2, the difference between the vaccine and placebo could be estimated with 99·99% confidence.
We first compiled statistics of the number and proportion of participants with adverse events or serious adverse events after vaccination and compared the difference among dose groups using Fisher's exact test. The RBD-binding antibodies and neutralising titres against live SARS-CoV-2 were calculated with seroconversion rates and GMTs. Cytokine production was calculated with GMTs. We used the Clopper-Pearson method to calculate 95% CIs, χ2 tests and the Fisher's exact probability method to compare differences in the proportion of adverse events among the dose groups, and Student's t test to analyse the significance of ELISpot GMTs between different timepoints and neutralising GMTs between groups. Statistical analyses were done with SAS, version 9.4, and GraphPad Prism, version 8.0.1.
These trials are registered with ClinicalTrials.gov (NCT04445194 and NCT04466085) and participant follow-up is ongoing.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
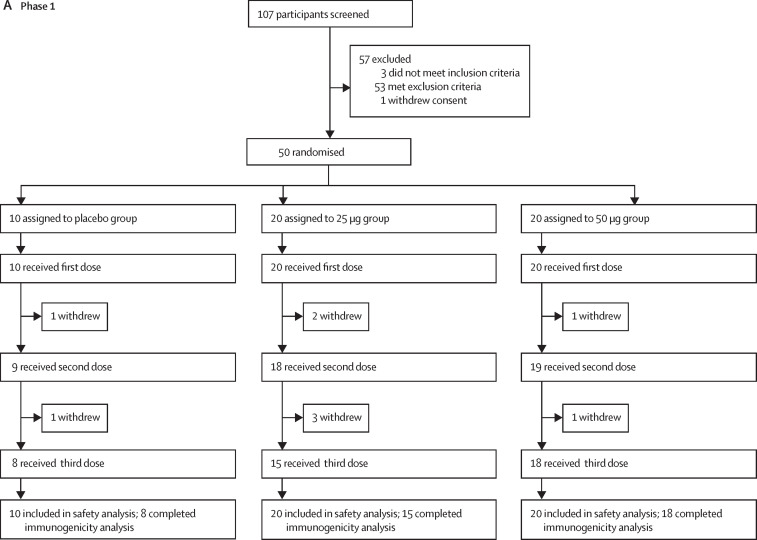
Between June 22 and July 3, 2020, 107 individuals were recruited and screened for phase 1 of the trial in Chongqing and Beijing, China (figure 1 ). After exclusion of 57 individuals, 50 eligible participants were randomly assigned into three groups to receive the 25 μg dose (n=20) or 50 μg dose (n=20) of the vaccine or placebo (n=10). The mean age of all participants was 32·6 years (SD 9·4; range 20·9–57·9), with balanced age distribution among vaccination groups (table 1 ). Eight of ten participants in the placebo group, 15 of 20 in the 25 μg group, and 18 of 20 in the 50 μg group completed the three-dose immunisation and follow-up visits as scheduled (figure 1; appendix 2 p 1).
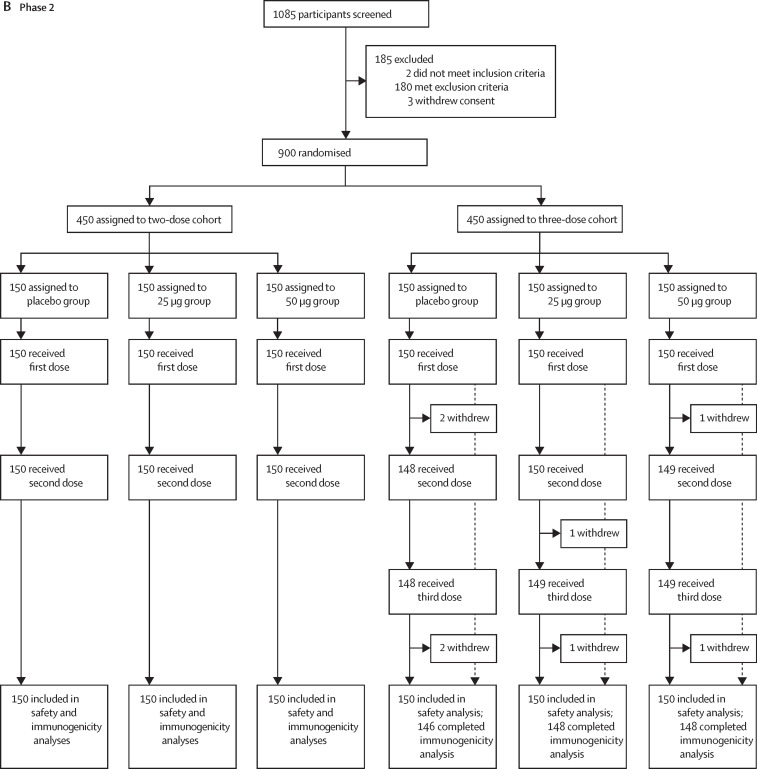
Figure 1.
Trial profiles
(A) Phase 1. (B) Phase 2. 17 participants (nine in phase 1, and eight in phase 2) were not included in the per-protocol cohort for safety and immunogenicity analysis; reasons for their withdrawal are listed in appendix 2 (p 1).
Table 1.
Baseline demographic characteristics of participants in phase 1 and phase 2 trials
|
Phase 1 trial (n=50) |
Phase 2 trial (n=900) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo, three-dose (n=10) | 25 μg, three-dose (n=20) | 50 μg, three-dose (n=20) | Placebo, two-dose (n=150) | 25 μg, two-dose (n=150) | 50 μg, two-dose (n=150) | Placebo, three-dose (n=150) | 25 μg, three-dose (n=150) | 50 μg, three-dose (n=150) | ||
| Age (years) | ||||||||||
| Mean (SD) | 32·5 (11·3) | 31·7 (9·7) | 33·6 (8·5) | 44·1 (9·3) | 43·04 (9·6) | 44·4 (8·4) | 43·4 (8·9) | 42·7 (9·4) | 43·2 (9·4) | |
| Median (IQR) | 29·4 (21·0–57·9) | 26·7 (22·9–54·7) | 34·1 (20·9–49·4) | 46·1 (20·7–59·4) | 43·7 (18·8–58·4) | 45·3 (19·9–59·1) | 44·4 (20·7–58·0) | 43·7 (20·0–59·7) | 43·7 (19·3–59·6) | |
| Ethnicity | ||||||||||
| Han Chinese | 9 (90%) | 20 (100%) | 18 (90%) | 149 (99%) | 150 (100%) | 150 (100%) | 149 (99%) | 150 (100%) | 148 (99%) | |
| Other | 1 (10%) | 0 | 2 (10%) | 1 (1%) | 0 | 0 | 1 (1%) | 0 | 2 (1%) | |
| Sex | ||||||||||
| Male | 5 (50%) | 14 (70%) | 11 (55%) | 68 (45%) | 65 (43%) | 57 (38%) | 74 (49%) | 71 (47%) | 63 (42%) | |
| Female | 5 (50%) | 6 (30%) | 9 (45%) | 82 (55%) | 85 (57%) | 93 (62%) | 76 (51%) | 79 (53%) | 87 (58%) | |
| Mean BMI kg/m2 (SD) | 22·5 (1·5) | 24·0 (2·8) | 22·6 (2·8) | NR | NR | NR | NR | NR | NR | |
Data are mean (SD), median (IQR), or n (%). BMI=body-mass index. NR=not recorded.
Between July 12 and July 17, 2020, 1085 individuals were recruited and screened for phase 2 of the trial in Xiangtan, China (figure 1). After exclusion of 185 individuals, 900 participants were enrolled into the trial and randomly assigned to six groups (n=150 per group) to receive the 25 μg or 50 μg dose of the vaccine or placebo, in either a two-dose or a three-dose schedule, 30 days apart. The mean age of participants was 43·5 years (SD 9·2; range 18·8–59·7), with balanced age and sex distribution among vaccination groups (table 1). All 450 participants on the two-dose schedule completed the full vaccination and follow-up visits. Among participants on the three-dose schedule, 146 (97%) of 150 in the placebo group, 148 (99%) of 150 in the 25 μg group, and 148 (99%) of 150 in the 50 μg group received the full vaccination and completed the follow-up visits (figure 1; appendix 2 p 1).
In the phase 1 trial, six (60%) of ten participants in the placebo group, 14 (70%) of 20 in the 25 μg group, and 18 (90%) of 20 in the 50 μg group reported at least one adverse event within 30 days after vaccination, with no significant between-group differences (table 2 ; appendix 2 pp 2–4). Within 7 days after vaccination, most of the local and systemic reactogenicity was mild or moderate (grade 1 or 2 adverse events). The most common solicited local adverse events were injection-site pain, redness, and itch (table 2). The most common solicited systemic adverse events were cough, fever, and headache (table 2). Two (10%) grade 3 or worse adverse events were reported in the 50 μg group. One was vaccine related (swelling and redness) and the other was a serious adverse event (rhabdomyolysis), but was assessed by the investigators as being unrelated to the vaccine (appendix 2 pp 14–19). No vaccine-related serious adverse events were reported.
Table 2.
Adverse events and reactions in the phase 1 trial
| Placebo (n=10) | 25 μg dose (n=20) | 50 μg dose (n=20) | p value | |
|---|---|---|---|---|
| Overall adverse events within 30 days | ||||
| Any | 6 (60%) | 14 (70%) | 18 (90%) | 0·1786 |
| Grade ≥3 | 0 | 0 | 2 (10%) | 0·3469 |
| Solicited adverse events within 7 days | ||||
| Any | 3 (30%) | 12 (60%) | 14 (70%) | 0·1286 |
| Grade ≥3 | 0 | 0 | 1 (5%) | 1·0000 |
| Solicited systemic adverse reactions | ||||
| Any | 1 (10%) | 4 (20%) | 5 (25%) | 0·7423 |
| Fever | 0 | 2 (10%) | 0 | 0·3469 |
| Headache | 0 | 1 (5%) | 1 (5%) | 1·0000 |
| Fatigue | 0 | 0 | 1 (5%) | 1·0000 |
| Weakness | 0 | 0 | 1 (5%) | 1·0000 |
| Cough | 0 | 1 (5%) | 3 (15%) | 0·5123 |
| Impaired appetite | 1 (10%) | 0 | 0 | 0·2000 |
| Nausea | 0 | 0 | 2 (10%) | 0·3469 |
| Muscle pain | 0 | 1 (5%) | 0 | 1·0000 |
| Solicited local adverse reactions | ||||
| Any | 2 (20%) | 9 (45%) | 12 (60%) | 0·1298 |
| Grade ≥3 | 0 | 0 | 1 (5%) | 1·0000 |
| Injection-site pain | 2 (20%) | 4 (20%) | 11 (55%) | 0·0527 |
| Swelling | 0 | 1 (5%) | 3 (15%) | 0·5132 |
| Grade ≥3 swelling | 0 | 0 | 1 (5%) | 1·0000 |
| Induration | 0 | 2 (10%) | 5 (25%) | 0·187 |
| Redness | 1 (10%) | 4 (20%) | 4 (20%) | 0·8016 |
| Grade ≥3 redness | 0 | 0 | 1 (5%) | 1·0000 |
| Rash | 0 | 0 | 1 (5%) | 1·0000 |
| Itch | 0 | 4 (20%) | 7 (35%) | 0·1012 |
| Unsolicited adverse reactions | ||||
| Any | 4 (40%) | 6 (30%) | 6 (30%) | 0·8535 |
Data are n (%). p values are calculated with Fisher's exact test.
In the phase 2 trial, the overall frequency of adverse events was low within 30 days after vaccination. Among participants receiving two doses, 37 (25%) of 150 in the placebo group, 43 (29%) of 150 in the 25 μg group, and 50 (33%) of 150 in the 50 μg group reported at least one adverse event. Among participants receiving three doses, 47 (31%) of 150 in the placebo group, 72 (48%) of 150 in the 25 μg group, and 65 (43%) of 150 in the 50 μg group reported at least one adverse event (table 3 ; appendix 2 pp 3, 5). Within 7 days after each vaccination, most of the local and systemic reactogenicity was mild or moderate (grade 1 or 2 adverse events). The most common solicited local adverse events in participants on the two-dose and three-dose schedules were injection-site pain, swelling, induration, redness, and itch (table 3). The most common solicited systemic adverse events in participants on the two-dose and three-dose schedules were fever, cough, headaches, and fatigue (table 3). 18 participants reported grade 3 or worse adverse events, and 11 (61%) of these were vaccine related: redness (six participants: four in the three-dose 50 μg group, one in the three-dose 25 μg group, and one in the two-dose 50 μg group), swelling (three participants in the three-dose 50 μg group), injection-site pain (one participant in the three-dose 25 μg group), induration (one participant in the three-dose 50 μg group), rash (one participant in the three-dose 50 μg group), fever (one participant in the two-dose 25 μg group), headaches (one participant in the two-dose 25 μg group), and cough (one participant in the three-dose placebo group). Seven participants reported serious adverse events (one [1%] in the two-dose 25 μg vaccine group, one [1%] in the two-dose 50 μg vaccine group, two [1%] in the three-dose placebo group, one [1%] in the two-dose 25 μg vaccine group, and two [1%] in the two-dose 50 μg vaccine group), but none was considered to be related to the study vaccine as assessed by the investigators (appendix 2 pp 14–19). No adverse events of special interest were reported.
Table 3.
Adverse events and reactions in the phase 2 trial
|
Two-dose schedule |
Three-dose schedule |
p value | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n=150) | 25 μg dose (n=150) | 50 μg dose (n=150) | Placebo (n=150) | 25 μg dose (n=150) | 50 μg dose (n=150) | ||
| Overall adverse events within 30 days | |||||||
| Any | 37 (25%) | 43 (29%) | 50 (33%) | 47 (31%) | 72 (48%) | 65 (43%) | <0·0001 |
| Grade ≥3 | 0 | 4 (3%) | 2 (1%) | 2 (1%) | 4 (3%) | 6 (4%) | 0·1871 |
| Solicited adverse reactions within 7 days | |||||||
| Any | 16 (11%) | 27 (18%) | 32 (21%) | 19 (13%) | 55 (37%) | 42 (28%) | <0·0001 |
| Grade ≥3 | 0 | 2 (1%) | 1 (1%) | 1 (1%) | 2 (1%) | 5 (3%) | 0·1460 |
| Solicited systemic adverse reactions | |||||||
| Any | 8 (5%) | 15 (10%) | 16 (11%) | 16 (11%) | 15 (10%) | 13 (9%) | 0·5890 |
| Grade ≥3 | 0 | 2 (1%) | 0 | 1 (1%) | 0 | 0 | 0·2189 |
| Fever | 6 (4%) | 8 (5%) | 10 (7%) | 12 (8%) | 12 (8%) | 11 (7%) | 0·6787 |
| Grade ≥3 fever | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0·4152 |
| Headache | 1 (1%) | 3 (2%) | 1 (1%) | 0 | 3 (2%) | 0 | 0·2160 |
| Grade ≥3 headache | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0·4152 |
| Fatigue | 0 | 4 (3%) | 3 (2%) | 4 (3%) | 0 | 0 | 0·0422 |
| Cough | 1 (1%) | 3 (2%) | 3 (2%) | 3 (2%) | 1 (1%) | 0 | 0·4310 |
| Grade ≥3 cough | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 0·4152 |
| Nausea | 1 (1%) | 1 (1%) | 0 | 1 (1%) | 0 | 1 (1%) | 0·8479 |
| Muscle pain | 0 | 1 (1%) | 0 | 0 | 1 (1%) | 0 | 0·5481 |
| Solicited local adverse reactions | |||||||
| Any | 9 (6%) | 17 (11%) | 19 (13%) | 6 (4%) | 45 (30%) | 35 (23%) | <0·0001 |
| Grade ≥3 | 0 | 0 | 1 (1%) | 0 | 2 (1%) | 5 (3%) | 0·0121 |
| Injection-site pain | 6 (4%) | 5 (3%) | 7 (5%) | 4 (3%) | 18 (12%) | 18 (12%) | 0·0003 |
| Grade ≥3 injection-site pain | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0·4152 |
| Swelling | 2 (1%) | 6 (4%) | 9 (6%) | 2 (1%) | 21 (14%) | 20 (13%) | <0·0001 |
| Grade ≥3 swelling | 0 | 0 | 0 | 0 | 0 | 3 (2%) | 0·0102 |
| Induration | 1 (1%) | 4 (3%) | 8 (5%) | 0 | 14 (9%) | 11 (7%) | <0·0001 |
| Grade ≥3 induration | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0·4152 |
| Redness | 2 (1%) | 12 (8%) | 12 (8%) | 1 (1%) | 24 (16%) | 21 (14%) | <0·001 |
| Grade ≥3 redness | 0 | 0 | 1 (1%) | 0 | 1 (1%) | 4 (3%) | 0·0337 |
| Rash | 0 | 3 (2%) | 4 (3%) | 0 | 2 (1%) | 1 (1%) | 0·1514 |
| Grade ≥3 rash | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0·4152 |
| Itch | 0 | 9 (6%) | 13 (9%) | 0 | 28 (19%) | 26 (17%) | <0·0001 |
| Unsolicited adverse reactions | |||||||
| Any | 6 (4%) | 5 (3%) | 4 (3%) | 8 (5%) | 7 (5%) | 4 (3%) | 0·7847 |
Data are n (%). p values are calculated with Fisher's exact test.
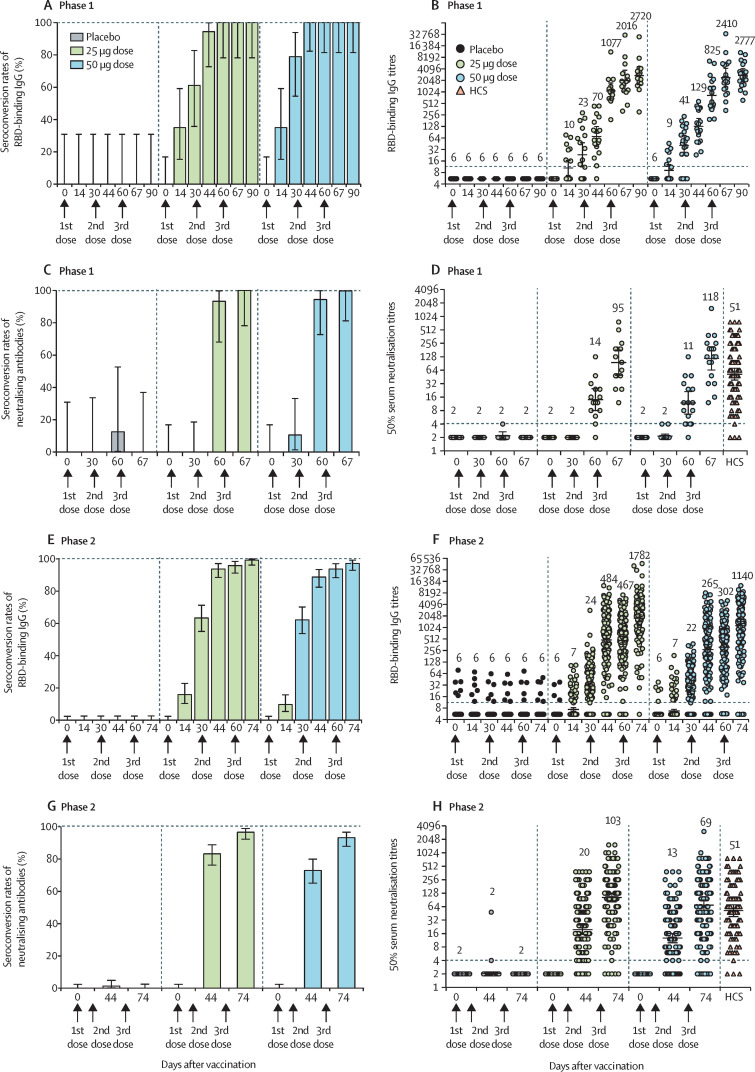
In the phase 1 trial, we measured the serological RBD-binding IgG titres by ELISA to assess antibody responses. Baseline antibody titres of participants are shown in appendix 2 (p 6). At day 30 after the first immunisation, seroconversion rates were 61% (11 of 18 participants) in the 25 μg group and 79% (15 of 19 participants) in the 50 μg group, and increased to 100% in both groups (15 of 15 participants in the 25 μg group and 18 of 18 in the 50 μg group) at day 30 after the second and third immunisations (figure 2A ; appendix 2 pp 7–8). At day 30 after the first dose, GMTs of vaccine-induced RBD-binding IgG were 23·1 (95% CI 11·2–47·9) in the 25 μg dose group and 40·8 (22·5–74·0) in the 50 μg dose group, and increased to 1077·0 (663·7–1747·5) in the 25 μg dose group and 825·5 (486·9–1399·4) in the 50 μg dose group at day 30 after the second dose. GMTs further increased to 2719·5 (95% CI 1584·0–4668·8) in the 25 μg group and 2776·8 (1875·5–4111·2) in the 50 μg group at day 30 after the third dose (figure 2B; appendix 2 pp 7–8).
Figure 2.
Humoral immune responses in phase 1 and phase 2 trials
Seroconversion rates (A) and GMTs (B) of RBD-binding antibodies at different timepoints after vaccination in phase 1. Seroconversion rates (C) and GMTs (D) of neutralising antibodies at different timepoints after vaccination in phase 1. Seroconversion rates (E) and GMTs (F) of RBD-binding antibodies at different timepoints after three-dose vaccination in phase 2. Seroconversion rates (G) and GMTs (H) of neutralising antibodies at different timepoints after three-dose vaccination in phase 2. Results for the two-dose groups are shown in appendix 2 (p 22). Error bars represent 95% CIs. The horizontal dashed lines in panels B, D, F, and H indicate the limit of detection. GMT=geometric mean titres. HCS=human convalescent serum. RBD=receptor-binding domain.
We also measured 50% neutralising titres in serum samples of participants. Baseline titres of neutralising antibodies of participants are summarised in appendix 2 (p 6). At day 30 after the second dose, seroconversion rates reached 93% (14 of 15 participants) in the 25 μg group and 94% (17 of 18 participants) in the 50 μg group. At day 7 after the third dose, seroconversion rates were 100% in both groups (figure 2C; appendix 2 p 9). The SARS-CoV-2-neutralising GMTs were 14·0 (95% CI 8·0–24·6) in the 25 μg group and 11·4 (6·6–19·8) in the 50 μg group at day 30 after the second dose, and increased to 94·5 (49·3–181·3) in the 25 μg group and 117·8 (64·6–214·9) in the 50 μg group at day 7 after the third dose (figure 2D; appendix 2 pp 9–12). Meanwhile, a panel of 89 SARS-CoV-2 human convalescent serum samples was tested for comparison. The GMT of neutralising antibodies for the SARS-CoV-2 convalescent serum panel was 51·2 (95% CI 38·3–70·5; figure 2D).
Baseline antibody titres of participants from the phase 2 trial are summarised in appendix 2 (p 6). At day 30 after the first immunisation, in participants on the two-dose schedule, seroconversion rates of RBD-binding IgG were 1% (one of 147) in the placebo group, 59% (87 of 147) in the 25 μg group, and 65% (96 of 147) in the 50 μg group (appendix 2 pp 10–12); in participants on the three-dose schedule, seroconversion rates of RBD-binding IgG were 0% (none of 141) in the placebo group, 63% (92 of 145) in the 25 μg group, and 62% (89 of 143) in the 50 μg group. At day 30 after the second dose, in participants on the two-dose schedule, seroconversion rates increased to 1% (two of 147) in the placebo group, 95% (140 of 147) in the 25 μg group, and 97% (142 of 147) in the 50 μg group; in participants on the three-dose schedule, seroconversion rates were 0% (none of 141) in the placebo group, 96% (139 of 145) in the 25 μg group, and 94% (134 of 143) in the 50 μg group. At day 14 after the third dose, in participants on the three-dose schedule, seroconversion rates were 0% (none of 140) in the placebo group, 99% (143 of 144) in the 25 μg group, and 97% (139 of 143) in the 50 μg group (figure 2E; appendix 2 pp 10–12). In participants on the two-dose schedule, RBD-binding IgG GMTs significantly increased from 19·4 (95% CI 16·0–23·6) 30 days after the first dose to 419·5 (325·8–540·1) 30 days after the second dose in the 25 μg group (p<0·0001); and from 22·6 (18·5–27·6) 30 days after the first dose to 344·8 (271·0–438·7) 30 days after the second dose in the 50 μg group (p<0·0001; appendix 2 pp 10–12). By contrast, GMTs in the placebo group were between 5·7 (95% CI 5·5–5·9) and 6·0 (5·4–6·6) after the first, second, and third doses. In participants on the three-dose schedule, RBD-binding IgG GMTs significantly increased after each dose, from 24·4 (95% CI 19·6–30·2) 30 days after the first dose to 467·3 (366·8–595·3) 30 days after the second dose and to 1782·3 (1440·2–2205·7) 14 days after the third dose in the 25 μg dose group; and from 22·2 (18·1–27·4) 30 days after the first dose to 302·5 (234·2–390·6) 30 days after the second dose to 1140·0 (882·2–1473·2) 14 days after the third dose in the 50 μg dose group. By contrast, GMTs in the placebo group were between 5·8 (95% CI 5·5–6·1) and 5·9 (5·6–6·2) after the first, second, and third doses (figure 2F; appendix 2 pp 10–12).
With regard to neutralising antibody titres against live SARS-CoV-2, seroconversion rates and GMTs were analysed. For participants on the two-dose schedule, seroconversion rates were 0% (none of 150) in the placebo group, 76% (114 of 150) in the 25 μg group, and 72% (108 of 150) in the 50 μg group 14 days after the second dose (appendix 2 p 13). For participants on the three-dose schedule, seroconversion rates were 1% (two of 147) 14 days after the second dose in the placebo group, 83% (124 of 149) in the 25 μg group, and 73% (108 of 148) in the 50 μg group; seroconversion rates 14 days after the third dose were 0% (none of 146) in the placebo group, 97% (143 of 148) in the 25 μg group, and 93% (138 of 148) in the 50 μg group (figure 2G, appendix 2 p 13). Neutralising GMTs against live SARS-CoV-2 14 days after the second dose in participants on the two-dose schedule were 17·7 (95% CI 13·6–23·1) in the 25 μg group and 14·1 (10·8–18·3) in the 50 μg group. In participants on the three-dose schedule, neutralising GMTs increased from 19·5 (95% CI 15·2–25·0) 14 days after the second dose to 102·5 (81·8–128·5) 14 days after the third dose in the 25 μg group; and from 12·6 (10·0–16·0) 14 days after the second dose to 69·1 (53·0–90·0) 14 days after the third dose in the 50 μg group. By contrast, neutralising GMTs in the placebo group were 2·1 (95% CI 2·0–2·1) 14 days after the second dose and 2·0 (2·0–2·0) 14 days after the third dose (figure 2H; appendix 2 p 13). After three doses, both the 25 μg and 50 μg groups showed neutralising GMTs exceeding the level of a panel of convalescent serum samples (figure 2H). The 25 μg group showed a significant increase in neutralising GMTs compared with the convalescent samples (p=0·0004) in the phase 2 trial, while the 50 μg group did not. We similarly observed no enhancement of neutralising GMTs for participants receiving the high dose (50 μg) compared with those receiving the low dose (25 μg).
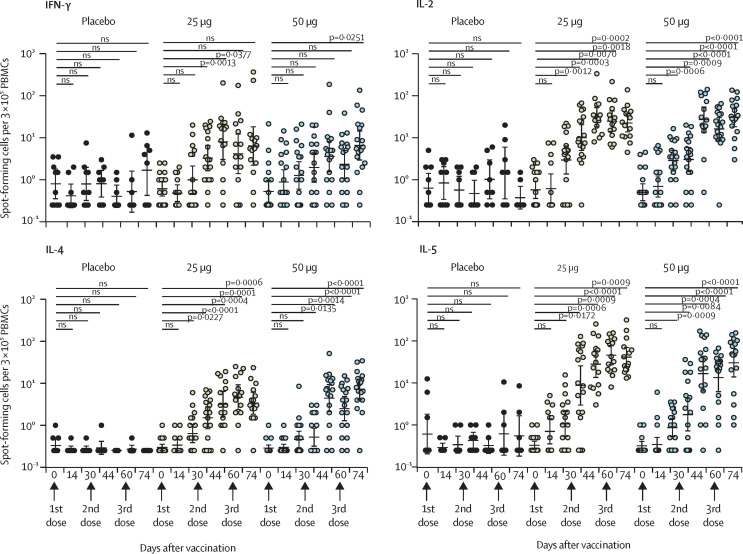
To assess T-cell responses, in the phase 1 trial we did ELISpot assays for PBMCs of participants against the SARS-CoV-2 RBD protein. T-helper 1 (Th1) and T-helper 2 (Th2) cell responses were measured by detection of the Th1 cytokines IFNγ and IL-2 and the Th2 cytokines IL-4 and IL-5. As expected, no increase in T-cell cytokines was observed in the placebo group. By contrast, the 25 μg and 50 μg doses elicited moderate levels of both Th1 (IFNγ and IL-2) and Th2 (IL-4 and IL-5) cytokine production after the immunisations (figure 3 ).
Figure 3.
Th1 and Th2 cell responses in the phase 1 trial
Cytokines IFNγ and IL-2 of Th1 cells and IL-4 and IL-5 of Th2 cells were measured with enzyme-linked immunospot assays. p values were calculated with Student's t test. IFNγ=interferon-γ. IL=interleukin. ns=not significant. PBMCs=peripheral blood mononuclear cells. Th1=T helper 1. Th2=T helper 2.
Discussion
The S protein RBD is an attractive vaccine target against COVID-19.17 To date, various vaccine candidates based on the RBD have shown efficacy in animal models against SARS-CoV, MERS-CoV, and SARS-CoV-2.4 Several RBD-based COVID-19 vaccines are being evaluated in clinical trials.4 Clinical data have been published for an RBD-based COVID-19 vaccine candidate, developed by BioNTech and Pfizer. This candidate, BNT162b1, is an mRNA-based vaccine and showed good immunogenicity in healthy adult volunteers, but it was not chosen for further development.18, 19 Here, we report, to the best of our knowledge, the first clinical data for an RBD-based protein subunit vaccine against COVID-19.
In these phase 1 and phase 2 trials, we found that vaccination with the 25 or 50 μg doses and two-dose or three-dose schedules was well tolerated. The frequency of adverse events between the vaccine and placebo groups was similar in both phase 1 and phase 2. Most adverse events were mild or moderate, with the most common symptoms being injection-site pain, redness, and swelling. These adverse events are anticipated for alum-adjuvanted protein subunit vaccines and were transient and resolved within 3–4 days after vaccination. Compared with other COVID-19 vaccine candidates, such as mRNA-based vaccines or adenovirus-vectored vaccines, the occurrences of fever and fatigue were lower with ZF2001.20, 21, 22, 23, 24, 25, 26, 27 Compared with another protein subunit vaccine, NVX-CoV2373, which used Matrix-M1 as an adjuvant, the occurrences of injection-site pain, fatigue, headache, and nausea were also lower with ZF2001.28 However, safety data in different clinical trials are affected by subjective factors, such as personal feelings, from participants. Our data demonstrate a potential application of the RBD protein in a COVID-19 vaccine. These results could be applicable to other RBD-based COVID-19 vaccines under development.
Humoral responses have been considered as immune correlates of protection against SARS-CoV-2.4 So far, humoral responses for several COVID-19 vaccine candidates in clinical trials have been reported. Notably, it is difficult to compare different vaccine candidates since there are no standardised neutralisation assays. We used microcytopathogenic effect assays to determine neutralising antibody titres for serum samples from vaccinees, with a panel of COVID-19 convalescent serum samples as the control. The comparative immunogenicity showed that three doses of ZF2001 elicited neutralising GMTs two times greater than the GMTs tested for the convalescent serum samples. Notably, two mRNA-based vaccines and two adenovirus-vectored vaccines have reported 70–95% efficacy against COVID-19 in phase 3 clinical trials, with their vaccine-induced humoral responses being reported to be similar to or higher than those from convalescent samples.5, 20, 23, 24, 27 Although interpretation of vaccine immunogenicity can be influenced by the severity of the illness of the convalescents selected for comparison, our findings indicate that ZF2001 is potentially effective against COVID-19. Three-dose vaccination substantially enhanced antibody responses compared with two-dose vaccination, but increasing the antigen dose from 25 μg to 50 μg did not improve immunogenicity. Therefore, the 25 μg antigen with the three-dose schedule has been selected for further efficacy evaluation in a phase 3 trial (NCT04646590).
Cellular immune responses also have a protective role in SARS-CoV-2 infection. Virus-specific T-cell responses were associated with milder disease in patients with COVID-19.4 Based on the levels of spot-forming cells per 0·3 million cells, cytokines representing both Th1 and Th2 responses were moderately elicited after vaccination. The moderate but balanced production of cytokines associated with Th1 and Th2 cells might be beneficial in promoting T-cell-mediated protection rather than resulting in vaccine-enhanced respiratory disease, which is presumably caused by predominantly Th2-biased responses.29
There were 50 volunteers in phase 1, and the mean age was 32·6 years, compared with 900 participants in phase 2, with a mean age of 43·5 years. The differences in mean age and the trial sizes might account for the variation in the frequency of adverse events and immunogenicity observed between phases 1 and 2. Additionally, the 25 μg dose showed higher immunogenicity than the 50 μg dose in the phase 2 trial. We speculate that the 25 μg dose is sufficient to elicit the desired immune response. A larger dose might not further increase immunogenicity; instead, it could decrease antibody responses.
These trials have several limitations. First, participants in both trials were young adults aged 18–59 years and we did not include children and older people; yet older people are known to be more vulnerable to SARS-CoV-2 infection. Moreover, the study populations were not ethnically diverse, with the majority of participants being Han Chinese. These limitations will be addressed by our outreach study, by including a cohort of older participants (aged >60 years; NCT04550351) and in phase 3 trials enrolling participants from a more diverse range of ethnic backgrounds (NCT04646590). Second, immunogenicity was tested at day 30 after full vaccination in phase 1 and at day 14 after full vaccination in phase 2; the full duration of the immune response cannot be assessed during this time period. Immune responses at later timepoints, including at least 6 months after vaccination, will be obtained in the follow-up visits and investigations. Third, there was no benchmark to evaluate protective immune responses against COVID-19. Therefore, although ZF2001 induced neutralising GMTs higher than those observed in the convalescent samples, the protective efficacy of the vaccine cannot yet be confirmed. Since no participant was subsequently exposed to SARS-CoV-2, we were unable to assess the protective efficacy of ZF2001. This limitation will be addressed in the ongoing international multicentre phase 3 trials. Fourth, we used the ELISpot assay to detect cytokine production, without distinguishing between CD4+ and CD8+ cells. Therefore, we cannot evaluate the cytotoxic T lymphocyte response at present. Additionally, compared with the two-dose regimen reported for the other vaccines, a three-dose schedule is more time consuming for mass immunisation. However, given that current vaccine supplies are inadequate to meet the global demand, a three-dose schedule is still a viable alternative for large-scale immunisation because of ZF2001's promising safety profile, scalable production, and low-cost transportation requirements (2–8 °C).
Data sharing
The individual participant-level data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices). This clinical trial is ongoing, and all individual participant-level data will not be available until after the immune persistence assessments have been done. The data will be made available immediately after publication and finalisation of the complete clinical study report, for at least 6 months. Supporting clinical documents, including the study protocol, statistical analysis plan, and the informed consent form will be made available immediately after publication of this Article for at least 1 year. Information about how to access the supporting clinical documents is available in appendix 2. Researchers who provide a scientifically sound proposal will be allowed to access the de-identified individual participant data. Proposals should be sent to the corresponding authors. These proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.
Declaration of interests
YLi, LD, JY, and GFG are listed in the patent as the inventors of the RBD dimer as a betacoronavirus vaccine. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work is funded by the National Program on Key Research Project of China (grant number 2020YFC0842300), National Science and Technology Major Projects of Drug Discovery (grant number 2018ZX09101001), Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB29010202), and Anhui Zhifei Longcom Biopharmaceutical. We thank Yuhai Bi for providing SARS-CoV-2 and the staff of Biosafety Level 3 Laboratory (Institute of Microbiology, Chinese Academy of Science) for their help in live virus experiments. We thank Zhifang Ying and Zhen Chen (National Institute for Food and Drug Control) for their technical support in neutralisation experiments.
Contributors
GFG, LD, and JY initiated and designed the vaccine. SY, YLiu, and JY contributed to the protocol and design of the trials. XY, HR, and LL were the study site principal investigators in the phase 1 trial. FL was the study site principal investigator in the phase 2 trial. LG and JL were responsible for the organisation and supervision of the phase 2 trial. ZZ participated in the implementation of trials. YLi, ZH, JY, JW, PH, CL, SM, XZ, XF, CWa, XD, ST, FW, and LW contributed to laboratory testing and assay development. ZC was responsible for the collection of convalescent serum samples. EH and CWu were responsible for vaccine manufacture. YLuo did the statistical analysis. ZH, LD, JY, and GFG participated in data collection and data analysis. LD, YLi, and GFG contributed to the writing of the manuscript. All authors reviewed all the data and approved the final version of the manuscript. All authors reviewed and verified the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang S, Shi Z, Shu Y, et al. A distinct name is needed for the new coronavirus. Lancet. 2020;395:949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei Q, Wang Y, Ma J, et al. Description of the first strain of 2019-nCoV, C-Tan-nCoV Wuhan strain—National Pathogen Resource Center, China, 2020. China CDC Wkly. 2020;2:81–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–83. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, EI Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore JP, Klasse PJ. COVID-19 vaccines: “warp speed” needs mind melds, not warped minds. J Virol. 2020;94:e01083–e01120. doi: 10.1128/JVI.01083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai L, Zheng T, Xu K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182:722. doi: 10.1016/j.cell.2020.06.035. 33.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An Y, Li S, Jin X, et al. A tandem-repeat dimeric RBD protein-based COVID-19 vaccine ZF2001 protects mice and nonhuman primates. bioRxiv. 2021 doi: 10.1101/2021.03.11.434928. published online March 11. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Wang Q, Gao GF. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 19.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 20.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su S, Du L, Jiang S. Learning from the past: development of safe and effective COVID-19 vaccines. Nat Rev Microbiol. 2020;19:211–219. doi: 10.1038/s41579-020-00462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant-level data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices). This clinical trial is ongoing, and all individual participant-level data will not be available until after the immune persistence assessments have been done. The data will be made available immediately after publication and finalisation of the complete clinical study report, for at least 6 months. Supporting clinical documents, including the study protocol, statistical analysis plan, and the informed consent form will be made available immediately after publication of this Article for at least 1 year. Information about how to access the supporting clinical documents is available in appendix 2. Researchers who provide a scientifically sound proposal will be allowed to access the de-identified individual participant data. Proposals should be sent to the corresponding authors. These proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.