Abstract
G-protein-gated inwardly rectifying potassium (GIRK) channels are essential regulators of cell excitability in the brain. While implicated in a variety of neurological diseases in both human and animal model studies, their therapeutic potential has been largely untapped. Here, we review recent advances in the development of small molecule compounds that specifically modulate GIRK channels, and compare with first-generation compounds that exhibit off-target activity. We describe the method of discovery of these small molecule modulators, their chemical features, and their effects in vivo. These studies provide a promising outlook on future development of subunit-specific GIRK modulators to regulate neuronal excitability in a brain region-specific manner.
Keywords: G-protein-gated inwardly rectifying potassium channel, small molecule modulator, addiction, neurological disease, alcoholism
GIRK Channel Physiology and its Role in Disease
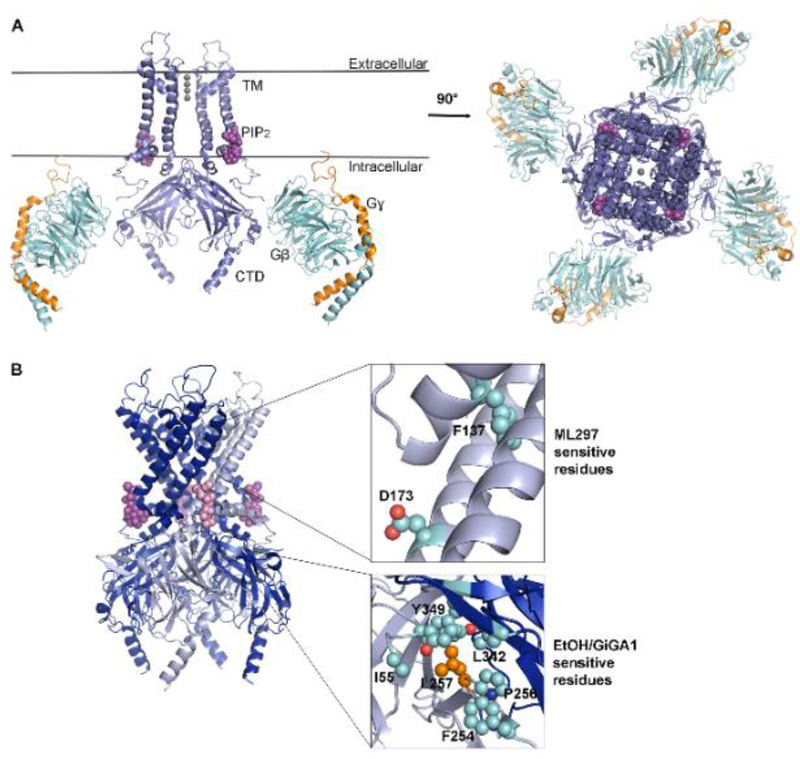
G-protein-gated inwardly rectifying potassium (GIRK, also referred to as Kir3) channels play an important role in maintaining the resting membrane potential in brain and cardiac cells. Their ability to conduct potassium ions (K+) into the cell more easily than out of the cell is referred to as the property of “inward rectification” (see Glossary). Under physiological conditions, the small outward K+ current through GIRK channels decreases the excitability of the cell [1]. Three types of GIRK subunits (GIRK1, GIRK2, and GIRK3) are expressed in the brain and form either homotetramers of GIRK2, or heterotetramers (e.g., GIRK1 and GIRK2) in different brain regions [2–4]. Although the GIRK1/GIRK2 combination is abundant in the majority of brain regions, dopaminergic neurons in the ventral tegmental area (VTA) express only GIRK2 and GIRK3 subunits, and substantia nigra (SNc) dopaminergic neurons express only GIRK2 [4–6]. In the heart, IKACh GIRKs are comprised of GIRK1/GIRK4 heterotetramers and GIRK4 homotetramers [7]. In native tissues, G-protein-coupled receptor (GPCR) activation leads to the dissociation of Gβγ subunits from the heterotrimeric G protein complex, and activation of GIRK channels via the binding of Gβγ to the channel (Figure 1A) [8, 9]. In addition to this canonical activation pathway, GIRK channels can be modulated by both exogenous and endogenous small molecules, such as ethanol [10–12] and cholesterol [13, 14], respectively.
Figure 1: GIRK Channel Structure and Modulatory Sites.
A) Left: Side view model of the GIRK2 channel and the G-protein β (cyan) and γ subunits (orange) (PDB: 4kfm). Two subunits are removed for clarity. Transmembrane (TM) domains are in contact with PIP2 (magenta spheres) near the intracellular interface. The cytoplasmic domain (CTD) interacts directly with the Gβγ subunits. Right: view of the top extracellular side of all four subunits. B) Model of GIRK2 tetramer with four subunits. A single subunit is highlighted (light blue) with its corresponding PIP2 molecule (magenta). Expansion of the regions containing key residues implicated in activation by ML297 (top) and GiGA1 (bottom) are shown on the right. Amino acids involved in ML297 activation of GIRK1/GIRK2 channels: GIRK1 F137 and D173 (top). Amino acids involved in GiGA1 interactions, GIRK1 R43 and L246 (not shown), and GIRK2 D346 and L257; in EtOH and GIRK2 I55, F254, P256, L342, L257 and Y349 (bottom). A key residue for EtOH/GiGA1 interaction, L257, is shown in orange. All residues numbers provided are for mouse GIRK1 and GIRK2 isoforms.
As important inhibitory downstream effectors of the G-protein-mediated signaling pathway, GIRK channels are implicated in a number of human diseases, such as epilepsy, cardiac arrhythmias, addiction, and alcohol abuse [15–19]. For instance, GIRK2 KO (knockout) mice are more susceptible to spontaneous and pharmacologically induced seizures [15]. A loss-of-function mutation in GIRK4 (Kir3.4 gene) is associated with long QT syndrome [17]. GIRK3 KO mice show increased ethanol binge-like drinking behavior [19]. These and other studies [3] (review article) point to the therapeutic potential of targeting GIRK channels for treating diseases, and the need for developing potent and specific GIRK modulators. Over the last two decades, several studies have demonstrated modulation of GIRK channels by drugs designed for other targets. The availability of high-resolution atomic structures of GIRK2 channels and related inwardly rectifying K+ channels has helped by facilitating structural mechanisms of action and providing a scaffold for structure-based drug design [10, 20–23]. Recently, a number of small molecules have been developed that specifically modulate GIRK channels, and a majority of them show in vivo effects in mitigating the impact of diseases.
First Generation Compounds
GIRK channels are increasingly recognized as important targets for drug design focused on diseases such as epilepsy and addiction. While current studies employed methods that specifically target GIRK channels, historically that was not the case. Typically, drugs developed for other diseases, such as FDA-approved drugs, were found to have an effect on GIRK channels. The off-target effects of drugs with other primary targets or multiple targets was a common theme among the first generation of compounds that modulate GIRK channels, as described for channel activators (Box 1) and inhibitors (Box 2). These channel modulators provided important information about GIRK channel function and pharmacology. As the lack of specificity could limit their potential clinical use, this review will focus on more GIRK-selective compounds.
Box 1: First Generation GIRK Channel Activators.
Volatile anesthetics
More than twenty years ago, some of the first studies on GIRK modulators showed that volatile anesthetics, such as halothane and isoflurane, acted on GIRK channels [58]. Subsequent studies further characterized the effects of these two volatile anesthetics, as well as others, such as F3 (1-chloro-1,2,2-trifluorocyclobutane), toluene, chloroform, and nitrous oxide [59–63]. Additionally, a recent study showed that chloroform activated GIRK1/GIRK2 and GIRK1/GIRK4 channels in a G-protein-independent manner, albeit at millimolar concentrations, while having no effect on Kir2 inward-rectifiers [63].
However, their clinical usefulness is limited due to negative side effects, and the sites and mechanisms by which volatile anesthetics modulate GIRK channels are diverse and not well characterized. Understanding the sites and mechanisms of action will help in the design of therapeutics for GIRK channels.
Naringin
Naringin, a bioflavonoid found in grapefruit, has been identified as a GIRK channel agonist. Studies suggest that naringin might exert its agonist effects extracellularly, within a site containing aromatic tyrosine residues on the GIRK1 extracellular vestibule (mGIRK1 Y148 and Y150), which are known to be important in the binding of the inhibitor toxin tertiapin [64]. Naringin and tertiapin may share a binding site, although there is no proposed mechanism as to how naringin might lead to channel activation [65].
Ivermectin
Ivermectin is an FDA-approved drug for treating parasitic infections by activating glutamate-gated Cl− channels in the parasite [66]. Recent studies demonstrated that ivermectin also activated GIRK channels expressed in the heterologous system in a PIP2-dependent, but G-protein-independent manner, with an EC50 of 3–8 μM. Ivermectin showed a higher potency for activating GIRK2 over GIRK4, the primary GIRK subunit in the heart. Recent mutagenesis studies and electrophysiological recordings revealed a critical residue for the action of ivermectin on GIRK channels, I82, which is localized at the slide helix of the N-terminus [67, 68].
Flupirtine
Flupirtine is a widely used non-opioid analgesic agent with muscle-relaxant properties [69]. It has been shown that therapeutic concentrations of flupirtine potentiate both KV7 channels and GABAA receptors in neurons, which might be responsible for its analgesic property [70]. Flupirtine also has neuroprotective and antiepileptic effects in preclinical and clinical studies [71]. Recent studies suggested that flupirtine may indirectly inhibit NMDA receptor activity through GIRK channels [72]. Although the precise mechanism is still not clear, the effect of flupirtine on GIRK channels seems to be G-protein-dependent.
Box 2: First Generation GIRK Channel Inhibitors.
Antipsychotic drugs
Several classes of antipsychotic drugs inhibit both cardiac and neuronal GIRK channels with different potency and efficacy [73, 74]. Primary among these is clozapine, an atypical antipsychotic drug used for treating schizophrenia or similar disorders [75]. Clozapine inhibited both GIRK1/GIRK2 (neuronal type) and GIRK1/GIRK4 (cardiac type) GIRK channels [73]. The inhibitory effect of clozapine on GIRK channels may underlie some of the side effects from its clinical use, such as sedation, seizures, and sinus tachycardia.
Selective Serotonin Reuptake Inhibitors (SSRIs)
Various SSRIs, including fluoxetine and paroxetine, modulate GIRK channels [76, 77]. Fluoxetine inhibits the functions of various receptors and ion channels [78–80]. Further studies showed that fluoxetine inhibited GIRK1/GIRK2, GIRK2, and GIRK1/GIRK4 channels expressed in Xenopus oocytes [76].
Antitussives
Antitussive drugs, or cough suppressants, including dextromethorphan, tipepidine, cloperastine, and caramiphen inhibit the currents caused by activation of GIRK channels in neurons [81–84]. For example, tipepidine activated VTA dopamine neurons via inhibition of D2 mediated inwardly-rectifying potassium channel current [83]. Tipepidine also increased dopamine levels in the nucleus accumbens (NAc) without increases in locomotor activity seen in the use of methamphetamine [85]. These studies point to the inhibition of GIRK channels as an additional therapeutic in depression.
Tertiapin
In 1998, Jin and Lu searched for high-affinity inhibitors against inwardly-rectifying potassium channels. From this study they identified a particular honey bee venom, tertiapin (TPN), which had particularly high affinity for cardiac GIRK channels and Kir1.1 (ROMK1) channels [86]. TPN has proven a useful tool in studying GIRK channels in cells and animals. For example, induced atrial fibrillation (AF) in dogs was inhibited by the administration of TPN [87].
Schering-Plough (SCH) compounds
A screening study identified an H+/K+ ATPase inhibitor, SCH28080, that inhibited GIRK channels in pituitary (AtT-20) cells and cardiac (HL-1) cells at nanomolar concentrations [88]. Another compound, SCH23390, a dopamine D1 antagonist, blocked endogenous GIRK currents in AtT-20 cells with nanomolar affinity [89]. To identify the chemical features that contribute to GIRK channel block, several structurally related compounds were tested and identified a halide atom that is critical for GIRK channel blockade [89].
Benzopyrane derivatives
Several studies evaluated benzopyrane derivatives developed to treat atrial fibrillation. Initially, NIP-142 was shown to suppress atrial fibrillation via inhibition of IKACh block [90]. Further optimization resulted in the synthesis of NTC-801, which blocked the GIRK1/GIRK4 channel more selectively than GIRK1/GIRK2 [91]. Preliminary studies in dogs suggested clinical efficacy in treating certain forms of atrial fibrillation [92].
Non-sedative antihistamines
A recent study identified that terfenadine, a piperidine-based antihistamine, inhibits the activity of GIRK1-containing GIRK channels [93]. Terfenadine was generated to target the histamine H1 receptor to treat allergic diseases with no sedative side-effects [94]. However, it was withdrawn from clinical use due to its off-target effect on blocking hERG channels [95]. This study suggested that an amino acid (F137) in mGIRK1 is responsible for the inhibitory effect of terfenadine, residue also critical for ML297 activity [26].
ML297: a Potent and Selective GIRK Activator
The paucity of specific GIRK channel modifiers motivated a de novo search for new GIRK modulators. In 2013, ML297 was identified in a high-throughput screening (HTS) assay, which was based on a thallium-flux measurement in HEK293 cells expressing GIRK1/GIRK2 channels [24]. Kaufmann and colleagues performed chemical optimization of an initial promising compound identified in the HTS assay (VU0032230), which led to the discovery of ML297 (Figure 2A, Table 1) [25]. ML297 was the first potent activator of GIRK1/GIRK2, with an EC50 of 160 nM. ML297 exhibited modest selectivity towards GIRK1/GIRK2 subunits, relative to GIRK1/GIRK3 and GIRK1/GIRK4 (around 6-fold range), and an absence of activity on GIRK1-lacking channels. Two amino acids in mouse GIRK1 (mGIRK1), F137 in the pore helix and D173 in the second membrane-spanning domain (Figure 1B), were subsequently identified as required for ML297 activity [26]. ML297 activation of GIRK1/GIRK2 required the presence of the membrane phospholipid, Phosphatidylinositol 4,5-bisphosphate (PIP2), but not G protein Gβγ subunits, therefore exhibiting a GPCR-independent mechanism of channel activation [25]. ML297 activated native GIRK channels expressed in hippocampal mouse neurons in acutely prepared brain slices (most likely GIRK1/GIRK2 channels) (Table 1) [26, 27].
Figure 2: Discovery of New Small Molecule GIRK Modulators.
A) ML297 was initially discovered through high-throughput screening flux assays with the molecular libraries small molecule repository (MLSMR) and followed by structure-activity relationship (SAR)-based optimization [25]. ML297 is an agonist for GIRK1-containing channels. The second generation of compounds were discovered by structure modification of ML297. The essential chemical groups of ML297 are highlighted. The flow chart shows both the urea and non-urea scaffold compounds [31, 34, 35, 38, 40]. Chemical structures and names of the compounds are indicated. B) The flow chart shows the discovery of GiGA1 through structure-based virtual screening method. A structural model of an alcohol pocket in GIRK2 was built based on the GIRK2 crystal structure [20, 21] and alcohol-bound IRK1 structure [10]. The screening was conducted by docking molecules from a ZINC15 library and National Center for Advancing Translational Sciences (NCATS) small-molecule library to the alcohol pocket in GIRK2. Top hits were further screened with whole-cell patch-clamp recordings on HEK cells expressing different GIRK channels. NCATS_2 was discovered to activate both GIRK2 and GIRK1/GIRK2 channels. Following screenings tested NCATS_2 analogs, and found that NCATS_2.3 (GiGA1) showed selectivity for activating GIRK1-containing channels [46]. Chemical structures and names of the compounds are indicated.
Table 1:
Biological Activity of Small Molecule GIRK Modulators Observed in Heterologous Expression Systems and in Native GIRK-expressing Neurons.
| Compound | GIRK selectivity | Biological GIRK-related activity | |
|---|---|---|---|
| GIRK heterologous expression | GIRK native expression | ||
| ML-297 | GIRK1-containing channels (GIRK1/GIRK2 preference) | ↑ current in GIRK1/GIRK2 and GIRK1/GIRK4 expressing HEK293 [25] | ↑ baclofen-sensitive currents in mouse hippocampal neurons [26] ↓ neuronal excitability in mouse hippocampal neurons [27] ↓ neuronal excitability in mouse hypothalamic and hippocampal brain slices [28] |
| VU0466551 | GIRK1-containing channels (GIRK1/GIRK2 preference) | TBD | TBD |
| LOGO5 | GIRK1-containing channels (GIRK1/GIRK2, GIRK1/GIRK4) | ↑ current in GIRK1/GIRK2 expressing HEK293 [34] | ↓ excitability in rat hippocampal neurons [34] |
| VLOGO | GIRK1-containing channels (GIRK1/GIRK2, GIRK1/GIRK4) | ↑ current in GIRK1/GIRK2 expressing HEK293 [35] | ↓ neuronal excitability in mouse hippocampal slices [35] |
| CLOGO | GIRK1-containing channels (GIRK1/GIRK2, GIRK1/GIRK4) | ↑ current in GIRK1/GIRK2 expressing HEK293 [36] | ↓ action potential firing in mouse hippocampal neurons [36] |
| VU0810464 | GIRK1-containing channels (GIRK1/GIRK2 preference) | TBD | ↑ barium-sensitive current in mouse hippocampal neurons [38] |
| VU0529331 | GIRK1-containing and non-containing channels | ↑ current in GIRK1/GIRK2 and GIRK2 expressing HEK293 [45] | TBD |
| GAT1508 | GIRK1/GIRK2 | ↑ current in GIRK1/GIRK2 expressing HEK293 [40] | ↑ outward current in rat BLA brain slices [40] |
| GiGA1 | GIRK1-containing channels (preference for GIRK1/GIRK2) | ↑ current in GIRK1/GIRK2, GIRK1/3 and GIRK1/GIRK4 expressing HEK293 [46] | ↓ neuronal excitability in mouse hippocampal brain slices [46] |
Abbreviations used: TBD- to be determined.
The effect of ML297 in vivo was investigated in several different behavioral assays, including those for epilepsy, anxiety, and insomnia (Figure 3). ML297 (60 mg/kg i.p.) exhibited improved efficacy compared to the anticonvulsant sodium valproate in both an electroshock and a pentylenetetrazol-induced mouse model of epilepsy, increasing the latency of seizure onset and preventing convulsions and lethality [25]. Huang and collaborators further demonstrated the antiepileptic effect of ML297 (30 mg/kg i.p.) [27]. ML297 diminished cyclothiazide-induced enhancement of neuronal excitability in cultured hippocampal neurons (Table 1), and reduced Racine score and extended seizure latency in a pilocarpine-induced mouse model of epilepsy [27]. In the elevated plus maze (EPM), ML297 (30 mg/kg i.p.) effectively reduced anxiety-like behavior in mice, and reduced stress-induced hyperthermia (Figure 3) [26]. These effects were absent in GIRK1 KO mice, implicating the action of ML297 on GIRK1-containing channels [26]. Moreover, ML297 did not affect locomotor activity in the open-field test, and did not show significant reinforcing effects in the conditioned place preference test at the studied dose (30 mg/kg i.p.). This suggested that the anxiolytic effects were not related to reduced locomotion due to sedation, nor to a rewarding effect of ML297 [26]. Recently, Zou et al investigated the potential for ML297 to treat insomnia, under the hypothesis that direct GIRK activation would avoid side-effects associated with the use of GABAB receptor agonists [28]. In hypothalamic brain slices, ML297 (10 μM) produced long-lasting hyperpolarization and blocked spontaneous firing of action potentials in hypocretin neurons (Table 1). Systemic injection of ML297 (30 mg/kg i.p.) reduced wake activity and locomotion and increased non-rapid eye movement (NREM) sleep in mice, without inducing spike-wave discharges (an absence seizure activity) or memory impairment (Figure 3) [28].
Figure 3: In vivo Biological Activities of Small Molecule GIRK Modulators.
A) Antiepileptic effect of GIRK activators ML297 and GiGA1 in chemically and/or electroshock induced epilepsy mouse models. B) Sleep-inductive effect of ML297 in mice, reducing wake activity, and increasing the nonrapid eye movement (NREM) sleep. C) Analgesic effect of VU0466551 in the hot-plate nociception test in mice. D) Reduction of the swimming motility in zebrafish larvae by LOGO5 and CLOGO under 365 nm or 420 nm light irradiation (respectively). E) Anxiolytic effect of ML297 in mice in the elevated plus maze (EPM) test, increasing the time spent in the open arm. F) Anxiolytic effect of ML297 and VU0810464 measured as reduction of stress-induced hyperthermia in mice. G) Fear extinction enhancement (reducing freezing behavior) of GAT1508 in rats trained in a Pavlovian conditioned fear paradigm.
Although ML297 was discovered with a GIRK-specific screening assay, it exhibits some modest off-target activity. ML297 was found to inhibit the hERG potassium channel (KV11.1), albeit at relatively high concentrations (IC50 ≈ 10 μM) [25]. In a radioligand binding assay, ML297 at 10 μM displayed modest activity on 5-HT2b, sigma σ1, and GABAA receptors [25]. Importantly, whole-cell electrophysiology on HEK293 cells expressing GABAA receptors showed no activation with ML297 [25]. ML297 exhibited modest (~3x’s) preference for activating neuronal GIRK1/GIRK2 over cardiac GIRK1/GIRK4 channels, which could produce undesired cardiovascular effects, but had no effect on GIRK2 homotetramers or Kir2.1 channels.
ML297-related GIRK Modulators
In recent years, a structure-based search of small molecule modulators of GIRK channels derived from ML297 yielded an extensive family of GIRK activators, with variable potency and selectivity (Figure 2A). The N-phenyl substituted pyrazole and the NH moieties of the urea linkage in ML297 appeared to be essential for GIRK activation (Figure 2A) [25]. Therefore, the first efforts focused on structure-activity relationship (SAR) studies and iterative parallel synthesis to generate a myriad of urea-bearing chemical families that behaved identically with regard to GIRK selectivity, activating only GIRK1-containing channels [29, 30]. For example, the N-benzyl pyrazole derivative VU0466551 was a more potent activator of GIRK1/GIRK2 channels (EC50 of 70 nM for VU0466551 vs. 160 nM for ML297), and retained selectivity for GIRK1-containing channels [29, 31].
VU0466551 was recently tested in vivo as a potential supplement to opioid analgesics in a mouse pain model [31, 32]. Abney and collaborators examined the potential analgesic efficacy of VU0466551 [31]. In two murine models of nociception, a 30 mg/kg injection of VU0466551 produced analgesic efficacy, either alone (formalin test) or in combination with sub-maximally effective doses of morphine (formalin and hotplate tests), enhancing the analgesia exerted by the opioid (Figure 3). Despite the weak off-target effect of VU0466551 on NaV1.7 channels [31], these findings highlight GIRK activation as a potential therapeutic approach for analgesia.
With the aim of designing improved pharmacological tools to dissect the function of GIRK channels, a photo-pharmacological approach led to the development of a series of photoswitchable versions of VU0259369, a hit molecule identified in a previous thallium-flux assay based HTS [33]. The new photoswitchable modulators enabled light control of GIRK activation [34, 35]. These GIRK activating compounds, based on the photoswitchable azobenzene moiety, were named LOGO for Light-Operated GIRK channel Opener [34], and VLOGO (Visible LOGO), a red-shifted variant [35]. Both act as photochromic GIRK1-containing channel openers in the dark, and the channel inactivates upon exposure to light of 365 (LOGO) or 420 nm (VLOGO). Photoswitching of LOGO5 or VLOGO reduced neuronal excitability in rodent hippocampal neurons, and diminished the motility of zebrafish larvae in vivo (Table 1, Figure 3) [34, 35]. In 2019, Trads et al described a diazozine version of photoswitchable GIRK openers, CLOGO (Cyclic azobenzene LOGO) [36]. CLOGO has an inverted pharmacological sign (inactive in the dark and active upon light exposure) which makes it more suitable as pharmacological tool. CLOGO reduced action potential firing in mouse neurons (Table 1) [36]. The main drawback of these photoswitchable GIRK modulators, however, is their lack of selectivity, since they act with similar potency on both GIRK1/GIRK2 and GIRK1/GIRK4 in the thallium flux assay [34–36].
Also based on ML297, Wieting and collaborators generated a novel library of GIRK1/GIRK2 activators that used an acetamide moiety instead of the urea scaffold to improve the selectivity and the pharmacokinetic properties [37]. The new acetamide VU0810464 showed nanomolar potency on GIRK1/GIRK2 (EC50 of 165 nM) and enhanced brain penetration [37, 38]. VU0810464 showed nine times more selectivity for GIRK channels expressed in mice hippocampal neurons than for those in cardiac sino-atrial node cells, indicating a stronger preference for GIRK1/GIRK2 over cardiac GIRK1/GIRK4 heterotetramers [38]. In vivo, 30 mg/kg VU0810464 reduced stress-induced hyperthermia in mice in a GIRK1-dependent manner, although it showed no anxiolytic effect in the EPM test, unlike ML297 [38] (Figure 3). The authors considered that this disparity in the anxiolytic effect of VU0810464 might be caused by its shorter half-life compared to ML297 (≈20 min for VU0810464, ≈40 min for ML297) [38], and by the complementary assessments of anxiety-related behavior provided by these tests. Nevertheless, VU0810464 shows promise for selectively targeting GIRK1/GIRK2 channels, and ongoing studies are using this scaffold to further improve this selectivity profile.
In an attempt to improve GIRK1/GIRK2 selectivity, Sharma et al generated a family of tetrazol-acetamides [39], based on a molecule from their first HTS [25]. These tetrazol-acetamides were shown to be GIRK1/GIRK2 activators in the thallium flux assay [39]. However, neither the selectivity nor the pharmacokinetic properties were significantly improved with this change of scaffold [39].
Recently, Xu and collaborators carried out a chemical optimization of ML297 and generated GAT1508, a bromo-substituted thiophene derivative that conserves the urea and the N-phenyl-pyrazole moieties, and improved the potency and selectivity for GIRK1/GIRK2 channels [40]. In electrophysiological recordings from HEK293 cells expressing either GIRK1/GIRK2 or GIRK1/GIRK4, GAT1508 activated GIRK1/GIRK2 more than ML297 (EC50 of 75 nM versus 110 nM for ML297) and showed little activation of GIRK1/GIRK4. Computational modeling and mutagenesis studies performed by Xu and collaborators [40] suggested that GAT1508 binds to the same residues in GIRK1 as ML297 [26] (Figure 1). Importantly, GAT1508 did not affect cardiovascular parameters in mice, including the atrial action potential duration, consistent with its lack of activity on cardiac GIRK1/GIRK4 channels [40]. GAT1508 perfusion increased barium-sensitive outward currents in basolateral amygdala neurons in brain slices (Table 1), and potentiated the effects of baclofen, supporting the action of GAT1508 on GIRK1/GIRK2 channels [40]. Given the role that the amygdala plays in fear conditioning, Xu and collaborators evaluated GAT1508 in rats using a Pavlovian conditioned fear paradigm, a preclinical model of post-traumatic stress disorder [41]. GAT1508 (10 and 30 mg/kg i.p.) facilitated fear extinction, without affecting cognitive or motor functions (Figure 3) [40]. In short, this new small molecule shows increased selectivity for GIRK1/GIRK2 channels, overcomes the undesired cardiac effects, and has great promise as a potential therapeutic for the treatment of post-traumatic stress disorders.
Although the predominant GIRK channels in the CNS are GIRK1/GIRK2 heterotetramers, other combinations of GIRK1-lacking channels, such as homomeric GIRK2 and heteromeric GIRK2/GIRK3, have been described in dopamine neurons of the substantia nigra and VTA, respectively, and play a key role in reward and addiction [42–44]. In light of the absence of GIRK1-lacking channels modulators, Kozek and colleagues conducted a HTS based on a thallium flux assay with HEK293 cells expressing homomeric GIRK2 [45]. This assay led to the discovery of VU0529331, the first synthetic small molecule that activates GIRK1-lacking channels [45]. In whole-cell patch-clamp recordings, VU0529331 increased GIRK2 and GIRK1/GIRK2 currents, though exerting a greater activation in homomeric GIRK2-expressing HEK293 cells (Table 1) [45]. VU0529331 also activated homomeric GIRK4 and GIRK1/GIRK4 channels with micromolar potencies. Given this lack of selectivity, the assessment of VU0529331 effects in cells naturally expressing these different GIRK channels would have been desired, but its low potency (around 5 μM) limited further studies. Furthermore, the authors observed off-target activity on ATP-gated Kir6.1/SUR2a and Kir6.1/SUR2b channels [45]. Nonetheless, the discovery of VU0529331 represents the first step in the development of GIRK1-lacking channels activators, such as homomeric GIRK2 or GIRK2/GIRK3, and could provide new insights into the role of these channels in addiction. Future SAR studies and chemical optimization of VU0529331 should be performed to achieve these goals.
Structure-based Discovery of GIRK Modulator, GiGA1
Recently, a GIRK1-specific modulator, named G-protein-independent GIRK activator 1 (GiGA1), was discovered through virtual screening against the alcohol pocket in GIRK2 [46]. Alcohol (ethanol) consumption leads to widespread effects not only in the central nervous system (CNS) but also in peripheral organs, like liver and heart. The simplicity of its structure and the lack of a binding site favored an initial hypothesis that the effects of ethanol are due to an alteration in plasma membrane fluidity. However, ethanol has minimal effects on membrane lipids at clinically relevant concentrations [47]. Ethanol interacts with potassium and calcium channels, as well as receptors for glutamate, γ-amino butyric acid (GABA), dopamine, and opioids [48]. In 1999, two different groups demonstrated that ethanol activates brain and cardiac GIRK channels [11, 12]. These researchers also provided evidence that other short-chain alcohols, such as methanol and propanol activated GIRK channels, but longer chain alcohols (> four-carbons) led to channel inhibition [11, 12], suggesting a finite capacity or size of a physical ethanol binding site [47].
In addition to the finding of direct activation of GIRK channels by alcohol, multiple animal studies have implicated GIRK channels, particularly GIRK2 and GIRK3 subunits, in alcohol abuse, as well as in psychostimulant addiction [19, 42]. In vivo studies with GIRK2 KO and GIRK2 weaver mice showed loss of ethanol-induced behaviors, such as anxiolytic and withdrawal effects [49], as well as analgesic effects [11, 50, 51]. GIRK2 KO mice did not develop a conditioned place preference for ethanol, exhibiting less sensitivity to its motivational effects [52]. GIRK3 KO mice, on the other hand, displayed conditioned place preference for ethanol [53] and enhanced ethanol binge drinking [19]. The activation of GIRK channels by alcohol and their demonstrated role in alcohol-related behaviors highlighted GIRK channels as a potential target for the study and treatment of alcohol abuse.
In support of direct activation of GIRK channels by alcohol, a crystal structure of an alcohol bound IRK1 (Kir2.1) channel enabled the elucidation of an alcohol binding pocket in GIRK2 [10, 54] (Figure 1B). The alcohol pocket is located in the subunit interface region and is formed by the N-terminal domain and the βD-βE sheets from one subunit and the βL-βM sheets from the other subunit (Figure 1B). A residue, mGIRK2 L257 in the βD-βE sheets, is critical for alcohol-dependent activation of GIRK channel [10].
The characterization of the alcohol pocket in GIRK2 provided for a new strategy to virtually screen and design new drugs based on the binding properties of alcohol to its pocket. Employing this strategy, Zhao et al built a homology model of alcohol-bound GIRK2 to perform virtual screening for identifying new GIRK modulators that target the alcohol pocket [46]. The initial screening identified a small molecule, NCATS_2, which activated both GIRK2 and GIRK1/GIRK2 channels in heterologous expression systems. Subsequent analog screening revealed a variant of NCATS_2, named GiGA1, that preferentially activated GIRK1-containing GIRK channels (Figure 2B). Like ML297, GiGA1 activation is G-protein-independent, does not alter inward rectification of GIRK1/GIRK2, and exhibits preference for activating GIRK1/GIRK2 channels, as compared to GIRK1/GIRK4 or GIRK1/GIRK3 (Table 1) [46].
Although ML297 also activates GIRK1-containing channels [25], there are several important differences to consider. In addition to differences in the method of discovery (Figure 2), GiGA1 showed different kinetics of modulation. While ML297 has a slow off-rate (t1/2 ≈ 20 s), GiGA1 exhibits fast (alcohol-like) activation and deactivation rates (2–3 s). This difference might be attributable to different binding mechanisms and affinity. It has been previously demonstrated that two amino acids in the membrane-spanning region of GIRK1 are critical for ML297 action on GIRK1/GIRK2 channels [26] (Figure 1B). Mutation of two amino acids in GIRK2 to the equivalent amino acid in GIRK1 enabled activation by ML297, though only in the context of co-expression with wild-type GIRK2 [26]. However, neither of these positions appeared to be important for GiGA1 activation. Since GiGA1 identification was based on the GIRK2 alcohol pocket, mutagenesis studies and related molecular dynamics simulations revealed critical residues in the alcohol pocket of GIRK1/GIRK2 channel for GiGA1 action (Figure 1B). In particular, a pair of amino acids (R43 on mGIRK1 and D346 on mGIRK2) located in the subunit interface region seems to contribute to the subunit specificity of GiGA1 [46].
Hippocampal slice recordings revealed that GiGA1 activated natively expressed GIRK channels in hippocampal pyramidal neurons and decreased neuronal excitability (Table 1) [46]. In an acute epilepsy mouse model, the systemic application of GiGA1 (40 and 60 mg/kg) significantly reduced the severity and duration of seizures (Figure 3) [46]. Systemic GiGA1 exhibited good brain penetration. The fairly rapid metabolism (i.e., ≈30-min half-life) makes GiGA1 well-suited for acute treatments and perhaps limits the potential side-effects of GiGA1. At high doses, GiGA1 produced a sedative effect, which may need to be mitigated in future studies. Nonetheless, GiGA1 exhibits potential in controlling abnormal neuronal excitability and preventing overexcitation-induced neuronal damage. Moreover, the screening method in this study can be used in the future to identify subunit-specific GIRK modulators, such as targeting GIRK2 and GIRK3 subunits expressed in SNc and VTA dopamine neurons. A GIRK2 homotetramer or GIRK2/GIRK3 heterotetramer-specific activator is highly desirable as a potential anti-addictive drug, given their role in controlling dopamine release in drug addiction [55].
Concluding Remarks and Future Perspectives
Over the past 25 years, there have been significant contributions to the understanding of GIRK channel pharmacology and the modulation of channel properties by various drugs. Until recently, however, a majority of the drugs which were found to modulate GIRK channels were developed for other targets, and were therefore difficult to study in vivo. The recent studies, detailed here, employed both structure-based and structure-activity based screening methods for identifying GIRK channel-specific drugs and represent a significant advance.
Moving forward, there are three aims for future studies; improve specificity, reduce off-target activity, and improve potency (see Outstanding Questions). Current studies on ML297-related SAR and GiGA1 analog analysis revealed the essential chemical groups on these two molecules. The initial optimization of ML297 has been focused on the modification of the aryl/heteroaryl group while leaving the essential urea linker and N-pyrazole intact. Subsequent studies determined that the substitution of urea linker improved subunit specificity. GiGA1 was developed by varying the number of methyl groups on the phenyl ring of NCATS_2. This small modification surprisingly increased the subunit specificity and potency of GiGA1. Despite the structural similarities between ML297 and GiGA1, the mutagenesis studies performed indicate different binding sites for these molecules. Further high-resolution structural studies of these drugs bound to GIRK1 containing channels will help further elucidate the structural features underlying their activity and selectivity. Moreover, future SAR studies and chemical optimization of the existing templates can be performed to improve the specificity of the drugs as well as targeting heterotetramers, such as GIRK1/GIRK3 or GIRK2/GIRK3, for which there are currently no known specific drugs. Additionally, drugs specific for GIRK1/GIRK4 could be developed to treat atrial fibrillation (AF). Thus far, most of the drugs developed are GIRK1/GIRK2 specific. Although these are the most prominent isoforms in the brain, drugs targeting GIRK2/GIRK3 channels in the VTA DA neurons could be useful for treating addiction. Mapping the effects of these drugs on the specific brain circuits will be important for tuning the targeting of these drugs, as well as to develop strategies to achieve not only subunit specificity, but also brain region specificity, in order to avoid undesired side-effects. Similarly, the potential side-effects due to non-cardiac peripheral expression of GIRK channels [56, 57] represent a challenge that should be addressed in the future regarding drugs targeting cardiac GIRK1/GIRK4. In parallel with drug development, functional, biochemical, and structural studies should be employed to better understand the site of action and the mechanism by which drug binding alters channel function. For instance, the isoform specificity and whether they are activators or inhibitors has been elucidated for many of these drugs. However, the structural and molecular mechanisms underlying these actions still remain unclear. Currently, all mechanisms are based on site-directed mutagenesis with guidance by computational models in some instances. Biochemical assays, such as chemical crosslinking and mass spectrometry, or solving structures of the current drugs bound to their targets using cryo-electron microscopy (CryoEM) or X-ray crystallography would help improve drug design. These data could then be used in a feedback loop to inform the optimization).
Outstanding Questions.
What are the structural mechanisms for selectivity and activation with ML297 and GiGA1 on GIRK1-containing channels, and are they different?
Will small molecules that act on endogenous modulators (e.g., Gβγ, cholesterol, PIP2) be more efficacious than compounds directly targeting the GIRK channel subunit?
Will the current screening methods for identifying GIRK modulators be successful in obtaining highly specific compounds for GIRK2 homotetramers and/or GIRK2/GIRK3 heterotetramers?
Can GIRK subunit-specific compounds be targeted to specific brain regions?
What are the next steps for improving the pharmacokinetics of GIRK channel modulators?
Lastly, improving pharmacokinetics will be essential for the next generation of drugs. Some of the drugs being developed are quickly metabolized or difficult to target to their desired brain region. A better understanding of the molecular and structural basis of how these drugs interact with GIRK channels and how they alter gating will be fundamentally important for providing new insights into the development of novel strategies that can be explored for future drug discovery.
Highlights.
GIRK channels are important regulators of cellular excitability and have been largely overlooked as potential therapeutic targets for treating neurological diseases.
Recent efforts have led to the identification of potent and selective small molecule modulators of GIRK channels.
GIRK-specific modulators display promising effects in vivo.
Identifying drugs for specific GIRK channel subunits remains a challenge.
Acknowledgements:
Illustrations created with BioRender.com. Some of the studies reported in this review were supported by National Institutes of Health (R01AA018734) to PAS.
GLOSSARY
- γ-amino butyric acid (GABA)
a primary inhibitory neurotransmitter in the brain that is an agonist for ionotropic GABAA receptors and metabotropic GABAB receptors
- Baclofen
a selective high-affinity agonist for the GABAB receptor
- Elevated plus maze (EPM)
an apparatus used to monitor the anxiety-related behavior in rodents, consistent of four elevated arms, two of which are enclosed by opaque walls, and the other two are open, the latter allowing the animal to perceive the height of the arm
- G-protein-coupled receptor (GPCR)
a seven-transmembrane domain protein that couples to heterotrimeric G proteins comprising α, β, and γ subunits. The α subunits are functionally divided into αi/o, αs, αq/11, and α12/13 families
- GABAB receptor
an obligate heterodimer of GABAB R1a/b and GABAB R2 receptor subunits that couples to Gi/o proteins
- Inward rectification
the electrophysiological property of a channel in which the flow of ions is much larger in one direction (i.e. inward) than in the other direction (i.e. outward)
- Non-rapid eye movement (NREM) sleep
a dreamless state of synchronization of the electroencephalogram during sleep where slow oscillations predominate, characterized by a decrease in global cerebral blood flow
- Phosphatidylinositol 4,5-bisphosphate (PIP2)
a membrane-bound phospholipid that is required for function of inwardly rectifying potassium channels
- Structure-activity relationship (SAR)
a method used to predict the biological activity of a chemical-based on the molecular structure. This tool is often required to guide the optimization of current undesirable chemical groups
Footnotes
Disclaimer Statement: Yulin Zhao, Isabel Gamerio-Ros, Ian W. Glaaser and Paul A. Slesinger have no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kubo Y et al. (1993) Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364 (6440), 802–6. [DOI] [PubMed] [Google Scholar]
- 2.Lesage F et al. (1995) Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem 270 (48), 28660–7. [DOI] [PubMed] [Google Scholar]
- 3.Luscher C and Slesinger PA (2010) Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11 (5), 301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lujan R and Aguado C (2015) Localization and Targeting of GIRK Channels in Mammalian Central Neurons. Int Rev Neurobiol 123, 161–200. [DOI] [PubMed] [Google Scholar]
- 5.Liao YJ et al. (1996) Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci 16 (22), 7137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz HG et al. (2004) Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci 7 (2), 153–9. [DOI] [PubMed] [Google Scholar]
- 7.Wickman K et al. (1998) Abnormal heart rate regulation in GIRK4 knockout mice. Neuron 20 (1), 103–14. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis DE et al. (1987) The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325 (6102), 321–6. [DOI] [PubMed] [Google Scholar]
- 9.Reuveny E et al. (1994) Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature 370 (6485), 143–6. [DOI] [PubMed] [Google Scholar]
- 10.Aryal P et al. (2009) A discrete alcohol pocket involved in GIRK channel activation. Nat Neurosci 12 (8), 988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T et al. (1999) Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci 2 (12), 1091–7. [DOI] [PubMed] [Google Scholar]
- 12.Lewohl JM et al. (1999) G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci 2 (12), 1084–90. [DOI] [PubMed] [Google Scholar]
- 13.Glaaser IW and Slesinger PA (2017) Dual activation of neuronal G protein-gated inwardly rectifying potassium (GIRK) channels by cholesterol and alcohol. Sci Rep 7 (1), 4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bukiya AN et al. (2017) Cholesterol up-regulates neuronal G protein-gated inwardly rectifying potassium (GIRK) channel activity in the hippocampus. J Biol Chem 292 (15), 6135–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Signorini S et al. (1997) Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A 94 (3), 923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath GA et al. (2018) Gain-of-function KCNJ6 Mutation in a Severe Hyperkinetic Movement Disorder Phenotype. Neuroscience 384, 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y et al. (2010) Identification of a Kir3.4 mutation in congenital long QT syndrome. Am J Hum Genet 86 (6), 872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rifkin RA et al. (2018) GIRK currents in VTA dopamine neurons control the sensitivity of mice to cocaine-induced locomotor sensitization. Proc Natl Acad Sci U S A 115 (40), E9479–E9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman MA et al. (2015) GIRK3 gates activation of the mesolimbic dopaminergic pathway by ethanol. Proc Natl Acad Sci U S A 112 (22), 7091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whorton MR and MacKinnon R (2011) Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147 (1), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whorton MR and MacKinnon R (2013) X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498 (7453), 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pegan S et al. (2005) Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci 8, 279–287. [DOI] [PubMed] [Google Scholar]
- 23.Pegan S et al. (2006) Andersen’s syndrome mutation effects on the structure and assembly of the cytoplasmic domains of Kir2.1. Biochemistry 45 (28), 8599–606. [DOI] [PubMed] [Google Scholar]
- 24.Niswender CM et al. (2008) A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol 73 (4), 1213–24. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann K et al. (2013) ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci 4 (9), 1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wydeven N et al. (2014) Mechanisms underlying the activation of G-protein-gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proc Natl Acad Sci U S A 111 (29), 10755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y et al. (2018) GIRK1-mediated inwardly rectifying potassium current suppresses the epileptiform burst activities and the potential antiepileptic effect of ML297. Biomed Pharmacother 101, 362–370. [DOI] [PubMed] [Google Scholar]
- 28.Zou B et al. (2019) Direct activation of G-protein-gated inward rectifying K+ channels promotes nonrapid eye movement sleep. Sleep 42 (3), zsy244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen W et al. (2013) Discovery of ‘molecular switches’ within a GIRK activator scaffold that afford selective GIRK inhibitors. Bioorg Med Chem Lett 23 (16), 4562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen W et al. (2014) Discovery of potent and selective GIRK1/2 modulators via ‘molecular switches’ within a series of 1-(3-cyclopropyl-1-phenyl-1H-pyrazol-5-yl)ureas. Bioorg Med Chem Lett 24 (21), 5102–6. [DOI] [PubMed] [Google Scholar]
- 31.Abney KK et al. (2019) Analgesic Effects of the GIRK Activator, VU0466551, Alone and in Combination with Morphine in Acute and Persistent Pain Models. ACS Chem Neurosci 10 (3), 1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marker CL et al. (2004) Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci 24 (11), 2806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos-Hunter SJ et al. (2013) Discovery and SAR of a novel series of GIRK1/2 and GIRK1/4 activators. Bioorg Med Chem Lett 23 (18), 5195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barber DM et al. (2016) Optical control of neuronal activity using a light-operated GIRK channel opener (LOGO). Chem Sci 7 (3), 2347–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trads JB et al. (2016) Optical control of GIRK channels using visible light. Org Biomol Chem 15 (1), 76–81. [DOI] [PubMed] [Google Scholar]
- 36.Trads JB et al. (2019) Sign Inversion in Photopharmacology: Incorporation of Cyclic Azobenzenes in Photoswitchable Potassium Channel Blockers and Openers. Angew Chem Int Ed Engl 58 (43), 15421–15428. [DOI] [PubMed] [Google Scholar]
- 37.Wieting JM et al. (2017) Discovery and Characterization of 1H-Pyrazol-5-yl-2-phenylacetamides as Novel, Non-Urea-Containing GIRK1/2 Potassium Channel Activators. ACS Chem Neurosci 8 (9), 1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vo BN et al. (2019) VU0810464, a non-urea G protein-gated inwardly rectifying K(+) (Kir 3/GIRK) channel activator, exhibits enhanced selectivity for neuronal Kir 3 channels and reduces stress-induced hyperthermia in mice. Br J Pharmacol 176 (13), 2238–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S et al. (2019) Discovery, synthesis and characterization of a series of (1-alkyl-3-methyl-1H-pyrazol-5-yl)-2-(5-aryl-2H-tetrazol-2-yl)acetamides as novel GIRK1/2 potassium channel activators. Bioorg Med Chem Lett 29 (6), 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y et al. (2020) The small molecule GAT1508 activates brain-specific GIRK1/2 channel heteromers and facilitates conditioned fear extinction in rodents. J Biol Chem 295 (11), 3614–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahan AL and Ressler KJ (2012) Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35 (1), 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rifkin RA et al. (2017) G Protein-Gated Potassium Channels: A Link to Drug Addiction. Trends Pharmacol Sci 38 (4), 378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotecki L et al. (2015) GIRK Channels Modulate Opioid-Induced Motor Activity in a Cell Type- and Subunit-Dependent Manner. J Neurosci 35 (18), 7131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCall NM et al. (2019) GIRK Channel Activity in Dopamine Neurons of the Ventral Tegmental Area Bidirectionally Regulates Behavioral Sensitivity to Cocaine. J Neurosci 39 (19), 3600–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozek KA et al. (2019) Discovery and Characterization of VU0529331, a Synthetic Small-Molecule Activator of Homomeric G Protein-Gated, Inwardly Rectifying, Potassium (GIRK) Channels. ACS Chem Neurosci 10 (1), 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y et al. (2020) Identification of a G-Protein-Independent Activator of GIRK Channels. Cell Rep 31 (11), 107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris RA et al. (2008) Ethanol’s molecular targets. Sci Signal 1 (28), re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abrahao KP et al. (2017) Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 96 (6), 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blednov YA et al. (2001) Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther 298 (2), 521–30. [PubMed] [Google Scholar]
- 50.Blednov YA et al. (2003) A pervasive mechanism for analgesia: Activation of GIRK2 channels. Proc Natl Acad Sci U S A 100 (1), 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda K et al. (2002) Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys? Neurosci Res 44 (2), 121–131. [DOI] [PubMed] [Google Scholar]
- 52.Hill KG et al. (2003) Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology (Berl) 169 (1), 108–14. [DOI] [PubMed] [Google Scholar]
- 53.Tipps ME et al. (2016) G Protein-Gated Inwardly Rectifying Potassium Channel Subunit 3 Knock-Out Mice Show Enhanced Ethanol Reward. Alcohol Clin Exp Res 40 (4), 857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bodhinathan K and Slesinger PA (2013) Molecular mechanism underlying ethanol activation of G-protein-gated inwardly rectifying potassium channels. Proc Natl Acad Sci U S A 110 (45), 18309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luscher C and Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69 (4), 650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwanir S and Reuveny E (2008) Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels. Pflugers Arch 456 (6), 1097–108. [DOI] [PubMed] [Google Scholar]
- 57.Shankar H et al. (2004) Role of G protein-gated inwardly rectifying potassium channels in P2Y12 receptor-mediated platelet functional responses. Blood 104 (5), 1335–43. [DOI] [PubMed] [Google Scholar]
- 58.Magyar J and Szabo G (1996) Effects of volatile anesthetics on the G protein-regulated muscarinic potassium channel. Mol Pharmacol 50 (6), 1520–8. [PubMed] [Google Scholar]
- 59.Milovic S et al. (2004) The sensitivity of G protein-activated K+ channels toward halothane is essentially determined by the C terminus. J Biol Chem 279 (33), 34240–9. [DOI] [PubMed] [Google Scholar]
- 60.Yamakura T et al. (2001) Differential effects of general anesthetics on G protein-coupled inwardly rectifying and other potassium channels. Anesthesiology 95 (1), 144–53. [DOI] [PubMed] [Google Scholar]
- 61.Weigl LG and Schreibmayer W (2001) G protein-gated inwardly rectifying potassium channels are targets for volatile anesthetics. Mol Pharmacol 60 (2), 282–9. [DOI] [PubMed] [Google Scholar]
- 62.Nimitvilai S et al. (2016) Differential Effects of Toluene and Ethanol on Dopaminergic Neurons of the Ventral Tegmental Area. Front Neurosci 10, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kollert S et al. (2020) Chloroform is a potent activator of cardiac and neuronal Kir3 channels. Naunyn Schmiedebergs Arch Pharmacol 393 (4), 573–580. [DOI] [PubMed] [Google Scholar]
- 64.Patel D et al. (2020) Structural Determinants Mediating Tertiapin Block of Neuronal Kir3.2 Channels. Biochemistry 59 (7), 836–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yow TT et al. (2011) Naringin directly activates inwardly rectifying potassium channels at an overlapping binding site to tertiapin-Q. Br J Pharmacol 163 (5), 1017–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell WC et al. (1983) Ivermectin: a potent new antiparasitic agent. Science 221 (4613), 823–8. [DOI] [PubMed] [Google Scholar]
- 67.Su Z et al. (2016) Novel cell-free high-throughput screening method for pharmacological tools targeting K+ channels. Proc Natl Acad Sci U S A 113 (20), 5748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen IS and Kubo Y (2018) Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol 596 (10), 1833–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osborne NN et al. (1998) Flupirtine, a nonopioid centrally acting analgesic, acts as an NMDA antagonist. Gen Pharmacol 30 (3), 255–63. [DOI] [PubMed] [Google Scholar]
- 70.Klinger F et al. (2012) Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine. Br J Pharmacol 166 (5), 1631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedel HA and Fitton A (1993) Flupirtine. A review of its pharmacological properties, and therapeutic efficacy in pain states. Drugs 45 (4), 548–69. [DOI] [PubMed] [Google Scholar]
- 72.Kornhuber J et al. (1999) Flupirtine shows functional NMDA receptor antagonism by enhancing Mg2+ block via activation of voltage independent potassium channels. Rapid communication. J Neural Transm (Vienna) 106 (9–10), 857–67. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi T et al. (2000) Inhibition by various antipsychotic drugs of the G-protein-activated inwardly rectifying K(+) (GIRK) channels expressed in xenopus oocytes. Br J Pharmacol 129 (8), 1716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kobayashi T et al. (2004) Modulators of G protein-activated inwardly rectifying K+ channels: potentially therapeutic agents for addictive drug users. Ann N Y Acad Sci 1025, 590–4. [DOI] [PubMed] [Google Scholar]
- 75.Khan AH and Zaidi S (2017) Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus 9 (12), e1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobayashi T et al. (2003) Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac). Br J Pharmacol 138 (6), 1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kobayashi T et al. (2006) Inhibition of G protein-activated inwardly rectifying K+ channels by the antidepressant paroxetine. J Pharmacol Sci 102 (3), 278–87. [DOI] [PubMed] [Google Scholar]
- 78.Wong DT et al. (1995) Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci 57 (5), 411–41. [DOI] [PubMed] [Google Scholar]
- 79.Perchenet L et al. (2001) Effects of anorexinogen agents on cloned voltage-gated K(+) channel hKv1.5. J Pharmacol Exp Ther 298 (3), 1108–19. [PubMed] [Google Scholar]
- 80.Thomas D et al. (2002) The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J Pharmacol Exp Ther 300 (2), 543–8. [DOI] [PubMed] [Google Scholar]
- 81.Takahama K (2012) Multiple pharmacological actions of centrally acting antitussives--Do they target G protein-coupled inwardly rectifying K(+) (GIRK) channels? J Pharmacol Sci 120 (3), 146–51. [DOI] [PubMed] [Google Scholar]
- 82.Sasaki T et al. (2014) Tipepidine in children with attention deficit/hyperactivity disorder: a 4-week, open-label, preliminary study. Neuropsychiatr Dis Treat 10, 147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamasaki R et al. (2013) Tipepidine activates VTA dopamine neuron via inhibiting dopamine D(2) receptor-mediated inward rectifying K(+) current. Neuroscience 252, 24–34. [DOI] [PubMed] [Google Scholar]
- 84.Soeda F et al. (2016) Centrally acting non-narcotic antitussives prevent hyperactivity in mice: Involvement of GIRK channels. Pharmacol Biochem Behav 144, 26–32. [DOI] [PubMed] [Google Scholar]
- 85.Hamao K et al. (2015) Tipepidine increases dopamine level in the nucleus accumbens without methamphetamine-like behavioral sensitization. Behav Brain Res 284, 118–24. [DOI] [PubMed] [Google Scholar]
- 86.Jin W and Lu Z (1998) A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 37 (38), 13291–9. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto N et al. (2006) Tertiapin, a selective IKACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacol Res 54 (2), 136–41. [DOI] [PubMed] [Google Scholar]
- 88.Walsh KB (2011) Targeting GIRK Channels for the Development of New Therapeutic Agents. Front Pharmacol 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuzhikandathil EV and Oxford GS (2002) Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol 62 (1), 119–26. [DOI] [PubMed] [Google Scholar]
- 90.Matsuda T et al. (2006) Blockade by NIP-142, an antiarrhythmic agent, of carbachol-induced atrial action potential shortening and GIRK1/4 channel. J Pharmacol Sci 101 (4), 303–10. [DOI] [PubMed] [Google Scholar]
- 91.Machida T et al. (2011) Effects of a highly selective acetylcholine-activated K+ channel blocker on experimental atrial fibrillation. Circ Arrhythm Electrophysiol 4 (1), 94–102. [DOI] [PubMed] [Google Scholar]
- 92.Yamamoto W et al. (2014) Effects of the selective KACh channel blocker NTC-801 on atrial fibrillation in a canine model of atrial tachypacing: comparison with class Ic and III drugs. J Cardiovasc Pharmacol 63 (5), 421–7. [DOI] [PubMed] [Google Scholar]
- 93.Chen IS et al. (2019) Non-sedating antihistamines block G-protein-gated inwardly rectifying K(+) channels. Br J Pharmacol 176 (17), 3161–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woodward JK and Munro NL (1982) Terfenadine, the first non-sedating antihistamine. Arzneimittelforschung 32 (9a), 1154–6. [PubMed] [Google Scholar]
- 95.Taglialatela M et al. (1999) Cardiac ion channels and antihistamines: possible mechanisms of cardiotoxicity. Clin Exp Allergy 29 Suppl 3, 182–9. [DOI] [PubMed] [Google Scholar]