Abstract
BACKGROUND AND PURPOSE:
Middle ear surgery is often performed through the external auditory canal, and the CT appearance of the external auditory canal after transcanal middle ear surgery can mimic erosive pathology such as carcinoma, external auditory canal cholesteatoma, or necrotizing external otitis. We reviewed the CT findings in a group of patients following transcanal surgery to highlight this potential pitfall in interpretation.
MATERIALS AND METHODS:
Twenty-seven temporal bones in 25 patients with a history of a transcanal approach to the middle ear and available postoperative CT imaging were identified. Images were assessed for changes along or involving the walls of the external auditory canal, including widening, irregularity, bony defects, and soft tissue opacification.
RESULTS:
Osseous changes along the floor of the external auditory canal were demonstrated in 25 of 27 (92.6%) temporal bone CT scans. Similar changes were present in the superior and anterior walls of the external auditory canal in 21 and 18 temporal bones, respectively. The anterior wall was the most common site for complete bony defects (10 of 27 temporal bones). The posterior wall was the least often involved, with osseous changes in 15 of 27 temporal bones and bony defects in 3 cases. Soft tissue thickening was seen most commonly along the floor. No patient was found to have a superimposed pathologic process of the external auditory canal.
CONCLUSIONS:
CT findings in the external auditory canal after transcanal surgery include thinning, irregularity and/or flattening of the bone, soft tissue thickening, and bony wall defects. Although these changes may be subtle, they may mimic pathology and should be included in the differential diagnosis of osseous abnormality of the external auditory canal.
Middle ear surgery performed through the external auditory canal (EAC) often involves drilling a portion of the bony canal wall to provide access and necessary exposure.1–3 In the absence of associated transmastoid surgery (such as a canal wall down mastoidectomy), the postoperative status may not be immediately obvious to the interpreting radiologist, and relevant history may not be provided. On CT, such postoperative changes in the external canal can mimic bony and soft tissue changes typically associated with neoplasms, external canal cholesteatoma, or aggressive infections of the EAC. Prior literature has predominantly focused on the appearance of the middle ear after surgery.4–11 We describe the CT appearance of the EAC after transcanal surgery so that postoperative change can be included in the differential diagnosis, even in the absence of available history, and erroneous diagnoses may be avoided.
Materials and Methods
Patients
This retrospective study was performed in accordance with the Health Insurance Portability and Accountability Act. Twenty-five patients with a history of transcanal middle ear surgery (including 2 patients with a history of bilateral surgery) and subsequent postoperative CT imaging were retrospectively identified from an imaging data base. Postoperative imaging was performed during a 7-year period from July 2007 to April 2014. Confirmation of transcanal surgery with an operative report and/or clinical surgical note describing the alterations/drilling of the EAC was necessary for inclusion. Patients with a history of prior canal wall down mastoidectomy were excluded, given the distinctive appearance of the removal of the posterosuperior wall of the EAC. Patients with a history of surgery for a primary EAC indication (such as exostosis removal or repair of EAC stenosis) rather than middle ear surgery were also excluded. The electronic medical record was reviewed for otologic history, clinical examination findings, and operative reports, in addition to demographic data.
CT Technique
All 25 patients underwent dedicated temporal bone CT without intravenous contrast. Of 27 temporal bones, 19 were imaged on a 40-section multidetector CT scanner (Somatom Sensation; Siemens, Erlangen, Germany). Scanning parameters were 120 kV(peak), 320 mAs at 0.6-mm collimation, and a 0.55 pitch with helical acquisition extending from just superior to the petrous ridge through the inferior skull base. In pediatric patients, 120 mAs was used to decrease the radiation dose. Data from each ear were reconstructed into 0.6 (section thickness) × 0.2 mm (reconstruction interval) axial images in a bone algorithm at a display FOV of 100 mm and a matrix of 512 × 512. The technologist then created standardized axial and coronal reformatted images along the plane of the lateral semicircular canal at the scanner console by using the sagittal images as a reference. The remaining 8 of 27 temporal bones were imaged on a digital volume tomography (conebeam) scanner (3D Accuitomo 170; J. Morita, Osaka, Japan). Scanning parameters were 90 kVp, 8 mA, 30.8-second rotation time, with a 60 × 60 mm FOV. The raw data voxel size was 0.5 × 0.08 × 0.08 mm, and the same standardized reformatted images were created in the axial and coronal planes, as described above.
Image Analysis
The axial and coronal reformatted images from each temporal bone were reviewed on the PACS of our institution. All CT images were independently reviewed by 2 neuroradiologists (V.M. with 4 years of experience and board-certified in Thailand; H.R.K. with 7 years of experience with a Certificate of Added Qualification in neuroradiology). The images were reviewed for cortical change (including flattening of the floor of the EAC with apparent loss of the tympanic sulcus, thinning, and/or irregularity), bony defects, and soft tissue thickening along each EAC wall (anterior, posterior, superior, and inferior). Preoperative imaging was used for comparison if available. Comparison was also made with the contralateral side if imaged and if asymptomatic by history. A bony defect was defined as focal discontinuity of the anterior and/or inferior EAC wall. For the superior and posterior walls, a bony defect was defined as a loss of the bony plate covering the mastoid air cells. Measurement of the maximal defect size of each wall was also performed, by using the axial plane for the anterior and posterior walls and the coronal plane for the superior and inferior walls. Any soft tissue opacity along the bony EAC walls was also recorded. The tympanic membrane was also assessed for thickening and/or calcification. The absence of any ossicles or the presence of an ossicular prosthesis or both were also recorded. Interobserver agreement was calculated by using the κ statistic. Discrepancies were resolved by consensus and with additional adjudication by an experienced head and neck radiologist (H.D.C., board-certified, with >30 years of experience and a Certificate of Added Qualification in neuroradiology).
Results
Of the 25 patients included in the study, 14 were male and 13 were female, with ages ranging from 8 to 87 years (median age, 47 years). The time interval between operative intervention and CT ranged from 2 months to 30 years (median interval, 4 years). The surgical procedure in 25 of the 27 temporal bones was tympanoplasty, with 7 of these patients also undergoing canal wall up mastoidectomy, 4 undergoing additional canaloplasty, and 3 undergoing additional atticotomy. One operation included canal wall up mastoidectomy and canaloplasty, while a single patient was status/post transcanal resection of a glomus tympanicum. Other than this last patient, the indications for surgery included chronic otitis media, middle ear cholesteatoma, and chronic tympanic membrane perforation. Indications for imaging (as indicated by the imaging requisition submitted by the referring surgeon) included chronic otitis media, evaluate cholesteatoma (n = 14); hearing loss with or without additional history of chronic otitis media included (n = 7); ear pain and drainage (n = 2); tympanic membrane not visible on examination with external canal stenosis, evaluate middle ear involvement (n = 1); possible middle ear mass on examination (n = 1); otorrhea, evaluate CSF leak (n = 1); and recurrent pulsatile tinnitus and feeling of blockage in ear, evaluate recurrent glomus tympanicum (n = 1).
Preoperative imaging was only available for comparison in 4 of the 25 patients/27 temporal bones. All 27 temporal bones demonstrated osseous changes along at least 1 wall of the EAC. A widened appearance of the canal was also observed in all 27 temporal bones. The most common site for osseous changes in the EAC was the floor (inferior wall), found in 25 of 27 temporal bones (92.6%). These changes included mild flattening and/or slight irregularity with thinning of the floor and loss of the normal tympanic sulcus (Fig 1) or more extensive bony irregularity mimicking erosive change (Fig 2). The relatively flat and wide appearance of the canal with predominant involvement of the EAC floor and loss of the tympanic sulcus was present in 17 of 25. This was the most common overall finding in the cohort, seen in 63% of cases (17 of 27 total temporal bones). In the 8 cases with more extensive bony irregularity along the floor of the EAC, focal bony defects were present, ranging from 1 to 8.1 mm (average, 3.3 mm). Similar osseous changes were seen in the superior and anterior walls of the EAC in 21 and 18 temporal bones (77.8% and 66.7%, respectively). The anterior wall was the most common location for osseous defects, occurring in 10 of 27 temporal bones (37%). The posterior wall was the least common site for osseous findings, found in 15 of 27 temporal bones (55.6%), with only 3 (11.1%) demonstrating osseous defects.
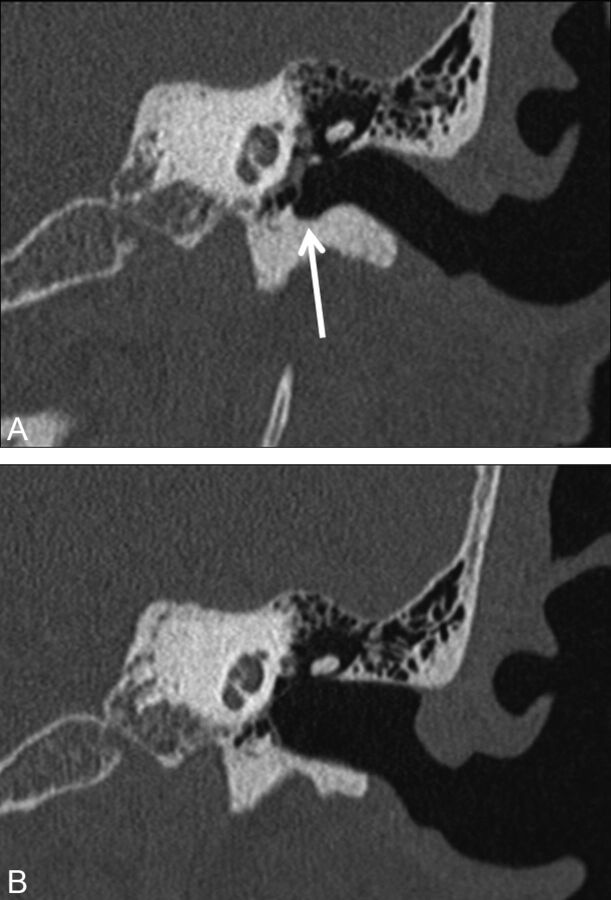
Fig 1.
A 59-year-old woman with history of left chronic otitis media and recurrent cholesteatoma status/post left-sided transcanal tympanoplasty. Images were obtained before surgery and 2 years after surgery. The preoperative coronal image (A) demonstrates the normal curvature of the walls of the external auditory canal and the normal tympanic sulcus (arrow), while the postoperative coronal image (B) demonstrates flattening and smoothing of the superior wall and floor of the external canal, with loss of the normal tympanic sulcus inferiorly.
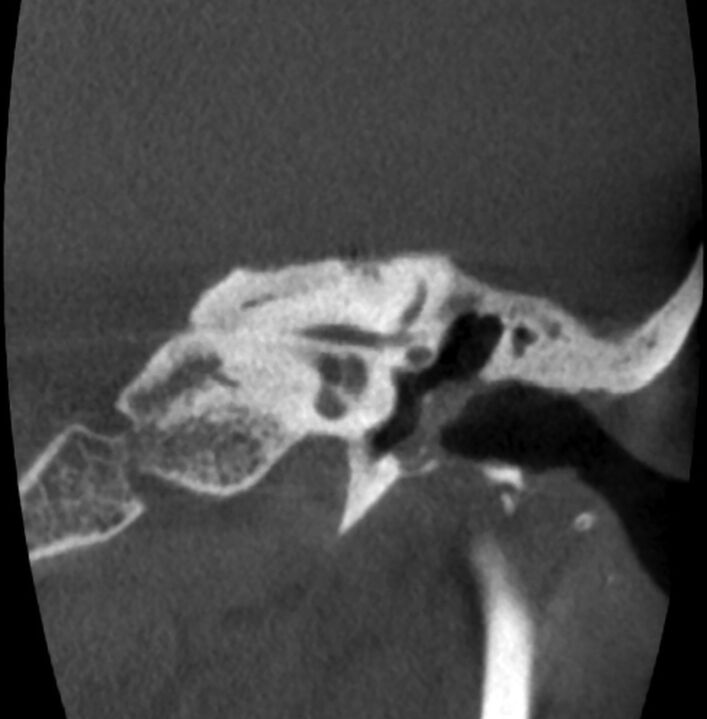
Fig 2.
A 59-year-old woman with a history of left chronic otitis media status/post tympanoplasty 8 years before conebeam CT imaging. Irregularity and loss of the normal bony cortex are demonstrated along the EAC floor, with a focal bony defect and mild adjacent soft tissue opacification abutting the tympanic membrane. No active infection or cholesteatoma was present on otologic follow-up.
Soft tissue thickening was also most common along the inferior EAC wall, present in 18 of 27 temporal bones (66.7%). Soft tissue thickening was seen along the anterior, superior, and posterior walls slightly less commonly (14, 12, and 12 temporal bones, respectively). The presence of soft tissue thickening within the EAC did not correlate with the time interval from surgery and was seen in instances of both recent and remote operative interventions.
Thickening of the tympanic membrane was also observed in 17 of 27 temporal bones and, in all cases, was associated with prior tympanoplasty. In 4 of these 17, calcification of the tympanic membrane was also present. In the remaining cases, no thickening was demonstrated or the thickness of the tympanic membrane could not be assessed adequately due to adjacent soft tissue attenuation in the external canal or middle ear (3 cases).
In 3 cases, partial ossicular prostheses were in place, with the stapes present but absence of the malleus and incus in all 3. In 2 cases, total ossicular prostheses were present, with absence of all ossicles. In 1 case, the incus was not in the normal position and an osseous structure seen between the stapes and the tympanic membrane was postulated to represent an incus interposition graft. In the remaining 21 temporal bones, the ossicles were present and no prostheses were identified.
Interobserver agreement was excellent (κ = 0.80; 95% CI, 0.7–0.9). Discrepancies regarding the presence of bony change and/or soft tissue attenuation along ≥1 wall of the EAC involved a miss by 1 of the 2 readers for all cases.
In 3 of the official radiology reports, the prior surgical history was not known or postulated by the interpreting radiologist (Fig 3). In the remaining reports, prior surgery in the external canal was postulated, though additional differential diagnostic considerations were included in many cases. In these cases, pathologic processes of the EAC were included in the differential diagnosis, including cholesteatoma, keratosis obturans, and external otitis.
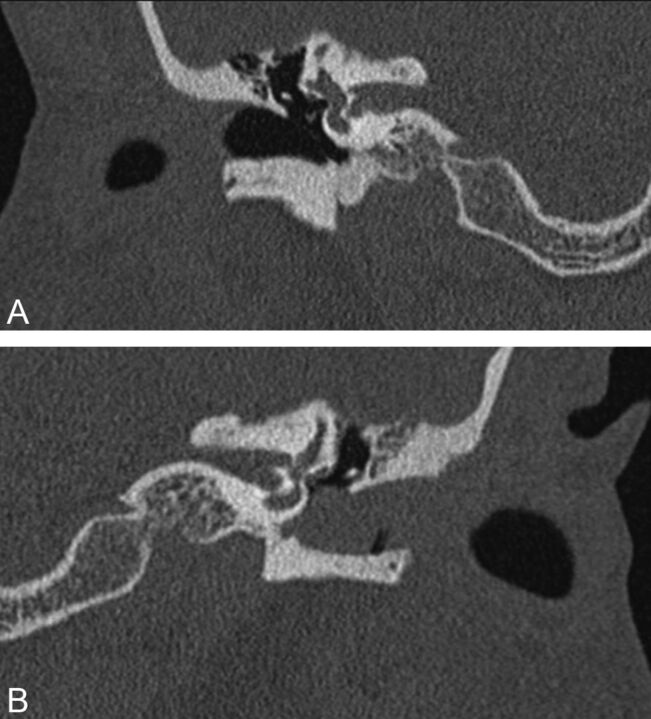
Fig 3.
A 51-year-old man with a history of chronic otitis media status/post left tympanoplasty (with total drum replacement) 6 months before multidetector row CT imaging. The normal right temporal bone is shown in the coronal image on the right (A). Coronal image of the left temporal bone (B) demonstrates soft tissue filling the EAC, with smooth “erosion” of the anterior and inferior EAC walls. The patient developed soft tissue stenosis of the EAC postoperatively, related to a hypersensitivity reaction to antibiotic drops, without infection or cholesteatoma on clinical follow-up. The interpreting radiologist did not have the clinical history and interpreted the findings as probable EAC cholesteatoma.
Although a few patients required additional procedures after CT for recurrent middle ear disease, none of the patients included in our study had pathologic findings in the EAC at clinical/surgical follow-up. Only chronic postoperative changes were observed in the EAC in all patients by their referring otologists per the electronic medical record. One patient developed chronic soft tissue stenosis of the EAC after surgery (Fig 3).
Discussion
The EAC is an S-shaped tubular structure, typically 16–25 mm in length and 7–10 mm in diameter, extending from the meatus of the pinna to the tympanic membrane. The lateral one-third is fibrocartilaginous, while the medial two-thirds is osseous, formed by 3 segments of the temporal bone: the squamous, mastoid, and tympanic segments. There are 2 physiologic narrowings in the EAC: one at the isthmus (the junction between fibrocartilaginous and bony portions) and another medially adjacent to the tympanic membrane. At the medial end of the EAC is a rise in the floor of the EAC adjacent to a narrow furrow, the tympanic sulcus.12–14
Surgery is widely used to treat various middle ear conditions, including inflammatory disease, congenital malformations, trauma, and tumors.4,5 Surgical approaches to the middle ear through the EAC may involve drilling the bony EAC walls to provide adequate exposure.1–3 Extensive literature describes the postoperative appearance of the middle ear after such surgeries4–11 and more extensive surgeries involving the external canal, such as canal wall down mastoidectomy. We undertook a detailed description of the CT findings in the EAC after transcanal surgery. Patients with canal wall down mastoidectomy were excluded. The surgeries performed included total drum replacement and atticotomy, with changes that are much more subtle than more extensive tympanomastoid surgeries.
The typical sites for bony removal of the EAC walls at transcanal surgery are the anterosuperior and anteroinferior walls (Fig 4), usually with further dissection of the inferior wall.1,2 In our series, osseous changes were observed along the inferior EAC margin most frequently, followed by the superior and anterior walls. The posterior wall was the least commonly observed site for osseous changes, bony defects, and soft tissue opacity. These findings correlate with the typical surgical approaches and may be subtle, with only mild bony changes seen. In these cases, we have found comparison with the contralateral normal side helpful, especially if preoperative images are not available.
Fig 4.
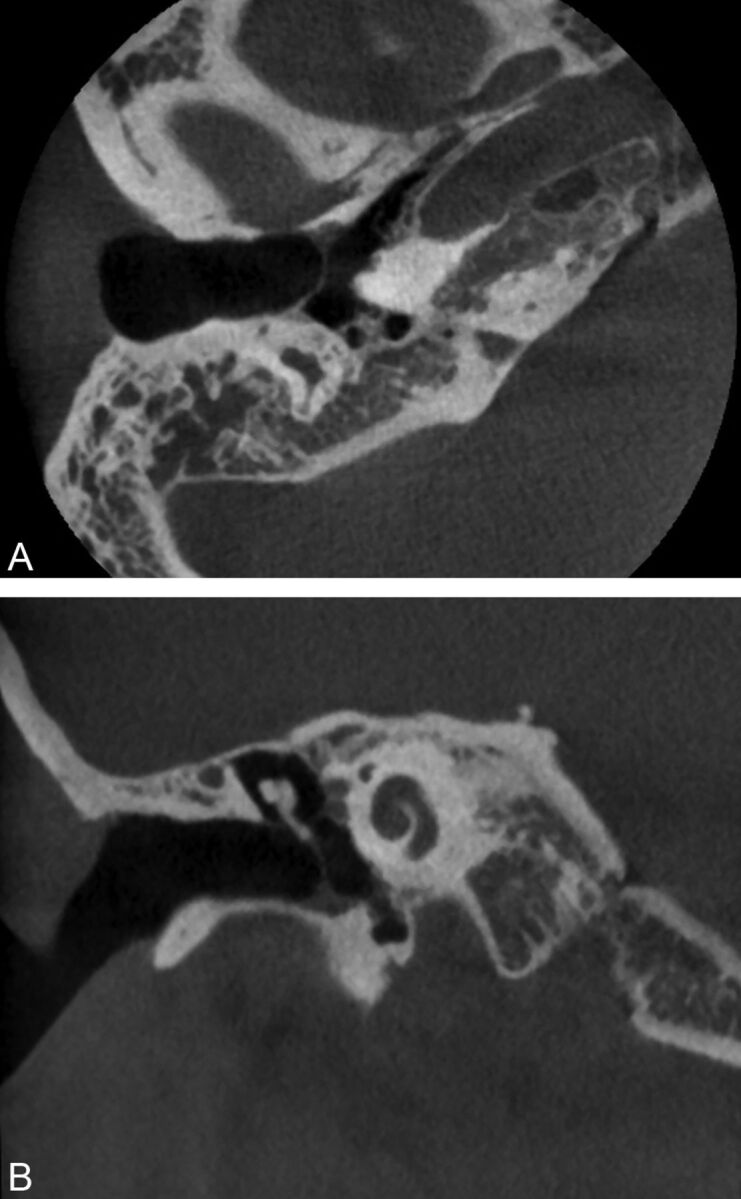
A 20-year-old woman with a history of bilateral chronic otitis media status/post right-sided transcanal tympanoplasty 10 years before conebeam CT. The axial image (A) demonstrates a small bony defect along the anterior wall, while the coronal image (B) demonstrates rounded bony change along floor with soft tissue at the base of the tympanic membrane. No infection or cholesteatoma was present at follow-up.
In our experience, postoperative findings in the EAC may mimic pathologic processes, including cholesteatoma, infection (including malignant [necrotizing] otitis externa), malignancy, radiation necrosis, and granulomatosis with polyangiitis (Wegener granulomatosis). The subtle osseous irregularities may be misinterpreted if the radiologist is not aware of the typical appearance of the EAC after transcanal surgery and/or does not have the clinical history at the time of imaging.
At CT, EAC cholesteatoma commonly presents as a soft tissue mass with associated bony erosion and may be either smooth or irregular, with intramural bony fragments.14–16 The inferior and posterior EAC walls are the most common sites of origin of the soft tissue mass, though they can be circumferential.15 The amount of soft tissue attenuation material associated with the osseous changes may be minimal because cholesteatoma extends under the periosteum. The osseous changes in EAC cholesteatoma are typically focal, whereas the postoperative changes observed in our cohort typically involved a longer segment of the EAC margin, though findings may overlap significantly and the operative history may help distinguish these 2 entities.
Malignant tumors of the EAC are rare, with squamous cell carcinoma the most common histologic type. The inferior EAC wall is the most common site of disease, followed by the anterior and posterior walls.17,18 On CT imaging, EAC squamous cell carcinoma commonly presents as a soft tissue mass, usually with associated bony destruction.14,18 In the early stages, however, squamous cell carcinoma may be impossible to radiologically distinguish from benign disease such as malignant otitis externa and EAC cholesteatoma.14
Malignant otitis externa is a chronic progressive infection with extensive involvement of tissues beyond the EAC, typically due to Pseudomonas aeruginosa infection in elderly patients with diabetes.19 This entity usually begins along the EAC floor at the osseous-cartilaginous junction as a small area of granulation tissue, followed by chondritis with subsequent involvement of the adjacent soft tissues beneath the skull base. Unlike chronic postoperative changes, at CT there are typically edematous changes of the soft tissue surrounding the EAC and pinna, with obliteration of the normal fat planes inferior to the temporal bone and skull base. The CT appearance of malignant otitis externa may be indistinguishable from malignancy.14,19 The changes beyond the EAC and the clinical history should distinguish this entity from postoperative changes.
In our study, osseous changes and soft tissue opacity were most commonly observed along the inferior wall of the EAC in patients postoperatively, with similar findings less commonly demonstrated along the other margins of the EAC. The most common postoperative appearance observed was subtle irregularity and flattening of the external canal, predominantly involving the floor, with loss of the tympanic sulcus (63%). The posterior canal was the least commonly involved, though osseous changes were present in 55.6% of the temporal bones. Such findings along any margin of the EAC may be subtle but, when observed, should not necessarily prompt concern for a pathologic erosive process, particularly after confirmation of the appropriate surgical history. In other cases, a differential diagnosis may be appropriate; however, the interpreting radiologist may be the first to suggest postoperative changes as a possibility and prompt further investigation into the clinical history. None of the patients included in this study had pathologic findings in the EAC on clinical follow-up, other than chronic postoperative changes such as granulation tissue and acquired EAC stenosis.
Additional clues to prior transcanal surgery include thickening and/or calcification of the tympanic membrane, absence of ≥1 ossicle, and/or the presence of an ossicular prosthesis. However, these findings were only present in a minority of the cases in our cohort.
Limitations to our study include the retrospective design and the relatively small number of patients. Another possible limitation is that postoperative imaging is usually only performed in symptomatic patients. However, our sample likely reflects a typical referral pattern of an otologic practice and the patients most likely to be seen at temporal bone CT by a radiologist. Preoperative imaging was only available for comparison in 4 of 25 patients. Lack of preoperative imaging is a common occurrence at a large referral center where patients may have undergone surgery elsewhere. In our series, this was a common reason for lack of preoperative imaging. Patients in our cohort were also scanned on either multidetector row CT or conebeam CT, and differences between the 2 techniques could limit the generalizability of our results. Although conebeam CT has a higher resolution for fine bony detail, most patients in this cohort were evaluated with multidetector row CT and this technique is likely sufficient to detect the bony changes observed in this study.
Conclusions
CT findings in the EAC after transcanal surgery include smooth thinning, irregularity and/or focal defects of the bony walls, and soft tissue thickening. The inferior wall is the most common site for these postoperative changes; however, any of the margins of the EAC may be involved. Such changes at CT should not necessarily prompt concern for a pathologic process, and interpretation should include a thorough review of the clinical examination and surgical history. Knowledge of this typical postoperative appearance may help the radiologist suggest prior surgery and avoid misdiagnosis of external canal disease at CT imaging.
ABBREVIATION:
- EAC
external auditory canal
Footnotes
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology, May 17–22, 2014; Montréal, Québec, Canada.
REFERENCES
- 1. Fisch U, May JS, Linder T. Tympanoplasty. In: Fisch U, May JS, Linder T. Tympanoplasty, Mastoidectomy, and Stapes Surgery. 2nd ed. New York: Thieme; 2008:2–47 [Google Scholar]
- 2. Gurr A, Sudhoff H, Hildmann H. Approaches to the middle ear. In: Hildmann H, Sudhoff H, eds. Middle Ear Surgery. New York: Springer-Verlag; 2006:19–22 [Google Scholar]
- 3. Rodrigues S, Fagan P, Doust B, et al. A radiologic study of the tympanic bone: anatomy and surgery. Otol Neurotol 2003;24:796–99 [DOI] [PubMed] [Google Scholar]
- 4. Williams MT, Ayache D. Imaging of the postoperative middle ear. Eur Radiol 2004;14:482–95 [DOI] [PubMed] [Google Scholar]
- 5. Kösling S, Bootz F. CT and MR imaging after middle ear surgery. Eur J Radiol 2001;40:113–18 [DOI] [PubMed] [Google Scholar]
- 6. Rao AG, Weissman JL. Imaging of postoperative middle ear, mastoid, and external auditory canal. Semin Ultrasound CT MR 2002;23:460–65 [DOI] [PubMed] [Google Scholar]
- 7. Mukherji SK, Mancuso AA, Kotzur IM, et al. CT of the temporal bone: findings after mastoidectomy, ossicular reconstruction, and cochlear implantation. AJR Am J Roentgenol 1994;163:1467–71 [DOI] [PubMed] [Google Scholar]
- 8. Swartz JD, Goodman RS, Russell KB, et al. High-resolution computed tomography of the middle ear and mastoid. Part III. Surgically altered anatomy and pathology. Radiology 1983;148:461–64 [DOI] [PubMed] [Google Scholar]
- 9. Miracle AC, Mukherji SK. Conebeam CT of the head and neck. Part 2. Clinical applications. AJNR Am J Neuroradiol 2009;30:1285–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larson TL, Wong ML. Imaging of the mastoid, middle ear, and internal auditory canal after surgery: what every radiologist should know. Neuroimaging Clin N Am 2009;19:307–20 [DOI] [PubMed] [Google Scholar]
- 11. Ginat DT, Mukherji SK. Imaging of the postoperative ear and temporal bone. In: Ginat DT, Westesson PA. Atlas of Postsurgical Neuroradiology. New York: Springer-Verlag; 2012:287–340 [Google Scholar]
- 12. Curtin HD, Gupta R, Bergeron RT. Embryology, anatomy and imaging of the temporal bone. In: Som PM, Curtin HD, eds. Head and Neck Imaging. 5th ed. Philadelphia: Elsevier Mosby; 2011:1063–64 [Google Scholar]
- 13. Fatterpekar GM, Doshi AH, Dugar M, et al. Role of 3D CT in the evaluation of the temporal bone. Radiographics 2006;26(suppl 1):S117–32 [DOI] [PubMed] [Google Scholar]
- 14. Castillo M, Jewells VL, Buchman C. The external auditory canal and pinna. In: Swartz JD, Loevner LA, eds. Imaging of the Temporal Bone. 4th ed. New York: Thieme; 2009:25–57 [Google Scholar]
- 15. Heilbrun ME, Salzman KL, Glastonbury CM, et al. External auditory canal cholesteatoma: clinical and imaging spectrum. AJNR Am J Neuroradiol 2003;24:751–56 [PMC free article] [PubMed] [Google Scholar]
- 16. Malcolm PN, Francis IS, Wareing MJ, et al. CT appearances of external ear canal cholesteatoma. Br J Radiol 1997;70:959–60 [DOI] [PubMed] [Google Scholar]
- 17. Lobo D, Llorente JL, Suarez C. Squamous cell carcinoma of the external auditory canal. Skull Base 2008;18:167–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ong CK, Pua U, Chong VF. Imaging of carcinoma of the external auditory canal: a pictorial essay. Cancer Imaging 2008;8:191–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curtin HD, Wolfe P, May M. Malignant external otitis: CT evaluation. Radiology 1982;145:383–88 [DOI] [PubMed] [Google Scholar]