Abstract
BACKGROUND AND PURPOSE:
Safety analyses in the French Observatory have shown that treatment of intracranial aneurysms by using flow disruption with the Woven EndoBridge Device (WEB) is safe, with low morbidity and no mortality. The objective of this study was to analyze treatment feasibility, complications, and safety results in patients treated with the Woven EndoBridge Device Dual-Layer (WEB DL) and Woven EndoBridge Device Single-Layer/Single-Layer Sphere (WEB SL/SLS) in the French Observatory.
MATERIALS AND METHODS:
Patients with bifurcation aneurysms were included in this prospective, multicenter good clinical practices study. A medical monitor independently analyzed procedural and clinical data. The study started with the WEB DL, and secondarily, the WEB SL/SLS was authorized in the study.
RESULTS:
Between November 2012 and January 2014, 10 French centers included 62 patients with 63 aneurysms. Thirty patients with 31 aneurysms were treated with the WEB DL, and 32 patients with 32 aneurysms, with the WEB SL/SLS. The percentage of anterior communicating artery aneurysms treated with WEB SL/SLS was significantly higher (37.5%) compared with WEB DL (12.9%) (P = .04). The WEB SL/SLS was more frequently used in aneurysms of <10 mm than the WEB DL (respectively, 96.9% and 67.7%; P = .002). Morbidity was similar in both groups (WEB DL, 3.3%; WEB SL/SLS, 3.1%), and mortality was 0.0% in both groups.
CONCLUSIONS:
This comparative study shows increased use of WEB treatment in ruptured, small, and anterior communicating artery aneurysms when using WEB SL/SLS. There was a trend toward fewer thromboembolic complications with the WEB SL/SLS. With both the WEB DL and WEB SL/SLS, the treatment was safe, with low morbidity and no mortality.
Endovascular treatment is the preferred therapeutic option for ruptured aneurysms that are anatomically suitable for endovascular coil treatment, supported by randomized studies, especially in locations less suitable for surgery.1,2 It also has an important place in the management of unruptured aneurysms that are judged appropriate for treatment.3 Complex aneurysms (fusiform, wide-neck, large, or giant) are often untreatable or difficult to treat with standard coiling. For these complex cases, endovascular techniques such as balloon-assisted coiling, stent-assisted coiling, or flow diversion have been used with good results.4–9
Flow disruption is a new endovascular approach, which involves placement of a Woven EndoBridge Device (WEB; Sequent Medical, Aliso Viejo, California), which modifies the blood flow at the level of the neck and induces intra-aneurysmal thrombosis. The WEB was designed initially to treat wide-neck and bifurcation aneurysms. The initial clinical results have shown that treatment is feasible with a low level of complications, low morbidity, and no mortality.10–14 The device has been progressively developed from a dual-layer version (WEB Dual-Layer [DL] aneurysm embolization system; Sequent Medical) to single-layer versions (WEB Single-Layer [SL] and WEB Single-Layer Sphere [SLS]).
The French Observatory is a prospective, multicenter observational study of consecutive cases, with independent monitoring, across 10 French centers.
It has 2 major objectives:
To carefully evaluate the safety of this treatment with an independent assessment of all adverse events and morbidity/mortality.
To evaluate the efficacy of this treatment at 12 and 24 months with independent core lab adjudication.
Patients treated with both WEB DL and WEB SL/SLS were included in the French Observatory. The present analysis reports the feasibility of treatment, adverse events, and morbidity/mortality at 1 month in patients treated with WEB DL and WEB SL/SLS.
Materials and Methods
The study received required national regulatory authorization: approval from the Reims Institutional Review Board, the Consultative Committee of Information Processing in Health Care Research program, and the National Commission for Data Processing and Freedom. Written informed consent was obtained for all patients. The study has been declared on Clinicaltrials.gov (NCT01975233).
Trial Design
The French Observatory is a single-arm, prospective, consecutive, multicenter, observational, French study confined to the evaluation of WEB treatment for bifurcation aneurysms.
The study protocol had the following inclusion criteria:
Patients of 18–75 years of age, able to consent and comply with 30-day, 1-year, and 2-year follow-up.
Aneurysm status: ruptured (Hunt and Hess 1, 2, or 3), unruptured, and recanalized.
Aneurysm location: bifurcation aneurysms located at the basilar artery, middle cerebral artery, anterior communicating artery, and internal carotid artery terminus.
Aneurysm morphology: able to be treated with available WEB sizes, and dome-to-neck ratio greater ≥1.
In each participating institution, the treatment decision and its technique (surgery or endovascular treatment) were decided on a case-by-case basis by a local multidisciplinary team, including neurosurgeons, neuroanesthesiologists, neurologists, and neuroradiologists. The selection of aneurysms treated with the WEB device was performed autonomously in each center by the interventional neuroradiologists according to the study protocol.
Study follow-up was conducted at 30 days (safety/mRS), 12 months (safety/mRS and imaging per standard of care), and 24 months (safety/mRS and imaging per standard of care). All imaging was evaluated by an independent core lab to assess the adequacy of occlusion.
The study was initiated with the WEB DL device, and an amendment was approved when the WEB SL and SLS were available, making these devices usable in the study.
WEB Devices
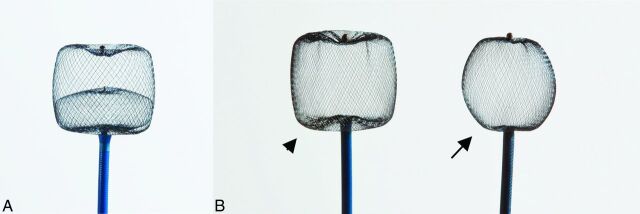
WEB devices (Figure) are retrievable, electrothermally detachable, nitinol braids that are placed within the sac of the aneurysm to disrupt the flow at the level of the neck and create aneurysmal thrombosis.
Figure.
A, WEB DL. B, WEB SL (arrowhead) and SLS (arrow).
WEB DL, which received CE Mark in 2010, contains a second nitinol braid that is proximally placed inside the first nitinol braid and provides a double-layer high-attenuation mesh coverage at the neck to achieve rapid intraprocedural stasis. The WEB DL is available in diameters between 5 and 11 mm and heights between 3 and 9 mm and has a barrel shape designed to treat wide-neck bifurcation aneurysms (ie, the WEB is wider than it is tall). With the double layers, WEB DL devices contain 216 or 288 total wires, depending on device size. More important, a given WEB DL contains 2 or 3 different-diameter wires braided into the device in a proprietary process called MicroBraid (Sequent Medical).
WEB SL and WEB SLS, which received CE Mark in 2013, represent an evolution of the MicroBraid technology. WEB SL and SLS are available in an expanded range of diameters between 4 and 11 mm and heights between 3 and 9 mm. Markedly different from the WEB DL, the WEB SL and SLS devices vary from 144 wires in 4-mm-diameter devices up to 216 wires in 11-mm-diameter devices. With a more spheric shape, WEB SLS is designed to treat ∼1.5–2 dome-to-neck-ratio aneurysms and aneurysms with V-shaped or tapered necks. Unlike the WEB SL, which is available in multiple heights at any given width, WEB SLSs are singularly available in sizes between 4 and 11 mm (ie, the heights of WEB SLSs are slightly less than their diameters due to the inclusion of the distal and proximal marker recesses). The increasing wire count in a single, braided layer—combined with multiple wire diameters—allows the WEB SL and SLS to achieve rapid contrast stasis and to balance radial force with conformability.15 This uniform response may be important clinically in that small and large WEB devices must have an appropriate radial force to remain where they are deployed (ie, WEB radial force is greater than the parent artery blood flow force) but must remain soft enough to conform safely to the aneurysm (ie, WEB radial force is less than the compressive force on the aneurysm from the subarachnoid space).
Since late 2012, VIA catheters have been available from Sequent Medical, designed specifically for the WEB. During 2010 through late 2012, WEB DL 5- to 7-mm diameters were used primarily with Rebar-27 (Covidien, Irvine, California), and WEB DL 8- to 11-mm diameters were used with DAC 038 (Stryker, Fremont, California). From late 2012 to the present, WEB procedures were performed with the VIA-27 (WEB DL 5–7 mm, WEB SL/SLS 4–9 mm), VIA-33 (WEB DL 8–9 mm, WEB SL/SLS 10–11 mm), and DAC 038 (WEB DL 10 and 11 mm). Taken together, the WEB SL/SLS and the VIA catheters provide complete systems for WEB delivery, retrieval, deployment, and detachment in aneurysms ∼3 to ∼10 mm in diameter.
Procedural Modalities
The treatment of aneurysms with the WEB was performed with techniques similar to those used in the treatment of aneurysms with coils (eg, general anesthesia, intraoperative treatment with intravenous heparin, single or double femoral approach). Pre-, intra-, and postoperative antiplatelet therapy was managed in each center as indicated for their standard endovascular treatment with coils (or stent and coils if this approach was a potential alternative treatment).
After accurate evaluation of aneurysm anatomy (aneurysm morphology, aneurysm transverse diameter and height, and neck size) by the treating physician by using MRA and DSA, it was determined whether the treatment with the WEB was indicated and device sizing was appropriate.
We usually used a triaxial access: a long introducer sheath placed in the internal carotid artery or vertebral artery, a distal access catheter placed in the intracranial portion of the ICA or vertebral artery, and a microcatheter placed in the aneurysm. The WEB device chosen according to aneurysm measurements was then positioned in the aneurysmal sac. A control angiogram was performed to check the position of the device in the aneurysm and to evaluate flow stagnation inside the aneurysm. If the position was not satisfactory, the device was resheathed and repositioned. If the size was not appropriate, the device was resheathed and another device was deployed into the aneurysm. When the right-sized device was correctly positioned, a final DSA run was performed. Treatment with ancillary devices (balloon, coils, and stent) was authorized if deemed necessary by the treating physician.
Data Collection
Each center completed a patient file with the following data: patient age and sex; aneurysm status; aneurysm characteristics, including location, size, and neck size; date of the procedure; type of device used (DL or SL/SLS); occurrence of a complications during or after the procedure; and use of an additional device during the procedure (coils, remodeling balloons, stents, or flow diverters). A preoperative Hunt and Hess grade was collected in case of ruptured aneurysms. mRS was collected before treatment (unruptured/recanalized aneurysms) and at 1 month (all patients).
All adverse events were collected in this good clinical practices series, even if no specific treatment was needed. Thromboembolic events were diagnosed intraoperatively by angiography regardless of type (clotting near the neck of the aneurysm, clotting in the distal branches, and parent vessel occlusion). Postoperative thromboembolic events were diagnosed by MR imaging and/or digital subtraction angiography performed in cases of sudden neurologic compromise. Intraoperative rupture was diagnosed by the exit of the tip of the coil or the microcatheter outside the limit of the aneurysmal sac and/or extravasation of contrast media. Adverse events were reported even if no clinical modification was associated with them.
Data Analysis
Clinical data were independently monitored and analyzed including all adverse events. Morbidity was defined as mRS of >2 when the preoperative mRS was ≤2 (or in case of ruptured aneurysm) and as an increase of 1 point when the preoperative the mRS was >2. Population, adverse events, and morbidity/mortality were compared in the DL and SL/SLS groups.
Statistical Analysis
Continuous variables were described as mean ± SD with extreme values and categoric variables as a number and percentage. Categoric variables and quantitative variables were compared between patients with WEB DL and WEB SL/SLS devices by using Mann-Whitney U tests, χ2 tests, or Fisher exact tests, as appropriate. A P value < .05 was considered significant. Analyses were conducted by using MedCalc statistical software for Windows (Version 11.4.3.0; MedCalc Software, Mariakerke, Belgium).
Results
Population
Patients were included in French Observatory between November 2012 and January 2014.
Thirty patients (women, 23; 76.7%) aged 33–71 years (mean, 55.6 ± 8.9 years) with 31 aneurysms were treated with the WEB DL. Thirty-two patients (women, 16; 50.0%) aged 33–74 years (mean, 57.4 ±10.3 years) with 32 aneurysms were treated with WEB SL or WEB SLS.
In the DL group, aneurysms were ruptured (2/31, 6.5%), unruptured (26/31, 83.9%), and recanalized (3/31, 9.6%). They were located on the MCA (19/31, 61.3%), basilar artery (6/31, 19.4%), ICA terminus (2/31, 6.5%), and anterior communicating artery (AcomA) (4/31, 12.9%). Aneurysm size was <10 mm in 21/31 aneurysms (67.7%). Neck size was >4 mm in 29/31 aneurysms (93.5%). Four patients (13.3%) received single (3 patients) or dual (1 patient) antiplatelet treatment before the procedure. During the procedure, 22 patients (73.3%) received antiplatelet treatment (single, 14; dual, 8). After the procedure, 26 patients (86.7%) received antiplatelet treatment (single, 18; dual, 8).
In the SL/SLS group, aneurysms were ruptured (5/32, 15.6%), unruptured (25/32, 78.1%), and recanalized (2/32, 6.3%). They were located on the MCA (13/32, 40.6%), basilar artery (3/32, 9.4%), ICA terminus (4/32, 12.5%), and AcomA (12/32, 37.5%). Aneurysm size was <10 mm in 31/32 aneurysms (96.9%). Neck size was >4 mm in 28/32 aneurysms (87.5%). Eleven patients (34.4%) received single (4 patients) or dual (7 patients) antiplatelet treatment before the procedure. During the procedure, 20 patients (62.5%) received antiplatelet treatment (single, 12; dual, 8). After the procedure, 22 patients (68.8%) received antiplatelet treatment (single, 13; dual, 9).
Patient and aneurysm populations are statistically compared in the Table.
Aneurysm characteristics, treatment complications, and morbidity and mortality in patients treated with WEB DL and WEB SL (and SLS)
| WEB DL | WEB SL/SLS | P Value | |
|---|---|---|---|
| Patients | 30 | 32 | |
| Age (mean) (yr) | 55.6 ± 8.9 | 57.4 ± 10.3 | .86 |
| Female | 24/30 (80.0%) | 16/32 (50.0%) | .20 |
| Aneurysms | 31 | 32 | |
| Ruptured | 2/31 (6.5%) | 5/32 (15.6%) | .42 |
| AcomA | 4/31 (12.9%) | 12/32 (37.5%) | .04 |
| Aneurysm <10 mm | 21/31 (67.7%) | 31/32 (96.9%) | .002 |
| Neck >4 mm | 29/31 (96.5%) | 28/32 (87.5%) | .67 |
| Antiplatelet treatmenta | |||
| Before treatment | 4/30 (13.3%) | 11/32 (34.4%) | .07 |
| During treatment | 22/30 (73.3%) | 20/32 (62.5%) | .42 |
| After treatment | 26/30 (86.7%) | 22/32 (68.8%) | .13 |
| Treatment feasibility | 30/31 (96.8%) | 32/32 (100.0%) | .49 |
| Adjunctive treatment | 4/30 (13.3%) | 3/32 (9.4%) | .70 |
| Adverse events | |||
| Device problems | 3/30 (10.0%) | 2/32 (6.3%) | .66 |
| TEb | 7/30 (23.3%) | 3/32 (9.4%) | .17 |
| IOR | 1/30 (3.3%) | 0/32 (0.0%) | .48 |
| Morbidity/mortality | |||
| Morbidity | 1/30 (3.3%) | 1/32 (3.1%) | 1 |
| Mortality | 0/30 (0.0%) | 0/32 (0.0%) | 1 |
Note:—TE indicates thromboembolic event/appearance of thrombus; IOR, intraoperative rupture.
One or 2 medications.
DL group: no clinical deficit in 3, transient deficits in 3, permanent deficit in 1. SL group: no deficit in 1, transient deficits in 2.
Treatment Feasibility
Technical success (deployment of the WEB in the target aneurysm) was achieved in 30/31 aneurysms (96.8%) in the DL group and in 32/32 aneurysms (100.0%) in the SL/SLS group. One treatment failure occurred in the DL group: it was impossible to deploy the WEB, which was stuck in the microcatheter. The aneurysm was treated with coils.
Adjunctive Treatments
Adjunctive devices were used in 4/30 aneurysms (13.3%) in the DL group (coiling in 3 cases and stent placement in 1 case) and in 3/32 aneurysms (9.4%) in the SL/SLS group (coiling in 1 case and stent placement in 2 cases).
Technical Problems and Adverse Events
Technical problems were encountered in 3/30 patients (9.7%) in the DL group (detachment problem, 1; WEB protrusion, 1; WEB stuck in microcatheter, 1) and 2/32 patients (6.3%) in the SL/SLS group (WEB protrusion, 2). All events were clinically asymptomatic.
Thromboembolic events or any appearance of thrombus was reported in 7/30 patients (23.3%) in the DL group (with no clinical deficit in 3, transient deficits in 3, and a permanent deficit in 1) and in 3/32 patients (9.4%) in SL/SLS group (with no deficit in 1 and a transient deficit in 2). In both groups, there was no evidence of a statistical relationship between antiplatelet medication and the occurrence of thromboembolic events.
Intraoperative rupture occurred in 1 patient (3.3%) in the DL group and zero patients in the SL/SLS group. The intraoperative rupture was not symptomatic.
One patient (3.3%) had an intracranial hemorrhage 2 days after WEB DL treatment related to dual antiplatelet therapy with no clinical worsening.
Statistical comparison for technical complications and adverse events between the DL and SL/SLS groups is shown in the Table.
Mortality/Morbidity at 1 Month
There was no mortality in the series. One patient in each group had mRS >2 at 1 month, leading to a morbidity of 3.3% in the DL group and 3.1% in the SL/SLS group. Morbidity was related to a thromboembolic event in the DL group and to an increase of a pre-existing aneurysm mass effect in the SL group.
Discussion
The present analysis shows that indications for the WEB DL and WEB SL/SLS are slightly different. Fewer thromboembolic complications or any appearance of thrombus was observed after treatment with the WEB SL/SLS (9.4% versus 23.3% in the DL group), but with such small numbers, this difference was not significant (P = .14). Morbidity was low and similar in both groups, (3.3% with the WEB DL and 3.1% with the WEB SL/SLS). There was no mortality.
For similar-sized devices, moving from a dual-layer to a single-layer device improves the device profile with several potential advantages, including a decrease in size of the microcatheter used for the placement of the device and improved navigability of the device in the microcatheter. Other potential advantages are easier deployment of the device in the aneurysm sac, better conformability of the device to the aneurysm, and improved retrievability of the device.
The present analysis shows a change in the way the WEB device is used. Of note, there was a trend toward an increased percentage of ruptured aneurysms treated in the cohort, 6.5% with WEB DL and 15.6% with WEB SL/SLS, though again, the trend was not significant (P = .42). The use of antiplatelet drugs is not mandatory when using this technique, making the WEB potentially suitable for use in the treatment of ruptured aneurysms, which present difficulties for standard coil treatment or stent-assisted coiling.16,17 Larger series are needed to determine the exact place of WEB treatment in the management of ruptured aneurysms.
The percentage of AcomA aneurysms treated with the WEB SL/SLS was significantly higher (37.5%) in this series compared with the WEB DL (12.9%) (P = .04). The lower device profile offered by the WEB SL/SLS is the single most important factor making it more suitable for aneurysms in this location. Entering the A1 segment of the anterior cerebral artery with a 0.027- to 0.033-inch microcatheter is not always simple, and the anatomy of the anterior communicating artery complex is transiently modified when the microcatheter is in the A1 segment and when the device is pushed into the microcatheter, making correct positioning of the device sometimes more difficult. The improvement of the WEB SL/SLS device profile consequently makes the procedure easier and increases the feasibility of treating more wide-neck anterior communication artery aneurysms.
In this study, both the WEB DL and WEB SL/SLS devices were used in predominantly wide-neck aneurysms (respectively, 96.5% and 87.5%). The WEB SL/SLS was more frequently used in aneurysms of <10 mm than the WEB DL (respectively, 96.9% and 67.7%; P = .002), probably as a result of reduced catheter profile and better conformability of the device.
Treatment success was high with both the WEB DL and WEB SL/SLS (respectively, 96.8% and 100.0%). Adjunctive devices were used in a similar percentage of cases in both the DL and SL/SLS groups (respectively, 13.3% and 9.4%). Coiling was used in 3 aneurysms after WEB DL treatment and in 1 aneurysm after WEB SL/SLS treatment, in case of incomplete treatment with the WEB. In all these cases, the device was undersized, with inappropriate filling of the aneurysm creating 2 different sometimes combined situations: 1) The device was not completely applied against the aneurysm wall; in this case, it was possible to catheterize the space between the aneurysm wall and the device and to deploy some coils. 2) An aneurysm remnant was left in place close to the neck; in this case, it was possible to fill the aneurysm remnant with coils by using the remodeling technique.4
Stent placement was used in 1 aneurysm after WEB DL treatment and in 2 aneurysms after WEB SL in case of WEB protrusion.
Device problems were encountered in a similar percentage of aneurysms with the WEB DL (10.0%) and WEB SL (6.3%). Fewer thromboembolic events were reported in the WEB SL/SLS group (9.4%) than in the WEB DL group (20.0%), but the difference was not statistically significant (P = .14). This phenomenon probably has several explanations, including the learning curve with the WEB device relating singularly to the procedure and WEB sizing (the WEB DL was used at the beginning of the experience and the WEB SL/SLS, after the WEB DL) and refinement of the antiplatelet medication protocol (because antiplatelet treatments were slightly different between the WEB DL and WEB SL, but not significantly).17
Very important, the observed clinical morbidity in patients treated with the WEB DL and WEB SL/SLS was similar and low with both devices (respectively, 3.3% and 3.1%). Mortality was 0.0% in both groups. Given the population of complex aneurysms treated, regardless of the device used, the treatment is safe.
This comparative study has some limitations. First, both devices were not used during the same period. The French Observatory started with the WEB DL at the beginning of the clinical experience with WEB, and the WEB SL/SLS was introduced half-way through the study. When using the WEB SL/SLS, the physicians were more familiar (learning curve) with flow-disruption procedures and WEB sizing. Second, both groups are small, with a small number of patients. This size limits any statistical comparison between the groups, but to date, the French Observatory is the largest good clinical practices study and the only study in which patients were treated with both the WEB DL and the WEB SL/SLS. Third, the number of ruptured aneurysms was not sufficient to perform subgroup analysis of ruptured-versus-unruptured aneurysms. Fourth, because the reason for using the WEB was not collected in the series, it is not possible to analyze precisely the framework of situations in which the WEB was used.
Conclusions
This comparative study suggests that there is increasing use of flow disruption with the WEB SL/SLS in ruptured, small, and AcomA aneurysms. With both the WEB DL and the WEB SL/SLS, treatment is safe, with low morbidity (respectively, 3.3% and 3.1%) and no mortality. The next step is to evaluate the efficacy of the WEB DL and WEB SL/SLS in terms of protection against bleeding and rebleeding and the stability of aneurysm occlusion.
ABBREVIATIONS:
- AcomA
anterior communicating artery
- DL
Dual-Layer
- SL
Single-Layer
- SLS
Single-Layer Sphere
- WEB
Woven EndoBridge Device
Footnotes
Disclosures: Laurent Pierot—RELATED: Consulting Fee or Honorarium: Sequent; UNRELATED: Consultancy: Codman, Covidien, MicroVention, Stryker; OTHER: proctoring and consultant agreement (Sequent), Principal Investigator for WEBCAST and the French Observatory. Jacques Moret—UNRELATED: Board Membership: Covidien; Consultancy: Covidien, MicroVention; OTHER RELATIONSHIPS: My son works for Covidien. Francis Turjman—UNRELATED: Consultancy: Codman, Covidien, Balt; Grants/Grants Pending: Covidien*; Payment for Lectures (including service on Speakers Bureaus): Codman,* Covidien*; Payment for Development of Educational Presentations: Codman,* Covidien*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Codman, Covidien, Stryker. Denis Herbreteau—RELATED: Other: Sequent (proctor); UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Sequent,* Comments: World Federation of Interventional and Therapeutic Neuroradiology (Buenos Aires, Val d'Isère). Xavier Barreau—RELATED: Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees, and the Like: Sequent; UNRELATED: Consultancy: MicroVention, Stryker, Covidien. Anne-Christine Januel—RELATED: Support for Travel to Meetings for the Study or Other Purposes: Sequent,* Comments: support occasionally for meetings; OTHER: Sequent (proctoring). Jean Yves Gauvrit—RELATED: core lab agreement for a European WEB series (Sequent). Christopher Cognard—RELATED: Consulting Fee or Honorarium: Sequent (proctoring); UNRELATED: Consultancy: Stryker, Codman, Sequent, MicroVention, Covidien. Andrew Molyneux—RELATED: Support for Travel to Meetings for the Study or Other Purposes: Sequent, Comments: in connection with study design and adverse event adjudication (adverse event adjudication agreement); Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees, and the Like: Sequent Medical, Comments: for time conducting independent evaluation of adverse events and study-design advice. Laurent Spelle—UNRELATED: Consultancy: Sequent, Stryker, Covidien; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Stryker, Covidien, Sequent; OTHER: Sequent (proctoring). *Money paid to the institution.
REFERENCES
- 1. Molyneux A, Kerr R, Stratton I, et al. ; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 2. Cognard C, Pierot L, Anxionnat R, et al. ; Clarity Study Group. Results of embolization used as the first treatment choice in a consecutive nonselected population of ruptured aneurysms: clinical results of the Clarity GDC study. Neurosurgery 2011;69:837–41; discussion 842 [DOI] [PubMed] [Google Scholar]
- 3. Pierot L, Spelle L, Vitry F; ATENA Investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008;39:2497–504 [DOI] [PubMed] [Google Scholar]
- 4. Pierot L, Cognard C, Spelle L, et al. Safety and efficacy of balloon remodeling technique during endovascular treatment of intracranial aneurysms: critical review of the literature. AJNR Am J Neuroradiol 2012;33:12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pierot L, Spelle L, Leclerc X, et al. Endovascular treatment of unruptured intracranial aneurysms: comparison of safety of remodeling technique and standard treatment with coils. Radiology 2009;251:846–55 [DOI] [PubMed] [Google Scholar]
- 6. Pierot L, Cognard C, Anxionnat R, et al. ; CLARITY Investigators. Remodeling technique for endovascular treatment of ruptured intracranial aneurysms had a higher rate of adequate postoperative occlusion than did conventional coil embolization with comparable safety. Radiology 2011;258:546–53 [DOI] [PubMed] [Google Scholar]
- 7. Shapiro M, Becske T, Sahlein D, et al. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol 2012;33:159–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pierot L. Flow diverter stents in the treatment of intracranial aneurysms: where are we? J Neuroradiol 2011;38:40–46 [DOI] [PubMed] [Google Scholar]
- 9. Berge J, Biondi A, Machi P, et al. Flow-diverter Silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 2012;33:1150–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pierot L, Liebig T, Sychra V, et al. Intrasaccular flow-disruption treatment of intracranial aneurysms: preliminary results of a multicenter clinical study. AJNR Am J Neuroradiol 2012;33:1232–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lubicz B, Mine B, Collignon L, et al. WEB device for endovascular treatment of wide-neck bifurcation aneurysms. AJNR Am J Neuroradiol 2013;34:1209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pierot L, Klisch J, Cognard C, et al. Endovascular WEB flow disruption in middle cerebral artery aneurysms: preliminary feasibility, clinical, and anatomical results in a multicenter study. Neurosurgery 2013;73:27–34; discussion 34–35 [DOI] [PubMed] [Google Scholar]
- 13. Lubicz B, Klisch J, Gauvrit JY, et al. WEB-DL endovascular treatment of wide-neck bifurcation aneurysms: short- and midterm results in a European study. AJNR Am J Neuroradiol 2014;35:432–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papagiannaki C, Spelle L, Januel AC, et al. WEB intrasaccular flow disruptor—prospective, multicenter experience in 83 patients with 85 aneurysms. AJNR Am J Neuroradiol 2014;35:2106–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mine B, Pierot L, Lubicz B. Intrasaccular flow-diversion for treatment of intracranial aneurysms: the Woven EndoBridge. Expert Rev Med Devices 2014;11:315–25 [DOI] [PubMed] [Google Scholar]
- 16. Caroff J, Mihalea C, Dargento F, et al. Woven Endobridge (WEB) Device for endovascular treatment of ruptured intracranial wide-neck aneurysms: a single-center experience. Neuroradiology 2014;56:755–61 [DOI] [PubMed] [Google Scholar]
- 17. Spelle L, Liebig T. Letter to the editor. Neuroradiol J 2014;27:369. [DOI] [PMC free article] [PubMed] [Google Scholar]