Abstract
A 46-year-old woman with uveitis was referred to our respiratory diseases department in July 2018. Her medical history included transient bilateral hilar mediastinal lymphadenopathy (BHL) and multiple pulmonary nodules in May 2013 during pegylated interferon-alpha and ribavirin treatment for chronic hepatitis C infection. Five years post-treatment, chest X-ray revealed BHL and nodular recurrence. A biopsy of the subcutaneous buttock nodules revealed scattered non-caseating epithelioid granulomas with positive PAB immunohistochemical staining. This seem to be the first report of Propionibacterium acnes-associated sarcoidosis possibly initially triggered by interferon-alpha therapy. Understanding the mechanisms underlying interferon-triggered P. acnes-associated sarcoidosis may clarify the sarcoidosis immunopathogenesis.
Keywords: sarcoidosis, interferon, drug-induced sarcoidosis-like reaction, bilateral hilar and mediastinal lymphadenopathy
Introduction
Sarcoidosis is a systemic granulomatous disease in which causative antigens, including Propionibacterium acnes (reclassified to Cutibacterium acnes; to avoid confusion with the earlier nomenclature we have used P. acnes within this report) (1), invade the thoracic lymph nodes via the respiratory tract and remain latent or spread to other organs throughout the body via the lymphatic system (2). Genetically susceptible individuals with colluding environmental factors are likely to develop this condition. These environmental factors vary and include age (3), sex, ovarian insufficiency (4), and viral infections, which are thought to trigger the T-helper (Th) 1 cell granulomatous reaction against causative antigens, leading to the development of sarcoidosis.
There is currently no consensus on the role of drugs in the immunopathogenesis of sarcoidosis. However, four common categories of drugs are known to be associated with the development of a systemic granulomatous tissue reaction, a drug-induced sarcoidosis-like reaction (DISR) that is indistinguishable from sarcoidosis (5). These include interferons (IFNs), tumor necrosis factor (TNF)-inhibitors, highly active antiretroviral therapy, and immune checkpoint inhibitors. Furthermore, the role of drugs in the development of P. acnes-associated sarcoidosis remains obscure, although the number of reports on this condition have increased, particularly in Japan, where cases have been detected using immunohistochemistry with a specific monoclonal antibody against P. acnes lipoteichoic acid (PAB antibody) (6,7). Only one case of P. acnes-associated sarcoidosis that developed during etanercept therapy has been reported (8).
We herein report a case of P. acnes-associated sarcoidosis initially triggered by IFN-alpha therapy.
Case Report
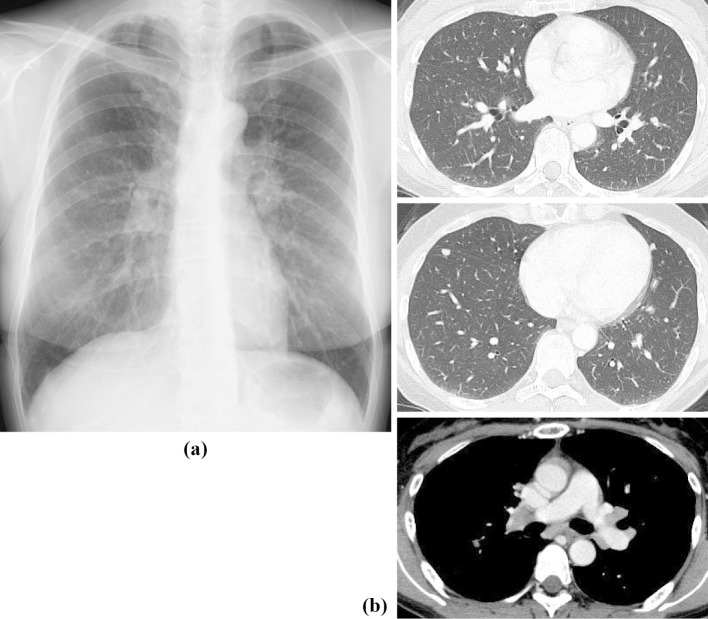
A 46-year-old woman with uveitis visited the ophthalmology department of a regional hospital in July 2018 and was referred to our respiratory department for further management 1 month later. She had a 25-pack-year smoking history. Her medical history included chronic hepatitis C, for which she had received pegylated IFN-alpha and ribavirin therapy from August 2012 (Fig. 1a) to June 2013. Chest X-ray (Fig. 1b) in May 2013 during a follow-up examination revealed transient bilateral hilar and mediastinal lymphadenopathy and multiple pulmonary nodules without any definite diagnosis; however, these improved after the withdrawal of therapy (Fig. 1c). No other causes were detected, such as mycobacterial or fungal infections or malignancy.
Figure 1.
Changes in chest X-ray findings during pegylated IFN-alpha and ribavirin therapy. The patient received pegylated IFN-alpha and ribavirin therapy for chronic hepatitis C infection from August 2012 (a) to June 2013. Chest X-ray in May 2013 (b) revealed transient bilateral hilar and mediastinal lymphadenopathy and multiple pulmonary nodules; however, these improved in June 2015 (c) after withdrawal of therapy. IFN: interferon
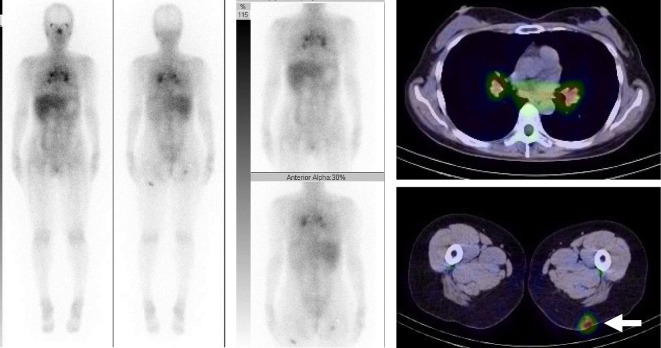
Repeat chest X-ray (Fig. 2a) and contrast-enhanced computed tomography (CT) (Fig. 2b) in July 2018 revealed recurrence of the bilateral hilar mediastinal lymphadenopathy and multiple small pulmonary nodules bilaterally. Gallium scintigraphy showed the 67Ga uptake in subcutaneous nodules in the buttocks bilaterally, as well as in the hilar and mediastinal lymph nodes and lacrimal and sublingual glands, also bilaterally (Fig. 3). Laboratory investigation findings included soluble interleukin 2 receptor 769 (normal ≤613) U/mL and serum calcium 10.3 (normal ≤10.1) mg/dL. A physical examination revealed hard elastic subcutaneous nodules with hyperpigmentation on the left buttock (Fig. 4), and a skin biopsy revealed scattered non-caseating epithelioid granulomas. Additional immunohistochemistry using a specific monoclonal antibody against P. acnes lipoteichoic acid (PAB antibody) (9) detected positively stained granuloma content (Fig. 5). She was diagnosed with P. acnes-associated sarcoidosis initially triggered by IFN-alpha therapy more than five years earlier.
Figure 2.
Chest X-ray and computed tomography (CT) images. Chest X-ray (a) and contrast-enhanced CT (b) in July 2018 revealed recurrence of bilateral hilar and mediastinal lymphadenopathy and multiple small pulmonary nodules bilaterally.
Figure 3.
Gallium scintigraphy. The 67Ga uptake is shown in subcutaneous nodules in the buttocks bilaterally (arrow), as well as in the hilar and mediastinal lymph nodes and lacrimal and sublingual glands, also bilaterally.
Figure 4.
Subcutaneous nodules with hyperpigmentation on the left buttock. Hard elastic subcutaneous nodules with hyperpigmentation evident on the left buttock.
Figure 5.
A skin biopsy of the subcutaneous nodules. (a) A skin biopsy of the subcutaneous nodules showing scattered non-caseating epithelioid granulomas (Hematoxylin and Eosin staining). Additional immunohistochemistry of the granuloma content using a specific monoclonal antibody against Propionibacterium acnes, lipoteichoic acid (PAB antibody), showing positive staining (b); however, no Tuberculous bacilli-associated staining is evident (c).
Discussion
To our knowledge, this is the first report on P. acnes-associated sarcoidosis initially triggered by IFN-alpha therapy. This case highlights two important observations. First, bilateral hilar mediastinal lymphadenopathy and pulmonary involvement were observed within nine months after the initiation of IFN-alpha therapy and were alleviated without specific treatment two years after cessation of IFN-alpha therapy. However, flare up of subcutaneous nodules on the buttocks was observed, as was ocular involvement and lacrimal and sublingual gland involvement five years after the cessation of IFN-alpha therapy, which led to the diagnosis of sarcoidosis triggered by IFN-alpha therapy. Second, PAB staining of biopsy specimens from subcutaneous nodules revealed sarcoid granulomas with positively stained content, based on which we judged that sarcoidosis was associated with P. acnes.
Currently, 100 or more cases describing the occurrence of DISRs with IFN-alpha therapy have been published in the English literature (5). Among these DISR cases, IFN-alpha was most commonly used to treat hepatitis C. The exact pathogenesis of DISR with IFN is not presently understood, but increased production of IFN-alpha has been linked to Th 1 polarization with an increased level of granuloma-promoting cytokines, such as IL-2, IL-8, IL-12, IL-18, and IFN-gamma (5). Indeed, the immunologic etiology of the disequilibrium between Th1 and Th17 responses and regulatory mechanisms is common to sarcoidosis and psoriasis, both of which can be triggered by IFN-alpha as well as TNF-inhibitors (4). However, whether these drugs truly cause sarcoidosis or cause conditions distinct from sarcoidosis is unclear (5).
In relation to the first observation mentioned above, DISRs induced by IFN-alpha have been detected from 6 to 104 weeks after starting therapy (5). The most commonly involved organs were the lung and mediastinal lymph nodes (70%) (5,10) and skin (60%) (5,10), particularly subcutaneous nodules (10). In our case, symptoms were alleviated by the cessation of IFN-alpha therapy, but the subsequent flare up indicated persistent symptoms of sarcoidosis, so we judged her to have sarcoidosis triggered by IFN-alpha.
In relation to the second observation, although cases of P. acnes-associated sarcoidosis have been increasingly frequently reported, particularly in Japan, the role of drugs in the immunopathogenesis of the condition are poorly understood. This case revealed the possibility that P. acnes-associated sarcoidosis could be triggered by IFN in addition to the TNF-inhibitor etanercept (8). Similar to TNF-inhibitors, IFN may reactivate latent P. acnes endogenously, potentially resulting in intracellular proliferation and granuloma formation in patients with immune system susceptibility to the development of sarcoidosis (11). The further accumulation of cases of P. acnes-associated sarcoidosis triggered by IFN-alpha therapy with histological, radiological, and laboratory findings will be necessary to show strong evidence for a causal link between this therapy and the disease onset.
In conclusion, we reported a case of P. acnes-associated sarcoidosis initially triggered by IFN-alpha therapy. Understanding the mechanisms by which IFN trigger a systemic granulomatous tissue reaction under these conditions may give important insight into the immunopathogenesis of sarcoidosis.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labour and Welfare, Japan. It was also partly supported by Jichi Medical University Alumni “2019 Itokai Research Project Grant” in 2018 (MS).
References
- 1. Alexeyev OA, Dekio I, Layton AM, et al. Why we continue to use the name Propionibacterium acnes. Br J Dermatol 179: 1227, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Sawahata M, Sugiyama Y. An epidemiological perspective on the pathology and etiology of sarcoidosis. Sarcoidosis Vasc Dis 33: 112-116, 2016. [PubMed] [Google Scholar]
- 3. Sawahata M, Sugiyama Y, Nakamura Y, et al. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Resp Med 109: 272-278, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Sawahata M, Sugiyama Y, Yamasawa H, et al. Sarcoidosis during etanercept treatment for rheumatoid arthritis in women with a history of bilateral oophorectomy. Sarcoidosis Vasc Dis 33: 178-181, 2016. [PubMed] [Google Scholar]
- 5. Chopra A, Nautiyal A, Kalkanis A, et al. Drug-induced sarcoidosis-like reactions. Chest 154: 664-677, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Inoue Y, Teraki Y. Association of Propionibacterium acnes with the efficacy of minocycline therapy for cutaneous sarcoidosis. Int J Dermatol 59: 704-708, 2020. [DOI] [PubMed] [Google Scholar]
- 7. Sasaki S, Kato M, Nakamura K, et al. Management of skin sarcoidosis with minocycline monotherapy. Respirol Case Rep 7: e00413, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Issiki T, Matsuyama H, Sakamoto S, et al. Development of Propionibacterium acnes-associated sarcoidosis during etanercept therapy: a case report. Intern Med 58: 1473-1477, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Negi M, Takemura T, Guzman J, et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol 25: 1284-1297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alazemi S, Campos MA. Interferon-induced sarcoidosis. Int J Clin Pract 60: 201-211, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Eishi Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes. Biomed Res Int 2013: 935289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]