Abstract
Keishibukuryogan is a Kampo medicine that induces vasodilation and improves the blood flow velocity in subcutaneous blood vessels. We herein report two cases in which keishibukuryogan completely diminished subcutaneous hematoma after cardiac resynchronization therapy pacemaker implantation and defibrillator battery replacement within a month. Keishibukuryogan can be a good option for treating or preventing subcutaneous hematoma after surgical procedures for devices.
Keywords: keishibukuryogan, subcutaneous hematoma, implantable cardiac device surgery, Kampo, Kampo medicine, herbal medicine
Introduction
Pocket hematoma formation is a common complication of surgery in implantable device procedures, such as pacemaker implantation, implantable cardioverter-defibrillator (ICD), and cardiac resynchronization therapy (CRT) (1). Severe hematomas may cause device infections, require blood transfusions, and sometimes require second surgery, including hematoma removal (2). In addition, even mild complications may extend the hospitalization due to skin problems of inflammation, which can worsen the activities of daily living (ADL). Hemostasis during surgical procedures is essential in order to prevent hematoma and depends on the skill of the operator. However, many patients, who require device treatment, take antiplatelets and/or anticoagulants due to coronary artery diseases and/or atrial fibrillation, which increases the incidence of hematoma formation (3,4). In our experience, it usually takes at least a few months until spontaneous improvement in large hematomas. There have been no previous reports regarding the period for the reduction of large hematomas.
Keishibukuryogan is a Kampo medicine that induces vasodilation and improves the blood flow velocity in the subcutaneous blood vessels (5) and may be useful for ameliorating microvascular inflammation in patients with skin diseases, such as chronic pigmented purpura (6), which may also be effective in managing subcutaneous hematomas after device surgery.
We herein report two cases of hematoma formation after CRT pacemaker (CRT-P) implantation and CRT-defibrillator (CRT-D) battery replacement in whom keishibukuryogan diminished hematoma within a month. This case report was approved by the Ethics Committee of Kurume University Hospital, and the patients gave their informed consent.
Case Reports
Case 1
A 79-year-old Japanese man with chronic heart failure (150.0 cm in height and 37.5 kg in weight), who had undergone percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) and aortic valve replacement for aortic regurgitation was admitted to our hospital for CRT-P implantation because he had been repeatedly hospitalized due to exacerbation of heart failure despite optimal medications.
On admission, his blood pressure was 102/70 mmHg, and his heart rate was 86 beats per minute and regular. He had jugular venous distention, but no abnormalities were observed in the skin or eyes or in the heart or breath sounds. Chest X-ray showed cardiomegaly (Fig. 1) with mild pulmonary congestion. An electrocardiogram (ECG) showed sinus rhythm (heart rate 76 beats/min) with complete left bundle branch block findings (Fig. 2). Echocardiography revealed a reduced left ventricular ejection fraction (LVEF, 22.4%) with diffuse severe hypokinesis (particularly akinesis in the anterior-septal region). Blood tests showed elevated N-terminal pro-brain natriuretic peptide (NTpro-BNP, 9987.3 pg/mL) (Table 1).
Figure 1.

Chest X-ray (Case 1).
Figure 2.
An electrocardiogram (Case 1).
Table 1.
Blood Tests on Admission (Case 1).
| WBC | 5,700 | /mL | Na | 139 | mmol/L | ALP | 312 | U/L | CRP | 1.22 | mg/dL | |||||||
| RBC | 436×104 | /mL | K | 4.4 | mmol/L | LDH | 229 | U/L | NT pro-BNP | 9,987.3 | pg/mL | |||||||
| Hb | 11.0 | g/dL | Cl | 104 | mmol/L | CPK | 58 | IU/L | PT-INR | 1.08 | ||||||||
| Ht | 35.7 | % | Fe | 59 | μg/dL | TG | 55 | mg/dL | APTT | 30.5 | ||||||||
| Plt | 30.4×104 | /mL | AST | 32 | U/L | HDL-C | 56 | mg/dL | ||||||||||
| BUN | 12 | mg/dL | ALT | 22 | U/L | LDL-C | 62 | mg/dL | ||||||||||
| Crea | 0.97 | mg/dL | γGTP | 58 | U/L | T.Bil | 0.8 | mg/dL |
WBC: white blood cells, RBC: red blood cells, Hb: hemoglobin, Ht: hematocrit, Plt: platelets, BUN: blood urea nitrogen, Cr: creatinine, Na: sodium, K: potassium, Cl: chloride, Fe: ferritine, AST: aspartate aminotransferaze, ALT: alanine aminotransferaze, γGTP: gamma-glutamyl transpeptidase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, CPK: creatine phosphokinase, TG: triglyceride, HDL-C: high density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, T.Bil:total bilirubin, CRP: C-reactive protein, NT pro-BNP: N-terminal pro-B-type natriuretic peptide, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time
We discontinued 75 mg/day of clopidogrel 10 days before the surgical procedure but continued other medications of 100 mg/day of aspirin, 7.5 mg/day of tolvaptan, 2.5 mg/day of bisoprolol, 2.5 mg/day of enarapril, 30 mg/day of azosemide, and 2.5 mg/day of pimobendane, and performed CRT-P implantation. Although we discontinued clopidogrel, but not aspirin, we observed substantial bleeding during the CRT-P implantation procedure and compressed the pocket using gauze immediately after the implantation to prevent hematoma. Gauze compression was released five days after the procedure, but hematoma formation accompanied by color change of the epidermis at the device placement site was observed (Fig. 3). On the same day, we started keishibukuryogan at 7.5 g/day orally, but no remarkable changes were observed after 3 days (Fig. 4). We re-administered 75 mg/day of clopidogrel at hospital discharge. On day 35 of keishibukuryogan (41 days after the procedure), hematoma had completely diminished (Fig. 5), so keishibukuryogan was discontinued.
Figure 3.

Subcutaneous hematoma five days after the procedure (Case 1).
Figure 4.
Subcutaneous hematoma three days after the administration of keishibukuryogan, eight days after the procedure (Case 1).
Figure 5.

Subcutaneous hematoma 36 days after the administration of keishibukuryogan, 41 days after the procedure (Case 1).
Case 2
An 85-year-old Japanese man with chronic heart failure (155.5 cm of height and 44.0 kg of weight) who had undergone PCI for AMI and subsequent CRT-D implantation for repeated exacerbations of chronic heart failure with a low cardiac function was admitted to our hospital to replace his low CRT-D battery.
On admission, his blood pressure was 100/60 mmHg, and his heart rate was 80 paces per minute and regular. He had an implanted CRT-D in his right precordial chest but no abnormalities in the skin or eyes as on in the heart or breath sounds. Chest X-ray showed cardiomegaly with no evidence of pulmonary congestion, and CRT-D was found in his right precordial chest (Fig. 6). An ECG showed pacing rhythm (heart rate 70 pacing/min) with atrial flutter (Fig. 7). Echocardiography revealed a reduced LVEF of 18%, which showed diffuse severe hypokinesis (particularly akinesis in the septal regions from base to middle and apical regions). Blood tests showed elevated NTpro-BNP of 4,506.8 pg/mL and creatinine (Cr, 1.35 mg/dL) (Table 2).
Figure 6.

Chest X-ray (Case 2).
Figure 7.
An electrocardiogram (Case 2).
Table 2.
Blood Tests on Admission (Case 2).
| WBC | 4,000 | /mL | Na | 141 | mmol/L | LDH | 224 | U/L | NT pro-BNP | 4,506.8 | pg/mL | |||||||
| RBC | 427×104 | /mL | K | 4.2 | mmol/L | CPK | 61 | IU/L | PT-INR | 1.60 | ||||||||
| Hb | 13.7 | g/dL | Cl | 105 | mmol/L | TG | 95 | mg/dL | APTT | 36.4 | ||||||||
| Ht | 42.6 | % | AST | 25 | U/L | HDL-C | 44 | mg/dL | ||||||||||
| Plt | 10.4×104 | /mL | ALT | 10 | U/L | LDL-C | 107 | mg/dL | ||||||||||
| BUN | 22 | mg/dL | γGTP | 60 | U/L | T.Bil | 1.3 | mg/dL | ||||||||||
| Crea | 1.35 | mg/dL | ALP | 246 | U/L | CRP | 2.40 | mg/dL |
WBC: white blood cells, RBC: red blood cells, Hb: hemoglobin, Ht: hematocrit, Plt: platelets, BUN: blood urea nitrogen, Cr: creatinine, Na: sodium, K: potassium, AST: aspartate aminotransferaze, ALT: alanine aminotransferaze, γGTP: gamma-glutamyl transpeptidase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, CPK: creatine phosphokinase, TG: triglyceride, HDL-C: high density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, T.Bil: total bilirubin, CRP: C-reactive protein, NT pro-BNP: N-terminal pro-B-type natriuretic peptide, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time
We continued 10 mg/day of rivaroxaban, 3.75 mg/day of tolvaptan, 0.625 mg/day of bisoprolol, 50 mg/day of losartan, 20 mg/day of furosemide, and 1.25 mg/day of pimobendane, and performed CRT-D battery replacement. During the procedure, the amount of bleeding was small, but we observed hematoma formation four days after the surgery, with an exacerbating tendency. We continued his medication, and on day 4 after the procedure, we started keishibukuryogan at 7.5 g/day orally (Fig. 8). On day 37 of keishibukuryogan (41 days after surgery), hematoma had completely diminished (Fig. 9), and we discontinued keishibukuryogan.
Figure 8.
Subcutaneous hematoma four days after the procedure (Case 2).
Figure 9.
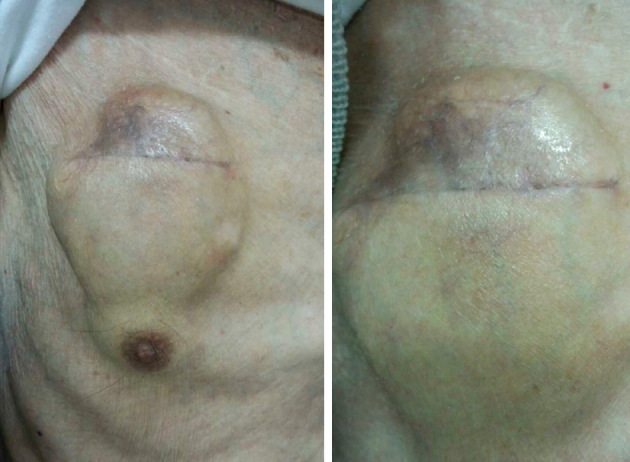
Subcutaneous hematoma 37 days after the administration of keishibukuryogan, 41 days after the procedure (Case 2).
Discussion
To our knowledge, this is the first report of keishibukuryogan completely diminishing subcutaneous hematomas after implantable device surgical procedures within a month. Compared with the usual clinical experience, this course was very rapid, especially in patients treated with antithrombotic agents. Unfortunately, we have no pictures from the first two weeks after the procedure in these patients, as we did not expect the large hematoma to be diminished so rapidly. However, we consider keishibukuryogan to be very useful to treat or prevent subcutaneous hematomas in high-risk patients.
Pocket hematomas during device implantation, a relatively common complication, are associated with an increased risk of device infection (7). It has been reported that the use of a post-surgical vest effectively suppresses the formation of hematomas (2). However, this pocket compression technique may not be comfortable and may affect the quality of life. Therefore, easier and more effective therapies, including pharmacotherapies for pocket hematoma, are sought. Keishibukuryogan is a low-cost drug and conventional Japanese formula that improves impaired microcirculation (8). It has been reported that keishibukuryogan was useful for improving the skin perfusion pressure and discomfort associated with varicose veins of the lower extremities (8), and consequently, keishibukuryogan was expected help reduce pocket hematoma, probably via vasodilation of the arterioles, increased blood velocity, and resolution of erythrocyte congestion in relation to enhanced endothelial nitric oxide production (5).
The two presently reported cases, both of whom continued their antithrombotic drugs, showed dramatically diminished pocket hematomas within a month after the start of administration of keishibukuryogan without major adverse events, such as a second surgical procedure, blood transfusion, or pocket infection. Furthermore, pocket hematoma showed no relapse in our two cases, suggesting that keishibukuryogan can be beneficial for treating or preventing hematoma after device surgical procedures. These two cases suggest that it may be best to administer keishibukuryogan just after the surgical procedures in high-risk patients in order to prevent hematoma.
However, the present case report has a major limitation. We have no data on how long it generally takes for spontaneous improvement to occur in large hematomas, as there have been no reports regarding this issue. We therefore cannot deny that this is the natural course of the improvement in these patients. Therefore, in order to confirm the effects of keishibukuryogan on hematoma, further prospective clinical studies with a control group will be required.
Conclusion
Keishibukuryogan was effective for reducing hematoma after device implantation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Sridhar AR, Yarlagadda V, Yeruva MR, et al. Impact of haematoma after pacemaker and CRT device implantation on hospitalization costs, length of stay, and mortality: a population-based study. Europace 17: 1548-1554, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Turagam MK, Nagarajan DV, Bartus K, et al. Use of a pocket compression device for the prevention and treatment of pocket hematoma after pacemaker and defibrillator implantation (STOP-HEMATOMA-I). J Interv Card Electrophysiol 49: 197-204, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Wiegand UK, Lejeune D, Bogushewaski F, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. CHEST 126: 1177-1186, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Demir GG, Guier GB, Guler E, et al. Pocket haematoma after cardiac electronic device implantation in patients receiving antiplatelet and anticoagulant treatment: a single-centre experience. Acta Cardiol 72: 47-52, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Tomita T, Hirayama A, Matsui H, Aoyagi K. Effect of keishibukuryogan, a japanese traditional kampo prescription, on improvement of microcirculation and oketsu and induction of endothelial nitric oxide: a live imaging study. Evid Based Completment Alternat Med. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshihisa Y, Furuichi M, Ur Rehman M, et al. The traditional Japanese formula keishibukuryogan inhibits the production of inflammatory cytokines by dermal endothelial cells. Mediators Inflamm. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Notaristefano F, Angeli F, Verdecchia P, et al. Device-pocket hematoma after cardiac implantable electronic devices. Circ Arrhythm Electrophysiol 13: e008372, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Hayashi S, Shibutani S, Okubo H, et al. Examination of clinical efficacy of keishibukuryogan on non-specific complaints associated with varicose veins of the lower extremity. Ann Vasc Dis 7: 266-273, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]






