Abstract
In triple A (Allgrove) syndrome, motor neuron disease is a co-morbid condition. We herein report a 38-year-old Japanese man with triple A (Allgrove) syndrome and novel tandem mutations: a novel c.881delT deletion mutation and c.835C>T localized to the AAAS gene. A nerve conduction study revealed marked axonal damage in several motor nerves. Tandem mutations in the AAAS gene may be involved in co-morbid motor neuron disease and aberrant electrophysiological findings.
Keywords: triple A (Allgrove) syndrome, ALADIN, motor neuron disease, nerve conduction study, c.881delT, c.835C>T
Introduction
First identified by Allgrove et al. in 1978 (1), triple A (Allgrove) syndrome is a rare autosomal recessive disorder characterized by the triad of achalasia, alacrimia, and adrenal insufficiency caused by mutations in the AAAS gene on chromosome 12q13, which encodes the nuclear pore complex protein ALADIN. Triple A syndrome may present with variable neurological findings, such as muscle weakness, hyperreflexia, ataxia, dysarthria, parkinsonism, sensory impairment, and mental retardation (2,3). Several cases of comorbid triple A (Allgrove) syndrome and motor neuron disease have already been reported (4-6).
We herein report a patient with triple A (Allgrove) syndrome and co-morbid motor neuron disease who had novel tandem mutations of the AAAS gene.
Case Report
A 38-year-old Japanese man was referred to us due to progressive muscle weakness of his extremities. None of his family members presented the same symptoms. He had adrenal insufficiency and received glucocorticoid replacement therapy from five years old. He noticed muscle atrophy of his extremities and trunk at 14 years old and difficulty in swallowing at 15 years old. The patient’s dysarthria was noticed by his family when he was 28 years old. Muscle weakness in the patient’s distal left upper limb appeared when he was 30 years old. He had repeatedly suffered from aspiration pneumonia at 34 years old. Muscle atrophy, muscle weakness, dysarthria, and dysphagia had progressively worsened thereafter. The patient was diagnosed with esophageal achalasia at 38 years old, and surgical treatment was recommended to the patient. He visited our department for a preoperative neurological evaluation of his physical condition.
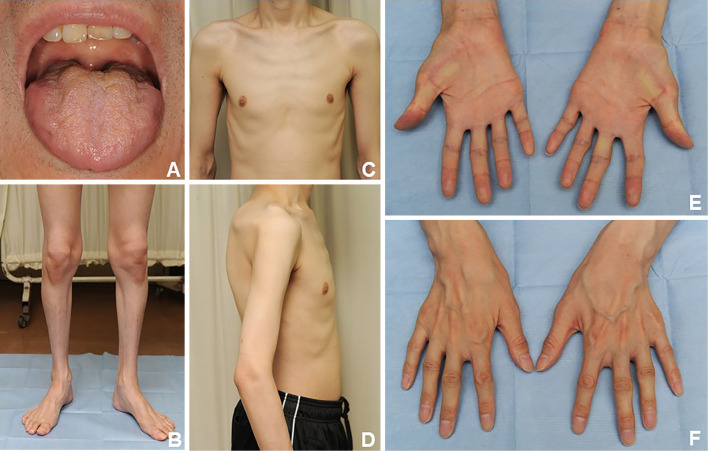
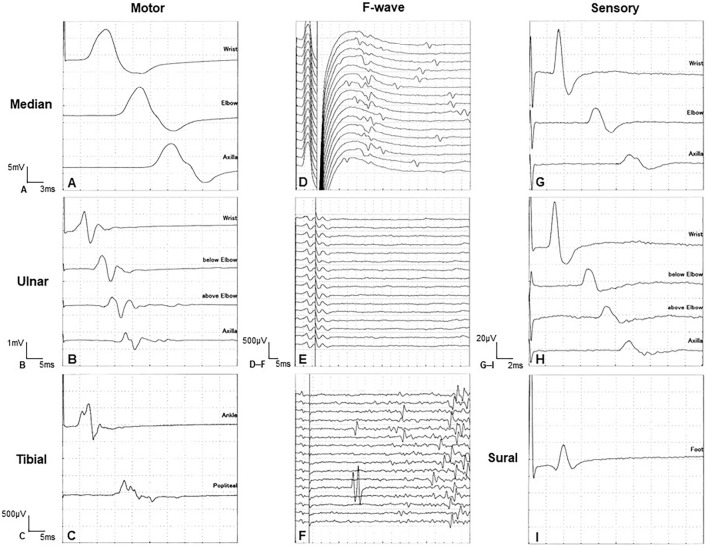
A neurological workup revealed dysarthria, dysphagia, tongue atrophy and fasciculations, muscle atrophy of all limbs and the body trunk, weakness and fasciculations in the distal muscles of both upper limbs, hyperreflexia in the extremities, and positive Wartenberg reflexes in both hands (Fig. 1). Blood tests showed adrenal hypofunction [plasma level of cortisol in the early morning, 5.42 μg/dL; maximum plasma level of cortisol in adrenocorticotropic hormone (ACTH) stimulation test, 9.8 μg/dL; plasma level of ACTH in the early morning, 131 pg/mL] and no other notable abnormalities. Computed tomography revealed systemic volume loss of skeletal muscle without fatty degeneration. Brain and whole-spinal-cord magnetic resonance imaging showed no abnormal signals or structural changes. Nerve conduction study (NCS) revealed markedly decreased amplitudes of compound muscle action potentials, slightly decreased motor conduction velocities, and polyphasic waveform patterns in the ulnar and tibial nerves. The distal latency and motor conduction velocities were normal in any nerves. The population of F-waves was decreased in all nerves. The sensory conduction study revealed no abnormalities. These findings indicated axonal damage (Table, Fig. 2).
Figure 1.
Physical findings. Tongue atrophy (A). Muscle atrophy in the four limbs and body trunk (B-F).
Table.
Nerve Conduction Study in the Right Limbs.
| Nerve stimulated, Right | Latency (ms) | Amplitude (motor in mV, sensory in µV) | Velocity (m/s) | Normative Data (m/s) |
F-wave Occurrence (%) | |
|---|---|---|---|---|---|---|
| Motor | ||||||
| Medinan | Wrist | 3.03 | 13.06 | 0 | ||
| Elbow | 8.43 | 12.99 | 45.4 | 52.7–62.7 | ||
| Axilla | 13.59 | 11.42 | 46.5 | 57.3–69.7 | ||
| Ulnar | Wrist | 2.80 | 1.88 | |||
| Below elbow | 8.75 | 1.53 | 37.0 | 53.5–63.9 | 0 | |
| Above elbow | 11.95 | 1.24 | 37.5 | 55.4–66.6 | ||
| Axilla | 16.35 | 1.01 | 34.1 | 60.1–72.9 | ||
| Tibial | Ankle | 3.80 | 1.06 | 0 | ||
| Popliteal | 14.35 | 0.64 | 36.5 | 44.9–52.1 | ||
| Sensory | ||||||
| Medinan | Wrist | 2.64 | 25.90 | 56.8 | 50.4–62.0 | |
| Elbow | 6.52 | 8.70 | 63.1 | 57.7–66.1 | ||
| Axilla | 10.22 | 5.50 | 64.9 | |||
| Ulnar | Wrist | 2.26 | 27.20 | 53.1 | 49.4–60.2 | |
| Below elbow | 5.90 | 9.60 | 60.4 | 59.3–70.1 | ||
| Above elbow | 7.92 | 7.30 | 59.4 | 60.3–73.1 | ||
| Axilla | 10.36 | 5.70 | 61.5 | |||
| Sural | Foot | 3.08 | 14.6 | 45.5 | 39.3–62.9 | |
Figure 2.
Nerve conduction findings in the right limbs showed a slightly decreased motor nerve conduction velocity (A-C), decreased compound muscle action potentials (B, C), and polyphasic patterns (B, C). Decreased population of F-waves (D-F). Normal sensory conductions (G-I). A, D, and G show the median nerve. B, E, and H show the ulnar nerve. C and F show the tibial nerve. I shows the sural nerve.
Needle electromyography (nEMG) revealed motor unit potentials with long durations, polyphasic patterns, and high amplitudes, but no positive sharp waves, fibrillations, or fasciculation potentials, in the first dorsal, bicep, or the vastus lateralis of the quadricep muscles. These findings indicated chronic denervation changes. An ophthalmological examination detected alacrima. We performed a DNA sequencing analysis that revealed novel tandem mutations (c.881delT and c.835C>T) in the AAAS gene. The final diagnosis was triple A (Allgrove) syndrome with co-morbid motor neuron disease.
Discussion
While triple A (Allgrove) syndrome has been reported in western and north African populations, it is extremely rare in Japan (7). Our patient had already been diagnosed with adrenal insufficiency and achalasia before visiting our institution, and alacrima became evident upon a systemic examination; the characteristic triad of triple A (Allgrove) syndrome was thus confirmed. Progressive muscle atrophy and weakness similar to that induced by amyotrophic lateral sclerosis (ALS) has already been recognized as a clinical phenotype of co-morbid neurological abnormalities in triple A (Allgrove) syndrome, also called 4A syndrome (8,9). NCS revealed normal sensory conduction and axonal damage in motor nerves. Amplitude-dependent slowing of nerve conduction has also been reported in patients with ALS (10), so it is difficult to distinguish triple A (Allgrove) syndrome from ALS using NCS alone. However, these compound muscle action potentials in the median nerve were preserved, while those in the ulnar nerve were markedly decreased. These electrophysiological findings were contrary to the split hand observed in ALS patients as predominant atrophy of abductor pollcis brevis and first dosal interossei compared with abductor digiti minimi (11). Using nEMG, chronic denervation changes were observed in several muscles of our patient. These findings, along with the clinical and neurological findings, supported a diagnosis of co-morbid motor neuron disease.
Patients with triple A (Allgrove) syndrome and non-truncating mutations are reportedly more likely to show neurological dysfunction and to be late onset than their counterparts with truncating mutations (12). Our patient had a c.881delT deletion mutation and a c.835C>T mutation in the AAAS gene. This report is the first to describe a c.881delT mutation in a patient with triple A (Allgrove) syndrome (13-15). Located in the active locus of the AAAS gene, the c.881delT mutation may influence the pathogenicity of triple A (Allgrove) syndrome. However, the clinical presentation and course of our patient-including the clinical features and electrophysiological results indicative of a co-morbid motor neuron disease-were typical of triple A (Allgrove) syndrome. We therefore could not confirm whether or not the c.881delT mutation had a definite pathogenic influence on our patient's clinical course.
The neurobiological mechanisms underlying the pathogenesis of triple A (Allgrove) syndrome remain unknown (3). Mutations in ALADIN selectively impair the nuclear import of proteins, resulting in hypersensitivity to oxidative stress (16). Nuclear transport of the DNA repair proteins aprataxin and DNA ligase is selectively reduced, which increases the vulnerability of DNA to oxidative stress damage, potentially leading to various pathological conditions. Co-morbid motor neuron disease reportedly progresses slowly with other complications (17). In the present case, adrenal insufficiency had been observed when the patient was 5 years old, and the patient became symptomatic at 15 years old, just after muscle atrophy had been noticed 9 years following the diagnosis of adrenal insufficiency. The patient recognized his own muscle weakness at 30 years old. The slow progressive clinical course may be a feature of co-morbid motor neuron disease in cases of triple A (Allgrove) syndrome, which should be differentiated from ALS. Clarifying the pathogenic relationship between the AAAS mutation and motor neurons may provide some beneficial evidence for the treatment of ALS as well.
The present case featured several novel tandem mutations in the AAAS gene. Some reports have documented the c.835C>T mutation in patients with Fabry disease or episodic ataxia type 2 (18,19). However, the other gene mutation observed in this case, c.881delT, has never been reported in association with any disease. Either of the two AAAS variants (c.881delT and c.835C> T) may have been involved in our patient’s disease.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Allgrove J, Clayden GS, Grant DB, Macaulay JC. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet 1: 1284-1286, 1978. [DOI] [PubMed] [Google Scholar]
- 2. Clark AJL, Weber A. Adrenocorticotropin insensitivity syndromes. Endocr Rev 19: 828-843, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Flokas ME, Tomani M, Agdere L, Brown B. Triple A syndrome (Allgrove syndrome): improving outcomes with a multidisciplinary approach. Pediatr Health Med Ther 10: 99-106, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. deFreitas MRG, Orsini M, Araújo APQC, et al. Allgrove syndrome and motor neuron disease. Neurol Int 10: 7436, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vishnu VY, Modi M, Prabhakar S, Bhansali A, Goyal MK. ‘A’ motor neuron disease. J Neurol Sci 336: 251-253, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Vallet AE, Verschueren A, Petiot P, et al. Neurological features in adult Triple-A (Allgrove) syndrome. J Neurol 259: 39-46, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Ikeda M, Hirano M, Shinoda K, et al. Triple A syndrome in Japan. Muscle Nerve 48: 381-386, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Gazarian M, Cowell CT, Bonney M, Grigor WG. The ”4A” syndrome: adrenocortical insufficiency associated with achalasia, alacrima, autonomic and other neurological abnormalities. Eur J Pediatr 154: 18-23, 1995. [DOI] [PubMed] [Google Scholar]
- 9. Tullio-Pelet A, Salomon R, Hadj-Rabia S, et al. Mutant WD-repeat protein in trile-A syndrome. Nat Genet 26: 332-335, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Feinberg DM, Preston DC, Shefner JM, Logigian EL. Amplitude-dependent slowing of conduction in amyotrophic lateral sclerosis and polyneuropathy. Muscle Nerve 22: 937-940, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Eisen AA. Comment on the lower motor hypothesis. Muscle Nerve 16: 380-384, 1993. [DOI] [PubMed] [Google Scholar]
- 12. Patt H, Koehler K, Lodha S, et al. Phenotype-genotype spectrum of AAA syndrome from Western India and systematic review of literature. Endocr Connect 6: 901-913, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurnaz E, Duminuco P, Aycan Z, et al. Clinical and genetic characterization of a series of patients with triple A syndrome. Eur J Pediatr 177: 363-369, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Berrani H, Meskini T, Zerkaoui M, et al. Clinical and molecular report of c.1331 + 1G > A mutation of the AAAS gene in a Moroccan family with Allgrove syndrome: a case report. BMC Pediatr 18: 184, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanghvi J, Asati AA, Kumar R, Huebner A. Novel mutation in a patient with triple A syndrome. Indian Pediatr 52: 805-806, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Hirano M, Furiya Y, Asai H, Yasui A, Uneno S. ALADINI482S causes selective failure of nuclear protein import and hypersensitivity to oxidative stress in stress in triple A syndrome. Proc Natl Acad Sci U S A 103: 2298-2303, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura K, Yoshida K, Yoshinaga T, et al. Adult or late-onset triple A syndrome: case report and literature review. J Neurol Sci 297: 85-88, 2010. [DOI] [PubMed] [Google Scholar]
- 18. Koulousios K, Stylianou K, Pateinakis P, et al. Fabry disease due to D313Y and novel GLA mutations. BMJ Open. 7: e017098, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomlinson SE, Tan SV, Burke D, et al. In vivo impact of presynaptic calcium channel dysfunction on motor axons in episodic ataxia type 2. Brain 139: 380-391, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]