Abstract
Objective
This study was conducted to clarify the prevalence of short segment Barrett's esophagus (SSBE) using endoscopic observations with linked color imaging (LCI). In addition, the relationship between the presence of Barrett's epithelium (BE) and the status of H. pylori infection was investigated.
Methods
The study subjects were 3,353 individuals (2,186 men, 1,167 women; mean age 55.2±9.4 years old) whose status of H. pylori infection had been determined. An endoscopic observation using LCI was performed to examine the distal margin of palisade vessels and confirm the area of BE. The prevalence of BE ≥5 mm in length was investigated.
Results
BE was diagnosed in 1,884 (56.2%) subjects, with lengths of <10, 10-19, 20-29, and ≥30 mm found in 1,005, 851, 27, and 1, respectively. Its prevalence in H. pylori-negative, H. pylori-positive, and post-eradicated subjects was 41.7%, 64.4%, and 69.9%, respectively (p<0.001). The duration since successful eradication of H. pylori did not affect the prevalence of BE. The degree of gastric mucosal atrophy was higher in cases with BE (p<0.001), although negativity for H. pylori infection and mild gastric mucosal atrophy were significant factors for the development of longer BE.
Conclusion
A high prevalence of SSBE was noted when LCI was used to determine the area of BE, as the distal end of the palisade vessels was easily visualized. Negativity for H. pylori infection and mild gastric mucosal atrophy were not correlated with SSBE prevalence.
Keywords: Barrett's esophagus, SSBE, prevalence, LCI, Helicobacter pylori
Introduction
Barrett's esophagus is recognized as an important disease condition, as cases of esophageal adenocarcinoma originating from it have been increasing in both Western and Asian countries (1-3). In Japan, the prevalence of long segment Barrett's esophagus (LSBE) is rare, although cases of adenocarcinoma developing from short segment Barrett's esophagus (SSBE) are increasing (3-5). As a result, patients with SSBE are considered to be the main population requiring endoscopic surveillance for the detection of esophageal adenocarcinoma in Asian countries.
Determination of the esophago-gastric junction (EGJ) is necessary for the diagnosis of the presence of Barrett's epithelium, especially SSBE. Based on criteria presented by The Japan Esophageal Society, the EGJ is defined as the distal margin of the palisade vessels of the lower esophagus, with columnar-appearing mucosa between the squamocolumnar and EGJ diagnosed as Barrett's epithelium (6). Recent advances in endoscopy equipment have made the detection of palisade vessels much easier. Notably, endoscopic observation using linked color imaging (LCI) more clearly reveals the presence of palisade vessels as well as the area of Barrett's epithelium than that using white light imaging (WLI) (7).
The national health insurance system of Japan began providing coverage for Helicobacter pylori eradication therapy to treat H. pylori-associated chronic gastritis in February 2013, which has led to a rapid increase in the number of patients with H. pylori infection undergoing that therapy. Successful eradication has been shown to increase the prevalence of reflux esophagitis, which is recognized as being closely associated with the development of Barrett's epithelium (8-12).
We conducted the present study to examine the prevalence of Barrett's epithelium using endoscopic observations with LCI in individuals who came to our medical center for an annual medical checkup. In addition, the relationship between the presence of Barrett's epithelium and the status of H. pylori infection was investigated.
Materials and Methods
Study subjects
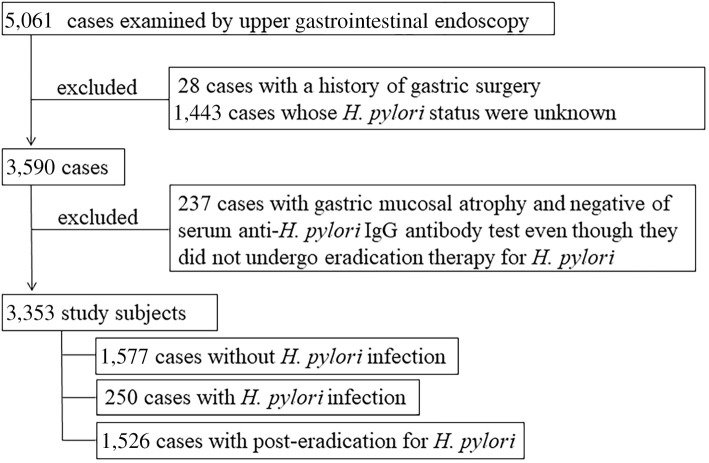
The study subjects were enrolled from among individuals who visited the Health Center of Shimane Environment and Health Public Corporation for a detailed medical checkup between April 2019 and March 2020, the majority of whom were socially active and productive and considered to be socioeconomically middle class. During the study period, 5,061 underwent an upper gastrointestinal (GI) endoscopic examination. Those with a history of gastric surgery or whose status of H. pylori infection could not be determined based on medical records and/or serum antibody test findings were excluded from enrollment.
Information regarding habitual drinking and smoking and the usage of anti-secretory drugs was also obtained. Details related to the kind, duration, or dosage of anti-secretory drugs could not be determined, since our study subjects only visited our medical center for a detailed medical checkup. Therefore, antisecretory drug usage was determined as positive when the subject reported the administration of a proton pump inhibitor (PPI) or H2 receptor antagonist (H2RA) within the preceding three months.
Individuals with endoscopic gastric mucosal atrophy classified as C2-O3 in the classification of Kimura and Takemoto (13) and negative on an anti-H. pylori IgG antibody test were excluded if they had not been treated for eradication of H. pylori, since those were considered to possibly have post-eradication status even though they had not previously undergone eradication therapy or were currently positive for H. pylori infection (14,15). As a result, the present study cohort was composed of 3,353 subjects (2,186 men, 1,167 women; mean age 55.2±9.4 years old) in whom the H. pylori infection status had been determined (Fig. 1).
Figure 1.
Protocol for subject selection.
Determination of the H. pylori infection status
Serum anti-H. pylori IgG antibody detection was performed using a SphereLight H. pylori antibody JⓇ kit (FUJIFILM Wako Pure Chemical, Osaka, Japan) (14,16). The antibody titer was automatically determined using a chemiluminescent enzyme immunoassay method, with a value ≥4.0 U/mL defined as positive, according to the manufacturer's instructions. In addition, a precise medical history concerning the status of H. pylori infection (negative, positive, post-eradication) was obtained in an interview with the subject conducted by a public health nurse. Those who had undergone therapy but without successful eradication were included in the group with H. pylori infection. When eradication therapy was confirmed to be not successful, we recommended the subject undergo an H. pylori stool antigen test at our institution. The presence or absence of H. pylori infection, and results of eradication therapy were also confirmed based on endoscopic findings obtained in an upper GI endoscopic examination (17-20), which was performed for all of the subjects.
Endoscopic findings
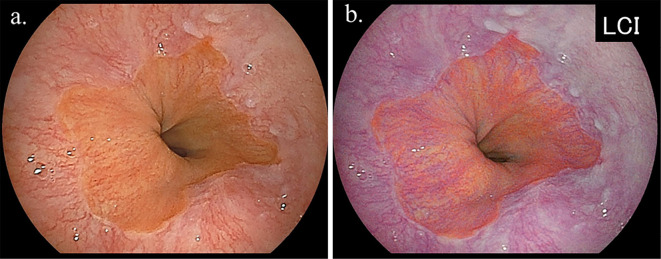
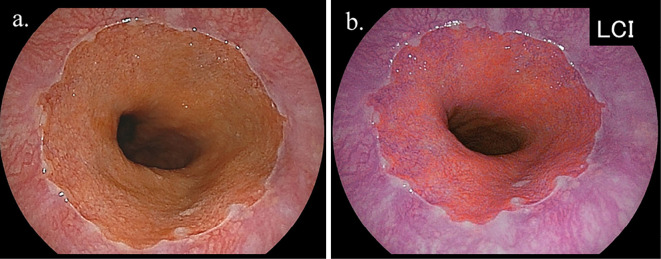
All upper GI endoscopic examinations were performed by experienced licensed endoscopists using an EG-L580NW endoscope (Fujifilm, Tokyo, Japan). At our institution, an upper GI endoscopic examination is performed with the subject in an unsedated condition without anti-cholinergic drug administration, and the endoscope is typically inserted in a transnasal manner. The area of the EGJ was investigated using endoscopy during deep inspiration. When the presence of Barrett's epithelium was suspected, an endoscopic observation with LCI was performed to determine the distal margin of the palisade vessels and confirm the area of Barrett's epithelium (Fig. 2, 3). In this study, Barrett's epithelium was diagnosed as positive when ≥ 5 mm in length halfway around the lower esophagus, since a shorter length of Barrett's epithelium was observed in nearly all cases that underwent an endoscopic observation with LCI, and Barrett's epithelium often exists non-circumferentially.
Figure 2.
Representative endoscopic findings of short segment Barrett’s esophagus (SSBE) in subject without H. pylori infection [a: white light imaging (WLI), b: linked color imaging (LCI)]. The presence of palisade vessels in the area of columnar lined epithelium was shown, and these vessels were revealed to be sequentially connected to the palisade vessels in the area of squamous epithelium, which was easily visualized by LCI. SSBE length was classified as 10-19 mm in this case.
Figure 3.
Representative endoscopic findings of short segment Barrett’s esophagus (SSBE) in subject without H. pylori infection [a: white light imaging (WLI), b: linked color imaging (LCI)]. The presence of palisade vessels in the area of columnar lined epithelium was easily diagnosed by LCI, although it was not easily recognized by WLI. The SSBE length was classified as <10 mm in this case.
Endoscopic findings of reflux esophagitis were evaluated using the Los Angeles (LA) classification (21), and individuals with a grade of A, B, C, or D were diagnosed as positive for reflux esophagitis. The diaphragmatic hiatus size was assessed using endoscopy by comparing the width of the cardiac opening with the diameter of the shaft at the cardiac portion; these findings were used to divide the subjects into 3 groups: <1.0, 1.0-2.0, and >2.0 cm. Gastric mucosal atrophy was evaluated based on endoscopic findings using the classification of Kimura and Takemoto, in which gastric mucosal atrophy is classified into 6 groups (C1, C2, C3, O1, O2, O3) (13). This classification has been shown to correlate well with the histological features of atrophy (22). Cases without gastric mucosal atrophy were classified as C1 according to the classification of Kimura and Takemoto. For the present study, C1-C2 was defined as mild, C3-O1 as moderate, and O2-O3 as severe gastric mucosal atrophy.
All endoscopic images from each subject were simultaneously reviewed by three expert endoscopists to determine the presence of Barrett's epithelium, with decisions made by consensus. In addition, endoscopic findings indicating positivity for H. pylori infection, such as nodular gastritis, spotty and/or diffuse redness of fundic gland mucosa, and sticky mucus (17), were carefully examined in all investigated cases. The longest length of Barrett's epithelium in each subject was determined to be <10 mm, 10-19 mm, 20-29 mm, or ≥30 mm, with the decision based on consensus. When there were inconsistencies in the judgment of the endoscopic images among the endoscopists, the final diagnosis was decided by the lead endoscopist (K.A.).
Statistical analyses
Statistical analyses were performed using a chi-squared test, Mann-Whitney's U test, and a Kruskal-Wallis test. All calculations were performed using the StatView 5.0 software program for Macintosh (Abacus Concepts, Berkeley, USA), with a p level <0.05 considered to indicate statistical significance.
Research ethics
This study was performed in accordance with the Declaration of Helsinki, and the protocol was approved by the ethics committee of the Shimane Environment and Health Public Corporation. Written informed consent indicating that obtained clinical data would be used for a clinical study without the release of individual information was received from all subjects before performing the medical checkups.
Results
Of the 3,353 study subjects, the presence of Barrett's epithelium was endoscopically determined in 1,884 (56.2%), with lengths of <10 mm, 10-19 mm, 20-29 mm, and ≥30 mm noted in 1,005, 851, 27, and 1, respectively. Subjects with lengths of 20-29 mm and ≥30 mm were combined into a single group designated as ≥20 mm, as relatively few people had a long Barrett's epithelium. None were diagnosed with Barrett's cancer during the study period.
Subject characteristics after dividing into those with and without Barrett's epithelium are shown in Table 1. Those with Barrett's epithelium showed a higher percentage of men, older age, and higher body mass index (BMI) as well as a greater proportion with habitual drinking and smoking than those without Barrett's epithelium. The rate of using anti-secretory drugs was not markedly different between the subjects with and without Barrett's epithelium. Reflux esophagitis and a larger diaphragmatic hiatus were frequently observed in cases with Barrett's epithelium, and the degree of gastric mucosal atrophy was also higher in those cases (p<0.001). The prevalence of Barrett's epithelium in H. pylori-negative, H. pylori-positive, and post-eradicated cases was 41.7%, 64.4%, and 69.9%, respectively (p<0.001).
Table 1.
Characteristics of Study Subjects with and without Barrett’s Epithelium.
| With BE (n=1,884) |
Without BE (n=1,469) |
p value | ||||
|---|---|---|---|---|---|---|
| Gender (male/female) | 1,309/575 | 877/592 | <0.001 | |||
| Age | 55.5±9.2 | 54.7±9.7 | 0.007 | |||
| BMI | 23.4±3.4 | 23.1±3.6 | <0.001 | |||
| Habitual drinking | 873 (46.3%) | 620 (42.2%) | 0.017 | |||
| Habitual smoking | 353 (18.7%) | 233 (15.9%) | 0.030 | |||
| Anti-secretory drug usage | 139 (7.4%) | 114 (7.8%) | 0.677 | |||
| Reflux esophagitis | 350 (18.6%) | 144 (9.8%) | <0.001 | |||
| Size of diaphragmatic hiatus | <0.001 | |||||
| <1.0 cm | 1,399 (74.3%) | 2,034 (85.6%) | ||||
| 1.0-2.0 cm | 416 (22.1%) | 381 (11.8%) | ||||
| >2.0 cm | 69 (3.7%) | 59 (2.5%) | ||||
| Gastric mucosal atrophy | <0.001 | |||||
| mild | 1,275 (67.7%) | 1,242 (84.5%) | ||||
| moderate | 486 (25.8%) | 173 (11.8%) | ||||
| severe | 123 (6.5%) | 54 (3.7%) | ||||
| Helicobacter pylori infection | <0.001 | |||||
| negative | 657 (34.9%) | 920 (62.6%) | ||||
| positive | 161 (8.5%) | 89 (6.1%) | ||||
| post-eradication | 1,066 (56.6%) | 460 (31.3%) |
Data are expressed as the mean±standard deviation or number of subjects.
BE: Barrett’s epithelium, BMI: body mass index. Habitual drinking: alcohol drinking 3 or more times per week, anti-secretory drug usage, usage of proton pump inhibitor or H2 receptor antagonist within preceding 3 months. Gastric mucosal atrophy was evaluated using the classification of Kimura and Takemoto (C1-C2: mild, C3-O1: moderate, O2-O3: severe).
When comparisons were performed among the subjects with different lengths of Barrett's epithelium, the ≥20 mm group showed a higher percentage of older age than others. Male predominance was observed in all Barrett's epithelium cases, regardless of length. The rate of using anti-secretory drugs tended to be higher, and reflux esophagitis, a large diaphragmatic hiatus, and mild degree of gastric mucosal atrophy were more frequently observed in cases with a longer Barrett's epithelium than in shorter cases. None of the cases with Barrett's epithelium ≥20 mm in length had H. pylori infection (Table 2).
Table 2.
Characteristics of Subjects Based on the Length of Barrett’s’ Epithelium.
| Length of Barrett’s’ epithelium | ||||||||
|---|---|---|---|---|---|---|---|---|
| <10 mm (n=1,005) |
10-19 mm (n=851) |
≥20 mm (n=28) |
p value | |||||
| Gender (male/female) | 689/316 | 597/254 | 23/5 | 0.259 | ||||
| Age | 54.9±9.1 | 56.2±9.4 | 57.3±7.8 | 0.004 | ||||
| BMI | 23.4±3.4 | 23.4±3.4 | 24.0±2.9 | 0.560 | ||||
| Habitual drinking | 452 (45.0%) | 406 (47.8%) | 15 (53.6%) | 0.371 | ||||
| Habitual smoking | 178 (17.7%) | 167 (19.6%) | 8 (28.6%) | 0.233 | ||||
| Anti-secretory drug usage | 62 (6.2%) | 74 (8.7%) | 3 (10.7%) | 0.092 | ||||
| Reflux esophagitis | 157 (15.6%) | 182 (21.4%) | 11 (39.3%) | <0.001 | ||||
| Size of diaphragmatic hiatus | <0.001 | |||||||
| <1.0 cm | 776 (77.2%) | 612 (72.9%) | 11 (39.3%) | |||||
| 1.0-2.0 cm | 207 (20.6%) | 199 (23.4%) | 10 (35.7%) | |||||
| >2.0 cm | 22 (2.2%) | 40 (4.7%) | 7 (25.0%) | |||||
| Gastric mucosal atrophy | <0.001 | |||||||
| mild | 741 (73.7%) | 515 (60.5%) | 19 (67.9%) | |||||
| moderate | 221 (22.0%) | 259 (30.4%) | 6 (21.4%) | |||||
| severe | 43 (4.3%) | 77 (9.1%) | 3 (10.7%) | |||||
| Helicobacter pylori infection | <0.001 | |||||||
| negative | 409 (40.7%) | 235 (27.6%) | 13 (46.4%) | |||||
| positive | 78 (7.8%) | 83 (9.7%) | 0 | |||||
| post-eradication | 518 (51.5%) | 533 (62.6%) | 15 (53.6%) | |||||
Data are expressed as the mean±standard deviation or number of subjects.
BMI: body mass index. Habitual drinking: alcohol drinking 3 or more times per week, anti-secretory drug usage: usage of proton pump inhibitor or H2 receptor antagonist within preceding 3 months. Gastric mucosal atrophy was evaluated using the classification of Kimura and Takemoto (C1-C2: mild, C3-O1: moderate, O2-O3: severe).
When the prevalence of Barrett's epithelium in subjects who had undergone H. pylori eradication was analyzed, there was no significant association with the duration since eradication. In addition, the distribution of Barrett's epithelium length did not change markedly after dividing the subjects based on the duration since eradication (Fig. 4).
Figure 4.
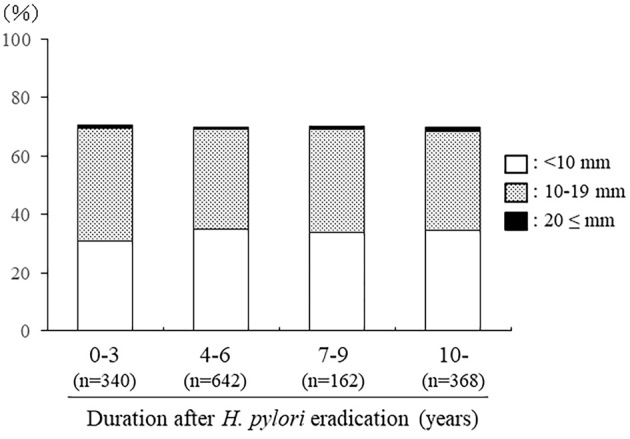
Prevalence of Barrett’s epithelium in subjects divided by the duration since the eradication of H. pylori.
Discussion
Barrett's esophagus is considered an important disease condition, as it is recognized to be the origin of esophageal adenocarcinoma (1-5). In addition, the length of Barrett's epithelium has been demonstrated to be an important factor affecting an increased risk of adenocarcinoma in patients with Barrett's esophagus (23-25). In Japan, however, the prevalence of LSBE is rare, and esophageal adenocarcinoma has been repeatedly demonstrated to mainly occur in cases with SSBE (3-5). Several Japan-based investigators have explored the prevalence of Barrett's epithelium, including SSBE, and shown it to range from 7.9% to 43.0% in the general population (Table 3) (26-34), although the criteria used for the SSBE diagnosis were not identical among those studies, especially with regard to length. In the present study, the prevalence of Barrett's epithelium using endoscopic observation with LCI was investigated. Our results showed that the prevalence of Barrett's epithelium with a length of ≥5 mm of 56.2%, while that of Barrett's epithelium with a length of ≥10 mm was 26.2%. Therefore, the prevalence of Barrett's epithelium in the Japanese population shown here was highest among the reported studies. We previously investigated the circumferential location of different shapes of SSBE and found the prevalence of Barrett's epithelium ≥10 mm in length in 5.2% of individuals who visited the same medical center (34). In contrast, that prevalence was 26.2% in the present study. This marked difference may have been caused by different methods being used to detect the presence of Barrett's epithelium, although the study subjects were not the same in the previous and present studies. In both studies, the diagnosis was based on columnar-appearing mucosa between the squamocolumnar and EGJ, with the latter defined as the distal margin of the palisade vessels in the lower esophagus based on the criteria of The Japan Esophageal Society (6).
Table 3.
Prevalence of Barrett’s Epithelium in Studies Conducted in Japan.
| Reference | Year | Number of cases | Total prevalence | Length of Barrett’s epithelium | |||
|---|---|---|---|---|---|---|---|
| 0-4 mm | 5-9 mm | 10-29 mm | ≥30 mm | ||||
| 26 | 2000 | 650 | 15.7% | 15.1% | 0.6% | ||
| 27 | 2003 | 548 | 12.2% | * | 12.0% | 0.2% | |
| 28 | 2005 | 2,577 | 20.8% | 17.5% | 3.1% | 0.2% | |
| 29 | 2008 | 5,338 | 37.6% | 37.4% | 0.2% | ||
| 30 | 2008 | 6,504 | 10.3% | 9.4% | 1.7% | 0.5% | |
| 31 | 2009 | 869 | 43.0% | ||||
| 32 | 2012 | 832 | 22.1% | 22.1% | 0% | ||
| 33 | 2013 | 18,792 | 7.9% | ||||
| 34 | 2016 | 3,788 | 5.2% | * | * | 5.2% | 0.03% |
| Present study | 2020 | 3,353 | 56.2% | * | 30.0% | 26.2% | 0.03% |
*Cases with this length were not considered to be positive for Barrett’s epithelium.
However, in our previous investigation, the distal margin was examined using WLI, as LCI was not available. LCI, a specific color-enhancing technology that processes endoscopic images to improve color separation in the red regions of mucosal blood vessels, was shown by Takeda et al. to improve the visibility of Barrett's esophagus (7), which was also seen in the present study. Nevertheless, the high prevalence of Barrett's epithelium demonstrated here should be confirmed by a comparative study of endoscopic observations between WLI and LCI as part of a large-scale multicenter study.
Barrett's esophagus is an acquired condition resulting from gastroesophageal reflux disease (35,36), and the prevalence of reflux esophagitis has often been demonstrated to be increasing in Japan due to the westernization of eating habits and lifestyle (37-39). The present subjects with Barrett's epithelium included a higher percentages of men, older subjects, and subjects with a high BMI, as well as a greater proportion with habitual drinking and smoking than those without Barrett's epithelium. In addition, a larger diaphragmatic hiatus was frequently observed in those with Barrett's epithelium. These factors have been shown to be associated with the occurrence of reflux esophagitis (37-41). Indeed, reflux esophagitis was more frequently observed in the present subjects with Barrett's epithelium than in those without it. Therefore, our findings confirm that gastroesophageal reflux has an important role in the development of Barrett's epithelium, including SSBE.
The results of this study showed that an H. pylori-positive or post-eradicated status was more predominant in the group with Barrett's epithelium than in the group without it. In addition, the prevalence of Barrett's epithelium was similar between the H. pylori-positive and post-eradicated subjects. Therefore, continuous H. pylori infection is considered to be related to the development of Barrett's epithelium, and the role of H. pylori infection in the incidence of SSBE might disappear after its successful eradication. In the present study, the subjects with a higher degree of gastric mucosal atrophy were also shown to be more predominant in the group with Barrett's epithelium than in the group without it, and the degree of gastric mucosal atrophy was higher in cases with Barrett's epithelium of 10-19 mm than in those with a length <10 mm. Therefore, the elongation of SSBE might be induced by a longer duration of H. pylori infection, since the progression of gastric mucosal atrophy is also caused by long-term H. pylori infection. At present, we cannot clearly explain how the presence and duration of H. pylori infection influence the prevalence and elongation of Barrett's epithelium. H. pylori infection was repeatedly shown to cause the inflammation of the EGJ portion (carditis) (42-44). Therefore, we speculated that carditis induced by H. pylori infection has some important role in the formation of SSBE. A further prospective study with a histological examination should be performed to clarify whether or not H. pylori-induced carditis correlates with the formation of SSBE.
Our analysis of cases with Barrett's epithelium ≥20 mm in length demonstrated that negativity for H. pylori infection and a milder degree of gastric mucosal atrophy were more commonly observed in these patients than in those with a shorter length, suggesting that the acidity of the gastroesophageal reflux contents may be an important factor in the development of LSBE. The eradication of H. pylori infection has been reported to increase the gastric acidity in patients with a high degree of gastric mucosal atrophy, while the prevalence of reflux esophagitis has been shown to also be increased after successful eradication (45,46). However, we were unable to demonstrate the relationship between the duration since the eradication of H. pylori and the length of Barrett's epithelium. The recovery of acidity following eradication might be too small to induce LSBE.
Several limitations associated with the present study warrant mention. It was not performed in a population-based manner, as the subjects visited our center for a medical check-up, but rather as a cross-sectional study at a single medical center. All endoscopic images were simultaneously reviewed by three expert endoscopists to determine the distal margin of the palisade vessels and presence of Barrett's epithelium, and inter-observer differences regarding the diagnosis were not examined. Additional studies are needed to determine the significance of endoscopic observations with LCI for diagnosing Barrett's epithelium. In addition, a long-term prospective study is recommended to determine the effects of H. pylori eradication on Barrett's epithelium progression.
In conclusion, Barrett's epithelium with a length of ≥5 mm was observed in 56.2% of 3,353 subjects by endoscopic observation with LCI, with nearly all of those cases determined to be SSBE. Negativity for H. pylori infection and mild gastric mucosal atrophy were not correlated with the SSBE prevalence, although they were shown to be significant factors for the development of longer Barrett's epithelium. In addition, the duration since the eradication of H. pylori did not have an effect on the prevalence of SSBE.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We wish to thank Noriko Hara, Marie Ishida, Yukari Inoue, Nanako Miura, Yuki Funaki, Noriko Yamauchi, and Naoyuki Notsu of the Shimane Environment and Health Public Corporation, as well as Keiko Masuzaki of the Second Department of Internal Medicine, Shimane University Faculty of Medicine, for their helpful technical support.
References
- 1. Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97: 142-146, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 136: 376-386, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol 24: 729-735, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Amano Y, Kinoshita Y. Barrett esophagus: perspectives on its diagnosis and management in Asian populations. Gastroenterol Hepatol (N Y) 4: 45-53, 2008. [PMC free article] [PubMed] [Google Scholar]
- 5. Kusano C, Gotoda T, Khor CJ, et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol 23: 1662-1665, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Japanese Classification of Esophageal Cancer. (The 10th Edition/Revised Version). The Japan Esophageal Society, ed. Kanehara Shuppan, Tokyo, 2008: 40-42. [Google Scholar]
- 7. Takeda T, Nagahara A, Ishizuka K, et al. Improved visibility of Barrett's esophagus with linked color imaging: inter- and intra-rater reliability and quantitative analysis. Digestion 97: 183-194, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Murai T, Miwa H, Ohkura R, et al. The incidence of reflux oesophagitis after cure of Helicobacter pylori in a Japanese population. Aliment Pharmacol Ther 14 (Suppl 1): 161-165, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Sasaki A, Haruma K, Manabe N, Tanaka S, Yoshihara M, Chayama K. Long-term observation of reflux oesophagitis developing after Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 17: 1529-1534, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Yamamori K, Fujiwara Y, Shiba M, et al. Prevalence of symptomatic gastro-oesophageal reflux disease in Japanese patients with peptic ulcer disease after eradication of Helicobacter pylori infection. Aliment Pharmacol Ther 20 (Suppl 1): 107-111, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Sugimoto M, Uotani T, Ichikawa H, Andoh A, Furuta T. Gastroesophageal reflux disease in time covering eradication for all patients infected with Helicobacter pylori in Japan. Digestion 93: 24-31, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Adachi K, Notsu T, Mishiro T, Kinoshita Y. Long-term effect of Helicobacter pylori eradication on prevalence of reflux esophagitis. J Gastroenterol Hepatol 34: 1963-1967, 2019. [DOI] [PubMed] [Google Scholar]
- 13. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1: 87-97, 1969. [Google Scholar]
- 14. Adachi K, Mishiro T, Tanaka S, Kinoshita Y. Analysis of negative result in serum anti-H. pylori IgG antibody test in cases with gastric mucosal atrophy. J Clin Biochem Nutr 59: 145-148, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toyoshima O, Nishizawa T, Arita M, et al. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol 24: 1419-1428, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karasawa H, Sugiyama A, Takeda M, et al. ABC classification of gastric cancer risk based on a new kit to detect serum Helicobacter pylori antibodies: comparison with another antibody detection kit. J Gastrointestinal Cancer Screen 54: 18-29, 2016(in Japanese with English abstract). [Google Scholar]
- 17. Kato T, Yagi N, Kamada T, et al. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: a multicenter prospective study. Dig Endosc 25: 508-518, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe K, Nagata N, Nakashima R, et al. Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol 19: 4374-4379, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kato M, Terao S, Adachi K, et al. Changes in endoscopic findings of gastritis after cure of H. pylori infection: multicenter prospective trial. Dig Endosc 25: 264-273, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Kyoto classification of gastritis. Haruma K, Ed. Nihon Medical Center, Tokyo, 2017. [Google Scholar]
- 21. Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 45: 172-180, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miki K, Ichinose M, Shimazu A. Serum pepsinogen as a screening test of extensive chronic gastritis. Gastroenterol Jpn 22: 133-141, 1987. [DOI] [PubMed] [Google Scholar]
- 23. Weston AP, Badr AS, Hassanein RS. Prospective multivariate analysis of clinical, endoscopic, and histological factors predictive of the development of Barrett's multifocal high-grade dysplasia or adenocarcinoma. Am J Gastroenterol 94: 3413-3419, 1999. [DOI] [PubMed] [Google Scholar]
- 24. Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett's esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am 44: 203-231, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pohl H, Pech O, Arash H, et al. Length of Barrett's oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut 65: 196-201, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Azuma N, Endo T, Arimura Y, et al. Prevalence of Barrett's esophagus and expression of mucin antigens detected by a panel of monoclonal antibodies in Barrett's esophagus and esophageal adenocarcinoma in Japan. J Gastroenterol 35: 583-592, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Fujiwara Y, Higuchi K, Shiba M, et al. Association between gastroesophageal flap valve, reflux esophagitis, Barrett's epithelium, and atrophic gastritis assessed by endoscopy in Japanese patients. J Gastroenterol 38: 533-539, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Kawano T, Kouzu T, Ohara S, Kusano M. The prevalence of Barrett's mucosa in the Japanese. Gastroenterol Endosc 47: 951-961, 2005(in Japanese with English abstract). [Google Scholar]
- 29. Okita K, Amano Y, Takahashi Y, et al. Barrett's esophagus in Japanese patients: its prevalence, form, and elongation. J Gastroenterol 43: 928-934, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Yamagishi H, Koike T, Ohara S, et al. Tongue-like Barrett's esophagus is associated with gastroesophageal reflux disease. World J Gastroenterol 14: 4196-4203, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akiyama T, Inamori M, Akimoto K, et al. Risk factors for the progression of endoscopic Barrett's epithelium in Japan: a multivariate analysis based on the Prague C & M Criteria. Dig Dis Sci 54: 1702-1707, 2009. [DOI] [PubMed] [Google Scholar]
- 32. Shimoyama S, Ogawa T, Toma T, Hirano K, Noji S. A substantial incidence of silent short segment endoscopically suspected esophageal metaplasia in an adult Japanese primary care practice. World J Gastrointest Endosc 4: 38-44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fujimoto A, Hoteya S, Iizuka T, et al. Obesity and gastrointestinal diseases. Gastroenterol Res Pract 2013: 760574, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adachi K, Mishiro T, Tanaka S, Kinoshita Y. Circumferential locations of different shapes of short-segment Barrett's esophagus. Intern Med 56: 1937-1942, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett's esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 61: 226-231, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Sharma P. Clinical practice. Barrett's esophagus. N Engl J Med 361: 2548-2556, 2009. [DOI] [PubMed] [Google Scholar]
- 37. Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol 44: 518-534, 2009. [DOI] [PubMed] [Google Scholar]
- 38. Kinoshita Y, Adachi K, Hongo M, Haruma K. Systematic review of the epidemiology of gastroesophageal reflux disease in Japan. J Gastroenterol 46: 1092-1103, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Mizuta A, Adachi K, Furuta K, et al. Different sex-related influences of eating habits on the prevalence of reflux esophagitis in Japanese. J Gastroenterol Hepatol 26: 1060-1064, 2011. [DOI] [PubMed] [Google Scholar]
- 40. Adachi K, Mishiro T, Tanaka S, Hanada K, Kinoshita Y. Gender differences in the time-course changes of reflux esophagitis in Japanese patients. Intern Med 54: 869-873, 2015. [DOI] [PubMed] [Google Scholar]
- 41. Niigaki M, Adachi K, Hirakawa K, Furuta K, Kinoshita Y. Association between metabolic syndrome and prevalence of gastroesophageal reflux disease in a health screening facility in Japan. J Gastroenterol 48: 463-472, 2013. [DOI] [PubMed] [Google Scholar]
- 42. Chen YY, Antonioli DA, Spechler SJ, Zeroogian JM, Goyal RK, Wang HH. Gastroesophageal reflux disease versus Helicobacter pylori infection as the cause of gastric carditis. Mod Pathol 11: 950-956, 1998. [PubMed] [Google Scholar]
- 43. Voutilainen M, Färkkilä M, Mecklin JP, Juhola M, Sipponen P. Chronic inflammation at the gastroesophageal junction (carditis) appears to be a specific finding related to Helicobacter pylori infection and gastroesophageal reflux disease. Am J Gastroenterol 94: 3175-3180, 1999. [DOI] [PubMed] [Google Scholar]
- 44. Goldblum JR. Inflammation and intestinal metaplasia of the gastric cardia: Helicobacter pylori, gastroesophageal reflux disease, or both. Dig Dis 18: 14-19, 2000. [DOI] [PubMed] [Google Scholar]
- 45. Iijima K, Ohara S, Sekine H, et al. Changes in gastric gcid secretion assayed by endoscopic gastrin test before and after Helicobacter pylori eradication. Gut 46: 20-26, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamada H, Haruma K, Mihara M, et al. High incidence of reflux oesophagitis after eradication therapy for Helicobacter pylori: impacts of hiatal hernia and corpus gastritis. Aliment Pharmacol Ther 14: 729-735, 2000. [DOI] [PubMed] [Google Scholar]