Abstract
Background:
5-HT4 receptor (5-HT4R) agonists exert prokinetic actions in the GI tract, but non-selective actions and potential for stimulation of non-target 5-HT4Rs have limited their use. Since 5-HT4Rs are expressed in the colonic epithelium and their stimulation accelerates colonic propulsion in vitro, we tested whether luminally acting 5-HT4R agonists promote intestinal motility.
Methods:
Non-absorbed 5-HT4R agonists, based on prucalopride and naronapride, were assessed for potency at the 5-HT4R in vitro, and for tissue and serum distribution in vivo in mice. In vivo assessment of prokinetic potential included whole gut transit, colonic motility, fecal output, and fecal water content. Colonic motility was also studied ex vivo in mice treated in vivo. Immunofluorescence was used to evaluate receptor distribution in human intestinal mucosa.
Key Results:
Pharmacological screening demonstrated selectivity and potency of test agonists for 5-HT4R. Bioavailability studies showed negligible serum detection. Gavage of agonists caused faster whole gut transit and colonic motility, increased fecal output, and elevated fecal water content. Prokinetic actions were blocked by a 5-HT4R antagonist, and were not detected in 5-HT4R knockout mice. Agonist administration promoted motility in models of constipation. Evaluation of motility patterns ex vivo revealed enhanced contractility in the middle and distal colon. Immunoreactivity for 5-HT4R is present in the epithelial layer of the human small and large intestines.
Conclusions and Inferences:
These findings demonstrated that stimulation of epithelial 5-HT4Rs can potentiate propulsive motility, and support the concept that mucosal 5-HT4Rs could represent a safe and effective therapeutic target for the treatment of constipation.
Keywords: constipation, serotonin, epithelial target, peristalsis, 5-HT4 receptor, prokinetic
Serotonin (5-hydroxytryptamine, 5-HT) is an important signaling molecule within the gastrointestinal (GI) tract that can influence motility, secretion, perception of visceral pain or nausea, and vasodilation1. These actions are mediated by a diverse group of 5-HT receptors expressed by neurons and many other cell types within the gut. Because of 5-HT’s important roles in GI functions and sensation, its receptors are targeted to treat GI disorders. For example, antagonists of the 5-HT3 receptor and agonists of the 5-HT4 receptor (5-HT4R) are used to treat various GI-related conditions including nausea, diarrhea, gastroparesis, constipation and pain in irritable bowel syndrome1.
5-HT4R agonists have prokinetic actions, and as a result, several compounds, have been developed and tested for their abilities to alleviate constipation in constipation-predominant irritable bowel syndrome (IBS-C) and chronic constipation, and provide pain relief in IBS-C2–4. Considerable physiological data demonstrate that 5-HT4R stimulation accelerates propulsive motility5–11. Application of 5-HT4R agonists to the myenteric plexus ex vivo increases acetylcholine release through a presynaptic facilitatory mechanism12–15, and as a result, it has been presumed that the prokinetic effect of these compounds involves activation of 5-HT4Rs on enteric nerve terminals. Thus, these drugs have been formulated for distribution via the blood stream. Given that the 5-HT4R is widely distributed outside of the GI tract, including the heart, bladder, adrenal gland and CNS the possibility of off-target actions is a necessary consideration16.
We have previously demonstrated the existence of an epithelial target for 5-HT4R agonists that could generate clinical benefit while minimizing the risk of adverse effects17. Molecular and genetic evidence shows 5-HT4R expression in the colonic epithelium of mouse, rat, guinea pig, and human, with highest expression in murine distal colon, and RNA expression was also detected in the human small and large intestes17. In ex vivo motility and peristaltic reflex assays, our work along with others found that 5-HT4R agonists applied into the lumen of the colon accelerate propulsive motility8,11,17–19. However, application of the agonists to the bathing solution, where they have access to the myenteric plexus, did not enhance motility17, suggesting the existence of 5-HT4Rs in the mucosa. Mucosal application of 5-HT4R agonists also activated 5-HT release from enterochromaffin (EC) cells, mucus discharge from goblet cells and chloride secretion by enterocytes; these effects were abrogated with 5-HT4R antagonists17. More recent findings indicate that activation of epithelial 5-HT4Rs promotes epithelial proliferation and migration, as well as resistance to oxidative stress20,21, and a recent study demonstrated that intraluminal administration of prucalopride elicits propulsive motility patterns in human subjects19. Therefore, the concept of targeting luminal 5-HT4 receptors to alleviate constipation is gaining momentum22.
In the present study, we tested the hypothesis that 5-HT4R agonists that are designed to act within the lumen, with minimal absorption and systemic distribution, could elicit pro-kinetic actions. In these experiments, we used assays that have been previously described in mice to evaluate the actions of these agents both in vivo and ex vivo23–25, and we used immunofluorescence to evaluate 5-HT4R expression in human intestinal epithelium. The results of these investigations support the concept that use of a luminally-acting 5-HT4R agonist could alleviate constipation by selectively targeting mucosal receptors without being systemically absorbed, resulting in a safer treatment for IBS-C and chronic functional constipation.
METHODS
See Supplementary Methods section for strains and sources of animals used, cAMP assay and compound selectivity profiling, experimental autoimmune encephalomyelitis Induction, details of immunohistochemistry protocols, and physiological solution recipes and reagents.
Animals:
All experimental protocols were approved by the University of Vermont or Takeda Pharmaceuticals Institutional Animal Care and Use Committee.
Patient and public involvement:
The public and patients were not involved in the design, recruitment or conduct of this study.
Whole GI Transit:
Mice received a 300 μL oral (PO) gavage of vehicle: 0.5% methylcellulose (Sigma Aldrich), 1% TWEEN® 80 (Sigma Aldrich), 5 mM citric acid monohydrate (Sigma Aldrich), and 6% carmine red (Sigma Aldrich, St. Louis, MO) in tap water ± drug added at various dosages. Mice were then placed in individual cages without bedding but allowed access to food and Napa Nectar™ ad libitum. Fecal pellets were monitored, and whole GI transit time was determined when carmine red dye was detectible in the first fecal pellet. On day one of experimentation, animals received a vehicle gavage, and on day 4 the assay was repeated with drug included in the gavage.
In vivo Motility of the Distal Colon:
Mice received a 100 μL oral gavage of either vehicle alone or with drug dissolved. After a 1-hour delay, mice were then lightly anesthetized with isoflurane, and a 2.3 mm diameter glass bead was inserted 20 mm into the distal colon using a blunt gavage needle. Colonic motility was measured as the time from animal awakening, based on when the animal could become upright independently, until bead expulsion, with shorter times indicating faster motility. On day one of experimentation, animals received a vehicle gavage, and on day 3 the assay was repeated with drug included in the gavage.
Fecal Output and Water Content:
For assessment of fecal output and water content in 7–15 week old mice, mice received a 100 μL oral gavage of vehicle alone or with drug dissolved. Mice were subsequently placed in individual cages for 1 hour without bedding but allowed access to food and water ad libitum. Fecal pellets were collected immediately after passage and placed in sealed 1.5 mL microcentrifuge tubes and weighed. The pellets were counted and dried for 24 hours at 50°C and re-weighed to determine fecal water content. On the first day of experimentation, animals received a vehicle gavage. After a recovery period of one day, the experiment was repeated on day 3 with drug included in gavage.
For assessment of fecal output and water content in aged mice, all animals were single housed in regular cages with wire mesh bottoms and plastic fitted bottoms, the night before dosing with free access to food and water. Each animal was administered freshly prepared vehicle or drug (10mL/kg) by gavage after acclimating to wire bottoms in a cage for at least 12 hours. Mice were returned to their wire bottom cage after dosing, with free access to food and water for an additional hour while fecal pellets were collected. Animals were removed and euthanized after completing the study.
At one-hour post dose, the number of fecal pellets present was recorded. Wet weight was obtained, and pellets were immediately placed in a lylophilizer for eight hours at 20°C, then an additional four hours at 25°C. Dry weight was then recorded.
Ex Vivo Motility Assays:
Mice received a 100 μL enema of either vehicle or drug compound (10 mg/kg) and were then euthanized at 30 min. Entire murine colon was then removed and pinned loosely in a black-bottomed organ bath containing aerated 37°C Krebs solution. Ten-minute videos were recorded (3.75 fps, 1280 × 960, 8-bit) with a digital camera (DMK 41AF02, The Imaging Source, Charlotte, NC) running Astro IIDC software (Aupperle Services and Contracting, Calgary, Alberta). Analysis was performed using custom-written software (VolumetryG9a, Grant Hennig).
Reagents:
Prucalopride and GR113808 were acquired from Takeda Pharmaceutical Company (San Diego, CA) or Sigma Aldrich (St. Louis, MO), and compounds 5HT4-LA1 and 5HT4-LA2 were both acquired from Takeda. For all in vivo studies, drug compounds were either dissolved in a standard vehicle consisting of 0.5% methylcellulose (Sigma Aldrich) and 1% TWEEN® 80 (Sigma Aldrich) in tap water, or dissolved in a final concentration of 5 mM citric acid monohydrate buffer (Sigma Aldrich) within the standard vehicle. Ex vivo motility assays used drugs dissolved in standard Krebs solution: (NaCl, 121 mmol/L; KCl, 5.9 mmol/L; CaCl2, 2.5 mmol/L; MgCl2, 1.2 mmol/L; NaHCO3, 25 mmol/L; NaH2PO4, 1.2 mmol/L; and glucose, 8 mmol/L; Sigma Aldrich). When used, antagonist was administered 30 min prior to agonist for whole gut transit studies, and 10 min prior to agonist for colonic motility studies.
Data Analysis:
Data are presented as means ± standard error of the mean (SEM) for N animals or standard deviation (SD) for pharmacokinetic data. Statistical analyses were calculated using GraphPad Prism software (v8.0a; GraphPad Software, La Jolla, CA). Data between two groups were analyzed by paired Student t test for all in vivo experiments. Data containing three or more groups were analyzed by 1- or 2- way ANOVA, as appropriate, with Tukey’s or Dunnett’s multiple comparisons test, respectively. Data that were not normally distributed were analyzed using the Wilcoxon matched-pairs signed rank test for paired data. Outliers were determined by a Q test, and values greater than 2 standard deviations (SD) from the mean were disregarded. Significance was determined as a P value ≤ 0.05.
RESULTS
In vitro activity and pharmacokinetics of modified 5-HT4 receptor agonists:
Two compounds, 5HT4-LA1 (based on prucalopride) and 5HT4-LA2 (based on naronapride), were generated to test the hypothesis that the prokinetic actions of 5-HT4R agonists can be elicited by activating epithelial 5-HT4Rs. These compounds are 5-HT4R agonists that exhibit minimal systemic absorption. In a cellular assay measuring agonist activity in CHO cells engineered to over-express 5-HT4R, these compounds were potent 5-HT4R agonists with EC50 of 1.1 nM (5HT4-LA1) and 18.8 nM (5HT4-LA2) (Fig. 1A). Importantly, these compounds were highly selective 5-HT4R agonists and did not exhibit activity at concentrations that would be expected to confound results of this study across a diverse panel of G-protein coupled receptors, nuclear hormone receptors, ion channels, kinase and non-kinase enzymes and transporters (see supplementary results).
Figure 1. Modified 5-HT4R agonists activate the 5-HT4R and are minimally absorbed.
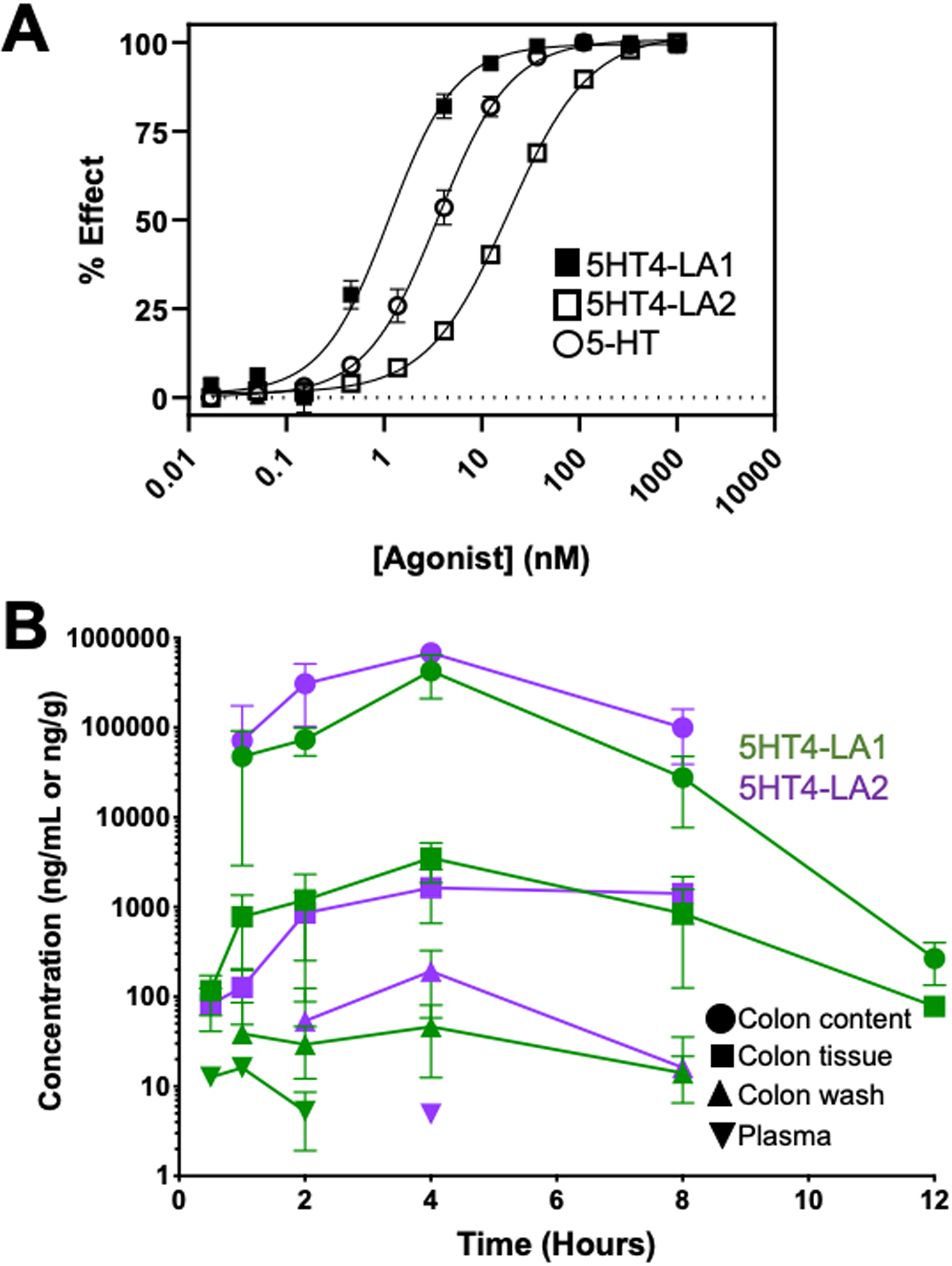
A. Data from CHO cells engineered to over-express the 5-HT4R, and investigated in a cAMP assay. The compounds were potent agonists, with EC50 concentrations of 1.1 nM (5HT4-LA1) and 18.8 nM (5HT4-LA2), as compared to an EC50 of 3.6 nM for 5-HT. B. Mean concentration of 5HT4-LA1 and 5HT4-LA2 after PO administration, in mg/mL or mg/g, in log scale, respectively. Graph shows mean concentration of drug over time (12 hours for 5HT4-LA1 and 8 hours for 5HT4-LA2) in fasted male B6 mice. Drug concentrations for both compounds are significantly higher in colon content versus plasma (P<0.0001). Colon content indicates drug concentrations in chyme fluid; colon tissue indicates drug concentrations in homogenized whole murine colon; colon washings are concentrations from washed colonic mucosa. High levels of drug are detectible as early as 0.5 hours in the colon, with peak concentrations at 4-hour time point. Negligible plasma concentrations are detected from 0.5–2 hours for 5HT4-LA1, but drug is not detectible thereafter. 5HT4-LA2 is only detectible in plasma at the 4-hour time point at a negligible dose. Plasma concentrations were measured at all time points. Data represented as N=3 animals per time point with mean ± standard deviation (SD).
Bioavailability studies were conducted to assess whether these compounds when administered orally remained in the lumen of the colon with minimal systemic distribution. Following oral gavage, both compounds were detectible in the colon as early as 0.5 hours and peaked at 4 hours. Negligible concentrations of the drugs were detected in plasma between 0.5 – 2 hours, but no drug was detected in plasma thereafter. For compound 5HT4-LA1, the mean colon content drug concentration was over 14,000-fold higher than mean plasma drug concentration at the 2-hour time point, and for compound 5HT4-LA2, the colon content was over 140,000-fold higher than plasma at the 4-hour time point (P < 0.001 and P < 0.0001, respectively; Fig. 1B).
Luminally acting 5-HT4R agonists accelerate whole GI transit
Whole gut transit time was determined as the time elapsed for carmine red dye administered by gastric gavage to appear in the feces. In each animal, transit was tested following administration of vehicle gavage on day 1, and then again three days later on day 4, following administration of vehicle, agonist, or agonist plus antagonist. There was no significant difference in transit time when vehicle was administered on both day 1 and day 4 (Fig. 2A).
Figure 2. Luminally acting 5-HT4R agonists accelerate whole gut transit.
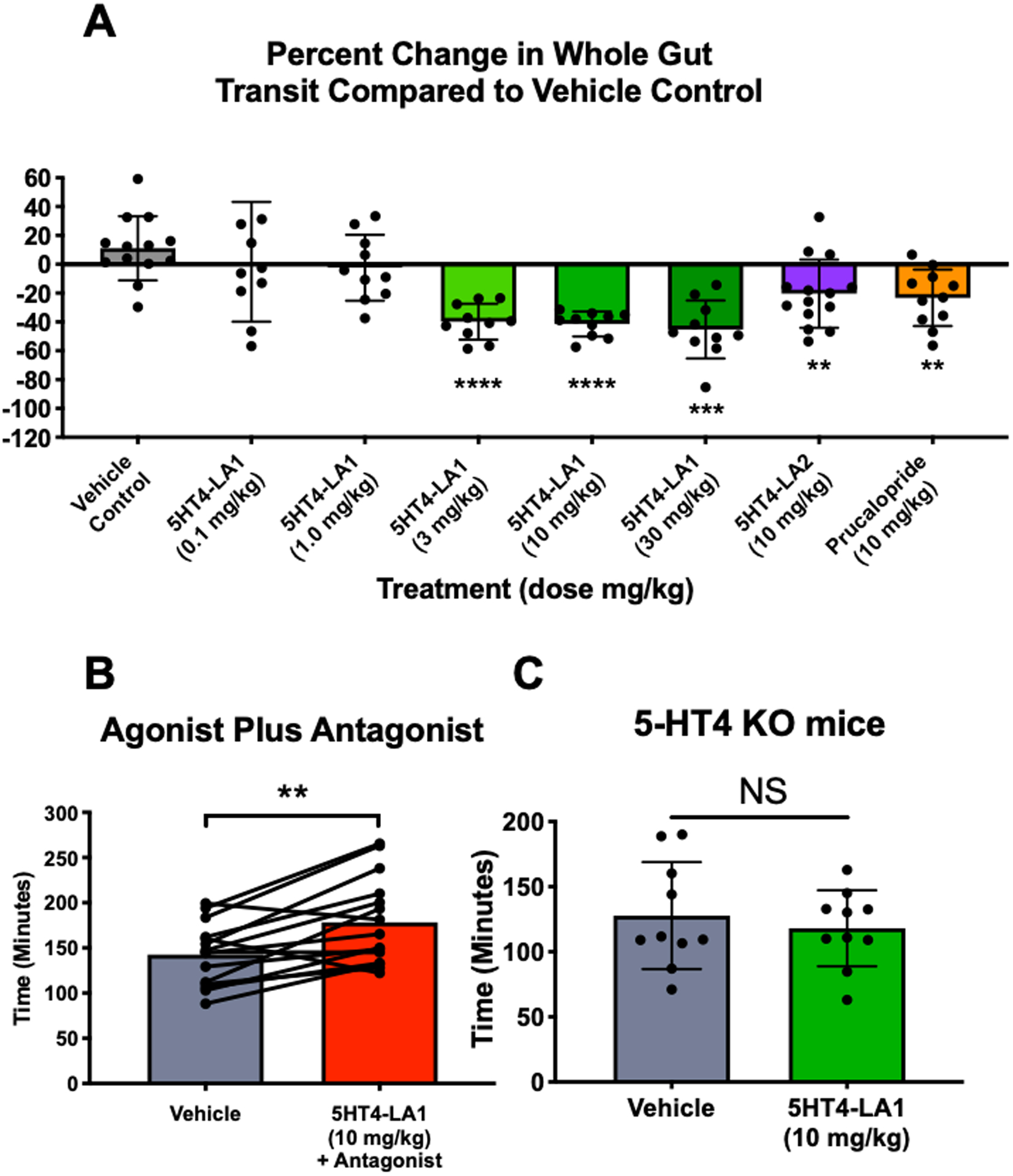
A. Graph demonstrating percent change in whole gut transit time, relative to data from vehicle trials in the same animals three days earlier. Whole gut transit time was not significantly altered when vehicle gavage was administered on day one compared to day four (first bar), while transit time was significantly faster in animals treated with 5HT4-LA1 at doses of 3–30 mg/kg, with 5HT4-LA2 (10 mg/kg) and with prucalopride (10 mg/kg) (vehicle, N=10; all other groups, N=13). B. The prokinetic effect was inhibited when animals were treated with the 5-HT4R antagonist, GR113808 (1 mg/kg), in combination with the agonist, 5HT4-LA1, and in fact, gut transit was significantly slower following antagonist plus agonist administration (N=15). C. The prokinetic effect of 5HT4-LA1 (10 mg/kg) was not detected in mice lacking 5-HT4R (N=4 per group). Data represented as mean ± SEM and analyzed by 2-tailed paired Student t test between vehicle control and treatment. **p≤0.01; ***p≤0.001; ****, p≤0.0001.
Both luminally acting agonists accelerated whole GI transit time following oral gavage of carmine red dye, but not change was detected in vehicle controls. Compound 5HT4-LA1, significantly decreased whole gut transit time when administered at doses of 3 mg/kg and above (Fig. 2A), and compound 5HT4-LA2 also accelerated whole gut transit at the one dose that was tested (10 mg/kg; Fig. 2A). Figure 2A illustrates the data as percent change from paired vehicle control trials, where each bar represents a separate experiment comparing paired transit times from vehicle to drug. As expected, prucalopride, a systemic 5-HT4R agonist, also significantly accelerated whole GI transit.
When administered in combination with the 5-HT4R antagonist, GR113808 (1 mg/kg), 5HT4-LA1 (10 mg/kg) failed to accelerate whole gut transit, and in fact, transit was significantly slowed in this condition (Fig. 2B). In further support of the role of 5-HT4R activation in mediating the prokinetic actions of 5HT4-LA1, whole gut transit was not faster in 5-HT4R KO mice that were treated with the agonist (Fig. 2C).
Luminally acting 5-HT4R Agonists Accelerate Colonic Motility
The bead expulsion assay was used to evaluate changes in colonic propulsive motility, in vivo, in response to vehicle administration in each animal, and then again two days later following administration of agonist or agonist plus antagonist. Animals were treated orally with vehicle or experimental compounds one hour prior to bead placement. No difference in bead expulsion time was detected when vehicle was administered on the first and third days (Fig. 3C).
Figure 3. Colonic motility is significantly faster, in vivo, following administration of luminally acting 5-HT4R agonists.
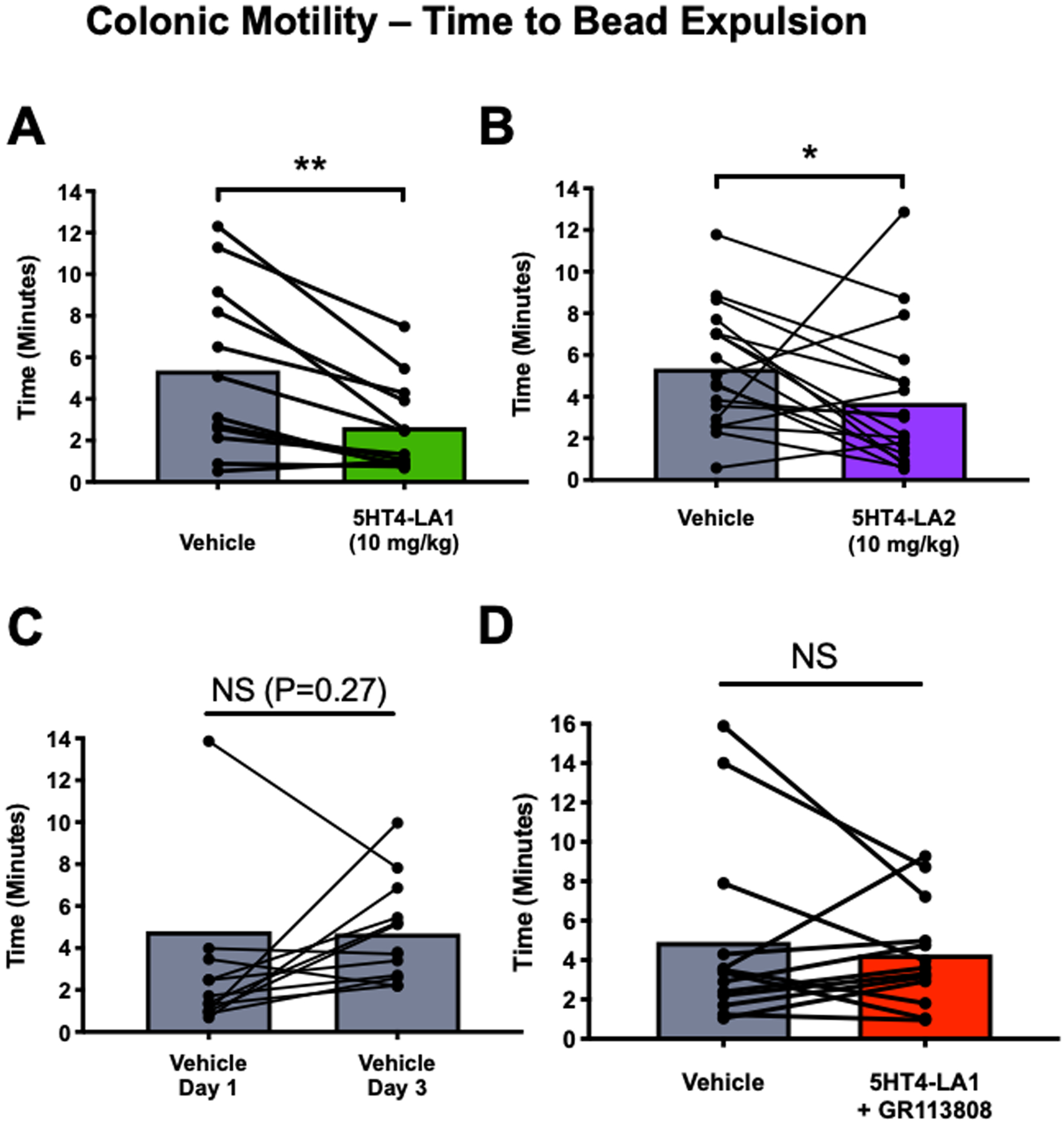
Both 5HT4-LA1 (A; N=12) and 5HT4-LA2 (B; 10 mg/kg; N=18) decreased the time to expulsion of a glass bead place 2 cm into the distal colon, as compared to vehicle trials two days earlier, whereas no difference was detected between trials when the vehicle was administered instead of an agonist (C; N=13). D. The agonist, 5HT4-LA1 did not alter the rate of colonic motility when administered in the presence of the 5-HT4R antagonist, GR113808 (1 mg/kg; N=13). *p≤0.05; **p≤0.01. Data represented as mean ± SEM and analyzed by 2-tailed paired Student t test.
The time elapsed to bead expulsion after administration of 5HT4-LA1 or 5HT4-LA2 (10 mg/kg) was significantly faster as compared to vehicle (Figs. 3A and 3B), suggesting that colonic propulsive motility is accelerated by agonist treatment. This effect was blocked by treatment with the 5-HT4R antagonist GR113808 10 min prior to agonist administration (1 mg/kg; Fig. 3D).
Luminally acting 5-HT4R agonist increases fecal pellet output and water content
Decreased rate of fecal output and decreased fecal water content are features of constipation. Agents that have prokinetic actions in the colon can lead to increased fecal output and fecal water content due to a decrease in the time available for water absorption and/or increased secretion. To determine whether a luminally acting 5-HT4R agonist could affect fecal output and/or water content, mice were placed in individual cages for one hour after oral gavage of vehicle or agonist, and fecal pellets were counted and weighed to calculate water content. Mice were tested following administration of vehicle, and then again two days later following administration of vehicle, 5HT4-LA1, or prucalopride. Fecal pellet count and water content were significantly higher in mice receiving the 5-HT4R agonists versus controls (Figs. 4A and B), although the effects of the luminally acting agonist were not dose dependent in the range that was tested.
Figure 4. Luminally acting 5-HT4R agonist administration increases fecal output and water content.
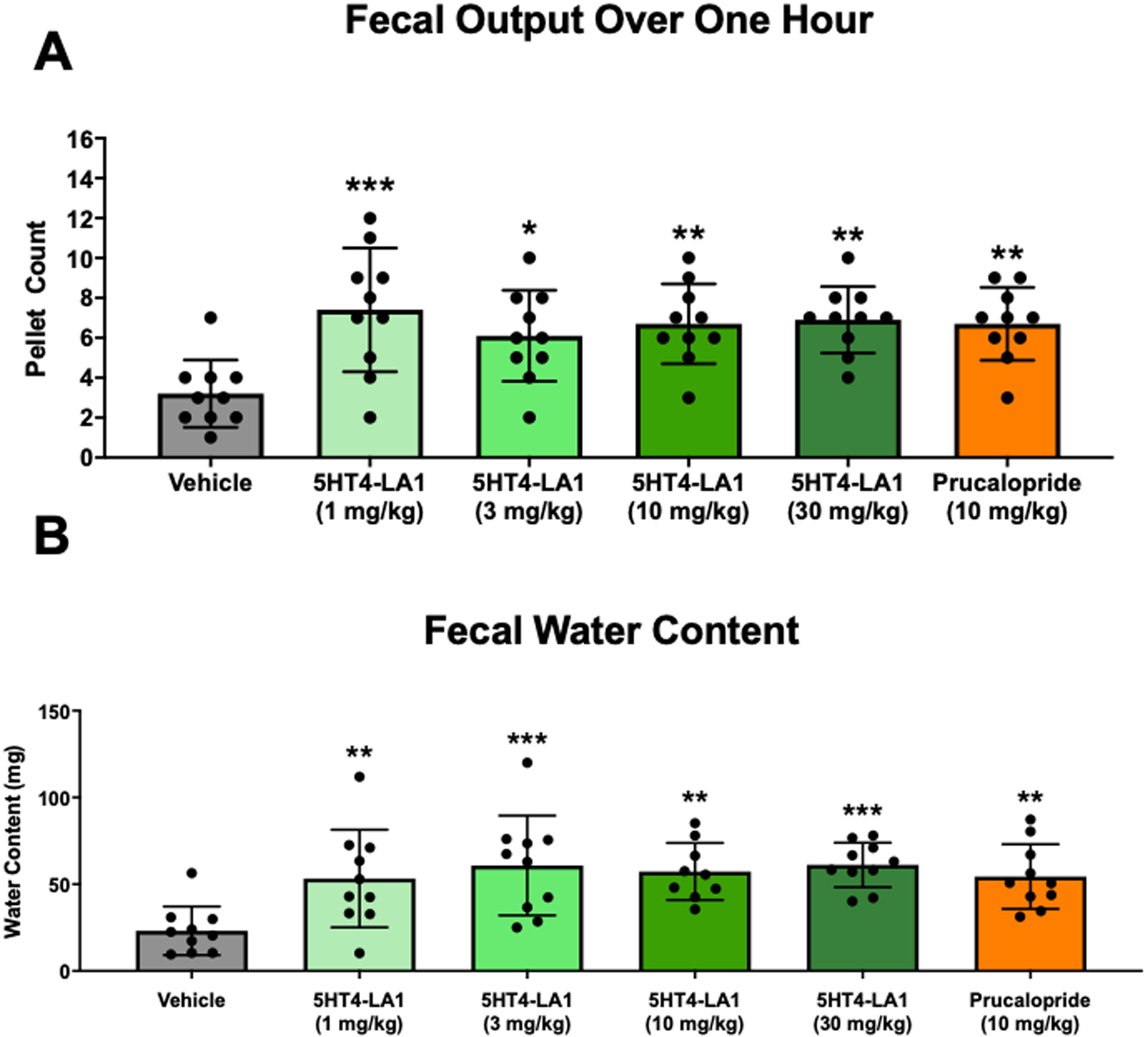
A. Fecal output measured in the first hour following agonist administration by oral gavage. B. Water content of feces collected during the first hour after treatment. N = 10 mice / treatment dose, One-Way ANOVA Compared to Vehicle. *p≤0.05; **p≤0.01; ***p≤0.001
Effects of luminally acting 5-HT4R agonists in models of constipation
The luminally acting 5-HT4R agonist was also tested for prokinetic activity as measured by change in fecal pellet output in a model of chronic constipation. Aging is well known to be associated with altered function of the GI tract in human and often manifests as development of chronic constipation. Aged rodents develop constipation and serve as a model for this phenotype in human26,27. When administered to aged mice that exhibited decreased fecal pellet output compared to young mice at baseline, 5HT4-LA1 caused an increase in fecal pellet output, reversing age-induced constipation (Fig. 5A).
Figure 5. Administration of luminally acting 5-HT4R agonist enhanced motility in two models of constipation.
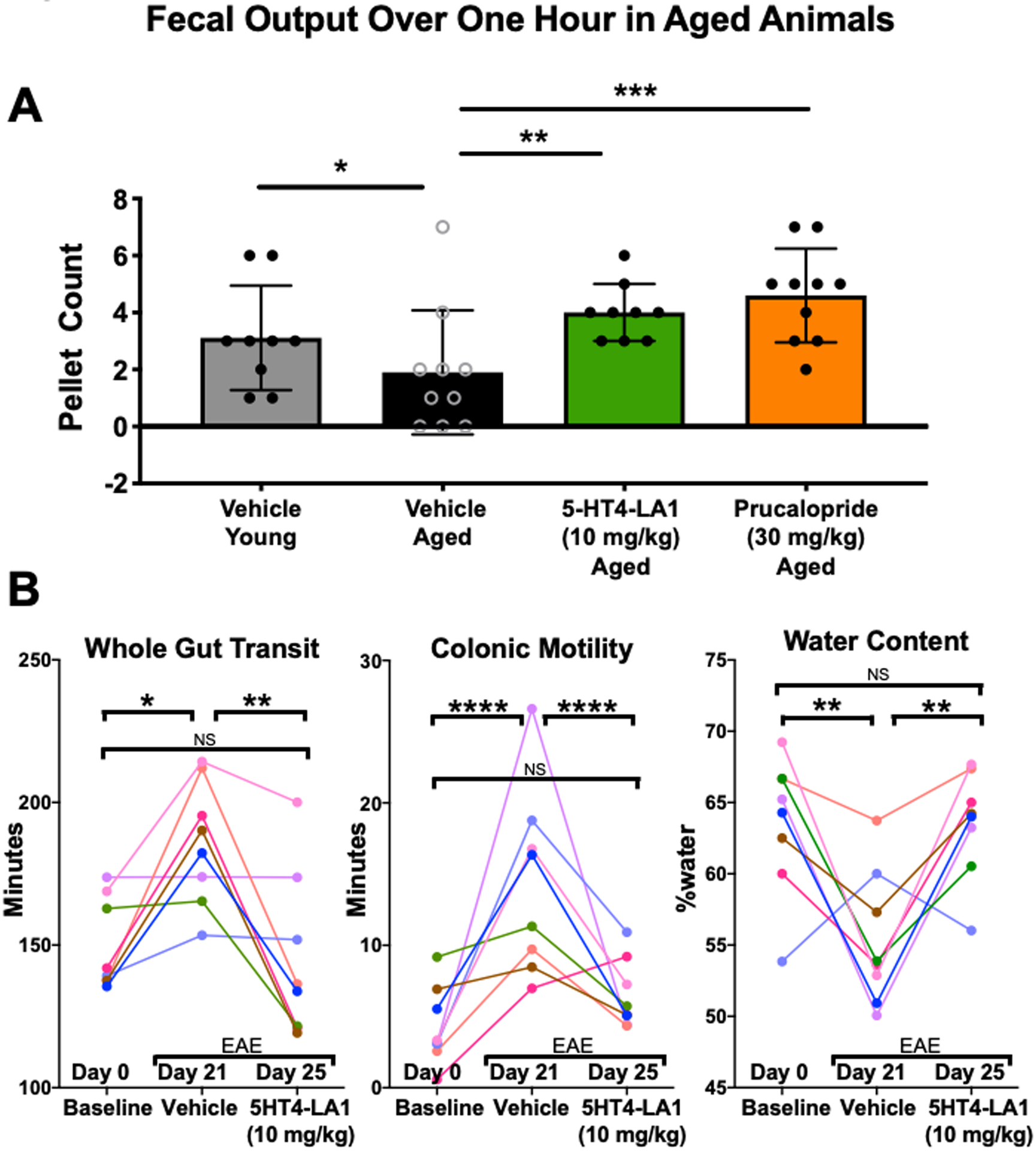
A. Pellet output was decreased in aged animals, as compared to younger mice. In aged mice, fecal output was increased by 5HT4-LA1 and by prucalopride. B. Graphs showing paired data from mice tested for whole gut transit, colonic motility and water content at three different time points: Day 0, before induction of EAE, at Day 21–22, when EAE symptoms had developed, and Day 25–26, when EAE symptoms were still present. Changes in intestinal function consistent with constipation were evident at 21–22 days, and they were reversed to normal levels by treatment with 5HT4-LA1. 2-way ANOVA. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
We have previously demonstrated that mice with experimental autoimmune encephalomyelitis, a commonly used animal model of multiple sclerosis, exhibit alterations in intestinal function that are consistent with constipation23. These include slower whole gut transit, slower colonic motility and drier feces. We therefore tested whether treatment with 5HT4-LA1 would reverse these symptoms. Animals were tested prior to immunization, at 21 days, when GI changes are detectable, and again at 25 days following treatment with the agonist. Agonist treatment significantly improved whole gut transit and colonic motility, and restored fecal water content, to the control levels (Fig. 5B).
Luminally acting 5-HT4R Agonist Increases Colonic Contractility Ex Vivo
Previous work has shown that systemically acting 5-HT4R agonists applied into the lumen of the colon accelerate propulsive motility in isolated ex vivo segments of guinea pig distal colon17. To examine whether the enhancing effects of the 5-HT4R compounds on colonic motility persisted ex vivo, we examined the effect of prior drug application (enema 30 minutes before animals were euthanized) in an ex vivo model of whole murine colon. Spatiotemporal analysis of spontaneous activity was used to measure overall contractile activity, quantified as the proportion of the recording time the colon was undergoing active (≥ 0.16 mm.s−1) contractions. For this study, the type of contractile behavior, such as CMMCs, myogenic “ripples” and longitudinal motions was not specifically considered. Mice treated with 5HT4-LA1 displayed an increased prevalence of contractile activity, particularly in the distal colon (~3x increase; p≤ 0.05; see Fig. 6). This effect was not due to changes in the amount of contents applying different distension forces on the wall of the colon (Suppl. Fig. 1).
Figure 6. Luminally acting 5-HT4R agonist administration enhances contractile activity of isolated colons.
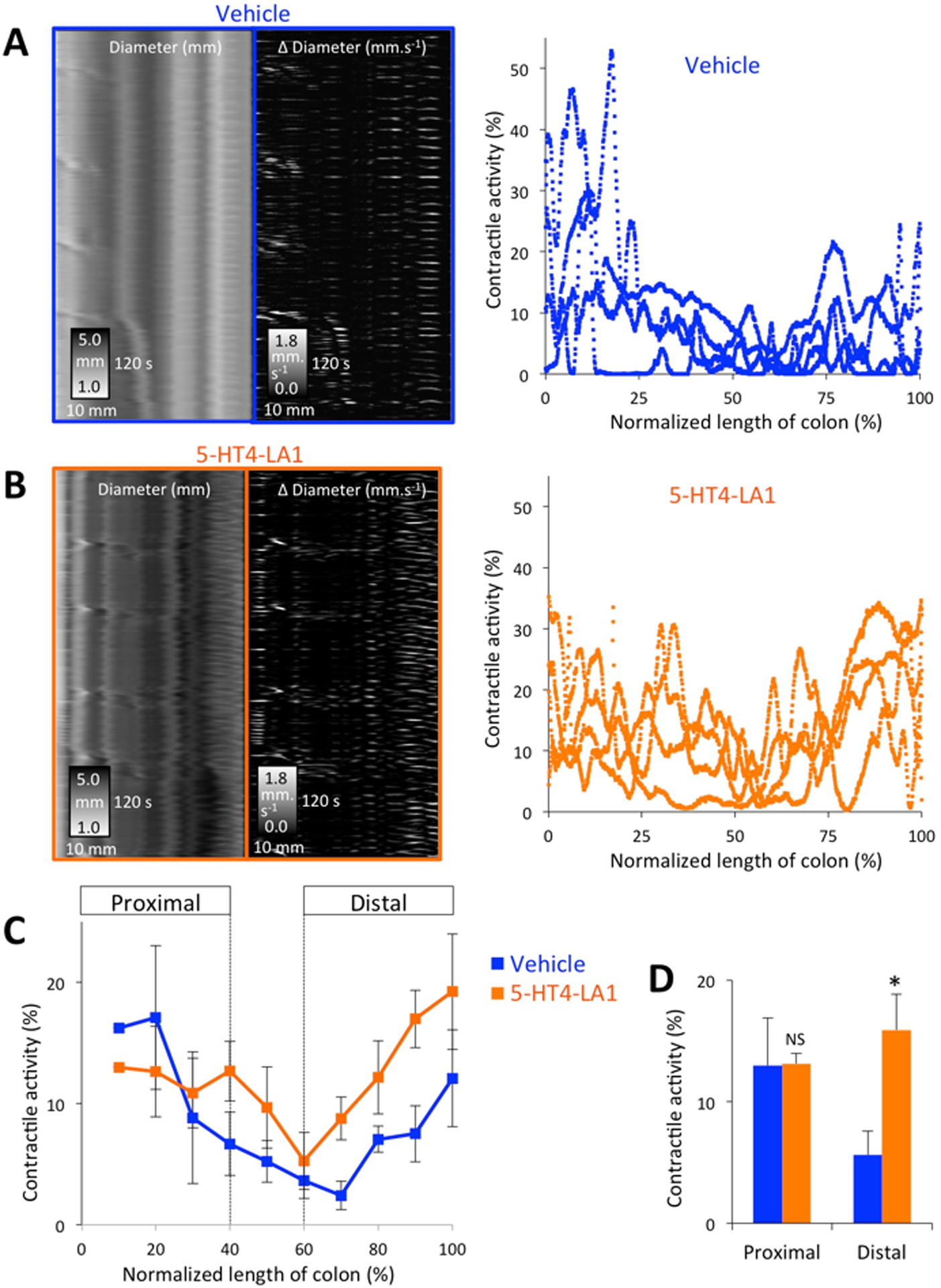
A. Examples of motility patterns (left panels = diameter ST maps, middle panels = Δ diameter ST maps) after vehicle administration and quantified prevalence of contractile activity along the length of the colon (right panel n=4). B. Prior 5-HT4R agonist administration significantly enhanced the degree of contractile activity in isolated colons, particularly in the distal regions of the preparations, as shown in the summary data (C) and (D) where activity was averaged into proximal (0–40% length) and distal (60–100% length) colonic regions. * p<0.05.
5-HT4 receptor immunoreactivity in the human intestine
Morphological evidence for expression of 5-HT4R in the intestinal epithelium was previously obtained indirectly by evaluating enhanced green fluorescent protein (eGFP) fluorescence in sections of intestine from BAC transgenic mice in which the promotor for the 5-HT4R gene drives eGFP fluorescence17. This was due to a lack of a selective and effective 5-HT4R antibody at the time. To investigate whether 5-HT4R is expressed in the human intestine, a newly developed rabbit anti-5-HT4R polyclonal antiserum was used. Control experiments demonstrated that no immunoreactivity was detected with pre-immune serum, with antiserum pre-incubated with the antigen peptide, or in secondary controls (supplemental Fig. 2A). Furthermore, as expected, immunoreactivity was detected in enteric neurons (supplemental Fig. 2B). 5-HT4R immunoreactivity was detected throughout the epithelial layer of the duodenum, ileum, and colon, with the most intense immunoreactivity present in the ileum (Fig. 7). This is consistent with RT-PCR results from human mucosal biopsy samples demonstrating 5-HT4R RNA expression in all intestinal regions tested, with highest levels in the ileum17.
Figure 7. 5-HT4R-immunoreactivity is detectable in the epithelial layer of the human intestine.
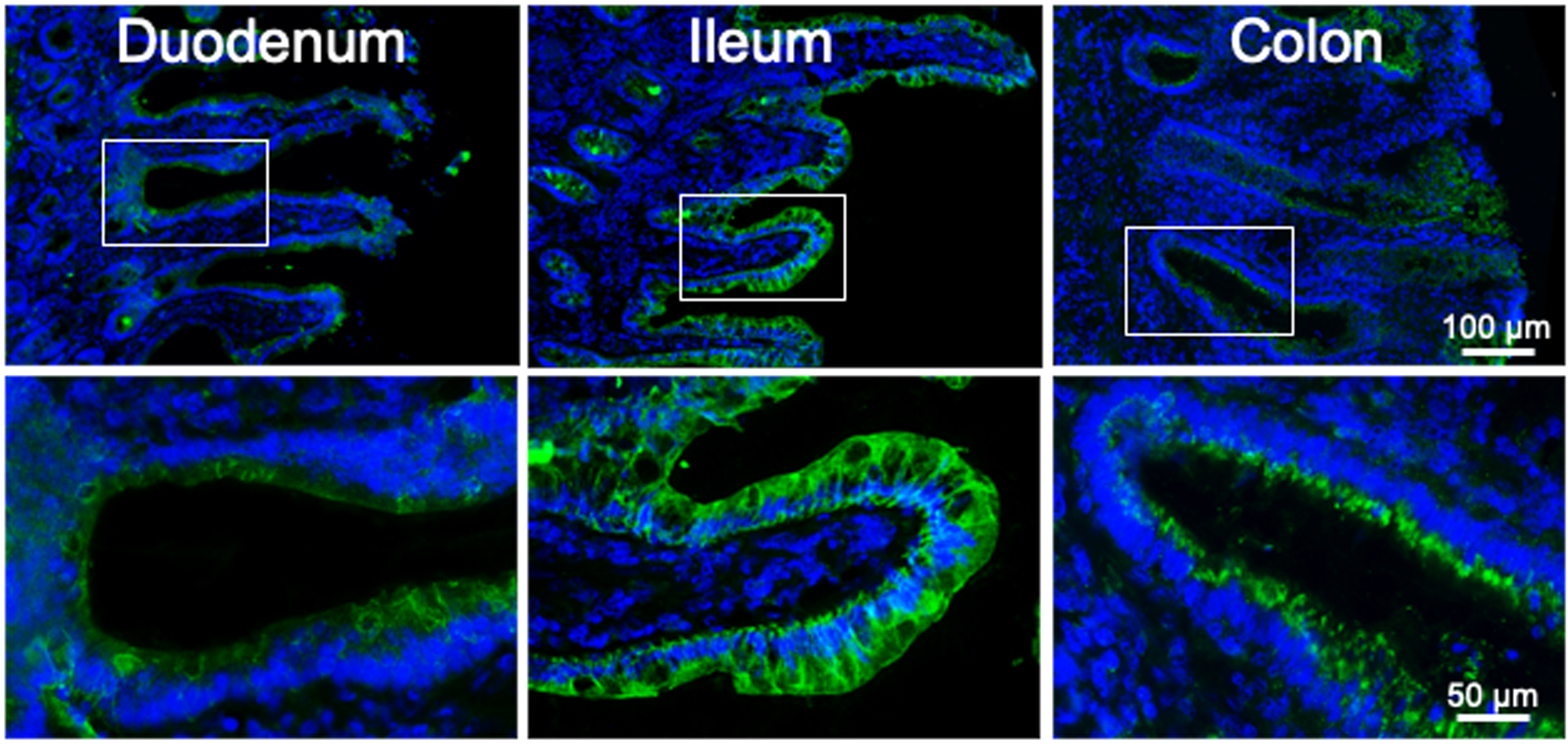
Examples of immunostaining detected in the mucosal layer in human duodenum, ileum and colon. Boxes in the micrographs of the upper row indicate regions shown at higher magnification in the lower row.
DISCUSSION
This study was conducted to test the hypothesis that luminally acting 5-HT4R agonists are at least as effective in eliciting pro-kinetic actions within the GI tract as their systemically absorbed counterparts. In this manuscript, we describe the actions of two novel 5-HT4R agonist compounds, which have negligible systemic absorption and reach the colon at high concentrations following gastric gavage. Our findings demonstrate that these luminally acting compounds are prokinetic and evoke a number of actions that are consistent with the alleviation of constipation, including acceleration of both whole gut transit and colonic motility, increased fecal pellet output, and increased fecal water content. Additionally, responses to the luminally acting 5-HT4R agonists were blocked by the 5-HT4R antagonist GR113808, or were absent when an agonist was administered in 5-HT4R KO mice. We also show, for the first time, that epithelial cells are 5-HT4R-immunoreactive in the human small and large intestines. Collectively, these data demonstrate that activation of epithelial 5-HT4Rs is prokinetic, supporting the emerging concept that gut mucosal 5-HT4Rs could represent an important therapeutic target for the treatment of constipation, as they have similar efficacy while minimizing chances of systemic side-effects.
5-HT4R agonists have been shown to relieve constipation and visceral pain in diseases such as chronic idiopathic constipation (CIC) and IBS-C1,28–33. They were initially identified on nerve terminals of enteric neurons, and stimulation of these receptors has a presynaptic facilitatory effect leading to increased release of neurotransmitters, including acetylcholine, when they are activated17. To reach these terminals, 5-HT4R agonists were developed as systemic compounds such that they could access the enteric neural plexuses of the stomach and intestines via the blood stream. As a result, these compounds had access to tissues throughout the body, where they had the potential to mediate off-target actions, as was the case with cisapride’s inhibition of ERG K+ channels in cardiac muscle. Furthermore, the ability of agonists distributed by the circulation to interact with 5-HT4Rs outside of the GI tract, could present the possibility of adverse reactions to the drugs. In addition to the GI tract, 5-HT4Rs are present in a number of peripheral tissues, including the heart, the adrenal cortex, and the urinary bladder, as well as blood and lymphatic vessels34. Therefore, the ability to limit distribution of 5-HT4R agonists as much as possible to the desired targets may improve both for efficacy and safety.
The impetus for developing and testing luminally acting 5-HT4R agonists came from our previous study demonstrating the extensive expression of 5-HT4Rs in the colonic epithelial layer, and that stimulation of these receptors mediated actions that could help alleviate constipation17. One of the motivations for examining the epithelium as a locus of 5-HT4Rs was the finding that propulsive motility in the guinea pig colon was accelerated when agonists were infused into the lumen8,17, but not when they were added to the bathing solution17. Data were obtained indicating that the 5-HT4R is expressed by enterocytes, goblet cells and enterochromaffin cells, and that 5-HT4R agonists cause Cl−, mucus, and serotonin secretion from these cell types, respectively17. Furthermore, infusion of 5-HT4R agonists into the large intestine inhibited nociceptive responses to distal colon distension17.
Evidence for functional 5-HT4Rs in the epithelium has been reported in other studies. In a study of potential protective effects of enema administration of 5-HT4R agonists, we found that agonist treatment decreased the extent of colitis, and accelerated recovery from established colitis, in conjunction with increased epithelial proliferation, wound healing and resistance to oxidative stress21. In the mouse ileum, systemic administration of prucalopride by osmotic pumps was shown to stimulate mucosal growth and increase carbohydrate absorption20. Furthermore, a recent study by Kashyap and colleagues35 identified gut microbiota-derived tryptamine as a ligand for the epithelial 5-HT4R, which induces accelerated whole gut transit and colonic secretion in mice, supporting the concept that the pro-kinetic actions of the 5-HT4R lie within the epithelium and not necessarily in the muscularis propria. More recently, intraluminal 5-HT4 agonist administration has been shown to enhance propulsive motor activity in ex vivo preparations of rabbit colon19. Additionally, a recent case study involved administration of prucalopride intraluminally into the proximal colon of a human subject and used high-resolution colonic manometry to demonstrate increased propulsive motor patterns that were associated with sphincter relaxation and urge to defecate36. Collectively, these studies support the existence of 5-HT4Rs in the colonic mucosa, and demonstrate that stimulation of these receptors elicits prokinetic responses.
While targeting 5-HT4Rs with luminally-acting agonists to promote motility and alleviate constipation is novel, luminally acting compounds, working through pro-secretory mechanisms, have been developed as constipation therapies2. Lubiprostone activates water and electrolyte secretion via activation of ClC-2 chloride channels. Linaclotide and Plecanatide are agonists of the guanylate cyclase-C receptor, which, when stimulated, increases cGMP levels in intestinal epithelial cells, and, in turn, increases the open state probability of chloride channels. While luminally acting 5-HT4R agonists might work, at least in part, through increased chloride secretion by enterocytes, since this action has been demonstrated17, the finding that propulsive motility is enhanced in open ended distal colon preparations by intraluminal infusion indicates that other mechanisms play a contributing role. For example, goblet cell mucus secretion could promote fecal propulsion by decreasing surface friction, and serotonin secretion by EC cells could activate intrinsic peristaltic and secretory reflexes.
In addition to demonstrating that luminally acting 5-HT4R agonists can exert prokinetic actions in normal mice, findings reported here from two different models of constipation show that 5HT4-LA1 improved colonic motility when it was compromised. These included aged mice, which have previously been shown to exhibit reduced fecal output26,27, and mice with experimental autoimmune encephalomyelitis, which can be used as a model for the constipation symptoms experienced by individuals with multiple sclerosis23.
In the current study, 5-HT4R-immunoreactivity is demonstrated in epithelial cells of the human duodenum, ileum and colon, with the most intense immunofluorescence in the ileum. These findings are consistent with our previous demonstration that 5-HT4R RNA is present in human biopsy samples from the small and large intestines, with highest levels, as measured by quantitative RT-PCR, in the ileum17. Together with the demonstration that intraluminal administration of prucalopride enhances propulsive motor patterns36, several lines of evidence support the possibility that luminally acting 5-HT4R agonists could mediate pro-kinetic actions in the human, as they do in the mouse.
In the cAMP assay to test the affinity of the test compounds for the 5-HT4 receptor, a classic sigmoidal concentration effect relationship was detected. This was not the case for the dose-response relationship in the in vivo whole gut transit and fecal output studies reported here, but this was not necessarily unexpected based on previous studies of this receptor. For example a bell shape curve responses were reported in study investigating the effects of a 5-HT4 agonist in a guinea pig model of postoperative ileus37.
In conclusion, the concept that activation of luminal 5-HT4 receptors could provide a safe and effective approach for the treatment of constipation is gaining momentum22. The findings described here reveal that novel, luminally acting 5-HT4R agonist compounds can generate pro-kinetic actions in the colon. This further supports effort to selectively target the epithelium within GI tract to generate safe and effective therapies for constipation by avoiding systemic side effects.
Supplementary Material
Acknowledgments
The authors wish to thank Yasuo Itomi and Antonio Guy for assistance with in vivo studies conducted at the Takeda laboratories.
Funding information This work was supported by a grant from Takeda Pharmaceuticals, a grant from the National Institutes of Health (R01DK113800 to GMM), and DFG Grant GU 1521/4–1 to DG
Footnotes
Conflict of interest statement Some of the authors of this investigation are employees of Takeda Pharmaceuticals. Dr. Mawe has received research funding from Takeda Pharmaceuticals that has supported this investigation.
References:
- 1.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nature Reviews Gastroenterology and Hepatology. 2013;10(8):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers. 2017;3:17095. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD, Pare P, Viegas A, Ligozio G, Shetzline MA. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103(5):1217–1225. [DOI] [PubMed] [Google Scholar]
- 4.De Maeyer J, Lefebvre R, Schuurkes J. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterology & Motility. 2008;20(2):99–112. [DOI] [PubMed] [Google Scholar]
- 5.Craig DA, Clarke DE. Pharmacological characterization of a neuronal receptor for 5-hydroxytryptamine in guinea pig ileum with properties similar to the 5-hydroxytryptamine receptor. J Pharmacol Exp Ther. 1990;252(3):1378–1386. [PubMed] [Google Scholar]
- 6.Tonini M, Candura SM, Onori L, Coccini T, Manzo L, Rizzi CA. 5-hydroxytryptamine4 receptor agonists facilitate cholinergic transmission in the circular muscle of guinea pig ileum: antagonism by tropisetron and DAU 6285. Life Sci. 1992;50(21):PL173–178. [DOI] [PubMed] [Google Scholar]
- 7.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111(5):1281–1290. [DOI] [PubMed] [Google Scholar]
- 8.Jin J-G, Foxx-Orenstein AE, Grider J. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. Journal of Pharmacology and Experimental Therapeutics. 1999;288(1):93–97. [PubMed] [Google Scholar]
- 9.Grider JR. Desensitization of the peristaltic reflex induced by mucosal stimulation with the selective 5-HT4 agonist tegaserod. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G319–327. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Amer J Physiol-Gastrointest L. 1996;34(5):G849–G857. [DOI] [PubMed] [Google Scholar]
- 11.Grider JR, Foxx-Orenstein AE, Jin J-G. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115(2):370–380. [DOI] [PubMed] [Google Scholar]
- 12.Nemeth PR, Ort CA, Zafirov DH, Wood JD. Interactions between serotonin and cisapride on myenteric neurons. Eur J Pharmacol. 1985;108(1):77–83. [DOI] [PubMed] [Google Scholar]
- 13.Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Physiol. 1994;266(2 Pt 1):G230–238. [DOI] [PubMed] [Google Scholar]
- 14.Galligan J, Pan H, Messori E. Signalling mechanism coupled to 5-hydroxytryptamine4 receptor-mediated facilitation of fast synaptic transmission in the guinea-pig ileum myenteric plexus. Neurogastroenterology & Motility. 2003;15(5):523–529. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;289(6):G1148–G1163. [DOI] [PubMed] [Google Scholar]
- 16.Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5-HT4 agonists developed for gastrointestinal disorders. Alimentary pharmacology & therapeutics. 2012;35(7):745–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman JM, Tyler K, MacEachern SJ, et al. Activation of colonic mucosal 5-HT4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142(4):844–854. e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foxx-Orenstein AE, Jin J-G, Grider J. 5-HT4 receptor agonists and δ-opioid receptor antagonists act synergistically to stimulate colonic propulsion. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1998;275(5):G979–G983. [DOI] [PubMed] [Google Scholar]
- 19.Shokrollahi M, Chen JH, Huizinga JD. Intraluminal prucalopride increases propulsive motor activities via luminal 5-HT4 receptors in the rabbit colon. Neurogastroenterol Motil. 2019;31(10):e13598. [DOI] [PubMed] [Google Scholar]
- 20.Park CJ, Armenia SJ, Zhang L, Cowles RA. The 5-HT4 Receptor Agonist Prucalopride Stimulates Mucosal Growth and Enhances Carbohydrate Absorption in the Ileum of the Mouse. J Gastrointest Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 21.Spohn SN, Bianco F, Scott RB, et al. Protective Actions of Epithelial 5-Hydroxytryptamine 4 Receptors in Normal and Inflamed Colon. Gastroenterology. 2016;151(5):933–944 e933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwynne RM, Bornstein JC. Luminal 5-HT4 receptors-A successful target for prokinetic actions. Neurogastroenterol Motil. 2019;31(10):e13708. [DOI] [PubMed] [Google Scholar]
- 23.Spear E, Holt E, Joyce E, et al. Altered gastrointestinal motility involving autoantibodies in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neurogastroenterology & Motility. 2018:e13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman JM, Brooks EM, Mawe GM. Gastrointestinal motility monitor (GIMM). Journal of visualized experiments: JoVE. 2010(46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. Journal of pharmacological methods. 1981;6(3):211–217. [DOI] [PubMed] [Google Scholar]
- 26.West CL, Amin JY, Farhin S, Stanisz AM, Mao YK, Kunze WA. Colonic Motility and Jejunal Vagal Afferent Firing Rates Are Decreased in Aged Adult Male Mice and Can Be Restored by an Aminosterol. Front Neurosci. 2019;13:955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel BA, Patel N, Fidalgo S, et al. Impaired colonic motility and reduction in tachykinin signalling in the aged mouse. Exp Gerontol. 2014;53:24–30. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Ma T. Luminally Acting Agents for Constipation Treatment: A Review Based on Literatures and Patents. Frontiers in pharmacology. 2017;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tougas G, Snape W Jr, Otten M, et al. Long-term safety of tegaserod in patients with constipation-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2002;16(10):1701–1708. [DOI] [PubMed] [Google Scholar]
- 30.Quigley EM, Wald A, Fidelholtz J, Boivin M, Pecher E, Earnest D. Safety and tolerability of tegaserod in patients with chronic constipation: pooled data from two phase III studies. Clinical Gastroenterology and Hepatology. 2006;4(5):605–613. [DOI] [PubMed] [Google Scholar]
- 31.Tack J, Van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58(3):357–365. [DOI] [PubMed] [Google Scholar]
- 32.Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterology & Motility. 2014;26(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tack J, Camilleri M, Dubois D, Vandeplassche L, Joseph A, Kerstens R. Association between health-related quality of life and symptoms in patients with chronic constipation: an integrated analysis of three phase 3 trials of prucalopride. Neurogastroenterology & Motility. 2015;27(3):397–405. [DOI] [PubMed] [Google Scholar]
- 34.Hegde SS, Eglen RM. Peripheral 5-HT4 receptors. The FASEB journal. 1996;10(12):1398–1407. [DOI] [PubMed] [Google Scholar]
- 35.Bhattarai Y, Williams BB, Battaglioli EJ, et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe. 2018;23(6):775–785 e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shokrollahi M, Wang X-Y, Milkova N, Huizinga JD, Chen J-H. Intraluminal Prucalopride Increases Propulsive Motor Activities in the Human Colon. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 37.Hussain Z, Lee YJ, Yang H, Jeong EJ, Sim JY, Park H. YH12852, a potent and highly selective 5-HT4 receptor agonist, significantly improves both upper and lower gastrointestinal motility in a guinea pig model of postoperative ileus. Neurogastroenterol Motil. 2017;29(10):1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


