Abstract
Pilot studies have hinted that serotonergic psychedelics such as psilocybin may relieve depression, and could possibly do so by promoting neural plasticity. Intriguingly, another psychotomimetic compound, ketamine, is a fast-acting antidepressant and induces synapse formation. The similarities in behavioral and neural effects have been puzzling, because the compounds target distinct molecular receptors in the brain. In this Opinion article, we develop a conceptual framework that suggests the actions of ketamine and serotonergic psychedelics may converge at the dendrites, to both enhance and suppress membrane excitability. We speculate that mismatches in the opposing actions on dendritic excitability may relate to these compounds’ cell-type and region selectivity, their moderate range of effects and toxicity, and their plasticity-promoting capacities.
Keywords: calcium signaling, serotonin receptor, neural plasticity, antidepressant, depression, psilocybin
Towards a shared basis for rapid-acting antidepressants
Psychedelics are compounds that produce an atypical state of consciousness characterized by altered perception, cognition, and mood [1]. Drugs with these properties include serotonergic psychedelics, such as psilocybin and lysergic acid diethylamide (LSD), and dissociatives, such as ketamine. Research interest in these compounds has grown due to their therapeutic potential. At subanesthetic dose, ketamine relieves depression with a rapid onset (within 4 hours) and sustained positive effects (for at least a week) [2, 3]. The antidepressant effect of ketamine is supported by two decades of studies, culminating in successful clinical trials and the approval of esketamine nasal spray ([3, 4, 5]). For serotonergic psychedelics, their potential as a treatment for mood disorders has long been recognized but historically less studied [6, 7]. Clinical trials examining psilocybin, for example, are still underway, though a few studies with small sample sizes suggest the compound may relieve symptoms of depression and anxiety with rapid onset and perhaps longer duration (weeks if not months) [8, 9].
Many psychedelics are broken down in the body rapidly (plasma half-life after intravenous injection in humans is 79 min for ketamine [10], and 74 min for psilocybin [11]), yet behavioral improvements are reported to last for weeks. A current theory for how the short half-life can translate into enduring benefits is that the drugs engage neurotrophic factors to promote neural plasticity [12, 13]. Supporting this idea, in rodents, a single dose of ketamine elevates the expression of synaptic proteins [14] and increases the formation rate of new dendritic spines in the medial frontal cortex [15, 16]. Likewise, serotonergic psychedelics and related agonists enhance the expression of neurotrophic factors and genes associated with synaptic plasticity [17, 18], as well as induce remodeling of dendritic arbors [19].
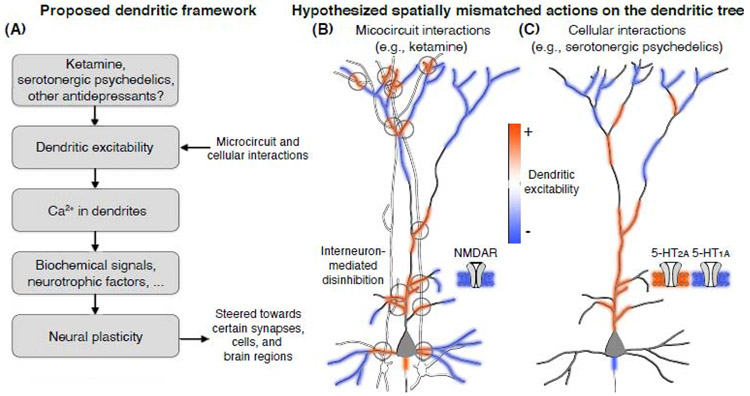
We are not the first to note the comparable effects of serotonergic psychedelics and ketamine in terms of neural and behavioral consequences [20, 21]. However, ketamine is an N-methyl-D-aspartate glutamate receptor (NMDAR) antagonist, whereas serotonergic psychedelics act primarily on serotonergic receptors. The mechanisms for how the disparate molecular targets converge to drive similar plasticity and behavioral effects remain unknown. In this Opinion article, we propose these drugs may share a common ability to both enhance and suppress the excitability of dendrites. What is the consequence of a drug that can drive opposing actions on dendritic excitability? We hypothesize that spatial mismatches in the opposing actions may account for the plasticity-promoting capacities of the drugs as well as their cell-type and brain-region specificity. In other words, competition at the dendrites might steer which synapse, which neuron, and which brain region will undergo plasticity and be modified, thereby positioning dendrites as an important substrate for understanding the actions of ketamine and serotonergic psychedelics (Figure 1A, Key Figure).
Figure 1. Dendritic excitability as a plausible shared substrate of compounds with fast-acting antidepressant properties.
(A) A flowchart outlining the intuition behind a dendrite-focused framework of antidepressant drug actions: ketamine, serotonergic psychedelics, and potentially other drugs with rapid-acting antidepressant effects could acutely modulate dendritic excitability through idiosyncratic ligand-receptor interactions, inducing local gradients of Ca2+ influx that drive neurotrophic factors (e.g., BDNF) and biochemical cascades (e.g., mTOR) to bias certain synapses for the favorable effects of long-term neural plasticity. Based on this view, schematic illustrations show how (B) ketamine is hypothesized to leverage the microcircuit architecture to drive competing actions on pyramidal cell dendrites: inhibition from NMDAR antagonism directly on pyramidal cells (blue shading) and excitation from interneuron-mediated disinhibition (red shading). By contrast, (C) serotonergic psychedelics may take advantage of compartmentalized distributions of serotonin receptor subtypes to drive competing actions on dendritic excitability: agonism of 5-HT1A receptors, likely along the axonal initial segment or in somato-dendritic distribution, decreases excitability (blue shading), while agonism of 5-HT2A receptors, primarily along the proximal apical dendritic trunk, leads to increased excitability (red shading). The hypothesized spatially mismatched actions illustrated in (B) & (C) are supported by some evidence on interneuron connectivity [62, 63] and receptor localization [41-43, 46, 47], but the scheme remains to be validated.
Ketamine – competition for dendritic excitability through microcircuit interactions
Ketamine is a noncompetitive NMDAR antagonist with complex pharmacology [22]. One notable characteristic is that both the induction and reversal of NMDAR blockade are use-dependent. That is, ketamine binds only when the receptor is in its agonist-bound form, but the compound also becomes trapped in a closed channel and cannot unbind until the receptor is reopened by an agonist [23]. Accordingly, although ketamine exhibits similar micromolar affinities for NMDARs of various subunit compositions [24], it may favor receptor subtypes with slower deactivation times [25]. Because blockade decreases the open time and frequency of NMDARs, the anticipated effect of ketamine on dendrites of pyramidal neurons is to reduce membrane excitability (Figure 1B, blue shading).
However, in cortical microcircuits, ketamine antagonizes not only NMDARs on pyramidal neurons, but also NMDARs on GABAergic inhibitory neurons. From the perspective of pyramidal neurons, the consequence is a loss of inhibition, which is the basis of the disinhibition framework of NMDAR antagonism [26]. The disinhibition framework is in agreement with the elevated glutamate efflux [27] and heighted firing rates of pyramidal neurons [28] observed in medial frontal cortex in vivo following the systemic administration of NMDAR antagonists. The heightened spike rates, in particular, suggest decreased inhibition on the cell body. Indeed, NMDAR antagonists have been shown to attenuate the activity of soma-targeting, parvalbumin-expressing (PV) GABAergic neurons [29-31]. A recent study showed that knockdown of NMDARs in PV interneurons can block ketamine’s antidepressant-like effects in mice [32], although the behavioral effects of interneuron manipulation can be complicated [33]. Nonetheless, this finding and other lines of evidence [32, 34] indicate PV interneurons may be involved in ketamine’s antidepressant action.
Extending the disinhibition framework, a recent study demonstrated that ketamine has a substantial impact on inhibition mediated by the dendrite-targeting, somatostatin-expressing (SST) GABAergic interneurons [35]. Using subcellular-resolution two-photon imaging to monitor cellular and synaptic Ca2+ signals, it was shown that the activity of frontal cortical SST interneurons was markedly reduced in awake mice within an hour of ketamine administration. The diminished dendritic inhibition was accompanied by elevated Ca2+ influx in apical dendritic spines, indicative of increased synaptic excitability. Blocking ketamine’s actions on prefrontal SST interneurons prevented drug-induced behavioral outcomes. Therefore, an indirect effect of ketamine on dendrites of pyramidal neurons, through disinhibition, is an increase of membrane excitability (Figure 1B, red shading). Of note, disinhibition by PV interneurons may also contribute to elevating dendritic excitability, as the PV interneurons may influence the proximal portion of the dendritic tree. Taken together, these findings suggest that the direct and indirect effects of ketamine produce opposing actions on the dendritic excitability of pyramidal neurons.
Serotonergic psychedelics – competition for dendritic excitability through co-expressing receptors
Serotonergic psychedelics, also referred to as serotonergic hallucinogens (e.g., psilocybin, LSD, mescaline), have high affinity for serotonin receptors. Take for example psilocybin: after entering the body, it is rapidly converted in the liver into multiple metabolites including psilocin [36]. Psilocin, a structural analog of serotonin, has affinity for many serotonin receptor subtypes and select adrenergic, dopaminergic, and histaminergic receptors (Table 1). The listed serotonin receptors are G protein-coupled receptors that engage a wide range of intracellular signal transduction pathways that can influence neuronal excitability. Here we will focus mainly on 5-HT2A and 5-HT1A receptors because these subtypes have been most heavily characterized in the cortex for their potential roles in mediating the actions of serotonergic psychedelics.
Table 1. The binding affinities of ketamine and psilocin for various receptor types.
| Ki values, binding affinity (nM) | |||
|---|---|---|---|
| Ketamine | Psilocin | Radioligand | |
| 5-HT1A | Low affinity | 567 | [3H]-8-OH-DPAT |
| 5-HT1B | Low affinity | 220 | [3H]-GR-125743 |
| 5-HT1D | Low affinity | 36 | [3H]-GR-125743 |
| 5-HT1E | Low affinity | 52 | [3H]-5HT |
| 5-HT2A | Low affinity | 107 | [3H]-Ketanserin |
| 5-HT2B | Low affinity | 5 | [3H]-LSD |
| 5-HT2C | Low affinity | 97a | [3H]-Mesulergine |
| 5-HT3 | Low affinity | Low affinity | [3H]-LY 278584 |
| 5-HT5 | Low affinity | 84 | [3H]-LSD |
| 5-HT6 | Low affinity | 57 | [3H]-LSD |
| 5-HT7 | Low affinity | 4 | [3H]-LSD |
| 5-HT transporter | N.A. | 3,801 | [3H]-Citalopram |
| NMDA | 661a | N.A. | [3H]-MK-801 |
| Adrenergic α2A | Low affinity | 1,379 | Ketamine: [3H]-Rauwolscine; Psilocin: [125I]-Clonidine |
| Adrenergic α2B | Low affinity | 1,894 | Ketamine: [3H]-Rauwolscine; Psilocin: [125I]-Clonidine |
| Dopamine D3 | Low affinity | 2,645a | Ketamine: [3H]-N-Methylspiperone; Psilocin: [3H]-NMSP |
| Histamine H1 | Low affinity | 305 | [3H]-Pyrilamine |
Low affinity, Ki >10,000 nM
N.A., not available
Source: NIMH Psychoactive Drug Screen Program (PDSP) [129]. Ki values are PDSP certified values (human data except where a indicates value from rats when human data were unavailable). Note that values for ketamine are for the racemic mixture, which consists of (S)- and (R)-ketamine that are converted into multiple metabolites. The enantiomers and metabolites have varying affinities to NMDAR and other receptors [22].
As expected from their respective coupling to Gq and Gi/o protein pathways, activations of 5-HT2A and 5-HT1A receptors have contrasting effects on neuronal excitability. For 5-HT2A receptors, electrophysiological recordings from layer 5 pyramidal neurons in the rat frontal cortex in vitro indicate that activation leads acutely to membrane depolarization [37], and facilitates spiking activity by reducing afterhyperpolarization and decreasing spike frequency accommodation [38]. By contrast, activation of 5-HT1A receptors is associated with membrane hyperpolarization [37, 38]. Similar opponent actions have been reported for human neocortical neurons [39].
Where are these receptors located in the frontal cortex? In one early study, it was estimated that about 60% of frontal cortical neurons in the rat have detectable levels of Htr1a or Htr2a transcripts and, among these cells, about 80% showed co-expression in the same cell [40]. The co-expression is in line with electrophysiological observations of within-neuron competition: some pyramidal neurons have a biphasic firing response to serotonin, where the addition of a 5-HT2A or 5-HT1A receptor-specific antagonist could diminish the excitatory or suppressive component respectively [38].
Although 5-HT2A and 5-HT1A receptors can be present in the same pyramidal neuron, their subcellular localization appears to differ. Immunohistochemical stains revealed a strikingly high density of 5-HT2A receptors in the proximal apical dendritic trunk of pyramidal neurons in rat and macaque frontal cortex [41,42]. The postsynaptic localization to dendritic shafts and dendritic spines was confirmed by an ultrastructural characterization [43], as well as identification of molecular partners that drive the preferential sorting of 5-HT2A receptors [44]. In a study using microiontophoresis to target different dendritic locations, it was shown that adding serotonin locally at the apical dendrite was sufficient to elevate the frequency and amplitude of spontaneous excitatory postsynaptic currents, an effect that was notably absent when the same manipulation was performed on basilar dendrites [45]. Converging evidence therefore indicates that 5-HT2A agonism leads to increased membrane excitability, most strongly in the proximal apical dendrite (Figure 1C, red shading).
There is less consensus on the subcellular localization of 5-HT1A receptors. An immunohistochemical study indicated a somato-dendritic distribution for 5-HT1A receptors on pyramidal neurons in the rat hippocampus [46]. However, subsequent work, which relied on an antibody recognizing a different epitope, reported a concentration of 5-HT1A receptors on the axon initial segment of cortical pyramidal cells [47]. Despite uncertainty in the subcellular localization, what is clear is that the effect of 5-HT1A agonism is a decrease in membrane excitability (Figure 1C, blue shading).
Putting it all together, multiple lines of evidence suggest ketamine and serotonergic psychedelics exert competing actions on dendritic excitability. Although the principal mechanisms—microcircuit interactions for ketamine and receptor co-expression for serotonergic psychedelics—may differ, we reason that the ability of the compounds to drive opposing effects on dendritic function in the same cell is similar. In the following sections, we will explore five ways in which the proposed opposing actions could account for some of the neural and behavioral features of ketamine and serotonergic psychedelics.
Opposing actions as a mechanism to promote long-term neural plasticity
An increase of membrane excitability will boost Ca2+ influx through calcium-permeable channels including NMDARs and voltage-gated Ca2+ channels. Once in the dendritic compartment, Ca2+ acts as a second messenger to initiate signaling cascades and engage neurotrophic factors responsible for spine growth [48]. The essential role of Ca2+ in synaptic plasticity has been demonstrated thoroughly by studies involving bidirectional manipulation of postsynaptic Ca2+ levels during protocols of synaptic potentiation [49, 50]. The relationship is further supported by how the peak Ca2+ accumulation in dendritic spines tracks the magnitude of long-term changes in synaptic efficacy [51]. Overall, our perspective (Figure 1A) is in line with the prevailing neurotrophic model for stress-related mood disorders and antidepressant actions [12, 52], because postsynaptic Ca2+ influx is expected to upregulate neurotrophins [53], such as brain-derived neurotrophic factor (BDNF), which act on tropomyosin receptor kinase B (TrkB) receptors to stimulate molecular target of rapamycin (mTOR) signaling crucial to synapse formation. The plasticity actions of ketamine [54] and serotonergic psychedelics [19] have been linked to BDNF expression and mTOR activation, suggesting these cascades may underlie the shared capacity to drive sustained antidepressant effects. What differs is that the proposed framework emphasizes the acute drug actions on dendritic excitability and Ca2+ influx as leading factors and equally important contributors to the plasticity effects.
Through their actions on dendritic excitability, ketamine and serotonergic psychedelics are expected to initiate plasticity. Plasticity is predicted, by our framework, to occur only for select dendritic branches and spines because the impact of the drugs on the dendritic tree is likely to be heterogeneous, due to spatial mismatches in the opposing actions. In particular, ‘hot spots’ of elevated excitability may be potentiated to enhance synaptic coupling, congruent with the excitatory synapse hypothesis of depression [55, 56]. Although the idea seems intuitive, details remain open for experimental confirmation, because precise measurements of the pharmacological effect across the entire dendritic tree are currently lacking (see Outstanding Questions). For example, there could be differences in the exact dendritic locations targeted by ketamine and serotonergic psychedelics, which would allow for differences in time course and phenotype of the drugs’ antidepressant actions.
Outstanding Questions:
What is the impact of psychedelics on membrane excitability across the entire dendritic tree? The proposed framework predicts that select dendritic locations could be targeted due to spatial mismatches in the microcircuit or cellular interactions, yet measurements across the totality of an individual neuron’s dendrites remains technically challenging.
To the extent that exposure to ketamine and serotoninergic psychedelics may open a ‘critical window of plasticity’ in the frontal cortex, can this period be used to steer and augment the long-term plasticity effects towards connections that would yield therapeutic effects?
Ketamine appears to engage at least two forms of disinhibition via PV and SST interneurons. What are the relative contributions of these disinhibitory mechanisms for the psychotomimetic and fast-acting antidepressant effects of ketamine?
A small but notable fraction of 5-HT1A and 5-HT2A receptors are expressed in GABAergic interneurons. Do serotonergic psychedelics also engage microcircuit interactions (e.g., SST neuron-mediated disinhibition) as ketamine does?
Several neuronal subclasses express serotonin receptor subtypes that are far less understood than 5-HT1A and 5-HT2A receptors, but for which psilocin shows affinity. How do these additional receptor subtypes contribute to the actions of serotonergic psychedelics?
Selective serotonin reuptake inhibitors (SSRI) target also the serotonergic system and promote antidepressant effects. Do they recruit opposing actions at dendrites? Why don’t SSRIs have comparable rapid or durable antidepressant effects?
During acute intoxication, ketamine distorts sensory perception whereas classical psychedelics generate vivid hallucinations. The compounds may also differ in their duration of antidepressant action. What is the neural basis underlying these differences?
Although a full picture of each drug’s actions on dendrites is lacking, one can still appreciate the powerful impact of how local control of dendritic excitability can sculpt Ca2+ signaling and plasticity, by surveying experiments in which dendritic excitability is perturbed directly using electrical or optical methods. At the dendritic trunk, raising excitability by current injection increases the occurrence of dendritic Ca2+ spikes that lead to widespread Ca2+ influx in apical dendritic tufts in vivo [57]. By contrast, reducing excitability by evoking an unitary inhibitory input is sufficient to short-circuit backpropagating regenerative events [58]. These empirical findings are in agreement with computational models showing that inhibition at the dendrites modifies the threshold and amplitude of dendritic electrogenesis [59]. At the dendritic tuft, dendrite-targeting interneurons have a propensity to inhibit cortical apical dendrites in a branch-specific fashion [60, 61]. The inhibitory inputs are primarily located on shafts [62, 63], and the impact of SST interneuron-mediated inhibition on Ca2+ signaling varies greatly across individual dendritic spines [64, 65]. The specificity in dendritic inhibitory innervation suggests that ketamine-induced disinhibition may apply to only a subset of dendritic spines. In one study, the impact of individual GABAergic synapses on dendritic Ca2+ signals was measured and the attenuation had a narrow spatial window of ~25 μm [66]. Collectively, diverse forms of local and widespread dendritic Ca2+ signals are associated with a multitude of plasticity mechanisms [67, 68], which we suspect are targeted by spatially confined changes in excitability arising from the actions of ketamine and serotonergic psychedelics.
We focused on dendritic locations with increased excitability, but is there also a role for other locations in the same dendritic tree with concomitant reductions in excitability? One possibility is that the balance of excitatory and inhibitory effects serves to stabilize the overall excitability of the neuron, preventing aberrant spiking activity. Intriguingly, higher doses of ketamine, where it acts as an anesthetic, dramatically reduce the propagation of electrical signals from the apical dendritic tuft to the cell body of layer 5 pyramidal neurons, leading to an electrical decoupling of the dendritic tree from the somatic compartment [69].
Opposing actions as a mechanism to acutely alter synaptic integration
A central function of the dendrite is synaptic integration, where thousands of inputs are transformed into a (typically) all-or-none output. The integration process is regulated by a balance of excitatory and inhibitory synapses along dendrites [70], which is tuned by homeostatic mechanisms that can calibrate excitability in a branch-specific manner [71] or even at the level of local GABAergic inputs [72]. Because ketamine and serotonergic psychedelics acutely perturb dendritic excitability, the drugs are expected to impair the ability of dendrites to receive and filter inputs. In the frontal cortex, inputs impinging on dendrites carry behaviorally relevant information including sensory- and reinforcement-related signals [61, 73, 74].
The behavioral alterations during the short period when ketamine or a serotonergic psychedelic is bioavailable are consistent with altered synaptic integration. For psychedelics, a core symptom is a warped awareness of the surroundings, corresponding to a disruption of sensory input filtering [1]. The dendritic origin of this phenotype is supported by experiments that have knocked out the dendrite-localized 5-HT2A receptors in neocortex [75] and, more specifically, compromised the dendritic targeting of 5-HT2A receptors [44]. These manipulations eliminated head-twitch responses, a drug-induced motor stereotype in rodents that correlates closely with the potency of hallucinogen exposure in humans [76]. Furthermore, a neural signature for diminished input filtering would be an aberrant increase in functional connectivity with the frontal cortex. Using electrical microstimulation to excite long-range inputs and two-photon imaging to record from frontal cortical dendrites, it was demonstrated that ketamine administration in mice elicits hypersensitivity to long-range cortical inputs [35].
Reinforcement-related signals arriving at the apical dendrites could serve as a substrate for forming new associations or calculating credit assignments during reward-guided learning [58, 77]. In this instance, it is instructive to consider one of the more carefully designed studies that have characterized effects of subanesthetic ketamine on cognitive flexibility. In this study, the authors instructed participants to play the Wisconsin Card Sorting Task twice, one week apart [78]. Administration of ketamine immediately prior to the first task exposure induced perseverative deficits, whereas the same treatment before the second task exposure had no noticeable effect on performance. These results suggest that cognitive rigidity due to subanesthetic ketamine may be ascribed to a learning deficit, because the effect is absent when subjects merely have to re-implement a learned rule.
Opposing actions as a mechanism to influence select cortical regions
Thus far, we have discussed the actions of ketamine and serotonergic psychedelics with an emphasis on the medial frontal cortex. In part, this is because numerous preclinical studies have implicated neural plasticity in the medial frontal cortex as essential for the antidepressant-like effects of ketamine [14, 16]. Another reason is that although the drug would be broadly present in the brain following systemic administration, mapping studies indicated higher metabolic activity in select regions, which included the medial frontal cortex for ketamine in humans [79, 80] and rodents [81], and for psilocybin in humans [82]. These results indicate that the drugs act on certain brain regions more than others (see Box 1 for a brief discussion of other targeted brain regions).
Box 1. Future work informed by the dendritic framework.
The dendritic framework has a number of gaps and predictions that compel further study. We focused the discussion of ketamine on frontal cortex, but regions such as lateral habenula may respond through mechanisms other than dendritic excitability, and play important roles in the antidepressant effects [130]. Serotonergic psychedelics can inhibit spontaneous activity in subcortical nuclei, for example through direct actions on dorsal raphe [131] and indirect actions on locus coeruleus [132]. These neuromodulatory effects may underpin reduced sensory drive in primary cortex [133], contributing to the unique subjective effects of these compounds such as visual hallucinations, which are distinct from ketamine’s effect of distorting sensory perception.
Given the myriad target regions, one intriguing hypothesis is that ketamine and serotonergic psychedelics open a ‘critical window of plasticity’ in the frontal cortex, with concomitant inputs from other regions as necessary ingredients to strengthen specific long-range pathways. If true, this would suggest that purposeful, pathway-specific stimulation during the acute phase of drug administration or within the time window of neurotrophin induction could be beneficial, and may be leveraged to augment plasticity actions. It remains unclear how the direct receptor-level actions of ketamine and serotonergic psychedelics contribute to the acute dissociative or hallucinogenic effects, and whether these psychotomimetic effects are related to the antidepressant action (e.g., [134, 135]). High-fidelity behavioral phenotyping during and following drug administration may help uncover the relations between the on- and off-target behavioral effects. To this end, it may be fruitful to examine whether psychedelics affecting other receptors (e.g., salvinorin A acting on κ-opioid receptors [136]) exhibit similar competing actions on dendritic excitability and rapid antidepressant effect [137].
For serotonergic psychedelics, novel insights might be gained by characterizing the relative expression of serotonin receptor subtypes, rather than their absolute abundances, in relation to dendritic responses, neural pathways [138], and behavioral outcomes [139]. For ketamine, current evidence suggests that only a subset of dendritic spines are under the influence of inhibitory inputs, and therefore sensitive to ketamine-induced disinhibition. Future experiments that focus on these plastic connections, perhaps to identify the source of their presynaptic inputs (see [63, 140]), will be informative. Relatedly, visualizing the acute and sustained drug actions across the entire dendritic tree will help address the nature of the predicted spatial mismatches in dendritic excitability. Current optical methods are limited in terms of relatively small fields of view and poor temporal resolution [141]. New imaging approaches, for instance those relying on remote focusing and Bessel beam technologies [73, 142], open the possibility to measure calcium and other biochemical signals over a large fraction of the dendritic field following drug administration.
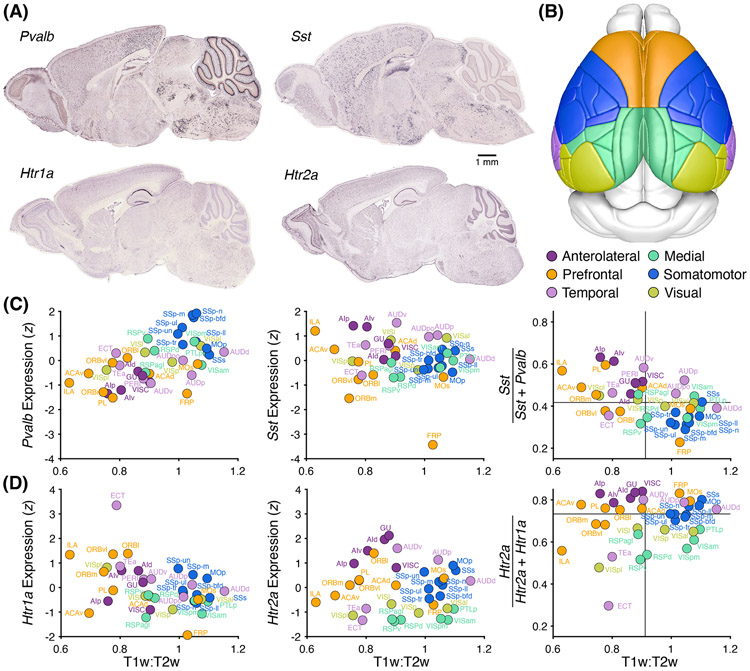
For ketamine, we have proposed that the competing actions coalesce at the apical dendrites of pyramidal cells due to interneuron-mediated disinhibition that opposes the direct effects of NMDAR antagonism. Thus, one prediction is that ketamine should have a stronger influence in regions with a high abundance of SST interneurons relative to the overall inhibitory tone (PV interneurons used as a proxy in the following analyses), because of the relative preponderance of sites available for dendritic disinhibition. This interneuron distribution is indeed the case for medial frontal cortex, in terms of transcript expression in humans [83] as well as cell density in mice [84, 85]. To reproduce these earlier findings but for transcript expression in mice, we plotted the relative levels of Sst and Pvalb mRNA from in situ hybridization data [86] against neuroimaging-based estimates of cortical hierarchy [85] (Figure 2A-C). As the visualization indicates, prefrontal and anterolateral regions have increased expression of Sst relative to Pvalb, consistent with recent measures of cell density [84, 85]. This pattern, we speculate, may render these brain areas more susceptible to drug-induced dendritic disinhibition relative to, for example, motor regions. Such region specificity for ketamine-induced disinhibition is consistent with recent measurements [35], although will require further testing. We note that, in addition to neocortex, similar dendrite-targeting interneurons and microcircuit motifs exist in the hippocampus, another location that is activated robustly by ketamine [81, 87].
Figure 2. Regional differences in the expression of Sst, Pvalb, Htr1a, and Htr2a in the adult mouse neocortex.
(A) For each gene, an example of the mRNA transcripts detected from in situ hybridization in a near-midsagittal section of an adult C57BL/6J mouse, from the Allen Institute for Brain Science database [86]. (B) Cortical regions as demarcated in the Allen Mouse Common Coordinate Framework, and further color-coded based on six groupings. (C) Regional expression of Pvalb and Sst as well as their ratios, obtained from [86], plotted against the T1w:T2w parameter (inversely related to cortical hierarchy), obtained via [85] from the Scalable Brain Atlas [143] in Waxholm space [144]. Lines, medians. (D) Similar to (C), but for Htr1a and Htr2a.
Similarly, the relative abundance of 5-HT1A and 5-HT2A receptors might determine the regional selectivity of serotonergic psychedelics. In a study using high-resolution positron emission tomography in humans, it was found that while 5-HT1A and 5-HT2A receptors showed enrichment in frontal cortex, entorhinal cortex, temporal cortex and the insula, the ratio of expression differs across the regions [88]. Moreover, LSD-induced functional connectivity matches the HTR2A gene expression in human neocortex [89]. To investigate the relationship in mice, we mined in situ hybridization data for Htr1a and Htr2a (Figure 2D). Prefrontal regions tend to have higher Htr2a:Htr1a expression ratio than posterior cortical regions (i.e. medial and visual areas in Figure 2B). The extent to which these differences relate (for instance in mice) to the effect of serotonergic psychedelics on brain-wide activity remains to be measured. It is worth noting that regions important for drug actions are often assumed to have elevated firing or metabolic activity, but this may not be the case for serotonergic psychedelics (see [90]), as plasticity arising from dendritic electrogenesis could occur independent of spiking output [91, 92].
Opposing actions as a mechanism to target subpopulations of neurons
Within the frontal cortex, there are numerous subtypes of pyramidal neurons, and each subtype’s response to ketamine and serotonergic psychedelics may depend on its sensitivity to the hypothesized opposing actions. Take serotonergic psychedelics as an example, subpopulations of pyramidal neurons that have high levels of 5-HT2A relative to 5-HT1A receptors may exhibit pronounced excitation, whereas other cells with favored expression of 5-HT1A receptors will display the opposite response.
There are many anatomical and molecular differences that could contribute to differential sensitivity across subtypes of pyramidal neurons. For example, pertaining to ketamine, supragranular pyramidal neurons have more inhibitory inputs near the main dendritic bifurcation, but fewer inhibitory inputs in distal tufts, relative to deep-layer pyramidal neurons [63]. Among layer 5 pyramidal neurons, the thick- and slender-tufted pyramidal neurons (putatively pyramidal tract (PT) and intratelencephalic (IT) subtypes) have different amounts of inhibitory innervations [63]. Similarly, with regard to their sensitivity to serotonin, most IT neurons exhibit 5-HT2A receptor-dependent increase in firing, whereas many PT neurons display 5-HT1A receptor-dependent activity suppression in the mouse frontal cortex [93, 94], although differences across cell types may be species-specific and developmentally regulated [94].
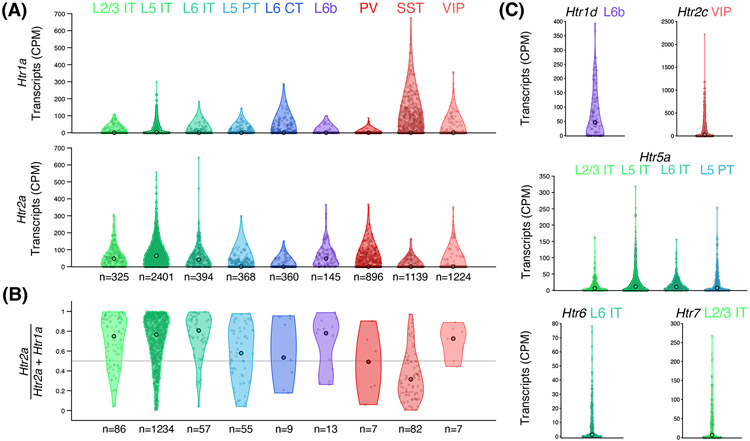
To seek insight into the cell types likely responsive to serotonergic psychedelics, we took advantage of a public database of single-cell RNA sequencing data from >10,000 cells sampled from the anterolateral motor cortex in mice [95]. Focusing on serotonin receptor subtypes with high affinity to psilocin (Table 1), this visualization reveals that although most neuronal subclasses have low levels of Htr1a, IT neurons have enriched expression of Htr2a (Figure 3A). Consistent with prior literature [93, 94], we suggest that the high Htr2a:Htr1a expression ratio in IT neurons should render these cells susceptible to psychedelic-induced increases in membrane excitability (Figure 3B). Other intriguing observations include: a Htr1a bias for SST interneurons suggesting serotonergic psychedelics may also induce dendritic disinhibition, the near absence of co-expression in layer 6 pyramidal neurons and non-SST interneuron subtypes, as well as considerable levels of several other serotonin receptor subtypes whose cell-type-specific functions are unknown (Figure 3C). Overall, the gradient of receptor composition in different cells should correspond to a spectrum of pharmacological responses (Figure 4A-C), with the implication that through a balance of receptor expression ratios, plasticity is steered towards select subpopulations of neurons.
Figure 3. Cell-type differences in the expression of Htr1a, Htr2a, and other serotonin receptor genes in the adult mouse frontal cortex.
(A) Expression of Htr1a and Htr2a transcripts in cortical cell types, based on analyzing single-cell RNA sequencing (SMART-Seq v4) data from 7,252 neurons sampled from the anterolateral motor cortex of adult C57BL/6J mice of both sexes by the Allen Institute for Brain Science [95]. Open circle, median value. n, count of the cell subclass. (B) The Htr2a:Htr1a expression ratio, for neurons with non-zero expression values for both Htr1a and Htr2a. Open circle, median value. n, count of the cell subclass. (C) Additional serotonin receptor subtypes (among those listed in Table 1) that show enriched expression (median CPM > 0) in select neuronal subclasses. Abbreviations: CPM, counts per million reads; L2/3, layer 2/3; L5, layer 5; L6, layer 6; IT, intratelencephalic; PT, pyramidal tract; CT, corticothalamic; PV, parvalbumin; SST, somatostatin; VIP, vasoactive-intestinal protein.
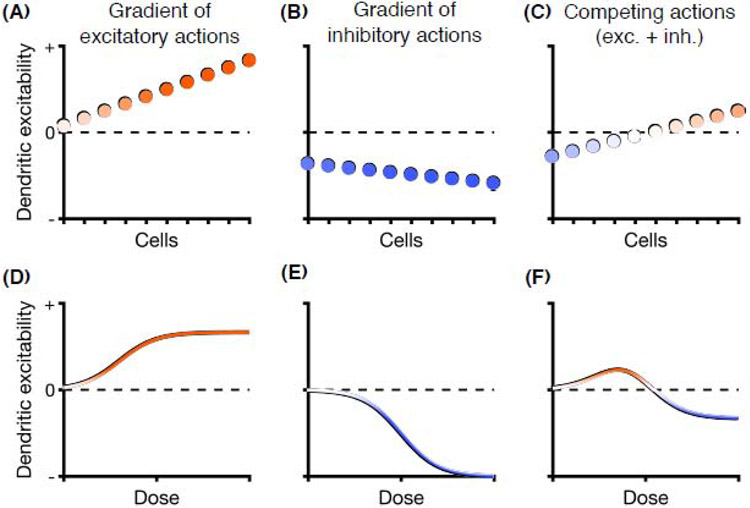
Figure 4. Mismatches in excitatory and inhibitory actions can mediate drug selectivity: a schematic illustration.
A drug may exert a gradient of (A) excitatory effects (e.g., interneuron-mediated disinhibition for ketamine, 5-HT2A receptor agonism for serotonergic psychedelics) and (B) inhibitory effects (e.g., NMDAR antagonism, 5-HT1A receptor agonism) on dendritic excitability across cells or brain regions. (C) The summative effect of the competing actions may steer increases in dendritic plasticity towards a subpopulation of cells or select brain regions, while leaving others unaffected or even suppressed. (D-F) Similarly, for dose-response curves, the summative effect of the competing actions could constrain the effects of a drug on dendritic excitability, limiting the positive effects to a restricted dose range and safeguarding against high-intensity responses associated with drug toxicity.
Opposing actions as a mechanism to mitigate toxicity
Although transient imbalance in dendritic excitability can promote plasticity, prolonged and excessive dendritic alterations may be deleterious and underpin various neuropsychiatric disorders [96, 97]. Confronted with excitotoxicity, dendrites appear to be particularly vulnerable to overactivation [98], perhaps because of their morphology and limited ability to invoke intracellular pathways to counter the excitotoxic challenge [99].
For ketamine and serotonergic psychedelics, the concurrent push-and-pull actions are expected to influence dendritic excitability with a dose-response curve that depends on the precise nature of excitatory and inhibitory responses to each dose. In an illustrated example (Figure 4D-F), the response is tempered at high dose because both actions are engaged. At a lower dose, depending on the potency (i.e., receptor affinity) and efficacy (i.e., maximum biological response) of the competing actions, the excitatory actions can exceed inhibitory effects to generate maximal increase in dendritic excitability for the compound. An inverted U-shaped dose-response curve safeguards against high-intensity responses associated with drug toxicity [100]. This, in combination with the rapid pharmacokinetics, may be crucial for inducing neural plasticity while avoiding dendritic damage. Consistent with this idea, while low-dose ketamine has fast-acting antidepressant effects, higher doses produce general anesthesia. Likewise, although chronic exposure may be neurotoxic [101], serotonergic psychedelics are typically tolerated at high doses. For example, the therapeutic ratio of LSD in humans is 280 (effective dose = 50 mg; lethal dose = 14,000 mg), making the therapeutic dose remarkably distant from doses carrying any lethality risk [1]. By contrast, the hallucinogen NBOMe, which has much higher selectivity for 5-HT2A compared to 5-HT1A receptors, presents negative side effects and has been linked to fatalities [102].
The mitigation of toxicity is a core appeal of non-selective pharmacological therapies. Specifically, ketamine and serotonergic psychedelics may generate a summative therapeutic action, while limiting side effects stemming from excessively agonizing or antagonizing a single target. This idea has been championed as the ‘magic shotgun’ [103], in contrast to highly selective agents which would be ‘magic bullets’.
Limitations of the proposed framework
It is important to consider features of ketamine and serotonergic psychedelics that may be inconsistent with the dendritic framework. Here we proposed that ketamine’s opposing actions on dendritic excitability are due to NMDAR antagonism. Yet other NMDAR antagonists (e.g., dizocilpine, phencyclidine, memantine, rapastinel, and lanicemine) have not consistently produced antidepressant effects [104]. What sets ketamine apart? A parsimonious explanation is that subanesthetic ketamine may induce just the right amount of delicate balance of suppressive and disinhibitory actions on dendritic excitability, varying from other NMDAR antagonists due to differences in pharmacological properties. Indeed, ketamine differs from other NMDAR antagonists in binding site affinity [105], NMDAR trapping [106], potency of effect on intracellular cascades [107] and even preference for particular receptor states and subcellular locations [108]. Still, the exact reasons remain unknown.
Ketamine has additional intriguing characteristics and off-target effects, but these features generally align with the hypothesized push and pull on dendritic excitability. For example, at a slightly higher dose, ketamine appears to suppress HCN1-containing channels, which would relieve shunting in dendrites and also enhance excitability [109]. Recent data indicate that (R)-ketamine may exert more potent antidepressant effect with fewer side effects than (S)-ketamine [110, 111], though both enantiomers are suspected to antagonize NMDARs albeit with slightly different affinity [22]. Moreover, the metabolite hydroxynorketamine (HNK) has been shown to mediate rapid antidepressant-like action without antagonizing NMDARs ([112], but see [113]). Although the effects of ketamine metabolites on dendritic excitability are not yet known, and therefore cannot be fully explained by the present framework, they appear to drive antidepressant-like effects in mice through similar neurotrophic cascades as ketamine [22].
Serotonin receptor signaling is complex, and the pharmacological features distinguishing psychedelics from other serotonin-related agents are not well understood [6]. On the one hand, although serotonin can promote neurite growth in cortical neurons, the effect is minimal compared to psychedelics [19]. On the other hand, the selective 5-HT2A agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) may promote neurite growth [19] and anxiolytic action [114] without agonizing 5-HT1A receptors. Moreover, selective serotonin-reuptake inhibitors (SSRIs) exert antidepressant effects on a different time scale. Some of the complexity may arise because 5-HT2A receptors can form heteromeric complexes (e.g., with metabotropic glutamate 2 receptor (mGluR2) [115]) to recruit additional signal transduction pathways. Furthermore, the effect of serotonin on its receptors may not fully recapitulate the actions of serotonergic psychedelics, as agonist-directed signaling of the 5-HT2A receptor can involve different intracellular partners and transduction pathways [75, 116-118] leading to unique effects on Ca2+ mobilization [119]. Beyond the acute drug actions, there is substantial adaptation in the receptors following agonist exposure, including the well-documented desensitization and downregulation in 5-HT2A receptors [120]. A key challenge will be to reconcile how the various effects at the molecular, cellular, and circuit levels contribute to the antidepressant actions of serotonergic psychedelics.
Dysfunctional signaling of monoamines, including glutamate [5] and serotonin [121], is thought to play a major role in the etiology of depression. Ideally, the actions of an antidepressant should be viewed through the lens of the dysfunction, as the goal of pharmacological treatments is to restore function. In particular, the excitability of dendritic branches and spines may be regulated with homeostatic set points [122]. By nudging excitability in both directions, ketamine and serotonergic psychedelics could act to restore the homeostasis required for proper dendritic function [13]. However, the interaction of dysfunction and pharmacological actions on the dendritic substrate remains poorly understood. In this article, we focused on discussing how psychedelics may converge to prompt selective plasticity actions, but it will also be important to know why the selected plasticity actions are beneficial.
To develop a comprehensive understanding of ketamine’s and serotonergic psychedelics’ antidepressant effects, one will need to consider these compounds’ actions in various brain regions and their potential off-target effects. Even within the frontal cortex, it should be underscored that pyramidal neurons are embedded in cortical microcircuits, therefore considering the drugs’ actions at the individual cell level leads to an incomplete picture. The reverberation of excitatory activity and influences from other cell types is expected to lead to higher-order, downstream effects that further shape dendritic excitability. Serotonergic psychedelics, for example, while preferentially exciting IT pyramidal neurons (based on [93] and Figure 3B), are likely to have second-order effects on PT pyramidal neurons as well, because of the biased connectivity between the two cell types [123]. Moreover, a minor fraction of 5-HT1A and 5-HT2A receptors resides in interneurons (Figure 3A), which can modify GABAergic signaling [124, 125]. There are also possibly 5-HT2A receptors in thalamocortical axons in the frontal cortex [126], although the presynaptic localization is at odds with other evidence [43, 75]. In a similar vein, drug actions on other brain regions are expected to regulate the long-range synaptic inputs arriving at the dendrites. In the medial frontal cortex, inputs impinging on the apical dendritic tufts can arise from a variety of sources (e.g., thalamus, amygdala, other cortical regions) [127], and neuromodulatory inputs such as dopaminergic terminals can play a crucial role [128]. The extent to which these additional layers of micro- and mesoscale circuit interactions relate to the plasticity actions of ketamine and serotonergic psychedelics will be a key question for future research (see Box 1).
Concluding remarks
In summary, ketamine and serotonergic psychedelics have sparked interest as potential groundbreaking neuropsychiatric therapies. Our current understanding of these compounds suggests that the diverse drug actions converge around dendritic signaling. Given that the hypothesized mismatches in opposing actions can have important ramifications for plasticity and selectivity of drug targets, a promising avenue for future exploration will be to clarify the details of the competing mechanisms (see Outstanding Questions). By uncovering the neurobiology for how drug actions translate into sustained symptom improvement, basic science research can reveal critical insights into how to use and innovate on these emerging pharmacological therapies to best serve patients.
Highlights:
Ketamine can relieve symptoms of depression and anxiety, therefore filling a critically unmet psychiatric need. A few small-scale clinical studies suggest serotonergic psychedelics may have similar therapeutic effects.
Ketamine may both enhance and suppress dendritic excitability, through microcircuit interactions involving disinhibition.
Serotonergic psychedelics may both enhance and suppress excitability, through targeting co-expressed receptors.
Spatial mismatch in the opposing drug actions on dendritic excitability is predicted to steer plasticity actions towards certain synapses and cell types.
We present a dendrite-focused framework as a novel lens to view the actions of ketamine and serotonergic psychedelics on cortical circuits.
Acknowledgements
We thank John Murray for early discussions of presented ideas; John Krystal and Farhan Ali for comments on the manuscript; Ben Fulcher for sharing code to analyze the T1w:T2w and in situ hybridization data; Clara Liao for help with the analysis; the Allen Institute for Brain Science for the public resources of single-cell ribonucleic acid sequencing and in situ hybridization data. This work was supported by the Yale Center for Psychedelic Science, NIH/NIMH grants R01MH112750 (A.C.K.) and R01MH121848 (A.C.K.), Simons Foundation Autism Research Initiative Pilot Award (A.C.K.), and NIH/NIGMS Medical Scientist Training grant T32GM007205 (N.K.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
A.C.K. has received investigational materials from the drug supply program at Usona Institute. The authors declare no other competing interests.
References
- 1.Julien RM et al. (2010) A Primer of Drug Action. Twelfth Edition., Worth Publishers. [Google Scholar]
- 2.Berman RM et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47 (4), 351–4. [DOI] [PubMed] [Google Scholar]
- 3.Zarate CA Jr. et al. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63 (8), 856–864. [DOI] [PubMed] [Google Scholar]
- 4.Popova V et al. (2019) Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am J Psychiatry 176 (6), 428–438. [DOI] [PubMed] [Google Scholar]
- 5.Singh JB et al. (2020) Approval of esketamine for treatment-resistant depression. Lancet Psychiatry 7(3), 232–235. [DOI] [PubMed] [Google Scholar]
- 6.Nichols DE (2016) Psychedelics. Pharmacol Rev 68 (2), 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollenweider FX and Preller KH (2020) Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci (in press). [DOI] [PubMed] [Google Scholar]
- 8.Carhart-Harris RL et al. (2016) Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. The Lancet Psychiatry 3 (7), 619–627. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths RR et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30 (12), 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idvall J et al. (1979) Ketamine infusions: pharmacokinetics and clinical effects. Br J Anaesth 51 (12), 1167–1173. [DOI] [PubMed] [Google Scholar]
- 11.Hasler F et al. (1997) Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv 72 (3), 175–184. [DOI] [PubMed] [Google Scholar]
- 12.Duman RS and Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338 (6103), 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavalali ET and Monteggia LM (2020) Targeting Homeostatic Synaptic Plasticity for Treatment of Mood Disorders. Neuron 106 (5), 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N et al. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 (5994), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phoumthipphavong V et al. (2016) Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro 3 (2), e0133–15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moda-Sava RN et al. (2019) Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364 (6436), eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaidya VA et al. (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17 (8), 2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols CD and Sanders-Bush E (2002) A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26 (5), 634–642. [DOI] [PubMed] [Google Scholar]
- 19.Ly C et al. (2018) Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 23 (11), 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghajanian GK and Marek GJ (2000) Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev 31 (2-3), 302–312. [DOI] [PubMed] [Google Scholar]
- 21.Vollenweider FX and Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11 (9), 642–651. [DOI] [PubMed] [Google Scholar]
- 22.Zanos P et al. (2018) Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev 70 (3), 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald JF et al. (1987) Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol 58 (2), 251–266. [DOI] [PubMed] [Google Scholar]
- 24.Ogden KK and Traynelis SF (2011) New advances in NMDA receptor pharmacology. Trends Pharmacol Sci 32 (12), 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paoletti P et al. (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14 (6), 383–400. [DOI] [PubMed] [Google Scholar]
- 26.Lisman JE et al. (2008) Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 31 (5), 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghaddam B et al. (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17 (8), 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson ME et al. (2004) NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A 101 (22), 8467–8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homayoun H and Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27 (43), 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan LZ et al. (2018) All-optical synaptic electrophysiology probes mechanism of ketamine-induced disinhibition. Nat Methods 15 (10), 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widman AJ and McMahon LL (2018) Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A 115 (13), E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerhard DM et al. (2020) GABA interneurons are the cellular trigger for ketamine's rapid antidepressant actions. J Clin Invest 130 (3), 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JA et al. (2015) Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Mol Psychiatry 20 (12), 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng LHL et al. (2018) Ketamine and selective activation of parvalbumin interneurons inhibit stress-induced dendritic spine elimination. Transl Psychiatry 8 (1), 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali F et al. (2020) Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun 11 (1), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passie T et al. (2002) The pharmacology of psilocybin. Addict Biol 7 (4), 357–364. [DOI] [PubMed] [Google Scholar]
- 37.Davies MF et al. (1987) Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res 423 (1-2), 347–52. [DOI] [PubMed] [Google Scholar]
- 38.Araneda R and Andrade R (1991) 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40 (2), 399–412. [DOI] [PubMed] [Google Scholar]
- 39.Newberry NR et al. (1999) Actions of 5-HT on human neocortical neurones in vitro. Brain Res 833 (1), 93–100. [DOI] [PubMed] [Google Scholar]
- 40.Amargos-Bosch M et al. (2004) Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex 14 (3), 281–299. [DOI] [PubMed] [Google Scholar]
- 41.Willins DL et al. (1997) Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 27 (1), 79–82. [DOI] [PubMed] [Google Scholar]
- 42.Jakab RL and Goldman-Rakic PS (1998) 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A 95 (2), 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miner LAH et al. (2003) Ultrastructural localization of serotonin2a receptors in the middle layers of the rat prelimibic prefrontal cortex. Neuroscience 116, 107–117. [DOI] [PubMed] [Google Scholar]
- 44.Abbas AI et al. (2009) PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci 29 (22), 7124–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aghajanian GK and Marek GJ (1997) Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36 (4-5), 589–599. [DOI] [PubMed] [Google Scholar]
- 46.Riad M et al. (2000) Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417 (2), 181–194. [PubMed] [Google Scholar]
- 47.DeFelipe J et al. (2001) Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. J Comp Neurol 433, 148–155. [DOI] [PubMed] [Google Scholar]
- 48.Nishiyama J and Yasuda R (2015) Biochemical Computation for Spine Structural Plasticity. Neuron 87 (1), 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsch JC and Crepel F (1992) Postsynaptic calcium is necessary for the induction of LTP and LTD of monosynaptic EPSPs in prefrontal neurons: an in vitro study in the rat. Synapse 10 (2), 173–175. [DOI] [PubMed] [Google Scholar]
- 50.Malenka RC et al. (1998) Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science 242 (4875), 81–84. [DOI] [PubMed] [Google Scholar]
- 51.Nevian T and Sakmann B (2006) Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci 26 (43), 11001–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duman RS and Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59 (12), 1116–1127. [DOI] [PubMed] [Google Scholar]
- 53.West AE et al. (2001) Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A 98 (20), 11024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou W et al. (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29 (7), 419–423. [DOI] [PubMed] [Google Scholar]
- 55.Cai X et al. (2013) Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci 16 (4), 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson SM et al. (2015) An excitatory synapse hypothesis of depression. Trends Neurosci 38 (5), 279–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu NL et al. (2012) Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 492 (7428), 247–251. [DOI] [PubMed] [Google Scholar]
- 58.Larkum ME et al. (1999) A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398 (6725), 338–341. [DOI] [PubMed] [Google Scholar]
- 59.Jadi M et al. (2012) Location-dependent effects of inhibition on local spiking in pyramidal neuron dendrites. PLoS Comput Biol 8 (6), e1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stokes CC et al. (2014) Single dendrite-targeting interneurons generate branch-specific inhibition. Front Neural Circuits 8, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cichon J and Gan WB (2015) Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature 520 (7546), 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwon T et al. (2019) Ultrastructural, Molecular and Functional Mapping of GABAergic Synapses on Dendritic Spines and Shafts of Neocortical Pyramidal Neurons. Cereb Cortex 29 (7), 2771–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karimi A et al. (2020) Cell-type specific innervation of cortical pyramidal cells at their apical dendrites. Elife 9, e46876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiu CQ et al. (2013) Compartmentalization of GABAergic inhibition by dendritic spines. Science 340 (6133), 759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marlin JJ and Carter AG (2014) GABA-A receptor inhibition of local calcium signaling in spines and dendrites. J Neurosci 34 (48), 15898–15911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mullner FE et al. (2015) Precision of Inhibition: Dendritic Inhibition by Individual GABAergic Synapses on Hippocampal Pyramidal Cells Is Confined in Space and Time. Neuron 87 (3), 576–589. [DOI] [PubMed] [Google Scholar]
- 67.Magee JC and Grienberger C (2020) Synaptic Plasticity Forms and Functions. Annu Rev Neurosci 43, 95–117. [DOI] [PubMed] [Google Scholar]
- 68.Sjostrom PJ et al. (2008) Dendritic excitability and synaptic plasticity. Physiol Rev 88 (2), 769–840. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki M and Larkum ME (2020) General Anesthesia Decouples Cortical Pyramidal Neurons. Cell 180 (4), 666–676 e13. [DOI] [PubMed] [Google Scholar]
- 70.Liu G (2004) Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 7 (4), 373–379. [DOI] [PubMed] [Google Scholar]
- 71.Losonczy A et al. (2008) Compartmentalized dendritic plasticity and input feature storage in neurons. Nature 452 (7186), 436–441. [DOI] [PubMed] [Google Scholar]
- 72.Chiu CQ et al. (2018) Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron 97 (2), 368–377 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerlin A et al. (2019) Functional clustering of dendritic activity during decision-making. eLife 8, e46966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacefield CO et al. (2019) Reinforcement Learning Recruits Somata and Apical Dendrites across Layers of Primary Sensory Cortex. Cell Rep 26 (8), 2000–2008 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez-Maeso J et al. (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53 (3), 439–452. [DOI] [PubMed] [Google Scholar]
- 76.Halberstadt AL et al. (2020) Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guerguiev J et al. (2017) Towards deep learning with segregated dendrites. Elife 6, e22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krystal JH et al. (2000) Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry 47 (2), 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vollenweider FX et al. (1997) Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 7 (1), 9–24. [DOI] [PubMed] [Google Scholar]
- 80.Ionescu DF et al. (2018) Ketamine-Associated Brain Changes: A Review of the Neuroimaging Literature. Harv Rev Psychiatry 26 (6), 320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duncan GE et al. (1999) Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res 843 (1-2), 171–183. [DOI] [PubMed] [Google Scholar]
- 82.Vollenweider FX et al. (1997) Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16 (5), 357–372. [DOI] [PubMed] [Google Scholar]
- 83.Burt JB et al. (2018) Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci 21 (9), 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim Y et al. (2017) Brain-wide Maps Reveal Stereotyped Cell-Type-Based Cortical Architecture and Subcortical Sexual Dimorphism. Cell 171 (2), 456–469 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fulcher BD et al. (2019) Multimodal gradients across mouse cortex. Proc Natl Acad Sci U S A 116 (10), 4689–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lein ES et al. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445 (7124), 168–176. [DOI] [PubMed] [Google Scholar]
- 87.Schobel SA et al. (2013) Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78 (1), 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beliveau V et al. (2017) A High-Resolution In Vivo Atlas of the Human Brain's Serotonin System. J Neurosci 37 (1), 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Preller KH et al. (2018) Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wood J et al. (2012) Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci 32 (9), 3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golding NL et al. (2002) Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature 418, 326–331. [DOI] [PubMed] [Google Scholar]
- 92.Kampa BM et al. (2006) Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J Physiol 574 (Pt 1), 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Avesar D and Gulledge AT (2012) Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits 6 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elliott MC et al. (2018) Serotonin Differentially Regulates L5 Pyramidal Cell Classes of the Medial Prefrontal Cortex in Rats and Mice. eNeuro 5 (1), ENEURO.0305–17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tasic B et al. (2018) Shared and distinct transcriptomic cell types across neocortical areas. Nature 563 (7729), 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ali F et al. (2020) Inhibitory regulation of calcium transients in prefrontal dendritic spines is compromised by a nonsense Shank3 mutation. Mol Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Penzes P et al. (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14 (3), 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greenwood SM and Connolly CN (2007) Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology 53 (8), 891–898. [DOI] [PubMed] [Google Scholar]
- 99.Hasel P et al. (2015) Selective dendritic susceptibility to bioenergetic, excitotoxic and redox perturbations in cortical neurons. Biochim Biophys Acta 1853 (9), 2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nestler EJ et al. (2009) Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. Second Edition., McGraw-Hill. [Google Scholar]
- 101.Cameron LP et al. (2019) Chronic, Intermittent Microdoses of the Psychedelic N,N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem Neurosci 10 (7), 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lawn W et al. (2014) The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample. J Psychopharmacol 28 (8), 780–788. [DOI] [PubMed] [Google Scholar]
- 103.Roth BL et al. (2004) Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3, 353–359. [DOI] [PubMed] [Google Scholar]
- 104.Gould TD et al. (2019) Molecular Pharmacology and Neurobiology of Rapid-Acting Antidepressants. Annu Rev Pharmacol Toxicol 59, 213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daniell LC (1990) The noncompetitive N-methyl-D-aspartate antagonists, MK-801, phencyclidine and ketamine, increase the potency of general anesthetics. Pharmacol Biochem Behav 36 (1), 111–115. [DOI] [PubMed] [Google Scholar]
- 106.Bolshakov KV et al. (2003) Determinants of trapping block of N-methyl-d-aspartate receptor channels. J Neurochem 87 (1), 56–65. [DOI] [PubMed] [Google Scholar]
- 107.Gideons ES et al. (2014) Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A 111 (23), 8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glasgow NG et al. (2017) Memantine and Ketamine Differentially Alter NMDA Receptor Desensitization. J Neurosci 37 (40), 9686–9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen X et al. (2009) HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci 29 (3), 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hashimoto K (2019) Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psychiatry Clin Neurosci 73 (10), 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leal GC et al. (2020) Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci (in press). [DOI] [PubMed] [Google Scholar]
- 112.Zanos P et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533 (7604), 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki K et al. (2017) Effects of a ketamine metabolite on synaptic NMDAR function. Nature 546 (7659), E1–E3. [DOI] [PubMed] [Google Scholar]
- 114.Nic Dhonnchadha BA et al. (2003) Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav Brain Res 140 (1-2), 203–214. [DOI] [PubMed] [Google Scholar]
- 115.Fribourg M et al. (2011) Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147 (5), 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmid CL et al. (2008) Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A 105 (3), 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shan J et al. (2012) Ligand-dependent conformations and dynamics of the serotonin 5-HT(2A) receptor determine its activation and membrane-driven oligomerization properties. PLoS Comput Biol 8 (4), e1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Banerjee AA and Vaidya VA (2020) Differential signaling signatures evoked by DOI versus lisuride stimulation of the 5-HT2A receptor. Biochem Biophys Res Commun 531 (4), 609–614. [DOI] [PubMed] [Google Scholar]
- 119.Cussac D et al. (2008) Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol 594 (1-3), 32–8. [DOI] [PubMed] [Google Scholar]
- 120.Gray JA and Roth BL (2001) Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull 56 (5), 441–451. [DOI] [PubMed] [Google Scholar]
- 121.Nautiyal KM and Hen R (2017) Serotonin receptors in depression: from A to B. F1000Res 6, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davis GW (2013) Homeostatic signaling and the stabilization of neural function. Neuron 80 (3), 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kiritani T et al. (2012) Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci 32 (14), 4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Llado-Pelfort L et al. (2012) 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex 22 (7), 1487–1497. [DOI] [PubMed] [Google Scholar]
- 125.Foehring RC et al. (2002) Serotonergic modulation of supragranular neurons in rat sensorimotor cortex. J Neurosci 22 (18), 8238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barre A et al. (2016) Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A 113 (10), E1382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barthas F and Kwan AC (2017) Secondary Motor Cortex: Where 'Sensory' Meets 'Motor' in the Rodent Frontal Cortex. Trends Neurosci 40 (3), 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu M et al. (2020) Ketamine restores escape behavior by re-engaging dopamine systems to drive cortical spinogenesis. biorxiv. [Google Scholar]
- 129.Roth BL et al. (2000) The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrassment of riches. Neuroscientist 6, 252–262. [Google Scholar]
- 130.Yang Y et al. (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554 (7692), 317–322. [DOI] [PubMed] [Google Scholar]
- 131.Aghajanian GK et al. (1968) Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science 161 (3842), 706–8. [DOI] [PubMed] [Google Scholar]
- 132.Rasmussen K and Aghajanian GK (1986) Effect of hallucinogens on spontaneous and sensory-evoked locus coeruleus unit activity in the rat: reversal by selective 5-HT2 antagonists. Brain Res 385 (2), 395–400. [DOI] [PubMed] [Google Scholar]
- 133.Michaiel AM et al. (2019) A Hallucinogenic Serotonin-2A Receptor Agonist Reduces Visual Response Gain and Alters Temporal Dynamics in Mouse V1. Cell Rep 26 (13), 3475–3483 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Acevedo-Diaz EE et al. (2020) Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord 263, 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grabski M et al. (2020) Ketamine as a mental health treatment: Are acute psychoactive effects associated with outcomes? A systematic review. Behav Brain Res 392, 112629. [DOI] [PubMed] [Google Scholar]
- 136.Roth BL et al. (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A 99 (18), 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taylor GT and Manzella F (2016) Kappa Opioids, Salvinorin A and Major Depressive Disorder. Curr Neuropharmacol 14 (2), 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ju A et al. (2020) Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex. Molecular Brain 13 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Halberstadt AL and Geyer MA (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61 (3), 364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kubota Y et al. (2007) Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J Neurosci 27 (5), 1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ali F and Kwan AC (2020) Interpreting in vivo calcium signals from neuronal cell bodies, axons, and dendrites: a review. Neurophotonics 7 (1), 011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu R et al. (2017) Video-rate volumetric functional imaging of the brain at synaptic resolution. Nat Neurosci 20 (4), 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bakker R et al. (2015) The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics 13 (3), 353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson GA et al. (2010) Waxholm space: an image-based reference for coordinating mouse brain research. Neuroimage 53 (2), 365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]