Abstract
Background
Most of the in vivo neurovascular imaging studies are performed in anesthetized animals. However, anesthesia significantly affects cerebral hemodynamics.
New method
We applied optical coherence tomography (OCT) methods such as optical microangiography (OMAG) and Doppler optical microangiography (DOMAG) to quantitatively evaluate the effect of anesthesia in cerebral vasculature and blood flow in mouse brain.
Results
The OMAG results indicated the increase of large vessel diameter and capillary density induced by ketamine-xylazine and isoflurane, meaning that both anesthetics caused vasodilation. In addition, the preliminary results from DOMAG showed that isoflurane increased the baseline cerebral blood flow.
Comparison with existing methods
In comparison with other in vivo imaging modalities, OCT can provide label-free assessment of cortical tissue including tissue morphology, cerebral blood vessel network and flow information down to capillary level, with a large field of view and high imaging speed.
Conclusions
OCT angiography methods demonstrated the ability to measure the differences in the baseline morphological and flow parameters of both large and capillary cerebrovascular networks between awake and anesthetized mice.
Keywords: optical coherence tomography, optical microangiography, anesthetized mouse, awake mouse, cerebral blood flow, microvasculature
Graphical Abstract

1. Introduction
The use of in vivo animal models for imaging cerebral blood flow (CBF) is crucial for understanding the mechanisms of CBF regulation and brain function. Several in vivo optical imaging studies have been done in anesthetized animals due to the necessity to suppress motion artifacts during image acquisition. However, different anesthetics have proven to disrupt the baseline (prestimulus resting) hemodynamics and functional response to stimuli. [1] As a consequence, anesthesia greatly impacts the baseline CBF, which can potentially cause discrepancies in interpreting the experimental results of hemodynamic response and cerebrovascular disease studies. [2] For example, under α-chloralose/urethane anesthesia, the cerebrovascular response to ethanol caused vasoconstriction, while under halothane it induced vasodilation. [2,3] Therefore, it is important to study the effect of anesthesia on baseline cerebral hemodynamics in animal models. Moreover, the research study showed that the upstream arteries and intracortical microvessels might have separate or independent mechanisms for the regulation of local CBF [4], indicating a need for investigating the vessel responses at both large and microcirculatory (capillary) levels.
Previous studies report the difference in hemodynamic response to functional stimulation between anesthetized and awake animals. [5–7] Optical imaging results from mouse visual cortex showed that neural activity was followed by much stronger and quicker hemodynamic response during wakefulness than during anesthesia. [5] The functional magnetic resonance imaging (fMRI) study with rats demonstrated that the temporal profile of hemodynamic response to whisker stimulation under urethane was different from that at the awake state. [7] Some research studies provided an evaluation of baseline CBF. In the positron emission tomography (PET) study, an increased resting CBF due to the effect of ketamine anesthesia on occipital lobe of baboon was observed, but due to the limited spatial resolution no vascular morphological changes were explored. [8] The two-photon imaging quantified the vasodilatory effect of isoflurane anesthesia on basal cerebral microvasculature in marmosets and revealed a reduction in baseline blood flow speed. However, because of the limited field of view (FOV) the analysis was performed at the individual- vessel level only. [10] The alternation of the baseline hemodynamics and oxygen metabolism under isoflurane was assessed with quantitative photoacoustic microscopy in mouse brain, yet the study was focused on big vessels and excluded the capillary response assessment. [11] Lack of quantitative information on basal hemodynamic parameters according to experimental regime and technical limitations of the current imaging modalities invoke more detailed analysis of anesthesia effect at both macro- and microvascular levels.
Here we report the effect of anesthetics on baseline vessel dimensions and CBF parameters measured with optical coherence tomography (OCT). In contrast to other non-invasive in vivo imaging modalities, OCT provides fast three-dimensional (3D) visualization of biological tissues up to 2 mm in depth with micron-level resolution. [12] Optical microangiography (OMAG) is an OCT advanced extension which allows for acquiring blood vessel and flow information down to capillary level. [13] Using the key advantage of OMAG to extract blood flow signal making it almost free of background tissue noises, another OCT angiography method named Doppler OMAG (DOMAG) has been developed. [14] DOMAG detects a wide range of axial blood flow velocity in penetrating vessels, which reveals detailed information on cerebral blood flow. [15] The combination of OMAG and DOMAG techniques has been extensively used in various cerebrovascular studies including studies on stroke [16,17], arteriogenesis [18], and aging [19]. The main objective of this study is to demonstrate that OCT dual image processing approach enables complementary and more comprehensive analysis of anesthesia effect on cerebral vascular morphology and blood flow.
Here, we applied the combination of OMAG and DOMAG methods to compare the vessel and flow parameters in mouse cortex between anesthetized and awake regimes. The effects of isoflurane and ketamine-xylazine on cerebral vascular tone and flow dynamics were systematically evaluated by measuring the changes in artery and vein diameter, capillary area density, capillary flux, axial flow velocity and total blood flow.
2. Materials and methods
2.1. Animal preparation
Animal surgeries and experimental procedures in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Washington and conduced in accordance with University of Washington guidelines and ARRIVE guidelines. Six C57BL/6 mice (Charles River Laboratories, 3- month-old, 23–25 g) were used in this study. One day prior the surgery, each mouse was weighed and housed in a separate cage. Next, each animal underwent a surgical procedure to implant a cranial window for optical access [20] followed by a 7-day recovery. To acclimate mice to experimental environment and imaging setup, they were trained for at least 4 days in the laboratory.
2.2. Awake imaging
For experiments in awake mice, we placed the animal in a Mobile HomeCage (Neurotar Oy Ltd) which included a head-restraining apparatus and an air-floating cage. Although the mouse was head-restrained, it was able to freely walk/run or remain stationary during imaging. The detailed description of awake imaging protocol can be found in [21]. Each imaging session lasted for about 20 minutes.
2.3. Anesthetized imaging
Immediately following the awake imaging, the mouse was removed from the head-restraining device and anesthetized with isoflurane (5%) in oxygen-enriched gas in an induction chamber. After confirming an adequate anesthesia level by toe-pinching test, the animal was transferred to the heated stereotaxic frame with a nose cone through which isoflurane was supplied and maintained at 1.5–2% throughout imaging. After the imaging session (~20min), the animal was recovered from isoflurane anesthesia for 24 hours. On the next day (24 hours later), the mouse was anesthetized again by intraperitoneal injection of ketamine/xylazine cocktail (0.1 ml/25 g mouse) and imaged (~20min). After this imaging session, while still being anesthetized, the animal was euthanized. Physiological parameters such as adequate anesthesia depth (no hindpaw reflexes), heart rate and body temperature (36.8±0.2 °C) were monitored throughout all anesthetized imaging procedures.
2.4. OCT system configuration
In this study, we used an in-house built spectral domain OCT (SD-OCT) system to image the mouse cerebral cortex in-vivo (Figure 1). The system was equipped with a superluminescent diode (SLD) (LS2000B, Thorlabs Inc.) with a center wavelength of 1310 nm and a spectral bandwidth of 110 nm which provided ~7.5-μm axial resolution in air (~5.1 μm in mouse brain tissue). In the sample arm, divided by a 2 × 2 optical coupler, one part of SLD light passed through the optical system composed of a collimator, X-Y galvanometer and an 10X objective lens with 10-μm lateral resolution. The rest of the light was directed into the reference arm through a collimator and lens. The light reflected from the sample interfered with the reference light. Next, to detect the interference signal, the output light was transmitted to a spectrometer with a spectral resolution of 0.141 nm, which provided 2.22 mm imaging depth into the sample. The spectrometer was equipped with an InGaAs line scan camera (SUI, Goodrich Corp) capable of 92 kHz A- line rate to capture the interference signal.
Figure 1.
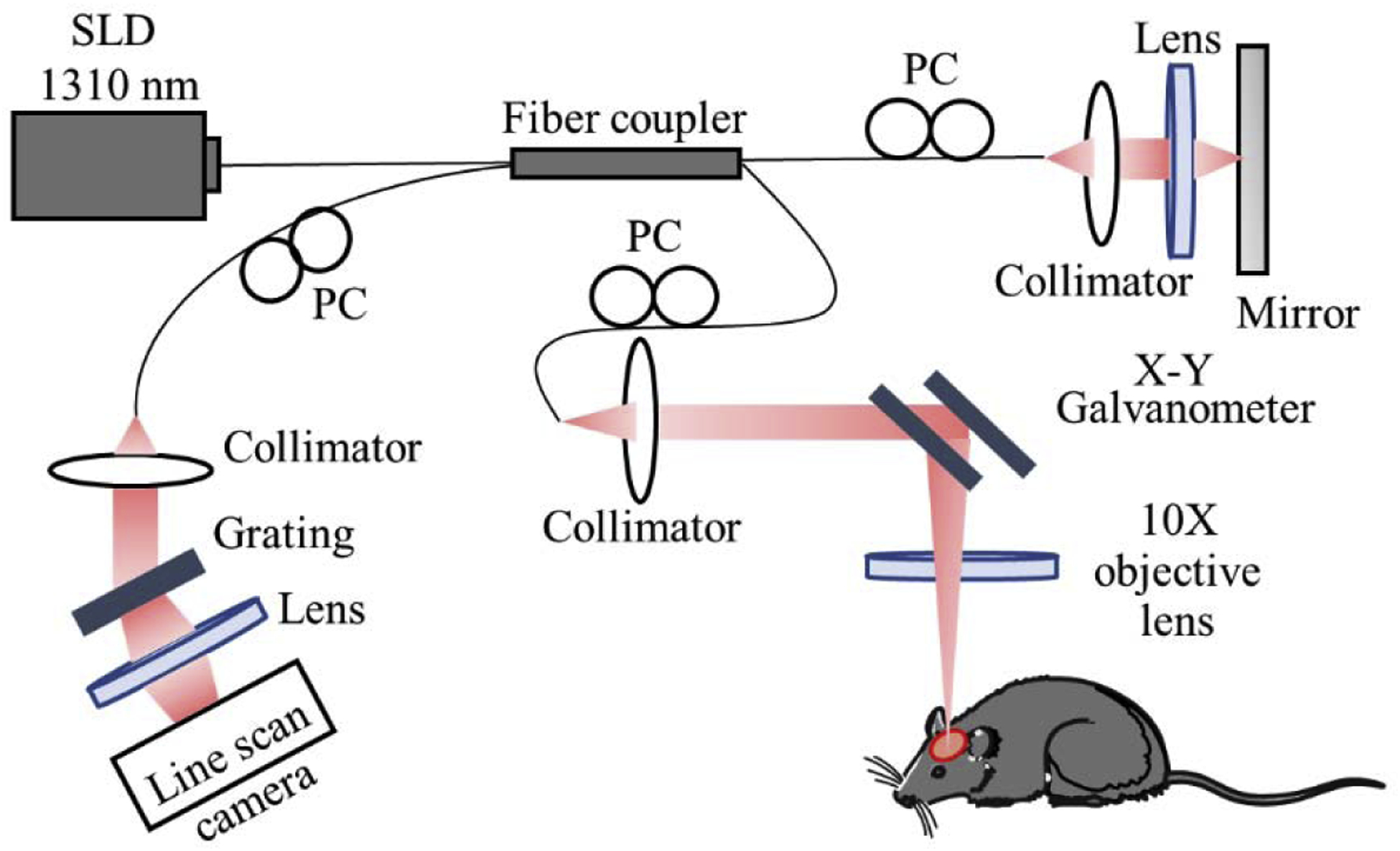
Schematic diagram of OCT system. SLD: superluminescent diode, PC: polarization controller.
2.5. OCTA scanning protocols
To obtain information about vascular morphology and flow in mouse cerebral cortex, we used two scanning protocols per single imaging session as described below.
2.5.1. Optical microangiography
To obtain 3D OMAG images, a clustered B-scan protocol [22,23] was applied to image the volumetric vasculature. In this protocol, every B-frame was formed by 400 A-lines (z axis) at A-scan rate of 92 kHz. B-frame was repeated 8 times at each transverse location (x axis), and a total of 400 locations was recorded in the C-scan direction (y axis) with a scan rate of 180 frames/sec (fps). Thus, the final 3D volumetric data set consisted of 3200 B-frames taking ~15 seconds to acquire and forming a cube of approximately 2.5 mm (x axis) × 2.5 mm (y axis) × 2.22mm (z axis). Four quadrants were acquired, and the final image (4mm × 4 mm) was automatically stitched from 4 angiograms with ~1-mm overlap, using a proprietary software package developed in house (originally developed for retinal imaging).
2.5.2. Doppler microangiography
To obtain 3D DOMAG images, the repeated A-scan protocol [15] was used. A-line was repeated 25 times at each depth location to form one M-scan (z axis) at A-scan rate of 45 kHz for axial velocity calculation based on Doppler principle. Each B-frame consisted of 300 M-scans, and a total of 300 B-frames (x axis) was acquired in the C-scan direction (y axis) with a scan rate of 6 fps. Thus, the final 3D volumetric data set took ~50 seconds to acquire and formed a cube of approximately 2 mm (x axis) × 2 mm (y axis) × 2.22mm (z axis).
2.6. Data analysis
To analyze multiple morphological and flow parameters in cerebral vessel networks, the methods described below were used. All the data processing was performed in customized MATLAB (MathWorks) programs and was visualized in the 3D software AMIRA (Visual Imaging).
2.6.1. Vein and artery diameter measurement
The vessel diameter was measured from OMAG angiograms obtained from enface maximum intensity projection (MIP) of 3D OMAG data within ~50 μm below the cortical surface. The detailed process description of producing OMAG angiograms can be found in [23]. In brief, the backscattered OCT signal was presented as a superposition of three independent components: clutter (static and slowly moving tissue structures), blood flow signal (moving RBCs), and noise (system and shot noise). After applying eigendecomposition method to signal correlation matrix, blood flow signal was distinguished from tissue bulk motion and noise to generate OMAG enface images. The angiograms were converted into black-and-white binary masks to accurately detect the vessel edges. To keep the vessel segment selection consistent for comparing among different experimental regimes, vein and artery branches were classified according to branching order, in which segment 1 (Seg 1) was the most proximal and segment 3 (Seg 3) was the most distal (Fig. 2A). The diameters were calculated using MATLAB interactive distance tool that displayed the distance between line endpoints and compared among awake, ketamine-xylazine and isoflurane states. Finally, the diameter change index, ΔD, defined as
where Danest is the vessel diameter value at anesthetized state (isoflurane or ketamine-xylazine) and Daw is the vessel diameter value at awake state, was compared for every vein and artery segment among three different regimes.
Figure 2.
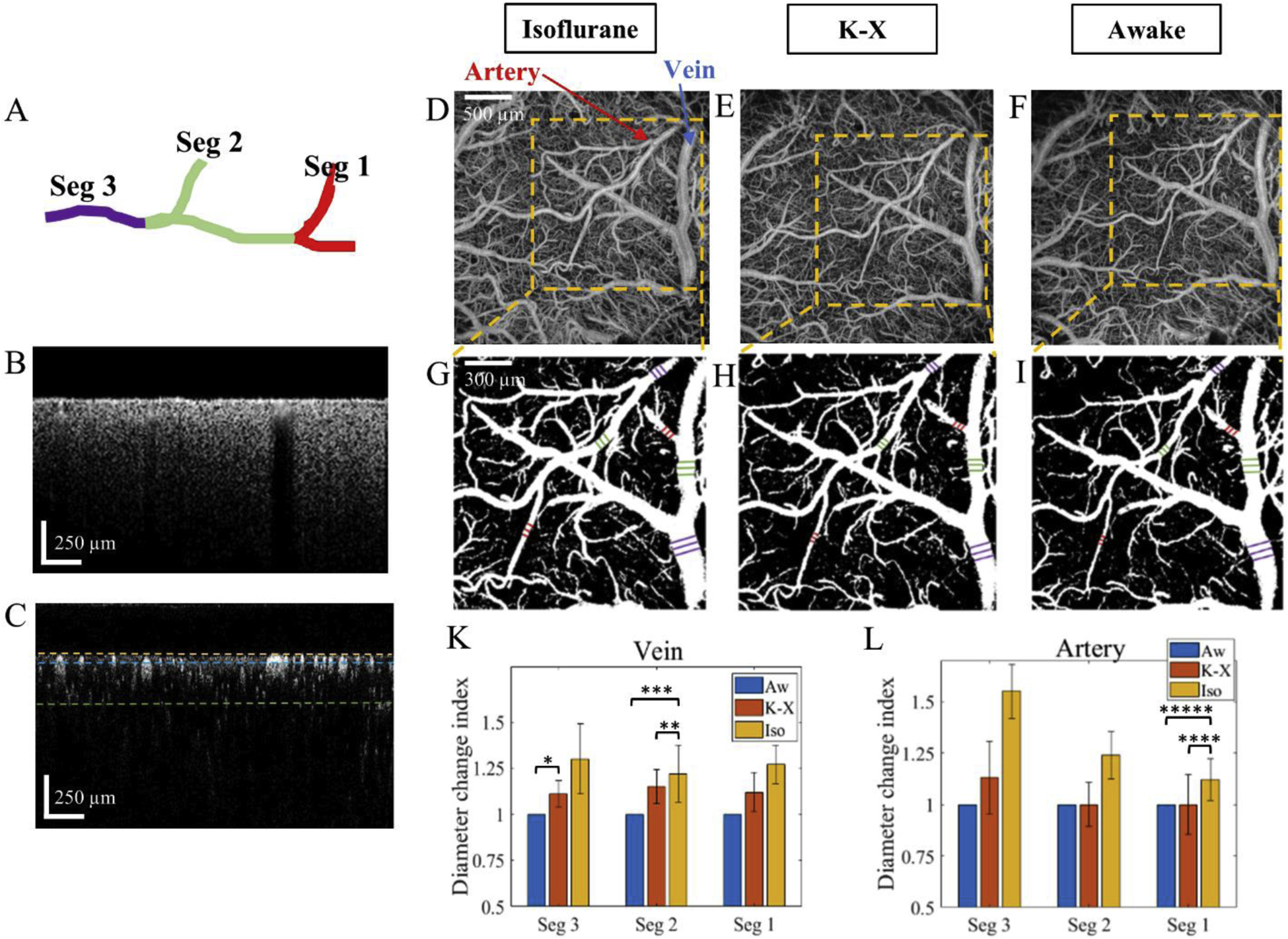
Comparison of artery and vein segment diameter. A. Classification of vessel branches with the most proximal branch assigned as segment 1 (Seg 1) and the most distal one as segment 3 (Seg 3). B. B-scan structural cross-section OCT image. C. B-scan flow cross-section OCT image. The yellow line indicates the cortical surface, while the blue and green lines are located at the depths of 50 and 300 μm from the cortical surface, respectively. D-F. OMAG angiograms corresponding to isoflurane, ketamine-xylazine, and awake states, respectively, obtained from enface MIP of 3-D OMAG datasets within a slab between yellow and blue dotted lines shown in C. Artery and vein are indicated in D with red and blue arrows, respectively. G-I. Vessel masks corresponding to D-F angiograms, respectively, with diameter length indicated in violet (Seg 3), green (Seg 2), and red (Seg 1). K-L. Comparison of diameter change index of vein and artery, respectively, for Seg 3, Seg 2 and Seg 1 among three different states. Aw – awake, K-X – ketamine/xylazine, Iso – isoflurane. *p-value=0.0498, **p-value=0.0430, ***p-value=0.0198, ****p-value=0. 0352, *****p-value=0.0104. The values are mean ± std. (standard deviation of group mean, N=6 animals per group).
2.6.2. Vessel area density and flux in capillaries
To quantify vessel area density (VAD) for capillaries, we used the method previously validated in OCT retina imaging [24]. First, to exclude the meningeal layer and isolate the cortical layers of interest, MIP images were reconstructed from 3D OMAG data set between 50–300 μm thick slab below the cortical surface. Second, large vein and arterial vessels were excluded, so that only capillary network remained for the analysis. Next, with the application of global thresholding, Hessian filter and adaptive thresholding, the images were then converted into binary vessel area maps. The capillaries with higher flux which survived the image binarization were indicated by a white pixel (value=1), while the capillaries with lower flux which disappeared after binarization were presented as a black pixel (value=0). The VAD maps were generated by using a Kernel moving across the entire binary image showing the variation of local vessel pixel ratios. Mean capillary VAD was calculated as a ratio of the region occupied by vessels (white pixels) to a given image region (2.5 mm×2.5 mm).
Capillary flux was calculated based on flow signal strength varying in the full dynamic range of 0–255. The signal was divided by 255, thus normalized to the range from 0 to 1, and multiplied by binary matrix. Mean capillary flux index (CFI) was obtained by averaging the normalized blood flow signal over the given area (2.5 mm×2.5 mm). [25, 25] Both mean capillary VAD and mean CFI were compared among isoflurane, ketamine-xylazine and awake conditions.
2.6.3. CBF analysis
To assess CBF in penetrating vessels, we used the acquired DOMAG data. First, the axial RBC velocity, Vaxial, was calculated using a phase-resolved technique described in [15]:
where λ is the light source center wavelength, φ is the phase difference, n is the tissue refractive index, and T is the time interval between adjacent A-lines. An axial velocity range of −6mm/s to 6 mm/s was achieved by increasing T to a 3 A-line interval. To extract Doppler flow signal, a phase variance mask was used to eliminate the noisy background. Bidirectional axial velocity maps were constructed based on this signal. Next, an x-y orthogonal slice (2 mm×2 mm) ~50 μm below the cortical surface was selected from the 3D DOMAG data set and divided into 4 regions to quantify flow lateral cross-sections at each region. Finally, the regional CBF was calculated by multiplying velocity and flow area measured within each region. [27] The values were than normalized to a unit region (1 mm2) and compared between awake and isoflurane groups in one animal.
2.6.4. Statistical analysis
The differences between awake, ketamine-xylazine and isoflurane conditions were tested with One-way ANOVA followed by Tukey’s honestly significant difference test for multiple comparisons. p<0.05 was considered as statistically significant.
3. Results
3.1. Effect of anesthesia on vasodilation
Vessel dilations induced by isoflurane and ketamine-xylazine were revealed by OMAG (Fig. 2). To make the comparison more accurate, the vein and artery branches were categorized according to branching order: segment 1 (Seg 1) as the most proximal, segment 2 (Seg 2) in the middle, and segment 3 (Seg 3) as the most distal (Fig. 2A). Cross-sectional images of cortex structure and blood flow obtained with OCT are shown in Fig.2B and Fig.2C, respectively. OMAG angiograms were obtained from ~50 μm-thick slab indicated in Fig. 2C between yellow and blue lines. Angiograms in Fig. 2D–F correspond to isoflurane, ketamine-xylazine and awake states, accordingly. The vein and artery diameter values for Seg 1 (red), 2 (green) and 3 (purple) were measured from binary masks in Fig. 2G–I. ΔD was compared for every vein and artery segment among three different regimes (Fig.2K–L). Overall, there was an increasing trend from awake to isoflurane states. ΔD for vein Seg 3 at ketamine-xylazine was higher by 11% (p-value=0.0498) than at awake regime. ΔD for vein Seg 2 at isoflurane was higher by 6% (p-value=0.0430) than at ketamine- xylazine, and 22% higher (p-value=0.0198) than at awake state. ΔD for artery Seg 1 at isoflurane was higher by 12% (p-value=0.0352) than at ketamine-xylazine, and 29% higher (p-value=0.0104) than at awake state. These results showed that the vein and artery diameter increased at anesthetized states comparing to awake states, meaning that isoflurane and ketamine-xylazine caused significant vessel dilation.
3.2. Vessel area density and flux in capillaries
To compare capillary VAD and flux among three different states, OMAG angiograms (Fig. 3A–C) were processed to exclude big arteries and veins (Fig. 3D–F). The remaining capillary vessels generated capillary VAD maps (Fig. 3G–I) and flux maps (Fig. 3K–M) at isoflurane, ketamine-xylazine and awake conditions. Fig. 3N demonstrates the flux index distributions at three different states fitted to Kernel distribution, where it is observed that flux index distribution at isoflurane had the largest population of flux index over the mean, while awake state had the smallest one. The comparisons of mean VAD and mean CFI over the given region among three conditions are presented in Fig. 3O–P, respectively. The results showed that mean VAD at awake state was lower by 23% than at ketamine-xylazine (p-value=0.0368) and by 25% than at isoflurane (p-value=0.0065). Mean CFI at awake state was lower by 5% than at ketamine-xylazine (p-value=0.0369) and by 15% than at isoflurane (p-value=0.0131). Overall, both capillary VAD and mean CFI increased from awake to anesthetized conditions.
Figure 3.
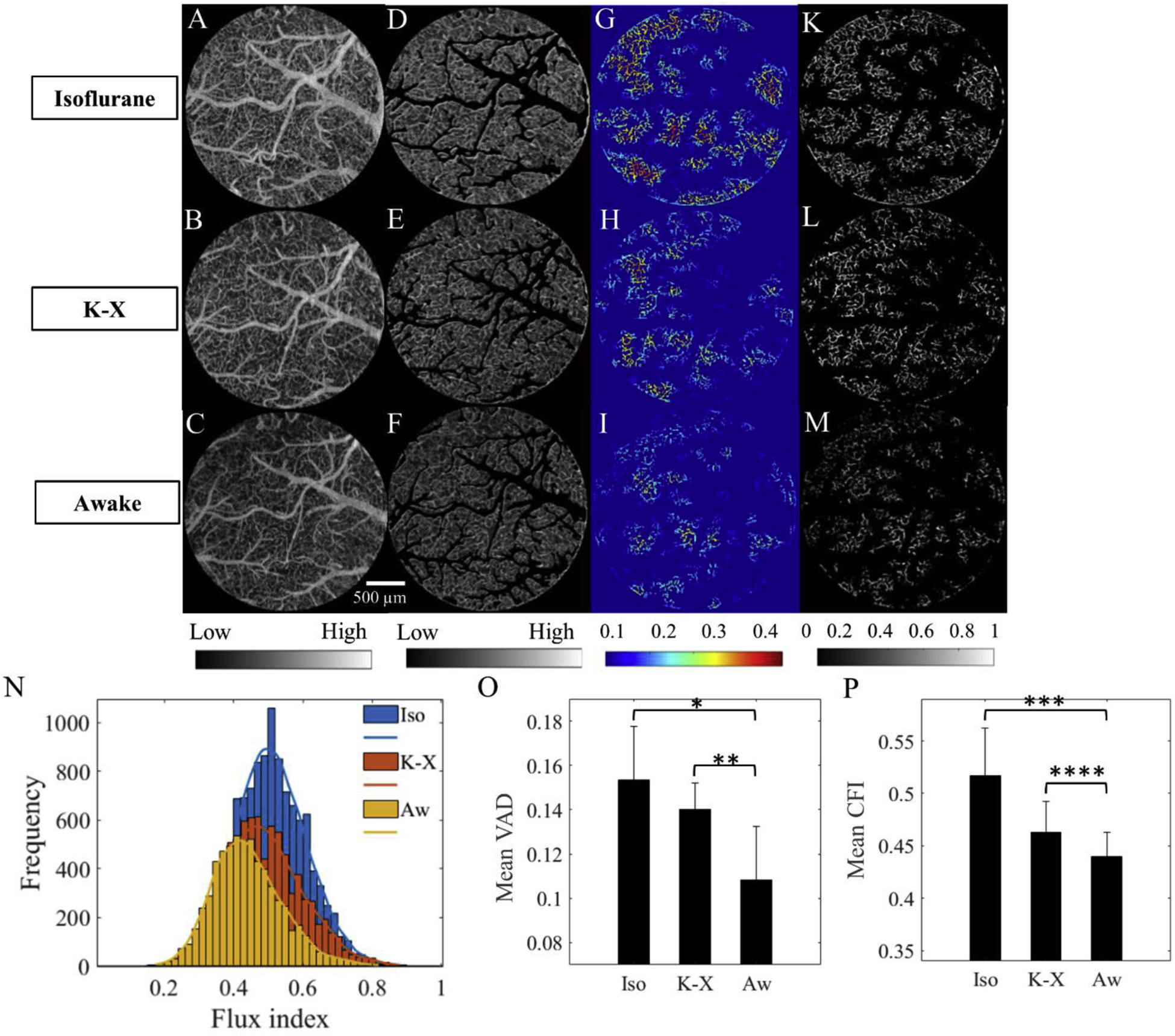
Comparison of VAD and flux in capillaries. A-C. OMAG angiograms corresponding to isoflurane, ketamine- xylazine, and awake states, respectively, obtained from enface MIP of 3-D OMAG datasets within a 50–300 μm slab. Color bar represents low and high intensity signal. D-F. OMAG angiograms with arteries and veins excluded. G-I. Capillary VAD maps at three states. Color bar represents local white pixels ratio where blue is smaller, and red is larger. K-M. CFI maps at three states. Color bar represents the intensity signal in the range from 0 to 1. N. Capillary flux histogram distributions at three states fitted to Kernel distribution. O. Comparison of mean VAD among three states. *pvalue=0.0065, **p-value=0.0368. P. Comparison of mean CFI among three states. ***p-value=0.0131, ****p-value=0.0369. The values are mean ± std. (standard deviation of group mean, N=6 animals per group).
3.3. CBF measurements from penetrating vessels
To assess the CBF difference between awake and anesthetized regimes, we quantified the axial velocity of RBCs, flow cross-section area, and total blood flow from DOMAG velocity maps (Fig. 4) in one animal. Figures 4A–B reveal the bidirectional enface DOMAG axial velocity maps at isoflurane and awake regimes. Diving arterioles and rising venules are displayed in green and red colors, respectively, and the axial flow velocity information is coded with a color bar in a range of ±6.0 mm/s. Orthogonal slices extracted from 3D slab in Fig. 4C were produced (Fig.4D–E) to compare the mean axial velocity (Fig. 4F), flow cross- section area (Fig. 4G) and total blood flow (Fig. 4H) over four regions between awake and isoflurane regimes. While mean velocity value increased only by 3% from awake to isoflurane state, the flow cross- section area value increased by 57% (p<0.04) and total blood flow increased by 55% (p<0.04). In addition, from Fig. 4A–B and Fig. 4D–E, the penetration vessel density increased from awake to isoflurane state, which supports the results related to capillary VAD in Fig. 3G–I.
Figure 4.

Comparison of CBF parameters in one animal. A-B. Bidirectional axial CBF velocity maps of mouse cortex at isoflurane and awake regimes, correspondingly, generated by enface maximum intensity projection of 3D DOMAG datasets within a slab demonstrated in C. Color bar represents the axial velocity of RBCs moving down (negative, green) and up (positive, red) in a range of ±6.0 mm/s. The yellow color is a mix of green and red signals from the projection effect. C. 3D visualization of 300-μm thick slab generated from 3D DOMAG dataset with descending and ascending vessels. D-E. Orthogonal slices from C at an x-y plane ~50 mm below the cortical surface at isoflurane and awake regimes, respectively. Green and red dots represent the cross sections of vessel flows. The slices are divided into four equal regions, F-H. Comparison of mean axial velocity, flow cross-section area, and total blood flow, accordingly, between awake and isoflurane regimes. The values are mean ± std. (standard deviation of group mean, N=4 regions per group).
4. Discussion
Imaging CBF in in vivo animal models plays a key role in neurovascular studies. Although the use of anesthetized animals is a simple and convenient approach to suppress the motion artifact during imaging, there is evidence that anesthetic agents have a profound effect on the animal physiology.[1] As a result, the CBF regulation is considerably influenced by anesthesia which might affect the experimental outcome. [2] Previous imaging studies demonstrated the effect of anesthesia on vessel dimension and CBF. [5–11] However, due to their technical limitations, there has been little quantitative analysis at the microcirculatory level. In this study, we used the advanced OMAG and DOMAG techniques to quantitatively compare the cerebral vasodilation and blood flow at both large and capillary vessels between the awake and the regimes of the most used anesthetics in biomedical research - ketamine-xylazine and isoflurane anesthesia.
To assess the effect on anesthetics on vascular tone, we measured the diameters of vein and artery segments under ketamine-xylazine and isoflurane anesthesia and compared them to that at the awake state. Our results (Fig.2K–L) showed an increase of vessel diameter under anesthetics with the largest increase under isoflurane. Ketamine-xylazine cocktail also induced the increase of vessel dimensions, yet to a lesser degree. The observed diameter increase supports the evidence of anesthesia-caused dilation of large vessels. A possible explanation for this might be that both isoflurane [28,29] and ketamine [30,31] induce the relaxation of cerebral blood vessels via their actions on the intracellular calcium dynamics of smooth muscle cells. [2]
OMAG technique was also shown feasible to reveal the increase of vessel perfusion and dilation at the capillary level. OMAG vessel images are based on blood flow signal strength which is proportional to the number of RBCs passing through the vessels per unit time, i.e. flux. [25, 25]. “New” capillary segments appeared at anesthetized states are not exactly new, but the capillaries with an increased flux (or flow) which are not visible at awake state because of the lower flux. Thus, VAD describes the overall capillary perfusion and also reflects the change of “existing” vessel size giving information about capillary dilation. We found that mean VAD increased from awake to anesthetized conditions (Fig. 3O) indicating increased capillary perfusion and capillary dilation. In addition, mean CFI increased from awake to anesthetized conditions (Fig. 3P) meaning that anesthesia also elevated the capillary flux.
Cerebral vasodilation results in decreased arterial pressure, which subsequently elevates CBF and peripheral blood perfusion. [32,33] We performed a preliminary experiment in one animal to check if the flow parameters vary between awake and isoflurane conditions, which had the most pronounced vascular differences. While mean axial velocity of RBCs did not change significantly from awake to isoflurane state (Fig.4F), the flow cross-section area increased by 57% (Fig.4G) presumably caused by vessel dilation observed in Fig.2. Consequently, total blood flow also escalated by 55% (Fig.4H). Number of studies on anesthesia effect support the given result reporting the increase of baseline CBF due to isoflurane [34,35] and ketamine [36]. Although the given study demonstrated that isoflurane could elevate CBF, the experiment was conducted with one sample only. To validate our result, the observation must be tested on repeatability with a valid sample size which could be a subject for future research. In addition, monitoring physiological parameters such as breathe and pulse rate and comparing them across the regimes could provide additional information.
Although in contrast to other imaging methods, OCT allows measuring relatively large FOV down to capillary level without a need for contrast agents, there are still some limitations. First, our system’s lateral resolution was ~10μm, which might be larger than the size of some individual capillaries. However, we would argue that OMAG was still able to detect the changes in capillary network because our measurements were an average over more than hundreds of capillary vessels within 2.5mm × 2.5mm FOV. In addition, we assume that there is an interstitial tissue space between capillary vessels. Second, despite the system’s detectable depth range of ~3mm, the system’s focus depth (DOF) was limited to ~200 μm since we used a high numerical aperture (NA) objective lens to provide high lateral resolution (~10μm). The DOF can be increased using a lower NA lens, but lateral resolution would be then sacrificed. Lastly, DOMAG technique is mainly sensitive to the axial velocity of RBCs, whereas the velocity and flow in the vessels perpendicular to the optical axis are excluded due to their small axial velocities.
Our study showed that the use of anesthetics such as isoflurane and ketamine-xylazine can cause abnormal cerebrovascular response and alter the baseline CBF. However, despite the rapid development of head-restraining techniques, the transition to fully awake imaging can still be challenging. Even though the animal was given a 7-day training before the imaging started, awake imaging still produced significant restraint-related stress and discomfort to animals, which can contaminate the obtained signals. [37] Anesthesia-effect studies report that the contribution of anesthetic agents is dose dependent. For example, low isoflurane concentration (1.3%−1.5%) maintains CBF values close to those of the awake condition. [2] Hence, the decision on an experimental regime should arise from experiment type and the purpose of study. In addition, it is suggested to perform experiments under different experimental conditions and compare the obtained results to produce reliable results. [33]
5. Conclusion
In this study, we used OMAG and DOMAG techniques to measure and compare the baseline morphological and flow parameters of both large and capillary cerebral vascular networks between awake and anesthetized mice. Our results showed that isoflurane and ketamine-xylazine caused vasodilation in both large and capillary vessels. The increase of vessel dimensions resulted in increased CBF. Overall, we demonstrated that our OCT dual image processing approach allows for complementary and more comprehensive analysis of anesthesia effect on cerebral vasculature and blood flow. We believe that our quantitative comparison study will help researchers to choose an appropriate experimental protocol for neurovascular studies with optical imaging.
Highlights.
OCT/OMAG reveals vascular changes in mouse cortex between anesthetized and awake regimes
Isoflurane and ketamine-xylazine induce the vasodilation in large and capillary vessels
OCT/DOMAG demonstrates the increase of cerebral blood flow due to isoflurane effect
Anesthetics cause abnormal cerebrovascular response and alter the baseline blood flow
Acknowledgements
This study is supported in part by National Heart, Lung, and Blood Institute (R01HL093140), Washington Research Foundation, and Research to Prevent Blindness. The funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of conflict of interest
None.
References
- 1.Slupe AM, Kirsch JR, 2018. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J. Cereb. Blood Flow Metab 38 (12), 2192–2208. doi: 10.1177/0271678x18789273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masamoto K, Kanno I, 2012. Anesthesia and the Quantitative Evaluation of Neurovascular Coupling. J. Cereb. Blood Flow Metab 32 (7), 1233–1247. doi: 10.1038/jcbfm.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon EL, Meno JR, Ngai AC, Lam AM, Winn HR, 1995. Anesthetic-dependent pial arteriolar response to ethanol. J. Neurosurgery 83 (5), 875–877. doi: 10.3171/jns.1995.83.5.0875. [DOI] [PubMed] [Google Scholar]
- 4.Masamoto K, Obata T, Kanno I, 2010. Intracortical microcirculatory change induced by anesthesia in rat somatosensory cortex. Oxyg. Transp. to Tissue XXXI. Advances in Experimental Medicine and Biology 662, 57–61. doi: 10.1007/978-1-4419-1241-1_7. [DOI] [PubMed] [Google Scholar]
- 5.Pisauro MA, Dhruv NT, Carandini M and Benucci A, 2013. Fast hemodynamic responses in the visual cortex of the awake mouse. J. of Neuroscience 33(46), 18343–18351. doi: 10.1523/JNEUROSCI.2130-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharp PS, Shaw K, Boorman L, Harris S, Kennerley AJ, Azzouz M and Berwick J, 2015. Comparison of stimulus-evoked cerebral hemodynamics in the awake mouse and under a novel anesthetic regime. Sci. Rep 5, 12621. doi: 10.1038/srep12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao YR, Ma Y, Zhang Q, Winder AT, Liang Z, Antinori L, Drew PJ and Zhang N, 2017. Time to wake up: Studying neurovascular coupling and brain-wide circuit function in the un-anesthetized animal. Neuroimage 153, 382–398. doi: 10.1016/j.neuroimage.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin C, Martindale J, Berwick J and Mayhew J, 2006. Investigating neural–hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 32(1), 33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Szabó CÁ, Narayana S, Franklin C, Knape KD, Davis MD, Fox PT, Leland MM and Williams JT, 2008. “Resting” CBF in the epileptic baboon: correlation with ketamine dose and interictal epileptic discharges. Epilepsy Res. 82(1), 57–63. doi: 10.1016/j.eplepsyres.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santisakultarm TP, Kersbergen CJ, Bandy DK, Ide DC, Choi SH and Silva AC, 2016. Two-photon imaging of cerebral hemodynamics and neural activity in awake and anesthetized marmosets. J. of Neuroscience Methods 271, 55–64. doi: 10.1016/j.jneumeth.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao R, Li J, Ning B, Sun N, Wang T, Zuo Z and Hu S, 2017. Functional and oxygen-metabolic photoacoustic microscopy of the awake mouse brain. Neuroimage 150, 77–87.doi: 10.1016/j.neuroimage.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR and Gruber A, 2007. Three dimensional optical angiography. Optics Express 15(7), 4083–4097. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
- 13.Wang RK, 2009. Optical microangiography: a label-free 3-D imaging technology to visualize and quantify blood circulations within tissue beds in vivo. IEEE J. of Sel. Top. in Quantum Electronics 16(3), 545–554. doi: 10.1109/jstqe.2009.2033609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang RK and An L, 2009. Doppler optical micro-angiography for volumetric imaging of vascular perfusion in vivo. Optics Express 17(11), 8926–8940. doi: 10.1364/oe.17.008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L, Qin J, Reif R and Wang RK, 2013. Wide velocity range Doppler optical microangiography using optimized step-scanning protocol with phase variance mask. J. of Biomedical Optics 18(10), 106015. doi: 10.1117/1.jbo.18.10.106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baran U, Li Y and Wang RK, 2015. Vasodynamics of pial and penetrating arterioles in relation to arteriolo-arteriolar anastomosis after focal stroke. Neurophotonics 2(2), 025006. doi: 10.1117/1.nph.2.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi WJ, Li Y and Wang RK, 2019. Monitoring acute stroke progression: multi-parametric OCT imaging of cortical perfusion, flow, and tissue scattering in a mouse model of permanent focal ischemia. IEEE Transactions on Med. Imaging 38(6), 1427–1437. doi: 10.1109/tmi.2019.2895779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Choi WJ, Qin W, Baran U, Habenicht LM and Wang RK, 2016. Optical coherence tomography based microangiography provides an ability to longitudinally image arteriogenesis in vivo. J. of Neuroscience Methods 274, 164–171. doi: 10.1016/j.jneumeth.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Choi WJ, Wei W, Song S, Zhang Q, Liu J and Wang RK, 2018. Aging-associated changes in cerebral vasculature and blood flow as determined by quantitative optical coherence tomography angiography. Neurobiology of Aging 70, 148–159. doi: 10.1016/j.neurobiolaging.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Baran U and Wang RK, 2014. Application of thinned-skull cranial window to mouse cerebral blood flow imaging using optical microangiography. PLoS One 9(11), e113658. doi: 10.1371/journal.pone.0113658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Rakymzhan A, Tang P and Wang RK, 2020. Procedure and protocols for optical imaging of cerebral blood flow and hemodynamics in awake mice. Biomedical Optics Express 11(6), 3288–3300. doi: 10.1364/boe.394649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang RK, An L, Francis P and Wilson DJ, 2010. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Optics Lett. 35(9), 1467–1469. doi: 10.1364/ol.35.001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousefi S, Zhi Z and Wang RK, 2011. Eigendecomposition-based clutter filtering technique for optical microangiography. IEEE Transactions on Biomedical Eng. 58(8), 2316–2323. doi: 10.1109/tbme.2011.2152839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Z, Lin J, Gao C, Xin C, Zhang Q, Chen CL, Roisman L, Gregori G, Rosenfeld PJ and Wang RK, 2016. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. of Biomedical Optics 21(6), 066008. doi: 10.1117/1.jbo.21.6.066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CL, Zhang A, Bojikian KD, Wen JC, Zhang Q, Xin C, Mudumbai RC, Johnstone MA, Chen PP and Wang RK, 2016. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography–based microangiography. Investigative Ophthalmology & Vis. Sci 57(9), OCT475–OCT485. doi: 10.1167/iovs.15-18909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CL, Bojikian KD, Gupta D, Wen JC, Zhang Q, Xin C, Kono R, Mudumbai RC, Johnstone MA, Chen PP and Wang RK, 2016. Optic nerve head perfusion in normal eyes and eyes with glaucoma using optical coherence tomography-based microangiography. Quantitative Imaging in Med. and Surg 6(2), 125. doi: 10.21037/qims.2016.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan VJ, Sakadžić S, Gorczynska I, Ruvinskaya S, Wu W, Fujimoto JG and Boas DA, 2010. Quantitative cerebral blood flow with optical coherence tomography. Optics Express 18(3), 2477–2494. doi: 10.1364/oe.18.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iida H, Ohata H, Iida M, Watanabe Y and Dohi S, 1998. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiol.: The J. of the Am. Society of Anesthesiol 89(4), 954–960. doi: 10.1097/00000542-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Flynn NM, Bulijubasic N, Bosnjak ZJ and Kampine JP, 1992. Isoflurane Produces Endothelium-independent Relaxation inCanine Middle Cerebral Arteries. Anesthesiol.: The J. of the Am. Society of Anesthesiol 76(3), 461–467. doi: 10.1097/00000542-199203000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Oren RE, Rasool NA and Rubinstein EH, 1987. Effect of ketamine on cerebral cortical blood flow and metabolism in rabbits. Stroke 18(2), 441–444. doi: 10.1161/01.str.18.2.441. [DOI] [PubMed] [Google Scholar]
- 31.Akata T, Izumi K and Nakashima M, 2001. Mechanisms of direct inhibitory action of ketamine on vascular smooth muscle in mesenteric resistance arteries. Anesthesiol.: The J. of the Am. Society of Anesthesiol 95(2), 452–462. doi: 10.1097/00000542-200108000-00030. [DOI] [PubMed] [Google Scholar]
- 32.Cudmore RH, Dougherty SE and Linden DJ, 2017. Cerebral vascular structure in the motor cortex of adult mice is stable and is not altered by voluntary exercise. J. Cereb. Blood Flow Metab 37(12), 3725–3743. doi: 10.1177/0271678x16682508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremoleda JL, Kerton A and Gsell W, 2012. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfare. EJNMMI research 2(1), 44. doi: 10.1186/2191-219x-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond JC, Todd MM, Scheller MS and Shapiro HM, 1986. A comparison of the direct cerebral vasodilating potencies of halothane and isoflurane in the New Zealand white rabbit. Anesthesiol.: The J. of the Am. Society of Anesthesiol 65(5), 462–467. doi: 10.1097/00000542-198611000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA and Duong TQ, 2003. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J. Cereb. Blood Flow Metab 23(4), 472–481. doi: 10.1097/00004647-200304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeiler FA, Sader N, Gillman LM, Teitelbaum J, West M and Kazina CJ, 2016. The cerebrovascular response to ketamine: a systematic review of the animal and human literature. J. of Neurosurg. Anesthesiol 28(2), 123–140. doi: 10.1097/ana.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 37.Lahti KM, Ferris CF, Li F, Sotak CH and King JA, 1999. Comparison of evoked cortical activity in conscious and propofol‐ anesthetized rats using functional MRI. Magn. Resonance in Med.: An Off. J. of the International Society for Mag. Resonance in Med. 41(2), 412–416. doi:. [DOI] [PubMed] [Google Scholar]


