Abstract
In the United States (US), rural areas have a higher burden of type 2 diabetes mellitus (T2DM) compared to urban areas. However, there is limited information on risk factors and interventions that improve the primary prevention and management of T2DM in rural areas. To synthesize current knowledge on T2DM in rural areas and to guide healthcare providers and policy makers, we reviewed five scientific databases and the gray literature over the last decade (2010 to 2020). We described classification systems for rurality and the T2DM burden based on rurality and region (West, South, Midwest, and Northeast). We highlighted risk factors for T2DM in rural compared to urban areas, and summarized interventions to screen and manage T2DM based on opportunistic screening, T2DM self-management, community-based initiatives, as well as interventions targeting comorbidities and T2DM. Several studies identified the co-existence of T2DM and depression/psychological symptoms, which could reduce adherence to non-pharmacologic and pharmacologic management of T2DM. We highlighted the role of technology in education and counseling of patients with geographic and financial barriers to accessing care, which is exacerbated by the SARS-CoV-2 coronavirus disease-19 (COVID-19) pandemic. We identified knowledge gaps and next steps in improving T2DM care in rural areas. There is an urgent need for interventions tailored to rural areas given that rural Americans currently experience a disproportionate burden of T2DM and are encumbered by its associated morbidity, mortality, and loss in economic productivity.
Keywords: type 2 diabetes mellitus, rural health, urban-rural disparities, primary prevention
INTRODUCTION
In the United States (US), approximately 34 million people have diabetes mellitus, with striking health disparities in rural, compared to urban, areas.1 Of these 34 million people, approximately 32 million have type 2 diabetes mellitus (T2DM). Compared to urban populations, rural populations have a 16% higher prevalence of T2DM, a 20% higher T2DM-related hospital mortality, and a smaller improvement in overall T2DM-related mortality rates from 1999 to 2016.1–5 Despite the many studies on T2DM, none of the published reports compared T2DM incidence in urban and rural areas. In 2017, the cost of managing diabetes mellitus (type 1 and type 2 diabetes, combined) was $327 billion, which was 26% higher than costs five years prior, and largely resulted from medical expenses and reduced productivity.6 Most of these costs was attributed to T2DM. The reasons for rural-urban inequalities for T2DM are largely unknown but represent a major public health problem for the 60 million Americans living in rural areas.
Over the last four decades, the US burden of diabetes mellitus has changed. Based on self-reported diabetes mellitus status (type 1 and type 2, combined) from the US National Health Interview Survey, the incidence of age-adjusted diagnosed diabetes mellitus plateaued from 1980 to1990, increased from 1990 to 2007, and decreased annually by 3.1% to reach an annual incidence rate of 6.0% in 2017. This decrease was driven by non-Hispanic whites.7 The national prevalence of diabetes mellitus (type 1 and type 2, combined) mirrored national trends in incident diabetes mellitus, except for the period from 2009 to 2017, during which, the prevalence plateaued rather than declined.7 Despite the somewhat reassuring plateau in temporal trends of the national prevalence and incidence of diabetes mellitus, there are notable temporal disparities when analyzed by subgroup (gender, race/ethnicity, and region).8,9 For example, men had a higher age-adjusted prevalence of diabetes mellitus compared to women, although the incidence rates were comparable. From 2007 to 2018, the age-adjusted prevalence of diabetes mellitus based on race/ethnicity ranged from 7.5% (non-Hispanic whites) to 14.7% (American Indians/Alaska Natives), whereas age-adjusted incidence ranged from 5.0% (non-Hispanic whites) to 9.7% (Hispanics).9 In the US, Hispanic populations face a disproportionate burden of T2DM attributed to genetic factors (e.g., higher susceptibility to obesity and insulin resistance), sociocultural factors (e.g., lower income) reduced access to healthcare, among other factors.10
In comparison to information on the national prevalence and incidence of diabetes mellitus (type 1 and type 2, combined) by gender and race/ethnicity, less information is available for the epidemiology of diabetes mellitus by region and rurality. In 2016, among adults 20 years or older, the prevalence of age-adjusted diabetes mellitus across US counties ranged from 1.5% to 33%, and annual age-adjusted incidence ranged from 1.2 to 46.2 per 1000 persons.9 Despite these data, to our knowledge, no studies have synthesized the multi-dimensional perspectives of rurality and T2DM. To address this knowledge gap, we conducted a literature review study11 to obtain a multi-dimensional perspective on rurality and T2DM including rural-urban classification systems, risk factors for T2DM, comorbidities associated with T2DM, interventions to reduce the rural burden of T2DM, and knowledge gaps to guide future research. This study highlights the effort required to fulfill the Health People 2020 goal of improving the “quality of life for all persons who have, or are at risk for” T2DM diabetes.12 We did not focus on type 1 diabetes mellitus because its epidemiology, genetics, and pathophysiology differ from T2DM and may be less amenable to primary prevention interventions compared to T2DM. However, the human and healthcare burden of type 1 diabetes mellitus is important and should be reviewed in a separate study.
MATERIALS and METHODS
This study was classified as a literature review, based on the proposed analytic framework.11 The search strategy was designed by the study investigators in consultation with an academic librarian at Mayo Clinic, Rochester, MN. We searched CINAHL, Embase, MEDLINE, Scopus, and Web of Science databases and the gray literature using a combination of database index terms and key words ‘diabetes mellitus’, ‘rural’, ‘nonmetropolitan’, ‘United States’, and their variants. We focused on articles from the last decade to emphasize the contemporary burden of risk factors for T2DM as well as interventions/strategies and, thereby, maximize relevance to healthcare providers. Thus, we limited our search to English language articles published from 2010 to June 5, 2020 (date of literature search). We included full-length original and review articles, and excluded other article types (conference abstracts, editorials, brief reports, letters to the editor, and theses). Our search generated 1016 articles; duplicate articles were excluded and 431 unique articles were exported to EndNote®, a reference management software package.13 To identify articles in the gray literature, we used Google® to search government resources (site: .gov) as well as broader organizational and educational websites for T2DM in rural populations. The search string ‘rural AND type 2 diabetes’ was used and weblinks on the first five pages of results were reviewed.
One investigator (SBD) screened the titles and/or abstracts of the unique articles (n=431) and identified 122 articles for full-text review. We included articles on the primary prevention and management of T2DM in US rural populations. From these articles, we summarized definitions of rurality, described the burden of T2DM in rural areas, compared risk factors for T2DM in rural and urban areas, and discussed interventions to reduce the burden of T2DM in rural areas. We also identified knowledge gaps to reduce urban-rural disparities for the primary prevention of T2DM. This work is relevant to healthcare providers and policy makers who deliver or design care for rural populations.
This article summarizes published studies and did not require Institution Ethics approval.
RESULTS
Major Classification Systems for Rurality
Defining rurality is nuanced, and depends on the context, as briefly summarized below and in Table 1.
Table 1.
US† rural-urban classification systems
| Source | Description of rural and urban areas |
|---|---|
| 2010 US Census Bureau14,84 |
Urban: territory, population, and housing units located within UAs‡ (population ≥50,000) and UCs§ (2,500≤ population <50,000) Rural: all non-urban areas |
| USDA Economic Research Service15 | Frontier and Remote Area (FAR) codes ‘Frontier and remote’ describes territory characterized by combination of low population size and high geographic remoteness. Based on 2010 US census, four codes are defined in relation to the time it takes to travel by car to the edges of nearby UAs. i. Level one FAR approximates remoteness from UAs of ≥50,000 people ii. Level two FAR approximates remoteness from UAs of ≥25,000 people iii. Level three FAR approximates remoteness from UAs of ≥10,000 people iv. Level four FAR shows remoteness from UA of ≥2,500 people |
| Office of Management and Budget (OMB)16 | County-level designation based on population density and commuting: Metropolitan statistical areas: i. contain a core urban area with one or more UAs (population ≥50,000), and ii. adjacent counties with high degree of social and economic integration to the core UAs, measured by labor-force commuting Nonmetropolitan areas outside of the boundaries of metropolitan areas and subdivided in to: i. micropolitan statistical areas centered on UCs (10,000≤ population <50,000) ii. noncore areas not included in metropolitan and micropolitan areas Metropolitan areas are generally considered urban, and nonmetropolitan areas are generally considered rural. |
| National Center for Health Statistics (Centers for Disease Control and Prevention)17 | County-level designation Metropolitan counties i. Large central metropolitan: counties in metropolitan statistical areas (MSA) of ≥1 million population that: • contain the entire population of the largest principal city of the MSA, or • are completely contained within the largest principal city of the MSA, or • contain at least 250,000 residents of any principal city in the MSA ii. Large fringe metropolitan: counties in MSAs of ≥1 million population that do not qualify as large central metro counties iii. Medium metropolitan: counties in MSAs of 250,000–999,999 population iv. Small metropolitan: counties in MSAs of <250,000 population Nonmetropolitan counties i. Micropolitan: counties in micropolitan statistical areas ii. Noncore: nonmetropolitan counties that do not qualify as micropolitan |
| United States Department of Agriculture (USDA) Economic Research Service18 | Rural-urban continuum codes (RUCC) based on OMB codes, and developed in 2013. Metropolitan counties: i. counties in metropolitan areas with population ≥1 million ii. counties in metropolitan areas with population 250,000 – 1 million iii. counties in metropolitan areas with population <250,000 Nonmetropolitan counties: i. urban population ≥20,000, adjacent to a metropolitan area ii. urban population ≥20,000, not adjacent to a metropolitan area iii. urban population 2,500 – 19,999, adjacent to a metropolitan area iv. urban population 2,500 – 19,999, not adjacent to a metropolitan area v. completely rural or <2,500 urban population, adjacent to a metropolitan area vi. completely rural or <2,500 urban population, not adjacent to a metropolitan area Unknown-Alaska/Hawaii State/not official USDA Rural-Urban Continuum code Unknown/not official USDA Rural-Urban Continuum code |
| Health Resources and Services Administration’s (HRSA’s) Office of Rural Health Policy; USDA’s Economic Research Service; WWAMI Rural Health Research Center19 | Rural-Urban Commuting Area (RUCA) codes are an alternative to the county-level classification system. RUCA codes classify US census tracts using measures of population density, urbanization, and daily commuting. Metropolitan area core: primary flow within a UA Metropolitan area high commuting: primary flow 30% or more to a UA Metropolitan area low commuting: primary flow 10% to 30% to a UA Micropolitan area core: primary flow within a UC of 10,000 to 50,000 (large UC) Micropolitan high commuting: primary flow 30% or more to a large UC Micropolitan low commuting: primary flow 10% to 30% to a large UC Small town core: primary flow within a UC of 2,500 to 9,000 (small UC) Small town core: primary flow 30% or more to a small UC Small town low commuting: primary flow 10% to 30% to a small UC Rural areas: primary flow to a tract outside a UA or UC Sub-codes present within each category, as described.19 |
| USDA Economic Research Service20 | Urban Influence Codes Metropolitan counties i. In large metropolitan area of ≥1 million population ii. In small metropolitan area of <1 million population Nonmetropolitan counties i. Micropolitan area adjacent to large metropolitan area ii. Noncore adjacent to large metropolitan area iii. Micropolitan area adjacent to small metropolitan area iv. Noncore adjacent to small metropolitan area and contains a town of ≥2,500 population v. Noncore adjacent to small metropolitan area and contains a town of <2,500 population vi. Micropolitan area not adjacent to a metro area vii. Noncore adjacent to micro area and contains a town of at least 2,500 residents viii. Noncore adjacent to micro area and does not contain a town of at least 2,500 residents ix. Noncore not adjacent to metro or micro area and contains a town of at least 2,500 residents x. Noncore not adjacent to metro or micro area and does not contain a town of at least 2,500 residents |
US: United States;
UA: urbanized area;
UC: urban cluster
The 2010 US Census Bureau system classifies geographic areas into urban and rural based on population density.14 Census tracts and/or census blocks are classified as urbanized areas, urban clusters, and rural areas, the latter encompassing non-urban areas. Frontier and Remote Area (FAR) codes identify remote and sparsely populated areas that are predominant in the Great Plains and Intermountain West.15 FAR codes are characterized by population size and geographic remoteness, and are particularly relevant when describing the availability of health services, food, and publicly available social services.
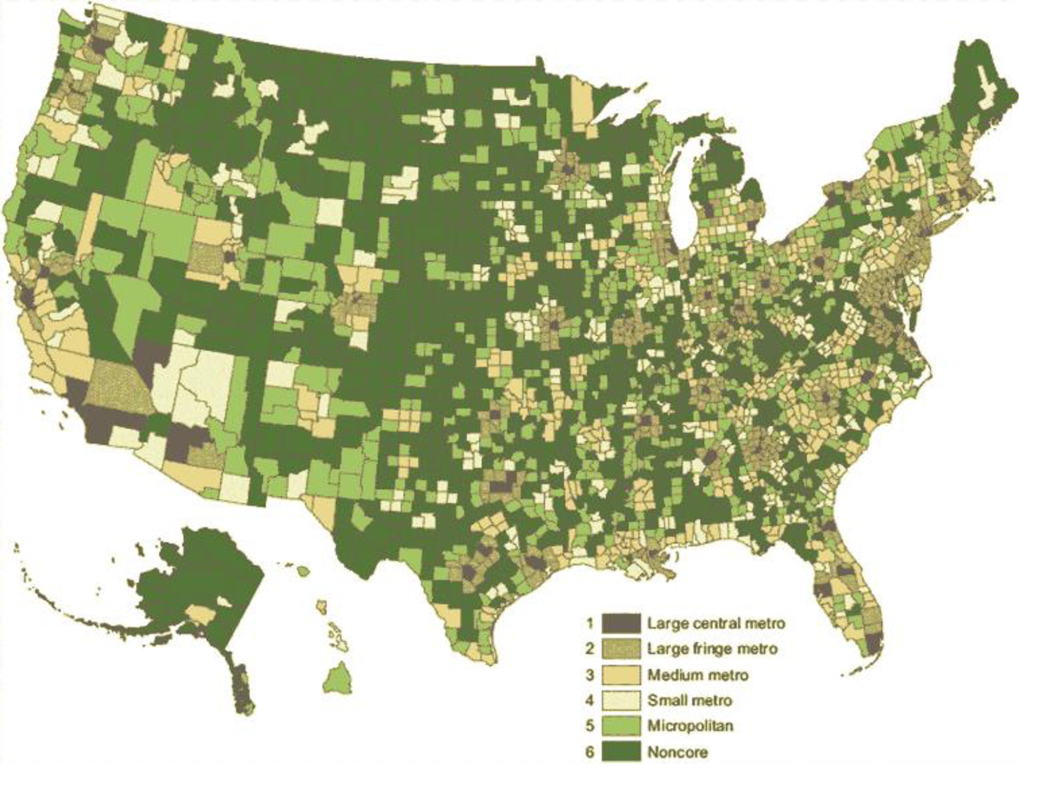
The 2013 Office of Management and Budget system classifies counties as metropolitan statistical areas or nonmetropolitan statistical areas based on population density and commuting.16 Nonmetropolitan areas are outside of metropolitan area boundaries and are subdivided into micropolitan and noncore areas. Metropolitan areas are generally considered urban, and nonmetropolitan areas are generally considered rural. To increase granularity of the 2013 Office of Management and Budget classification system and to study health differences across the urban-rural continuum, the National Center for Health Statistics (NCHS) developed a six-level system to classify rurality at the county level.17 The NCHS system has four levels of metropolitan counties (large central metropolitan, large fringe metropolitan, medium metropolitan and small metropolitan) and two levels of nonmetropolitan counties (micropolitan and noncore) (Figure 1).
Figure 1.
Distribution of counties using the 2013 National Center for Health Statistics (NCHS) system for urban-rural classification at the county level. The six-level system comprises metropolitan counties (large central metro, large fringe metro, medium metro, and small metro) and nonmetropolitan counties (micropolitan and noncore). Reproduced from publicly available report.17
The Rural-urban continuum code (RUCC) provides granularity beyond the NCHS system by distinguishing metropolitan counties by the metropolitan area population (three levels) and nonmetropolitan counties by the degree of urbanization and adjacency to a metropolitan area (six levels).18 In this classification system, each county is assigned one of nine codes. The advantage of the RUCC over the NCHS is that counties can be divided into finer residential groups to study the influence of population density and metropolitan influence on health outcomes.18 The RUCC will be updated in mid-2023.18 Rural-Urban Commuting Area (RUCA) codes characterize census tracts based on US Census Bureau definitions (for urbanized areas and urbanized clusters)14 in combination with work commuting information.19 In addition to RUCA codes, a ZIP code based RUCA approximation was also developed and is publicly available.19 Urban Influence Codes divide counties and county equivalents into 12 groups (Table 1).20 Similar to most classification systems, this allows for granular gradation of the rural-urban continuum to study the design and delivery of health services.
Of these seven rural classification systems, the system used depends on the economic, health, or policy issue under consideration. Although an increased rural-urban granularity may reveal health disparities, it is challenging to obtain and use such granular information. With individual rural-urban mobility, possibly living and working in areas of different rurality, it is difficult to attribute area-level characteristics to individual health outcomes. Finally, health policy decisions may not be made at the level where health disparities are identified, making it challenging to increase health resources or implement change if the proposed changes align differently from political priorities.
Urban-Rural Disparities in Diabetes-related Mortality
The US Census Bureau delineates the US into four census regions: Northeast (9 states), Midwest (12 states), South (16 states and District of Columbia) and the West (13 states).14,21 From 1999 to 2016, the crude diabetes (type 1 and type 2, combined)-related mortality rate across US regions differed by rurality.5,14 Using the NCHS system for county-level rurality, in 1999, the Midwest showed lower diabetes-related mortality in noncore counties (i.e., most rural; 24.6 deaths per 100,000) compared to central metropolitan counties (i.e., most urban; 28.1 deaths per 100,000). However, by 2016, urban counties showed marked improvement in diabetes-related mortality rates (21.8 deaths per 100,000) whereas there was little reduction in rural counties (23.9 deaths per 100,000). This resulted in a higher mortality burden in rural counties.5 The South showed a similar trend: from 1999 to 2016, mortality in central metropolitan counties reduced from 29 deaths per 100,000 to 21 deaths per 100,000. However, over the same time period, mortality in noncore (rural) regions was unchanged from 29.3 deaths per 100,000 to 28.6 deaths per 100,000.5 The differences were less pronounced in the Northeast and West.
Across US regions, there are urban-rural disparities in diabetes-related hospital mortality. Analysis from the Nationwide Inpatient Sample, from 2009 to 2015, showed 29% higher odds of mortality in noncore vs. large central metropolitan counties in the South (odds ratio, 95% confidence interval 1.29 [1.25–1.33]), Midwest (1.23 [1.18–1.28]) and West (1.10 [1.02–1.17], but not Northeast.4 The reasons for the urban-rural disparities are incompletely understood.
Risk Factors for T2DM in Rural Areas
The higher overall mortality in rural versus urban areas may be a reflection of the higher incidence in rural versus urban areas. However, there are no published reports comparing the incidence rates of T2DM in rural and urban areas, due to paucity in data. Most data are self-reported and/or do not distinguish type 1 from type 2 diabetes mellitus, precluding an analysis of the incidence of T2DM in rural and urban areas. Importantly, as lifestyle changes can avert or delay T2DM,22 T2DM prevention strategies optimized for rural populations could reduce the T2DM burden and, thereby, urban-rural health disparities. Several studies have focused on comparing risk factors in rural and urban areas.
Traditional metabolic risk factors
Risk factors for T2DM across the general population include age ≥45 years, family history of diabetes, overweight or obesity, inadequate physical activity, hypertension, and dyslipidemia.22,23 The Centers for Disease Control and Prevention (CDC) has comprehensive data on risk factors for T2DM in rural populations. In 2013, CDC data showed that the prevalence of five optimal lifestyle factors (sufficient sleep, nonsmoking, normal body weight, nondrinking/moderate alcohol, and physical activity), most of which are linked to a higher risk of T2DM, was lower in rural than urban populations (27% vs. 32%).24 The analysis of National Health Interview Survey data from 2008 to 2017 also showed that the age-standardized prevalence of meeting physical activity guidelines (150 minutes per week of vigorous- and light or moderate intensity leisure-time physical activity) differed among adults in urban and rural areas.25
Similar to physical activity, there were urban-rural differences in the prevalence of obesity.26 Using 2016 Behavioral Risk Factor Surveillance System (BRFSS) data, there was a higher unadjusted obesity prevalence in nonmetropolitan areas (34.2%) than metropolitan areas (28.7%).26 The nonmetropolitan-metropolitan difference was 5.6% (South; 36.6% vs. 31.0%), 5.4% (Northeast; 31.8% vs. 26.4%), 3.7% (Midwest; 34.2% vs. 30.5%), and 2.9% (West; 28.6% vs. 25.7%). The higher frequency of inadequate physical activity and obesity in rural compared to urban areas is concerning given their association with higher risk of T2DM.
Other studies have examined the association of diet and risk of T2DM. The analysis of 2009 BRFSS data showed that rural compared to non-rural adults were less likely to consume at least five daily servings of fruits and vegetables.27 Using 2012 BRFSS data that included adults aged ≥65 years, rural compared to urban areas were characterized by higher obesity rates and lower fruit consumption.28 Among urban adults, there was an inverse association between obesity and fruit consumption, an association not observed among rural adults.28 In addition, among 96 American Indian families living in rural areas, the higher intake of processed meat (top vs. lowest quintile of consumption) was associated with a 1.6-fold higher risk of incident T2DM.27 However, the association between dietary choices and T2DM is nuanced and may vary by region and availability of affordable healthful food nutrition.
Novel Rural-enriched Risk Factors
In addition to traditional metabolic risk factors, there is growing interest in the association between novel rural-enriched factors and risk of T2DM. Exposure to pesticides, organic pollutants, and fertilizers is associated with a higher risk of T2DM.29–32 Although the use of these organic chemicals may be higher in rural than urban populations,33 the association between these factors and T2DM risk in rural populations has not been examined directly. It is unknown if exposure to these risk factors is associated with higher risk of T2DM in rural populations, and if they increase risk beyond traditional risk factors.
T2DM Risk Prediction Scores for Rural Areas
There are several risk prediction scores for T2DM; the vast majority were developed in Europe and have not been examined in US rural populations.34,35 Most of these risk prediction scores incorporate traditional risk factors, as described in an earlier section. Many risk prediction scores incorporate biomarkers such as cholesterol and triglycerides, which may limit people in rural areas with geographic and financial barriers for laboratory testing.34,35 As a result, there is emerging interest in the use of non-invasive (i.e., non-laboratory) T2DM risk scores to possibly overcome geographic and financial barriers for risk prediction. Also, rural-enriched factors have not been incorporated into risk prediction scores, and it is unknown if they improve risk prediction beyond traditional risk factors. The paucity of T2DM risk prediction scores validated and optimized in rural populations may limit the ability of healthcare providers to determine T2DM risk and provide preventive counseling in these areas.
Comorbidities and Other Factors Associated with T2DM
A meta-analysis of 102 prospective studies (n ~700,000 participants) showed that diabetes mellitus (type 1 and type 2, combined) is associated with a 1.5–2 fold higher risk of cardiovascular events and accounted for approximately 10% of vascular deaths.36 Effective management of T2DM requires non-pharmacologic and pharmacologic interventions to mitigate the risk of cardiovascular events. However, adherence to these interventions may be influenced by comorbidities and social factors. For example, among veterans with T2DM and depression, medication non-adherence (<80% medication possession ratio for T2DM medications) was higher among rural compared with urban Hispanic patients, but lower among rural compared with urban non-Hispanic whites and non-Hispanic blacks.37 This and other studies showed that people with T2DM and concomitant depressive symptoms, high psychosocial stress of having T2DM, or low social supports, have lower adherence to lifestyle and pharmacologic management of T2DM.38–42 Further, perceptions of the neighborhood can affect management of comorbidities. A study of 250 Latino adults with T2DM in a rural agricultural community (San Joaquin Valley, California) showed that neighborhood perceptions (e.g., crime, access to exercise facilities, transportation) were independently associated with higher body mass index and blood pressure, after adjusting for demographics and comorbidities.43 In addition, the workplace can be a significant barrier to effective self-management. Rural areas tend to have employment directed toward services, trades, and government organizations. People with T2DM working in rural compared to urban areas may have inadequate food choices, reduced ability to regulate timing of meals and medications, and lower flexibility in work schedules to meet with healthcare providers.44 These factors can affect the management of T2DM and associated comorbidities.
Screening and Management of T2DM in Rural Areas
Residents of rural areas, compared to urban counterparts, receive inadequate diabetes education and care (e.g., dilated eye examination).45,46 Two systematic reviews of factors that influence T2DM self-management in rural communities showed that personal factors (e.g., cost of medications), cultural factors (e.g., lack of culturally competent guidelines/recommendations), and infrastructure (e.g., transportation, distance to health facilities) were important barriers to self-management.47,48 Various strategies have been implemented to try to improve the screening and management of T2DM in rural areas, as briefly described below.
Opportunistic Screening
The availability of healthcare services is essential to the screening and management of T2DM. A cross-sectional study of health providers in the ‘diabetes belt’ Appalachian region (including 410 counties in West Virginia and 12 other states) showed that most health facilities did not have adequate diabetes specialists: less than 15% had an endocrinologist, only 37% had a certified diabetes educator, and some facilities reported inadequate funding.49,50 While rural areas may have limited access to a primary care provider, providers have leveraged opportunistic nontargeted screening for prediabetes and T2DM. At the Northern Navajo Medical Center, a rural Indian Health Service emergency department in Shiprock, New Mexico, providers screened people presenting to the emergency department. Of 924 people tested for T2DM, 26.2% (n=242) had undiagnosed prediabetes and 2.6% (n=24) had undiagnosed T2DM.51 Similarly, diabetes screening and education at a local festival (Pioneer Day) in Loachapoka, Alabama, allowed for the increased detection of previously undiagnosed prediabetes and T2DM.52
T2DM Self-Management
In-person and telehealth interventions with clear goal-setting (e.g., for diet, physical activity, blood pressure) have been associated with improvement in T2DM self-management.47 There have been nine studies of in-person interventions including group classes (n=7 studies), group and individual classes (n=1 study), and in-home education with a nurse (n=1 study) of duration ranging from 8 weeks to 12 months. Six studies on telehealth interventions used videoconference sessions of varying duration (6 months to 3 years); of these, one study offered videoconference and secure messaging to a nurse care manager.47 These studies compared the intervention to usual care, but there were no studies comparing the effectiveness of different interventions.
Community-based Initiatives
Community-based initiatives may be effective in improving T2DM management. In a rural county in South Carolina, adults with diabetes were given access to a federally qualified health center-based farmers’ market and personal financial incentives. Over the 22-week intervention and pre/post evaluation, adults with diabetes increased their consumption of fruits and vegetables.53 Similarly, other lifestyle improvement programs based on recommendations from the American Diabetes Association and YMCA (28 sessions over a 12-month period) were associated with improvement in body-mass index, hemoglobin A1c, and medication adherence.54 In another study, participants randomized to a 3-month community health worker intervention vs. usual care showed improvement in adherence to self-management recommendations.55
Interventions Targeting Comorbidities and T2DM
Given the frequent co-existence of T2DM and depression/psychological symptoms, studies have evaluated whether the treatment of depression/psychological symptoms improves T2DM management. The ACTIVE (Appalachians Coming Together to Increase Vital Exercise) program enrolled 50 adults with T2DM and depression in a single-arm intervention of aerobic activity (150 minutes per week) and cognitive behavioral therapy (10 sessions). After the 12-week intervention period, there was an improvement in major depressive disorder symptoms, hemoglobin A1c (lowered by 0.4%), and fasting glucose (lowered by 4.7 mg/dL), although the hemoglobin A1c changes were not sustained at 3-months following the intervention.56 Similarly, among 129 rural African-American women in the EMPOWER study, women were randomized to telephone-based peer-support (intervention) or mail-based educational material (control) arms to manage diabetes-related distress. The intervention was associated with greater improvement in hemoglobin A1c and medication adherence compared to those with worsened/unchanged diabetes distress.57 The COMRADE study randomized 139 rural adults to 16 sessions of severity-tailored cognitive behavioral therapy plus lifestyle counseling compared with usual care.58 The intervention was associated with improvement in depressive symptoms, self-care behaviors, medication adherence, and a non-significant decrease in hemoglobin A1c (–0.92 vs. –0.31; p=0.06).58
While these studies highlight the importance of treating depression/psychological symptoms, it is also important to screen for these comorbidities. One study showed adults may not have received adequate treatment for depression, which in turn, may have affected the non-pharmacologic and pharmacologic management of T2DM.59 Additional studies are required to evaluate novel strategies to treat comorbidities and improve T2DM self-management. Ultimately, engaging a patient in shared decision making may improve decisional outcomes and, possibly, clinical outcomes.60
Role of Technology in Managing T2DM
As many rural patients experience geographic barriers to access healthcare, there has been a growing interest to use technology-based interventions to improve T2DM management.61–65 These studies showed that telemedicine interventions can improve T2DM knowledge,62 reduce waist circumference, 62 lower body mass index,62 and lower hemoglobin A1c65. In the ENHANCED randomized controlled trial, a dietician nutritionist-led telemedicine program resulted in improvement in diabetes care measures (hemoglobin A1c <8%, blood pressure <140/90 mmHg, tobacco cessation, statin use, and aspirin use appropriate) compared to the control arm.63 Importantly, studies have shown that telemedicine delivery of a T2DM prevention program was non-inferior to an in-person program, suggesting that telemedicine could become an important mechanism to deliver care in areas with reduced access to providers.64,66 These results require replication in other cohorts, but are reassuring for providers and facilities implementing telemedicine based care for rural patients.
T2DM Primary Prevention Initiatives in Rural Areas
Several studies examined interventions to improve risk factors for T2DM among rural populations.67–70 In a study in rural North Carolina, African-American adults at high-risk of T2DM participated in a community-based ‘Power to Prevent’ T2DM prevention program. Individuals were defined as high-risk based on the 7-item risk calculator from the American Diabetes Association. This program was associated with an improvement in knowledge on diabetes and lifestyle, but not in blood glucose, weight, or blood pressure.68 Similarly, other community programs involving diabetes education,68 nutrition education with behavioral coaching,70 and faith-based programs at local churches,69 have shown success in improving risk factors associated with T2DM. Future work is required to evaluate if these programs reduce incident T2DM and if the health benefits are sustained after completion of the program.
Guidelines and T2DM in Rural Areas
The American Diabetes Association identified telemedicine as an effective strategy to manage T2DM among rural patients.71 The American Heart Association and American Stroke Association released a Call to Action highlighting the growing burden of cardiovascular disease in rural areas.72 This Call to Action highlights the burden of risk factors, including T2DM, and the urgent need for strategies to improve rural health. Adults across rural populations vary by age, race/ethnic composition, desire to incorporate technology for T2DM self-management, and preference for individual vs. group interventions. The success of interventions will also rely on their cultural competence, as shown for T2DM self-management programs.73–78
Knowledge Gaps and Next Steps
Improving the health of rural populations, an important minority group, is a priority for national organizations including the CDC and the National Institutes of Health.9,79–81 At present, there is sparse information on the incidence rates of T2DM in rural compared to urban areas. In addition, the determinants of the high burden of T2DM in rural areas have not been well studied. Based on publicly reported information, there are very few clinical trials of interventions to improve T2DM primary prevention in rural areas.
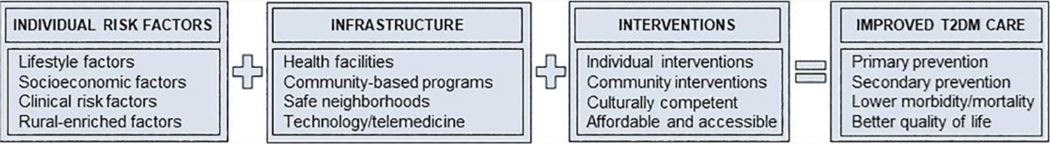
Future studies on rural areas should evaluate the incidence rates and temporal trends of T2DM and its major determinants at the individual level (e.g., obesity, dietary lifestyle, physical activity) and community level (e.g., access to healthful food, community programs, health services) by gender, sex, and race/ethnicity. In this study, we focused on T2DM given its link to metabolic risk factors (e.g., obesity, metabolic syndrome) and vascular (micro- and macro-) complications; however, these topics should be reviewed in separate studies. To guide health disparities research, the National Institute on Minority Health and Health Disparities (NIMHD) developed a research framework that outlines levels of influence (individual, interpersonal, community, and societal) and domains of influence (biological, behavioral, physical/built environment, sociocultural environment, and health care system) to improve health outcomes at the individual, family/organizational, community, and population levels.80 There is an urgent need for systematic efforts to assess these levels and domains of influence to develop targeted interventions to improve health outcomes (Figure 2). This is particularly relevant as a recent report showed rural areas lag urban areas in their progress toward reducing mortality from T2DM and other conditions.82
Figure 2.
Schematic outlining importance of individual risk factors, infrastructure, and tailored interventions to improve type 2 diabetes mellitus (T2DM) care in rural population.
CONCLUSIONS
In the US, the burden of T2DM is higher in rural compared to urban areas. The rural burden of T2DM varies by gender, race/ethnicity, region, infrastructure, and access to health services. Given the diversity of rural populations, it will be challenging and unrealistic to design a single strategy for widespread implementation. Instead, culturally competent tailored strategies will be more effective in improving the primary prevention of T2DM. Recognizing this, the Federal Office of Rural Health Policy, Health Resources & Services Administration (US Department of Health and Human Services) funds at least 10 rural health research center and policy initiatives that focus on the burden and local needs of people.83 A concerted effort is required to engage patients, providers, and governmental and non-governmental stakeholders, design patient-centered approaches to reduce the burden of T2DM in rural areas, and achieve the Health People 2020 goal for diabetes.12
ACKNOWLEDGMENTS
The authors are grateful to Ms Dana Gerberi MLS AHIP, academic librarian at Mayo Clinic Libraries, for assistance in developing the scientific literature search and managing the references; and, Ms. Marla Battey, Mayo Clinic Libraries, for assistance in obtaining full-texts of articles.
FINANCIAL SUPPORT
SBD was supported by the Robert and Elizabeth Strickland Career Development Award, Mayo Clinic, Rochester, MN, USA; MMM was supported by National Institute on Aging grant R01 AG49704.
Footnotes
CONFLICT of INTEREST DISCLOSURE
None of the authors reports any conflict of interest.
DATA AVAILABILITY
There were no original data or datasets used in this manuscript. All cited literature is publicly available.
REFERENCES
- 1.Centers for Disease Control and Prevention. Type 2 Diabetes. 2019. https://www.cdc.gov/diabetes/basics/type2.html (1 September 2019)
- 2.Rutledge SA, Masalovich S, Blacher RJ, Saunders MM. Diabetes Self-Management Education Programs in Nonmetropolitan Counties - United States, 2016. MMWR Surveill Summ 2017;66:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferdinand AO, Akinlotan MA, Callaghan TH, Towne SD Jr, Bolin JN. Diabetes-Related Hospital Mortality in Rural America: A Significant Cause for Concern. 2018. https://srhrc.tamhsc.edu/docs/srhrc-pb3-ferdinand-diabetes.pdf [Google Scholar]
- 4.Ferdinand AO, Akinlotan MA, Callaghan T, Towne SD, Bolin J. Diabetes-related hospital mortality in the U.S.: A pooled cross-sectional study of the National Inpatient Sample. J Diabetes Complications 2019;33:350–355. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan T, Ferdinand AO, Akinlotan MA, Towne SD, Bolin J. The Changing Landscape of Diabetes Mortality in the United States Across Region and Rurality, 1999–2016. J Rural Heal 2019;0:1–6. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care 2019;7:e000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, Fujimoto WY, Imperatore G. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA 2019;322:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. National Diabetes Statistics Report 2020. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 10.Aguayo-Mazzucato C, Diaque P, Hernandez S, Rosas S, Kostic A, Caballero AE. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metab Res Rev 2019;35:e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant MJ, Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91–108. [DOI] [PubMed] [Google Scholar]
- 12.Bolin J, Schulze A, Helduser J, Ory M. The Burden of Diabetes in Rural America. In Bolin JN, Bellamy G, Ferdinand AO, et al. eds. (2015). Rural Healthy People 2020. Vol. 1. College Station, Texas: Texas A&M Health Science Center School of Public Health, Southwest Rural Health Research Center. 2015. p. 43–53. [Google Scholar]
- 13.EndNote. EndNote. https://endnote.com/ [Google Scholar]
- 14.US Census. United States Census Bureau. 2019. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- 15.United States Department of Agriculture. Frontier and Remote Area Codes. 2019. https://www.ers.usda.gov/amber-waves/2012/december/data-feature-mapping-frontier-and-remote-areas-in-the-us/
- 16.OMB. Office of Management and Budget. http://www.ruralhome.org/storage/documents/rrbriefs/rpb_omb_outside_metro.pdf
- 17.Ingram D, Franco S. 2013 NCHS urban–rural classification scheme for counties. National Center for Health Statistics. Vital Health Stat 2(166). 2014. [PubMed] [Google Scholar]
- 18.US Department of Agriculture. Rural-Urban Continuum Codes. 2019. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/.aspx
- 19.Center Rural Health Research. Rural-Urban Commuting Area Codes. http://depts.washington.edu/uwruca/
- 20.USDA. Urban Influence Codes. https://www.ers.usda.gov/data-products/urban-influence-codes/documentation/
- 21.United States Census Bureau. Geography Program. 2019. https://www.census.gov/programs-surveys/geography.html (12 September 2019)
- 22.American Diabetes Association. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41:S51–S54. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S13–S27. [DOI] [PubMed] [Google Scholar]
- 24.Matthews KA, Croft JB, Liu Y, Lu H, Kanny D, Wheaton AG, Cunningham TJ, Khan LK, Caraballo RS, Holt JB, Eke PI, Giles WH. Health-Related Behaviors by Urban-Rural County Classification — United States, 2013. MMWR Surveill Summ 2017;66:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitfield GP, Carlson SA, Ussery EN, Fulton JE, Galuska DA, Petersen R. Trends in Meeting Physical Activity Guidelines Among Urban and Rural Dwelling Adults — United States, 2008–2017. MMWR Morb Mortal Wkly Rep 2019;68:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundeen EA, Park S, Pan L, O’Toole T, Matthews K, Blanck HM. Obesity Prevalence Among Adults Living in Metropolitan and Nonmetropolitan Counties — United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fretts AM, Howard B V., McKnight B, Duncan GE, Beresford SAA, Mete M, Eilat-Adar S, Zhang Y, Siscovick DS. Associations of processed meat and unprocessed red meat intake with incident diabetes: The Strong Heart Family Study. Am J Clin Nutr 2012;95:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen SA, Greaney ML, Sabik NJ. Assessment of dietary patterns, physical activity and obesity from a national survey: Rural-urban health disparities in older adults. PLoS One 2018;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DH, Porta M, Jacobs DR, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr. Rev. 2014. p. 557–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup Environ Med 2014;71:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, Sun Q. Persistent Organic Pollutants and Type 2 Diabetes: A Prospective Analysis in the Nurses’ Health Study and Meta-analysis. Environ Health Perspect 2013;121:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zong G, Valvi D, Coull B, Göen T, Hu FB, Nielsen F, Grandjean P, Sun Q. Persistent organic pollutants and risk of type 2 diabetes: A prospective investigation among middle-aged women in Nurses’ Health Study II. Environ Int 2018;114:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein JN, Geller A, Negussie Y, Baciu A, eds. Communities in Action. Washington, D.C.: National Academies Press; 2017. https://www.nap.edu/catalog/24624 (30 September 2019) [Google Scholar]
- 34.Kengne AP, Beulens JW, Peelen LM, Moons KG, Schouw YT van der, Schulze MB, Spijkerman AM, Griffin SJ, Grobbee DE, Palla L, Tormo M-J, Arriola L, Barengo NC, Barricarte A, Boeing H, Bonet C, Clavel-Chapelon F, Dartois L, Fagherazzi G, Franks PW, Huerta JM, Kaaks R, Key TJ, Khaw KT, Li K, Mühlenbruch K, Nilsson PM, Overvad K, Overvad TF, Palli D, et al. Non-invasive risk scores for prediction of type 2 diabetes (EPIC-InterAct): a validation of existing models. Lancet Diabetes Endocrinol 2014;2:19–29. [DOI] [PubMed] [Google Scholar]
- 35.Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ 2011;343:d7163–d7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, Angelantonio E Di, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CDA, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J, Tipping RW, Ford CE, Pressel SL, Folsom AR, Chambless LE, Wagenknecht LE, Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C, Knuiman M, Whincup PH, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Axon RN, Gebregziabher M, Hunt KJ, Lynch CP, Payne E, Walker RJ, Egede LE. Comorbid depression is differentially associated with longitudinal medication nonadherence by race/ethnicity in patients with type 2 diabetes. Medicine (Baltimore) 2016;95:e3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharya G. Psychosocial impacts of type 2 diabetes self-management in a rural African-American population. J Immigr Minor Heal 2012;14:1071–1081. [DOI] [PubMed] [Google Scholar]
- 39.Bell RA, Andrews JS, Arcury TA, Snively BM, Golden SL, Quandt SA. Depressive symptoms and diabetes self-management among rural older adults. Am J Health Behav 2010;34:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter RD, Theeke LA, Mallow JA, Theeke E, Gilleland D. Relationships among Distress, Appraisal, Self-Management Behaviors, and Psychosocial Factors in a Sample of Rural Appalachian Adults with Type 2 Diabetes. Online J Rural Nurs Heal Care 2017;17:34–64. [Google Scholar]
- 41.Hunt CW, Wilder B, Steele MM, Grant JS, Pryor ER, Moneyham L. Relationships among self-efficacy, social support, social problem solving, and self-management in a rural sample living with type 2 diabetes mellitus. Res Theory Nurs Pract 2012;26:126–141. [DOI] [PubMed] [Google Scholar]
- 42.Miller ST. Diabetes and psychological profile of younger rural African American women with type 2 diabetes. J Heal Care Poor Underserved 2011;22:1239–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno G, Morales L, Nuñez de Jaimes F, Tseng C-H, Isiordia M, Noguera C, Mangione C. Neighborhood Perceptions and Health-Related Outcomes Among Latinos with Diabetes from a Rural Agricultural Community. J Community Health 2014;39:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant JS, Steadman LA. Barriers to diabetes self-management among rural individuals in the workplace. Work Heal Saf 2016;64:243–248. [DOI] [PubMed] [Google Scholar]
- 45.Hale NL, Bennett KJ, Probst JC. Diabetes care and outcomes: Disparities across rural America. J Community Health 2010;35:365–374. [DOI] [PubMed] [Google Scholar]
- 46.Brown-Guion SY, Youngerman SM, Hernandez-Tejada MA, Dismuke CE, Egede LE. Racial/ethnic, regional, and rural/urban differences in receipt of diabetes education. Diabetes Educ 2013;39:327–334. [DOI] [PubMed] [Google Scholar]
- 47.Lepard MG, Joseph AL, Agne AA, Cherrington AL. Diabetes Self-Management Interventions for Adults with Type 2 Diabetes Living in Rural Areas: A Systematic Literature Review. Curr Diab Rep 2015;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross S, Benavides-Vaello S, Schumann L, Haberman M. Issues that impact type-2 diabetes self-management in rural communities. J Am Assoc Nurse Pract 2015;27:653–660. [DOI] [PubMed] [Google Scholar]
- 49.Denham SA, Remsberg K, Wood L. Diabetes education in the Appalachian region: providers’ views. Rural Remote Health 2010;10:1321. [PubMed] [Google Scholar]
- 50.Denham SA, Wood LE, Remsberg K. Diabetes care: provider disparities in the US Appalachian region. Rural Remote Heal 2010;10:1320. [PubMed] [Google Scholar]
- 51.Anderson ES, Dworkis DA, DeFries T, Emery E, Deegala C, Mohs K. Nontargeted Diabetes Screening in a Navajo Nation Emergency Department. Am J Public Health 2019;109:270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown LG, Prather C. A Diabetes Screening and Educational Event in Rural Alabama. Am J Nurs 2020;120:61–63. [DOI] [PubMed] [Google Scholar]
- 53.Freedman DA, Choi SK, Hurley T, Anadu E, Hebert JR. A farmers’ market at a federally qualified health center improves fruit and vegetable intake among low-income diabetics. Prev Med (Baltim) 2013;56:288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freeman K, Hanlon M, Denslow S, Hooper V. Patient Engagement in Type 2 Diabetes: A Collaborative Community Health Initiative. Diabetes Educ 2018;44:395–404. [DOI] [PubMed] [Google Scholar]
- 55.Glenn LE, Nichols M, Enriquez M, Jenkins C. Impact of a community-based approach to patient engagement in rural, low-income adults with type 2 diabetes. Public Health Nurs 2020;37:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Groot M de, Doyle T, Kushnick M, Shubrook J, Merrill J, Rabideau E, Schwartz F. Can lifestyle interventions do more than reduce diabetes risk? Treating depression in adults with type 2 diabetes with exercise and cognitive behavioral therapy. Curr Diab Rep 2012;12:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummings DM, Lutes LD, Littlewood K, Solar C, Hambidge B, Gatlin P. Impact of Distress Reduction on Behavioral Correlates and A1C in African American Women with Uncontrolled Type 2 Diabetes: Results from EMPOWER. Ethn Dis 2017;27:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cummings DM, Lutes LD, Littlewood K, Solar C, Carraway M, Kirian K, Patil S, Adams A, Ciszewski S, Edwards S, Gatlin P, Hambidge B. Randomized Trial of a Tailored Cognitive Behavioral Intervention in Type 2 Diabetes With Comorbid Depressive and/or Regimen-Related Distress Symptoms: 12-Month Outcomes From COMRADE. Diabetes Care 2019;42:841–848. [DOI] [PubMed] [Google Scholar]
- 59.Groot M de, Doyle T, Averyt J. Program ACTIVE: Cognitive Behavioral Therapy to Treat Depression in Adults With Type 2 Diabetes in Rural Appalachia. J Cogn Psychother 2017;31:158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Branda ME, LeBlanc A, Shah ND, Tiedje K, Ruud K, Houten H Van, Pencille L, Kurland M, Yawn B, Montori VM. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res 2013;13:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McIlhenny C V, Guzic BL, Knee DR, Demuth BR, Roberts JB. Using technology to deliver healthcare education to rural patients. Rural Remote Health 2011;11. [PubMed] [Google Scholar]
- 62.Izquierdo R, Lagua CT, Meyer S, Ploutz-Snyder RJ, Palmas W, Eimicke JP, Kong J, Teresi JA, Shea S, Weinstock RS. Telemedicine intervention effects on waist circumference and body mass index in the IDEATel project. Diabetes Technol Ther 2010;12:213–220. [DOI] [PubMed] [Google Scholar]
- 63.Benson GA, Sidebottom A, Hayes J, Miedema MD, Boucher J, Vacquier M, Sillah A, Gamam S, VanWormer JJ. Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) Telemedicine Randomized Controlled Trial on Diabetes Optimal Care Outcomes in Patients with Type 2 Diabetes. J Acad Nutr Diet 2019;119:585–598. [DOI] [PubMed] [Google Scholar]
- 64.Vadheim LM, Patch K, Brokaw SM, Carpenedo D, Butcher MK, Helgerson SD, Harwell TS. Telehealth delivery of the diabetes prevention program to rural communities. Transl Behav Med 2017;7:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Egede LE, Williams JS, Voronca DC, Knapp RG, Fernandes JK. Randomized Controlled Trial of Technology-Assisted Case Management in Low Income Adults with Type 2 Diabetes. Diabetes Technol Ther 2017;19:476–482. [DOI] [PubMed] [Google Scholar]
- 66.Ciemins EL, Coon PJ, Coombs NC, Holloway BL, Mullette EJ, Dudley WN. Intent-to-treat analysis of a simultaneous multisite telehealth diabetes prevention program. BMJ Open Diabetes Res Care 2018;6:e000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kramer L, Rabanizada N, Haasenritter J, Bosner S, Baum E, Donner-Banzhoff N. Do guidelines on first impression make sense? Implementation of a chest pain guideline in primary care: a systematic evaluation of acceptance and feasibility. BMC Fam Pr 2011;12:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cene CW, Haymore LB, Ellis D, Whitaker S, Henderson S, Lin FC, Corbie-Smith G. Implementation of the Power to Prevent Diabetes Prevention Educational Curriculum Into Rural African American Communities: A Feasibility Study. Diabetes Educ 2013;39:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boltri JM, Davis-Smith M, Okosun IS, Seale JP, Foster B. Translation of the National Institutes of Health Diabetes Prevention Program in African American churches. J Natl Med Assoc 2011;103:194–202. [DOI] [PubMed] [Google Scholar]
- 70.Radcliff TA, Cote MJ, Whittington MD, Daniels MJ, Bobroff LB, Janicke DM, Perri MG. Cost-Effectiveness of Three Doses of a Behavioral Intervention to Prevent or Delay Type 2 Diabetes in Rural Areas. J Acad Nutr Diet 2019;30:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ADA. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020. p. S7–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harrington RA, Califf RM, Balamurugan A, Brown N, Benjamin RM, Braund WE, Hipp J, Konig M, Sanchez E, Joynt Maddox KE. Call to action: Rural health: a presidential advisory from the american heart association and american stroke association. Circulation. 2020. p. E615–E644. [DOI] [PubMed] [Google Scholar]
- 73.Brown SA, Perkison WB, Garcia AA, Cuevas HE, Velasquez MM, Winter MA, Hanis CL. The Starr County Border Health Initiative: Focus Groups on Diabetes Prevention in Mexican Americans. Diabetes Educ 2018;44:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown SA, Garcia AA, Winter M, Silva L, Brown A, Hanis CL. Integrating education, group support, and case management for diabetic Hispanics. Ethn Dis 2011;21:20–26. [PMC free article] [PubMed] [Google Scholar]
- 75.Soto SC, Louie SY, Cherrington AL, Parada H, Horton LA, Ayala GX. An ecological perspective on diabetes self-care support, self-management behaviors, and hemoglobin A1C among Latinos. Diabetes Educ 2015;41:214–223. [DOI] [PubMed] [Google Scholar]
- 76.Paz-Pacheco E, Sandoval MA, Ardena GJR, Paterno E, Juban N, Lantion-Ang FL, Jimeno C, Patal P, Bongon J. Effectiveness of a community-based diabetes self-management education (DSME) program in a rural agricultural setting. Prim Heal Care Res Dev 2017;18:35–49. [DOI] [PubMed] [Google Scholar]
- 77.Williams IC, Utz SW, Hinton I, Yan G, Jones R, Reid K. Enhancing diabetes self-care among rural African Americans with diabetes: results of a two-year culturally tailored intervention. Diabetes Educ 2014;40:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brunk DR, Taylor AG, Clark ML, Williams IC, Cox DJ. A Culturally Appropriate Self-Management Program for Hispanic Adults With Type 2 Diabetes and Low Health Literacy Skills. J Transcult Nurs 2017;28:187–194. [DOI] [PubMed] [Google Scholar]
- 79.CDC. National Diabetes Fact Sheet. 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf
- 80.NIMHD. NIMHD Research Framework,. https://nimhd.nih.gov/about/overview/research-framework/nimhd-framework.html
- 81.National Institutes of Health. National Institute on Minority Health and Health Disparities (NIMHD). 2019. https://www.nih.gov/about-nih/what-we-do/nih-almanac/national-institute-minority-health-health-disparities-nimhd
- 82.Yaemsiri S, Alfier JM, Moy E, Rossen LM, Bastian B, Bolin J, Ferdinand AO, Callaghan T, Heron M. Healthy People 2020: Rural Areas Lag In Achieving Targets For Major Causes Of Death. Health Aff (Millwood) NLM (Medline); 2019;38:2027–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rural Health Research Gateway. Rural Health Research Centers and Analysis Initiatives. https://www.ruralhealthresearch.org/centers
- 84.Bureau UC. What is Rural America? 2019. https://www.census.gov/library/stories/2017/08/rural-america.html