Abstract
The aim of the study is to identify reliable quantitative fetal echocardiographic predictors for postnatal development of coarctation (CoA). In this retrospective study, we included 65 fetuses with a prenatally suspected, isolated CoA, born 2010–2018. Dimensions of the cardiac structures, aortic, and ductal arches expressed as ratios and Z-scores were analyzed in relation to outcome. Fetuses that developed CoA postnatally (34%) exhibited significantly smaller Z-scores of left cardiac structures from the mitral valve to the aortic isthmus. The most sensitive and specific predictors were a carotid-subclavian artery index (CSAI) of < 0.78 (92.3% sensitivity, 96.8% specificity) or a product of isthmus-to-duct ratio in the three-vessel trachea view (3VT) and the mitral-to-tricuspid valve ratio (I/D3VTxMV/TV) of < 0.37 (100% sensitivity, 94.6% specificity). When comparing different Z-score datasets, we observed large and highly significant differences. Postnatal CoA can be predicted with high accuracy during fetal life using CSAI or I/D3VTxMV/TV. The latter may be particularly useful if adequate sagittal aortic arch images cannot be obtained. As significant and clinically unacceptable differences in Z-scores were observed for the same measurements, this calls for a large multi-center collaboration to generate reliable fetal echocardiographic Z-scores.
Keywords: Fetus, Coarctation, Fetal echocardiography, Carotid-subclavian artery index, Prenatal diagnosis
Introduction
Coarctation of the aorta (CoA) is one of the most common congenital heart defects (CHDs) accounting for 8% of all CHDs [1]. It is defined as a circumscribed narrowing of the aortic isthmus, often combined with a tubular hypoplasia of the aortic arch. CoA may occur in isolation or in association with other CHDs, most commonly ventricular septal defects (VSD). If not detected prenatally or shortly post partum, critical narrowing of the aortic isthmus may develop as the arterial duct closes. This leads to underperfusion of the lower body and subsequently to multiorgan failure, shock, and even death. If CoA is diagnosed prior to clinical decompensation, however, the prognosis following surgical repair is generally good [2].
Thus, a timely, preferably prenatal diagnosis is essential in order to facilitate delivery planning and avoid unnecessary morbidity and mortality [3].
Unfortunately, the prenatal diagnosis of CoA remains challenging in spite of technical advances. Fetal CoA is often suspected in case of a disproportion of the ventricles and great vessels with smaller left heart structures. Overall, fewer than 50% of fetuses with a ventricular disproportion develop CoA postnatally, leading to high false-positive detection rates associated with unnecessary parental anxiety and use of healthcare-related resources [4–7]. On the flipside, prenatal detection rates for CoA are only approximately 20–35% and thereby among the lowest of all critical CHDs [8–10]. This low prenatal detection rate is related to the fact that the actual narrowing of the isthmus typically does not develop until the arterial duct closes postnatally. Even pulse oximeter newborn screening has only marginally improved the early detection rate for CoA [11, 12].
It was the primary aim of this study to identify reliable quantitative fetal echocardiographic predictors of postnatal CoA, using both dimension ratios and Z-scores of cardiac structures and the secondary aim to compare Z-scores calculated from various normative datasets.
We hypothesized that development of CoA postnatally can be predicted with high diagnostic accuracy.
Methods
This retrospective study was conducted at Skane`s University Hospital at Lund University, one of two tertiary referral centers for pediatric cardiac surgery in Sweden. The institutional fetal cardiology database and “The Swedish Registry of Congenital Heart Defects” (SWEDCON) were searched for infants born 2010–2018 with a prenatal suspicion of CoA. Fetuses with prenatally suspected CoA with or without borderline hypoplasia of the left heart structures were included. Exclusion criteria were prenatally suspected hypoplastic left heart syndrome, complex CHD other than associated aortic arch hypoplasia, VSD, mild aortic (AS) or mitral valve stenosis (MS) or persistent left superior vena cava (LSVC), and insufficient technical quality including suboptimal imaging of the sagittal aortic arch. This study was approved by the Regional Ethical Review Board according to the Helsinki declaration.
Demographic pre- and postnatal variables were obtained from the medical record and operative reports. If multiple echocardiograms were available, the study with the most optimal imaging technique was used for analysis.
Fetal echocardiograms were analyzed using SyngoDynamics (Siemens, Germany). All measurements were conducted by an experienced fetal echocardiographer (K.F.).
Fetal Echocardiographic Measurements
Quantitative measurements of fetal echocardiograms included dimensions of the left and right cardiac structures and function as well as standard and non-standard measurements of the aortic arch and arterial duct. According to published guidelines, fetal Z-scores corresponding to gestational age were computed [13–16].
The following ratios between left and right cardiac structures were determined: left-to-right ventricular (LV/RV) width and length ratios and mitral-to-tricuspid valve (MV/TV) dimension ratio examined in the four-chamber view; aortic-to-pulmonary valve (AoV/PV) ratio; ascending-to-descending aorta (Ao asc/DAo) ratios measured in the outflow tract and sagittal views; and isthmus aortae-to-arterial duct (I/D) diameter ratios examined in the three-vessel trachea (3VT) and sagittal views. In addition, we calculated the carotid-subclavian artery index (CSAI), defined as the ratio of the aortic arch diameter at the left subclavian artery, to the distance between the left carotid artery and the left subclavian artery [17].
Moreover, we examined anatomy and shunt direction through the interatrial communication and aortic arch. Morphological and functional features of the ventricles and aortic arch as well as the presence of associated minor CHDs were noted.
In addition, we validated the most important study results prospectively in 16 fetuses with prenatally suspected CoA born in 2019.
Post Partum Approach if CoA is Suspected in Fetal Life
Newborns with prenatally suspected CoA are delivered vaginally in our tertiary center with unimpeded access to cardiac surgery if needed. In the majority of cases, infants are monitored post partum for the development of coarctation without starting prostaglandins. When developing CoA is confirmed clinically and echocardiographically, prostaglandin infusion is started and maintained until surgical repair. In isolated cases, when there is marked hypoplasia of the isthmus and/or aortic arch, we refrain from a trial-off prostaglandin.
Statistics
Data are presented as median (inter-quartile range) or mean (standard deviation). Group-wise comparisons of fetuses with and without postnatal CoA were performed using the Chi2- or Fisher´s Exact test for categorical variables and Mann–Whitney-U-test for continuous variables. T-test for related samples was used when comparing different Z-score datasets. Logistic regression analyses were performed using postnatal development of CoA as the dependent variable and quantitative echocardiographic measures as independent variables. Odds ratios (95% confidence interval) are provided. Receiver operating characteristic (ROC) curves and area under the curve (AUC) were calculated for variables significantly associated with the postnatal development of CoA. Cut-off points with corresponding sensitivity and specificity are provided. Study data were collected and managed using REDCap electronic data capture tools hosted at Lund University [18, 19]. Statistical analyses were performed using Statistical Package for Social Sciences, version 25 (IBM SPSS, Chicago, IL).
Results
Seventy-seven fetuses with prenatally suspected CoA born between 2010 and 2018 were identified. There was no intrauterine death. Twelve cases were excluded due to technically inadequate documented sagittal clips of the aortic arch. Of the remaining 65 fetuses, 22 (34%) developed CoA postnatally, whereof 4 underwent univentricular palliation due to associated mitral and/or aortic valve pathologies. All patients with postnatally confirmed CoA or univentricular circulation were operated in the neonatal period: 4 with end-to-end anastomosis, 11 with end-to-side anastomosis, 3 with arch reconstruction, and 4 with stage I Norwood palliation.
The most common indication for the initial fetal echocardiogram was an abnormal second or third trimester obstetrical screening ultrasound (n = 60), usually due to a disproportion of the ventricles or great vessels. Other indications included an abnormal first trimester screening (n = 2), maternal diabetes (n = 5), fetal arrhythmias (n = 2), or family history of CHD (n = 2). Forty-five fetuses (69.2%) had a high suspicion for CoA in the final echocardiogram prior to delivery according to the evaluating fetal cardiologist.
The mean gestational age at the time of the fetal echocardiogram was 34.1 weeks (range 26.3–39.3). Forty-four fetuses (68%) exhibited a moderately underdeveloped left ventricle (borderline left ventricle), defined as Z-scores for width of −2 to −4 and with antegrade flow across the mitral and aortic valve. Associated cardiac anomalies included a hypoplastic aortic arch based on qualitative assessment (n = 22), LSVC (n = 7), mild MS (n = 1) or AS (n = 2), and VSD (n = 10). Three fetuses had associated extracardiac anomalies, and one had a genetic anomaly (Down`s syndrome).
Qualitative Fetal Echocardiographic Assessment
A bidirectional or retrograde flow in the aortic arch (p < 0.001) or bidirectional or left–right shunt across the interatrial communication (p = 0.002) was linked to postnatal CoA (Table 1). Furthermore, postnatal CoA was significantly associated with qualitative assessment of a borderline hypoplastic left ventricle, hypoplastic aortic arch, posterior shelf, and VSDs detected on the fetal echocardiogram (Table 1).
Table 1.
Categorical parameters in fetuses with and without postnatal CoA
| Categorical parameters | Postnatal CoA n/N (%) | No postnatal CoA n/N (%) | P value |
|---|---|---|---|
| Interatrial shunt direction | |||
| Right-left | 2/17 (11.8) | 22/34 (64.7) | 0.002 |
| Left-right | 1/17 (5.9) | 0/34 (0) | |
| Bidirectional | 13/17 (76.5) | 12/34 (35.3) | |
| No shunt | 1/17 (5.9) | 0/34 (0) | |
| Flow direction aortic arch | |||
| Antegrade | 6/20 (30) | 32/43 (74.4) | < 0.001 |
| Bidirectional | 9/20 (45) | 11/43 (25.6) | |
| Retrograde | 5/20 (25) | 0/43 (0) | |
| Borderline left ventricle | 21/22 (95.5) | 23/43 (53.5) | 0.001 |
| Hypoplastic aortic arch | 20/22 (90.9) | 2/43 (4.7) | < 0.001 |
| Posterior Shelf | 14/15 (93) | 12/39 (30.8) | < 0.001 |
| Left superior vena cava | 4/22 (18.2) | 3/43 (7) | 0.17 |
| Ventricular septal defect | 8/22 (36.4) | 2/43 (4.7) | 0.002 |
CoA coarctation, n number of patients for given variable, N total number of patients
N = 65, for interatrial shunt direction = 51; for flow direction aortic = 63; for posterior shelf = 54
Quantitative Fetal Echocardiographic Measurements
Fetuses with a postnatally confirmed CoA exhibited significantly smaller left cardiac structures from the mitral valve to the aortic isthmus when adjusted for gestational age (Table 2). Notably, both groups had median Z-scores below average, i.e., Z-score < 0. Tricuspid and pulmonary valve Z-scores as well as right ventricular length and arterial duct Z-scores were not significantly different between the groups. Of the right cardiac structures, only right ventricular width was significantly larger in fetuses with postnatally confirmed CoA. Lastly, all ratios between left and right cardiac structures, except for LV/RV length ratio, were significantly lower in fetuses with postnatally confirmed CoA (Table 2).
Table 2.
Z-scores and ratios of continuous fetal echocardiographic parameters
| Continuous parameters | Postnatal CoA median (IQR) |
No postnatal CoA median (IQR) |
P value |
|
|---|---|---|---|---|
| Left heart structures (Z-score sets) | ||||
| Mital valve annulus | Krishnan et al. [14] | –3.35 (–4.68 to –2.20) | –1.05 (–1.80 to –0.17) | < 0.001 |
| Schneider et al. [16] | –4.00 (–5.43 to –3.07) | –1.85 (–2.63 to –1.00) | < 0.001 | |
| Left ventricular length | Krishnan et al. [14] | –3.95 (–4.78 to –2.69) | –3.00 (–3.60 to –2.50) | 0.009 |
| Schneider et al. [16] | –1.70 (–2.30 to –0.65) | –0.90 (–1.20 to –0.40) | 0.002 | |
| Left ventricular width | Gabbay–Benziv et al. [13] | –2.25 (–3.40 to –1.87) | –0.80 (–1.45 to –0.45) | < 0.001 |
| Schneider et al. [16] | –1.70 (–2.70 to –1.20) | –0.60 (–1.10 to– 0.00) | < 0.001 | |
| Aortic valve annulus | Krishnan et al. [14] | –2.55 (–2.95 to –1.00) | –0.06 (–0.70 to– 0.60) | < 0.001 |
| Schneider et al. [16] | –2.75 (–3.13 to –1.53) | –0.70 (–1.20 to –0.10) | < 0.001 | |
| Ascending aorta | Krishnan et al. [14] | –3.10 (–4.45 to –1.95) | –1.50 (–1.90 to –0.75) | < 0.001 |
| Schneider et al. [16] | –3.60 (–4.45 to –2.15) | –1.40 (–1.90 to–0.90) | < 0.001 | |
| Isthmus aortae (sag.) | Krishnan et al. [14] | –4.70 (–6.4 to –3.95) | –2.60 (–3.70 to –1.40) | < 0.001 |
| Pasquini et al. [15] | –2.40 (–4.15 to –1.85) | –1.00 (–1.80 to –0.50) | < 0.001 | |
| Isthmus aortae (3VT) | Pasquini et al. [15] | –3.00 (–4.05 to –2.70) | –1.70 (–2.40 to –0.68) | < 0.001 |
| Descending aorta | Krishnan et al. [14] | –1.05 (–1.47 to –0.63) | –0.90 (–1.40 to –0.52) | 0.26 |
| Schneider et al. [16] | –0.35 (–0.78–0.28) | –0.03 (–0.60–0.35) | 0.22 | |
| Right heart structures (Z-score sets) | ||||
| Tricuspid valve annulus | Krishnan et al. [14] | 0.30 (–0.70–0.85) | 0.50 (–0.12–0.90) | 0.34 |
| Schneider et al. [16] | –0.40 (–1.13–0.33) | –0.10 (–0.80–0.43) | 0.33 | |
| Right ventricular length | Krishnan et al. [14] | –2.40 (–3.35 to –1.75) | –1.90 (–2.90 to –1.20) | 0.11 |
| Schneider et al. [16] | –0.70 (–1.33 to –0.08) | –0.20 (–0.90–0.25) | 0.06 | |
| Right ventricular width | Gabbay–Benziv et al. [13] | 0.95 (0.00–2.12) | 0.08 (–0.35–1.00) | 0.02 |
| Schneider et al. [16] | 0.99 (0.15–1.85) | 0.19 (–0.23–1.00) | 0.02 | |
| Pulmonary valve annulus | Krishnan et al. [14] | 1.40 (0.50–2.60) | 1.70 (1.00–2.55) | 0.79 |
| Schneider et al. [16] | 0.60 (–0.05–1.90) | 1.00 (0.25–1.90) | 0.74 | |
| Arterial duct (sag.) | Krishnan et al. [14] | –1.30 (–2.24– 0.10) | –2.30 (–2.70 to –1.50) | 0.05 |
| Schneider et al. [16] | 1.15 (1.00– 1.92) | 1.00 (0.50–1.50) | 0.07 | |
| Arterial duct (3VT) | Pasquini et al. [15] | 1.00 (0.07–1.58) | 0.25 (–0.60–1.10) | 0.12 |
| Ratios | ||||
| Left/right ventricular length | 0.96 (0.85–1.04) | 1.00 (0.92–1.11) | 0.13 | |
| Left/right ventricular width | 0.60 (0.52–0.72) | 0.82 (0.73–0.88) | < 0.001 | |
| Mitral/tricuspid valve annulus | 0.55 (0.47–0.52) | 0.73 (0.67–0.82) | < 0.001 | |
| Aortic/pulmonary valve annulus | 0.54 (0.46–0.59) | 0.66 (0.60–0.71) | < 0.001 | |
| Carotid- subclavian artery index | 0.51 (0.43–0.58) | 1.35 (1.05–1.88) | < 0.001 | |
| Ascending/descending aorta | 0.84 (0.61–0.94) | 0.96 (0.92–1.01) | < 0.001 | |
| Isthmus/arterial duct (sag.) | 0.43 (0.38–0.54) | 0.66 (0.59–0.73) | < 0.001 | |
| Isthmus/arterial duct (3VT) | 0.48 (0.43–0.53) | 0.67 (0.61–0.75) | < 0.001 | |
CoA: coarctation, 3VT view: three-vessel trachea view; sag. sagittal view
Measurements of the arterial duct in sagittal (n = 55) or 3VT view (n = 57). All other cardiac structures could be measured in 62 or more cases
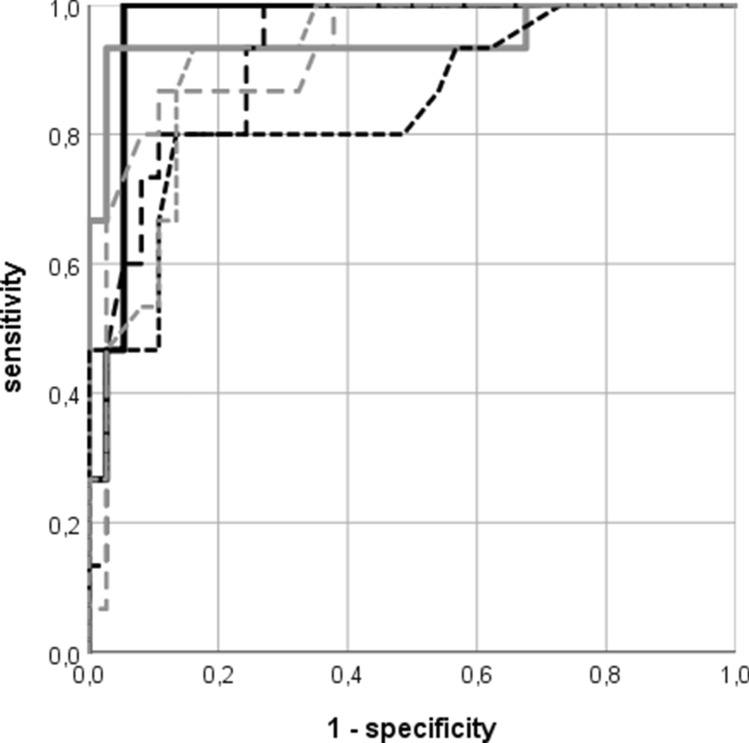
For parameters that were significantly different in the group-wise comparison, logistic regression analyses and receiver operation characteristics were carried out (Table 3). Due to the high number of parameters significantly associated with postnatal CoA, we present data on specificity and sensitivity as well as suggested cut-off points only for variables with an area under the curve of at least 0.90 (Table 4). The parameter with the highest sensitivity and specificity was the CSAI under 0.78 with 92.3% and 96.8%, respectively. Alternatively, combining the I/D ratio in the 3VT view with the MV/TV ratio in the four-chamber view allows for comparable, if not superior sensitivities and specificities (Table 4, Fig. 1). Both ratios were measureable in the majority of fetuses (n = 53 of 65, 81.5%).
Table 3.
Odds ratios and area under the curve for significant Z-scores and ratios
| Continuous parameters | Postnatal CoA | ||||
|---|---|---|---|---|---|
| OR (95% CI) | P (OR) | AUC (95% CI) | P (AUC) | ||
| Left heart structures (Z-score sets) | |||||
| Mitral valve annulus | Krishnan et al. [14] | 0.16 (0.06–0.44) | < 0.001 | 0.94 (0.89–0.99) | < 0.001 |
| Schneider et al. [16] | 0.39 (0.23–0.65) | < 0.001 | 0.88 (0.77–0.99) | < 0.001 | |
| Left ventricular length | Krishnan et al. [14] | 0.49 (0.30–0.82) | 0.006 | 0.68 (0.53–0.83) | 0.019 |
| Schneider et al. [16] | 0.36 (0.18–0.70) | 0.003 | 0.72 (0.58–0.87) | 0.004 | |
| Left ventricular width | Gabbay-Benziv et al. [13] | 0.26 (0.13–0.51) | < 0.001 | 0.82 (0.67–0.97) | 0.001 |
| Schneider et al. [16] | 0.27 (0.14–0.55) | < 0.001 | 0.78 (0.62–0.94) | 0.004 | |
| Aortic valve annulus | Krishnan et al. [14] | 0.28 (0.14–0.53) | < 0.001 | 0.79 (0.60–0.97) | 0.003 |
| Schneider et al. [16] | 0.22 (0.10–0.47) | < 0.001 | 0.78 (0.59–0.97) | 0.004 | |
| Ascending aorta | Krishnan et al. [14] | 0.28 (0.14–0.55) | < 0.001 | 0.82 (0.69–0.95) | < 0.001 |
| Schneider et al. [16] | 0.21 (0.09–0.47) | < 0.001 | 0.85 (0.72–0.97) | < 0.001 | |
| Isthmus aortae (sag.) | Krishnan et al. [14] | 0.45 (0.29–0.68) | < 0.001 | 0.81 (0.67–0.95) | 0.001 |
| Pasquini et al. [15] | 0.25 (0.12–0.52) | < 0.001 | 0.85 (0.73–0.97) | < 0.001 | |
| Isthmus aortae (3VT) | Pasquini et al. [15] | 0.21 (0.09–0.52) | 0.001 | 0.80 (0.66–0.96) | 0.001 |
| Right heart structures Z-score sets | |||||
| Right ventricular width | Gabbay-Benziv et al. [13] | 1.72 (1.07–2.75) | 0.024 | 0.68 (0.53–0.82) | 0.019 |
| Schneider et al. [16] | 1.93 (1.09–3.40) | 0.025 | 0.68 (0.53–0.82) | 0.016 | |
| Ratios | |||||
| Left/right ventricular width | 1 ×10−6(1.2 × 10–9−0.001) | < 0.001 | 0.89 (0.79–0.99) | < 0.001 | |
| Mitral/tricuspid valve annulus | 1.08 ×10-8(9.4 × 10−13-1.2 ×10−4) | < 0.001 | 0.90 (0.8–0.99) | < 0.001 | |
| Aortic/pulmonary valve annulus | 3.1×10−10(2.6×10−15–(2.6×10−153.8×10−5) | < 0.001 | 0.79 (0.66–0.93) | 0.002 | |
| Ascending/descending aorta | 1.3×10−(2 × 10−8−0.009) | 0.001 | 0.69 (0.52–0.86) | 0.05 | |
| Carotid-subclavian artery index | 0.001 (3.2 ×10−5–0.034) | < 0.001 | 0.94 (0.85–1) | < 0.001 | |
| Isthmus/arterial duct (sag.) | 4×10−7(2×10−10−0.001) | < 0.001 | 0.90 (0.81–0.99) | < 0.001 | |
| Isthmus/arterial duct (3VT) | 4×10−9(1.3×10−13–1.2×10−4) | < 0.001 | 0.93 (0.85–1) | < 0.001 | |
OR (95% CI): Odds ratio with 95% confidence interval, AUC (95% CI): area under the curve with 95% confidence interval, sag. sagittal view, 3VT: three-vessel trachea view
Table 4.
Cut-off points, sensitivity, and specificity for highly significant continuous parameters
| Continuous parameters | Cut-off points | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Left heart structures (Z-score sets) | |||
| Mitral valve annulus: Krishnan et al. [14] | –2.03 | 90.5 | 88.1 |
| Ratios | |||
| Mitral/tricuspid valve annulus | 0.61 | 84.6 | 87.1 |
| Carotid-subclavian artery index | 0.78 | 92.3 | 96.8 |
| Isthmus/arterial duct (sag.) | 0.59 | 92.3 | 77.4 |
| Isthmus/arterial duct (3VT) | 0.57 | 84.6 | 90 |
| I/D3VTxMV/TV | 0.37 | 100 | 94.6 |
I/D3VTxMV/TV: product of isthmus/arterial duct in the three-vessel trachea view and mitral/tricuspid valve in the four-chamber view
Fig. 1.
Receiver operation characteristic (ROC) curve for selected fetal echocardiographic variables using postnatal development of aortic coarctation as the outcome: black continuous line: product of isthmus/arterial duct in the three-vessel trachea view and mitral/tricuspid valve in the four-chamber view (I/D3VTxMV/TV); gray continuous line: carotid-subclavian artery index; black dashed line: isthmus-ductus ratio in the three-vessel trachea view; gray dashed line: mitral/tricuspid valve annulus index; black dotted line: isthmus Z-score in the three-vessel trachea view; gray dotted line: mitral valve annulus Z-score (Krishnan et al. [14])
In a logistic regression model including both the I/D3VTxMV/TV and the CSAI, no interaction was noted (p = 0.6).
Prospective Validation
Furthermore, we validated the CSAI and I/D3VTxMV/TV indices prospectively in 16 fetuses with prenatal suspicion of CoA born in 2019, four of which developed CoA postnatally. A CSAI < 0.78 detected postnatal development of CoA with a sensitivity of 100% and a specificity of 91.7%. The I/D3VTxMV/TV < 0.37 exhibited both a 100% sensitivity and specificity.
Variability Between Z-Score Datasets
Lastly, we compared the reliability of different Z-score datasets on 12 parameters from the current study (Table 5). Significant differences between Z-score datasets were detected for right and left cardiac structures with mean differences up to 2.91 Z-scores for the same measurement (Table 5). Intraclass correlations coefficients comparing different Z-score datasets though were consistently above 0.93 (p < 0.001) except for arterial duct dimensions from the sagittal view (0.89, p < 0.001).
Table 5.
Differences of Z-score sets for cardiac structures
| Z-score sets | Mean difference (SD) | P value |
|---|---|---|
| Left heart structures | ||
| Mitral valve annulus (Krishnan-Schneider) | 0.68 (0.49–0.87) | < 0.001 |
| Left ventricular length (Krishnan-Schneider) | –2.20 (–2.29 to –2.11) | < 0.001 |
| Left ventricular width (Gabbay-Benziv-Schneider) | –0.42 (–0.49 to –0.36) | < 0.001 |
| Aortic valve annulus (Krishnan-Schneider) | 0.53 (0.46–0.59) | < 0.001 |
| Ascending aorta (Krishnan-Schneider) | 0.05 (–0.02–0.13) | 0.17 |
| Isthmus aortae (sag.) (Krishnan-Pasquini) | –1.59 (–1.83 to –1.36) | < 0.001 |
| Descending aorta (Krishnan-Schneider) | –0.78 (–0.82 to –0.74) | < 0.001 |
| Right heart structures | ||
| Tricuspid valve annulus (Krishnan-Schneider) | 0.57 (0.51–0.62) | < 0.001 |
| Right ventricular length (Krishnan-Schneider) | –1.73 (–1.83 to –1.63) | < 0.001 |
| Right ventricular width (Gabbay-Benziv-Schneider) | –0.003 (–0.06- 0.06) | 0.92 |
| Pulmonary valve annulus (Krishnan-Schneider) | 0.68 (0.65–0.72) | < 0.001 |
| Ductus arteriosus (sag.) (Krishnan-Schneider) | –2.91 (–3.10 to –2.71) | < 0.001 |
SD: standard deviation
Discussion
Fetal prediction of postnatal CoA continues to be a challenge. We have demonstrated in this retrospective study of fetuses with suspected CoA that postnatal CoA can be predicted with a high degree of accuracy using indices with the fetus as the internal control. Specifically, those were the CSAI and a combined score of I/D in the 3VT view and MV/TV ratio in the four-chamber view. The use of Z-scores—using normal fetuses as controls—was limited by its lower predictive value, and potential differences related to “normal” cohorts and/or observers.
Z-Scores
Our study showed that fetuses with postnatally confirmed CoA exhibited significantly smaller left cardiac structures and diameters of the isthmic region normalized for gestational age. The best Z-scores for prediction of postnatal CoA were those of the MV-annulus, ascending aorta, and isthmus aortae in the sagittal or 3VT views, which have been described in other studies [6, 20–24]. Most studies to date have used Z-scores from Pasquini et al. for isthmic or ductal diameters and Schneider et al. for intracardiac structures or the ascending aorta, often with a pre-defined cut-off point of < −2 [15, 16]. The cut-off points calculated in our study are deviated for most anatomic structures from −2. Furthermore, we only included fetuses with prenatally suspected CoA in our study, which likely explains why left cardiac structures generally had Z-scores < 0 (−0.6 to −3) even in fetuses who did not develop postnatal CoA.
Ratios
Various ratios of the left-to-right cardiac structures have been reported as sensitive indicators for postnatal development of CoA [6, 25–27]. In our study, the best predictors for postnatal CoA were the aortic-arch-related ratios (CSAI and I/D ratios) and MV/TV ratio.
Our study showed that the application of the sagittal arch view for depiction of the aortic and ductal arch is important for risk stratification of fetuses with prenatal suspicion of CoA. The CSAI had the highest sensitivity and specificity for detection of postnatal CoA (Table 4). The CSAI has previously been described to predict the development of CoA in newborns [17]. Further, two fetal studies have suggested that CSAI is a reliable predictor for postnatal CoA [23, 28]. However, both studies have smaller sample sizes and did not specify cut-off points with corresponding sensitivity and specificity, making it difficult to guide risk stratification for postnatal development of CoA in those fetuses. The current study is the first to demonstrate superiority of CSAI over Z-scores and other ratios in isolation for prediction of postnatal CoA.
The I/D ratio, which has previously been associated with the development of postnatal CoA, had a lower sensitivity and specificity compared to CSAI [5, 6, 24, 29].
CSAI can only be demonstrated from a sagittal view, which can be difficult to obtain due to fetal position or shadowing of the echogenic spine late in gestation. We therefore sought to combine measurements that do not require a sagittal aortic arch view to predict postnatal CoA. In fact, combining I/D ratio from the 3VT view with the MV/TV ratio in the four-chamber view, is an excellent alternative for prenatal prediction of CoA. The I/D3VTxMV/TV might be slightly superior to the CSAI, a finding we even could validate prospectively in fetuses with suspected CoA born in 2019. Its high accuracy might derive from combining both ventricular and isthmic-to ductal discrepancies in only one index. Because the I/D3VTxMV/TV index is technically easier obtained in pregnant women with suboptimal acoustic windows, this might reduce the frequency of clinical consultations and in its turn healthcare costs and anxiety of prospective parents.
Overall, ratios predicted postnatal CoA more reliably than Z-scores. We believe that using the individual fetuses cardiac structure as an internal control has the advantage of eliminating a) potential confounders related to comparison with “normal” cohorts (cardiac Z-scores are based on gestational age, not actual somatic size) and/or b) observer related subtle differences in measurement technique (ratios have the individual observers measurements as an internal control; Z-scores are based on measurements by other observers).
Qualitative variables have previously been associated with CoA and were not the focus of this study. Borderline left ventricular hypoplasia, a hypoplastic aortic arch, or posterior shelf are known predictors for postnatal CoA [6, 20, 29, 30]. We observed similar findings in our study. In the clinical setting, however, subjective criteria may generally be subject to higher inter-observer variability than quantitative data.
In addition, the association between the flow direction across the atrial septum or in the aortic arch and postnatal CoA has been reported [22, 23]. However, in our study, they had a relatively low specificity of 65–75%, leading in some cases to the misjudgment by the fetal cardiologist that there might exist high risk for postnatal CoA or even univentricular outcome, if the flow direction was bidirectional or left–right at the atrial level or bidirectional in the aortic arch.
A prenatally detected VSD in association with a suspicion for CoA was associated with postnatal CoA with a high specificity (90.7%) but a low sensitivity (36.4%). While the presence of a VSD in the setting of a suspected CoA is a red flag, its absence should not provide false reassurance [30].
Differences Between Z-Scores
Perhaps most importantly clinically, we demonstrate significant differences between Z-scores based on Krishnan et al. [14] vs. Schneider et al. [16] or Pasquini et al. [15] and Gabbay-Benziv et al. [13] (Tables 2, 5) [13–16]. This was particularly evident for right and left ventricular length as well as sagittal isthmus aortae and arterial duct dimension Z-scores. Overall, the number of fetuses in each Z-score dataset is rather limited. The large volume of up to 414 fetuses has been published by Krishnan et al. [14]. We suggest that larger multi-center fetal echocardiographic data need to be analyzed in order to generate more reliable Z-score datasets. Meanwhile, for the individual patient, we recommend comparing the results of different Z-score datasets and interpreting them with caution in the context of the qualitative impression by the experienced fetal echocardiographer.
Limitations
This study has several limitations including that it is a retrospective single-center study of small to moderate size with some missing data points (Table 1). The single fetal echocardiographer performing the measurements (K.F.) had clinical contact with some of the patients, so that bias could not be excluded. However, we were able to prospectively validate the findings using a completely blinded approach.
Moreover, we included fetal echocardiograms at later gestational age than the usual screening echocardiogram (18–24 weeks of gestation). Prospective multicenter validation of our findings, even in younger fetuses, is necessary prior to implementing our findings into clinical practice. In the future, we may test those indices even in fetuses with suspicion of CoA in more complex cases of CHD.
Conclusion
It may be possible to predict postnatal CoA in third trimester fetuses with a prenatal suspicion with high accuracy. In the study presented herein, ratios were more reliable indicators than Z-scores, whereof the CSAI or alternatively a combination of the isthmus aortae-to-arterial duct ratio in the 3VT view with the mitral-to-tricuspid valve ratio was most predictive. The I/D3VTxMV/TV index is a new index that might be superior to the CSAI when sagittal arch views are difficult to obtain. Its high accuracy might derive from combining both ventricular and isthmic-ductal discrepancies in one index. In addition, using the available fetal Z-score datasets, significant and clinically unacceptable differences in Z-scores were observed for the same measurements. This calls for a large multi-center collaboration to generate reliable fetal echocardiographic Z-scores.
Acknowledgements
We thank our fetal cardiac team consisting of specialized obstetricians, fetal cardiologist, contact nurses and specialized midwifes for a superb collaboration, which guarantees the best care of our patients and made this study possible.
Funding
Open access funding provided by Lund University. This study was supported by the Swedish Heart-Lung Foundation (#20170397; C.G.W.) and Avtal om Läkarutbildning och Forskning (ALF; C.G.W.).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenthal E. Coarctation of the aorta from fetus to adult: curable condition or life long disease process? Heart. 2005;91:1495–1502. doi: 10.1136/hrt.2004.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farag ES, Kluin J, de Heer F, Ahmed Y, Sojak V, Koolbergen DR, Blom NA, de Mol B, Ten Harkel ADJ, Hazekamp MG. Aortic coarctation repair through left thoracotomy: results in the modern era. Eur J Cardiothorac Surg. 2019;55:331–337. doi: 10.1093/ejcts/ezy241. [DOI] [PubMed] [Google Scholar]
- 3.Holland BJ, Myers JA, Woods CR., Jr Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis. Ultrasound Obstet Gynecol. 2015;45:631–638. doi: 10.1002/uog.14882. [DOI] [PubMed] [Google Scholar]
- 4.Anuwutnavin S, Satou G, Chang RK, DeVore GR, Abuel A, Sklansky M. Prenatal sonographic predictors of neonatal coarctation of the aorta. J Ultrasound Med. 2016;35:2353–2364. doi: 10.7863/ultra.15.06049. [DOI] [PubMed] [Google Scholar]
- 5.Beattie M, Peyvandi S, Ganesan S, Moon-Grady A. Toward improving the fetal diagnosis of coarctation of the aorta. Pediatr Cardiol. 2017;38:344–352. doi: 10.1007/s00246-016-1520-6. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Montes E, Herraiz I, Gomez-Arriaga PI, Escribano D, Mendoza A, Galindo A. Gestational age-specific scoring systems for the prediction of coarctation of the aorta. Prenat Diagn. 2014;34:1198–1206. doi: 10.1002/pd.4452. [DOI] [PubMed] [Google Scholar]
- 7.van Nisselrooij AEL, Rozendaal L, Linskens IH, Clur SA, Hruda J, Pajkrt E, van Velzen CL, Blom NA, Haak MC. Postnatal outcome of fetal isolated ventricular size disproportion in the absence of aortic coarctation. Ultrasound Obstet Gynecol. 2018;52:593–598. doi: 10.1002/uog.17543. [DOI] [PubMed] [Google Scholar]
- 8.Evers PD, Ranade D, Lewin M, Arya B. Diagnostic approach in fetal coarctation of the aorta: a cost-utility analysis. J Am Soc Echocardiogr. 2017 doi: 10.1016/j.echo.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Lytzen R, Vejlstrup N, Bjerre J, Petersen OB, Leenskjold S, Dodd JK, Jorgensen FS, Sondergaard L. Live-born major congenital heart disease in Denmark: incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiol. 2018;3:829–837. doi: 10.1001/jamacardio.2018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordenstam F, Sonesson SE 2017 [Fetal Cardiology. Experiences from a tertiary referral centre in Stockholm, Sweden]. Lakartidningen 114:ELZU [PubMed]
- 11.de Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganas L, Eriksson M, Segerdahl N, Agren A, Ekman-Joelsson BM, Sunnegardh J, Verdicchio M, Ostman Smith I. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley JL, Soni NB, Wilson D, Sen S. Effectiveness of pulse-oximetry in addition to routine neonatal examination in detection of congenital heart disease in asymptomatic newborns. J Matern Fetal Neonatal Med. 2015;28:1736–1739. doi: 10.3109/14767058.2014.967674. [DOI] [PubMed] [Google Scholar]
- 13.Gabbay-Benziv R, Turan OM, Harman C, Turan S. Nomograms for fetal cardiac ventricular width and right-to-left ventricular ratio. J Ultrasound Med. 2015;34:2049–2055. doi: 10.7863/ultra.14.10022. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan A, Pike JI, McCarter R, Fulgium AL, Wilson E, Donofrio MT, Sable CA. Predictive models for normal fetal cardiac structures. J Am Soc Echocardiogr. 2016;29:1197–1206. doi: 10.1016/j.echo.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Pasquini L, Mellander M, Seale A, Matsui H, Roughton M, Ho SY, Gardiner HM. Z-scores of the fetal aortic isthmus and duct: an aid to assessing arch hypoplasia. Ultrasound Obstet Gynecol. 2007;29:628–633. doi: 10.1002/uog.4021. [DOI] [PubMed] [Google Scholar]
- 16.Schneider C, McCrindle BW, Carvalho JS, Hornberger LK, McCarthy KP, Daubeney PE. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. 2005;26:599–605. doi: 10.1002/uog.2597. [DOI] [PubMed] [Google Scholar]
- 17.Dodge-Khatami A, Ott S, Di Bernardo S, Berger F. Carotid-subclavian artery index: new echocardiographic index to detect coarctation in neonates and infants. Ann Thorac Surg. 2005;80:1652–1657. doi: 10.1016/j.athoracsur.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arya B, Bhat A, Vernon M, Conwell J, Lewin M. Utility of novel fetal echocardiographic morphometric measures of the aortic arch in the diagnosis of neonatal coarctation of the aorta. Prenat Diagn. 2016;36:127–134. doi: 10.1002/pd.4753. [DOI] [PubMed] [Google Scholar]
- 21.Marginean C, Marginean CO, Muntean I, Toganel R, Voidazan S, Gozar L. The role of ventricular disproportion, aortic, and ductal isthmus ultrasound measurements for the diagnosis of fetal aortic coarctation, in the third trimester of pregnancy. Med Ultrason. 2015;17:475–481. doi: 10.11152/mu.2013.2066.174.rvd. [DOI] [PubMed] [Google Scholar]
- 22.Morgan CT, Mueller B, Thakur V, Guerra V, Jull C, Mertens L, Friedberg M, Golding F, Seed M, Miner SES, Jaeggi ET, Manlhiot C, Nield LE. Improving prenatal diagnosis of coarctation of the aorta. Can J Cardiol. 2019;35:453–461. doi: 10.1016/j.cjca.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Sivanandam S, Nyholm J, Wey A, Bass JL. Right ventricular enlargement in utero: is It coarctation? Pediatr Cardiol. 2015;36:1376–1381. doi: 10.1007/s00246-015-1168-7. [DOI] [PubMed] [Google Scholar]
- 24.Toole BJ, Schlosser B, McCracken CE, Stauffer N, Border WL, Sachdeva R. Importance of relationship between ductus and isthmus in fetal diagnosis of coarctation of aorta. Echocardiography. 2016;33:771–777. doi: 10.1111/echo.13140. [DOI] [PubMed] [Google Scholar]
- 25.Hornberger LK, Sahn DJ, Kleinman CS, Copel J, Silverman NH. Antenatal diagnosis of coarctation of the aorta: a multicenter experience. J Am Coll Cardiol. 1994;23:417–423. doi: 10.1016/0735-1097(94)90429-4. [DOI] [PubMed] [Google Scholar]
- 26.Quartermain MD, Cohen MS, Dominguez TE, Tian Z, Donaghue DD, Rychik J. Left ventricle to right ventricle size discrepancy in the fetus: the presence of critical congenital heart disease can be reliably predicted. J Am Soc Echocardiogr. 2009;22:1296–1301. doi: 10.1016/j.echo.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Slodki M, Rychik J, Moszura T, Janiak K, Respondek-Liberska M. Measurement of the great vessels in the mediastinum could help distinguish true from false-positive coarctation of the aorta in the third trimester. J Ultrasound Med. 2009;28:1313–1317. doi: 10.7863/jum.2009.28.10.1313. [DOI] [PubMed] [Google Scholar]
- 28.Patel C, Weeks B, Copel J, Fahey J, Song X, Shabanova V, Ferdman DJ. Fetal echocardiographic measures to improve the prenatal diagnosis of coarctation of the aorta. Pediatr Cardiol. 2018 doi: 10.1007/s00246-018-2040-3. [DOI] [PubMed] [Google Scholar]
- 29.Matsui H, Mellander M, Roughton M, Jicinska H, Gardiner HM. Morphological and physiological predictors of fetal aortic coarctation. Circulation. 2008;118:1793–1801. doi: 10.1161/CIRCULATIONAHA.108.787598. [DOI] [PubMed] [Google Scholar]
- 30.Familiari A, Morlando M, Khalil A, Sonesson SE, Scala C, Rizzo G, Del Sordo G, Vassallo C, Elena Flacco M, Manzoli L, Lanzone A, Scambia G, Acharya G, D'Antonio F. Risk factors for coarctation of the aorta on prenatal ultrasound: a systematic review and meta-analysis. Circulation. 2017;135:772–785. doi: 10.1161/CIRCULATIONAHA.116.024068. [DOI] [PubMed] [Google Scholar]