Abstract
Sinocarum is a Sino-Himalayan endemic genus of Apiaceae and distributed in high-elevations from Nepal to SW China. In this study, morphological characteristics were combined with nuclear internal transcribed spacer (ITS) and two chloroplast DNA (cpDNA) intron sequences (rpl16 and rps16) to determine the phylogenetic placement of Sinocarum and the infrageneric relationships between five Sinocarum species. The results confirmed that Sinocarum was a polyphyletic group separated into two clades, Acronema and East Asia clades. S. coloratum, the generic type of Sinocarum, S. cruciatum, S. vaginatum and S. filicinum are in the Acronema clade. Among them, the first three species are clustered into a subclade and are closely related to the genus Acronema. While S. filicinum has a close affinity with Meeboldia. S. schizopetalum did not ally with its congeners we collected and is allied closely with members of the distantly related East Asia clade. In addition, the fruit of the Acronema clade Sinocarum species is usually oblong-ovoid or ovoid, and the pollen is super-rectangular, while the Sinocarum species in the East Asia clade have broad-ovoid fruit and sub-rhomboidal pollen. This study has furnished cumulative evidence to reduce phylogenetic uncertainty and provide a more comprehensive description of the plant morphology, fruit morphology and anatomy, and pollen morphology of these five Chinese Sinocarum species.
Keywords: Apiaceae , morphology, phylogeny, Sinocarum
Introduction
Sinocarum H. Wolff ex R. H. Shan & F. T. Pu (1980: 374) was transferred from the genus Carum L. (1753: 263) by Wolff (1927), but formally described by Shan and Pu (1980). Sinocarum encompasses about 20 species, with eight species (four endemic) in China and is distributed at high-elevation in the Sino-Himalayan region from Nepal to SW China (Pu et al. 2005). It is usually classified by a suite of characteristics: elongate rhizome, expanded petiole sheaths, obtuse at apex and clawed at base petals and oblong-ovoid fruit (Shan et al. 1980; Pu et al. 2005). Despite several easily recognizable characteristics, there remains morphological and taxonomic confusion in the genus, including the lack of morphological description and specimens of mature fruit, unclear intergeneric boundary and excessive use of synonyms. Sinocarum is a taxonomically complex genus that is closely related to Acronema Falcon. ex Edgew. (1846: 51) and sometimes difficult to distinguish (Pu et al. 2005). Thus, further work and more extensive specimen collections are needed to clarify the situation.
Palynological study of Sinocarum mainly focused on seven species, these being S. coloratum (Diels) H. Wolff ex R. H. Shan & F. T. Pu (1985: 33), S. cruciatum (Franch.) H. Wolff ex R. H. Shan & F. T. Pu (1985: 33), S. dolichopodum (Diels) H. Wolff ex R. H. Shan & F. T. Pu (1985: 38), S. filicinum H. Wolff (1929: 182), S. pauciradiatum R. H. Shan & F. T. Pu (1980: 374), S. schizopetalum (Franch.) H. Wolff ex R. H. Shan & F. T. Pu (1985: 33) and S. vaginatum H. Wolff (1929: 183). The seven species were observed through light microscope (LM) and scanning electron microscope (SEM) (Wang 1998; Shu and She 2001). The study indicated that the equatorial view of Sinocarum pollen was usually broad-ellipsoidal, and the equatorial exine was rugulate (Shu and She 2001).
Previous studies have shown that fruit characteristics play a key role in the classification of subfamily Apioideae (Kljuykov et al. 2004; Lyskov et al. 2017; Guo et al. 2018; Jia et al. 2019). The fruit characteristics of Sinocarum species are described in Flora Reipublicae Popularis Sinicae and Flora of China. The fruit of Sinocarum is oblong-ovoid with 5 filiform ribs, but only young fruit is involved, and the mature fruit is unknown (Shan and Pu 1985; Pu et al. 2005). Hence, the definition of the fruit’s morphological and anatomical characteristics needs to be supplemented to allow for better identification.
Similarly, previous molecular studies have been limited and results ambiguous. Sinocarum was found to be polyphyletic based on ITS, cpDNA sequences and limited specimen materials (S. coloratum, S. cruciatum and S. dolichopodum) and there has been no consensus on its phylogenetic placement (Valiejo-Roman et al. 2002; Zhou et al. 2008; Zhou et al. 2009; Downie et al. 2010). Consequently, there is a gap in our understanding of Sinocarum’s phylogeny and infrageneric classification due to insufficient specimen sampling. Together with limited definitions of morphological characteristics, there is a need to study the phylogeny and morphology of this genus based on new, comprehensive materials.
Therefore, the objective of this study was to estimate the phylogenetic placement of Sinocarum and the infrageneric relationships of the five Sinocarum species we collected. This is the first comprehensive phylogenetic analysis of Sinocarum using morphology and three DNA regions data (i.e. ITS, rpl16 and rps16). Given this more comprehensive analysis, we also discuss the significance of using morphology in phylogenic analyses. In addition, we provide more comprehensive descriptions for the plant morphology, fruit morphology and anatomy, pollen morphology and identification of herbarium specimens of five accepted Sinocarum species. We believed that this study will contribute to a better understanding of the phylogenetic status, infrageneric relationships and morphological identification of Sinocarum.
Materials and methods
Field investigation, morphology study and specimen examination
Samples were obtained from type localities and adjacent areas of S. coloratum (Mt. Yulong, Yunnan), S. cruciatum (Mt. Jizu, Yunnan), S. filicinum (Mt. Cang; Mt. Jizu, Yunnan), S. schizopetalum (Mt. Cang, Yunnan) and S. vaginatum (Mt. Cang, Yunnan). The fruit of Meeboldia yunnanensis (H. Wolff) Constance & F. T. Pu (1998: 70) was obtained from Kunming, Yunnan. Photographs of specimens were made using a Nikon D5600 camera. Fruits were observed and photographed using a stereomicroscope, Nikon SMZ 25 (Japan), and five representative fruit samples were selected to observe characters and measure their size, and then calculate the average value. Pollen grains from the anthers of specimens were directly mounted on copper stubs with conductive carbon adhesive tabs using a needle, sputtercoated with gold, and observed with a Hitachi-SX-450 SEM (Japan). The continuous section of the middle transection of the mericarp was made by the normal paraffin section method. And the section was observed and photographed using stereomicroscope Nikon SMZ25 (Japan). A total of ten pollen grains were selected to measure their length of polar axis (P) and equatorial axis (E), and calculate their average value, ratio of polar axis to equatorial axis (P/E) and size index (). The micromorphological characteristics of pollen were described according to Shu and She (2001). Morphological characteristics were measured using Kayotype (Altınordu et al. 2016). Voucher specimens were deposited in the herbarium of Natural History Museum of Sichuan University (SZ) (Table 1).
Table 1.
Voucher details and GenBank accession numbers of taxa used in this study.
| Taxa | Voucher | Locality | ITS | rpl16 | rps16 |
|---|---|---|---|---|---|
| Acronema astrantiifolium H. Wolff | T2010093003(SZ) | Muli, Sichuan, China | KP940757 | KP940829 | KP940901 |
| A. muscicola (Hand.-Mazz.) Hand.-Mazz. | XZ2011081741(SZ) | Xizang, China | KP940756 | KP940828 | KP940900 |
| A. paniculatum (Franch.) H. Wolff | T2010100602(SZ) | Xiangcheng, Sichuan, China | KP940758 | KP940830 | KP940902 |
| A. schneideri H. Wolff | ZJ810826(KUN) | Shangri-La, Yunnan, China | EU236156 | FJ385070 | – |
| Anthriscus sylvestris (L.) Hoffm. | ZJ0566(KUN) | Daocheng-Litang, Sichuan, China | EU236159 | FJ385078 | FJ385176 |
| Chaerophyllum prescottii DC. | ZJ0744(KUN) | Habahe, Xinjiang, China | FJ385039 | FJ385084 | FJ385183 |
| Changium smyrnioides H. Wolff | J101(KUN) | Jiangsu Institute of Botany, China | DQ517340 | FJ385088 | FJ385187 |
| Chuanminshen violaceum M. L. Sheh & R. H. Shan | J105(KUN) | Xinlong, Sichuan, China | FJ385040 | FJ385089 | FJ385188 |
| Cyclorhiza peucedanifolia (Franch.) Constance | J034(KUN) | Lijiang, Yunnan, China | FJ385042 | FJ385092 | FJ385191 |
| C. waltonii (H. Wolff) M. L. Sheh & R. H. Shan | ZJ0536(KUN) | Derong, Sichuan, China | EU236165 | FJ385093 | FJ385192 |
| Ferula kingdon-wardii H. Wolff | ZJ810846(KUN) | Shangri-La, Yunnan, China | EU236166 | FJ385094 | FJ385193 |
| Halosciastrum melanotilingia (H. Boissieu) Pimenov & V. N. Tikhom. | Pimenov & Kljuykov 200 (MW) | Khasan distr., Primorsk Terr., Russia | AY328937, AY330503 | – | – |
| Hansenia forbesii (H. Boissieu) Pimenov et Kljuykov | 666939(SZ) | – | GU390407 | – | – |
| H. weberbaueriana (Fedde ex H. Wolff) Pimenov et Kljuykov | ZJ0697(KUN) | KIB nursery, Yunnan, China | EU236180 | FJ385115 | FJ385212 |
| Harrysmithia franchetii (M. Hiroe) M. L. Sheh | ZJ0748(KUN) | Luquan, Yunnan, China | FJ385044 | FJ385097 | FJ385195 |
| H. heterophylla H. Wolff | T2012052603 (SZ) | Baoxing, Sichuan, China | KP940763 | – | – |
| Haplosphaera phaea Hand.-Mazz. | ZJ0521(KUN) | Shangri-La, Yunnan, China | EU236167 | FJ385096 | FJ385194 |
| Heptaptera anisoptera (DC.) Tutin | Pimenov & Kljuykov 438 (MW) | Lorestan, Iran | AY941273, AY941301 | – | – |
| Komarovia anisosperma Korovin | – | – | AF077897 | AF094434 | AF110555 |
| Ligusticum delavayi Franch. | ZJ810841(KUN) | Shangri-La, Yunnan, China | EU236174 | FJ385106 | FJ385204 |
| Meeboldia achilleifolia (DC.) P. K. Mukh. & Constance | Pimenov & Kljuykov 28 (MW) | Langtang National Park, Nepal | AY038206, AY038220 | – | – |
| M. yunnanensis (H. Wolff) Constance & F. T. Pu | ZJ0673(KUN) | Fumin, Yunnan, China | EU236178 | FJ385110 | FJ385208 |
| Oenanthe hookeri C. B. Clarke | ZJ0519(KUN) | Shangri-La, Yunnan, China | EU236182 | – | – |
| Oreocomopsis stelliphora (Cauwet & Farille) Pimenov & Kljuykov | Farille 81-421 (G) | N Annapurna, Nepal | GQ379322 | – | – |
| Oreomyrrhis involucrata Hayata | J111(KUN) | Taiwan, China | FJ385052 | – | FJ385218 |
| Ostericum scaberulum (Franch.) C. Q. Yuan & R. H. Shan | YL757(KUN) | Lijiang, Yunnan, China | FJ385053 | FJ385121 | FJ385219 |
| Pachypleurum xizangense H. T. Chang & R. H. Shan | Watson & Gilbert 1580 (E, EBH) | Madoi, Qinghai, China, | KJ660841 | – | KJ660442 |
| Physospermopsis cuneata H. Wolff | J066(KUN) | Lijiang, Yunnan, China | FJ385055 | FJ385125 | FJ385221 |
| P. kingdon-wardii (H. Wolff) C. Norman | ZJ810822(KUN) | Sichuan, China | EU236190 | FJ385127 | FJ385223 |
| P. muliensis R. H. Shan & S. L. Liou | ZJ0686(KUN) | Ninglang, Yunnan, China | EU236191 | FJ385128 | FJ385224 |
| P. rubrinervis (Franch.) C. Norman | FED 378 (E) | Shangri-La, Yunnan, China | AF164836, AF164861 | – | – |
| P. shaniana C. Y. Wu & F. T. Pu | ZJ0678(KUN) | Ninglang, Yunnan, China | EU236192 | FJ385129 | FJ385225 |
| Pimpinella acuminata (Edgew.) C. B. Clarke | ZJ0503(KUN) | Lijiang, Yunnan, China | EU236193 | FJ385130 | FJ385226 |
| P. henryi Diels | ZJ0524(KUN) | Shangri-La, Yunnan, China | EU236195 | FJ385132 | FJ385228 |
| P. purpurea (Franch.) H. Boissieu | ZJ0527(KUN) | Shangri-La, Yunnan, China | EU236197 | FJ385133 | FJ385229 |
| Pleurospermum franchetianum Hemsl. | ZJ0573(KUN) | Yajiang-Kangding, Sichuan, China | EU236198 | FJ385137 | FJ385232 |
| P. hookeri var. thomsonii C. B. Clarke | ZJ0545(KUN) | Sichuan, China | EU236199 | FJ385138 | FJ385233 |
| P. wrightianum H. Boissieu | ZJ0669(KUN) | Shangri-La, Yunnan, China | EU236201 | FJ385140 | FJ385235 |
| P. yunnanense Franch. | ZJ091033(KUN) | Shangri-La, Yunnan, China | EU236202 | FJ385141 | FJ385236 |
| Pternopetalum botrychioides (Dunn) Hand.-Mazz. | ZJ04(KUN) | Suijiang, Yunnan, China | EU236203 | FJ385142 | FJ385237 |
| P. cardiocarpum (Franch.) Hand.-Mazz. | ZJ0581(KUN) | Luding-Mianning, Sichuan, China | EU236204 | FJ385143 | FJ385238 |
| P. davidii Franch. | ZJ06(KUN) | Suijiang, Yunnan, China | EU236205 | FJ385144 | FJ385239 |
| Pterygopleurum neurophyllum (Maxim.) Kitag. | – | – | AY509127 | – | – |
| Rupiphila tachiroei (Franch. & Sav.) Pimenov & Lavrova | Pimenov & Kljuykov 169 (MW) | Primorsk Terr., Russia | AY328952, AY330518 | – | – |
| Sinocarum bellum (C. B. Clarke) Pimenov & Kljuykov | Skvortzov & Proskurjakova (MHA) | West Bengal, India | MK309872 | – | – |
| S. coloratum (Diels) H. Wolff ex R. H. Shan & F. T. Pu | XYP19071901(SZ) | Lijiang, Yunnan, China | MN846685* | MN852960* | MN852964* |
| S. coloratum (Diels) H. Wolff ex R. H. Shan & F. T. Pu | YL561(KUN) | Lijiang, Yunnan, China | FJ385063 | FJ385154 | FJ385248 |
| S. coloratum (Diels) H. Wolff ex R. H. Shan & F. T. Pu | – | – | AY328927 | – | – |
| S. cruciatum (Franch.) H. Wolff ex R. H. Shan & F. T. Pu | XYP19080301(SZ) | Dali, Yunnan, China | MN846686* | MN852961* | MN852965* |
| S. cruciatum (Franch.) H. Wolff ex R. H. Shan & F. T. Pu | ZJ0672(KUN) | Shangri-La, Yunnan, China | EU236209 | FJ385155 | FJ385249 |
| S. cruciatum (Franch.) H. Wolff ex R. H. Shan & F. T. Pu | – | – | AY038199, AY038213 | – | – |
| S. dolichopodum (Diels) H. Wolff ex R. H. Shan & F. T. Pu | ZJ0548(KUN) | Sichuan, China | EU236208 | FJ385156 | FJ385250 |
| S. filicinum H. Wolff (JZS) | XYP19080302(SZ) | Dali, Yunnan, China | MT586806* | MT588116* | MT588118* |
| S. filicinum H. Wolff (CS) | XYP19091803(SZ) | Dali, Yunnan, China | MT586807* | MT588117* | MT588119* |
| S. schizopetalum (Franch.) H. Wolff ex R. H. Shan & F. T. Pu | XYP19080401(SZ) | Dali, Yunnan, China | MN846687* | MN852962* | MN852966* |
| S. vaginatum H. Wolff | XYP19080402(SZ) | Dali, Yunnan, China | MN846688* | MN852963* | MN852967* |
| S. wolffianum (Fedde ex H. Wolff) P. K. Mukh. & Constance | Pimenov & Kljuykov 62 (MW) |
Yumthang, Sikkim, India | MK309871 | – | – |
| Sinolimprichtia alpina H. Wolff | 0465919(KUN) | Xizang, China | FJ385064 | FJ385157 | FJ385251 |
| Sium frigidum Hand.-Mazz. | ZJ0520(KUN) | Shangri-La, Yunnan, China | EU236210 | – | – |
| S. ventricosum (H. Boissieu) Li S. Wang & M. F. Watson | – | – | AY038200, AY038214 | – | – |
| Spuriopimpinella arguta (Diels) X. J. He & Z. X. Wang | T2012091505 (SZ) | Songxian, Henan, China | KP940760 | – | – |
| S. brachycarpa (Kom.) Kitag. | T2012093001 (SZ) | Anshan, Liaoning, China | KP940761 | – | – |
| Tilingia ajanensis Regel & Til. | Pimenov & Kljuykov 139 (MW) | Saghalien, Russia | AY328939, AY330505 | – | – |
| Torilis japonica (Houtt.) DC. | ZJ0623(KUN) | Hongyuan, Sichuan, China | EU236214 | FJ385163 | AF123741 |
| Tongoloa elata H. Wolff | Pimenov et al. 180 (MW) |
Hongyuan-Barkam, Sichuan, China | AY038207, AY038221 | – | – |
| T. gracilis H. Wolff | ZJ0554 (KUN) | Daocheng, Sichuan, China | EU236211 | – | – |
| T. loloensis (Franch.) H. Wolff | ZJ0501(KUN) | Lijiang, Yunnan, China | EU236212 | FJ385160 | FJ385254 |
| T. silaifolia (H. Boissieu) H. Wolff | ZJ810821(KUN) | Sichuan, China | EU236213 | FJ385161 | FJ385255 |
| T. tenuifolia H. Wolff | J075(KUN) | Lijiang, Yunnan, China | FJ385066 | FJ385162 | FJ385256 |
| Trachydium simplicifolium W. W. Sm. | J091(KUN) | Lijaing, Yunnan, China | FJ385067 | FJ385164 | FJ385257 |
| Vicatia bipinnata R. H. Shan & F. T. Pu | ZJ0564(KUN) | Daocheng-Litang, Sichuan, China | EU236217 | FJ385167 | FJ385260 |
– unavailable sequences. * Newly generated sequences; otherwise, sequences were obtained from GenBank.
The related specimens in A, BM, CDBI, E, GB, GH, HNWP, IBSC, JAY, K, KATH, KUN, NAS, NWFC, NY, P, PE, SZ, USP and W were studied and presented in Table 2. Information and photographs of type specimens were gathered from Tropicos (http://www.tropicos.org), the International Plant Names Index (http://www. ipni.org) and JSTOR Global Plants (http://plants.jstor.org).
Table 2.
Sinocarum specimens examined in this study.
| Species | Type specimens | Additional specimens examined |
|---|---|---|
| S. coloratum | China. Yunnan: Grassy ledges of cliffs on the eastern flank of the Lichiang Range, 11–12000 ft, Lat. 27°25'N, September 1906, G. Forrest 3060 (lectotype: E!, designated by Watson, 1998: 382; isolectotype: BM0000574892). | China. Without specific locality, Lianda expedition 21341 (KUN); without specific locality, Lianda expedition 21612 (KUN). Sichuan Province: Konkaling, Tsungu, 3850 m, 30 August 1937, T. T. Yu 13026 (PE); Daocheng, Mt. Gongga, 3200 m, 1 September 1981, Qinghai-Xizang expedition 6025 (KUN); Daocheng, Mt. Gongga, 4500 m, 29 August 1981, Qinghai-Xizang expedition 5581 (KUN). Yunnan Province: Shangri-La, 3500 m, 16 August 1962, Zhongdian expedition 957 (PE); Lijiang, 3 September 1939, Z. G. Zhao 30577 (PE); Lijiang, 3000 m, 11 August 1937, T. T. Yu 15416A (PE); Binchuan, Ki-chan, 2900 m, 18 September 1929, R. C. Ching 24714 (PE); Mekong-Yangtze divide, 4000 m, August 1914, G. Forrest 12970 (PE); Likiang Snow Range, 3 September 1939, R. C. Ching 30577 (KUN); Fugong, Mt. Biluo, 4300 m, 12 September 1964, S. G. Wu 8780 (KUN); Fugong, Mt. Biluo, 12 September 1964, S. G. Wu 8807 (KUN); Shangri-La, Mt. Haba, 31 August 1962, Zhongdian expedition 1800 (KUN); Shangri-La, Mt. Haba, 8 September 1962, Zhongdian expedition 1923 (KUN); Lijiang, Mt. Yulong, 3800 m, 19 July 2019, Y. P. Xiao & Q. P. Jiang XYP19071901 (SZ); Lijiang, Mt. Yulong, 3800 m, 27 September 2019, Y. P. Xiao & Q. P. Jiang XYP19092701 (SZ). |
| S. cruciatum | China. Yunnan: Mt. Ki-chan, 2800 m, 10 September 1884, Delavay 182 (lectotype: P03224861!, designated by Pimenov, 2017: 219; isolectotypes: P02284823, P03224868). | China. Yunnan Province: Binchuan, Mt. Jizu, 3000 m, 3 August 2019, Y. P. Xiao & Q. P. Jiang XYP19080301 (SZ). |
| S. filicinum | China. Yunnan: Eastern flank of the Tali Range, 2540 m, G. Forrest 6963 (lectotype: E!, designated by M. Farille; isolectotype: K000685663!). | China. Without specific locality, Lianda expedition 12092 (KUN). Yunnan Province: Dali, 22 June 1945, H. C. Wang 4412 (PE); Dali-Hejiang, September 1941, H. C. Wang 1396 (PE); Binchuan, Mt. Jizu, 3200 m, R. C. Ching 24920 (PE); Binchuan, Mt. Jizu, Anonymous 2447 (PE); Binchuan, Mt. Jizu, 3000 m, 3 August 2019, Y. P. Xiao & Q. P. Jiang XYP19080302 (SZ); Dali, Mt. Cang, 3200 m, 18 September 2019, Y. P. Xiao & Q. P. Jiang XYP19091803 (SZ). |
| S. schizopetalum | China. Yunnan: Mt. Tsang-chan, 4000 m, 25 July 1884, Delavay 196 (lectotype P!, designated by Pimenov, 2017: 221; isolectotypes: K000685665!, PE). | Without specific locality, C. Y. Wu & D. Y. Liu 20581 (PE, PEY). Yunnan Province: Dali, Mt. Cang, 3800 m, 4 August 2019, Y. P. Xiao & Q. P. Jiang XYP19080401 (SZ). |
| S. vaginatum | China. Yunnan: Mt. Ghi Shan, 11000 ft. (About 3350 m), open pasture, August 1917, G. Forrest 15484 (lectotype: E!, designated by Pimenov, 2017: 221; isolectotype: K000685664). | China. Sichuan Province: Yanyuan, Mt. Xiaogao, 2150 m, October 1986, Z. H. Pan & Y. J. Li & F. T. Pu 964 (CDBI). Yunnan Province: Dali, Mt. Cang, 21 August 1944, H. C. Wang 4511 (PE); Dali, Mt. Cang, 4 August 2019, Y. P. Xiao & Q. P. Jiang XYP19080402 (SZ). |
DNA extraction, amplification and sequencing
Total genomic DNA was extracted from silica gel-dried leaves and herbarium materials according to the protocols of plant genomic DNA kit (Tiangen Biotech, Beijing, China). Nuclear ribosomal DNA (nrDNA) ITS sequences and two chloroplast DNA (cpDNA) intron sequences (rpl16 and rps16) were applied to phylogenetic analyses. The primers ITS4 (5’-TCC TCC GCT TAT TGA TAT GC-3’) and ITS5 (5’-GGA AGT AAA AGT CGT AAC AAG G-3’; White et al. 1990) were used for PCR-amplification of a complete ITS fragment. The rpl16 intron region was amplified with primers F71(5’-GCT ATG CTT AGT GTG TGA CTC GTT G-3’) and R1516 (5’-CCC TTC ATT CTT CTA TGT TG-3’; Jordan et al. 1996; Kelchner and Clark 1997). The rps16 intron was amplified using primers rps16 5’exon (5’-AAA CGA TGT GGN AGN AAR CA-3’) and rps16 3’exon (5’-CCT GTA GGY TGN GCN CCY TT-3’; Downie and Katz-Downie 1999). Amplification was undertaken in a 30 µL mixture of 2 µL plant total DNA, 10 µL ddH2O, 1.5 µL forward primer, 1.5 µL reverse primer and 15 µL 2 × Taq MasterMix (cwbio, Beijing, China). The amplification of the ITS region was obtained by initial denaturation for 3 min at 94 °C, followed by 30 cycles of 45 s at 94 °C, 60 s at 54 °C, and 90 s at 72 °C, and then a final extension of 10 min at 72 °C. The amplification of the rpl16 region was obtained by initial denaturation for 3 min at 94 °C, followed by 36 cycles of 45 s at 94 °C, 70 s at 58.5 °C, and 90 s at 72 °C, and then a final extension of 10 min at 72 °C. Whereas amplification of rps16 region was obtained by initial denaturation for 3 min at 94 °C, followed by 36 cycles of 45 s at 94 °C,70 s at 54 °C, and 90 s at 72 °C, and then a final extension of 10 min at 72 °C. All PCR products were separated using a 1.5% (w/v) agarose TAE gel and sent to Sangon (Shanghai, China) for sequencing. New sequences generated for this study have been deposited in GenBank (Table 1).
Sequence alignment and phylogenetic analysis
We used 53 nrDNAITS sequences obtained from GenBank, and six sequences newly sequenced for this study (Table 1), to infer the phylogenetic placement of Sinocarum. Seventy-four accessions obtained from GenBank for the nrDNA (ITS) and cpDNA (rpl16 and rps16), and 15 accessions newly sequenced (Table 1) represented 35 species from 21 genera of Apiaceae and were used to reconstruct the phylogenetic tree of the Acronema clade. Tribe Scandiceae was selected as the outgroup (Downie et al. 2000a; Zhou et al. 2008; Zhou et al. 2009). Eighty-three accessions obtained from GenBank for the nrDNA (ITS) and cpDNA (rpl16 and rps16), and three accessions newly sequenced (Table 1) represented 31 species from 15 genera of Apiaceae and were used to reconstruct the phylogenetic tree of the East Asia clade. Tribe Pleurospermeae was selected as the outgroup (Downie et al. 2000b; Zhou et al. 2008; Zhou et al. 2009). Sequence data for the ITS 5.8S region were excluded from the analysis because they were unavailable for several previously published taxa.
SeqMan (Burland 2000) was used to assemble DNA sequences and obtain consensus sequences. DNA sequences were aligned with ClustalX ver. 2.1 (Larkin et al. 2007) and then adjusted manually using MEGA7 (Kumar et al. 2016). Phylogenetic analyses of data were conducted by employing Maximum Likelihood (ML) and Bayesian Inference (BI) methods. Maximum Likelihood phylogenetic reconstruction was performed using RAxML-HPC ver. 8.2.10 under the GTR+G nucleotide substitution model and 1,000 rapid bootstraps. The BI analysis was performed in MrBayes version 3.2 (Ronquist et al. 2012). MrModeltest version 2.2 (Nylander 2004) was used for BI analysis to determine a best-fit model of nucleotide substitution. From a random starting tree, the BI analysis was run for 10 million generations and the trees were saved to a file every 1,000 generations. Posterior probabilities were approximated by sampling trees using a variant of the Markov Chain Monte Carlo (MCMC) method. The first 1,000 trees were discarded as “burn-in” and a majority-rule consensus tree was calculated based upon the remaining 9,000 trees resulting from Tracer 1.4 analysis (Drummond and Rambaut 2007).
Results
Plant morphology
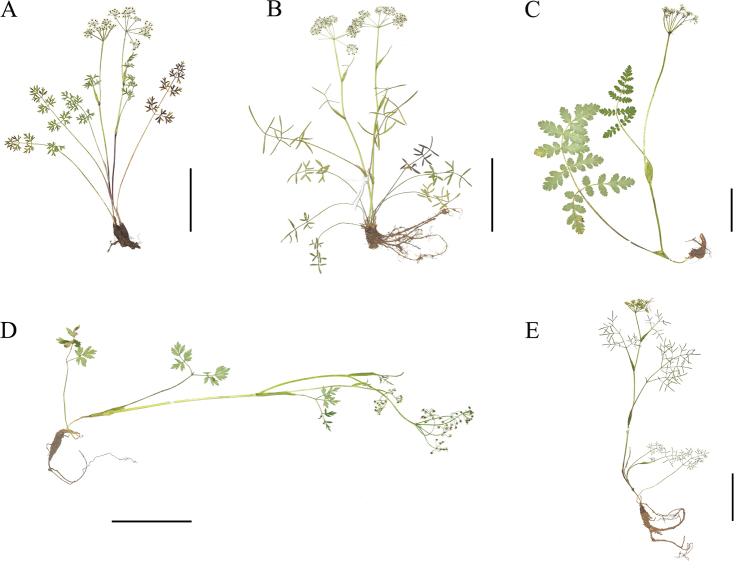
The plant morphological characteristics of Sinocarum species are shown in Table 3 and Fig. 1, and we can know that Sinocarum coloratum typically possesses purplish stems, oblong-ovate sheaths, lanceolate ultimate segments of blades and white petals (Fig. 1A). S. cruciatum generally has torulose roots, subequal rays, triangular in outline and ternate-1–2-pinnate basal leaves, and reduced upward to 1-pinnate or 3-lobed cauline leaves (Fig. 1B). S. filicinum develops broadly ovate sheaths, linear-lanceolate bracts and bracteoles, triangular in outline and 2-pinnate blades, oblong-ovate blade ultimate segments with serrated margins, and sparsely pubescent petioles, rachides and the abaxial surface of segments (Fig. 1C). S. schizopetalum typically has conic taproot, broad-lanceolate sheaths, triangular in outline and ternate-1–2-pinnate blades with oblong-lanceolate ultimate segments, and white or violet petals (Fig. 1D). S. vaginatum generally possess ovate sheaths, unequal rays, entire petals with acute apexes, triangular and ternate-2–3-pinnate blades with elongate-linear ultimate segments, and reduced upwards to 1–2-pinnate cauline leaves (Fig. 1E).
Table 3.
Comparison of plant morphological characteristics of Sinocarum.
| Characteristics | S. coloratum | S. cruciatum | S. filicinum | S. schizopetalum | S. vaginatum |
|---|---|---|---|---|---|
| Root | taproot elongate, thickened at apex, branched | rootstock short, thick; roots torulose | taproot elongate, stout, often branched | taproot conic | taproot elongate, thick, often branched |
| Stems | characteristically purplish | green | green | green | green |
| Basal petioles | 2–10 cm | 5–7 cm | 8–15 cm, sparsely pubescent | 5–8 cm | 5–18 cm |
| Sheath | oblong-ovate | oblong-ovate | broadly ovate | broadly lanceolate | ovate |
| Basal leaves | blade ovate-lanceolate in outline, 1–2-pinnate; pinnae 4–5 pairs | triangular in outline, ternate-1–2-pinnate; pinnae 3–5 pairs | triangular in outline, 2-pinnate; pinnae 3–7 pairs | triangular in outline, ternate to 1- or 2-pinnate | triangular in outline, ternate-2–3-pinnate; pinnae 4–6 pairs |
| Ultimate segments of blade | linear-lanceolate | linear-lanceolate | oblong-ovate, margins serrate, abaxially sparsely pubescent along veins | oblong-lanceolate | elongate-linear |
| Cauline leaves | similar to the basal leaves | elongate-linear, reduced upwards becoming 1-pinnate or 3-lobed | similar to the basal leaves; upper leaves 1-pinnate | similar to the basal leaves | elongate-linear, 1–2-pinnate, reduced upwards |
| Bracts | absent or occasionally 1–2, linear | absent, occasionally 1 | 1–4, linear-lanceolate | absent, occasionally 1, linear-lanceolate | absent or occasionally 1 |
| Bracteoles | absent, rarely 1, linear | absent, occasionally 1 | 5–8, linear-lanceolate | 3–5, linear-lanceolate | absent |
| Rays | 5–8(–12), unequal | 4–7(–10), subequal | 2–8, 1–3 cm, unequal | (3–)5–6(–8), unequal | 10–12, unequal |
| Petals | ovate or broadly obovate, apex usually entire, occasionally 2–3-lobed, white | oblong-ovate or broadly obovate, apex obtuse to subacute, greenish-white | ovate or broadly obovate, apex subacute, white | apex palmately 3–4-lobed, lobes lanceolate or oblanceolate, white or violet | oblong-ovate or broadly obovate, apex subacute, white |
Figure 1.
Specimens of SinocarumAS. coloratumBS. cruciatumCS. filicinumDS. schizopetalumES. vaginatum. Scale bars: 5 cm.
Fruit morphology and anatomy
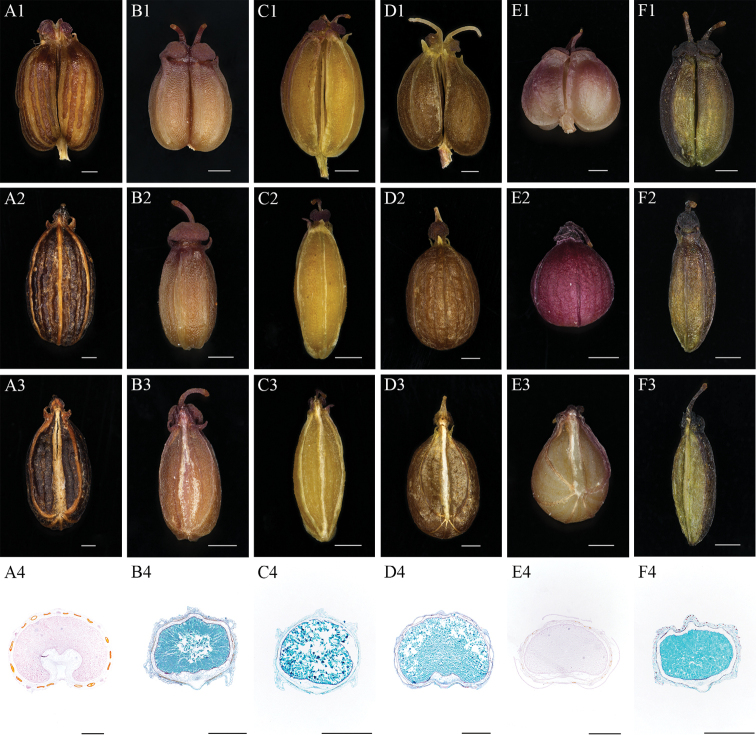
The fruit morphological and anatomical characteristics of Sinocarum species and Meeboldia yunnanensis were studied. The results are shown in Table 4 and Fig. 2. We found that the mature fruits of Meeboldia yunnanensis are ovoid with 5 filiform inconspicuous ribs, 2–3 vittae in each furrow and 4 on commissure, semicircle transection of mericarp and cordate concave endosperm concrescence (Fig. 2A1–A4). The ovoid mature fruits of S. coloratum have 1–3 vittae in each furrow and 2–4 on commissure, sub-pentagon transection and flat endosperm concrescence (Fig. 2B1–B4). S. cruciatum typically has oblong-ovoid fruits with 5 filiform ribs, 1–3 vittae in each furrow and 2–4 on commissure, and sub-pentagon transection and flat endosperm concrescence (Fig. 2C1–C4). The mature fruits of S. filicinum are generally ovoid with slightly constricted apex, obscure ribs, 2–3 vittae in each furrow and 4 on commissure, semicircle transection and sub-cordate concave endosperm concrescence (Fig. 2D1–D4). The mature fruits of S. schizopetalum are broad-ovoid with obscure ribs, 1–3 vittae in each furrow and 2 on commissure, semicircular transection of mericarp, and slightly concave endosperm concrescence (Fig. 2E1–E4). The mature fruits of S. vaginatum are typically oblong-ovoid with 1–3 vittae in each furrow and 2–4 on commissure, sub-pentagon transection and flat endosperm concrescence (Fig. 2F1–F4).
Table 4.
Mature fruit morphological and anatomical characteristics of Sinocarum species and Meeboldia yunnanensis.
| Species | Fruit shape | Transection | Endosperm concrescence | Development degree of ribs | Vittae number | ||
|---|---|---|---|---|---|---|---|
| L × W (mm) | Shape | Furrow | Commissure | ||||
| Meeboldia yunnanensis | 4.08 × 2.35 | ovoid | semicircle | cordate concave | unobvious | 2–3 | 4 |
| S. coloratum | 2.09 × 1.16 | ovoid | sub-pentagon | flat | obvious | 1–3 | 2–4 |
| S. cruciatum | 2.41 × 0.96 | oblong-ovoid | sub-pentagon | flat | obvious | 1–3 | 2–4 |
| S. filicinum | 2.81 × 1.97 | ovoid | semicircle | sub-cordate concave | unobvious | 2–3 | 4 |
| S. schizopetalum | 1.43 × 1.36 | broad-ovoid | semicircle | slightly concave | unobvious | 1–3 | 2 |
| S. vaginatum | 2.28 × 0.90 | oblong-ovoid | sub-pentagon | flat | obvious | 1–3 | 2–4 |
Figure 2.
Morphological and anatomical characteristics of mature fruit AMeeboldia yunnanensisBSinocarum coloratumCS. cruciatumDS. filicinumES. schizopetalumFS. vaginatum. Scale bars: 0.5 mm.
Palynology
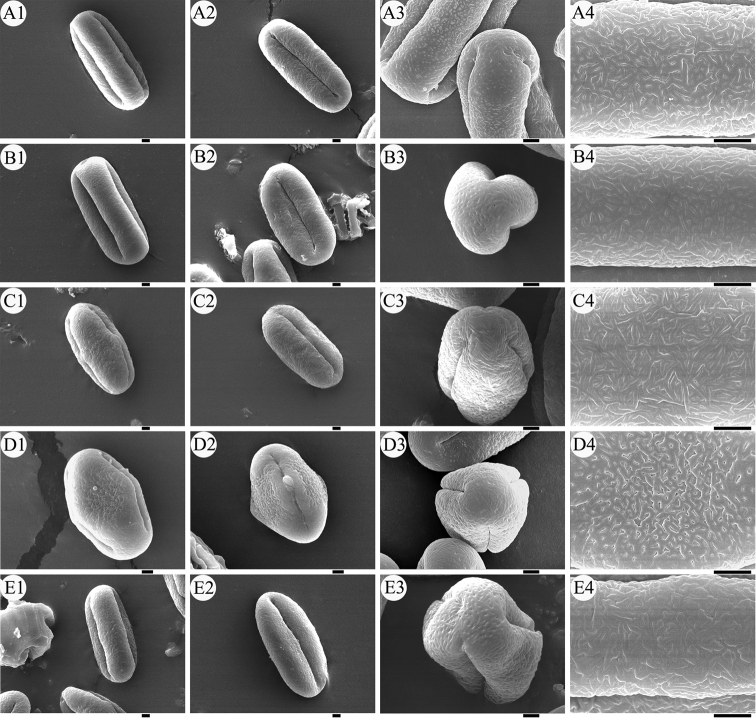
The pollen morphology of the five Sinocarum species was studied by SEM, as shown in Table 5 and Fig. 3. The average ratio of the polar axis to the equatorial axis (P/E) of the pollen grains of S. coloratum, S. cruciatum and S. vaginatum is greater than 2, and the average size index was greater than 19. The pollen grains of these three species are super-rectangular in equatorial view, trilobate circular in polar view (Fig. 3A1–A3, B1–B3, E1–E3). The exine ornamentation of the polar area is cerebroid with a few perforations, and the equatorial area is cerebro reticulate (Fig. 3A3–A4, B3–B4, E3–E4). The pollen grains of S. filicinum are super-rectangular in equatorial view, trilobate circular in polar view (Fig. 3C1–C3). The exine ornamentation of the polar area is striate reticulate with a few perforations, and the equatorial area is cerebro reticulate (Fig. 3C3–C4). Compared with other Sinocarum species, the pollen size of S. schizopetalum is smaller, and its size index is 15.11(14.15~16.62). And its pollen grains are sub-rhomboidal in equatorial view, obtuse triangled in polar view (Fig. 3D1–D3). The exine ornamentation of the polar area is cerebroid, and the equatorial area is pitted reticulate (Fig. 3D3–D4).
Table 5.
Pollen morphology of Sinocarum species in scanning electron microscope (SEM).
| Species | Type | Shape | Size(μm) | Polar axis/Equatorial axis(P/E) | Size index () | Exine ornamentation | ||
|---|---|---|---|---|---|---|---|---|
| Equatorial view | Polar view | Equatorial area | Polar area | |||||
| S. coloratum | super-rectangular | super-rectangular | trilobate circular | (27.20~32.90)30.36× (11.72~13.95)12.78 | 2.38 (2.16~2.59) | 19.69 (18.51~20.00) | cerebro reticulate | cerebroid with a few perforations |
| S. cruciatum | super-rectangular | super-rectangular | trilobate circular | (26.07~32.15)28.84× (11.42~15.33)13.24 | 2.19 (1.80~2.56) | 19.52 (17.25~20.92) | cerebro reticulate | cerebroid with a few perforations |
| S. filicinum | super-rectangular | super-rectangular | trilobate circular | (22.46~27.08)24.95× (11.12~13.57)12.56 | 1.99 (1.66~2.28) | 17.69 (15.80~18.68) | cerebro reticulate | striate reticulate with a few perforations |
| S. schizopetalum | sub-rhomboidal | sub-rhomboidal | obtuse triangle | (18.27~20.81)19.61× (10.40~13.31)11.66 | 1.69 (1.56~1.92) | 15.11 (14.15~16.62) | pitted reticulate | cerebroid |
| S. vaginatum | super-rectangular | super-rectangular | trilobate circular | (25.83~29.30)27.44× (10.92~14.62)13.58 | 2.03 (1.82~2.37) | 19.29 (16.79~20.20) | cerebro reticulate | cerebroid with a few perforations |
Figure 3.
Pollen morphology in scanning electron microscope (SEM) ASinocarum coloratumBS. cruciatumCS. filicinumDS. schizopetalumES. vaginatum. Scale bars: 2 μm.
Sequence characteristics
The characteristics of the three DNA regions are summarized in Table 6. These results indicated that the aligned length of the background tree using 59 ITS sequences from Apiaceae was 472, containing 73.94% average sequence divergence and 276 parsimony informative characters. In addition, average sequence divergence of ITS was more variable (60.50%; 56.76%) than cpDNA (16.76%; 13.06%) across the Acronema clade, East Asia clade and their outgroups. Overall, ITS was more variable than cpDNA, with greater average sequence divergence and more parsimony informative characters.
Table 6.
Statistical summary for ITS and the cpDNA regions used to infer phylogenetic relationships of Sinocarum.
| Apioideae | Acronema clade | East Asia clade | ||||
|---|---|---|---|---|---|---|
| ITS | ITS | cpDNA(rpl16+rps16) | ITS | cpDNA(rpl16+rps16) | ||
| No. of accessions | 59 | 44 | 45 | 34 | 52 | |
| Aligned length | 472 | 476 | 1897 | 444 | 2128 | |
| No. variable characters | 349 (73.94%) | 288 (60.50%) | 318 (16.76%) | 252 (56.76%) | 278 (13.06%) | |
| No. parsimony informative characters | 276 (58.47%) | 205 (43.07%) | 143 (7.54%) | 184 (41.44%) | 163 (7.66%) | |
| Model | ML | GTR+G | GTR+G | GTR+G | GTR+G | GTR+G |
| BI | – | GTR+G | GTR+I+G | GTR+G | GTR+G | |
ITS, internal transcribed spacer; ML, maximum likelihood; BI, Bayesian Inference.
Phylogenetic analyses
ITS trees (Figs 4, 5A, 6A)
Figure 4.
Phylogenetic relationships inferred from maximum likelihood (ML) analysis of 59 nrDNAITS sequences from Apiaceae subfamily Apioideae. The tree is rooted with Pleurospermeae. The names of the clades are identified by Zhou et al. (2008, 2009) and Downie et al. (2010).
Figure 5.
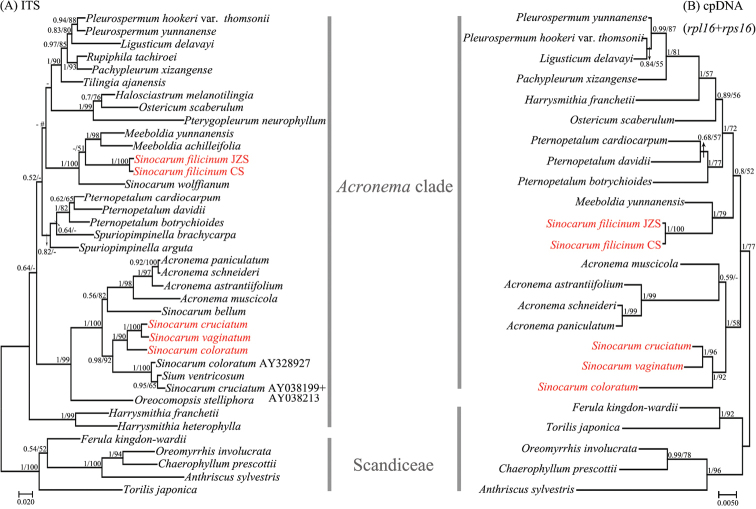
Bayesian 50% strict consensus trees of 44 nrDNAITS sequences (A) and 45 combined cpDNArpl16 and rps16 intron sequences (B) from Acronema clade and outgroups. Values on the branches indicate its support (Bayesian posterior probability/ bootstrap value). Those nodes not occurring in the ML strict consensus tree are indicated by pound symbols (#). Short line denotes values < 50%. The tree is rooted with Scandiceae. The names of the clades are identified by Zhou et al. (2008, 2009) and Downie et al. (2010).
Figure 6.
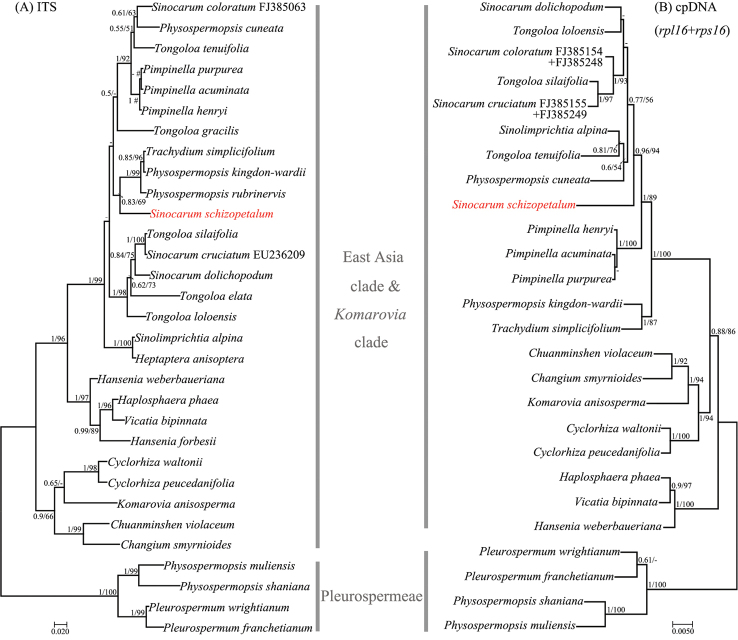
Bayesian 50% strict consensus tree of 34 nrDNAITS sequences (A) and 52 combined cpDNArpl16 and rps16 intron sequences (B) from East Asia clade & Komarovia clade and outgroups. Values on the branches indicate its support (Bayesian posterior probability/ bootstrap value). Those nodes not occurring in the ML strict consensus tree are indicated by pound symbols (#). Short line denotes values < 50%. The tree is rooted with Pleurospermeae. The names of the clades are identified by Zhou et al. (2008, 2009) and Downie et al. (2010).
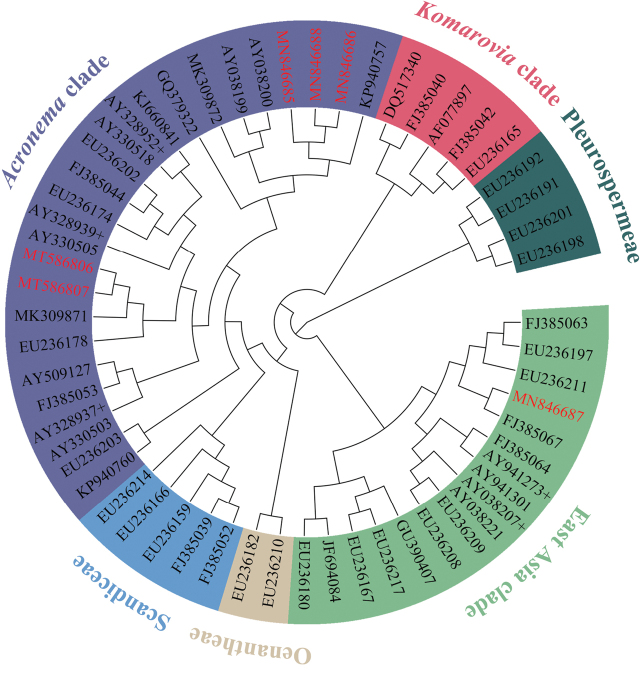
After phylogenetic analyses with comprehensive sequence data, we confirmed that the collected Sinocarum materials were from a polyphyletic group and fell into two different clades (i.e. Acronema clade, East Asia clade) according to the ITS tree inferred by ML approach (Fig. 4). The ITS trees of Acronema clade and its outgroups (Fig. 5A) inferred by ML and BI approaches were inconsistent for subclade topology. The ITS tree demonstrated again that Sinocarum was a polyphyletic group. Additionally, it indicated that the collected Sinocarum materials (S. coloratum, S. cruciatum and S. vaginatum), together with two previously sequenced Sinocarum species (S. coloratumAY328927; S. cruciatumAY038199; AY038213) and Sium ventricosum (H. Boissieu) Li S. Wang & M. F. Watson (2016: 266) (AY038200; AY038214) constituted a strongly supported group (Bayesian Inference (BI)–posterior probability (PP)=0.98/Maximum Likelihood (ML)–bootstrap value (BS)=92%), as sister group to four species of Acronema and Sinocarum bellum (C. B. Clarke) Pimenov & Kljuykov (2006: 122) (PP/BS = 1/100%). A newly recognized but weakly supported subclade in the ITS trees encompassed four species of Acronema and Sinocarum bellum (PP/BS = 0.56/82%). Of three Sinocarum species we collected, S. cruciatum was allied closer with S. vaginatum than S. coloratum (PP/BS = 1/100%). Two populations of S. filicinum, two species of Meeboldia H. Wolff (1924: 313) and S. wolffianum (Fedde ex H. Wolff) P. K. Mukh. & Constance (1991: 42) formed a strongly supported subclade (PP/BS = 1/100%), and S. filicinum had a closer relationship with Meeboldia. The ITS trees of the East Asia clade and its outgroups (Fig. 6A) inferred by ML and BI approaches were inconsistent for Pimpinella L. (1753: 263) subclade topology. In addition, the trees indicated that S. schizopetalum was allied distantly with other collected Sinocarum species, and closely related to TrachydiumLindley (1835: 232) subclade (PP/BS = 0.83/69%) in the strongly supported East Asia clade (PP/BS = 1/96%).
cpDNA trees (Figs 5B, 6B)
The cpDNA trees of Acronema clade and its outgroups (Fig. 5B) inferred by ML and BI approaches had consistent topologies. The cpDNA trees indicated that the generic type of Sinocarum, S. coloratum, together with S. cruciatum and S. vaginatum constituted a supported monophyletic group (PP/BS = 1/92%) as sister group to Acronema (PP/BS = 1/58%). Two populations of S. filicinum allied powerfully with the genus Meeboldia (PP/BS = 1/79%). The cpDNA trees of the East Asia clade and its outgroups (Fig. 6B) inferred by ML and BI approaches had consistent topologies and indicated that the position of S. schizopetalum differed from the ITS tree and was located in the East Asia clade.
Discussion
Morphology
We have studied the plant morphology, fruit morphological and anatomical characteristics, and palynology of five species of Sinocarum, and perfected the mature fruit characteristics of these species. Through the analysis of comprehensive morphological data, the five Sinocarum species can be divided into three groups. Group 1 includes S. coloratum, S. cruciatum and S. vaginatum. They are characterized by slender and glabrous plants, usually ovate or oblong-ovate sheath, mostly absent bracts and bracteoles, typically entire petals, ovoid or oblong-ovoid mature fruit with 1–3 vittae in each furrow and 2–4 on commissure, sub-pentagon transection and flat endosperm concrescence. And the pollen grains of these three species are super-rectangular in equatorial view, trilobate circular in polar view. Group 2 includes S. filicinum, whose morphological characteristics were significantly different from those of other Sinocarum species we collected, and the key identification features were the linear-lanceolate bracts and bracteoles, oblong-ovate blade ultimate segments with serration on the margins, sparsely pubescent petioles, rachides and the abaxial surface of segments. Group 3 includes S. schizopetalum, whose most prominent features are apex palmately 3–4-lobed and white or violet petals, broad-ovoid mature fruits and sub-rhomboidal pollen. Among them, petal characteristics are very special in the whole genus Sinocarum. It is concluded that plant morphology, fruit morphological and anatomical characteristics, and palynology have important taxonomic significance.
Phylogenetic placement of Sinocarum
Previous studies have shown that Sinocarum is not a monophyletic group and the phylogenetic placement remains unclear (Valiejo-Roman et al. 2002; Zhou et al. 2008; Zhou et al. 2009; Downie et al. 2010). S. coloratum is the generic type of Sinocarum, its phylogenetic placement represents the phylogenetic placement of Sinocarum. In this study, we confirmed that Sinocarum is not a monophyletic group and used three sequences of S. coloratum (MN846685, AY328927, FJ385063), our sequenced specimen and two downloaded sequences, to determine the phylogenetic placement of Sinocarum. We found that one of the downloaded (FJ385063) was located in the East Asia clade, while our accession and the other downloaded accession were located in the Acronema clade but these two were not clustered together. The results of our field investigation, morphological study and specimen verification of S. coloratum obtained from the type locality (Mt. Yulong) showed that our collected material is highly consistent with the type specimen and the original literature description of S. coloratum. In conclusion, the true phylogenetic placement of Sinocarum is within the Acronema clade and the genus has a close affinity with Acronema.
The relationship between Sinocarum and Acronema
This study’s phylogeny results indicated that there was a close and complex relationship between Sinocarum and Acronema. Fusiform or elongate roots and apex slightly obtuse or rarely lobed petals are easily recognizable characteristics of Sinocarum, and an apex long-linear or long-aristate petal is the most prominent feature of Acronema. In fact, within each genus there are species that deviate in one or more morphological characteristics from the typical and the generic boundaries are blurred with a few species being easily confused as belonging to the other genus (Watson 1996; Watson et al. 2004; Pu et al. 2005). For example, S. cruciatum has torulose roots and several species of the genus Acronema, A. chienii R. H. Shan & S. L. Liou (1980: 197), A. chinense H. Wolff (1926: 309) have apex acute or obtuse-acute petals, characteristics typically observed in the other genus. Through a literature review, field investigation, morphological study and specimen examination, we found that the plants of Sinocarum and Acronema are all slender. In addition, Sinocarum and Acronema are both distributed in the high-elevation Sino-Himalayan region from Nepal to SW China. The habitat of the two genera is extremely similar as they are distributed in the humid environment of rock crevices, alpine meadows or shady forests. These conditions provide further evidence for the close and complex affinity between the two genera. This study and others have provided cumulative evidence to reduce phylogenetic uncertainty. Despite recent collections, the range of materials for the two genera is still limited and more field specimens will be required to provide a comprehensive revision of the phylogeny of Sinocarum and Acronema across their geographic range.
Infrageneric relationships
Our ITS and cpDNA trees showed that S. coloratum (generic type), S. cruciatum and S. vaginatum clustered together, but S. cruciatum had a closer relationship with S. vaginatum, which was consistent with the results of morphological study. The closer relationship between S. cruciatum and S. vaginatum is supported by the ultimate segments of their blades being more slender than other Sinocarum species and forming a group of narrow-leaved taxa. However, S. vaginatum develops elongate-linear ultimate segments of basal leaves and cauline leaves, and more rays, about 10–12. Whereas S. cruciatum has subequal rays and torulose roots. These two species are recognizable by these major features. In addition, S. cruciatum and S. vaginatum were both collected from the Dali range, Dali, Yunnan, overlapping in their ranges. Consequently, the morphological evidence and geographical distribution are consistent with the phylogenetic analysis results.
Sinocarum filicinum H. Wolff (1929: 182) was originally described by Wolff (1929) based on G. Forrest n. 6863, 11691, 7230, and obtained from the eastern flank of the Dali Range in Yunnan. Since the description of S. schizopetalum, its phylogenetic placement has been controversial. Franchet (1894) originally described it as a new species as Carum schizopetalum Franch., and Wolff (1927) transferred it to Sinocarum, later Wu Zhengyi (1984) transferred this species to Dactylaea H. Wolff (1930: 304) as Dactylaea schizopetala (Franch.) Wu Zhengyi (1984: 910). Pu et al. (2005) accepted Wolff’s view in the description of Sinocarum in Flora of China. Pimenov (2017) also accepted Wolff’s view that this species belongs to Sinocarum after studying the specimens of Apiaceae, especially the type specimens. In addition, no molecular phylogenetic studies have been carried out on S. filicinum and S. schizopetalum.
The results showed that the two populations of S. filicinum were not related to S. coloratum (Sinocaurm generic type) and allied most closely with the genus Meeboldia, according to the ITS and cpDNA trees. Our morphological results indicated that the morphological characteristics of S. filicinum are distinct from the three other Sinocarum species in the Acronema clade that we collected and are very consistent with the characteristics of Meeboldia. Among them, fruit characteristics play a key role of subfamily Apioideae classification (Kljuykov et al. 2004; Lyskov et al. 2017; Guo et al. 2018; Jia et al. 2019). And the fruit characteristics of S. filicinum are similar to those of Meeboldia yunnanensis, they are all ovoid, with 5 filiform inconspicuous ribs, 2–3 vittae in each furrow and 4 on commissure, semicircle transection of mericarp and cordate concave or sub-cordate endosperm concrescence (Fig. 2A, D). The molecular data and morphological evidence indicated that S. filicinum is closely related to Meeboldia and should be isolated from Sinocarum, but due to the lack of comprehensive samples, the phylogenetic placement will not be revised at present.
Our phylogenetic results showed that S. schizopetalum was distantly related to the other Sinocarum species we collected (S. coloratum, S. cruciatum, S. filicinum and S. vaginatum). We found that the exact phylogenetic placement of S. schizopetalum was inconsistent between the ITS tree and the cpDNA tree, but was nevertheless located in the East Asia clade. Morphologically, S. schizopetalum has apex palmately 3–4-lobed petals, broad-ovoid mature fruits and sub-rhomboidal pollen, and these features are clearly distinct from other species of Sinocarum. Unlike the other studied Sinocarum species, the plant morphology, fruit and pollen morphology of S. schizopetalum are more similar to species of the East Asia clade. According to the results of phylogeny and morphology studies, it is suggested S. schizopetalum should be isolated from Sinocarum. However, due to the complex taxonomic problems among genera in the East Asia clade, the phylogeny of S. schizopetalum cannot be resolved. Thus, S. schizopetalum needs revision pending expanded sampling and phylogenetic analyses to include more East Asia clade species from the Sino-Himalayan region.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31872647, 32070221), National Specimen Information Infrastructure, Educational Specimen Sub-Platform (Grant No. 2005DKA21403-JK), the fourth national survey of traditional Chinese medicine resources (Grant No. 2019PC002). The authors thank Q. P. Jiang for her help in material collection; curators and staff of the following herbaria: A, BM, CDBI, E, GB, GH, HNWP, IBSC, JAY, K, KATH, KUN, NAS, NWFC, NY, P, PE, SZ, USP, W who allowed the authors to examine their specimens or get digital information via the network.
Citation
Xiao Y-P, Guo X-L, Price M, Gou W, Zhou S-D, He X-J (2021) New insights into the phylogeny of Sinocarum (Apiaceae, Apioideae) based on morphological and molecular data. PhytoKeys 175: 13–32. https://doi.org/10.3897/phytokeys.175.60592
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31872647, 32070221), National Specimen Information Infrastructure, Educational Specimen Sub-Platform (Grant No. 2005DKA21403-JK), the fourth national survey of traditional Chinese medicine resources (Grant No. 2019PC002).
Reference
- Altınordu F, Peruzzi L, Yu Y, He X. (2016) A tool for the analysis of chromosomes: Karyo Type. Taxon 65(3): 586–592. 10.12705/653.9 [DOI] [Google Scholar]
- Burland TG. (2000) DNASTAR’s Lasergene sequence analysis software. Methods in Molecular Biology (Clifton, N.J. ) 132: 71–91. 10.1385/1-59259-192-2:71 [DOI] [PubMed] [Google Scholar]
- Downie SR, Katz-Downie DS. (1999) Phylogenetic analysis of chloroplast rps16 intron sequences reveals relationships within the woody southern African Apiaceae subfamily Apioideae. Canadian Journal of Botany 77(8): 1120–1135. 10.1139/b99-086 [DOI] [Google Scholar]
- Downie SR, Katz-Downie DS, Spalik K. (2000a) A phylogeny of Apiaceae tribe Scandiceae: Evidence from nuclear ribosomal DNA internal transcribed spacer sequences. American Journal of Botany 87(1): 76–95. 10.2307/2656687 [DOI] [PubMed] [Google Scholar]
- Downie SR, Katz-Downie DS, Watson MF. (2000b) A phylogeny of the flowering plant family Apiaceae based on chloroplast DNA rpl16 and rpoC1 intron sequences: Towards a suprageneric classification of subfamily Apioideae. American Journal of Botany 87(2): 273–292. 10.2307/2656915 [DOI] [PubMed] [Google Scholar]
- Downie SR, Spalik K, Katz-Downie DS, Reduron JP. (2010) Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. Plant Diversity and Evolution 128(1–2): 111–136. 10.1127/1869-6155/2010/0128-0005 [DOI] [Google Scholar]
- Drummond AJ, Rambaut A. (2007) BEAST: Bayesian evolutionary analyses by sampling trees. BMC Evolutionary Biology 7(1): e214. 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed]
- Edgeworth MP. (1846) III. Descriptions of some unpublished Species of Plants from North‐Western India. Transactions of the Linnean Society of London 20(1): 23–91. 10.1111/j.1096-3642.1846.tb00410.x [DOI] [Google Scholar]
- Franchet A. (1894) Notes sur quelques Ombellifères du Yunnan. Bulletin de la Société Philomathique de Paris 8: 106–146. [Google Scholar]
- Guo XL, Wang CB, Wen J, Zhou SD, He XJ. (2018) Phylogeny of Chinese Chamaesium (Apiaceae: Apioideae) inferred from ITS, cpDNA and morphological characters. Phytotaxa 376: 001–016. 10.11646/phytotaxa.376.1.1 [DOI] [Google Scholar]
- Jia SB, Guo XL, Zhou SD, He XJ. (2019) Hansenia pinnatiinvolucellata is conspecific with H. weberbaueriana (Apiaceae) based on morphology and molecular data. Phytotaxa 418: 203–210. 10.11646/phytotaxa.418.2.5 [DOI] [Google Scholar]
- Jordan WC, Courtney MW, Neigel JE. (1996) Low levels of intraspecific genetic variation at a rapidly evolving chloroplast DNA locus in North American Duckweeds (Lemnaceae). American Journal of Botany 83(4): 430–439. 10.1002/j.1537-2197.1996.tb12724.x [DOI] [Google Scholar]
- Kelchner SA, Clark LG. (1997) Molecular evolution and phylogenetic utility of the chloroplast rpl16 intron in Chusquea and the Bambusoideae (Poaceae). Molecular Phylogenetics and Evolution 8(3): 385–397. 10.1006/mpev.1997.0432 [DOI] [PubMed] [Google Scholar]
- Kljuykov EV, Liu M, Ostroumova TA, Pimenov MG, Tilney PM, Wyk BEV. (2004) Towards a standardised terminology for taxonomically important morphological characters in the Umbelliferae. South African Journal of Botany 70(3): 488–496. 10.1016/S0254-6299(15)30233-7 [DOI] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution 33(7): 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, Mcwilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. (2007) Clustal W and Clustal X version 2.0. Bioinformatics (Oxford, England) 23(21): 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lindley J. (1835) Illustrations of the botany and other branches of the natural history of the Himalayan Mountains. Royle, John Forbes, London, 232 pp. [Google Scholar]
- Linnaeus C. (1753) Species Plantarum (ed. 2, Vol. 1). Laurentii Salvii, Holmiae, 263 pp. [Google Scholar]
- Liu SL, Shan RH. (1980) A preliminary study on the Chinese Acronema. Acta Phytotaxonomica Sinica 18: 194–204. [Google Scholar]
- Lyskov DF, Degtjareva GV, Samigullin TH, Pimenov MG. (2017) The revision of Prangos, subsections Koelzella and Fedtschenkoana, (Apiaceae) with some notes to phylogeny and biogeography of the genus: Molecular and morphological evidences. Plant Systematics and Evolution 313(7): 815–826. 10.1007/s00606-017-1412-0 [DOI] [Google Scholar]
- Mukherjee PK, Constance L. (1991) New taxa and transfers in Indian Umbelliferae. Edinburgh Journal of Botany 48(1): 41–44. 10.1017/S0960428600003589 [DOI] [Google Scholar]
- Nylander JAA. (2004) MrModeltest, a program to evaluate the fit of several models of evolution to a given data and unrooted tree (version 2.2). Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- Pimenov MG. (2017) Updated checklist of Chinese Umbelliferea: Nomenclature, synonymy, typification, distribution. Turczaninowia 20(2): 106–239. 10.14258/turczaninowia.20.2.9 [DOI] [Google Scholar]
- Pimenov MG, Kljuykov EV. (2006) Two new species, two new nomenclatural combinations and a new name in Himalayan Sinocarum (Umbelliferae). Komarovia 4: 116–123. [Google Scholar]
- Pu FT. (1998) New names in Chinese Umbelliferae. Novon 8(1): 70–71. 10.2307/3391897 [DOI] [Google Scholar]
- Pu FT, Watson MF, Holmes-Smith I. (2005) Sinocarum H. Wolff ex R. H. Shan & F. T. Pu, Apiaceae. In: Wu ZY, Raven PH. (Eds) Flora of China (Vol 14).Science Press, Beijing and Missouri Botanical garden Press, Saint Louis, 82–85.
- Ronquist F, Teslenko M, Mark PVD, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan RH, Pu FT. (1985) Sinocarum Wolff ex Shan & Pu. Flora Reipublicae Popularis Sinicae 55: 30–38. [Google Scholar]
- Shan RH, She ML, Yuan CC, Wang TS, Pu FT, Chang HT. (1980) New taxa of the Umbelliferae from Xizang (Tibet). Acta Phytotaxonomica Sinica 18: 374–379. [Google Scholar]
- Shu P, She ML. (2001) Pollen Photographs and Flora of Umbelliferae in China. Shanghai Science and Technology Press, Shanghai, 26 pp. [Google Scholar]
- Valiejo-Roman CM, Terentieva EI, Samigullin TH, Pimenov MG. (2002) nrDNA ITS sequences and affinities (Umbelliferae) of Sino-Himalayan Apioideae. Taxon 51: 685–701. 10.2307/3647333 [DOI] [Google Scholar]
- Wang PL. (1998) Practical Pollen in China. Sichuan Science and Technology Press, Chengdu, 110–111.
- Wang LS, Zhang HR, Watson MF, Knapp S. (2016) On the identity of Sium frigidum (Apiaceae). Phytotaxa 288(3): 265–272. 10.11646/phytotaxa.288.3.7 [DOI] [Google Scholar]
- Watson MF. (1996) Notes relating to the flora of Bhutan: XXXIII. Umbelliferae, I. Edinburgh Journal of Botany 53(1): 127–144. 10.1017/S0960428600002766 [DOI] [Google Scholar]
- Watson MF, She ML, Pu FT, Pan ZH. (2004) Nomenclatural novelties in the Apiaceae (Umbelliferae) for the Flora of China. Acta Phytotaxonomica Sinica 42: 561–565. 10.1088/1009-0630/6/5/011 [DOI] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR Protocols: A Guide to Methods and Applications.Academic Press Inc, San Diego, California, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wolff H. (1924) Meeboldia, genus novum Umbelliferarum Himalayicum. Repertorium novarum specierum regni vegetabilis 19(16–21): 313–313. 10.1002/fedr.19240191615 [DOI] [Google Scholar]
- Wolff H. (1926) Plantae sinenses a Dre. H. Smith annis 1921–22 lectae. XVI. Umbelliferae. Acta Horti Gothoburgensis 2: 288–328. [Google Scholar]
- Wolff H. (1927) Umbelliferae – Apioideae – Ammineae – Carinae, Ammineae-novemjugatae et genuinae. In: Engler A. (Ed.) Das Pflanzenreich.Wilhelm Engelmann, Berlin, 90: 1–398.
- Wolff H. (1929) Umbelliferae Asiatiae novae relictae. II. Repert. Spec. Nov. Regni Veget 27: 179–192. 10.1002/fedr.4870270909 [DOI] [Google Scholar]
- Wolff H. (1930) Umbelliferae asiaticae novae relictae. III. Repertorium novarum specierum regni vegetabilis 27(16–25): 301–335. 10.1002/fedr.4870271612 [DOI] [Google Scholar]
- Wu ZY. (1984) The catalogue of seed plants in Yunnan Province. Yunnan People’s Publishing House, Kunming, 910 pp. [Google Scholar]
- Zhou J, Peng H, Downie SR, Liu ZW, Gong X. (2008) A molecular phylogeny of Chinese Apiaceae subfamily Apioideae inferred from nuclear ribosomal DNA internal transcribed spacer sequences. Taxon 57: 402–416. 10.2307/25066012 [DOI] [Google Scholar]
- Zhou J, Gong X, Downie SR, Peng H. (2009) Towards a more robust molecular phylogeny of Chinese Apiaceae subfamily Apioideae: Additional evidence from nrdna its and cpDNA intron (rpl16 and rps16) sequences. Molecular Phylogenetics and Evolution 53(1): 56–68. 10.1016/j.ympev.2009.05.029 [DOI] [PubMed] [Google Scholar]