ABSTRACT
The peri-implantation window of mammalian development is the crucial window for primordial germ cell (PGC) specification. Whereas pre-implantation dynamics are relatively conserved between species, the implantation window marks a stage of developmental divergence between key model organisms, and thus potential variance in the cell and molecular mechanisms for PGC specification. In humans, PGC specification is very difficult to study in vivo. To address this, the combined use of human and nonhuman primate embryos, and stem cell-based embryo models are essential for determining the origin of PGCs, as are comparative analyses to the equivalent stages of mouse development. Understanding the origin of PGCs in the peri-implantation embryo is crucial not only for accurate modeling of this essential process using stem cells, but also in determining the role of global epigenetic reprogramming upon which sex-specific differentiation into gametes relies.
KEY WORDS: Germ cells, Peri-implantation, Primordial germ cells, Embryo, Implantation, Pluripotency
Summary: The peri-implantation window of mammalian development is crucial for primordial germ cell (PGC) specification. This Review discusses the induction of PGCs in key mammalian models, including the new stem cell-based embryo models.
Introduction
Germ cells are essential for inheritance because they are the only cell type capable of passing genetic and epigenetic information from parent to offspring. In some model organisms, such as Drosophila, C. elegans, Xenopus laevis and zebrafish, the germline is specified by a process known as pre-formation (Extavour and Akam, 2003). In these species, upon fertilization and cellularization/cleavage, the embryonic cells that inherit certain RNAs and proteins from the fertilized oocyte maintain germ cell fate, while the remaining cells differentiate into somatic cells. These first germline cells in the metazoan embryo are called primordial germ cells (PGCs). Unlike the model organisms that use pre-formation, mammals specify PGCs through a process called induction. One of the major distinguishing features of mammalian PGCs at the end of the PGC induction period is their unique epigenetic state characterized by global DNA demethylation, imprint erasure and, in female embryos, activation of both X chromosomes. Therefore, understanding the development of PGCs in mammals requires models that incorporate features that not only recapitulate the inductive signaling events, but also those that establish the characteristic epigenetic state.
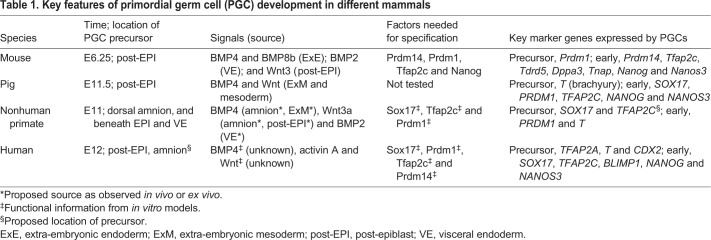
In mammals, PGCs are induced after the differentiation of the trophectoderm (TE) and primitive endoderm (PE) (Saitou and Yamaji, 2012; Sasaki et al., 2016). This window of mammalian development is very difficult to study due to the small number of cells in the embryo, combined with the dynamic changes in cell fate as the embryonic and extra-embryonic cell lineages heterogeneously develop to generate the conceptus. It is now appreciated that there are differences in the cell and molecular mechanisms involved in extra-embryonic lineage differentiation and PGC specification between mammals. For example, in mouse and pig (Box 1) models, PGCs are specified in a region of the peri-implantation embryo called the posterior proximal epiblast (Kobayashi et al., 2017; Lawson et al., 1999). However, in cynomolgus macaques (Macaca fascicularis; ‘cyno’), PGCs are first specified in an extra-embryonic tissue called the amnion and possibly the posterior epiblast (Sasaki et al., 2016). Whether PGCs in other nonhuman primates and humans are specified similarly in the amnion or have a dual origin for specification is an ongoing debate in the field, yet to be clarified by emerging models and model systems.
Box 1. Pig primordial germ cell specification.
The porcine (pig) embryo develops as a bilaminar disc during implantation similar to humans and monkeys (Keibel, 1897). Furthermore, porcine primordial germ cell (pPGC) precursors emerge from brachyury (T)-competent progenitors in the posterior post-epiblast (EPI) similar to mouse egg cylinder embryos (Kobayashi et al., 2017). Isolation of pig epiblasts reveals that post-EPI competency for pPGC specification requires Wnt (Kobayashi, et al., 2017). As detailed in this Review, BMP4 signaling from the extra-embryonic ectoderm (ExE) is required to specify mPGCs in egg cylinder embryos. In the pig embryo, BMP4 is expressed by extra-embryonic mesoderm (ExM) and nascent mesoderm cells (Magana et al., 2014). However, whether these tissues provide the source of BMP4 for pPGC specification has not yet been resolved. Once specified, pPGC precursors are identified as co-expressing the same transcription factors used to identify hPGCs. These include Sox17, Blimp1 and Nanog (Kobayashi et al., 2017). By E12.5, pPGC specification from precursors begins with expression of Tfap2c (Kobayashi et al., 2017). Competency of the post-EPI to specify additional pPGCs is reduced as gastrulation continues; however, given the lack of proliferation in newly specified pPGCs, it is speculated that recruitment of pPGCs from T-competent pPGC precursors continues until ∼E15.5, 4 days after pPGC specification is initiated (Kobayashi et al., 2017).
Given these differences in PGC specification among mammals, it is useful to re-evaluate the transition of embryonic progenitor cells into PGCs in the available mammalian model organisms as well as stem cell-based embryo models. These comparisons are essential to identify the most appropriate model(s) for studying human PGC specification. In this Review, we discuss what is known about PGC specification in mouse, nonhuman primates and humans, drawing primarily from in vivo evidence. We highlight similarities and differences in PGC specification between these systems, as well as current gaps in our understanding, particularly relating to the unique process of epigenetic reprogramming in PGCs. Finally, we discuss how in vitro and ex vivo models can address major unanswered questions in PGC specification.
PGC specification in the mouse embryo
For the past three decades, the mouse has been used as the major mammalian model to study early embryo development, including mouse PGC (mPGC) specification. In the mouse, a tissue called the extra-embryonic ectoderm (ExE) is primarily responsible for providing the crucial signals that induce mPGC from the proximal epiblast (Lawson et al., 1999). Therefore, understanding the origins of the ExE, and its physical relationship to the mPGC precursors, is essential for interpreting data from new stem cell-based embryo models, as well as the specification of mPGCs in vivo. Although the ExE differentiates after implantation, the immediate precursors to the ExE are specified before the embryo implants (Tanaka et al., 2013; Rossant, 1988). In this section, we describe how the ExE arises during pre- and peri-implantation development in the mouse. We discuss the tissues and signals that contribute to specifying mPGC precursors in the posterior proximal epiblast, and finally the factors needed for mPGC specification from these precursors.
Development of the extra-embryonic ectoderm in mouse
The blastocyst is the last embryonic stage before embryo implantation. Immediately before implantation, at embryonic day (E) 4.5, the inner cell mass (ICM) differentiates into two distinct cell types: the pluripotent pre-implantation epiblast (pre-EPI) and a new epithelial layer called the primitive endoderm (PE) (Rossant et al., 2003; Chazaud et al., 2006). TE cells furthest from the embryonic pole become mural TE cells, which contact the uterine wall to trigger implantation (Dickson, 1963; Kirby et al., 1967; Armant, 2005; Sutherland, 2003; Hu and Cross, 2010). Conversely, the polar TE cells associated with the pre-EPI and PE remain multipotent and differentiate into ExE at around E5.5 (Copp, 1978).
As the ExE is specified, additional major morphological and cellular changes occur (Bardot and Hadjantonakis, 2020; Rossant et al., 2003). These changes include conversion of the pre-EPI to the early post-implantation EPI (post-EPI) and specialization of the PE epithelium into parietal endoderm, which lines the blastocoel cavity and visceral endoderm (VE) that encapsulates the ExE and post-EPI of the embryo (Rossant et al., 2003; Gardner and Rossant, 1979; Lawson and Pedersen, 1987). Cavitation within the early post-EPI occurs simultaneously with VE encapsulation, creating a polarized columnar epithelium in a cup-like shape containing a central pro-amniotic cavity. The post-EPI continues to expand, converting into a pseudostratified epithelium with the conceptus resembling a cylindrical structure (Coucouvanis and Martin, 1995). At this time, the site of connection to the uterus defines the proximal pole of the proximodistal axis. The ExE also undergoes cavitation to create an ExE epithelium that is contiguous with the post-EPI and a cavity that becomes continuous with the amniotic cavity. The stages between E5.0, when the pro-amniotic cavity first appears, and E7.5, when the amnion and chorion are fully formed and gastrulation is almost complete, are called the ‘egg cylinder stages’ of mouse embryo development; it is during these stages that mPGCs are specified (Fig. 1A).
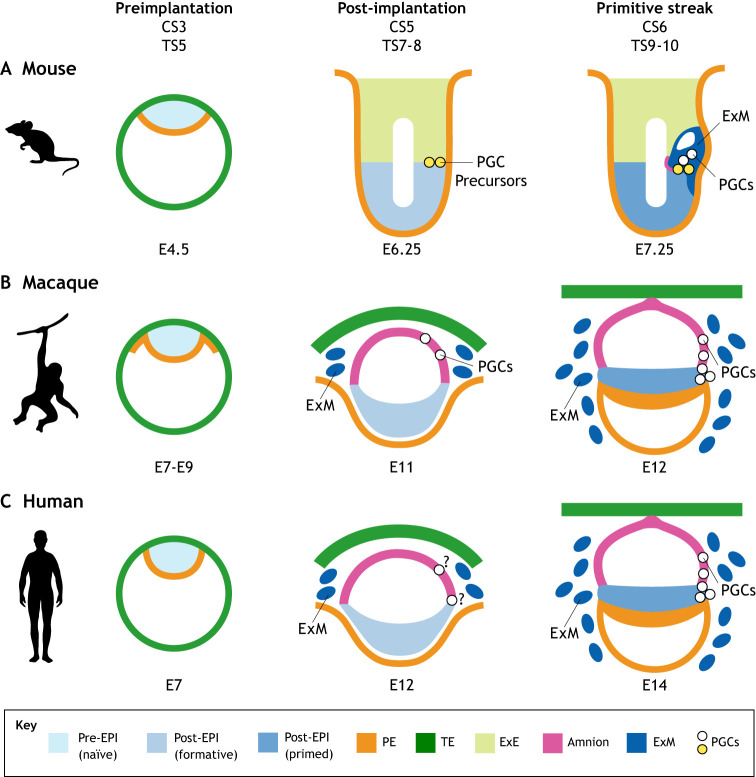
Fig. 1.
Representation of peri-implantation embryo development in mammals. Mouse (A), monkey (B) and human (C) development. Formative pluripotency is described only in mouse and is therefore inferred in the other species. (A) In mouse embryos, the egg cylinder stages begin at Theiler stage (TS) 7. A pre-streak embryo is shown at embryonic day E6.25, which can be assigned to late TS8 with the specification of Blimp1+ primordial germ cell (PGC) precursors (yellow). Primitive streak formation begins at TS9, with mPGC specification from the precursors occurring late in TS9 and continuing in TS10. (B) In the monkey (cyno), cyPGCs are first identified at E11 [Carnegie stage (CS) 5c] in the amnion, with morphological evidence of primitive streak formation occurring in CS6. (C) In humans, ex vivo culture indicates that hPGCs can be identified before primitive streak formation at E12. ‘?’ indicates that the cell layer of origin is not yet known for humans. At CS6, cyPGCs are found in the posterior primitive streak. In the mouse embryo, bone morphogenetic protein 4 (BMP4) and BMP8b are produced from extra-embryonic endoderm (ExE) to induce mPGC specification. In the nonhuman primate embryo, BMP4 is reported as being produced by amnion cells and possibly by extra-embryonic mesoderm (ExM). In the human embryo, the cell of origin is not yet known, and the source of BMP for inducing PGC specification is also unclear. Supporting embryonic and extra-embryonic tissue types are depicted as: preimplantation epiblast (pre-EPI), represented in culture by the naïve state; post-implantation epiblast (post-EPI), represented by the formative and primed states; primitive endoderm (PE); and trophectoderm (TE).
Specification of mouse PGCs
One of the earliest reports of mPGC specification identified alkaline phosphatase-positive mPGCs at E7.5, located outside the mouse embryo in the extra-embryonic mesoderm (ExM) (Ginsburg et al., 1990). However, using elegant transplantation experiments it was revealed that both the proximal and distal ends of the posterior post-EPI at E6.5 are equally competent for mPGC specification, provided that the epiblast is positioned adjacent to the ExE (Tam and Zhou, 1996), indicating that the specification of mPGC precursors is reliant on short-range signals from the adjacent ExE, before mPGCs migrate to the ExM (Saitou et al., 2002). Using lineage tracing, it has been revealed that the mPGC precursors in the posterior post-EPI originate at E6.25 as a small number of cells positive for B-lymphocyte-induced maturation protein 1 (Blimp1; also known as Prdm1) adjacent to the ExE (Ohinata et al., 2005). Indeed, subsequent single-cell analyses have revealed that the Blimp1-positive mPGC precursors progress through a Sox2-negative Hoxb1-positive state, with essentially all of them remaining in the germline to re-express Sox2 and repress Hoxb1 (Kurimoto et al., 2008) (Fig. 1A). From around E7.25, mPGCs upregulate early onset PGC specification genes, including transcription factor AP-2γ (Tfap2c), Prdm14 and Tdrd5 (Yamaji et al., 2008; Kurimoto et al., 2008; Weber et al., 2010). Upregulation of developmental pluripotency associated 3 (Dppa3; also known as Stella), tissue non-specific alkaline phosphatase (Tnap) and Nanos3 occur by E7.5 (Saitou et al., 2002; Kurimoto et al., 2008) (Table 1).
Table 1.
Key features of primordial germ cell (PGC) development in different mammals
The major signaling molecule secreted by the ExE that acts on the posterior post-EPI to initiate mPGC specification is bone morphogenetic protein 4 (BMP4) (Lawson et al., 1999; Ohinata et al., 2009). During egg cylinder formation, BMP4 not only signals to the posterior post-EPI through canonical BMP receptors and the downstream Smad family of signal transducers (Zhao, 2003; Saitou and Yamaji, 2010; Senft et al., 2019), but also through activin A receptor type 1 (Acvr1; also known as Alk2) on VE cells to indirectly facilitate specification of the mPGC precursors (de Sousa Lopes et al., 2004). In addition, BMP8b (expressed by the ExE) and BMP2 (expressed by the VE) also regulate mPGC specification, as shown by a lack of or a severe reduction in the number of mPGCs in BMP8b- and BMP2-null mutant mice, respectively (Ying et al., 2000; Ying and Zhao, 2001).
Although mPGCs are specified in the posterior post-EPI, the entire epiblast could, in theory, be competent to develop into mPGCs if inhibitory signals are not produced by the anterior visceral endoderm (AVE) to restrict the field of BMP signaling to the posterior region (Ohinata et al., 2009). The AVE is a derivative of the PE, with BMP8b secretion from the ExE responsible for regulating AVE development (Ohinata et al., 2009). In addition, Wnt3 secreted by the posterior post-EPI is necessary to establish competency for mPGC specification, because Wnt3-deficient embryos fail to express Blimp1 or Prdm14 in the post-EPI, despite expression of BMP4 in the ExE (Ohinata et al., 2009). Wnt3 also activates the mesodermal-associated transcription factor brachyury (T) in post-EPI cells, with T regulating expression of Blimp1 (Aramaki et al., 2013). Further insights into the role of these signaling effectors have been established from in vitro model systems discussed in subsequent sections. For a discussion on the timing of PGC specification in the mouse relative to other species, see Box 2.
Box 2. Primordial germ cell specification timing.
The length of primordial germ cell (PGC) specification from PGC precursors differs within the mammalian clade and may be related to the differing lengths of gestation. In mPGC specification, the process of recruiting the Blimp1-positive mPGC precursors (first identified at E6.25) ceases around E7.5 (Ohinata et al., 2009). In the pig, pPGC precursors are first identified at E11.5, with recruitment estimated to end by E15.5 (Kobayashi et al., 2017). In humans and nonhuman primates, the timing of PGC specification is referenced in Carnegie stages (CS), a system by which embryo development is ordered based upon the appearance of morphological structures rather than time. Staging embryos using the CS system enables human development to be measured against other species, including monkeys, as well as the Theiler staging (TS) used for the mouse. Importantly, PGC specification is reported to begin in CS5 in humans and CS5c in monkeys. This timing loosely corresponds to human and monkey PGC specification, beginning around E11-E13. It remains to be determined when PGC specification ends in humans; however, given that cyPGCs are still detected in the amnion at E16 (Sasaki et al., 2016), it could be speculated that, similar to pPGC specification, recruitment from cyPGC precursors occurs over an extended time before the window of cyPGC specification finally closes.
PGC specification in the monkey embryo
Models of peri-implantation and early post-implantation monkey embryo development have relied primarily on the use of rhesus and cyno macaques (Macaca mulatta and Macaca fascicularis) and, to a lesser extent, the common marmoset monkey (Callithrix jacchus). Morphological studies examining developmental stages from fertilization to formation of the blastocyst have suggested that the pre-implantation window is similar to the mouse, albeit with differences in timing (Nakamura et al., 2016; Boroviak and Nichols, 2017) (Box 2; Fig. 1B). Here, we describe and compare the stages of early development in rhesus, cyno and marmosets, highlighting similarities and differences from mouse development, as well as how these differences may impact the signaling environment in the peri-implantation embryo during primate PGC specification.
Pre-implantation development in nonhuman primates
As in the mouse, the inner cell mass cells (ICMs) of nonhuman primate blastocysts segregate into pre-EPI and PE immediately before implantation (Heuser and Streeter, 1941; Enders and Schlafke, 1981; Nakamura et al., 2016). Studies in the rhesus have indicated that TE/PE/pre-EPI lineage segregation occurs between E5 and E7 (Enders and Schlafke, 1981; Liu et al., 2018) (Fig. 1B). In contrast, in vitro culture of cyno pre-implantation embryos suggests that this lineage segregation occurs between E7 and E9, indicating some differences in developmental timing between macaque species (Nakamura et al., 2016). Similar dynamics to cyno pre-implantation have also been observed in marmoset embryos, though further stages of early embryogenesis are then delayed compared with other primates (Boroviak et al., 2015, 2018; Phillips, 1976). Interestingly, in cyno embryos, the PE begins to expand to cover the blastocoel cavity prior to hatching from the zona pellucida, which is required for implantation (Nakamura et al., 2016). This has not been observed in mice or humans (Fig. 1B).
Peri-implantation development in nonhuman primates
Despite relative similarities in the pre-implantation stages, the peri-implantation and early post-implantation stages are different between monkeys and mice. Starting at the point of embryo implantation into the uterine epithelium, it is the polar TE, rather than the mural TE, of monkey embryos that proliferates and differentiates into cytotrophoblast cells that then become the multinucleated invasive syncytiotrophoblast (Enders, 1989; Enders et al., 1983; Enders and Lopata, 1999; Smith et al., 1987; Wislocki and Bennett, 1943). Furthermore, as the embryo implants, monkey embryos develop into a bilaminar disc structure, rather than an egg cylinder (Boroviak and Nichols, 2017; Enders et al., 1986; Heuser and Streeter, 1941). Formation of the bilaminar disc involves cavitation of the post-EPI to form the amniotic cavity at around E10.5 in rhesus embryos, E11 in cyno embryos and by E16 in marmoset embryos (Enders et al., 1986; Moore, 1985). The post EPI-derived cells neighboring the polar TE become amnion cells, whereas the post-EPI cells on the floor of the amniotic cavity form the embryonic disc (Enders and King, 1988; Luckett, 1975; Moore, 1985). During amniotic cavity formation the PE cells continue to proliferate and line the mural TE with a layer of cells and extracellular matrix known as Heuser's membrane (Enders et al., 1986; Luckett, 1975; Sasaki et al., 2016). The PE cells will also form a secondary yolk sac structure that is unique to anthropoid primates (Luckett, 1978). Emergence of the ExM can be observed between E12 and E14 in the rhesus, and between E11 and E15 in cyno, immediately before and coincident with gastrulation (Enders and King, 1988; Luckett, 1978; Nakamura et al., 2016). In contrast to the mouse, where the ExM first emerges from the post-EPI, anatomical studies have suggested that the earliest monkey ExM originates from PE-derived visceral endoderm (Enders and King, 1988). However, this has yet to be confirmed using lineage-tracing methods. The rhesus and cyno ExM surrounds the bilaminar disc embryo in the space between the cytotrophoblast and the amniotic epithelial cells (Enders and King, 1988; Nakamura et al., 2016) (Fig. 1B).
Specification of nonhuman primate PGCs
Studying early post-implantation monkey embryos is scientifically challenging because of the highly specialized surgical and animal care requirements to work with pregnant monkeys in research. Yet careful single-cell immunofluorescence studies of early post-implantation cyno embryos have shown that cyno PGCs (cyPGCs) are first identified in the extra-embryonic dorsal amnion at E11. This discovery was based on co-expression of the cyPGC markers Sox17 and Tfap2c in a subset of dorsal amnion cells (Sasaki et al., 2016). As described previously, in egg cylinder mouse embryos the ExE is essential to generating the source of BMP4 required for mPGC specification. However, a structure equivalent to the ExE is not present in any primate studied to date. Given the lack of ExE in monkey embryos, a major unanswered question is where is the location of the signaling source for primate PGC specification? Strong BMP4 expression has been detected throughout the amnion in a few embryos at E11, prior to the emergence of Sox17/Tfap2c cyPGCs, with expression shifting to the proximal/posterior amnion at E16. As determined in mice, Wnt3 is necessary for cells to interpret the BMP4 signal and specify mPGCs (Ohinata et al., 2009); in monkey embryos, Wnt3a has been detected in the amnion at E11, as well as in the adjacent cytotrophoblast. Together, these results suggest that, in the absence of an ExE-like tissue, the amnion itself could be providing autocrine signals necessary for cyPGC specification. Alternatively, the ExM could be an extra-embryonic source of signals; weak BMP4 expression has been detected in the ExM of a single E11 embryo, although further investigations are required to draw robust conclusions (Sasaki et al., 2016). BMP2 has also been robustly detected in the VE at E11, which may indicate a putative role in cyPGC specification similar to mPGCs. Downstream targets of both BMP and Wnt signaling are expressed exclusively in the amnion compared to the epiblast at E11, while antagonists are expressed in the VE, suggesting that the amnion is the primary recipient of BMP signaling and further supporting the idea that the amnion is a source of cells at E11 that are receptive to signals for cyPGC specification (Sasaki et al., 2016) (Table 1).
In egg cylinder mouse embryos, mPGC specification is complete by E7.5 (Ohinata et al., 2009). In cyno embryos, cyPGCs can still be identified in the dorsal amnion at E17, 6 days after the first cyPGCs are identified, with additional cyPGCs located between the post-EPI and PE. Therefore, it could be speculated that cyPGC specification is an extended process similar to the pig (Box 2), with ongoing recruitment from PGC precursors before the window of PGC specification has closed. Alternatively, PGCs detected at the later stages could be due to mis-migration, and this remains to be resolved. In addition to Sox17/Tfap2c double-positive cells, the competency factor T is also broadly expressed in the dorsal amnion around the time of cyPGC specification. However, it remains to be shown through lineage-tracing experiments whether the Sox17/Tfap2c double-positive cells in the amnion found prior to gastrulation correspond to the earliest cyPGCs. Furthermore, the relationship between these cells and those identified between the post-EPI and PE 24 hours (h) later remains to be determined.
PGC specification in the human embryo
Immediately prior to implantation, the ICM of human blastocysts also segregates into pre-EPI and PE lineages. Upon implantation, similar to nonhuman primates, human embryos develop into a bilaminar disc (Boroviak and Nichols, 2017; Cockburn and Rossant, 2010; Wamaitha and Niakan, 2018) (Fig. 1C). In order to study human development during implantation, the Carnegie and Kyoto collections of human embryos have been invaluable resources for the scientific community (Hertig et al., 1956; Hill, 2018; Nishimura et al., 1968; Müller and O'Rahilly, 1987; O'Rahilly and Müller, 2010; Yamaguchi and Yamada, 2018). In humans, embryo development is measured in Carnegie Stages (CS), which measures development based upon the ordered formation of key features, rather than a strict reliance on time after fertilization (Box 2). In human embryos, human PGCs (hPGCs) have been identified as early as CS5.
Human peri-implantation development
During CS5, human embryo implantation occurs and the amniotic sac containing the amnion and bilaminar embryonic disc are established. These events involve differentiation of the blastocyst TE cells into syncytiotrophoblast, which invades the maternal uterine wall, while the cytotrophoblast cells self-renew (Burton et al., 1999). Inside the implanting blastocyst, the pre-EPI and PE of the pre-implantation embryo transition into the post-EPI and PE of the bilaminar disc, with the amniotic sac and the primary yolk sac also forming (Hertig et al., 1956; Luckett, 1975). However, unlike the egg-cylinder mouse embryo, PE cells in the human embryo do not take on the role of surrounding the EPI and TE, and, similar to monkeys, there is no equivalent tissue to the ExE.
Specification of human PGCs
The first widely cited description of hPGCs in the post-implantation human embryo was in the yolk sac endoderm at ∼E28, corresponding to CS12 (Witschi, 1948). However, additional histological evaluation of the ‘Harvard 55’ embryo in CS6a (∼E13 post-fertilization) identified hPGCs ‘stuffed with glycogen’ near the edge of the embryonic disc (Hertig et al., 1958). There has been a single report of putative hPGCs at CS5 in the so-called ‘Werner embryo’ (∼E12), where large round cells, presumed to be hPGCs, are identified in the post-EPI (Hertig et al., 1958; O'Rahilly and Müller, 2010). Single-cell transcriptome analyses of cells in the rostral, caudal and yolk sac regions of a single CS7 embryo has revealed that these earliest hPGCs are similar to PGCs in monkeys and pigs, and co-express the PGC marker genes SOX17 and NANOS3 (Tyser et al., 2020 preprint). The analysis of single embryos in each of these studies highlights the challenges of studying human development in the peri-implantation window. These analyses reinforce the reliance on comparative studies against other species and on new technologies in embryo culture, as well as stem cell models to study key developmental events that are specific to the germline and essential for human reproduction (Table 1; Fig. 1C).
DNA methylation remodeling and X-chromosome reactivation in PGCs
After specification, PGC development continues with epigenetic reprogramming. Completion of PGC epigenetic reprogramming occurs after entrance into the gonads and sex-specific differentiation. Here, we focus on two major epigenetic marks that are progressively removed soon after PGCs are induced: DNA methylation and the reactivation of the inactive X (Xi) in female embryos. Specifically in this section, we highlight the mouse embryo as a source of key information on the dynamics of DNA methylation remodeling. In addition, we highlight the need for stem cell and ex vivo embryo models to address key questions in DNA demethylation and X-chromosome reactivation, which cannot be easily studied using human tissue.
DNA methylation dynamics during PGC specification
The mouse has been used extensively to study the kinetics of DNA demethylation during and after mPGC specification (Guibert et al., 2012; Kobayashi et al., 2013; Seisenberger et al., 2012; Smith et al., 2012). In post-EPI mouse egg cylinder embryos ∼70% of cytosines in a CpG sequence context are methylated (Seisenberger et al., 2012; Smith et al., 2012). DNA methylation levels have not been quantified at single-base resolution in committed mPGCs between E7.25 and E8.0. However, targeted sequencing of a small number of promoters at E8.5 suggests that mPGCs are highly methylated; by E9.5 CpG DNA methylation levels precipitously drop to ∼30% (Guibert et al., 2012; Seisenberger et al., 2012; Seki et al., 2005). A further drop in DNA methylation is observed between E9.5 and E13.5, at which point the PGCs are located in the gonad (Kobayashi et al., 2013; Hill et al., 2018; Seisenberger et al., 2012), leading to the hypothesis that newly specified mPGCs inherit high levels of DNA methylation from the post-EPI, which is then erased from the mPGC genome during mPGC development.
In the monkey, immunofluorescence for 5-methylcytosine (5mC) has shown that, from at least E20 in the cyno (Sasaki et al., 2016) and from E28 in the rhesus (Sosa et al., 2018), the PGC genome has extremely low levels of 5mC relative to surrounding somatic cells. This is because 5mC has been oxidized to 5-hydroxymethyl cytosine (5hmC) (Sosa et al., 2018), a new epigenetic mark that serves as an intermediate in the DNA demethylation process. In the pig, pPGCs in the primitive streak have equivalent levels of 5mC to the soma, with 5mC demethylation occurring concomitantly with increasing levels of 5hmC at E14 (Kobayashi et al., 2017). In the early post-implantation human embryo, DNA demethylation has not been clearly defined and will require revisiting the international conversation on ex vivo culture of human embryos beyond 14 days post-fertilization and/or primitive streak formation (Hyun et al., 2016), which is when sufficient numbers of hPGCs could be recovered for analysis.
X-chromosome reactivation
In addition to DNA demethylation, female mPGCs also reactivate the Xi (de Sousa Lopes et al., 2008; Sangrithi et al., 2017; Sugimoto and Abe, 2007). X-chromosome inactivation (XCI) is associated with expression of Xi-specific transcript (Xist) (Brown et al., 1991). Around the time of mPGC specification, XCI is identified in >90% of post-EPI mouse cells (Rastan, 1982), and early reports indicated that Xist is expressed by mPGCs at E7.0 (Sugimoto and Abe, 2007). Reactivation of the Xi involves Xist repression in mPGCs after E9.5 (de Sousa Lopes et al., 2008; Sangrithi et al., 2017). In the pig, repression of Xist RNA and biallelic expression of X-linked genes occurs at E14 at the end of pPGC induction (Zhu et al., 2020). In female hPGCs, there is now strong evidence that X-linked genes are biallelically expressed (Chitiashvili et al., 2020; Migeon and Jelalian, 1977; Vértesy et al., 2018), at a similar time to when promoter CGI methylation on the X chromosome is reported to be low (Tang et al., 2015). However, unlike female mPGCs, female hPGCs continue to express XIST RNA (Chitiashvili et al., 2020; Gkountela et al., 2015).
Expression of XIST and biallelic expression of X-linked genes in hPGCs could represent a state of X-chromosome dose compensation, first described in human pre-implantation embryos, called X-chromosome dampening (XCD) (Chitiashvili et al., 2020; Petropoulos et al., 2016). XCD involves repression of X-linked genes from both X chromosomes and is associated with biallelic expression of XIST, as well as a hominid-specific long noncoding RNA called X active coating transcript (XACT) (Vallot et al., 2013, 2017). Recent work has revealed that XIST is expressed in female hPGCs whereas XACT is expressed in both male and female PGCs, with repression of XIST and XACT occurring as PGCs begin sex-specific differentiation (Chitiashvili et al., 2020). XACT is expressed in chimpanzees, but it is not found in more evolutionarily distant primates, such as macaques (Casanova et al., 2019). Therefore, studying X-chromosome dose compensation at the time of hPGC specification will rely on information from in vitro human pluripotent stem cell models, as well as ex vivo culture of human embryos beyond 14 days.
In vitro and ex vivo models of PGC specification
Recent studies using pluripotent stem cell models of embryo development and ex vivo embryo culture have provided further insights into the cell and molecular requirements for PGC specification (Fig. 2A,B). In the mouse, culturing trophoblast stem cells (TSCs) with mouse embryonic stem cells (ESCs) results in the formation of an organized egg cylinder-like embryo structure called an ESC- and TSC-derived stem cell embryo (ETS-embryo), with mPGC specification occurring at the trophoblast/posterior-EPI boundary (Harrison et al., 2017) (Fig. 2B). These ETS-embryos do not contain VE, supporting the concept that the VE is not necessary for mPGC specification (Ohinata et al., 2009).
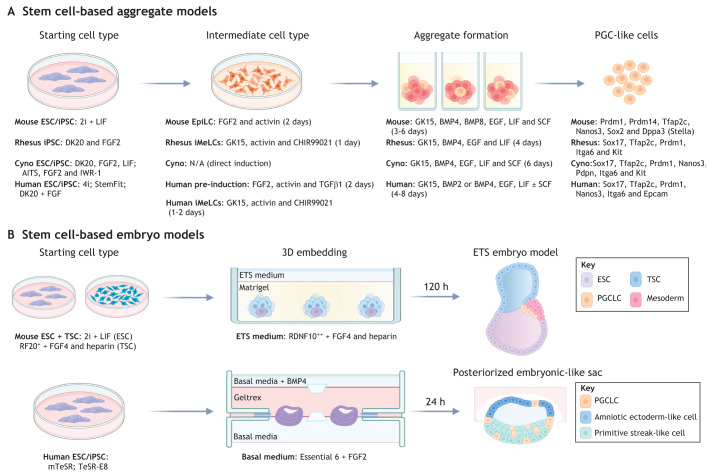
Fig. 2.
Stem cell models of primordial germ cell induction. Modeling primordial germ cell (PGC) development with embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) uses either aggregate models or embryo models. (A) Aggregate models involve the generation of disorganized three-dimensional aggregates with primordial germ cell (PGC)-like cells (PGCLCs) induced from either a progenitor intermediate or by direct induction in response to BMP4 and other cytosines. Aggregate models have been created in the mouse (Hayashi et al., 2011, 2012), rhesus (Sosa et al., 2018), cyno (Sakai et al., 2020) and human (Irie et al., 2015; Sasaki et al., 2015; Chen et al., 2017, 2019). Growth factors, inhibitors and media types used for each PGCLC induction protocol are depicted. Proteins used to identify PGCLCs in each species are shown. (B) Embryo models involve modeling the spatial organization of some lineages in the peri-implantation embryo; these models have identified PGCLCs in the embryonic stem cell (ESC) and trophoblast stem cell (TSC) embryo model generated with mouse cells (Harrison et al., 2017), as well as in the posteriorized embryonic-like sac model generated with human cells (Zheng et al., 2019). AITS, 50% advanced RPMI, 50% neurobasal medium, and 1× insulin, transferrin and selenoprotein; BMP, bone morphogenetic protein; cyno, cynomolgus; DK20, DMEM/F-12 and 20% KSR; EGF, epidermal growth factor; EpiLC, epiblast-like cell; ETS, ES- and TS-derived stem cell embryo; FGF, fibroblast growth factor; GK15, GMEM and 15% KSR; iMELCs, incipient mesoderm-like cell; iPSC, induced pluripotent stem cell; KSR, knockout serum replacer; mTeSR, commercial media; RDNF, 50% RPMI, 25% DMEM, 25% neurobasal medium and 10% fetal bovine serum (FBS); RF, RPM1 and 20% FBS; SCF, stem cell factor; TeSR-E8, TeSR-Essential 8 commercial media. Figure created with BioRender.com.
Mouse cell models of PGC development
ESCs are differentiated into PGC-like cells (PGCLCs) in vitro, a term that distinguishes them from endogenous PGCs. In the mouse, mPGCLC induction cannot occur directly from mouse ESCs cultured in 2i+LIF; instead, an intermediate epiblast-like cell (EpiLC) must first be induced (Hayashi et al., 2011). BMP4, BMP8 and other components are added to EpiLCs in order to robustly induce mPGCLCs within three-dimensional aggregates with an efficiency of ∼13% (Hayashi et al., 2011, 2012) (Fig. 2A). Importantly, EpiLCs are considered equivalent to post-EPI cells in the E5.5 embryo, and are thought to represent a ‘formative state’ of pluripotency (Smith, 2017). In contrast, mouse ESCs cultured in 2i+LIF are equivalent to pre-EPI cells of the blastocyst, and this pluripotent state is referred to as the ‘naïve’ or ‘ground state’ of pluripotency. A third state of pluripotency known as ‘primed’ is represented by in vitro cultured cells derived from egg cylinder mouse embryos called epiblast stem cells (EpiSCs) (Brons et al., 2007; Kojima et al., 2014; Tesar et al., 2007; Wu et al., 2015). Mouse PGCLC induction from mEpiSCs occurs at significantly lower efficiency (1.5%) compared with EpiLCs (Hayashi et al., 2011; Hayashi and Surani, 2009; Huang et al., 2012). Therefore, the transition from naïve to formative pluripotency (but before acquiring primed pluripotency) is likely to be another essential requirement for mPGC competency.
The differentiation of mPGCLCs from EpiLCs has revealed that ExE, ExM and VE cells are not required to specify mPGCLCs from male or female EpiLCs, provided that BMP4 and other molecules are present in the media (Hayashi et al., 2012, 2011). Using this in vitro system, the details of mPGC specification have been teased apart in even finer detail. For example, during mPGCLC induction, BMP4 and Wnt3 upregulate Blimp1 and T; the latter then directly targets and upregulates Blimp1, Prdm14 and Tfap2c for mPGC specification (Aramaki et al., 2013; Nakaki et al., 2013). Using this same model, overexpression of Prdm14, Blimp1 and Tfap2c in EpiLCs induces mPGCLC formation even if BMP4 is eliminated from the culture media (Aramaki et al., 2013; Magnúsdóttir et al., 2013). Doxycycline-induced overexpression of all three factors in the absence of cytokines causes robust expression of Blimp1 and Stella (34% of cells double positive) (Nakaki et al., 2013). Furthermore, induction of Prdm14 alone is capable of specifying mPGCLC fate in the absence of BMP signaling (3% Blimp1/Stella double-positive cells), as does Blimp1/Prdm14 (11.2%) and Blimp1/Tfap2c (2.6%) (Nakaki et al., 2013). Overexpression of the transcription factor Nanog can also induce mPGCLCs by promoting the expression of Blimp1 and Prdm14 (Murakami et al., 2016). With regard to DNA methylation, similar to the embryo, mPGCLCs are also highly methylated upon PGCLC specification and undergo X-chromosome reactivation (Hayashi et al., 2012; Shirane et al., 2016). In summary, combining information from in vivo and in vitro models of mPGC/PGCLC induction provides unprecedented details on the epigenome and on the relationships and networks of transcription factors that promote mPGC fate.
Nonhuman primate cell models of PGC development
In vitro nonhuman primate stem cell models
Monkey PGCLCs can also be induced from pluripotent stem cells using modified versions of the aggregate-based cytokine culture system developed in the mouse (Sakai et al., 2020; Sosa et al., 2018). Culture conditions include BMP4, but not BMP8, and use either an incipient-mesoderm intermediate (Sosa et al., 2018) or direct induction (Sakai et al., 2020). In the rhesus, cells triple positive for Sox17, Blimp1 and Tfap2c are observed in aggregates beginning 1 day after BMP4 exposure (Sosa et al., 2018). These three factors are crucial for both cyno and human PGC development (Irie et al., 2015; Sasaki et al., 2016). The newly specified rhesus PGCLCs have yet to initiate global epigenetic reprogramming and thus correspond to early in vivo PGCs, although partial reprogramming can be induced following xenotransplantation of rhesus PGCLCs into mouse testes (Sosa et al., 2018). In the cyno, Sox17, Blimp1 and Tfap2c are strongly upregulated in aggregates beginning 2 days after BMP4 exposure, with cyPGCLCs being transcriptionally similar to cyPGCs from E13 embryos onwards (Sakai et al., 2020) (Fig. 2A). Examining transcriptional dynamics during PGCLC induction in mice, cyno and human has revealed differences in gene expression between the mouse and the two primates, and further differences between human and cyno PGCLCs. For example, upregulation of Sox17, Blimp1 and Tfap2c occurs more rapidly during cyPGCLC induction compared with human, which likely explains the ability to dispense with the incipient-mesoderm intermediate, and genes associated with locomotion and migration are highly expressed in human PGCLCs compared with the cyno (Sakai et al., 2020). Although primate early embryogenesis models may show more similarity to humans, as would be predicted given the common bilaminar disc post-implantation embryo, these results indicate that there are still likely to be important differences.
Ex vivo nonhuman primate embryo culture
Technological advances in ex vivo primate embryo attachment culture models are crucially needed for progress in understanding mechanisms common to monkey and human PGC development ex vivo and in particular DNA demethylation and reactivation of Xi. Monkey embryo attachment culture models have recently been developed based on techniques to generate peri-implantation stage mouse or human embryos from blastocysts (Bedzhov et al., 2014; Deglincerti et al., 2016; Shahbazi et al., 2016). Using this approach, two different studies have shown that cyno bilaminar disc embryos self-assemble entirely in vitro (Ma et al., 2019; Niu et al., 2019).
Ma and colleagues plated cyno blastocysts onto Matrigel-coated dishes and cultured them in normoxic conditions according to a modified method used for mouse embryo culture (Bedzhov et al., 2014; Ma et al., 2019). This results in embryo ‘attachment’ in two-thirds (67%) of cyno blastocyts by E9-E10, and bilaminar disc formation in about one-quarter (27%) of embryos by E13-E14. Although the remaining embryos eventually attached by E11-E12, these had a much lower chance of successful disc formation. Meanwhile, Niu and colleagues used conditions that are more similar to the human embryo culture protocol (Deglincerti et al., 2016; Shahbazi et al., 2016). Cyno blastocysts were first cultured under hypoxia before plating onto tissue culture-treated plastic under normoxic conditions, following the rationale that, because in vivo embryos exist in a hypoxic environment, successful attachment may be better facilitated under these conditions (Niu et al., 2019). Indeed, here the majority (92%) of blastocysts attached by E10, forming a bilaminar disc by E13.
In both cases, cyno embryo attachment culture was performed up to E20, beyond the 14-day limit that applies to human embryos. Consequently, hallmarks of gastrulating cells could be observed (Niu et al., 2019). Ma and colleagues also observed neural structures in embryos at E19 (Ma et al., 2019). At earlier stages, Sox17/Tfap2c double-positive putative cyPGCs could be detected at E11-E12 in the amnion, as well as beneath the EPI and in the VE (Ma et al., 2019). Indeed, this timing correlates with in vivo observations for cyPGC specification reported at CS5c (Sasaki et al., 2016). Niu and colleagues also observed Sox17/Tfap2c double-positive cyPGCs within the amnion at E13, and in the junction between the amnion and post-EPI at E14-E16 (Niu et al., 2019). Curiously, transcriptome analysis identified two clusters of cells with cyPGC-like identity: one that clustered with EPI cells and one that did not, indicating possible heterogeneity in cyPGC development within the in vitro attachment culture (Niu et al., 2019). Transcriptome analysis by Ma and colleagues also designated a population of putative early amnion cells expressing BMP4 (which might be providing PGC specification signals), while Niu and colleagues identified Wnt3 in the EPI at E13 and E17 (Ma et al., 2019; Niu et al., 2019); however, further study is required to identify possible signaling effectors in these models. Together, these two studies provide useful tools to interrogate the transcriptional profiles of primate peri-implantation lineages, and future studies could focus on understanding DNA demethylation in PGCs.
Human cell models of PGC development
Ex vivo human embryo culture
Similar to the cyno, ex vivo human embryo attachment culture has been crucial to understanding the cell and molecular events in peri-implantation and early post-implantation development in the absence of a uterus (Deglincerti et al., 2016; Shahbazi et al., 2016). Initial studies cultured human embryos to E13 in vitro, and noted morphological similarities to peri-implantation embryos at CS5, including the emergence of prospective amniotic and yolk sac cavities, although these could not be maintained (Deglincerti et al., 2016; Shahbazi et al., 2016). These first papers did not describe hPGCs, but subsequent work has used human ex vivo embryo attachment culture to investigate these cells (Chen et al., 2019; Popovic et al., 2019).
Chen and colleagues thawed 127 E5, E6 or E7 blastocyst-stage embryos and placed these into attachment culture under normoxic conditions. Eighty-five embryos (67%) successfully adhered and were cultured to E10, E11 or E12. Of these, 78 were imaged, and 26 embryos analyzed at E12. Cells co-expressing the PGC markers Nanog, Tfap2c and Sox17 were identified at E12 in 10% of embryos (2/26) where the three major lineages (post-EPI, VE and TE) could simultaneously be detected using immunofluorescence (Chen et al., 2019). Consistent with these observations, Popovic and colleagues have identified Oct4/Sox17/Ifitm3/Pdpn-positive, Sox2-negative cells at E12, designated as putative hPGCs. These cells were present in 34.8% of embryos (8/23), in which Oct4 (encoded by POU5F1) expression could be detected by immunofluorescence (Popovic et al., 2019). For this study, 141 E6 blastocyst stage embryos (cultured from pre-implantation embryos thawed at E2 or E3) were plated and cultured to E12. Forty-nine embryos (35%) were viable at E12, and 20 of these were excluded from further analysis because OCT4 expression could not be detected (Popovic et al., 2019). Further improvements to ex vivo human embryo attachment culture are required to not only increase the likelihood of successful attachment, but also to minimize embryo deterioration. Doing so requires a source of human embryos consented for research, which is not always practical for ongoing research activities given the ethical considerations surrounding human embryo research, coupled with funding restrictions in some parts of the world.
In vitro human stem cell models
Cell and molecular mechanisms involved in hPGC specification are studied using human ESCs (hESCs) and human induced pluripotent stem cells (hiPSCs). Unlike mouse 2i+LIF ESCs, hESCs/hiPSCs are reported to be in the primed state of pluripotency given their similarity to mouse EpiSCs (Brons et al., 2007; Tesar et al., 2007). However, hESCs are derived from pre-implantation blastocysts, not post-implantation embryos, and their relatively flexible epigenome enables them to be reverted to the naïve ground state of pluripotency by simply changing the culture conditions, unlike mouse EpiSCs, which usually require transgene expression (Guo et al., 2009; Hanna et al., 2009; Silva et al., 2009; Takashima et al., 2014; Theunissen et al., 2014). Rare spontaneous reversions have been observed in a small proportion of dissociated single mEpiSCs cultured in stringent mouse ESC media; however, this varies widely between mEpiSC lines (Bao et al., 2009; Bernemann et al., 2011). Regardless, self-renewing mEpiSCs in vitro have low germline competency, while hESCs/hiPSCs can be differentiated efficiently into hPGCLCs (Irie et al., 2015; Hayashi et al., 2011; Sasaki et al., 2015). Collectively, this suggests that hESCs/hiPSCs and mEpiSCs are not functionally equivalent, even though they do share molecular characteristics. Instead, it is possible that hESCs/hiPSCs are more similar in function to mEpiLCs, which are highly competent for hPGCLC specification in vitro.
The first robust directed differentiation studies in which hESCs/hiPSCs were routinely differentiated into hPGCLCs revealed that, as in mouse, BMP4 is essential for specification. In addition, a new transcription factor network centered on SOX17, PRDM1 and TFAP2C was found to be required for hPGCLC formation in vitro (Chen et al., 2017; Irie et al., 2015; Kojima et al., 2017; Sasaki et al., 2015). Prdm14 is also involved in hPGCLC induction; however, the downstream targets are different when compared with the mouse (Sybirna et al., 2020). These early studies involved differentiating hESCs/hiPSCs either directly in the presence of BMP4 and other cytokines, or after brief exposure to activin and Wnt agonists to induce an incipient mesoderm-like cell type, followed by exposure to BMP4 as disorganized three-dimensional (3D) aggregates. Single-cell RNA sequencing and pseudotime lineage tracing in this system has indicated that hPGCLCs are specified within 24-48 h of aggregate formation in the presence of BMP4 (Chen et al., 2019) (Fig. 2A). hPGCLC precursors emerge from a Wnt3/activin A-primed transitional pluripotent state, which has also been identified in the E12 attachment culture (Chen et al., 2019). In future studies, it will be interesting to determine whether the Wnt3/activin exposed pluripotent cells resemble the formative pluripotency of E5.5 mouse epiblasts.
After exposure to BMP4, pseudotime tracking predicts that the transcription factor Tfap2a becomes rapidly expressed in hPGC precursors, together with other lineage transcription factors traditionally associated with amnion, trophoblast and posterior primitive streak, including T and Cdx2 (Chen et al., 2019). Crucially, all these cell types differentiate in the presence of high levels of BMP4. Therefore, Tfap2a may simply serve as a read-out for high levels of BMP4 exposure; whether it is functionally involved in hPGCLC progenitor formation remains to be determined. hPGCLC specification next involves the rapid repression of Tfap2a, alongside transcription factors that mark the somatic lineage, such as Gata3 and Eomes. Once specified, hPGCLCs express Sox17, Tfap2c and Nanog, as in the embryo attachment system, as well as Blimp1 and Nanos3 (Chen et al., 2019). DNA methylation remodeling using the differentiation of naïve hESCs into EpiLCs, followed by hPGCLC differentiation has revealed similarities to the mouse (von Meyenn et al., 2016). However, studying XCR in hPGCLCs is challenged by the phenomenon of X-chromosome erosion (Mekhoubad et al., 2012), which is characterized by the abnormal and irreversible reactivation of both X chromosomes (Mekhoubad et al., 2012; Patel et al., 2017). Improvement to naïve hESC/hiPSC stem cell culture could overcome this barrier in the future (Sahakyan et al., 2017).
More recently, stem cell-based embryo models have been developed. In these models, hESCs/hiPSCs are differentiated in microfluidics chambers to model amniotic sac formation (Zheng et al., 2019) or in 3D Matrigel to model luminogenesis of the amnion (Shahbazi et al., 2017, 2016). Gastruloids have also been modeled as 3D anteroposterior-organized aggregate structures, and bioengineered surfaces are used to model epiblast disc differentiation, demonstrating three germ layer specification and somitogenesis (Moris et al., 2020; Warmflash et al., 2014). The amniotic sac model (Fig. 2B) reveals that induction of hPGCLCs from hESCs occurs within 24 h of BMP4 exposure, with hPGCLCs first identified in presumptive amnion and pre-primitive streak-EPI. By 36 h, hPGCLCs are observed in much greater quantity in the EPI-like compartment. Given the amniotic sac model collapses between 36-72 h, new innovations are required to study the next steps in embryo development and hPGC formation.
Conclusions and future perspectives
Given the practicality of the mouse model, we have reached a comprehensive understanding of mPGC specification and epigenetic reprogramming. However, there are many apparent and important differences between rodent and primate PGC development. Through culturing human and primate embryos ex vivo, observing cyPGC, rhesus PGC and pig PGC development in vivo, and modeling human embryos in vitro using hESCs/hiPSCs, we are beginning to understand the cell(s) of origin for PGCs in primates and other bilaminate embryos relative to mice. Limitations to these methods include a scarcity of donated human tissue and high cost of nonhuman primate studies, resulting in low power and limited sample size in observational and high-throughput sequencing studies. Furthermore, ex vivo human embryo culture is limited by the ‘14-day' rule and by embryo availability, and the crucial peri-implantation window cannot otherwise be observed in human development unless this rule is changed. Encouragingly, in vitro human pluripotent stem cell models are rapidly improving, although additional optimization is required to generate an integrated stem cell-based embryo model that represents all the cell types of the peri-implantation human embryo. That said, given the pace of this research area, it is expected that these techniques will be invaluable in understanding this developmental period.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The authors' research was funded by grants from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD079546, R01HD098278 and R01HD058047). S.W. is supported by funds from the Iris Cantor-UCLA Women's Health Center Executive Advisory Board (NCATS UCLA CTS1 grant number UL1TR001881). Deposited in PMC for release after 12 months.
References
- Aramaki, S., Hayashi, K., Kurimoto, K., Ohta, H., Yabuta, Y., Iwanari, H., Mochizuki, Y., Hamakubo, T., Kato, Y., Shirahige, K.et al. (2013). A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Dev. Cell 27, 516-529. 10.1016/j.devcel.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Armant, D. R. (2005). Blastocysts don't go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev. Biol. 280, 260-280. 10.1016/j.ydbio.2005.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, S., Tang, F., Li, X., Hayashi, K., Gillich, A., Lao, K. and Surani, M. A. (2009). Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 461, 1292-1295. 10.1038/nature08534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardot, E. S. and Hadjantonakis, A.-K. (2020). Mouse gastrulation: coordination of tissue patterning, specification and diversification of cell fate. Mech. Dev. 163, 103617. 10.1016/j.mod.2020.103617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedzhov, I., Leung, C. Y., Bialecka, M. and Zernicka-Goetz, M. (2014). In vitro culture of mouse blastocysts beyond the implantation stages. Nat. Protoc. 9, 2732-2739. 10.1038/nprot.2014.186 [DOI] [PubMed] [Google Scholar]
- Bernemann, C., Greber, B., Ko, K., Sterneckert, J., Han, D. W., Araúzo–Bravo, M. J. and Schöler, H. R. (2011). Distinct developmental ground states of epiblast stem cell lines determine different pluripotency features. Stem Cells 29, 1496-1503. 10.1002/stem.709 [DOI] [PubMed] [Google Scholar]
- Boroviak, T. and Nichols, J. (2017). Primate embryogenesis predicts the hallmarks of human naïve pluripotency. Development 144, 175-186. 10.1242/dev.145177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroviak, T., Loos, R., Lombard, P., Okahara, J., Behr, R., Sasaki, E., Nichols, J., Smith, A. and Bertone, P. (2015). Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev. Cell 35, 366-382. 10.1016/j.devcel.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroviak, T., Stirparo, G. G., Dietmann, S., Hernando-Herraez, I., Mohammed, H., Reik, W., Smith, A., Sasaki, E., Nichols, J. and Bertone, P. (2018). Single cell transcriptome analysis of human, marmoset and mouse embryos reveals common and divergent features of preimplantation development. Development 145, dev167833. 10.1242/dev.167833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons, I. G. M., Smithers, L. E., Trotter, M. W. B., Rugg-Gunn, P., Sun, B., de Sousa Lopes, S. M. C., Howlett, S. K., Clarkson, A., Ahrlund-Richter, L., Pedersen, R. A.et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191-195. 10.1038/nature05950 [DOI] [PubMed] [Google Scholar]
- Brown, C. J., Ballabio, A., Rupert, J. L., Lafreniere, R. G., Grompe, M., Tonlorenzi, R. and Willard, H. F. (1991). A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349, 38-44. 10.1038/349038a0 [DOI] [PubMed] [Google Scholar]
- Burton, G. J., Jauniaux, E. and Watson, A. L. (1999). Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the boyd collection revisited. Am. J. Obstet. Gynecol. 181, 718-724. 10.1016/S0002-9378(99)70518-1 [DOI] [PubMed] [Google Scholar]
- Casanova, M., Moscatelli, M., Chauvière, L. É., Huret, C., Samson, J., Liyakat Ali, T. M., Rosspopoff, O. and Rougeulle, C. (2019). A primate-specific retroviral enhancer wires the XACT lncRNA into the core pluripotency network in humans. Nat. Commun. 10, 5652. 10.1038/s41467-019-13551-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud, C., Yamanaka, Y., Pawson, T. and Rossant, J. (2006). Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 10, 615-624. 10.1016/j.devcel.2006.02.020 [DOI] [PubMed] [Google Scholar]
- Chen, D., Liu, W., Lukianchikov, A., Hancock, G. V., Zimmerman, J., Lowe, M. G., Kim, R., Galic, Z., Irie, N., Surani, M. A.et al. (2017). Germline competency of human embryonic stem cells depends on eomesodermin. Biol. Reprod. 97, 850-861. 10.1093/biolre/iox138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., Sun, N., Hou, L., Kim, R., Faith, J., Aslanyan, M., Tao, Y., Zheng, Y., Fu, J., Liu, W.et al. (2019). Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep. 29, 4568-4582.e5. 10.1016/j.celrep.2019.11.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitiashvili, T., Dror, I., Kim, R., Hsu, F.-M., Chaudhari, R., Pandolfi, E., Chen, D., Liebscher, S., Schenke-Layland, K., Plath, K.et al. (2020). Female human primordial germ cells display X-chromosome dosage compensation despite the absence of X-inactivation. Nat. Cell Biol. 22, 1436-1446. 10.1038/s41556-020-00607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn, K. and Rossant, J. (2010). Making the blastocyst: lessons from the mouse. J. Clin. Investig. 120, 995-1003. 10.1172/JCI41229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp, A. J. (1978). Interaction between inner cell mass and trophectoderm of the mouse blastocyst. I. A study of cellular proliferation. J. Embryol. Exp. Morphol. 48, 109-125. [PubMed] [Google Scholar]
- Coucouvanis, E. and Martin, G. R. (1995). Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell 83, 279-287. 10.1016/0092-8674(95)90169-8 [DOI] [PubMed] [Google Scholar]
- Deglincerti, A., Croft, G. F., Pietila, L. N., Zernicka-Goetz, M., Siggia, E. D. and Brivanlou, A. H. (2016). Self-organization of the in vitro attached human embryo. Nature 533, 251-254. 10.1038/nature17948 [DOI] [PubMed] [Google Scholar]
- de Sousa Lopes, S. M. C., Roelen, B. A. J., Monteiro, R. M., Emmens, R., Lin, H. Y., Li, E., Lawson, K. A. and Mummery, C. L. (2004). BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev. 18, 1838-1849. 10.1101/gad.294004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Lopes, S. M. C., Hayashi, K., Shovlin, T. C., Mifsud, W., Surani, M. A. and McLaren, A. (2008). X chromosome activity in mouse XX primordial germ cells. PLoS Genet. 4, e30. 10.1371/journal.pgen.0040030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, A. D. (1963). TROPHOBLASTIC GIANT CELL TRANSFORMATION OF MOUSE BLASTOCYSTS. J. Reprod. Fertil. 6, 465-466. 10.1530/jrf.0.0060465 [DOI] [PubMed] [Google Scholar]
- Enders, A. C. (1989). Trophoblast differentiation during the transition from trophoblastic plate to lacunar stage of implantation in the rhesus monkey and human. Am. J. Anat. 186, 85-98. 10.1002/aja.1001860107 [DOI] [PubMed] [Google Scholar]
- Enders, A. C. and King, B. F. (1988). Formation and differentiation of extraembryonic mesoderm in the rhesus monkey. Am. J. Anat. 181, 327-340. 10.1002/aja.1001810402 [DOI] [PubMed] [Google Scholar]
- Enders, A. C. and Lopata, A. (1999). Implantation in the marmoset monkey: expansion of the early implantation site. Anat. Rec. 256, 279-299. [DOI] [PubMed] [Google Scholar]
- Enders, A. C. and Schlafke, S. (1981). Differentiation of the blastocyst of the rhesus monkey. Am. J. Anat. 162, 1-21. 10.1002/aja.1001620102 [DOI] [PubMed] [Google Scholar]
- Enders, A. C., Hendrickx, A. G. and Schlafke, S. (1983). Implantation in the rhesus monkey: initial penetration of endometrium. Am. J. Anat. 167, 275-298. 10.1002/aja.1001670302 [DOI] [PubMed] [Google Scholar]
- Enders, A. C., Schlafke, S. and Hendrickx, A. G. (1986). Differentiation of the embryonic disc, amnion, and yolk sac in the rhesus monkey. Am. J. Anat. 177, 161-185. 10.1002/aja.1001770205 [DOI] [PubMed] [Google Scholar]
- Extavour, C. G. and Akam, M. (2003). Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130, 5869-5884. 10.1242/dev.00804 [DOI] [PubMed] [Google Scholar]
- Gardner, R. L. and Rossant, J. (1979). Investigation of the fate of 4*5 day post-coitum mouse inner cell mass cells by blastocyst injection. J. Embryol. exp. Morph. 52, 141-152. [PubMed] [Google Scholar]
- Ginsburg, M., Snow, M. H. and McLaren, A. (1990). Primordial germ cells in the mouse embryo during gastrulation. Development 110, 521-528. [DOI] [PubMed] [Google Scholar]
- Gkountela, S., Zhang, K. X., Shafiq, T. A., Liao, W.-W., Hargan-Calvopiña, J., Chen, P.-Y. and Clark, A. T. (2015). DNA demethylation dynamics in the human prenatal germline. Cell 161, 1425-1436. 10.1016/j.cell.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert, S., Forné, T. and Weber, M. (2012). Global profiling of DNA methylation erasure in mouse primordial germ cells. Genome Res. 22, 633-641. 10.1101/gr.130997.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, G., Yang, J., Nichols, J., Hall, J. S., Eyres, I., Mansfield, W. and Smith, A. (2009). Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063-1069. 10.1242/dev.030957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna, J., Markoulaki, S., Mitalipova, M., Cheng, A. W., Cassady, J. P., Staerk, J., Carey, B. W., Lengner, C. J., Foreman, R., Love, J.et al. (2009). Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell 4, 513-524. 10.1016/j.stem.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, S. E., Sozen, B., Christodoulou, N., Kyprianou, C. and Zernicka-Goetz, M. (2017). Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356, eaal1810. 10.1126/science.aal1810 [DOI] [PubMed] [Google Scholar]
- Hayashi, K. and Surani, M. A. (2009). Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development 136, 3549-3556. 10.1242/dev.037747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, K., Ohta, H., Kurimoto, K., Aramaki, S. and Saitou, M. (2011). Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146, 519-532. 10.1016/j.cell.2011.06.052 [DOI] [PubMed] [Google Scholar]
- Hayashi, K., Ogushi, S., Kurimoto, K., Shimamoto, S., Ohta, H. and Saitou, M. (2012). Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 338, 971-975. 10.1126/science.1226889 [DOI] [PubMed] [Google Scholar]
- Hertig, A. T., Rock, J. and Adams, E. C. (1956). A description of 34 human ova within the first 17 days of development. Am. J. Anat. 98, 435-493. 10.1002/aja.1000980306 [DOI] [PubMed] [Google Scholar]
- Hertig, A. T., Adams, E. C., McKAY, D. G., Rock, J., Mulligan, W. J. and Menkin, M. F. (1958). A thirteen-day human ovum studied histochemically. Am. J. Obstet. Gynecol. 76, 1025-1038; discussion 1040-1043. 10.1016/0002-9378(58)90185-6 [DOI] [PubMed] [Google Scholar]
- Heuser, C. and Streeter, G. (1941). Development of the macaque embryo. Contributions to Embryology 29, 15-55. [Google Scholar]
- Hill, M. A. (2018). Two web resources linking major human embryology collections worldwide. Cells Tissues Organs 205, 293-302. 10.1159/000495619 [DOI] [PubMed] [Google Scholar]
- Hill, P. W. S., Leitch, H. G., Requena, C. E., Sun, Z., Amouroux, R., Roman-Trufero, M., Borkowska, M., Terragni, J., Vaisvila, R., Linnett, S.et al. (2018). Epigenetic reprogramming enables the transition from primordial germ cell to gonocyte. Nature 555, 392-396. 10.1038/nature25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. and Cross, J. C. (2010). Development and function of trophoblast giant cells in the rodent placenta. Int. J. Dev. Biol. 54, 341-354. 10.1387/ijdb.082768dh [DOI] [PubMed] [Google Scholar]
- Huang, Y., Osorno, R., Tsakiridis, A. and Wilson, V. (2012). In vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep. 2, 1571-1578. 10.1016/j.celrep.2012.10.022 [DOI] [PubMed] [Google Scholar]
- Hyun, I., Wilkerson, A. and Johnston, J. (2016). Embryology policy: revisit the 14-day rule. Nature 533, 169. 10.1038/533169a [DOI] [PubMed] [Google Scholar]
- Irie, N., Weinberger, L., Tang, W. W. C., Kobayashi, T., Viukov, S., Manor, Y. S., Dietmann, S., Hanna, J. H. and Surani, M. A. (2015). SOX17 is a critical specifier of human primordial germ cell fate. Cell 160, 253-268. 10.1016/j.cell.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keibel, F. (1897). Normentafel zur Entwicklungsgeschichte des Schweines, Sus scrofa domesticus. Fischer.
- Kirby, D. R. S., Potts, D. M. and Wilson, I. B. (1967). On the orientation of the implanting blastocyst. Development 17, 527-532. [PubMed] [Google Scholar]
- Kobayashi, H., Sakurai, T., Miura, F., Imai, M., Mochiduki, K., Yanagisawa, E., Sakashita, A., Wakai, T., Suzuki, Y., Ito, T.et al. (2013). High-resolution DNA methylome analysis of primordial germ cells identifies gender-specific reprogramming in mice. Genome Res. 23, 616-627. 10.1101/gr.148023.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T., Zhang, H., Tang, W. W. C., Irie, N., Withey, S., Klisch, D., Sybirna, A., Dietmann, S., Contreras, D. A., Webb, R.et al. (2017). Principles of early human development and germ cell program from conserved model systems. Nature 546, 416-420. 10.1038/nature22812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, Y., Kaufman-Francis, K., Studdert, J. B., Steiner, K. A., Power, M. D., Loebel, D. A. F., Jones, V., Hor, A., de Alencastro, G., Logan, G. J.et al. (2014). The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell 14, 107-120. 10.1016/j.stem.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Kojima, Y., Sasaki, K., Yokobayashi, S., Sakai, Y., Nakamura, T., Yabuta, Y., Nakaki, F., Nagaoka, S., Woltjen, K., Hotta, A.et al. (2017). Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell 21, 517-532.e5. 10.1016/j.stem.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Kurimoto, K., Yabuta, Y., Ohinata, Y., Shigeta, M., Yamanaka, K. and Saitou, M. (2008). Complex genome-wide transcription dynamics orchestrated by Blimp1 for the specification of the germ cell lineage in mice. Genes Dev. 22, 1617-1635. 10.1101/gad.1649908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, K. A. and Pedersen, R. A. (1987). Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development 101, 627-652. [DOI] [PubMed] [Google Scholar]
- Lawson, K. A., Dunn, N. R., Roelen, B. A. J., Zeinstra, L. M., Davis, A. M., Wright, C. V. E., Korving, J. P. W. F. M. and Hogan, B. L. M. (1999). Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424-436. 10.1101/gad.13.4.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D., Wang, X., He, D., Sun, C., He, X., Yan, L., Li, Y., Han, J.-D. J. and Zheng, P. (2018). Single-cell RNA-sequencing reveals the existence of naïve and primed pluripotency in pre-implantation rhesus monkey embryos. Genome Res. 28, 1481-–1493.. 10.1101/gr.233437.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckett, W. P. (1975). The development of primordial and definitive amniotic cavities in early rhesus monkey and human embryos. Am. J. Anat. 144, 149-167. 10.1002/aja.1001440204 [DOI] [PubMed] [Google Scholar]
- Luckett, W. P. (1978). Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am. J. Anat. 152, 59-97. 10.1002/aja.1001520106 [DOI] [PubMed] [Google Scholar]
- Ma, H., Zhai, J., Wan, H., Jiang, X., Wang, X., Wang, L., Xiang, Y., He, X., Zhao, Z.-A., Zhao, B.et al. (2019). In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science 366, eaax7890. 10.1126/science.aax7890 [DOI] [PubMed] [Google Scholar]
- Magaña, V. G., Rodríguez, A., Zhang, H., Webb, R. and Alberio, R. (2014). Paracrine effects of embryo-derived FGF4 and BMP4 during pig trophoblast elongation. Dev. Biol.387, 15-27. [DOI] [PubMed]
- Magnúsdóttir, E., Dietmann, S., Murakami, K., Günesdogan, U., Tang, F., Bao, S., Diamanti, E., Lao, K., Gottgens, B. and Azim Surani, M. (2013). A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat. Cell Biol. 15, 905-915. 10.1038/ncb2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhoubad, S., Bock, C., de Boer, A. S., Kiskinis, E., Meissner, A. and Eggan, K. (2012). Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 10, 595-609. 10.1016/j.stem.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon, B. R. and Jelalian, K. (1977). Evidence for two active X chromosomes in germ cells of female before meiotic entry. Nature 269, 242-243. 10.1038/269242a0 [DOI] [PubMed] [Google Scholar]
- Moore, N. W. (1985). The use of embryo transfer and steroid hormone replacement therapy in the study of prenatal mortality. Theriogenology 23, 121-128. 10.1016/0093-691X(85)90077-9 [DOI] [Google Scholar]
- Moris, N., Anlas, K., van den Brink, S. C., Alemany, A., Schröder, J., Ghimire, S., Balayo, T., van Oudenaarden, A. and Martinez Arias, A. (2020). An in vitro model of early anteroposterior organization during human development. Nature 582, 410-415. 10.1038/s41586-020-2383-9 [DOI] [PubMed] [Google Scholar]
- Müller, F. and O'Rahilly, R. (1987). The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat. Embryol. 176, 413-430. 10.1007/BF00310083 [DOI] [PubMed] [Google Scholar]
- Murakami, K., Günesdogan, U., Zylicz, J. J., Tang, W. W. C., Sengupta, R., Kobayashi, T., Kim, S., Butler, R., Dietmann, S. and Azim Surani, M. (2016). NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature 529, 403-407. 10.1038/nature16480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaki, F., Hayashi, K., Ohta, H., Kurimoto, K., Yabuta, Y. and Saitou, M. (2013). Induction of mouse germ-cell fate by transcription factors in vitro. Nature 501, 222-226. 10.1038/nature12417 [DOI] [PubMed] [Google Scholar]
- Nakamura, T., Okamoto, I., Sasaki, K., Yabuta, Y., Iwatani, C., Tsuchiya, H., Seita, Y., Nakamura, S., Yamamoto, T. and Saitou, M. (2016). A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537, 57-62. 10.1038/nature19096 [DOI] [PubMed] [Google Scholar]
- Nishimura, H., Takano, K., Tanimura, T. and Yasuda, M. (1968). Normal and abnormal development of human embryos: first report of the analysis of 1,213 intact embryos. Teratology 1, 281-290. 10.1002/tera.1420010306 [DOI] [PubMed] [Google Scholar]
- Niu, Y., Sun, N., Li, C., Lei, Y., Huang, Z., Wu, J., Si, C., Dai, X., Liu, C., Wei, J.et al. (2019). Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science 366, eaaw5754. 10.1126/science.aaw5754 [DOI] [PubMed] [Google Scholar]
- Ohinata, Y., Payer, B., O'Carroll, D., Ancelin, K., Ono, Y., Sano, M., Barton, S. C., Obukhanych, T., Nussenzweig, M., Tarakhovsky, A.et al. (2005). Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436, 207-213. 10.1038/nature03813 [DOI] [PubMed] [Google Scholar]
- Ohinata, Y., Ohta, H., Shigeta, M., Yamanaka, K., Wakayama, T. and Saitou, M. (2009). A signaling principle for the specification of the germ cell lineage in mice. Cell 137, 571-584. 10.1016/j.cell.2009.03.014 [DOI] [PubMed] [Google Scholar]
- O'Rahilly, R. and Müller, F. (2010). Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs 192, 73-84. 10.1159/000289817 [DOI] [PubMed] [Google Scholar]
- Patel, S., Bonora, G., Sahakyan, A., Kim, R., Chronis, C., Langerman, J., Fitz-Gibbon, S., Rubbi, L., Skelton, R. J. P., Ardehali, R.et al. (2017). Human embryonic stem cells do not change their X inactivation status during differentiation. Cell Rep. 18, 54-67. 10.1016/j.celrep.2016.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos, S., Edsgärd, D., Reinius, B., Deng, Q., Panula, S. P., Codeluppi, S., Plaza Reyes, A., Linnarsson, S., Sandberg, R. and Lanner, F. (2016). Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 165, 1012-1026. 10.1016/j.cell.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, I. R. (1976). The embryology of the common marmoset (Callithrix jacchus). Adv. Anat. Embryol. Cell Biol. 52, 3-47. [PubMed] [Google Scholar]
- Popovic, M., Bialecka, M., Gomes Fernandes, M., Taelman, J., Van Der Jeught, M., De Sutter, P., Heindryckx, B. and De Sousa Lopes, S. M. C. (2019). Human blastocyst outgrowths recapitulate primordial germ cell specification events. Mol. Hum. Reprod. 25, 519-526. 10.1093/molehr/gaz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastan, S. (1982). Timing of X-chromosome inactivation in postimplantation mouse embryos. J. Embryol. Exp. Morphol. 71, 11-24. [PubMed] [Google Scholar]
- Rossant, J. (1988). Development of extraembryonic cell lineages in the mouse embryo. In Experimental Approaches to Mammalian Embryonic Development (ed. Rossant J., and Pedersen R. A.) pp. 97-120. London: Cambridge University Press. [Google Scholar]
- Rossant, J., Chazaud, C. and Yamanaka, Y. (2003). Lineage allocation and asymmetries in the early mouse embryo. Philos. Trans. R. Soc. B Biol. Sci. 358, 1341-1349. 10.1098/rstb.2003.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakyan, A., Kim, R., Chronis, C., Sabri, S., Bonora, G., Theunissen, T. W., Kuoy, E., Langerman, J., Clark, A. T., Jaenisch, R.et al. (2017). Human naive pluripotent stem cells model X chromosome dampening and X inactivation. Cell Stem Cell 20, 87-101. 10.1016/j.stem.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou, M. and Yamaji, M. (2010). Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences. Reproduction 139, 931-942. 10.1530/REP-10-0043 [DOI] [PubMed] [Google Scholar]
- Saitou, M. and Yamaji, M. (2012). Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol. 4, a008375. 10.1101/cshperspect.a008375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou, M., Barton, S. C. and Surani, M. A. (2002). A molecular programme for the specification of germ cell fate in mice. Nature 418, 293-300. 10.1038/nature00927 [DOI] [PubMed] [Google Scholar]
- Sakai, Y., Nakamura, T., Okamoto, I., Gyobu-Motani, S., Ohta, H., Yabuta, Y., Tsukiyama, T., Iwatani, C., Tsuchiya, H., Ema, M.et al. (2020). Induction of the germ cell fate from pluripotent stem cells in cynomolgus monkeys†. Biol. Reprod. 102, 620-638. 10.1093/biolre/ioz205 [DOI] [PubMed] [Google Scholar]
- Sangrithi, M. N., Royo, H., Mahadevaiah, S. K., Ojarikre, O., Bhaw, L., Sesay, A., Peters, A. H. F. M., Stadler, M. and Turner, J. M. A. (2017). Non-canonical and sexually dimorphic X dosage compensation states in the mouse and human germline. Dev. Cell 40, 289-301.e3. 10.1016/j.devcel.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, K., Yokobayashi, S., Nakamura, T., Okamoto, I., Yabuta, Y., Kurimoto, K., Ohta, H., Moritoki, Y., Iwatani, C., Tsuchiya, H.et al. (2015). Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 17, 178-194. 10.1016/j.stem.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Sasaki, K., Nakamura, T., Okamoto, I., Yabuta, Y., Iwatani, C., Tsuchiya, H., Seita, Y., Nakamura, S., Shiraki, N., Takakuwa, T.et al. (2016). The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev. Cell 39, 169-185. 10.1016/j.devcel.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Seisenberger, S., Andrews, S., Krueger, F., Arand, J., Walter, J., Santos, F., Popp, C., Thienpont, B., Dean, W. and Reik, W. (2012). The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell 48, 849-862. 10.1016/j.molcel.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, Y., Hayashi, K., Itoh, K., Mizugaki, M., Saitou, M. and Matsui, Y. (2005). Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev. Biol. 278, 440-458. 10.1016/j.ydbio.2004.11.025 [DOI] [PubMed] [Google Scholar]
- Senft, A. D., Bikoff, E. K., Robertson, E. J. and Costello, I. (2019). Genetic dissection of Nodal and Bmp signalling requirements during primordial germ cell development in mouse. Nat. Commun. 10, 1089. 10.1038/s41467-019-09052-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi, M. N., Jedrusik, A., Vuoristo, S., Recher, G., Hupalowska, A., Bolton, V., Fogarty, N. M. E., Campbell, A., Devito, L. G., Ilic, D.et al. (2016). Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 18, 700-708. 10.1038/ncb3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi, M. N., Scialdone, A., Skorupska, N., Weberling, A., Recher, G., Zhu, M., Jedrusik, A., Devito, L. G., Noli, L., Macaulay, I. C.et al. (2017). Pluripotent state transitions coordinate morphogenesis in mouse and human embryos. Nature 552, 239-243. 10.1038/nature24675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane, K., Kurimoto, K., Yabuta, Y., Yamaji, M., Satoh, J., Ito, S., Watanabe, A., Hayashi, K., Saitou, M. and Sasaki, H. (2016). Global landscape and regulatory principles of DNA methylation reprogramming for germ cell specification by mouse pluripotent stem cells. Dev. Cell 39, 87-103. 10.1016/j.devcel.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Silva, J., Nichols, J., Theunissen, T. W., Guo, G., van Oosten, A. L., Barrandon, O., Wray, J., Yamanaka, S., Chambers, I. and Smith, A. (2009). Nanog is the gateway to the pluripotent ground state. Cell 138, 722-737. 10.1016/j.cell.2009.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. (2017). Formative pluripotency: the executive phase in a developmental continuum. Development 144, 365-373. 10.1242/dev.142679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. A., Moore, H. D. M. and Hearn, J. P. (1987). The ultrastructure of early implantation in the marmoset monkey (Callithrix jacchus). Anat. Embryol. 175, 399-410. 10.1007/BF00309853 [DOI] [PubMed] [Google Scholar]
- Smith, Z. D., Chan, M. M., Mikkelsen, T. S., Gu, H., Gnirke, A., Regev, A. and Meissner, A. (2012). A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 484, 339-344. 10.1038/nature10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa, E., Chen, D., Rojas, E. J., Hennebold, J. D., Peters, K. A., Wu, Z., Lam, T. N., Mitchell, J. M., Sukhwani, M., Tailor, R. C.et al. (2018). Differentiation of primate primordial germ cell-like cells following transplantation into the adult gonadal niche. Nat. Commun. 9, 5339. 10.1038/s41467-018-07740-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, M. and Abe, K. (2007). X chromosome reactivation initiates in nascent primordial germ cells in mice. PLoS Genet. 3, e116. 10.1371/journal.pgen.0030116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, A. (2003). Mechanisms of implantation in the mouse: differentiation and functional importance of trophoblast giant cell behavior. Dev. Biol. 258, 241-251. 10.1016/S0012-1606(03)00130-1 [DOI] [PubMed] [Google Scholar]
- Sybirna, A., Tang, W. W. C., Pierson Smela, M., Dietmann, S., Gruhn, W. H., Brosh, R. and Surani, M. A. (2020). A critical role of PRDM14 in human primordial germ cell fate revealed by inducible degrons. Nat. Commun. 11, 1282. 10.1038/s41467-020-15042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima, Y., Guo, G., Loos, R., Nichols, J., Ficz, G., Krueger, F., Oxley, D., Santos, F., Clarke, J., Mansfield, W.et al. (2014). Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254-1269. 10.1016/j.cell.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, P. P. L. and Zhou, S. X. (1996). The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev. Biol. 178, 124-132. 10.1006/dbio.1996.0203 [DOI] [PubMed] [Google Scholar]
- Tanaka, S. S., Nakane, A., Yamaguchi, Y. L., Terabayashi, T., Abe, T., Nakao, K., Asashima, M., Steiner, K. A., Tam, P. P. L. and Nishinakamura, R. (2013). Dullard/Ctdnep1 modulates WNT signalling activity for the formation of primordial germ cells in the mouse embryo. PLoS ONE 8, e57428. 10.1371/journal.pone.0057428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. W. C., Dietmann, S., Irie, N., Leitch, H. G., Floros, V. I., Bradshaw, C. R., Hackett, J. A., Chinnery, P. F. and Surani, M. A. (2015). A unique gene regulatory network resets the human germline epigenome for development. Cell 161, 1453-1467. 10.1016/j.cell.2015.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar, P. J., Chenoweth, J. G., Brook, F. A., Davies, T. J., Evans, E. P., Mack, D. L., Gardner, R. L. and McKay, R. D. G. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196-199. 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- Theunissen, T. W., Powell, B. E., Wang, H., Mitalipova, M., Faddah, D. A., Reddy, J., Fan, Z. P., Maetzel, D., Ganz, K., Shi, L.et al. (2014). Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471-487. 10.1016/j.stem.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyser, R. C. V., Mahammadov, E., Nakanoh, S., Vallier, L., Scialdone, A. and Srinivas, S. (2020). A spatially resolved single cell atlas of human gastrulation. bioRxiv 2020.07.21.213512. [Google Scholar]
- Vallot, C., Huret, C., Lesecque, Y., Resch, A., Oudrhiri, N., Bennaceur-Griscelli, A., Duret, L. and Rougeulle, C. (2013). XACT, a long noncoding transcript coating the active X chromosome in human pluripotent cells. Nat. Genet. 45, 239-241. 10.1038/ng.2530 [DOI] [PubMed] [Google Scholar]
- Vallot, C., Patrat, C., Collier, A. J., Huret, C., Casanova, M., Liyakat Ali, T. M., Tosolini, M., Frydman, N., Heard, E., Rugg-Gunn, P. J.et al. (2017). XACT noncoding RNA competes with XIST in the control of X chromosome activity during human early development. Cell Stem Cell 20, 102-111. 10.1016/j.stem.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértesy, Á., Arindrarto, W., Roost, M. S., Reinius, B., Torrens-Juaneda, V., Bialecka, M., Moustakas, I., Ariyurek, Y., Kuijk, E., Mei, H.et al. (2018). Parental haplotype-specific single-cell transcriptomics reveal incomplete epigenetic reprogramming in human female germ cells. Nat. Commun. 9, 1873. 10.1038/s41467-018-04215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenn, F., Berrens, R. V., Andrews, S., Santos, F., Collier, A. J., Krueger, F., Osorno, R., Dean, W., Rugg-Gunn, P. J. and Reik, W. (2016). Comparative principles of DNA methylation reprogramming during human and mouse in vitro primordial germ cell specification. Dev. Cell 39, 104-115. 10.1016/j.devcel.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamaitha, S. E. and Niakan, K. K. (2018). Human pre-gastrulation development. Curr. Top. Dev. Biol. 128, 295-338. 10.1016/bs.ctdb.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Warmflash, A., Sorre, B., Etoc, F., Siggia, E. D. and Brivanlou, A. H. (2014). A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847-854. 10.1038/nmeth.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, S., Eckert, D., Nettersheim, D., Gillis, A. J. M., Schäfer, S., Kuckenberg, P., Ehlermann, J., Werling, U., Biermann, K., Looijenga, L. H. J.et al. (2010). Critical function of AP-2 gamma/TCFAP2C in mouse embryonic germ cell maintenance. Biol. Reprod. 82, 214-223. 10.1095/biolreprod.109.078717 [DOI] [PubMed] [Google Scholar]
- Wislocki, G. B. and Bennett, H. S. (1943). The histology and cytology of the human and monkey placenta, with special reference to the trophoblast. Am. J. Anat. 73, 335-449. 10.1002/aja.1000730303 [DOI] [Google Scholar]
- Witschi, E. (1948). Migration of the Germ Cells of Human Embryos from the Yolk Sac to the Primitive Gonadal Folds. 575th edn. Carnegie Institution of Washington publication. [Google Scholar]
- Wu, J., Okamura, D., Li, M., Suzuki, K., Luo, C., Ma, L., He, Y., Li, Z., Benner, C., Tamura, I.et al. (2015). An alternative pluripotent state confers interspecies chimaeric competency. Nature 521, 316-321. 10.1038/nature14413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, Y. and Yamada, S. (2018). The kyoto collection of human embryos and fetuses: history and recent advancements in modern methods. Cells Tissues Organs 205, 314-319. 10.1159/000490672 [DOI] [PubMed] [Google Scholar]
- Yamaji, M., Seki, Y., Kurimoto, K., Yabuta, Y., Yuasa, M., Shigeta, M., Yamanaka, K., Ohinata, Y. and Saitou, M. (2008). Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat. Genet. 40, 1016-1022. 10.1038/ng.186 [DOI] [PubMed] [Google Scholar]
- Ying, Y. and Zhao, G.-Q. (2001). Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev. Biol. 232, 484-492. 10.1006/dbio.2001.0173 [DOI] [PubMed] [Google Scholar]
- Ying, Y., Liu, X.-M., Marble, A., Lawson, K. A. and Zhao, G.-Q. (2000). Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol. Endocrinol. 14, 1053-1063. 10.1210/mend.14.7.0479 [DOI] [PubMed] [Google Scholar]
- Zhao, G.-Q. (2003). Consequences of knocking out BMP signaling in the mouse. Genesis 35, 43-56. 10.1002/gene.10167 [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Xue, X., Shao, Y., Wang, S., Esfahani, S. N., Li, Z., Muncie, J. M., Lakins, J. N., Weaver, V. M., Gumucio, D. L.et al. (2019). Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421-425. 10.1038/s41586-019-1535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q., Sang, F., Withey, S., Tang, W., Dietmann, S., Klisch, D., Ramos-Ibeas, P., Zhang, H., Requena, C. E., Hajkova, P.et al. (2020). Specification and epigenetic resetting of the pig germline exhibit conservation with the human lineage. Cell Rep. 34, 108735. 10.1016/j.celrep.2021.108735 [DOI] [PMC free article] [PubMed] [Google Scholar]