Abstract
Introduction
This final analysis of a long-term extension (LTE) study assessed the safety, tolerability, and effectiveness of peficitinib (ASP015K), a pan-Janus kinase inhibitor, in Asian patients with rheumatoid arthritis (RA).
Methods
Patients had previously completed the 12-week phase 2b (RAJ1), or 52-week phase 3 (RAJ3 and RAJ4) peficitinib studies in Japan, Korea, and Taiwan, and received oral peficitinib 50 or 100 mg/day. Dose increase to 150 mg/day or reduction to 50 mg/day was permitted. Efficacy endpoints included American College of Rheumatology (ACR)20/50/70 response rates, 28-joint Disease Activity Score with C-reactive protein (DAS28-CRP), and ACR components. Safety endpoints included treatment-emergent adverse events (TEAEs), and incidence rates (IRs) of adverse events of special interest per 100 patient-years (PY).
Results
Overall, 843 patients received peficitinib for a mean 32.0 months (maximum 85.2 months), and most (64.4%) received peficitinib 100 mg/day as a maximum dose. Respective ACR20/50/70 response rates were maintained from baseline (week 0 of LTE; 71.6, 52.1, and 34.7%) to end of treatment (78.7, 63.3, and 44.1%); continuous improvements in ACR components and DAS28-CRP were observed from the baselines of preceding studies and throughout the LTE. Overall, 796/843 (94.4%) patients experienced TEAEs; most were severity grade 1/2. Most common TEAEs were nasopharyngitis (47.0%) and herpes zoster (17.3%). Drug-related TEAEs leading to permanent discontinuation occurred in 140 (16.6%) patients, and IRs (95% confidence interval) per 100 PY of serious infections, herpes zoster-related disease, and malignancies were 2.7 (2.1, 3.4), 7.3 (6.2, 8.6), and 1.2 (0.9, 1.8), respectively. Two deaths occurred during the study; one each from diffuse large B cell lymphoma and pneumonia, which were, respectively considered probably and possibly related to study drug.
Conclusions
Improvements in effectiveness variables were maintained during this long-term study of peficitinib in Asian patients with RA; peficitinib was generally well tolerated over a mean 32 months’ duration.
Trial Registration
ClinicalTrials.gov. NCT01638013, retrospectively registered on 11 July 2012 https://clinicaltrials.gov/ct2/show/NCT01638013.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-021-00280-5.
Keywords: Herpes zoster, Janus kinase inhibitors, Long-term extension study, Peficitinib, Rheumatoid arthritis, Serious infection, Targeted synthetic DMARDs
Key Summary Points
| Why carry out this study? |
| Rheumatoid arthritis (RA) is a chronic disease requiring long-term treatment; therefore an understanding of the long-term effectiveness, safety, and tolerability of an RA therapy is key. |
| Interim results from an open-label long-term extension (LTE) study of peficitinib (ASP015K), a pan-Janus kinase inhibitor, in Asian patients with RA, have been published. |
| This final analysis of the LTE study assessed the long-term safety, tolerability, and effectiveness of peficitinib in Asian patients with RA, over a mean 32 months of treatment. |
| What was learned from this study? |
| This study showed that improvements in American College of Rheumatology response and other effectiveness variables were maintained during long-term peficitinib treatment, and peficitinib was generally well tolerated in Asian patients with RA. |
| These final data support peficitinib use for the long-term management of RA in Asian patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13582829.
Introduction
Rheumatoid arthritis (RA) is a chronic condition characterized by systemic inflammation and joint destruction, leading to severe disability and premature mortality [1]. The RA treatment landscape has been strengthened by the development of the Janus kinase (JAK) inhibitors, a class of targeted synthetic disease-modifying antirheumatic drugs (DMARDs) [2]. JAK inhibitors target enzymes belonging to the JAK family with varying specificity and selectivity, thereby inhibiting a number of proinflammatory processes involved in the pathogenesis of RA [2]. The JAK inhibitors tofacitinib (preferential JAK 1 and 3 inhibitor), baricitinib (JAK 1 and 2 inhibitor), and upadacitinib (JAK 1 inhibitor) have undergone phase 3 clinical trials, and are approved for the treatment of RA in the USA, Europe, and Asia [2–12].
Peficitinib (ASP015K) is an oral, once-daily, pan-JAK inhibitor that has demonstrated efficacy and tolerability in Asian patients with RA, as a monotherapy in a 12-week phase 2b study (RAJ1), and in two 52-week phase 3 randomized controlled trials, in combination with DMARDs (RAJ3) or methotrexate specifically (RAJ4) [13–15]. Peficitinib has been approved in Japan, Korea, and Taiwan, for the treatment of RA [16–18].
RA is a chronic disease requiring long-term treatment; therefore an understanding of the long-term effectiveness, safety, and tolerability of an RA therapy is key. Interim results (over a mean treatment duration of 22.7 months) from an open-label long-term extension (LTE) study of peficitinib in RA patients who completed the RAJ1, RAJ3, or RAJ4 clinical trials have been published [19]. Here, we report the final safety and effectiveness data after completion of this LTE study of peficitinib.
Methods
Study Design
This open-label, LTE study was conducted at 165 sites in Japan, nine sites in Korea, and nine sites in Taiwan from June 2012 to September 2019. Eligible patients had previously completed the RAJ1, RAJ3, or RAJ4 study [13–15, 19], and consequently the duration of study treatment varied between patients (Supplementary Fig. S1). In the RAJ1 phase 2b study, patients were randomized (1:1) to receive peficitinib monotherapy or placebo for 12 weeks with a 4-week follow-up (without peficitinib treatment) [13]. RAJ3 was a phase 3 study in which patients with an inadequate response to DMARDs were randomized (1:1:1:2) to receive peficitinib 100 mg/day, peficitinib 150 mg/day, placebo or etanercept. The peficitinib and etanercept treatment duration was 52 weeks, while patients in the placebo arm were switched at week 12 to either dose of peficitinib. Patients in the etanercept control reference group of RAJ3 were not included in the extension study [14]. In the RAJ4 phase 3 study, patients with an inadequate response to methotrexate were randomized (1:1:1) to receive peficitinib 100 mg/day, peficitinib 150 mg/day or placebo in combination with methotrexate, with treatment duration of 52 weeks, and patients in the placebo arm switched to either dose of peficitinib at week 28 [15]. The LTE study design has been previously described [19]. Briefly, patients received oral peficitinib 50 mg/day (if transferring from RAJ1) or 100 mg/day (if transferring from RAJ3 or RAJ4) once daily after breakfast as the starting dose. The daily dose of peficitinib could be increased in patients with no safety issues from 50–100 mg/day at the investigator’s discretion, or from 100 to 150 mg/day in those with insufficient clinical response [28-joint Disease Activity Score (DAS28) erythrocyte sedimentation rate ≥ 3.2] after 4 weeks of peficitinib treatment. After the increase, the dose could be reduced from 100 mg/day or 150 mg/day to 50 mg/day at the discretion of the investigator. Subsequent to the publication of interim results, and following approval of peficitinib in Japan in March 2019, all Japanese patients receiving peficitinib 50 mg/day had their dose increased to 100 mg/day, or discontinued if the dose increase was not possible. The efficacy and safety of peficitinib were assessed by the investigator at each patient visit. Study drug administration could be suspended, interrupted, or discontinued based on certain criteria, as previously described [19].
Patients
Inclusion criteria have been described previously [19]. Briefly, patients were required to have completed treatment with peficitinib and undertaken assessments at week 16 for the RAJ1 study, and week 52 for the RAJ3 and RAJ4 studies. Patients were excluded if they were judged unsuitable to participate in the study for any reason by the investigator, or if they had taken any of the contraindicated therapies detailed in Supplementary Methods.
Concomitant Medications
Concomitant medications and therapies were permitted or prohibited as described in Supplementary Methods.
Outcomes
Efficacy Endpoints
Efficacy was evaluated in the overall patient population and in patients grouped by their preceding study. Assessment of the long-term efficacy of peficitinib included American College of Rheumatology (ACR)20, ACR50, and ACR70 response rates; changes from baselines of the preceding studies in DAS28 based on C-reactive protein (CRP); proportions of patients achieving DAS28-CRP-defined remission (< 2.6) and DAS28-CRP-defined low disease activity (LDA; ≤ 3.2); changes from the baselines of the preceding studies in Clinical (CDAI) and Simplified (SDAI) Disease Activity Indices; proportions of patients achieving CDAI- and SDAI-defined remission (≤ 2.8 and ≤ 3.3, respectively); ACR/European League Against Rheumatism (EULAR) Boolean-based definition of remission; and changes from the baselines of the preceding studies in the core set of ACR components (see Supplementary Methods) [20].
Safety
Safety outcomes included treatment-emergent adverse events (TEAEs), which were defined as any adverse event (AE) that started or worsened in severity after the first dose of study drug in RAJ2 to the end of the final observation. AEs of special interest were assessed per 100 patient-years (PY), and included serious infections, malignancies, herpes zoster-related disease (including varicella), and venous thromboembolism (VTE; post hoc analysis). Mean (standard deviation [SD]) changes from baseline in hematologic, biochemical, and select laboratory parameters were recorded throughout the LTE study. Vital signs were collected at each study visit, and an electrocardiogram was obtained every 48 weeks and at end of treatment (EOT)/early termination.
Patient Populations
Patient populations and statistical analyses have been defined previously [19]. Briefly, efficacy analyses were conducted on the full analysis set (FAS; all patients who received ≥ 1 dose of study drug and had measurements for any of the efficacy endpoints), and the safety analysis set (SAF) included all patients who received ≥ 1 dose of study drug.
Statistical Analysis
The planned sample size was approximately 800 patients treated with peficitinib. This was based on the number of patients from RAJ1 who participated in the LTE study, the number of patients planned to be enrolled in RAJ3 (excluding the etanercept reference arm), and the number of patients planned to be enrolled in RAJ4.
Statistical analyses have been defined previously [19]. Briefly, TEAEs were summarized according to the Medical Dictionary for Regulatory Activities (MedDRA, Version 11.1) System Organ Class (SOC) and Preferred Term (PT). Patients reporting ≥ 1 AE for a given MedDRA PT were counted only once for that PT. Patients reporting ≥ 1 AE within a SOC were counted only once for the SOC total. Other safety variables were analyzed descriptively.
Missing Data
ACR components, DAS28-CRP, and safety variables at EOT were summarized by the last observation carried forward (LOCF) method for each component available, and then calculated. All outliers were included in the analyses.
Ethics
This study was conducted in accordance with Good Clinical Practice, the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines, and local laws and regulations. The study protocol and amendments were reviewed and approved by an Institutional Review Board or Independent Ethics Committee (Supplementary Table S1) at each study site, and safety data were reviewed by an independent Data and Safety Monitoring Board. Each patient provided written informed consent prior to treatment initiation. This analysis, and the clinical trials from which data were included, followed the principles of the Declaration of Helsinki.
Results
Interim data have been previously reported for the RAJ2 study [19], and here we report the final data.
Patient Populations
Of 873 patients screened, 843 were enrolled and included in the SAF (201, 225, and 417 patients from RAJ1, RAJ3, and RAJ4 studies, respectively) (Supplementary Fig. S2). A total of 837 (99.3%) patients were included in the FAS.
Demographics and Characteristics at the Start of the Study
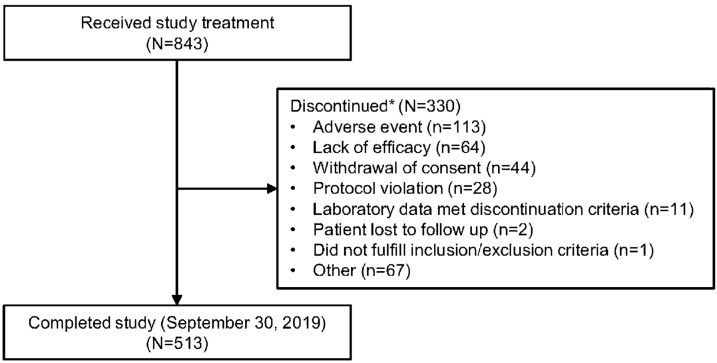
Demographics and baseline characteristics have been reported previously [19]. The majority of patients were female (619/843, 73.4%), from Japan (806/843, 95.6%), with a mean age of 55.7 years, and a mean (SD) duration of RA of 6.2 (5.6) years (Supplementary Table S2). Discontinuations were reported for 330 (39.1%) patients; the most frequently reported reasons were AE (13.4%), other (7.9%), and lack of efficacy (7.6%) (Fig. 1).
Fig. 1.
Patient flow through the long-term extension study. *Discontinued during overall period: discontinued at any time from start of initial dosing of study drug through the last dose day in the overall period
Treatment Exposure
Mean peficitinib exposure was 32.0 months (max. 85.2 months) (Table 1) and was longer in patients from the RAJ1 study (47.1 months) versus RAJ3 (28.1 months) and RAJ4 (26.9 months) (Supplementary Table S3). Total peficitinib exposure was 2277.9 PY. Dose increase or decrease was reported in 382 (45.3%) and 47 (5.6%) patients, respectively (Table 1), and the proportion of patients with dose increase was higher in RAJ1 (82.1%) than RAJ3 (34.7%) and RAJ4 (33.3%) (Supplementary Table S3). The proportions of patients who received peficitinib 50 mg/day, 100 mg/day, and 150 mg/day as the maximum dose were 4.3% (36/843), 64.4% (543/843), and 31.3% (264/843), respectively (Table 1). Overall, 52.2, 68.0, and 68.3% of patients from studies RAJ1, RAJ3 and RAJ4, respectively, received peficitinib 100 mg/day (Supplementary Table S3). Mean treatment compliance was 98.3% (Table 1) ranging from 97.7 to 98.9%, depending on preceding study (Supplementary Table S3).
Table 1.
Peficitinib treatment exposure and changes in peficitinib dose during the overall period in the long-term extension study (SAF)
| Total (N = 843) |
|
|---|---|
| Duration of peficitinib exposure, monthsa | |
| Mean (SD) | 32.0 (19.5) |
| Minimum | 0.1 |
| Median | 29.9 |
| Maximum | 85.2 |
| Duration of initial peficitinib dose, monthsb | |
| Mean (SD) | 17.5 (16.0) |
| Minimum | 0.1 |
| Maximum | 83.6 |
| Treatment compliance rate (%)c | |
| Mean (SD) | 98.3 (3.2) |
| Patients with dose increase, n (%) | |
| No | 461 (54.7) |
| Yes | 382 (45.3) |
| 1 dose increase | 309 (36.7) |
| 2 dose increases | 66 (7.8) |
| ≥ 3 dose increases | 7 (0.8) |
| Patients with dose decrease, n (%) | |
| No | 796 (94.4) |
| Yes | 47 (5.6) |
| 1 dose decrease | 41 (4.9) |
| 2 dose decreases | 6 (0.7) |
| ≥ 3 dose decreases | 0 |
| Maximum peficitinib dose, n (%) | |
| 50 mg | 36 (4.3) |
| 100 mg | 543 (64.4) |
| 150 mg | 264 (31.3) |
SAF safety analysis set, SD standard deviation
aDuration of exposure for overall period (days) was calculated as: date of the last dose of study drug–date of initial dose of study drug + 1
bDuration from first peficitinib taken (50 mg/day for patients from RAJ1, 100 mg/day for patients from RAJ3 and RAJ4) up to first dose change was calculated
cTreatment compliance for overall period (%) was calculated as: 100 × (total number of tablets actually received in the overall period/total number of tablets planned to receive in the overall period)
Efficacy
ACR Response
The ACR20, ACR50, and ACR70 response rates were maintained throughout the LTE study from baseline (71.6, 52.1, and 34.7%, respectively) to EOT (LOCF; 78.7, 63.3, and 44.1%, respectively) in the overall population (Fig. 2), and in patients from studies RAJ3 and RAJ4 (Supplementary Fig. S3). A sudden decrease in the ACR20 response rate was observed at week 192 in RAJ3, but the number of patients was considerably lower (n = 8) than at other time points (Supplementary Fig. S3). For patients from RAJ1, response rates initially increased from baseline and were then maintained until EOT (Supplementary Fig. S3).
Fig. 2.
Response rates for ACR20, ACR50, and ACR70 over time (FAS). *Includes LOCF. ACR American College of Rheumatology, EOT end of treatment, FAS full analysis set, LOCF last observation carried forward
Improvements (reductions) in the core set of ACR components [tender joint count at 68 joints (TJC68), swollen joint count at 66 joints (SJC66), Subject’s Global Assessment of Pain (SGAP), Subject’s Global Assessment of Disease Activity (SGA), Physician’s Global Assessment of Disease Activity [PGA], and Health Assessment Questionnaire–Disability Index (HAQ-DI)] were observed from the baselines of the preceding studies, and continued during the extension study (Supplementary Fig. S4).
ACR20
The ACR20 response rates were sustained during the LTE study for maximum peficitinib doses of both 100 mg/day and 150 mg/day. In patients with a maximum peficitinib dose of 50 mg/day, ACR20 response rates were maintained during the extension study after an initial increase from baseline of the LTE study (week 0) (Fig. 3).
Fig. 3.
ACR20 response at each visit by maximum peficitinib dose level (FAS). *Includes LOCF. ACR American College of Rheumatology, EOT end of treatment, FAS full analysis set, LOCF last observation carried forward
DAS28-CRP, CDAI, SDAI, and ACR/EULAR Responses
Mean changes from baseline in DAS28-CRP, CDAI, and SDAI scores remained consistent over time for the overall study population (Fig. 4), and in patients from RAJ3 and RAJ4 (Supplementary Fig. S4). Where RAJ1 was the preceding study, mean DAS28-CRP, CDAI, and SDAI scores decreased from baseline, and were then sustained until EOT (Supplementary Fig. S4). In the overall study population, the proportions of patients who achieved remission (DAS28-CRP < 2.6, CDAI ≤ 2.8, SDAI ≤ 3.3 or ACR/EULAR Boolean-based remission) or DAS28-CRP LDA (≤ 3.2) increased from the baseline of the LTE study (week 0), and were then generally maintained until EOT (Fig. 5). DAS28-CRP-defined remission was reported in 60.4% (504/835) of patients, with DAS28-CRP LDA in 75.3% (629/835) at EOT. CDAI-, SDAI-, and ACR/EULAR Boolean-based remission were observed in 36.1% (302/836), 36.8% (307/835), and 26.1% (218/835) of patients, respectively. There were marked increases in the proportions of patients who achieved remission or LDA when RAJ1 was the preceding study, but the corresponding proportions of patients from both the RAJ3 and RAJ4 studies remained relatively unchanged during the LTE study (Supplementary Fig. S5).
Fig. 4.
Mean changes from baseline over time in a DAS28-CRP, b CDAI, and c SDAI scores (FAS). *Includes LOCF. CDAI Clinical Disease Activity Index, DAS28-CRP Disease Activity Score in 28 joints based on C-reactive protein, EOT end of treatment, FAS full analysis set, LOCF last observation carried forward, SDAI Simplified Disease Activity Index
Fig. 5.
The proportion of patients achieving remission/LDA in relation to a DAS28-CRP < 2.6, b DAS28-CRP ≤ 3.2, c CDAI ≤ 2.8, d SDAI ≤ 3.3, and e ACR/EULAR Boolean-based definition of remission (FAS). *Includes LOCF. ACR American College of Rheumatology, CDAI Clinical Disease Activity Index, DAS28-CRP Disease Activity Score in 28 joints based on C-reactive protein, EOT end of treatment, EULAR European League Against Rheumatism, FAS full analysis set, LDA low disease activity, LOCF last observation carried forward, SDAI Simplified Disease Activity Index
Safety
Adverse Events
Overall, 796 of 843 (94.4%) patients experienced TEAEs during the LTE study, and serious adverse events (SAEs) were reported in 199 (23.6%) patients (Table 2). TEAEs were reported in 34/36 (94.4%), 505/543 (93.0%), and 257/264 (97.3%) patients who received peficitinib 50, 100, and 150 mg/day, respectively. Most TEAEs were grade 1 or 2 in severity, and the most common TEAEs by PT were nasopharyngitis (47.0%), herpes zoster (17.3%), and RA (16.1%) (Table 3). The proportions of patients reporting treatment-emergent nasopharyngitis were similar among peficitinib 50, 100, and 150 mg/day maximum dose groups (50.0, 45.9, and 48.9%, respectively), while occurrences of treatment-emergent herpes zoster were numerically higher for peficitinib 150 mg/day (22.7%) compared with 100 mg/day (15.3%) and 50 mg/day (8.3%) groups (Table 3). The proportions of patients with TEAEs and SAEs leading to permanent discontinuation of study drug were 16.6 and 8.9%, respectively (Table 2). Drug-related TEAEs and drug-related SAEs were reported in 656 (77.8%) and 113 (13.4%) patients, respectively (Table 2), and there were 92 (10.9%) patients who presented with drug-related TEAEs leading to permanent discontinuation (Table 2). The proportions of patients for each category of AE were generally higher in the RAJ1 study compared with RAJ3 and RAJ4 (Supplementary Table S4).
Table 2.
Overview of treatment-emergent adverse events in the overall period (SAF)
| Total (N = 843) n (%) |
|
|---|---|
| All TEAEs | 796 (94.4) |
| Drug-relateda TEAEs, | 656 (77.8) |
| ≥ Grade 3 TEAEb | 266 (31.6) |
| All SAEs | 199 (23.6) |
| Drug-relateda SAEs | 113 (13.4) |
| TEAEs leading to permanent discontinuation of study drug | |
| All | 140 (16.6) |
| Drug-relateda | 92 (10.9) |
| SAEs | 75 (8.9) |
| Drug-relateda SAEs | 50 (5.9) |
| Deathsc | 2 (0.2) |
TEAEs were defined as any AE that started or worsened in severity after initial dose of study drug in the extension study until the end of the final observation
AE adverse event, SAE serious adverse event, SAF safety analysis set, TEAE treatment-emergent adverse event
aPossibly or probably related to study drug, as assessed by the investigator or records where relationship was missing
bNational Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE): grade 3, severe or medically significant; grade 4, life-threatening; grade 5, death related to AE
cOne death during the study due to diffuse large B-cell lymphoma was considered probably related to the study drug. One death during the study due to pneumonia and one death after the end of the study due to uterine sarcoma were considered possibly related to the study drug
Table 3.
Overview of treatment-emergent adverse events occurring in ≥ 5% of patients in the overall period by maximum dose of peficitinib (50, 100, or 150 mg/day) (SAF)
| TEAEs | Maximum dose | Total (N = 843) n (%) |
||
|---|---|---|---|---|
| 50 mg (N = 36) n (%) |
100 mg (N = 543) n (%) |
150 mg (N = 264) n (%) |
||
| Nasopharyngitis | 18 (50.0) | 249 (45.9) | 129 (48.9) | 396 (47.0) |
| Herpes zoster | 3 (8.3) | 83 (15.3) | 60 (22.7) | 146 (17.3) |
| Rheumatoid arthritisa | 3 (8.3) | 54 (9.9) | 79 (29.9) | 136 (16.1) |
| Influenza | 5 (13.9) | 59 (10.9) | 37 (14.0) | 101 (12.0) |
| Blood creatine kinase increased | 5 (13.9) | 62 (11.4) | 30 (11.4) | 97 (11.5) |
| Bronchitis | 2 (5.6) | 43 (7.9) | 34 (12.9) | 79 (9.4) |
| Contusion | 2 (5.6) | 44 (8.1) | 29 (11.0) | 75 (8.9) |
| Hypertension | 5 (13.9) | 41 (7.6) | 26 (9.8) | 72 (8.5) |
| Pharyngitis | 2 (5.6) | 39 (7.2) | 30 (11.4) | 71 (8.4) |
| Upper respiratory tract infection | 2 (5.6) | 38 (7.0) | 24 (9.1) | 64 (7.6) |
| Dental caries | 2 (5.6) | 40 (7.4) | 20 (7.6) | 62 (7.4) |
| Gastroenteritis | 1 (2.8) | 36 (6.6) | 25 (9.5) | 62 (7.4) |
| Cystitis | 2 (5.6) | 37 (6.8) | 22 (8.3) | 61 (7.2) |
| Constipation | 3 (8.3) | 30 (5.5) | 27 (10.2) | 60 (7.1) |
| Back pain | 3 (8.3) | 37 (6.8%) | 19 (7.2) | 59 (7.0) |
| Cough | 3 (8.3) | 26 (4.8) | 23 (8.7) | 52 (6.2) |
| Abnormal hepatic function | 0 | 33 (6.1) | 11 (4.2) | 44 (5.2) |
| Headache | 2 (5.6) | 26 (4.8) | 16 (6.1) | 44 (5.2) |
| Lymphocyte count decreased | 1 (2.8) | 33 (6.1) | 9 (3.4) | 43 (5.1) |
| Eczema | 3 (8.3) | 22 (4.1) | 17 (6.4) | 42 (5.0) |
SAF safety analysis set; TEAE treatment-emergent adverse event
aThe reported terms ‘progression of rheumatoid arthritis’ and ‘rheumatoid arthritis aggravated’ were collated as the preferred term ‘rheumatoid arthritis’
Death was reported for two (0.2%) patients during the study; one patient died from diffuse large B-cell lymphoma and one patient from pneumonia. These events were considered by the investigator to be probably and possibly related to the study drug, respectively (Supplementary Results). After study completion, there was one death due to uterine sarcoma, and this was considered by the investigator to be possibly related to the study drug (Supplementary Results).
The overall incidence rates [IRs; 95% confidence intervals (CIs)] per 100 PY of serious infections, herpes zoster-related disease, and malignancies, were 2.7 (2.1, 3.4), 7.3 (6.2, 8.6), 1.2 (0.9, 1.8), respectively (Fig. 6). In a post hoc analysis of VTE, the IR (95% CI) was 0.1 (0.0, 0.4) per 100 PY in the overall period, which related to two cases (one case of pulmonary artery thrombosis and one case of deep vein thrombosis) between 24 and 36 months; there were no cases of pulmonary embolism. The proportions of patients with TEAEs related to AEs of special interest were analyzed according to the maximum doses of 50, 100, and 150 mg/day peficitinib for serious infections [4/36 (11.1%), 31/543 (5.7%), and 24/264 (9.1%), respectively], herpes zoster-related disease [3/36 (8.3%), 85/543 (15.7%), and 60/264 (22.7%), respectively], malignancies [1/36 (2.8%), 22/543 (4.1%), and 5/264 (1.9%), respectively], and VTE [0/36 (0.0%), 1/543 (0.2%), and 1/264 (0.4%)], respectively. The IRs (95% CIs) per 100 PY for serious infection-related TEAEs were slightly higher for peficitinib 150 mg/day [3.0 (2.0, 4.5)] than 100 mg/day [2.3 (1.6, 3.3)], but both IRs were lower than reported for peficitinib 50 mg/day [4.1 (1.5, 11.0)] (Fig. 7). For herpes zoster-related disease, IRs of TEAEs increased slightly with higher doses [50 mg/day: 3.1 (1.0, 9.5); 100 mg/day: 6.9 (5.6, 8.5); and 150 mg/day: 8.6 (6.7, 11.0)] (Fig. 7). The IRs of malignancy-related TEAEs were higher for peficitinib 100 mg/day [1.6 (1.1, 2.5)] compared with 50 mg/day [1.0 (0.1, 7.0) and 150 mg/day [0.6 (0.3, 1.5)] (Fig. 7). There was a low incidence (95% CI) of VTE-related TEAEs per 100 PY for all maximum dose groups [50 mg/day: 0.0; 100 mg/day: 0.1 (0.0, 0.5); and 150 mg/day: 0.1 (0.0, 0.9)] (Fig. 7). There were 16 patients with serious herpes zoster-related disease (Supplementary Table S5).
Fig. 6.

Incidence of adverse events of special interest per 100 PY during the overall period for a serious infections, b herpes zoster-related disease, and c malignancies (SAF). PY were calculated from initial dose up to first incidence of the event for patients who had ≥ 1 event, and from initial dose through follow-up for patients who had no events; incidence rate was calculated as (100 × number of patients who had ≥ 1 incidence)/total PY. CI confidence interval, IR incidence rate, PY patient-years, SAF safety analysis set
Fig. 7.
Incidence of treatment-emergent adverse events of special interest per 100 PY during the overall period (SAF). PY were calculated from initial dose up to first incidence of the event for patients who had ≥ 1 event, and from initial dose through follow-up for patients who had no events; incidence rate was calculated as (100 × number of patients who had ≥ 1 incidence)/total PY. CI confidence interval, IR incidence rate, PY patient-years, SAF safety analysis set
The total exposure to peficitinib among patients who had at least one AE of special interest was 2216.7, 2031.4, 2271.4, and 2277.8 PY for serious infections, herpes zoster-related disease, malignancy, and VTE, respectively.
Laboratory Measures
Increases in blood creatine kinase and decreases in lymphocytes were observed from baseline of the extension study (week 0), and were reported as TEAEs in 97/843 (11.5%) and 43/843 (5.1%) patients, respectively (Table 4). Among these, 14 patients had elevated creatine kinase of grade 3 or 4 severity and 22 patients had decreased lymphocyte counts of grade 3 severity. Other laboratory variables of interest remained generally stable over time (Table 4).
Table 4.
Mean changes from baseline (week 0) in laboratory measurements at end of treatment (SAF)
| Mean (SD) at baseline (week 0) | Mean (SD) at EOTa | Mean (SD) change from baseline at EOTa | |
|---|---|---|---|
| Absolute neutrophil count, 106/l | 4826.2 (1920.5) | 4609.6 (1989.2) | − 217.3 (1756.1) |
| Hemoglobin, g/l | 126.0 (14.3) | 126.4 (15.2) | 0.3 (11.1) |
| Lymphocytes, 106/l | 1429.8 (555.9) | 1193.9 (472.5) | − 233.9 (465.8) |
| Platelets, 109/l | 273.9 (71.0) | 273.6 (75.4) | 0.0 (52.8) |
| LDL cholesterol, mmol/l | 3.115 (0.858) | 3.145 (0.818) | 0.026 (0.782) |
| HDL cholesterol, mmol/l | 1.889 (0.547) | 1.917 (0.541) | 0.032 (0.367) |
| Creatinine, µmol/l | 58.17 (14.55) | 63.05 (16.50) | 4.83 (9.47) |
| Creatine kinase, U/l | 131.2 (124.8) | 169.8 (251.2) | 38.6 (246.0) |
| ALT, U/l | 22.9 (16.5) | 23.2 (15.7) | 0.3 (16.7) |
| AST, U/l | 27.1 (11.9) | 27.4 (12.0) | 0.4 (12.8) |
ALT alanine aminotransferase, AST aspartate aminotransferase, EOT end of treatment, HDL high-density lipoprotein, LDL low-density lipoprotein, SAF safety analysis set, SD standard deviation
aEOT assessments and tests were to be performed promptly once administration of peficitinib had ended; in cases of early termination of peficitinib administration, these assessments and tests were to be performed within 2 days of the last dose of peficitinib, if possible
Discussion
This open-label extension study, with mean 32.0 months of peficitinib treatment, and peficitinib exposure of 2277.9 PY, supports the results of the interim analysis (1615.3 PY of peficitinib exposure) [19], and provides further evidence of the effectiveness and safety of peficitinib for long-term use in Asian patients with RA.
Longer-term efficacy outcomes were either maintained or improved during this extension study in patients receiving peficitinib after completing the RAJ1, RAJ3, or RAJ4 studies [13–15, 19]. Both interim and final analyses of the extension study showed that ACR20, ACR50, and ACR70 response rates were maintained from baseline (71.6, 52.1, and 34.7%, respectively) to EOT (78.9, 61.4, and 42.7%, respectively, for the interim analysis [19], and 78.7, 63.3, and 44.1%, respectively, for the final analysis). The ACR20 response rate was lower at baseline (week 0) for patients transferring from RAJ1 compared with RAJ3 and RAJ4, which was likely due to some patients in RAJ1 receiving lower doses of peficitinib and/or the shorter treatment period in this study, as discussed previously [19]. Unsurprisingly, a similar trend was observed for the ACR20 response rate in patients receiving the maximum dose of 50 mg/day peficitinib. In patients who received maximum doses of 100 mg/day or 150 mg/day peficitinib, ACR20 response rates were maintained during the LTE study.
Other measures of efficacy, DAS28-CRP, CDAI, SDAI, TJC68, SJC66, SGAP, PGA, SGA, and HAQ-DI, showed an improvement from the baselines of the preceding studies, and continued to show improvement during the current study. This was consistent with trends seen in the interim analysis of this LTE study (CDAI and SDAI not reported) [19]. An improvement from baseline was also observed for measures of remission in the final analysis of this LTE study, and by EOT more than half of patients (60.4%) had achieved DAS28-CRP-defined remission, and 75.3% had achieved DAS28-CRP-defined LDA.
The safety profile of peficitinib in the final analysis of the LTE study was consistent with observations from the preceding studies and the interim LTE study analysis, indicating that peficitinib was generally well tolerated over a treatment duration of up to 7 years [13–15, 19]. Of note, the proportion of patients reporting each category of AE was generally higher in the RAJ1 study compared with RAJ3 and RAJ4, perhaps due to the longer mean treatment exposure in patients from the RAJ1 study (47.1 months) versus RAJ3 and RAJ4 (28.1 and 26.9 months, respectively). TEAEs were similar in frequency for each peficitinib dose, and most events were grade 1 or 2 in severity. The most commonly reported TEAE was nasopharyngitis, and its incidence did not increase with higher doses of peficitinib.
We compared the incidences of AEs of special interest observed in our study of Japanese, Korean, and Taiwanese patients with studies of other JAK inhibitors in Asian populations. There were no notable differences in the incidences of AEs of special interest between the phase 3 studies (RAJ3 and RAJ4) [14, 15], our LTE study, or published rates from studies of JAK inhibitors in Asian populations. The IRs (95% CI) per 100 PY of serious infections were 2.3 (1.6, 3.1) and 2.7 (2.1, 3.4) in the interim and final analyses of our extension study, respectively, and 3.7 (3.2, 4.3) and 4.2 (3.1, 5.4) in Asian patients receiving tofacitinib and baricitinib, respectively [19, 21, 22]. Studies have reported that JAK inhibitors can increase the risk of herpes zoster infection, particularly in Asian patients [21–24], so it was not surprising that the most frequently occurring serious infection in our study was herpes zoster-related disease, which had an IR (95% CI) of 7.3 (6.2, 8.6) per 100 PY. This was consistent with IRs (95% CI) per 100 PY from the interim analysis of the extension study [6.8 (5.6, 8.3)], and was similar to results reported for other JAK inhibitors in Asian populations [5.9 (5.2, 6.6) for tofacitinib and 6.2 (4.9, 7.7) for baricitinib] [19, 22, 25]. In our study, there were no major differences among the incidences of TEAEs for the different doses of peficitinib; however, IRs (95% CI) per 100 PY were slightly higher in patients receiving peficitinib 150 mg/day compared with 100 mg/day for serious infections [3.0 (2.0, 4.5) and 2.3 (1.6, 3.3), respectively] and herpes zoster-related disease [8.6 (6.7, 11.0) and 6.9 (5.6, 8.5), respectively]. These results indicate that the peficitinib dose should be carefully selected if general risks, such as advanced age, are identified. There were no major differences in malignancy IRs (95% CI) per 100 PY in our extension study [1.2 (0.9, 1.8)] and those reported previously for tofacitinib [0.8 (0.6, 1.1)] and baricitinib [1.0 (0.5, 1.7)] in Asian populations [21, 22]. It was difficult to interpret the IRs of malignancy-related TEAEs in our study, due to the low numbers of patients with malignancies; however, there appeared to be no evidence of dose-dependency.
There have been concerns around the potential for thromboembolic events in RA patients receiving tofacitinib or baricitinib, leading to dose restrictions in this group of patients [26]. The two cases of VTE reported for our extension study were considered not related to peficitinib.
The strengths and limitations of this study have been discussed in full previously [19]. Specifically, our study provides data from a large number of patients for a mean treatment duration of 32.0 months. Furthermore, the patients received a variety of peficitinib dosing regimens, which is likely to be more representative of routine clinical practice than the preceding trials. Key limitations include the lack of either a placebo or active comparator arm. It was also difficult to compare different dose groups due to the lack of randomization and the ability to adjust the dose, which may have resulted in individual patients experiencing different disease states during the course of treatment. Additionally, it should be taken into consideration that almost all patients were from Japan, a population known to be particularly susceptible to herpes zoster reactivation when treated with tofacitinib [24], and also to respond uniquely to biologic DMARDs [27].
Conclusions
Improvements in ACR response and other clinical effectiveness variables were maintained over a mean treatment duration of 32 months in Asian patients with RA, and peficitinib was generally well tolerated for a period of up to 7 years. These final data are consistent with the interim analysis of the LTE study, and support peficitinib use for long-term management of RA in Asian patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients involved in this study, the study investigators, and team staff.
Funding
Sponsorship for this study and Rapid Service fee were funded by Astellas Pharma, Inc. Medical writing support was funded by Astellas Pharma, Inc.
Medical Writing Assistance
Medical writing support was provided by Anne-Marie Edwards, MChem, of Cello Health MedErgy (Europe), and funded by Astellas Pharma, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Interim data from this study were published as Takeuchi and Tanaka et al. Safety and effectiveness of peficitinib (ASP015K) in patients with rheumatoid arthritis: interim data (22.7 months mean peficitinib treatment) from a long-term, open-label extension study in Japan, Korea, and Taiwan. Arthritis Research & Therapy (2020) 22:47. The final data from this study (32 months mean peficitinib exposure, as included in this manuscript) were presented in part as a virtual poster at the 22nd Asia-Pacific League of Associations for Rheumatology, 24–29 October 2020, Virtual Congress; and were accepted as an abstract to the 2020 JCRA Annual Meeting.
Disclosures
TT has received grants from Astellas Pharma, Inc., Chugai Pharma Co. Ltd, Daiichi Sankyo Co. Ltd, Takeda Pharma Co. Ltd, AbbVie G.K., Asahi Kasei Pharma Corp., Mitsubishi Tanabe Pharma Co., Pfizer Japan, Inc., Eisai Co. Ltd, AYUMI Pharma Corp., Nippon Kayaku Co. Ltd, and Novartis Pharma K.K.; speaking fees from AbbVie G.K., Bristol-Myers Squibb K.K., Chugai Pharma Co. Ltd, Mitsubishi Tanabe Pharma Co., Pfizer Japan, Inc., Astellas Pharma, Inc., Daiichi Sankyo Co. Ltd, Eisai Co. Ltd, Sanofi K.K., Teijin Pharma Ltd, Takeda Pharma Co. Ltd, and Novartis Pharma K.K.; consultancy fees from Astra Zeneca K.K., Eli Lilly Japan K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Co., AbbVie G.K., Nippon Kayaku Co. Ltd, Janssen Pharma K.K., Astellas Pharma, Inc., Taiho Pharma Co. Ltd, Chugai Pharma Co. Ltd, Taisho Toyama Pharma Co. Ltd, GlaxoSmithKline K.K., and UCB Japan Co. Ltd. YT reports speaking fees and/or honoraria from Daiichi Sankyo, Astellas, Eli Lilly, Chugai, Sanofi, AbbVie, Pfizer, YL Biologics, Bristol-Myers Squibb, GlaxoSmithKline, Mitsubishi-Tanabe, Novartis, Eisai, Janssen, Gilead, and Asahi-Kasei, and research grants from Mitsubishi Tanabe, Eisai, Chugai, Takeda, AbbVie, Astellas, Daiichi Sankyo, and Asahi-Kasei. ST reports personal fees from Asahi Kasei Pharma Co., Amgen, Astellas, BioPharma K.K., Ono Pharma Co. Ltd, KYOCERA Medical Co., Daiichi Sankyo Co. Ltd, Teijin Pharma Ltd, Eli Lilly Japan K.K., Pfizer Japan Inc., Astellas Pharma, Inc., AYUMI Pharma Co., Bristol-Myers Squibb, Chugai Pharma Co. Ltd, Eisai Co. Ltd, Hisamitsu Pharma Co. Inc., Mitsubishi Tanabe Pharma Co., AbbVie G.K., Taisho Toyama Pharma Co. Ltd; endowments from Astellas Pharma, Inc., Daiichi Sankyo Co. Ltd, Chugai Pharma Co. Ltd, Blanc Pharmacy, and Zimmer Biomet G.K.; and grants from Japan Agency for Medical Research and Development (AMED), Japan Society for the Promotion of Science (JSPS)/Grant-in-Aid for Scientific Research (A), and Japan Society for the Promotion of Science (JSPS)/Grant-in-Aid for Exploratory Research outside the submitted work. AK reports grants from AbbVie, Actelion, Asahi Kasei, Astellas, AYUMI, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa Hakko Kirin, MSD, Neopharma, Novartis, ONO, Sanofi, Taisho, Takeda Science Foundation, and Teijin; participation in speakers’ bureaux for AbbVie, Actelion, Asahi Kasei, Astellas, Boehringer Ingelheim, Celltrion, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Kowa, MedPeer, Mitsubishi Tanabe, Novartis, ONO, Pfizer, Taisho, and Takeda. YWS reports a grant from Astellas Pharma, Inc. YHC reports grants for research and clinical trials from Taiwan Ministry of Science and Technology, Taiwan Department of Health, Taichung Veterans General Hospital, National Yang-Ming University, GlaxoSmithKline, Pfizer, Bristol-Myers Squibb, and Astellas; and honoraria and consultant fees from Pfizer, Novartis, AbbVie, Johnson & Johnson, Bristol-Myers Squibb, Roche, Lilly, GlaxoSmithKline, AstraZeneca, Sanofi, MSD, Guigai, Boehringer Ingelheim, Astellas, Inova Diagnostics, UCB, and Thermo Fisher. MR, HI, SU, and YK are employees of Astellas Pharma, Inc.
Compliance with Ethics Guidelines
This study was conducted in accordance with Good Clinical Practice, the International Council on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines, and local laws and regulations. The study protocol and amendments were reviewed and approved by an Institutional Review Board or Independent Ethics Committee (Supplementary Table S1) at each study site, and safety data were reviewed by an independent Data and Safety Monitoring Board. Each patient provided written informed consent prior to treatment initiation. This analysis and the clinical trials from which data were included followed the principles of the Declaration of Helsinki.
Data Availability
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Footnotes
Tsutomu Takeuchi and Yoshiya Tanaka contributed equally to this article.
References
- 1.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 2.Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology. 2019;58:i17–26. doi: 10.1093/rheumatology/key225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duggan S, Keam SJ. Upadacitinib: first approval. Drugs. 2019;79:1819–1828. doi: 10.1007/s40265-019-01211-z. [DOI] [PubMed] [Google Scholar]
- 4.Pharmaceuticals and Medical Devices Agency (PMDA) Japan. New drugs approved in FY 2019 [Internet]. 2019. https://www.pmda.go.jp/english/about-pmda/index.html. Accessed 20 Aug 2020.
- 5.US Food and Drug Administration. FDA Approves Xeljanz [Internet]. 2012. https://www.drugs.com/newdrugs/fda-approves-xeljanz-rheumatoid-arthritis-3558.html. Accessed 6 Aug 2019.
- 6.Committee for Medicinal Products for Human Use (CHMP). Xeljanz-Assessment report [Internet]. 2017. https://www.ema.europa.eu/documents/assessment-report/xeljanz-epar-public-assessment-report_en.pdf. Accessed 6 Aug 2019.
- 7.Ministry of Health Labour and Welfare Japan. Report on the Deliberation Results: Xeljanz Tablets 5 mg report. 2013. https://www.pmda.go.jp/files/000153609.pdf. Accessed 6 Aug 2019.
- 8.US Food and Drug Administration. FDA Approves Olumiant. 2018. https://www.drugs.com/newdrugs/fda-approves-olumiant-baricitinib-2-mg-adults-moderately-severely-active-rheumatoid-arthritis-4760.html. Accessed 6 Aug 2019.
- 9.Committee for Medicinal Products for Human Use (CHMP). Olumiant-Assessment report. 2016. https://www.ema.europa.eu/en/documents/assessment-report/olumiant-epar-public-assessment-report_en.pdf. Accessed 6 Aug 2019.
- 10.Ministry of Health Labour and Welfare Japan. Report on the Deliberation Results: Olumiant Tablets 2 mg, 4 mg. 2017. http://www.pmda.go.jp/files/000226301.pdf. Accessed 6 Aug 2019.
- 11.Korea approves Olumiant pills for treatment of rheumatoid arthritis. 2017. http://www.koreabiomed.com/news/articleView.html?idxno=2098. Accessed 6 Aug 2019.
- 12.AbbVie. AbbVie receives European Commission approval of RINVOQTM (upadacitinib) for the treatment of adults with moderate to severe active rheumatoid arthritis [media release]. 2019. https://news.abbvie.com/news/press-releases/abbvie-receives-european-commission-approval-rinvoq-upadacitinib-for-treatment-adults-with-moderate-to-severe-active-rheumatoid-arthritis.htm. Accessed 20 Aug 2020.
- 13.Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75:1057–1064. doi: 10.1136/annrheumdis-2015-208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka Y, Takeuchi T, Tanaka S, Kawakami A, Iwasaki M, Song YW, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3) Ann Rheum Dis. 2019;78:1320–1332. doi: 10.1136/annrheumdis-2019-215163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi T, Tanaka Y, Tanaka S, Kawakami A, Iwasaki M, Katayama K, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. 2019;78:1305–1319. doi: 10.1136/annrheumdis-2019-215164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka Y, Izutsu H. Peficitinib for the treatment of rheumatoid arthritis: an overview from clinical trials. Expert Opin Pharmacother. 2020;21:1015–1025. doi: 10.1080/14656566.2020.1739649. [DOI] [PubMed] [Google Scholar]
- 17.Astellas Pharma Taiwan, Inc. Drug details: 50 mg Smyraf (peficitinib hydrobromide) [Internet]. 2020. https://info.fda.gov.tw/MLMS/H0001D.aspx?Type=Lic&LicId=52027856. Accessed Jun 4 2020.
- 18.Astellas Pharma Taiwan, Inc. Drug details: 100 mg Smyraf (peficitinib hydrobromide) [Internet]. 2020. https://info.fda.gov.tw/MLMS/H0001D.aspx?Type=Lic&LicId=52027857. Accessed 4 Jun 2020.
- 19.Takeuchi T, Tanaka Y, Tanaka S, Kawakami A, Song Y-W, Chen Y-H, et al. Safety and effectiveness of peficitinib (ASP015K) in patients with rheumatoid arthritis: interim data (22.7 months mean peficitinib treatment) from a long-term, open-label extension study in Japan, Korea, and Taiwan. Arthritis Res Ther. 2020;22:47. doi: 10.1186/s13075-020-2125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American college of rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36:729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 21.Lee EB, Yamanaka H, Liu Y, Tsai WC, Chen C, Kwok K, et al. Efficacy and safety of tofacitinib for the treatment of rheumatoid arthritis in patients from the Asia-Pacific region: post-hoc analyses of pooled clinical study data. Int J Rheum Dis. 2019;22:1094–1106. doi: 10.1111/1756-185X.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YC, Yoo DH, Lee CK, Li KJ, Won JE, Wu WS, et al. Safety of baricitinib in East Asian patients with moderate-to-severe active rheumatoid arthritis: an integrated analysis from clinical trials. Int J Rheum Dis. 2019;23:65–73. doi: 10.1111/1756-185X.13748. [DOI] [PubMed] [Google Scholar]
- 23.Winthrop KL, Curtis JR, Lindsey S, Tanaka Y, Yamaoka K, Valdez H, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 2017;69:1960–1968. doi: 10.1002/art.40189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675–2684. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: Integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253–1262. doi: 10.1136/annrheumdis-2016-210457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta P, Ciurtin C, Scully M, Levi M, Chambers RC. JAK inhibitors in COVID-19: need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919. doi: 10.1183/13993003.01919-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi T, Kameda H. The Japanese experience with biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2010;6:644–652. doi: 10.1038/nrrheum.2010.154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.