Abstract
Purpose of Review
Fractures of the proximal humerus (PHF) and distal radius (DRF) are among the most common upper extremity fractures in the elderly. Recent randomized controlled trials support non-surgical treatment. Evidence behind the best non-surgical treatment strategy has been sparse and raises questions as to when and how to initiate exercises. The purpose of this systematic review and meta-analysis was to assess the benefits and harms of early mobilization versus late mobilization and supervised versus non-supervised exercises therapy after PHF and DRF.
Recent Findings
15 published and 5 unpublished trials were included. Early mobilization after PHF resulted in better function with a mean difference (MD) of 4.55 (95% CI 0.00–9.10) on the Constant Shoulder Score. However, the MD was not found to be clinically relevant. No clear evidence showed that early mobilization after PHF had a positive effect on range of motion or pain. Neither did it lead to more complications. Furthermore, no eligible evidence was found supporting early mobilization to be superior to late mobilization after DRF, or that supervised exercise therapy was superior to non-supervised exercise therapy after PHF and DRF. The quality of evidence on all outcomes was found to be low or very low.
Summary
Early mobilization after PHF may have a beneficial effect on function. Due to the lack of clear evidence, there is an urgent need for future studies to determine the effect of early mobilization and supervised exercise therapy after PHF and DRF.
Prospero ID number: CRD42020167656, date of registration 28.04.2020
Supplementary Information
The online version contains supplementary material available at 10.1007/s12178-021-09697-5.
Keywords: Proximal humerus fracture, Distal radius fracture, Non-surgical treatment, Early mobilization, supervised exercise therapy
Introduction
Fractures of the proximal humerus (PHF) or distal radius (DRF) are among the most common fractures in the elderly population [1, 2]. The majority of patients suffering a PHF or DRF are aged 60 years or older, and the most representative patient is an elderly osteoporotic woman [2, 3]. Due to longer life expectancy in the elderly population, the incidence rates of these osteoporotic fractures are predicted to increase [2, 3]. In future, this increase will impose a substantial burden on healthcare systems and increase societal costs.
Both PHF and DRF can have a substantial impact on the patient´s physical function and independent living and are associated with higher morbidity and mortality [3, 4]. After sustaining a PHF or DRF, the main focus of treatment is to regain the best possible function of the shoulder or wrist. Recent evidence questions the benefit of surgical treatment compared with non-surgical treatment after PHF [5–7]. The same conclusion was reached in a recent review investigating the optimal treatment after DRF in which no clear benefit of surgical treatment was found in the elderly [8•]. Thus, the next important task is to address and optimize the non-surgical treatment strategy for these fractures.
The question of when to commence supervised exercise therapy is of high clinical importance. Sparse evidence suggests that there might be a preference for a short immobilization period after sustaining a PHF or DRF [9••].
Patients are usually referred to supervised rehabilitation after sustaining these types of fractures. However, supervised rehabilitation consumes considerable healthcare resources and raises the question as to what extent patients benefit from supervised exercise therapy [8, 10]. Bruder et al. have suggested that after PHF non-supervised exercises at home might be just as effective as exercises supervised by a therapist; however, this conclusion was based on scarse evidence [9••]. In 2015, the Cochrane review concluded that there is no evidence to determine the best possible non-surgical treatment after PHF [6].
Our aim therefore was to conduct a systematic review and meta-analysis to assess the benefits and harms of (1) early mobilization compared to late mobilization and of (2) supervised exercise therapy compared to non-supervised exercises after non-surgically treated PHF and DRF.
Materials and Methods
Study Design and Protocol
This is a systematic review with meta-analysis. Search strategy, trial selection, eligibility criteria, methodology assessment, data extraction, and analysis were performed in accordance with a predefined protocol (PROSPERO: CRD42020167656). Trial screening, selection of trials, data extraction, assessment of methodology, and quality of evidence were performed by two independent reviewers (H.K.Ø. and V.T.P.). Disagreements were resolved through a consensus process. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) was used as a checklist throughout the reporting [11].
Search Strategy and Trial Selection
Electronic databases were systematically searched for primary trials (Supplementary material). Searches were conducted in MEDLINE (OvidSP), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), CINAHL, Physiotherapy Evidence Database (PEDro), Scopus, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform (WHO ICTRP). Other sources involved the hand searching of reference lists of systematic reviews or guidelines. The search was limited to randomized controlled trials, non-randomized trials, and prospective observational studies. Searches were undertaken on September 20 and December 2, 2019 and repeated on June 11, 2020. Results were loaded into EndNote (version X9.2; Clarivate Analytics) software for deduplication. Full, database-specific search strategies are available in the online appendix. The identified trials were uploaded in systematic review management software (Covidence, Aus) and screened at title/abstract level. Eligible trials were then full-text screened for final inclusion. Reference lists from the full-text trials were also screened for supplementary relevant trials.
Eligibility Criteria
Eligibility criteria were defined according to the Population, Intervention, Comparison, and Outcome (PICO) method [12]. The population consisted of adults ≥ 18 years with a verified PHF or DRF due to recent trauma and referred to non-operative treatment. The interventions and comparisons were defined as (1) early mobilization (≤ within 2 weeks after time of fracture) compared to late mobilization or (2) supervised exercise therapy compared to non-supervised exercises. The included outcome measures were function, pain, and health-related quality of life (HRQoL). Function was defined as either function assessed by patient-reported outcome measures (PROMs), performance-based function including range of motion (ROM) and strength measures, or by questionnaires comprising both of these subjective and objective measurements.
Methodological Assessment
The included trials were evaluated using the Cochrane Risk of Bias tool (RoB 2.0) [13]. The risk of bias was rated as low, unclear, or high. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to rate the body of evidence for each outcome as either very low, low, moderate, or high [14].
Data extraction and statistical analysis
The following trial information was extracted: author, year, country, trial design, number of participants, population characteristics, description of intervention/control, outcome measures, and time to follow-up. Effect estimates were extracted as reported in the trials. In three cases, the corresponding authors were contacted for extended data details. If a minimum of two outcome measures were found eligible for comparison, a meta-analysis was undertaken and values were presented as either mean difference (MD) or standard mean difference (SMD) with 95% CIs, using the random-effect model. The I2 value was calculated and if higher than 50%, the heterogeneity was considered substantial and not eligible for meta-analysis. In cases where meta-analysis could not be undertaken, narrative synthesis was performed based on the conclusions reported in the trials. Statistics were performed using Stata 16 (TX, USA) and R 3.6.2 (R Foundation, Vienna).
Results
Search and Selection of Trials
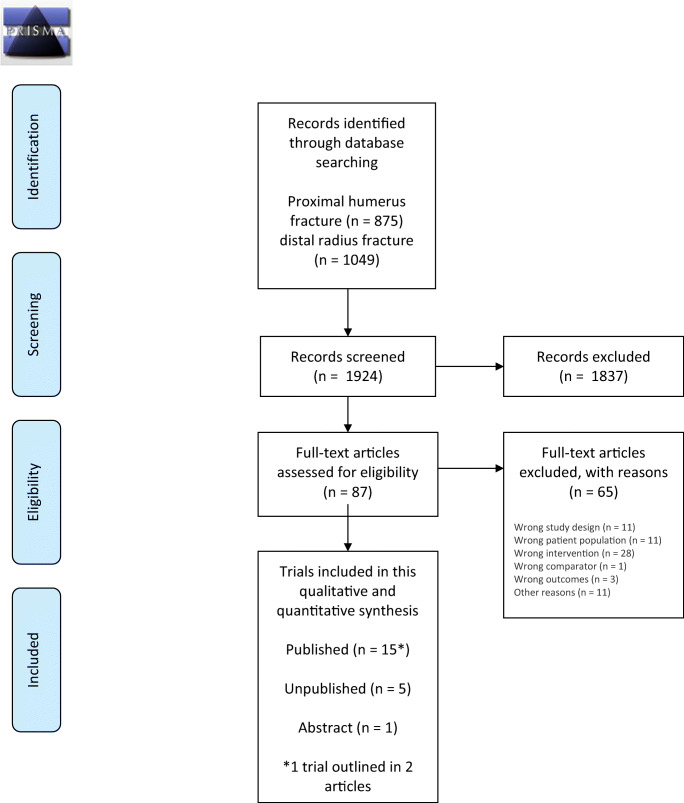
In total, 1924 trials were found eligible for screening. After screening and full-text reading, 15 RCTs (943 participants) were included. Furthermore, 1 abstract (98 participants) and 5 trials (736 participants) registered in ClinicalTrials.gov and WHO ICTRP were identified. A summary of the search results and trial selection process is provided in a PRISMA flow diagram (Fig. 1).
Fig. 1.
PRISMA flow chart of the included studies
Trial Characteristics
The mean age of the participants in the included trials ranged from 52.5 to 77.3 years. The follow-up times ranged from 2 weeks to 2 years. Specific details on the outcome measures used can be found in Tables 1, 2, and 3.
Table 1.
Study characteristics of the trials included in the systematic review (n = 15)
| Author Year Country Design |
Type of fracture | Number of included patients (N) Participants |
Intervention and comparison | Outcome measures and follow-up | Results |
|---|---|---|---|---|---|
|
Hodgson et al. (two papers) 2003 + 2007 England RCT |
Proximal humerus fracture (PHF) displaced (displacement ≤ 1 cm or angulation ≤ 45° Non-operatively treated |
N = 86 Age limit ≥ 40 years Mean age in years: Intervention group 70.7 and comparison group 69.6 Female/male: Intervention group 33/11, comparison group 37/5 |
Intervention: Early mobilization with immediate physiotherapy within 1 week Comparison: Physiotherapy after 3 weeks of immobilization in a sling Both groups had the same physiotherapy program |
Primary outcome • Constant shoulder score (CS) (as ratio between the fractured and non-fractured arm) Secondary outcomes • Croft shoulder disability questionnaire (Croft) • Short Form-36 (SF-36) Follow-up: 8 and 16 weeks + 1 and 2 years |
Early mobilization resulted in • Better function (CS) after 16 weeks (MD ratio 0.16, 95% CI 0.68–0.25) and 52 weeks (MD ratio 6.4, 95% CI 2.5–10.5) • Less pain (SF-36) 16 weeks (MD 12.2, 95% CI 3.2–21.02) and 52 weeks (MD AUC 486, 95% CI 83–889) • Higher role limitations score (SF-36) after 16 weeks (MD 22.2, 95% CI 3.4–40.9) Lower level of shoulder disability on the Croft score after 1 year (p ≤ 0.01) |
|
Lefevre-Colau et al. 2007 France RCT |
PHF Stable fracture of the metaphysis or epiphysis in which the fragments are driven into each other (AO classification) Non-operatively treated |
N = 74 Age limit ≥ 20 years Mean age in years: Intervention group 63.2 and comparison group 63.4 Female/male: Intervention group 24/14 and comparison group 30/7 |
Intervention: Early mobilization with exercise program initiated within 72 hours of fracture Comparison: Late mobilization with exercise program initiated 3 weeks post-fracture |
Primary outcome •CS Secondary outcomes • Visual Analog Scale (VAS) • Range of motion (ROM) (difference in abduction, anterior elevation, and lateral rotation between shoulders) • Non-unions • Adverse events Follow-up: 6 and 12 weeks + 6 months |
Early mobilization resulted in • Better function (CS) after 6 weeks (MD 10.1, 95% CI: 2.0–18.1) and after 3 months (MD 9.9, 95% CI 1.9–17.8) • Better ROM and strength score (CS) after 6 weeks (7.5, 95% CI 1.2–13.9) and 3 months (MD 7.0, 95% CI 1.4–12.5) • Reduced pain intensity (MD 15.7, 95% CI 0.52–30.8) after 3 months • Better abduction (MD 12, 95% CI 0.4–23.7) and anterior elevation (MD 12.1, 85, 95% CI 1.7–22.4) after 3 months No difference between groups was found after 6 months Complications: • One case of frozen shoulder in the late mobilization group • No cases of non-unions were reported |
|
Kristiansen et al. 1989 Denmark RCT |
PHF 74% of the fractures were minimally displaced Non-operatively treated |
N = 85 Median age in years: Intervention group 72 and comparison group 70 Female/male: Intervention group 30/12 and comparison group 30/12 |
Intervention: Early mobilization with pendulum exercises of the shoulder + active movement of the elbow after 1 week of immobilization in a sling and body bandage Comparison: Late mobilization with pendulum exercises of the shoulder + active movement of the elbow after 3 weeks of immobilization in a sling and body bandage |
• Modified Neer score (including pain, function, and ROM) Follow-up: 1, 3, 6, 12, and 24 months |
Early mobilization resulted in •Better Neer total score (median difference 9, p ≤ 0.001) after 3 months • Lower pain (Neer subscore) (median difference 10, p ≤ 0.01) after 1 month (median difference 9, p ≤ 0.001) after 3 months No difference between groups was found at any of the other follow-up Complications: 1 case of reflex dystrophy was seen in each group |
|
Christersson et al. 2018 Sweden RCT |
Distal radius fracture (DRF) Moderately displaced; initial dorsal angulation 5–40°, axial compression ≤ 4 mm, intra-articular step-off ≤ 1 mm Non-operatively treated All fractures had undergone closed reduction |
N = 109 Age limit ≥ 50 years Median age in years (range): Intervention group 67 (52–90) and comparison group 64.7 (50–92) Female/male: Intervention group 47/7 and comparison group 51/4 |
Intervention: Early mobilization with removal of plaster cast after 10 days Comparison: Immobilization in plaster cast for 4 weeks |
• Modifies De Bruijn wrist score • Modified Mayo wrist scoring chart • Gartland and Werley • Grip strength • Pincher strength • ROM (dorsal extension, volar flexion, forearm supination and pronation) • VAS • Treatment failures (problems leading to abandonment of given treatment and poor outcome leading to surgical treatment of malunion) Follow-up: 1, 4, and 12 months |
Early mobilization resulted in better dorsal extension (MD 14°, p ≤ 0.001) pronation (MD 12°, p = 0.002) and supination (MD 8°, p = 0.03) after 1 month No between-group differences was found in any other outcomes or follow-up 3 patients in the intervention group had treatment failures |
|
Stoffelen et al. 1998 Belgium RCT |
DRF Minimally displaced and stable fractures Non-operatively treated |
N = 52 No information about mean age or gender distribution |
Intervention: Early mobilization after immobilization in plaster cast for 1 week Comparison: Late mobilization after 3 weeks in dorsal plaster cast Plaster cast in neutral position |
• Cooney score (includes function, ROM, and grip strength) Follow-up: 6 weeks + 3, 6, and 12 months VAS was measured 1 and 3 weeks after cast removal |
No between-group difference on the Cooney score at any follow-up 4 cases of reflex dystrophy |
|
Jensen et al. 1997 Denmark RCT |
DRF Undisplaced or minimally displaced Non-operatively treated |
N = 62 Mean age in years: (range): Intervention group 59 (22–86) and comparison group 56 (19–82) Female/male: Intervention group 17/5 and comparison group 15/11 |
Intervention: Early mobilization after 1 week in dorsal plaster cast Comparison: Late mobilization after 3 weeks. in dorsal plaster cast |
• Gartland and Werley Follow-up: 12 and 26 weeks |
No between-group difference on the modified Gartland and Werley score |
|
Davis et al. 1987 England RCT |
DRF < than 10° of dorsal angulation Non-operatively treated |
N = 56 Mean age in years: Intervention group 56.6 and comparison group 55.5 Female/male: Intervention group 24/4 and comparison group 19/7 |
Intervention: Immobilization in below-elbow (backslab) plaster cast for 7–13 days followed by double tubigrip + encouraged to use the injured wrist within the limits of pain Comparison: Immobilization in below-elbow (back slab) plaster cast for 4–5 weeks |
• Gartland and Werley • Grip strength • Domestic abilities (handling coins, knife, or fork, turning a latch key, carrying a shopping bag and using a tin opener) • VAS (0–20) • Complications Follow-up: 2, 5, and 7 weeks after the fracture |
Early mobilization resulted in • Higher functional score (Gartland and Werley’s) (p < 0.05) after 5 and 7 weeks • Better recovery of domestic skills (p < 0.05) after 5 and 7 weeks No between-group difference was found in pain and grip strength |
|
Dias et al. 1987 England RCT |
DRF Undisplaced |
N = 97 (in the undisplaced group) Age limit ≥ 55 years No information provided on mean age or gender distribution in the undisplaced group |
Intervention: Crepe bandage was used and immediate mobilization was encouraged (no plaster cast used) Comparison: Immobilized in a conventional cast for 5 weeks |
• Functional score (by Steward et al.) • Anatomical score (by Lindstöm) • ROM (flexion, extension, radial and ulnar deviation of the wrist, forearm rotation, and metacarpophalangeal and interphalangeal movement) • Grip strength •VAS Follow-up: 1, 5, 9, and 13 weeks |
Early mobilization resulted in better grip strength after 5 (MD 9.6), 9 (MD 11.8), and 13 (17.9) weeks (p ≤ 0.005 for all) Early mobilization resulted in faster functional and movement recovery No between-group difference in pain |
|
Bertoft et al. 1984 Sweden RCT |
PHF Non-displaced or slightly displaced 7 patients had a fracture of the greater tubercle Non-operatively treated |
N = 20 Mean age in years (range): Intervention group 66 and comparison group 62 (50–75) Female/male: 17/1 (no group distribution reported) |
Intervention: Physiotherapist-supervised sessions during 10–12 weeks + manual therapy The intervention consisted of 9 sessions Comparison: Non-supervised training (3 instructions given) Both groups started exercises 10–12 days post-fracture, where sling was removed |
• ROM (hand on neck and hand on back) + pain measured during the movements (Borg 0–8) • ADL (subjective assessment of daily living) • Isometric strength (vertical and horizontal pushing) Follow-up: 3, 8, 16, 24 weeks and 1 year |
No between-group difference for any outcome at any follow-up |
|
Lundberg et al. 1979 Sweden RCT |
PHF Undisplaced with less than 1 cm displacement or 45° of angulation Non-operatively treated |
N = 42 Mean age in years (range): 65 (30–89) equal in both groups Female/male: 37/5 (no group distribution reported) |
Intervention: Physiotherapist supervised exercise 1–2 per week for 2–3 months Comparison: Non-supervised training (3 instructions given) Both groups started exercises 1 week post fracture |
• Neer’s numerical rating • ROM (active and passive elevation) • Muscle function • Grip strength • Pain (grouped as severe, moderate, or insignificant) Follow-up: 1 and 3 months and ≥ 1 year (mean 16 months) |
No between-group difference for any outcomes at any follow-up Complications: 2 cases of frozen shoulder in the supervised group and 1 case in the comparison group One case in the supervised group had a considerable degree of unexplained pain over a long period |
|
Gutierrez-Espinosa et al. 2017 Chile RCT |
DRF A3 extra-articular multifragmentary according to AO/ASIF classification Non-operatively treated |
N = 74 Age limit ≥ 60 Mean age in years: Intervention group 72 and comparison group 71.6 Female/male: Intervention group 35/2 and comparison group 36/1 |
Intervention: Supervised physiotherapy consisting of 15-min whirlpool exercises, manual therapy, and motor skill training The intervention consisted of 12 sessions, 2–3 times a week (1 h) Comparison: Non-supervised home exercise (1 instruction + leaflet given with exercises for 6 weeks) |
• Patient-Reported Wrist Evaluation (PRWE) • Active ROM (wrist flexion and extension) • Grip strength • VAS Follow-up: 6 weeks and 6 months |
Supervised exercise resulted in • Greater total score on the PRWE after 6 weeks (MD 17.7, 95% CI 23.7–11.6) and 6 months (MD 17.1, 95% CI 22.12–11.9) • Greater score on the PRWE function domain after 6 weeks (MD 15.2, 95% CI 21.5–12.6) and after 6 months (MD 14.5, 95% CI 20.9–11.4) • Improvement on the PRWE pain domain after 6 weeks (MD 5.6, 95% CI 2.1–10.9) and after 6 months (MD 2.5, 95% CI −1.2 to 6.3) • Lower degree of pain (VAS) after 6 weeks (MD 1.8, 95% CI 2.4–1.1) and after 6 months (MD 1.0, 95% CI 1.4–0.5) • Higher grip strength after 6 weeks (MD 21.2%, 95% CI 28.2–14.2) and after 6 months (MD 25.8, 95% CI 24.4–13.7) • Higher degree of active flexion after 6 weeks (MD 12.3, 95% CI 18.1–6.2) and after 6 months (17.7, 95% CI 23.1–12.3) • Higher degree of active extension after 6 weeks (MD 20.3, 95% CI 26.6–13.9) and after 6 months (MD 19.1, 95% CI 24.4–13.7) |
|
Krischak et al. 2009 Germany RCT |
DRF Non-operatively treated |
N = 42 Age ≥ 18 years Mean age in years (range): Intervention group 53.8 (18–90) and comparison group 52.5 (18–95) Female/male: Intervention group 13/8, comparison group 10/11 |
Intervention: Physiotherapist-supervised exercise (12 sessions of 20–30 min) Comparison: Non-supervised home exercise program |
• PRWE • ROM (supination, pronation, extension, and flexion) • Grip strength Follow-up: 6 weeks after cast removal |
Supervised exercise therapy resulted in • Less improvement of the functionality on the PRWE (MD 14.1, p = 0.003) • Lower grip strength (MD 16% of the starting base value; p = 0.02) No between-group difference in ROM |
|
Watt et al. 2000 Australia RCT |
DRF Non-operatively treated |
N = 18 Mean age in years: Intervention group 74.4 and comparison group 77.3 Female/male: Intervention group 9/0 and comparison group 8/1 |
Intervention: Physiotherapist-supervised exercise therapy (active exercise and passive joint mobilization + home exercise program and home advice passive joint) Mean number of visits was 5 times Comparison: Non-supervised home exercise (sheet + simple instruction) |
• Wrist extension • Grip strength Follow-up: 6 weeks after cast removal |
Supervised exercise therapy resulted in greater increases in wrist extension (MD 17.4, p = 0.01) and grip strength (MD 4.7, p = 0.026) after 6 weeks |
|
Wakefield et al. 2000 Scotland RCT |
DRF Non-operatively treated |
N = 96 Age ≥ 55 years Mean age in years: Intervention group 72 and comparison group 74 Female/male: Intervention group 44/5 and control 43/4 |
Intervention: Physiotherapist-supervised exercise therapy + instruction in standard sheet of home exercises Median number of physiotherapy treatment was 3 (range 1 to 22) Comparison: Non-supervised exercise therapy (after instructed in home exercises) |
•Functional assessment • ROM (flexion, extension, pronation, supination, and radial and ulnar deviation) • Grip strength • Pain (VAS) • SF-36 • X-ray for angulation Follow-up: 6 weeks + 3 and 6 months |
No between-groups difference in hand function, grips strength, pain, or HRQoL at any follow-up Supervised exercise therapy resulted in improved flexion/extension (MD 12.2 95% CI 5.4 to 19.2 ) at 6 months of follow-up |
|
Christensen et al. (2000) Denmark RCT |
DRF Non-operatively treated |
N = 30 Mean age in years(range): Intervention group 66.1 (46–82) and comparison group 65.9 (57–79) Female/male: Intervention group 14/2 and comparison group 13/1 |
Intervention: Occupational therapist supervised exercise therapy Mean time spent was 11.38 h Comparison: Non-supervised exercise therapy (after single instruction) Exercises in both groups were initiated after cast removal |
• Modified Gartland and Werley functional score Follow-up: 5 weeks, 3 and 9 months after cast removal |
No between-groups difference in Gartland and Werley functional score at any follow-up |
The table provides the reported information regarding the participants, the interventions and comparisons, the outcomes, the follow-up times, and results
Table 2.
Study characteristics of the unpublished trials registered in ClinicalTrials.gov or International Clinical Trials Registry Platform (ICTRP) included in the systematic review (n = 5)
| Author Identifier Date of registration Trial status Country Design |
Type of fracture | Number of included patients (N) Participants |
Intervention vs. comparison | Outcome measures and follow-up | Results |
|---|---|---|---|---|---|
|
Chen et al. February 22, 2007 Recruitment completed USA RCT |
PHF 82% non- or minimally displaced Non-operatively treated |
N = 62 Age limit ≥ 18 years Mean age in years: Intervention group 63 and control 62 Female/male: Intervention group 5/21 and comparison group 9/15 |
Intervention: Early mobilization with therapy starting immediately after injury Comparison: Late mobilization with therapy starting 3 weeks after injur |
Primary outcome • Shoulder flexion Secondary outcomes • Disability of the Arm, Shoulder, and Hand (DASH) • Abduction and external rotation • Shoulder Pain Likert scores (0–10) Follow-up: 3 and 6 months |
Numbers extracted from ClinicalTrials.gov No between-group difference in any of the outcomes at any follow-up |
|
Torrens et al. July 14, 2017 Recruitment completed Spain RCT |
PHF Non-operatively treated |
N = 130 Age limit: ≥ 60 years and < 85 years |
Intervention: Early mobilization with patients undergoing 1-week immobilization period in a sling Comparison: Late mobilization with patients undergoing a 3-week immobilization period in a sling |
Primary outcome • Visual Analog Scale (VAS) Secondary outcomes • Constant Shoulder Score (CS) • Fracture displacement • Simple Shoulder Test (SST) • Follow-up: 3 and 6 months and 1 year |
Numbers provided by author Carlos Torrens No between-group differences in any of the outcomes or at any follow-up |
|
Adolfsson et al. December 26, 2018 Not yet recruiting Sweden RCT |
PHF Non-operatively treated |
N = 400 Age limit ≥ 18 years |
Intervention: Early mobilization where patient are instructed to start rehabilitation 1 week after the trauma Control: Late mobilization after 4 weeks |
Primary outcome • Union of fracture Secondary outcomes • Oxford Shoulder Score • Numerical pain reporting scale • Quick DASH • Global assessment of improvement • Range of motion (ROM) Follow-up: 12 months |
No data available |
|
Østergaard et al. April 17, 2018 Recruiting Denmark RCT |
PHF 2-part fractures (Neer’s) Non-operatively treated |
N = 70 Age limit ≥ 60 years |
Intervention: Physiotherapist supervised exercise therapy once a week for 10 weeks + daily home exercises Comparison: Non-supervised exercise therapy at home (1 instruction given at inclusion) Both groups follow the same exercise protocol |
Primary outcome • Disabilities of the Arm, Shoulder, and Hand (DASH) Secondary outcomes • CS • Strength (isometric in 90° elevation in scapula plane) • Activity monitoring measured with accelerometer sensors • VAS • 15-dimensional score for HRQoL (15D) Follow-up: 3 and 12 months |
No data available |
|
Araya et al. RBR-59nbtf March 17, 2020 Recruitment completed Chile RCT |
DRF Extra-articular multifragmentary type FRD A3 after AO classification Non-operatively treated |
N = 74 Age limits ≥ 60 and ≤ 75 years |
Intervention: Physiotherapist-supervised exercise therapy + whirlpool and manual therapy (12 sessions, 2–3 times a week and approximately 1-h-long session) Comparison: Non-supervised home exercises program (all patients received a 30-min instruction by a physical therapist). They received a description of 6-week exercises |
Primary outcome • Patient-Rated Wrist Evaluation (PRWE) Secondary outcomes • Grip strength • VAS • ROM (wrist flexion and extension) Follow-up: 6 weeks, 6 and 12 months |
No data available |
The table provides available information regarding the participants, the interventions and comparisons, the outcomes, the follow-up times, and results
Table 3.
Study characteristics of the abstract included in the systematic review (n = 1)
| Author Date Country Design |
Type of fracture | Number of included patients (N) Participants |
Intervention vs. comparison | Outcome measures and follow-up | Results |
|---|---|---|---|---|---|
|
Bache et al. July 2000 England RCT |
DRF Non-operatively treated |
N = 98 Mean age in years: 69 Female/male: 82/16 |
Intervention: Supervised exercise therapy and advice Comparison: Non-supervised exercise therapy and advise |
Range of motion (ROM) Grip strength Visual Analog Scale (VAS) Follow-up: 4 and 12 weeks |
Supervised exercise therapy resulted in a greater increase of the supination (p = 0.04) No between-group difference in any of the other outcomes at any follow-up |
The table provides available information regarding the participants, the interventions and comparisons, the outcomes, the follow-up times, and results.
Benefits and harms of Early Mobilization after Proximal Humerus Fracture
Function
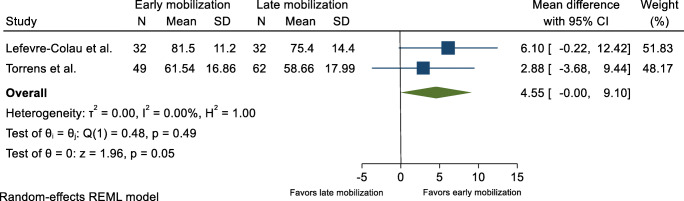
Functional scores were reported in three trials. [15–18]. One of the trials was outlined in two different papers [15, 16]. In addition, two unpublished trials (ClinicalTrials.gov) provided preliminary results [19, 20]. The outcome measures used are outlined in Tables 1 and 2. Data extracted from two of the trials [17, 20] allowed meta-analysis and an overall MD of 4.55 (95% CI 0.00–9.10) on the Constant Shoulder Score (CS) at 6-month follow-up in favor of early mobilization was found (Fig. 2). This finding was supported by two other trials concluding that early mobilization within 2 weeks after sustaining a PHF resulted in better function when compared to late mobilization [16, 18].
Fig. 2.
Function, 6 months after proximal humerus fracture. Displays pooled weighted mean difference for function (Constant shoulder score). 95% CI = 95% confidence interval
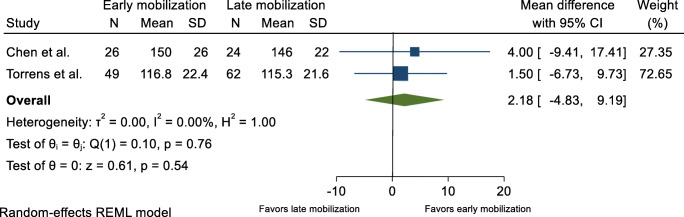
ROM was reported in two trials [17, 18]. In addition, two unpublished trials reported ROM figures on which a meta-analysis could be conducted [19, 20]. No eligible evidence of a difference between the groups was found at 6-month follow-up, either in flexion with a MD equal to 2.18° (95% CI −4.83° to 9.19°) or in abduction with a MD equal to 3.75° (95% CI −5.76° to 13.27°) (Figs. 3 and 4). The same conclusion was reached in a third trial that found no eligible evidence of a difference in ROM between groups [18]. A fourth trial reported a higher active and passive abduction and anterior elevation in favor of the early mobilization group [17] (Table 1).
Fig. 3.
Range of shoulder flexion, 6 months after proximal humerus fracture. Displays pooled weighted mean difference for shoulder flexion (°) of the fractured shoulder. 95% CI = 95% confidence interval
Fig. 4.
Range of shoulder abduction, 6 months after proximal humerus fracture. Displays pooled weighted mean difference for shoulder abduction (°) of the fractured shoulder. 95% CI = 95% confidence interval
Pain
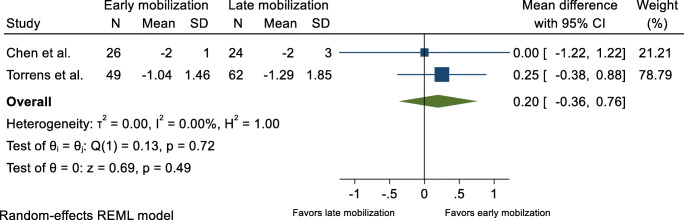
Pain scores were reported in two published trials [17, 18] and the two previously mentioned unpublished trials [19, 20]. Meta-analysis based on data from the two unpublished trials [19, 20] showed no evidence suggestive of between-group difference in pain 6 months after the fracture, with an MD equal to 0.20 (95% CI −0.36 to 0.76) (Fig. 5). In contrast, two other trials concluded individually that early mobilization resulted in a decrease of pain in favor of the early mobilization group [17, 18] (Table 1).
Fig. 5.
Patient-reported pain, 6 months after proximal humerus fracture. Displays pooled weighted mean difference for shoulder pain (VAS and Likert scale/0-10). 95% CI = 95% confidence interval
Health-Related Quality of Life
The Short Form-36 (SF-36) was used to measure HRQoL in one trial. The trial reported a positive effect in the role limitation and pain domains in favor of early mobilization. This positive effect was not, however, found between groups in any of the other domains [16].
Complications
One trial identified a single case (1/32) of frozen shoulder in the late mobilization group [17] while another trial found one case (1/39 and 1/41) of reflex dystrophy in both groups [18].
Benefits and Harms of Early Mobilization after Distal Radius Fracture
Function
Functional scores were reported in five trials. The outcomes used are described in Tables 1 and 2. Two trials reported an improvement of function in favor of early mobilization after DRF [21, 22]. This finding was not, however, in agreement with the results reported in three other trials, which concluded that early mobilization after DRF did not lead to improvement of function [23–25]. Recovery of domestic abilities was assessed in one trial that reported a higher number of patients in the early mobilization group regained their abilities within 5 weeks [21].
ROM was reported in two trials which stated that early mobilization improved movement of the wrist [22, 25]. Grip strength was assessed in three trials [21, 22, 25]. One trial reported grip strength being higher in the early mobilization group [22]. However, no increase in grip strength was reported in the other two trials [21, 25].
Pain
Pain scores were reported in two trials. The trials did not find eligible evidence of a difference between groups at any of the time points [21, 25].
Health-Related Quality of Life
HRQoL was not assessed in any of the trials investigating early versus late mobilization after DRF.
Complications
One trial identified three cases (3/54) of treatment failure in the early mobilization group, defined as problems leading to abandonment of given treatment or operation of malunited fracture [25]. Another trial reported four cases of reflex dystrophy, but they did not state in which groups [24].
Benefits and harms of supervised exercise therapy after proximal humerus fracture
Function
ROM was assessed in two trials. One trial measured movements comprising hand on back and hand on neck [26], and one trial measured active and passive elevation [27]. Both trials concluded that supervised exercise therapy did not result in a better ROM at any time point. Muscle strength was also assessed in the two trials. One trial measured isometric muscle strength by horizontal and vertical push [27], and the other trial measured shoulder and grip strength [26]. No eligible evidence of a difference between groups at any time point was detected.
Pain
Pain was assessed in two trials [26, 27]. One trial rated pain as insignificant, moderate, or severe and the other trial used a modified Borg scale (from 0 to 8). The trials did not show a higher degree of pain relief in the supervised exercise therapy group [26, 27].
Health-Related Quality of Life
HRQoL was not assessed in any of the trials investigating supervised exercise therapy after PHF.
Complications
One trial reported three cases of frozen shoulder. Two cases (2/20) were identified in the supervised group and one (1/22) case in the non-supervised group. In addition, one (1/22) patient in the non-supervised group had an unexplained pain over a longer period [26].
Benefits and Harms of Supervised Exercise Therapy after Distal Radius Fracture
Function
Patient-Rated Wrist Evaluation (PRWE) was used in two trials and the results were inconsistent. One trial found that supervised exercise therapy improved function [28••]. The second trial, however, reported a greater improvement in the non-supervised group [29], and a third trial did not find any eligible evidence of a between-group difference [30].
ROM was reported in four trials [28, 29, 31, 32] and in one trial abstract [33]. Only one of the trials found no eligible evidence of a difference in ROM between the two groups [29], whereas the remaining trials concluded that supervised exercise therapy after DRF leads to an increased ROM [28, 31–33].
Grip strength was reported in four trials and the results were inconsistent [28, 29, 31, 32]. Two trials found increased grip strength in the supervised group [28, 32], the third trial found no eligible evidence of a difference between the groups at any time point [31], and the fourth trial found grip strength to be greater in the non-supervised group [29].
Pain
VAS was used to measure pain in two trials [28, 31]. One trial reported a lower degree of pain in the supervised group [28••], but another trial found no eligible evidence of a clear difference between the groups at any time point [31].
Health-Related Quality of Life
HRQoL was assessed in one trial using SF-36. The trial concluded that supervised exercise therapy after DRF did not increase HRQoL [31].
Quality of Evidence
Risk of Bias
Randomization was applied in all 15 trials. However, only six trials provided sufficient details leading to low risk of bias (Table 4). Blinding of patients in these study settings was not possible, and therefore the risk of bias was rated high in this domain in all trials. Blinding of the outcome assessors was rated as unclear in seven of the trials. Based on adherence of the methods described in the trials, selective reporting of outcomes was not found to be an overall problem, and in most cases the risk of bias was rated as low. However, only a few trials had prior protocol registrations or published protocols, and thus the assessment of selective reporting of outcomes was not comprehensive.
Table 4.
Risk of bias assessment (RoB2) for the 15 included studies
| Sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel | Blinding of outcome assessors (detection bias) | Incomplete outcome data (attrition bias) | Selective outcome reporting (reporting bias) | Other sources of bias | |
|---|---|---|---|---|---|---|---|
|
Hodgson et al. 2003 and 2007 |
Unclear Randomly allocated, no further details |
Low Using sequentially numbered sealed envelopes |
High No blinding |
Low Blinded evaluator |
Low Loss to follow-up 16 weeks = 5.8% 1 year = 5% 2 years = 14% |
Low |
Unclear Baseline imbalance with more males in the early mobilization group (11 vs. 5) |
|
Lefevre-Colau et al. 2007 |
Low Block randomization involved choosing randomly from among blocks of lengths 4 and 2 to prevent the risk of predictability |
Low An independent researcher responsible for allocation was contacted by telephone |
High No blinding |
Low Blinded evaluator |
Low 13.5% withdrew after randomization and before baseline test No further loss to follow-up |
Low |
Low No apparent problems |
|
Kristiansen et al. 1989 |
Unclear Randomly allocated, no further details |
Unclear |
High No blinding |
Unclear |
High Substantial loss to follow-up: 1 month = 6% 3 months = 11% 6 months = 14% 12 months = 36% 24 months = 54% |
Unclear |
Unclear No sample size calculation |
|
Christersson et al. 2017 |
Low Randomization by using a log to ensure that envelopes were opened sequentially |
Low Randomization was done by ordered opaque and sealed envelopes |
High No blinding |
Unclear |
Low Little loss to follow-up: 1 month = 0.9% 4 months = 4.6% 12 months = 3.7% |
Unclear |
Low No apparent problems |
|
Jensen et al. 1997 |
Unclear Allocated at random, no further details |
Unclear |
High No blinding |
Unclear |
High Substantial loss to follow-up: 12 and 26 weeks = 22.6% |
Low |
Unclear Small sample and no sample size calculation |
|
Stoffelen et al. 1989 |
Unclear Randomly allocated, no further details |
Unclear |
High No blinding |
Unclear |
Unclear No loss to follow-up described |
Low |
High No sample size calculation Baseline imbalance due to significant age difference |
|
Davis et al. 1987 |
Unclear Randomly divided, no further details |
Unclear |
High No blinding |
High |
High Loss to follow-up not explained sufficiently |
Low |
Unclear No sample size calculation |
|
Dias et al. 1987 |
Unclear Randomized, no further details |
Unclear |
High No blinding |
Unclear Final review was undertaken by an independent observer. No information regarding blinding at the other follow-up visits |
Low Now loss to follow-up described |
Unclear |
Unclear No sample size calculation |
|
Lundberg et al. 1979 |
Unclear Randomly allocated, no further details |
Unclear |
High No blinding |
Unclear No information provided; however, final evaluation was done by a physiotherapist not previously engaged |
Unclear Loss to follow-up after 3 months = 5%, all in the control group |
Low |
Unclear Small sample and no sample size calculation |
|
Bertoft et al. 1984 |
Low Randomly assigned, mention permutation table |
Low Independent person had key to the permutation table |
High No blinding |
Low Evaluator blinded |
High High loss to follow-up 8 weeks (10%), 16 weeks (15%), 24 weeks (20%), and > 1 year (35%) |
Unclear |
Unclear Small sample and no sample size calculation |
|
Gutierrez-Espinoza et al. 2017 |
Low Randomized by sequence of numbers generated by computer |
Low Sealed envelopes |
High No blinding |
Low Evaluator blinded |
Low No reported loss to follow-up |
Low |
Unclear Two very different exercise programs are used in the two groups |
|
Krischak et al. 2009 |
Unclear Randomized by age |
Unclear |
High No blinding |
Unclear No information provided |
Low No reported loss to follow-up |
Low |
Unclear Weeks of immobilization in cast varied |
|
Watt et al. 2000 |
Unclear Randomly allocated |
Unclear |
High No blinding |
Low Evaluator blinded |
Unclear | Low |
Unclear Small sample and no sample size calculation |
|
Wakefield et al. 2000 |
Low Block randomization using a computer program |
Unclear |
High No blinding |
Low Evaluator blinded |
Low Loss to follow-up: 6.3% Due to preliminary analysis, only data on 66 patients were collected at 6 months of follow-up |
Low |
Unclear Median number of treatments in intervention group was only 3 but range was high (1–22) |
|
Christensen et al. 2000 |
Low Randomized |
Low Closed envelope method |
High No blinding |
Unclear | Unclear | Low |
Unclear Small sample |
GRADE assessment
As a result of incomparable outcome measures, difference in time to follow-up, and high I2 values, meta-analysis could only be undertaken on a limited number of outcomes. Thus, GRADE assessment was based on the meta-analysis and on the substance of the narrative synthesis. As a result of risk of bias, imprecision, and inconsistency of the trial results, the quality of evidence on all outcomes was found to be low or very low (Tables 5, 6, 7 and 8).
Table 5.
Summary of findings for each outcome. This table provides information on the effects of the intervention and the overall quality of evidence (GRADE) for each outcome
| Early mobilization after proximal humerus fracture | |||||
|---|---|---|---|---|---|
|
Population: Adults ≥ 18 years with a verified PHF, referred to non-operative treatment Intervention: Early mobilization within 2 weeks after the fracture Comparison: Late mobilization | |||||
| Outcome | Between-group difference | Effect, absolute MD, (95% CI) | Quality of the evidence (GRADE) | ||
| Early mobilization | |||||
| 0 to 3 months | 6 months | 1 to 2 years | |||
| Function (questionnaires including both subjective and objective measurements) | Low (due to risk of bias and imprecision) | ||||
| Constant Shoulder Score (CS) | |||||
| Hodgson et al. | + | + | |||
| Estimates (CS) poooled in meta-analysis: | |||||
| Lefevre-Colau et al. | = | 4.55 (0.00;9.10) | |||
| Torrens et al. | = | ||||
| The Croft score | |||||
| Hodgson et al. | + | ||||
| Modified Neer score | |||||
| Kristiansen et al. | + | = | = | ||
| Disabilities of the Arm Shoulder and Hand(DASH) | |||||
| Chen et al. | = | = | |||
| Performance-based function | Low (due to risk of bias and imprecision. | ||||
| ROM | |||||
| Lefevre-Colau et al. | + | ||||
| Kristiansen et al. | = | = | = | ||
| Estimates pooled in meta-analysis: | |||||
| Torrens et al. | |||||
| Chen et al. | |||||
| • Flexion | = | 2.18 (-4.83;9.19) | |||
| • Abduction | = | 3.75 (-5.76;13.27) | |||
| Pain | Low (due to risk of bias and imprecision) | ||||
| Neers pain subscore | |||||
| Kristiansen et al. | + | = | = | ||
| VAS | |||||
| Lefevre-Colau et al. | + | = | |||
| Estimates pooled in meta-analysis: | |||||
|
Torrens et al. Chen et al. |
= | 0.20 (-0.36;0.76) | |||
| Health-related quality of life | Very low (due to risk of bias and imprecision) | ||||
| SF-36 | |||||
|
Hodgson et al. Role limitations domain |
+ | = | |||
| Pain domains | + | + | |||
+ Beneficial effect on the listed outcome, = No effect on the listed outcome, - Harmful effect on the listed outcome, Empty field where no evidence could be located
Table 6.
Summary of findings for each outcome. This table provides information on the effects of the intervention and the overall quality of evidence (GRADE) for each outcome
| Early mobilization after distal radius fracture | |||||
|---|---|---|---|---|---|
|
Population: Adults ≥ 18 years with a verified DRF, referred to non-operative treatment Intervention: Early mobilization within 2 weeks after the fracture Comparison: Late mobilization | |||||
| Outcome | Between-group difference | Effect size Mean difference, (95% CI) |
Quality of the evidence (GRADE) | ||
| Early mobilization | |||||
| 0 to 3 months | 6 months | 1 to 2 years | |||
| Function (questionnaires including both subjective and objective measurements) | Low (due to risk of bias, imprecision) | ||||
| Gartland and Werley | |||||
| Jensen et al. | = | = | |||
| Davis et al. | + | ||||
| Christersson et al. | = | = | |||
| The Cooney score | |||||
| Stoffelen et al. | = | = | = | ||
| Modified de Bruijn wrist score | |||||
| Christersson et al. | = | = | |||
| Modified Mayo wrist scoring chart | |||||
| Christersson et al. | = | = | |||
| Functional score( by Steward et al.) | |||||
| Dias et al. | + | ||||
| Performance-based function | Low (due to risk of bias and imprecision) | ||||
| Grip strength | |||||
| Davis et al. | = | ||||
| Christersson et al. | = | = | |||
| Dias et al. | + | ||||
| Performance-based function | Low (due to risk of bias and imprecision) | ||||
| ROM | |||||
| Christersson et al. | + | = | |||
| Dias et al. | + | ||||
| Pain | Low (due to risk of bias and imprecision) | ||||
| VAS | |||||
| Davis et al. | = | ||||
| Christersson et al. | = | = | = | ||
+ Beneficial effect on the listed outcome, = No effect on the listed outcome, - Harmful effect on the listed outcome, Empty field where no evidence could be located
Table 7.
Summary of findings for each outcome. This table provides information on the effects of the intervention and the overall quality of evidence (GRADE) for each outcome
| Supervised exercise therapy after proximal humerus fracture | |||||
|---|---|---|---|---|---|
|
Population: Adults ≥ 18 years with a verified PHF, referred to non-operative treatment Intervention: Supervised exercise therapy Comparison: Non-supervised exercises | |||||
| Outcome | Between-group difference | Effect size Mean difference, (95% CI) |
Quality of the evidence (GRADE) | ||
| Supervised exercise therapy | |||||
| 0 to 3 months | 6 months | 1 to 2 years | |||
| Function (questionnaires including both subjective and objective measurements) | Very low (due to risk of bias and imprecision) | ||||
| Neers assessment | |||||
| Lundberg et al. | = | = | = | ||
| ADL | |||||
| Bertoft et al. | = | = | |||
| Performance-based function | Very low (due to risk of bias and imprecision) | ||||
| ROM | |||||
| Bertoft et al. | = | = | = | ||
| Lundberg et al. | = | = | |||
| Performance-based function | Very low (due to risk of bias and imprecision) | ||||
| Shoulder strength | |||||
| Bertoft et al. | = | = | = | ||
| Grip strength | |||||
| Lundberg et al. | = | = | = | ||
| Pain | Very low (due to risk of bias and imprecision) | ||||
| VAS | |||||
| Bertoft et al. | = | = | = | ||
| Lundberg et al. | = | = | |||
+ Beneficial effect on the listed outcome, = No effect on the listed outcome, - Harmful effect on the listed outcome, Empty field where no evidence could be located
Table 8.
Summary of findings for each outcome. This table provides information on the effects of the intervention and the overall quality of evidence (GRADE) for each outcome
| Supervised exercise therapy after distal radius fracture | |||||
|---|---|---|---|---|---|
|
Population: Adults ≥ 18 years with a verified DRF, referred to non-operative treatment Intervention: Supervised exercise therapy Comparison: Non-supervised exercises | |||||
| Outcome | Between-group difference | Effect size Mean difference, (95% CI) |
Quality of the evidence (GRADE) | ||
| Supervised exercise therapy | |||||
| 0 to 3 months | 6 months | 1 to 2 years | |||
| Function ( PROMs and questionnaires including both subjective and objective measurements) | Very low (due to risk of bias, inconsistency of results and imprecision) | ||||
| PRWE (PROM) | |||||
| Gutierrez-Espinoza et al. | + | + | |||
| Krischak et al. | - | ||||
| Modified Gartland and Werley | |||||
| Christiansen et al. | = | = | |||
| Performance-based function | Low (due to risk of bias and imprecision). | ||||
| ROM | |||||
| Gutierrez-Espinosa et al. | + | + | |||
| Watt et al. | + | ||||
| Krischak et al. | = | ||||
| Wakefield et al. | = | + | |||
| Performance-based function | Low (due to risk of bias and inconsistency). | ||||
| Grip strength | |||||
| Gutierrez-Espinosa et al. | + | + | |||
| Watt et al. | + | ||||
| Krischak et al. | - | ||||
| Wakefield et al. | = | = | |||
| Pain | Low (due to risk of bias, inconsistency of results) | ||||
| VAS | |||||
| Gutierrez-Espinosa et al. | + | + | |||
| Wakefield et al. | = | = | |||
| Health-related quality of life | Very low (due to risk of bias, imprecision) | ||||
| SF36 | |||||
| Wakefield et al. | = | = | |||
+ Beneficial effect on the listed outcome, = No effect on the listed outcome, - Harmful effect on the listed outcome, Empty field where no evidence could be located
Discussion
This systematic review and meta-analysis suggests that early mobilization within 2 weeks of fracture may result in better function after PHF. However, these findings are based on low quality evidence. Furthermore, the overall MD (4.55) on the CS is not considered to be a clinically important difference, which has been reported to be between 5.4 and 11.6 [34]. Moreover, no evidence showed early mobilization after PHF has a clear positive effect on ROM or pain. Neither did it lead to more complications. No eligible evidence was found supporting early mobilization to be superior to late mobilization after DRF in terms of improved wrist function, grip strength, HRQoL, or reduced pain. Finally, no clear evidence showed a clear benefit of supervised exercise therapy compared with non-supervised home exercises on function of the upper limb, HRQoL, or reduced pain after PHF or DRF.
These results confirm the conclusions of previous systematic reviews that have reported a lack of clear evidence to support the decision on when to commence exercise therapy and to what extent it should be supervised [6, 35].
Limitations
The low number of included trials is a limitation of the present study, and changes to the inclusion criteria may have resulted in a larger study sample. Several trials did not include PROMs as an outcome measure, and therefore most of the measurements were clinician assessed and do not necessarily reflect the patients’ own perception of function. Several trials did not provide sufficient information on the inclusion and exclusion criteria. Furthermore, sample size calculation was only reported in 6 out of the 15 trials [16, 17, 25, 28, 29, 31] and only 4 trials referred to a minimal clinical important difference for their main outcome [16, 17, 28, 31]. This adds additional uncertainty to the reported results.
Implications
The consequences of immobilizing elderly people who have sustained a PHF or DRF for longer than necessary is an important consideration. A longer period of immobilization may lead to physical inactivity, with an increased risk of compromising general health [36]. The present study identified three unpublished RCTs that had investigated the effects of early mobilization after PHF, leading us to believe that more evidence will be available in the future [19, 20, 37].
Rehabilitation after upper limb fractures must be viewed from a broader and more complex perspective than that investigated in this systematic review. Therefore, other independent risk factors for poor function after these type of fractures, such as social deprivation, low self-efficacy, and fear of movement, must be acknowledged [38, 39].
Conclusion
There is an urgent need for high-quality randomized controlled trials to substantiate the current evidence regarding the optimal time to initiate mobilization and the need for supervision after PHF and DRF.
Supplementary Information
(DOCX 57 kb)
Acknowledgments
We wish to thank Jaana Isojärvi for her assistance with the literature search. Furthermore, we wish to thank Carlos Torrens for sharing his unpublished data.
Author contributions
Conception and design of the work: H.K.Ø., I.M., A.P.L., V.M.M., and V.T.P. Data collection, selection, evaluation, and analysis: H.K.Ø. and V.T.P. Drafting the article: H.K.Ø. Guidance and critical revision of the manuscript: H.K.Ø., I.M., A.P.L., M.T.V., V.M.M., and V.T.P.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subject performed by any of the authors.
Conflict of Interest
H.K.Ø., I.M.,, A.P.L., M.T.V., V.M.M., and V.T.P. declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Helle K. Østergaard, Email: helle.oestergaard@viborg.rm.dk
Inger Mechlenburg, Email: Inger.mechlenburg@clin.au.dk.
Antti P. Launonen, Email: antti.launonen@pshp.fi
Marianne T. Vestermark, Email: marianne.t.vestermark@gmail.com
Ville M. Mattila, Email: ville.mattila@tuni.fi
Ville T. Ponkilainen, Email: ville.ponkilainen@tuni.fi
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691–697. doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 2.Launonen AP, Lepola V, Saranko A, Flinkkila T, Laitinen M, Mattila VM. Epidemiology of proximal humerus fractures. Arch Osteoporos. 2015;10:209. doi: 10.1007/s11657-015-0209-4. [DOI] [PubMed] [Google Scholar]
- 3.Rundgren J, Bojan A, Mellstrand Navarro C, Enocson A. Epidemiology, classification, treatment and mortality of distal radius fractures in adults: an observational study of 23,394 fractures from the national Swedish fracture register. BMC Musculoskelet Disord. 2020;21(1):88. doi: 10.1186/s12891-020-3097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lander ST, Mahmood B, Maceroli MA, Byrd J, Elfar JC, Ketz JP, Nikkel LE. Mortality rates of humerus fractures in the elderly: does surgical treatment matter? J Orthop Trauma. 2019;33(7):361–365. doi: 10.1097/bot.0000000000001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Launonen AP, Sumrein BO, Reito A, Lepola V, Paloneva J, Jonsson KB, et al. Operative versus non-operative treatment for 2-part proximal humerus fracture: a multicenter randomized controlled trial. PLoS Med. 2019;16(7):e1002855. doi: 10.1371/journal.pmed.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handoll HH, Brorson S. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2015;(11):Cd000434. 10.1002/14651858.CD000434.pub4. [DOI] [PubMed]
- 7.Rangan A, Handoll H, Brealey S, Jefferson L, Keding A, Martin BC, Goodchild L, Chuang LH, Hewitt C, Torgerson D. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. Jama. 2015;313(10):1037–1047. doi: 10.1001/jama.2015.1629. [DOI] [PubMed] [Google Scholar]
- 8.Mellstrand Navarro C, Brolund A, Ekholm C, Heintz E, Hoxha Ekstrom E, Josefsson PO, et al. Treatment of radius or ulna fractures in the elderly: a systematic review covering effectiveness, safety, economic aspects and current practice. PLoS One. 2019;14(3):e0214362. doi: 10.1371/journal.pone.0214362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruder AM, Shields N, Dodd KJ, Taylor NF. Prescribed exercise programs may not be effective in reducing impairments and improving activity during upper limb fracture rehabilitation: a systematic review. Aust J Phys. 2017;63(4):205–220. doi: 10.1016/j.jphys.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Handoll H, Brealey S, Rangan A, Keding A, Corbacho B, Jefferson L, Chuang LH, Goodchild L, Hewitt C, Torgerson D. The ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial – a pragmatic multicentre randomised controlled trial evaluating the clinical effectiveness and cost-effectiveness of surgical compared with non-surgical treatment for proximal fracture of the humerus in adults. Health Technol Assess. 2015;19(24):1–280. doi: 10.3310/hta19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, the PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 12.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson SA, Mawson SJ, Saxton JM, Stanley D. Rehabilitation of two-part fractures of the neck of the humerus (two-year follow-up) J Shoulder Elb Surg. 2007;16(2):143–145. doi: 10.1016/j.jse.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson SA, Mawson SJ, Stanley D. Rehabilitation after two-part fractures of the neck of the humerus. J Bone Joint Surg (Br) 2003;85(3):419–422. doi: 10.1302/0301-620x.85b3.13458. [DOI] [PubMed] [Google Scholar]
- 17.Lefevre-Colau MM, Babinet A, Fayad F, Fermanian J, Anract P, Roren A, Kansao J, Revel M, Poiraudeau S. Immediate mobilization compared with conventional immobilization for the impacted nonoperatively treated proximal humeral fracture. A randomized controlled trial. J Bone Joint Surg Am. 2007;89(12):2582–2590. doi: 10.2106/jbjs.F.01419. [DOI] [PubMed] [Google Scholar]
- 18.Kristiansen B, Angermann P, Larsen TK. Functional results following fractures of the proximal humerus. A controlled clinical study comparing two periods of immobilization. Arch Orthop Trauma Surg. 1989;108(6):339–341. doi: 10.1007/BF00932441. [DOI] [PubMed] [Google Scholar]
- 19.Early vs Delayed Physical Therapy (Exercises) for Non-Operatively-Treated Proximal Humerus Fractures: A Prospective Randomized Trial [database on the Internet]. National Library of Medicine (US). 2000 Feb 29, (cited July 2. 2020), Available at: https://clinicaltrials.gov/ct2/show/NCT00438633. Accessed 30 May 2020.
- 20.Conservative Treatment of Proximal Humeral Fractures – Immobilization for 1 Week Compared to Three Weeks: Prospective Randomized Study [database on the Internet]. National Library of Medicine (US). 2000 Feb 29 , (cited July 2, 2020), Available from: https://clinicaltrials.gov/ct2/show/study/NCT03217344. Accessed 30 May 2020.
- 21.Davis TR, Buchanan JM. A controlled prospective study of early mobilization of minimally displaced fractures of the distal radial metaphysis. Injury. 1987;18(4):283–285. doi: 10.1016/0020-1383(87)90015-5. [DOI] [PubMed] [Google Scholar]
- 22.Dias JJ, Wray CC, Jones JM, Gregg PJ. The value of early mobilisation in the treatment of Colles' fractures. J Bone Joint Surg (Br) 1987;69(3):463–467. doi: 10.1302/0301-620X.69B3.3584203. [DOI] [PubMed] [Google Scholar]
- 23.Stoffelen D, Broos P. Minimally displaced distal radius fractures: do they need plaster treatment? J Trauma. 1998;44(3):503–505. doi: 10.1097/00005373-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MR, Andersen KH, Jensen CH. Management of undisplaced or minimally displaced Colles' fracture: one or three weeks of immobilisation. J Orthop Sci. 1997;2(6):424–427. doi: 10.1007/BF02488930. [DOI] [Google Scholar]
- 25.Christersson A, Larsson S, Sandén B. Clinical outcome after plaster cast fixation for 10 days versus 1 month in reduced distal radius fractures: a prospective randomized study. Scand J Surg. 2018;107(1):82–90. doi: 10.1177/1457496917731184. [DOI] [PubMed] [Google Scholar]
- 26.Lungberg BJ, Svenungson-Hartwig E, Wikmark R. Independent exercises versus physiotherapy in nondisplaced proximal humeral fractures. Scand J Rehabil Med. 1979;11(3):133–136. [PubMed] [Google Scholar]
- 27.Bertoft ES, Lundh I, Ringqvist I. Physiotherapy after fracture of the proximal end of the humerus. Comparison between two methods. Scand J Rehabil Med. 1984;16(1):11–16. [PubMed] [Google Scholar]
- 28.Gutierrez-Espinoza H, Rubio-Oyarzun D, Olguin-Huerta C, Gutierrez-Monclus R, Pinto-Concha S, Gana-Hervias G. Supervised physical therapy vs home exercise program for patients with distal radius fracture: a single-blind randomized clinical study. J Hand Ther. 2017;30(3):242–252. doi: 10.1016/j.jht.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Krischak G, Krasteva A, Pandorf-Frediani S, Dehner C, Schneider F, Gebhard F, Kramer M. Effect of a home exercise program in rehabilitation of non operatively treated wrist fractures a prospectively randomized study. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin. 2009;19(4):185–192. doi: 10.1055/s-0029-1225336. [DOI] [Google Scholar]
- 30.Christensen OM, Kunov A, Hansen FF, Christiansen TC, Krasheninnikoff M. Occupational therapy and Colles' fractures. Int Orthop. 2001;25(1):43–45. doi: 10.1007/s002640000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakefield AE, McQueen MM. The role of physiotherapy and clinical predictors of outcome after fracture of the distal radius. J Bone Joint Surg Br Vol. 2000;82(7):972–976. doi: 10.1302/0301-620X.82B7.0820972. [DOI] [PubMed] [Google Scholar]
- 32.Watt CF, Taylor NF, Baskus K. Do Colles' fracture patients benefit from routine referral to physiotherapy following cast removal? Arch Orthop Trauma Surg. 2000;120(7-8):413–415. doi: 10.1007/pl00013772. [DOI] [PubMed] [Google Scholar]
- 33.Bache S. Two different approaches to the physiotherapeutic management of patients with distal radial fractures. Physiotherapy. 2000;86(7):383. doi: 10.1016/S0031-9406(05)60649-9. [DOI] [Google Scholar]
- 34.Dabija DI, Jain NB. Minimal clinically important difference of shoulder outcome measures and diagnoses: a systematic review. Am J Phys Med Rehabil. 2019;98(8):671–676. doi: 10.1097/phm.0000000000001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruder AM, Taylor NF, Dodd KJ, Shields N. Physiotherapy intervention practice patterns used in rehabilitation after distal radial fracture. Physiotherapy. 2013;99(3):233–240. doi: 10.1016/j.physio.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham C, O’Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30(5):816–827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 37.Non-operative Treatment in Sweden of Proximal Humeral Fractures, a Randomised Multicenter Trial. [database on the Internet]. National Library of Medicine (US). 2000 Feb 29 (cited July 2 2020), Available at: https://clinicaltrials.gov/ct2/show/NCT03786679. Accessed 30 May 2020.
- 38.Johnson NA, Dias JJ. The effect of social deprivation on fragility fracture of the distal radius. Injury. 2019;50(6):1232–1236. doi: 10.1016/j.injury.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Jayakumar P, Teunis T, Williams M, Lamb SE, Ring D, Gwilym S. Factors associated with the magnitude of limitations during recovery from a fracture of the proximal humerus: predictors of limitations after proximal humerus fracture. Bone Joint J. 2019;101-b(6):715–723. doi: 10.1302/0301-620x.101b6.Bjj-2018-0857.R1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 57 kb)