Abstract
This study aimed to elucidate the underlying molecular mechanism of photobiomodulation (PBM) in attenuating oxidative stress in diabetic wounded fibroblast cells. Cell models were exposed to PBM at a wavelength of 660 nm (fluence of 5 J/cm2, and power density of 11.2 mW/cm2) or 830 nm (fluence of 5 J/cm2, and power density of 10.3 mW/cm2). Non-irradiated cell models were used as controls. Cellular migration was determined at regular time intervals (0, 12, 24 and 48 h) using inverted light microscopy. Cell viability was determined by the Trypan blue exclusion assay. The levels of enzymic antioxidants superoxide dismutase (SOD), catalase (CAT), and heme oxygenase (HMOX1) were determined by the enzyme linked immunosorbent assay (ELISA). The alteration in the levels of AKT and FOXO1 was determined by immunofluorescence and western blotting. Upon PBM treatment, elevated oxidative stress was reversed in diabetic and diabetic wounded fibroblast cells. The reduced oxidative stress was represented by decreased FOXO1 levels and increased levels of SOD, CAT and HMOX1. This might be due to the activation of the AKT signaling pathway. This study concluded that treatment with PBM progressed diabetic wound healing by attenuating oxidative stress through inhibition of the FOXO1 signaling pathway.
Electronic supplementary material
The online version of this article (10.1007/s12079-020-00588-x) contains supplementary material, which is available to authorized users.
Keywords: Photobiomodulation, PBM, FOXO1, AKT, Antioxidants, Oxidative Stress
Introduction
Tissue repair is an interconnected process that involves cell proliferation, cell migration, angiogenesis, matrix deposition, and wound contraction and remodelling to re-establish the structural and functional integrity of injured skin/tissue. Several types of cells such as platelets, macrophages, fibroblasts, keratinocytes, and endothelial cells are involved in wound healing regulation. Among these cells, fibroblasts play a crucial role in wound bed formation and deposition (collagen formation/degradation), extracellular matrix (ECM) remodeling, and further wound healing (Wagner and Wehrmann 2007). Wound healing requires a normal physiologic environment to stimulate repair and regenerative processes. Various factors such as hypoxia, infection, tumors, and comorbid diseases such as diabetes mellitus (DM) disturb the normal physiological condition that results in delays in the healing process. Wounds which have failed to heal within their normal duration (typically 3 months) are known as chronic wounds, which have abnormal structural and functional integrity (Ayuk et al. 2017).
Many factors such as prolonged inflammation, high microbial invasion, excessive reactive oxygen species (ROS) generation, and increased protease synthesis contribute to delayed wound healing (Gangwar et al. 2015). One of the major consequences of diabetes is oxidative stress and nitrations of its mediated products (Cai et al. 2005; Khullar et al. 2010). Under normal physiological conditions, ROS is produced as necessary intermediates in picomolar concentrations and are involved as secondary messengers in cellular signaling, and are concerned with cell proliferation, differentiation, migration and apoptosis (Cong et al. 2013). High levels of ROS will damage various macromolecules including the ECM, and disturb the functioning of fibroblasts and keratinocytes. Under diabetic conditions, high levels of ROS mediate the transcription of proinflammatory cytokines and synthesis of matrix metalloproteases (MMPs) (Moseley et al. 2004). ROS interacts with proteins and causes several reactions that lead to peptide cleavage and amino acid oxidation, resulting in oxidation of proteins.
Transcription factors are involved in organizing the events for normal wound healing. Forkhead family of transcription factors class ‘O’ (FOXO) is a subgroup of the Forkhead family of transcription factors, of which there are over 100 members classified from FOXA to FOXR (Xu et al. 2015). FOXO1 (homeostatic factors) is involved in regulating the insulin/PI3K/AKT signaling pathway and wound healing, though their exact function in wound healing is not completely identified (Shaklai et al. 2015; Zhang et al. 2015). FOXO1 supports keratinocyte proliferation and mobilization that results in the upregulation of transforming growth factor-beta one (TGF-β1), integrin’s and MMPs. In addition, decreased FOXO1 levels directly attenuates oxidative stress to maintain keratinocyte proliferation and migration to promote wound healing (Ponugoti et al. 2013).
A serine/threonine kinase Protein Kinase B (PKB), commonly known as AKT plays a significant role in various cellular processes (glucose metabolism, cell death, apoptosis, fibroblast proliferation/migration and transcription). The principal role of the AKT signaling pathway is the regulation of tissue repair processes (Kern et al. 2011). AKT is regulated by oxidative stress for cell survival and proliferation. High glucose induced oxidative stress blocks the AKT signaling pathway, and in diabetic patients the downregulation of the PI3K/AKT pathway is common and leads to poor wound healing and tissue regeneration (Watson et al. 2009; Squarize et al. 2010). The use of drugs that activate and upregulate the PI3K/AKT pathway is beneficial for promoting healing in diabetic patients (Lima et al. 2017).
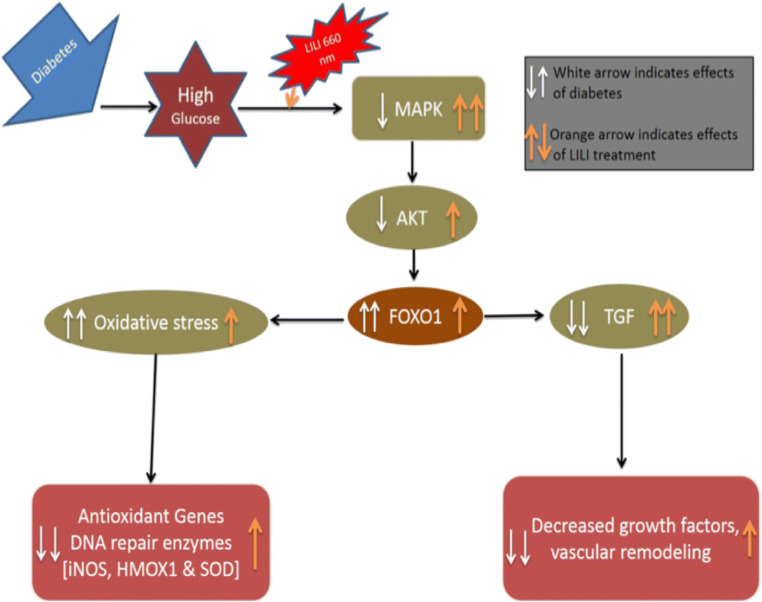
Photobiomodulation (PBM) is an alternative non-invasive treatment that is related with the use of low-energy light at specific doses and wavelengths to promote cellular and biochemical functions (Anders et al. 2015; Jere et al. 2018). PBM has the ability to alter ROS generation as well as antioxidant levels. PBM accelerates electron transport chain reactions to retain normal homeostasis for the activation of various cellular signaling pathways that leads to improved wound healing (Silveira et al. 2013). PBM plays a positive role in increasing cellular proliferation and viability of mesenchymal stem cells, endothelial cells, osteoblasts, lymphocytes, epithelial cells, and fibroblasts (Soares et al. 2015; Ayuk et al. 2017). Previous studies by this group have shown beneficial effects of PBM at both 660 and 830 nm (with 5 J/cm2) in diabetic wounded fibroblast cells. There has been an increase in cell migration rate, epidermal growth factor (EGF) release and activation / phosphorylation of its receptor (EGFR) and the JAK/STAT pathway (Jere et al. 2018). It was also shown that there was a decrease in MMP-3 and − 9 and an increase in MMP inhibitor TIMP-1 as well as collagen type I. There was also an alteration in the genes coding for these proteins, and the findings suggest that PBM promotes the remodeling phase of wound healing by decreasing matrix degradation and upregulating ECM synthesis (Ayuk et al. 2018). Irradiation also resulted in a decrease in pro-inflammatory cytokines interleukin (IL)-1β and tumour necrosis factor-alpha (TNF-a) (Houreld et al. 2010). Based on these findings we hypothesized that PBM will attenuate oxidative stress by decreasing FOXO1 levels (Fig. 8). The present study aims to explore the effect of PBM at 660 or 830 nm on oxidative stress and antioxidant activity in a diabetic wound model in vitro.
Fig. 8.
Schematic representation of possible mechanism of action of Photobiomodulation
Materials and methods
Cell culture
This study was carried out on WS1 human skin fibroblast cells (ATCC® Number: CRL-1502™), and received ethical clearance from the Faculty of Health Sciences, University of Johannesburg, Research Ethics Committee (REC-01-28-2017). Four cell models, namely normal (N), wounded (W), diabetic (D) and diabetic wounded (DW) were used as previously described (Houreld and Abrahamse 2010; Ayuk et al. 2012).
Laser irradiation
A continuous wave diode laser transmitting at a wavelength of 660 nm (Fremont, California, USA, RGBlase, TECIRL-70G-830SMA) with a power output of 102 mW or at 830 nm (Fremont, California, RGBlase, TECIRL-70G-830 SMA) with a power output of 94 mW was used for irradiation. The laser beam was directed from above towards cells via a fibre optic and had a spot size of 9.1 cm2, thus producing a power density of 11.2 mW/cm2 for 660 nm and a power density of 10.35 mW/cm2 for 830 nm. In order to irradiate cells with a fluence of 5 J/cm2, cells were irradiated in the dark for 445 s (45.39 J) or 485 s (45.59 J) respectively in an open culture dish containing 1 mL fresh culture media. Post-irradiation, cells were incubated for 0, 12, 24 and 48 h after which cell morphology, cell viability, nuclear damage, and expression of AKT, FOXO1 and enzymic antioxidants (SOD, CAT and HMOX1) were determined. Non-irradiated cells served as controls.
Cell viability
Cell viability was determined by the Trypan blue exclusion assay in all cell models using 0.4% Trypan blue (T8154, Sigma-Aldrich, South Africa). Equal amounts of stain and cells (10 µL) were counted on the Invitrogen Countess™ II FL Automated Cell Counter.
Hoechst staining
To determine nuclear damage, 6 × 105 cells were cultured on sterile cover slips for 12 h in 3.4 cm diameter culture dishes and irradiated with PBM at 660 or 830 nm. After 24 h, cells were incubated at room temperature with 1 µg/mL Hoechst 33,258 (Invitrogen, H21491, ThermoFisher Scientific, South Africa) for 15 min. Thereafter, cells were rinsed with phosphate buffered saline (PBS) and examined on the Carl Zeiss Axio Observer Z1 (352Ex/461Em).
Enzyme Linked Immunosorbent Assay (ELISA)
ELISA was used to determine enzymic antioxidants SOD, CAT and HMOX1 in cultured cells. Cells were detached 24 h post-irradiation using TrypLE™ Select (Gibco, 12563-029, ThermoFisher Scientific, South Africa) and 3 × 104 cells in 100 µL media were seeded into 96-well microplates (Corning®, Costar® 3596, Sigma-Aldrich, South Africa) and allowed to attach. Cells were fixed in 8% paraformaldehyde for 15 min at room temperature and washed three times with PBS. Cells were permeabilized with 1% Triton X-100 in PBS for 30 min at room temperature, and blocked in 2% blocking solution (10% bovine serum albumin, BSA, in PBS) for 2 h at room temperature. Plates were washed and incubated for 2 h with 1:50 primary antibody (Mouse monoclonal IgG; Santa Cruz Biotechnology, SOD-1: sc-101,523; CAT: sc-271,358; HMOX1: sc-136,960; Anatech Instruments (Pty) Ltd, South Africa). Plates were washed three times and incubated for 2 h with 1:5,000 secondary antibody (Goat anti-mouse IgG-HRP, Santa Cruz Biotechnology, sc-2005, Anatech (Pty) LTD, South Africa). Following rinsing, plates were incubated for 20 min with 3,3’,5,5’-tetramethylbenzidine substrate (TMB) and the reaction stopped with 1 M hydrochloric acid. Absorbance was read at 450 nm (Perkin-Elmer, Victor3™ multiplate reader).
Immunofluorescence
To examine the presence of AKT and FOXO1, 6 × 105 cells were cultured on sterile cover slips for 12 h and irradiated at 660 or 830 nm. Cells were fixed 24 h post-irradiation for 15 min in 4% formaldehyde at room temperature, washed twice in PBS, and permeabilized for 15 min at room temperature (0.01% v/v Triton X-100 in PBS). Cells were washed twice, blocked for 1 h (3% BSA in PBS), and incubated for 2 h at room temperature with 1:100 primary antibody (Mouse monoclonal IgG; AKT: AHO-01112, ThermoFisher Scientific, South Africa; Rabbit monoclonal IgG; FOXO1: mAb-2880, Cell Signalling Technology, South Africa). The cells were washed twice and incubated for 2 h with 1:2,500 conjugated secondary antibodies (Goat anti-mouse FITC: Santa Cruz Biotechnology, sc-2010, Anatech Instruments (Pty) Ltd, South Africa). Nuclei were stained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, D1306, ThermoFisher Scientific, South Africa) and slides examined using the Carl Zeiss Axio Observer Z1 (FITC 494Ex/518Em; and DAPI 358Ex/461Em).
Western blotting
The Pierce™ BCA Protein Assay Kit (23,225, ThermoFisher Scientific, South Africa) was used to determine protein concentration. Cell lysates were diluted to 1 µg/µL in sample buffer (Laemmli 2x buffer; S3401, Sigma-Aldrich, South Africa), and heated at 110 °C for 5 to 7 min. Protein samples (25 µL) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to an Immun-Blot PVDF membrane (162–0177, Bio-Rad, South Africa) for 3 h at 60 volts using a Semi-dry blotter (B2529, Sigma-Aldrich, South Africa). Once the protein was transferred, 5% BSA in Tris-buffered saline (TBS) was used for blocking for 20 min. Membranes were incubated at 4 °C overnight with primary antibody (Mouse monoclonal IgG; AKT: AHO-01112, ThermoFisher Scientific, South Africa; Rabbit monoclonal IgG; FOXO1: mAb-2880, Cell Signalling Technology, South Africa and GAPDH: sc-47,724, Santa Cruz Biotechnology, Anatech Instruments (Pty) Ltd, South Africa). After washing three times for 10 min with 0.1% Tween-20 in TBS, membranes were incubated with horseradish peroxidase conjugated secondary antibody (Goat anti-mouse HRP: Santa Cruz Biotechnology, sc-2005, Anatech Instruments (Pty) Ltd, South Africa). After 2 h incubation, membranes were washed with TBS and incubated in the dark for 5 min with 1% 3,3′-Diaminobenzidine (DAB) and 0.3% hydrogen peroxide in 5 mL PBS; developed bands were scanned. ImageJ software (National Institutes of Health, USA) was used for quantitative analysis of scanned densitometric values as ratios to GAPDH (used as a loading control).
Statistical analysis
Data is represented as the mean ± standard error of the mean (SEM) of three repeats done in duplicate (n = 3). Statistical significance was analyzed using SigmaPlot version 13.0. Significant differences was determined by the Student t-test and One-Way Analysis of Variance (ANOVA). Significant probability is shown as *p ≤ .05, **p ≤ .01 and ***p ≤ .001.
Results
Cell morphology
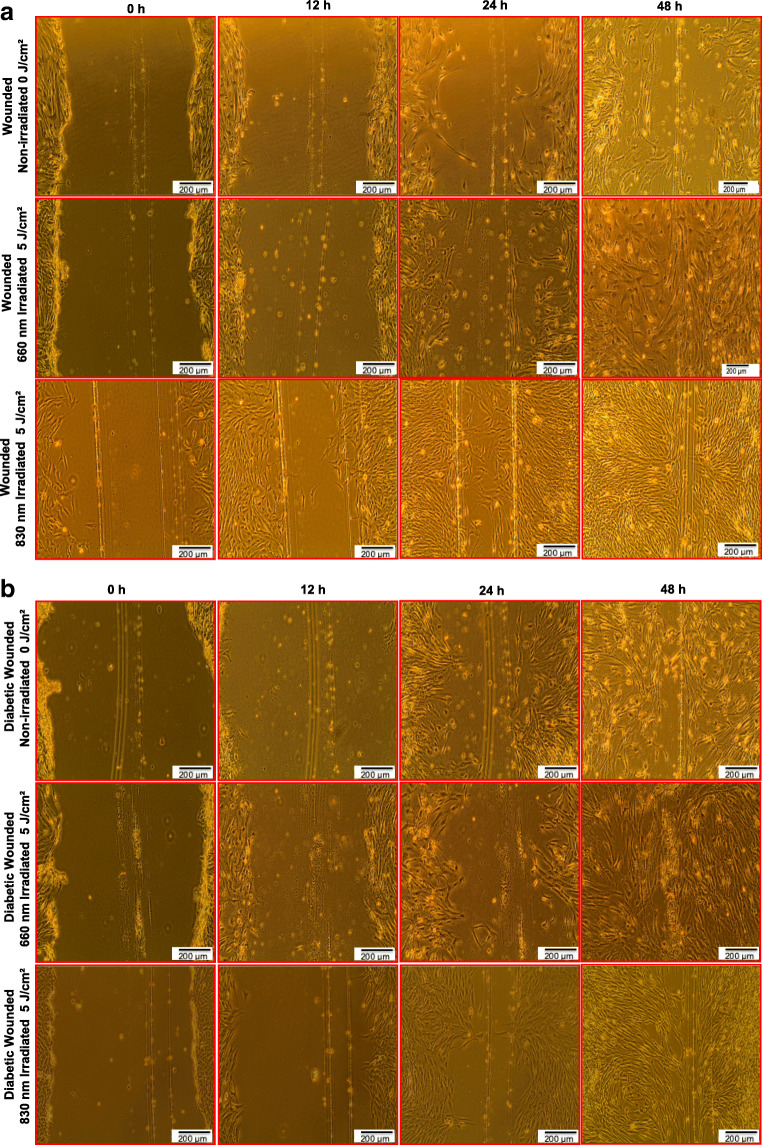
Figure 1a and b shows the morphological changes in non-irradiated and irradiated wounded (W) and diabetic wounded (DW) models. In the irradiated model (W and DW), cells looked healthy with their regular spindle shaped structure; increased cell proliferation and increased cell migration towards the central scratch was noted over time, and complete wound closure was observed at 48 h. On the other hand, non-irradiated models showed decreased cell proliferation, poor cell migration and a delay in wound closure at 48 h. In addition, non-irradiated diabetic wounded cells showed increased cell death and apoptotic bodies.
Fig. 1.
a Morphological changes observed in wounded human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2. In irradiated models, cell migration was rapid and more cells had made contact in the central scratch at 48 h. b Morphological changes observed in diabetic wounded human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2. In irradiated models, cell migration was rapid and more cells had made contact in the central scratch at 48 h
Cell viability
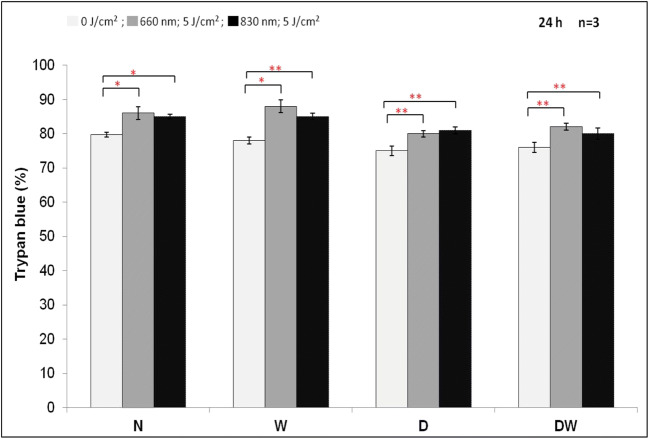
There was a significant increase at both wavelengths in all irradiated cells (Fig. 2). Comparison of the wavelengths showed that irradiation of W and DW cells at 660 nm produced a significant increase (P < .05) as compared to the same cells irradiated at 830 nm.
Fig. 2.
Effect of photobiomodulation on cell viability in normal (N), wounded (W), diabetic (D), and diabetic wounded (DW) human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2. Irradiated N, W, D and DW cells showed a significant increase in cell viability as compared to the non-irradiated cells. Data is expressed as the mean ± SEM (n = 3). Significant probability is shown as *P ≤ .05 and **P ≤ .01
Nuclear damage assessment by Hoechst staining
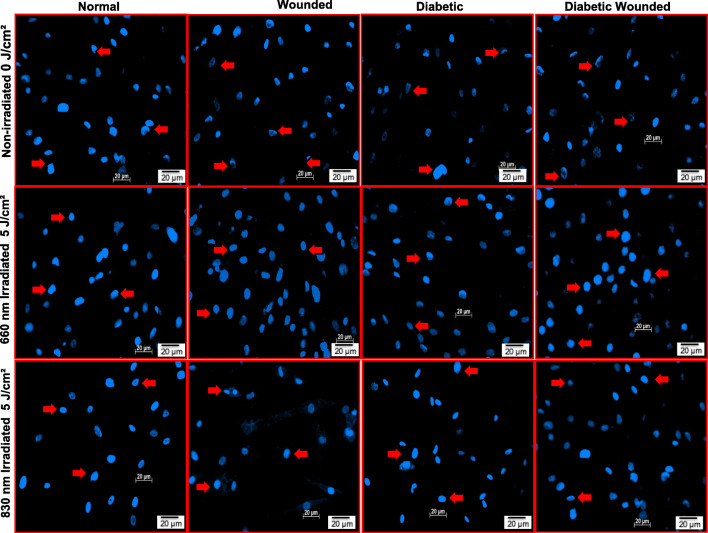
Nuclear damage in irradiated and non-irradiated cells was assessed 24 h post-irradiation (Fig. 3). In the irradiated models (5 J/cm2), nuclei stained uniformly signifying their dense nature; spherical shaped nuclei without any damage was observed. However, non-irradiated models (0 J/cm2) showed dark staining at the nuclear edges and hollow centers, reflecting irregular nuclear shape, fragmented nuclei, nuclear shrinkage and nuclear condensation.
Fig. 3.
Nuclear damage assessment by Hoechst 33,258 staining in normal, wounded, diabetic, and diabetic wounded human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2 and incubated for 24 h. In non-irradiated models, irregular shaped nuclei, fragmented nuclei and apoptotic cells were observed. In non-irradiated diabetic and diabetic wounded cells, there is an increase in the number of apoptotic cells and nuclear condensation. On the other hand, irradiated models showed uniformly stained nuclei signifying dense nuclei without any fragmentation. Red arrows indicate the irregular shaped nuclei, fragmented nuclei and apoptotic cells. Magnification = × 200
Analysis of AKT and FOXO1 levels
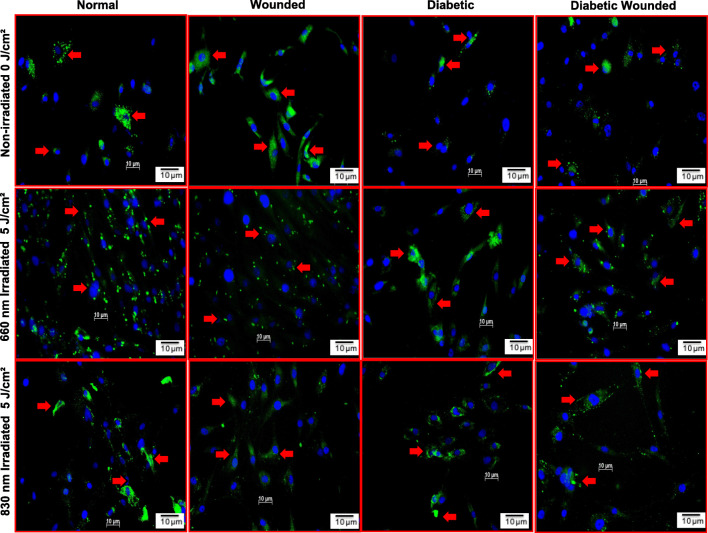
The impact of PBM on AKT was examined in N, W, D and DW non-irradiated and irradiated WS1 cells by immunofluorescence (Fig. 4). AKT fluorescence in non-irradiated D and DW cell models was decreased as compared to N and W cell models. This is due to the increase in oxidative stress induced by growing cells under hyperglycemic conditions. Following PBM at 660 or 830 nm AKT fluorescence in diabetic cell models increased and were comparable to those of normal cell models. There was also an increase in AKT in irradiated models as compared to their non-irradiated cell models (N, W, D and DW).
Fig. 4.
Effect of photobiomodulation on AKT in normal, wounded, diabetic, and diabetic wounded human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2. AKT is stained green with FITC and nuclei are stained blue with DAPI. Both the irradiated and non-irradiated cell models displayed positive staining for AKT proteins. Irradiated cells expressed more AKT protein compared to non-irradiated models. Red arrows indicate the presence of AKT. Magnification = × 200
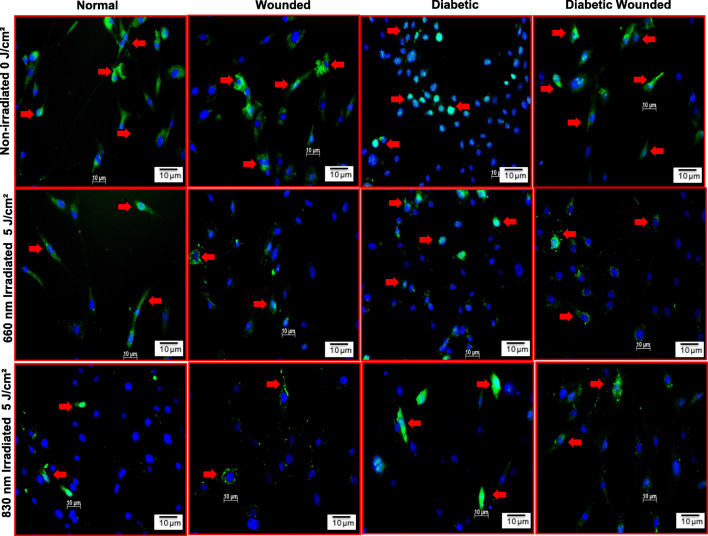
Figure 5 shows the impact of PBM on FOXO1 in N, W, D and DW non-irradiated and irradiated WS1 cells by immunofluorescence. FOXO1 fluorescence in non-irradiated D and DW cell models was increased as compared to N and W cell models. There was also an increase in nuclear translocation in D and DW models compared to N and W cell models, respectively. This is due to the increase in oxidative stress induced by growing cells under hyperglycemic conditions. After PBM at 660 or 830 nm FOXO1 levels in D and DW cell models decreased and were comparable to those of the N cell model. There was also a decrease in FOXO1 in irradiated models as compared to their non-irradiated models (N, W, D and DW).
Fig. 5.
Effect of photobiomodulation on FOXO1 levels in normal, wounded, diabetic, and diabetic wounded human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2. FOXO1 is stained green with FITC and nuclei are stained blue with DAPI. Both the irradiated and non-irradiated cell models displayed positive staining for FOXO1. Irradiated cells expressed less FOXO1 compared to non-irradiated cell models. In non-irradiated models there was an increase in nuclear translocation of FOXO1 in diabetic and diabetic wounded models compared to their normal counterparts. This is due to the increase in oxidative stress induced by growing cells under hyperglycemic condition. Red arrows indicate the presence of FOXO1. Magnification = × 200
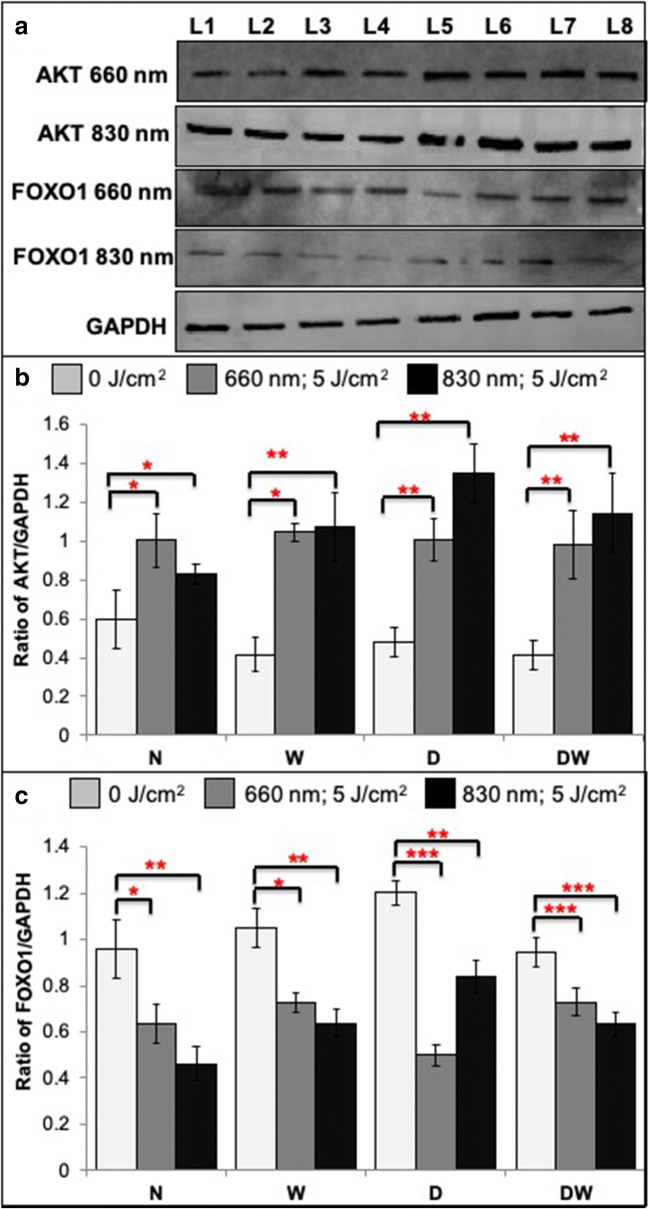
Semi quantitative analysis of AKT and FOXO1 was determined by western blotting 12 h post-irradiation (Fig. 6). There was a significant increase in AKT levels in all irradiated cell models (Fig. 6B). Comparison of the wavelengths showed that irradiation of N cells at 660 nm produced a significant increase in AKT levels (P < .05), whereas the D and DW cells showed a significant decrease in 660 nm irradiated cells (P < .05). These results confirm the results seen by immunofluorescence staining. The significant increase in AKT directly affects its downstream signaling, and there was a significant decrease in FOXO1 in all irradiated models (Fig. 6C). Comparison of the wavelengths showed that irradiation of N, W and DW cells at 830 nm produced a significant decrease in FOXO1 levels (P < .05), whereas the D cells showed a significant increase in 830 nm irradiated cells (P < .05). These results confirm the results observed by immunofluorescence staining of FOXO1.
Fig. 6.
Effect of photobiomodulation on AKT and FOXO1 in normal (N), wounded (W), diabetic (D), and diabetic wounded (DW) human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2. GAPDH was used as a loading control. L1- Normal (0 J/cm2); L2- Wounded (0 J/cm2); L3- Diabetic (0 J/cm2); L4- Diabetic wounded (0 J/cm2); L5- Normal (5 J/cm2); L6- Wounded (5 J/cm2); L7- Diabetic (5 J/cm2); L8- Diabetic wounded (5 J/cm2). A Representative blots. B & C Quantification of the ratio of the intensity of target protein (AKT & FOXO1) divided by the loading control protein (GAPDH). Irradiated N, W, D and DW cells showed a significant increase in AKT levels and decreased FOXO1 levels as compared to non-irradiated cells. Data was expressed as the mean ± SEM (n = 3). Significant probability compared to non-irradiated cell models are shown as *P ≤ .05, **P ≤ .01 and ***P ≤ .001
Expression of enzymic antioxidants
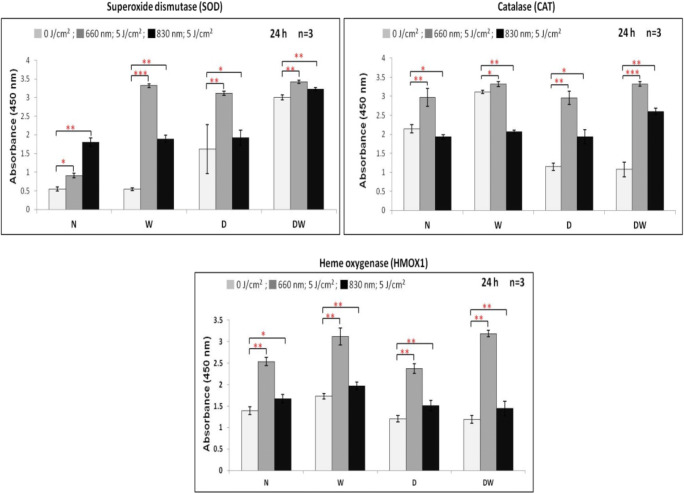
The levels of enzymic antioxidants (SOD, CAT and HMOX1) were determined by in-cell ELISA 24 h post-irradiation (Fig. 7). There was a significant increase in SOD in all cells irradiated at 660 or 830 nm. Comparison of the wavelengths showed that irradiation of W, D and DW cells at 660 nm produced a significant increase in SOD levels (P < .001, P < .001 and P < .05, respectively), whereas N cells displayed a significant decrease at 660 nm (P < .01). Comparison of CAT revealed a significant increase in all cell models when irradiated at 660 nm (Fig. 7). Irradiation at 830 nm produced a significant decrease in N and W cells, and a significant increase in D and DW cells. Comparison of the wavelengths showed that irradiation of N, W, D and DW cells at 660 nm produced a significant increase in CAT levels (P < .001, P < .001, P < .001, and P < .01, respectively) compared to the same cells irradiated at 830 nm. Irradiation at both wavelengths produced a significant increase in HMOX1 in all cell models (Fig. 7). Comparison of the wavelengths showed that irradiation of N, W, D and DW at 660 nm produced a significant increase (P < .001) in HMOX1 levels.
Fig. 7.
Effect of photobiomodulation on enzymic antioxidants in normal (N), wounded (W), diabetic (D), and diabetic wounded (DW) human skin fibroblasts irradiated at a wavelength of 660 or 830 nm with a fluence of 5 J/cm2. Irradiated N, W, D and DW cells showed a significant increase in all enzymic antioxidant levels as compared to their non-irradiated cells except N and W cells of 830 nm irradiated cells. Data was expressed as the mean ± SEM (n = 3). Significant probability compared to non-irradiated cell models are shown as *P ≤ .05, **P ≤ .01 and ***P ≤ .001
Discussion
A wound is defined as damage to the cellular and anatomic structure of tissue or skin. Wound healing is an exceedingly structured and controlled overlapping process of haemostasis, inflammation, proliferation and maturation (Rajendran et al. 2019). Chronic or non-healing wounds are those which fail to heal in the course of time due to a disturbance in the wound healing phases. Various factors such as hypoxia, infection, tumors, and diabetes disturb the normal physiological condition that results in a delay in the healing process (Rajendran et al. 2018). In the last decade many drugs have emerged to treat chronic wounds, however most of these chemical drugs have side effects and worsen the non-healing wound rather than promote healing (Jere et al. 2018). Many investigators are in search for potential therapeutic agents and treatment approaches with no or very minimal side effects.
PBM is widely used in biomedical applications to treat inflammation, pain, tissue and nerve regeneration, and to prevent tissue damage (Jere et al. 2018; Ayuk et al. 2012). It has been shown that in diabetic wounded fibroblast cells PBM promotes healing by increasing cell viability, proliferation, adenosine triphosphate (ATP), growth factors and cytokine production, and reduces pro-inflammatory cytokine levels (Avci et al. 2013). PBM at 600 and 700 nm with a fluence from 0.5 to 4.0 J/cm2 is proven effective in stimulating the proliferation of various cell lines that promotes tissue regeneration. PBM has the ability to alter the redox potential that leads to the increase in the generation of intracellular ROS (Farivar et al. 2014). This research work focuses on the effect of PBM at two wavelengths (one in the visible red and the other in the near infrared spectrum) on the AKT/FOXO1 signaling pathway in normal and diabetic cell models. The effect of PBM on oxidative stress and antioxidants was also investigated to determine the molecular mechanism in enhancing wound healing in diabetic wounded fibroblast cells.
In diabetic conditions, elevated free radicals and lowered antioxidant levels will aggravate cell death and prolong wound healing and closure. Therefore, it is necessary to remove these free radicals and to prevent biological macromolecule damage. Antioxidants play a significant role in removing these free radicals and ROS, and are involved in maintaining a normal redox balance (Srinivasan and Avadhani 2012; Bajpai et al. 2011). In the case of diabetes or under hyperglycemic conditions, there is increased generation of free radicals that creates an imbalance in the redox condition, resulting in damage to various tissues and organs. This elevated oxidative stress, irrespective of its precursor, promotes cellular dysfunction of smooth muscle and endothelial cells that affects angiogenesis and delays recovery (Rosenbaum et al. 2012).
Ahemd et al., reported that in diabetic wounded rats, elevated oxidative stress and inflammatory cytokine levels were reduced upon treatment with quercetin, PBM, and a combination of quercetin and PBM. They also showed that PBM (632.8 nm with a fluence of 6.36 J/cm2) promotes granular tissue formation, migration and proliferation of fibroblast cells, and formation of the ECM by increasing antioxidant levels (Ahmed et al. 2018). In the same regard, our results show that there is a significant increase in enzymatic antioxidant levels (SOD, CAT and HMOX1), which reflects that there is an elimination of free radicals and decrease in the oxidative stress condition. The increase in antioxidant levels indirectly modulates FOXO1 levels by reducing ROS levels. This shows that PBM at 660 and 830 nm has the ability to attenuate oxidative stress by increasing the levels of antioxidants and maintaining a normal redox balance.
Various cellular signaling pathways and cell-cell communications play a crucial role in the development of diabetic complications. G-protein-coupled receptors (GPCR) and receptor tyrosine kinases (RTKs) are activated by various ligands, growth factors, proteins, cytokines and hormones. Once RTKs and GPCRs are activated, they upregulate or downregulate various signaling pathways. One such pathway activated by RTKs is the AKT signaling pathway. The AKT signaling pathway is important for cell survival, cell growth, cell proliferation/ migration, angiogenesis and regeneration. The AKT pathway is highly regulated by multiple upstream signals and cross-talk with other signaling pathways. Alteration or dysregulation of the AKT signaling pathway is found in cancer, diabetes and in wound healing (Jere et al. 2019). In diabetic wounds the decreased migration and proliferation of fibroblasts is not only due to the increase in blood glucose levels, other factors such as increased oxidative stress, oxidation of proteins and downregulation of signaling pathways mainly the MAPK pathway are also responsible (Xiao et al. 2017). Poor wound healing in diabetes is due to the alteration in miRNA expression linked to several signaling pathways. Therefore, targeting the signaling pathway is an important aspect to promote wound healing (Kanazawa et al. 2010). Our results show that in non-irradiated diabetic fibroblast cells there is a significant decrease in AKT levels that resulted in delayed wound closure. On the other hand, irradiated fibroblast cells showed a significant increase in AKT levels, which hasten wound closure, as was seen morphologically. This shows that PBM has the ability to alter the AKT/FOXO1 signaling pathway.
Forkhead box transcription factors play a critical role in apoptosis, cell death and maintaining redox balance. It is well known that delayed wound healing in diabetes is due to decreased cell proliferation and cell migration, and increased cell death induced by increased oxidative stress and apoptosis. FOXO1 is responsible for regulating oxidative stress resistance and apoptosis. The activation/phosphorylation of FOXO1 positively influences inflammatory cytokines and apoptosis to reach their maximum levels, which damages cartilage in diabetic fracture conditions and prolongs the bone healing phase (Rajendran et al. 2019; Kayal et al. 2010) found that in diabetic mice increased levels of FOXO1 phosphorylation/nuclear translocation upregulates the TNF-α signaling pathway that induces chondrocyte apoptosis by upregulating pro-apoptotic genes and cell death, resulting in delays in the healing of the fracture. Therefore, it is necessary to attenuate or block FOXO1 nuclear translocation/activation to promote wound healing in diabetes. A recent in vivo study using mice confirms that the deletion of the FOXO1 gene significantly promotes wound closure as compared with normal wounds (Xu et al. 2015). Our study showed a significant decrease in FOXO1 in irradiated normal, wounded, diabetic and diabetic wounded cells compared to non-irradiated models, and the effect was still evident 12 h post-irradiation. In non-irradiated models, there was an increase in nuclear translocation of FOXO1 in diabetic and diabetic wounded models compared to their normal and wounded cell model counterparts. This is due to the increase in oxidative stress induced by growing cells under hyperglycemic conditions. This phenomenon is reduced in irradiated (5 J/cm2) diabetic and diabetic wounded cell models, where only a few cells display nuclear translocation of FOXO1. Based on the results, it may be assumed that PBM has the ability to dephosphorylate FOXO1 and increases the ubiquitination of FOXO1 that attenuates the transcription of apoptosis related genes and reduces oxidative stress. However, this needs to be confirmed by future research.
Various clinical studies have proven that PBM ranging from 600 to 850 nm has been found to be effective in regulating various cells and tissues. In our study, both wavelengths of 660 and 830 nm showed a positive role in promoting wound healing by attenuating oxidative stress. When the results for the wavelengths were compared, it is found that 660 nm was slightly more effective than 830 nm in regulating the AKT/FOXO1 pathway. The total radiant exposure for both wavelengths was virtually the same, with a 0.2 J difference (45.39 J for 660 nm and 45.59 J for 830 nm).
This research work focuses on exploring the molecular mechanism of action of PBM in promoting wound healing, more specifically diabetic wound healing. We demonstrated that under high glucose conditions PBM attenuates oxidative stress by increasing AKT and antioxidants levels, and decreasing FOXO1 levels in diabetic wounded fibroblast cells, which leads to increased cell viability and migration of diabetic wounded cells (Fig. 8).
Conclusions
In conclusion, delayed wound healing in diabetic fibroblasts was caused by increased oxidative stress and decreased antioxidants due to the alternation in the AKT/FOXO1 signaling pathway. PBM effectively regulates the AKT/FOXO1 pathway and maintains a normal redox balance and antioxidant levels. Irradiation in the visible red range at 660 nm with 5 J/cm2 is able to attenuate cellular oxidative stress better that irradiation at in the near infrared range at 830 nm, using the same radiant energy of 5 J/cm2. Although the present study revealed the molecular mechanism of PBM in accelerating wound healing, it focuses only on the protein changes occurred during PBM treatment. Further studies needed to find out the changes at mRNA levels focusing on FOXO1 signaling pathway.
Electronic supplementary material
(DOCX 66.2 KB)
Acknowledgements
This study was funded by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 98337), as well as grants received from the University of Johannesburg, the Council for Scientific and Industrial Research (CSIR), National Laser Centre (NLC), Laser Rental Pool Program, and the National Research Foundation (NRF) of South Africa. The Council for Scientific and Industrial Research (CSIR) - National Laser Centre (NLC) in South Africa provided and fitted the lasers.
Compliance with ethical standards
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed OM, Mohamed T, Moustafa H, Hamdy E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacother. 2018;101:58–73. doi: 10.1016/j.biopha.2018.02.040. [DOI] [PubMed] [Google Scholar]
- Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33:183–184. doi: 10.1089/pho.2015.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci P, Gupta A, Magesh S, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- Ayuk SM, Houreld NN, Abrahamse H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol Ther. 2012;14:1110–1117. doi: 10.1089/dia.2012.0125. [DOI] [PubMed] [Google Scholar]
- Ayuk SM, Abrahamse H, Houreld NN. Photobiomodulation alters matrix protein activity in stressed fibroblast cells in vitro. J Biopho. 2017;11(3):e201700127. doi: 10.1002/jbio.201700127. [DOI] [PubMed] [Google Scholar]
- Ayuk SM, Abrahamse H, Houreld NN (2018) Effect of 660 nm visible red light on cell proliferation and viability in diabetic models in vitro under stressed conditions. Lasers Med Sci 33:1085–1093. 10.1007/s10103-017-2432-2 [DOI] [PubMed]
- Bajpai S, Mishra M, Kumar H, Tripathi K, Singh SK. Effect of selenium on connexin expression, angiogenesis, and antioxidant status in diabetic wound healing. Biol Trace Elem Res. 2011;144:327–338. doi: 10.1007/s12011-011-9097-7. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang J, Li Y, Sun X, Wang L. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- Cong W, Ma W, Zhao T, Zhu Z, Wang Y. Metallothionein prevents diabetes-induced cardiac pathological changes, likely via the inhibition of succinyl-CoA:3-ketoacid coenzyme A transferase-1 nitration at Trp(374) Am J Physiol Endocrinol Metab. 2013;304:826–835. doi: 10.1152/ajpendo.00570.2012. [DOI] [PubMed] [Google Scholar]
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. Lasers Med Sci. 2014;5:58–62. [PMC free article] [PubMed] [Google Scholar]
- Gangwar M, Gautam MK, Ghildiyal S, Nath G, Goel RK. Mallotus philippinensis Muell. Arg fruit glandular hairs extract promotes wound healing on different wound model in rats. BMC Comp Alt Med. 2015;15:123–129. doi: 10.1186/s12906-015-0647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houreld NN, Abrahamse H. Low-intensity laser irradiation stimulates wound healing in diabetic wounded fibroblast cells (WS1) Diabetes Technol Ther. 2010;12:971–978. doi: 10.1089/dia.2010.0039. [DOI] [PubMed] [Google Scholar]
- Houreld N, Sekhejane P, Abrahamse H. Irradiation at 830 nm Stimulates Nitric Oxide Production and Inhibits Pro-Inflammatory Cytokines in Diabetic Wounded Fibroblast Cells. Lasers Surg Med. 2010;42(6):494–502. doi: 10.1002/lsm.20812. [DOI] [PubMed] [Google Scholar]
- Jere SW, Houreld NN, Abrahamse H. Photobiomodulation at 660 nm stimulates proliferation and migration of diabetic wounded cells via the expression of epidermal growth factor and the JAK/STAT pathway. Photochem Photobiol B: Biology. 2018;179:74–83. doi: 10.1016/j.jphotobiol.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;19:9–14. doi: 10.1016/j.cytogfr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Kanazawa S, Fujiwara T, Matsuzaki S, Shingaki K, Taniguchi M. bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS One. 2010;5:e12228. doi: 10.1371/journal.pone.0012228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal RA, Siqueira M, Alblowi J. TNF-α mediates diabetes enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J Bone Min Res. 2010;25:1604–1615. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F, Niault T, Baccarini M. Ras and Raf pathways in epidermis development and carcinogenesis. Br J Cancer. 2011;104:229–234. doi: 10.1038/sj.bjc.6606009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88:233–240. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- Lima MH, Caricilli AM, de Abreu LL, Araujo EP, Pelegrinelli FF, Thirone AC. Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One. 2017;7:e36974. doi: 10.1371/journal.pone.0036974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley R, Stewart JE, Stephens P, Waddington RJ, Thomas DW. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: lessons learned from other inflammatory diseases. Br J Dermatol. 2004;150:401–413. doi: 10.1111/j.1365-2133.2004.05845.x. [DOI] [PubMed] [Google Scholar]
- Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT. FOXO1 promotes wound healing through the up-regulation of TGF-beta1 and prevention of oxidative stress. J Cell Biol. 2013;203:327–343. doi: 10.1083/jcb.201305074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran NK, Dhilipkumar SS, Houreld NN, Abrahamse H. A review on nanoparticle-based treatment for wound healing. J Durg Deliv Sci Tech. 2018;44:421–430. doi: 10.1016/j.jddst.2018.01.009. [DOI] [Google Scholar]
- Rajendran NK, Dhilipkumar SS, Houreld NN, Abrahamse H. Understanding the perspectives of forkhead transcription factors in delayed wound healing. J Cell Commun Signal. 2019;13(2):151–162. doi: 10.1007/s12079-018-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum MA, Miyazaki K, Graham LM. Hypercholesterolemia and oxidative stress inhibit endothelial cell healing after arterial injury. J Vasc Surg. 2012;55(2):489–496. doi: 10.1016/j.jvs.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai G, Shefer N, Stern K. Glucose-dependent FOXO1 switch in healing wounds: a shred of hope for diabetic ulcers. Diabetes. 2015;64:6–8. doi: 10.2337/db14-1440. [DOI] [PubMed] [Google Scholar]
- Silveira PC, Silva LA, Pinho CA, Souza PS, Ronsani MM, Scheffer DL, Pinho RA. Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med Sci. 2013;28:431–436. doi: 10.1007/s10103-012-1075-6. [DOI] [PubMed] [Google Scholar]
- Soares DM, Ginani F, Henriques A, Barboza C. Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med Sci. 2015;30:1171–1174. doi: 10.1007/s10103-013-1436-9. [DOI] [PubMed] [Google Scholar]
- Squarize CH, Castilho RM, Bugge TH, Gutkind JS. Accelerated wound healing by mTOR activation in genetically definedmouse model. PLoS One. 2010;5:e10643. doi: 10.1371/journal.pone.0010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med. 2012;53:1252–1263. doi: 10.1016/j.freeradbiomed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Wehrmann M. Differential cytokine activity and morphology during wound healing in the neonatal and adult rat skin. J Cell Mol Med. 2007;11:1342–1351. doi: 10.1111/j.1582-4934.2007.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, Morris VL, Chan BM. Coordinated integrin and growth factor regulation of primary keratinocyte migration mediated through extracellular signal regulated kinase and phosphoinositide 3-kinase. Arch Dermatol Res. 2009;301:307–317. doi: 10.1007/s00403-009-0945-7. [DOI] [PubMed] [Google Scholar]
- Xiao W, Tang H, Wu M, Liao Y, Li K, Li L, et al. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci Rep. 2017;37:BSR20170658. doi: 10.1042/BSR20170658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Othman B, Lim J, Batres A, Ponugoti B, Zhang C, Yi L, Liu J, Tian C. FOXO1 inhibits diabetic mucosal wound healing but enhances healing of normoglycemic wounds. Diabetes. 2015;64:243–256. doi: 10.2337/db14-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ponugoti B, Tian C, Xu F, Tarapore R, Batres A, Alsadun S, Lim J, Dong G, Graves DT. FOXO1 differentially regulates both normal and diabetic wound healing. J Cell Biol. 2015;209:289–303. doi: 10.1083/jcb.201409032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 66.2 KB)