Abstract
Introduction
Ixekizumab, an interleukin-17A antibody, has shown efficacy in non-radiographic axial spondyloarthritis (nr-axSpA). The objectives of this analysis were (a) to measure improvement in ixekizumab-treated patients in Assessment of Spondyloarthritis International Society (ASAS) response domains and other patient-reported outcomes (PROs) and (b) to determine how ASAS responses were associated with changes in patient global disease activity (PtGA), spinal pain, function, stiffness, fatigue, and spinal pain at night.
Methods
COAST-X was a phase 3, 52-week multicenter, randomized, controlled trial investigating the efficacy and safety of 80-mg ixekizumab every 2 weeks (Q2W) and every 4 weeks (Q4W) in patients with active nr-axSpA. Changes from baseline in PROs were analyzed via mixed-effects models for repeated measures. Association analyses for ASAS responses used analysis of covariance with Scheffé’s method.
Results
Patients treated with ixekizumab Q2W and Q4W reported significantly greater improvements in PtGA, spinal pain, function, and stiffness at week 1, when these measures were first assessed, compared with placebo (p < 0.05). ASAS40 responders, in comparison to ASAS20 non-responders, had the highest correlations with improvements in all response domains (PtGA, spinal pain, function, and stiffness) as well as fatigue and spinal pain at night (p < 0.001). ASAS40 responses were associated with 3.5- to 48.0-fold greater improvements in these PROs, with the highest values for PtGA and function, compared to ASAS20 non-achievement.
Conclusions
As early as week 1, patients with nr-axSpA treated with ixekizumab reported significant improvements in PtGA, spinal pain, function, and stiffness compared with those taking placebo. ASAS40 responders reported significantly greater improvements in all ASAS response domains (PtGA, spinal pain, function, and stiffness) as well as fatigue and spinal pain at night than ASAS20 non-responders. Improvements in PtGA and function appear to be major drivers in achieving ASAS40 response in patients with nr-axSpA.
Trial Registration
Electronic supplementary material
The online version of this article (10.1007/s40744-020-00254-z) contains supplementary material, which is available to authorized users.
Keywords: Assessment of Spondyloarthritis International Society (ASAS) response, Biological therapy, Ixekizumab, Non-radiographic axial spondyloarthritis, Patient-reported outcomes
Key Summary Points
| Why carry out this study? |
| Patients with non-radiographic axial spondyloarthritis (nr-axSpA) experience negative impacts on health-related quality of life with pain and fatigue as notable symptoms. |
| This study measured improvement in ixekizumab-treated patients in patient-reported outcomes, including Assessment of Spondyloarthritis International Society (ASAS) treatment response domains to determine how ASAS responses were associated with changes in patient global disease activity, spinal pain, function, stiffness, fatigue, and spinal pain at night. |
| What was learned from the study? |
| Patients with nr-axSpA treated with ixekizumab reported greater improvements in patient-reported outcomes, and ASAS40 achievement had the highest correlation with improvements in patient global disease activity, spinal pain, function, stiffness, fatigue, and spinal pain at night. |
| Improvement in patient global disease activity and function may be drivers in achievement of ASAS40. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.13187009.
Introduction
Axial spondyloarthritis (axSpA), characterized by inflammation of the sacroiliac joints and axial skeleton, includes two subtypes: (1) ankylosing spondylitis (AS), also known as radiographic axSpA (r-axSpA), defined radiographically by substantial structural damage of the sacroiliac joints; and (2) non-radiographic axSpA (nr-axSpA), which lacks evidence of definite sacroiliitis on plain radiographs but may show evidence of inflammation on magnetic resonance imaging (MRI) in sacroiliac joints [1–4]. Of patients with axSpA, 40–60% may have nr-axSpA [5]. Patients with nr-axSpA experience similar negative impacts on health-related quality of life (HRQoL) to those in AS [1, 2, 6]. Spinal pain and fatigue particularly reflect the burden of disease in patients with axSpA [7].
Physical therapy and non-steroidal anti-inflammatory drugs (NSAIDs) are used as first-line treatments for nr-axSpA [8]. Biologic disease-modifying anti-rheumatic drugs (bDMARDs) are indicated for treatment of nr-axSpA patients with objective signs of inflammation who do not adequately respond to NSAID therapy [9]. While tumor necrosis factor inhibitors (TNFi) are efficacious in treating nr-axSpA, an unmet need remains since patients may not respond to TNFi or may only have partial responses [9–11].
Ixekizumab is a high-affinity monoclonal antibody that selectively targets interleukin (IL)-17A and has demonstrated efficacy in treating both AS and nr-axSpA [5, 11–13]. The Assessment of Spondyloarthritis International Society (ASAS) responses, ASAS20 and ASAS40 (defined by criteria including improvements of 20% and 40%, respectively) and partial remission, are primary endpoints in axSpA randomized controlled trials (RCTs). While ASAS responses are valuable in the context of RCTs, physicians in clinical practice focus on individual patient symptoms, such as patient global disease activity (PtGA), spinal pain, function, stiffness, fatigue, and spinal pain at night [14, 15]. The present study used data from the COAST-X trial to assess the impact of ixekizumab treatment over 52 weeks and explore how ASAS40 responses correlate with improvements in patient-reported outcomes (PROs).
Methods
Study Design
COAST-X (NCT02757352) was a phase 3 multicenter, 52-week RCT assessing the efficacy and safety of 80-mg ixekizumab every 2 weeks (Q2W) and every 4 weeks (Q4W) compared with placebo in bDMARD-naive patients with active nr-axSpA.
Patients
Inclusion criteria for COAST-X have been detailed previously [5]. Eligible patients were adults with an established diagnosis of axSpA by a physician and fulfilled the Assessment of SpondyloArthritis International Society (ASAS) classification criteria for axSpA, with at least 12 weeks of previous therapy with NSAIDs and an inadequate response, as determined by the investigator, to two or more NSAIDs at the therapeutic dose range for a total duration of at least 4 weeks. Centrally read X-rays excluded patients with radiographic criteria of definite sacroiliitis according to modified New York criteria [4, 5]. Key inclusion criteria were active disease at screening and baseline (defined as a Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] score of ≥ 4 and total back pain score of ≥ 4 on a 0–10 scale) and objective signs of inflammation, defined as evidence of elevated C-reactive protein (CRP > 5 mg/l) and/or presence of sacroiliitis on centrally read MRI, with sacroiliitis determined using the ASAS definition [16]. Enrolled patients could continue stable background medications including NSAIDs, conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), corticosteroids, and analgesics. Ethical review boards approved COAST-X at each site before the trial began. Procedures involving human participants were performed within the ethical standards of the institutional and national research committees at all sites. COAST-X was conducted in accordance with the standards of the Declaration of Helsinki. All patients gave written informed consent before undergoing procedures related to the trials. The master ethics committee was Schulman Associates IRB, Cincinnati, OH, USA; complete listings of sites and investigators are available in the supplements of previously published results from COAST-X [5].
Randomization and Blinding
We randomized 303 patients 1:1:1 to receive placebo Q2W (N = 105), 80-mg of ixekizumab Q4W (N = 96), or 80-mg of ixekizumab Q2W (N = 102), via subcutaneous injection [5]. To achieve comparability between groups, randomization was stratified by country and screening MRI/CRP status (positive MRI and elevated CRP, positive MRI and non-elevated CRP, negative MRI and elevated CRP). The randomized, blinded treatment period ranged from weeks 0–52. From weeks 16–44, if patients’ disease activity required and at investigator discretion (with no specific, pre-defined switch criteria), adjustment of background medications for axSpA was allowed and/or patients were able to switch to open-label ixekizumab Q2W or subsequent TNFi treatment. Patients who switched to open-label treatment continued to be followed during the trial, and both investigators and patients remained blinded to original treatment assignment.
Outcome Measures
The primary endpoints for the COAST-X trial were the proportions of ASAS40 responders at weeks 16 and 52 and have already been reported [5]. Secondary endpoints included ASAS20 responders, changes from baseline in the response domains of ASAS criteria (PtGA, spinal pain, function, and stiffness), as well as BASDAI fatigue, Fatigue Numeric Rating Scale (NRS), and spinal pain at night.
PtGA, spinal pain, BASFI, and BASDAI responses were assessed at weeks 0 (baseline), 1, and 2, every 4 weeks from weeks 4–36, and then at weeks 44 and 52. Stiffness was measured as the average score based on responses to BASDAI questions 5 and 6: intensity and duration of morning stiffness [14]. Function was measured as the average score from responses to the Bath Ankylosing Spondylitis Functional Index (BASFI) [14]. Fatigue was measured with the Fatigue Severity NRS [17]. Fatigue NRS was recorded at weeks 0 (baseline), 8, 16, 36, and 52. Fatigue was also measured by BASDAI question 1 [14]. Spinal pain at night scores were recorded at weeks 0 (baseline), 1, and 2, every 4 weeks from weeks 4–36, and then at weeks 44 and 52.
Statistical Analyses
Statistical analyses were performed on data from the intent-to-treat population comparing ixekizumab dosing regimens to placebo during the double-blind treatment period (weeks 0–52) prior to biologic switches to ixekizumab 80-mg Q2W or TNFi. In patients treated with open-label ixekizumab 80-mg Q2W following biologic switch, only data up to the time of switch were included in the analysis. Mixed-effects models for repeated measures (MMRM) were used to compare changes from baseline in treatment groups at each visit for continuous outcomes for each PRO. Each model included treatment, geographic region, screening MRI/CRP status, baseline value, visit, baseline value-by-visit, and treatment-by-visit interactions as fixed factors. No prior imputation for missing data was performed for the MMRM modeling. P values were nominal.
Post hoc association analyses between ASAS responses and changes from baseline to weeks 16 and 52 in PROs were conducted by pooling patients with fully observed ASAS and outcome data at baseline and weeks 16 or 52, respectively, into three ASAS response groups: ASAS20 non-responders, ASAS20 responders (but not ASAS40 responders), and ASAS40 responders. Changes from baseline to weeks 16 and 52 in the above PROs were compared between ASAS responder groups using analysis of covariance (ANCOVA) models that included responses, baseline values, age, and gender as factors. Pairwise comparisons between response groups were conducted with Scheffé’s method for multiplicity [18]. No imputation was performed on missing values in ASAS responders or the PROs comprising the ASAS response criteria (PtGA, spinal pain, function, and stiffness); observed values were reported for these measures’ data. Modified baseline observation carried forward was used to impute missing fatigue and spinal pain at night data for ASAS correlations. SAS 9.4 was used for all analyses.
Results
Baseline Characteristics
Baseline characteristics of patients enrolled in COAST-X are presented in Table 1 and were similar between treatment groups.
Table 1.
Baseline characteristics for patients enrolled in COAST-X
| PBO (N = 105) | IXE Q4W (N = 96) | IXE Q2W (N = 102) | |
|---|---|---|---|
| Age, years | 39.9 (12.4) | 40.9 (14.5) | 40.0 (12.0) |
| Male, n (%) | 44 (41.9) | 50 (52.1) | 49 (48.0) |
| Weight, kg | 75.8 (18.4)a | 79.5 (16.5) | 77.3 (16.6) |
| Duration of nr-axSpA symptoms, years | 10.1 (8.3) | 11.3 (10.7) | 10.6 (10.1) |
| Time since nr-axSpA diagnosis, years | 3.1 (4.5) | 4.2 (5.5) | 3.4 (4.6) |
| CRP, mg/l | 14.3 (24.4) | 12.4 (18.0) | 12.1 (17.8) |
| Screening MRI/CRP status, n (%) | |||
| Positive MRI and elevated CRP | 38 (36.2) | 30 (31.3) | 39 (38.2) |
| Positive MRI and non-elevated CRP | 40 (38.1) | 36 (37.5) | 34 (33.3) |
| Negative MRI and elevated CRP | 26 (24.8) | 30 (31.3) | 28 (27.5) |
| Therapy, n (%) | |||
| Current cDMARD use, including MTX | 36 (34.3) | 40 (41.7) | 42 (41.2) |
| Current MTX use | 17 (16.2) | 17 (17.7) | 15 (14.7) |
| ASDAS | 3.8 (0.9) | 3.8 (0.8) | 3.9 (0.8) |
| Patient global disease activity | 7.4 (1.7) | 7.1 (1.7) | 7.6 (1.5) |
| Spinal pain NRS | 7.4 (1.6) | 7.3 (1.7) | 7.4 (1.6) |
| Function, BASFI | 6.7 (2.0) | 6.4 (2.1) | 6.5 (1.8) |
| Stiffness, BASDAI questions 5 and 6 | 7.0 (1.9) | 6.8 (2.0) | 7.1 (1.5) |
| Fatigue NRS | 7.1 (1.8) | 7.2 (1.6) | 7.2 (1.8) |
| BASDAI fatigue | 7.4 (1.6) | 7.4 (1.6) | 7.5 (1.6) |
| Spinal pain at night NRS | 7.3 (1.7) | 7.0 (1.9) | 7.5 (1.8) |
aNx = 104
Data are mean (SD) unless otherwise stated. Percentage was calculated by n/N*100%
ASDAS Ankylosing Spondylitis Disease Activity Score, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, cDMARD conventional disease-modifying anti-rheumatic drug, CRP C-reactive protein, MRI magnetic resonance imaging, IXE Q2W 80-mg ixekizumab every 2 weeks, IXE Q4W 80-mg ixekizumab every 4 weeks, MTX methotrexate, N number of patients in the analysis category, n number of patients in subgroup, NRS numeric rating scale, nr-axSpA non-radiographic axial spondyloarthritis, PBO placebo, SD standard deviation
Changes from Baseline in Patient Global Disease Activity, Spinal Pain, Function, and Stiffness
Significant improvements in PtGA, spinal pain, function, and stiffness were reported as early as week 1 by patients treated with ixekizumab Q4W (p = 0.002, p < 0.001, p = 0.034, and p = 0.008, respectively) and ixekizumab Q2W (p < 0.001, p = 0.002, p = 0.037, and p = 0.005, respectively) compared with placebo (Fig. 1, Table 2). Significant improvements in PtGA were also reported at week 16 with ixekizumab Q4W and Q2W (p = 0.004 and p < 0.001, respectively) and week 52 with ixekizumab Q2W (p = 0.003) (Fig. 1a, Table 2). Similarly, significant improvements in spinal pain were reported at week 16 with ixekizumab Q4W and Q2W (p = 0.01 and p = 0.001, respectively) and week 52 with ixekizumab Q2W (p = 0.027) (Fig. 1b, Table 2). Significant improvements in function were also reported by patients treated with ixekizumab Q4W (p = 0.040) and Q2W (p = 0.004) at week 16 and sustained to week 52 (p = 0.018 and p = 0.008 for patients treated with ixekizumab Q4W and Q2W, respectively) (Fig. 1c, Table 2). Significant improvements in stiffness were reported by patients treated with ixekizumab Q4W (p = 0.004) and Q2W (p < 0.001) at week 16, and improvements were sustained to week 52 with ixekizumab Q4W (p = 0.007) and Q2W (p < 0.001) (Fig. 1d, Table 2).
Fig. 1.
Changes from baseline in patient global disease activity (a), spinal pain (b), function (BASFI) (c), and stiffness (BASDAI questions 5 and 6) through week 52 (d). Values are LSM from MMRM. Week 1: PBO, Nx = 103; IXE Q4W, Nx = 95; IXE Q2W, Nx = 99. Week 2: PBO, Nx = 102; IXE Q4W, Nx = 96; IXE Q2W, Nx = 102. Weeks 4 and 8: PBO, Nx = 101; IXE Q4W, Nx = 96; IXE Q2W, Nx = 101. Weeks 12 and 16: PBO, Nx = 99; IXE Q4W, Nx = 96; IXE Q2W, Nx = 98. Week 20: PBO, Nx = 55; IXE Q4W, Nx = 68; IXE Q2W, Nx = 73. Week 24: PBO, Nx = 50; IXE Q4W, Nx = 64; IXE Q2W, Nx = 64. Week 28: PBO, Nx = 43; IXE Q4W, Nx = 63; IXE Q2W, Nx = 61. Week 32: PBO, Nx = 43; IXE Q4W, Nx = 59; IXE Q2W, Nx = 60. Week 36: PBO, Nx = 39; IXE Q4W, Nx = 56; IXE Q2W, Nx = 58. Week 44: PBO, Nx = 36; IXE Q4W, Nx = 54; IXE Q2W, Nx = 56. Week 52: PBO, Nx = 34; IXE Q4W, Nx = 53; IXE Q2W, Nx = 52. P values were from MMRM (treatment vs. placebo). *p < 0.05, ‡p < 0.01, †p < 0.001. BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, IXE Q2W = 80-mg ixekizumab every 2 weeks, IXE Q4W 80-mg ixekizumab every 4 weeks, IXE Q2W 80-mg ixekizumab every 2 weeks, LSM least squares mean, MMRM mixed-effect model for repeated measures, Nx number of patients with non-missing values, PBO placebo
Table 2.
Changes from baseline in patient-reported outcomes at weeks 16 and 52
| PBO (N = 105) |
IXE Q4W (N = 96) | IXE Q2W (N = 102) | ||
|---|---|---|---|---|
| Patient global disease activity | ||||
| Week 16 | Nx | 99 | 96 | 98 |
| LSM (SE) | − 1.3 (0.25) | − 2.3 (0.25)‡ | − 2.6 (0.25)† | |
| Week 52 | Nx | 34 | 53 | 52 |
| LSM (SE) | − 1.8 (0.38) | − 2.8 (0.32) | − 3.3 (0.32)‡ | |
| Spinal pain | ||||
| Week 16 | Nx | 99 | 96 | 98 |
| LSM (SE) | − 1.5 (0.24) | − 2.4 (0.25)* | − 2.6 (0.24)‡ | |
| Week 52 | Nx | 34 | 53 | 52 |
| LSM (SE) | − 2.3 (0.35) | − 2.9 (0.31) | − 3.3 (0.30)* | |
| Function, BASFI | ||||
| Week 16 | Nx | 99 | 96 | 98 |
| LSM (SE) | − 1.3 (0.2) | − 2.0 (0.2)* | − 2.3 (0.2)‡ | |
| Week 52 | Nx | 34 | 53 | 52 |
| LSM (SE) | − 1.6 (0.3) | − 2.6 (0.3)* | − 2.8 (0.3)‡ | |
| Stiffness (BASDAI questions 5 and 6) | ||||
| Week 16 | Nx | 99 | 96 | 98 |
| LSM (SE) | − 1.4 (0.24) | − 2.4 (0.25)‡ | − 2.9 (0.24)† | |
| Week 52 | Nx | 34 | 53 | 52 |
| LSM (SE) | − 1.9 (0.33) | − 3.2 (0.29)‡ | − 3.5 (0.29)† | |
| Fatigue (NRS) | ||||
| Week 16 | Nx | 99 | 96 | 98 |
| LSM (SE) | − 1.4 (0.24) | − 2.1 (0.24)* | − 1.9 (0.24) | |
| Week 52 | Nx | 34 | 53 | 52 |
| LSM (SE) | − 2.1 (0.38) | − 2.6 (0.32) | − 2.7 (0.32) | |
| Fatigue (BASDAI question 1) | ||||
| Week 16 | Nx | 99 | 96 | 98 |
| LSM (SE) | − 1.7 (0.23) | − 2.2 (0.24) | − 2.3 (0.24) | |
| Week 52 | Nx | 34 | 53 | 52 |
| LSM (SE) | − 1.9 (0.34) | − 2.7 (0.29) | − 2.8 (0.29) | |
| Spinal pain at night | ||||
| Week 16 | Nx | 99 | 96 | 98 |
| LSM (SE) | − 1.7 (0.26) | − 2.4 (0.27) | − 2.8 (0.26)‡ | |
| Week 52 | Nx | 34 | 53 | 52 |
| LSM (SE) | − 2.3 (0.36) | − 3.0 (0.31) | − 3.6 (0.31)‡ | |
LSM (SE) is from MMRM
p values were from MMRM (treatment vs. placebo). *p < 0.05, ‡p < 0.01, †p < 0.001
BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, IXE Q2W 80-mg ixekizumab every 2 weeks, IXE Q4W 80-mg ixekizumab every 4 weeks, LSM least squares mean, MMRM mixed-effect model repeated measure, N number of patients in the treatment group, NRS numeric rating scale, Nx number of patients with non-missing values, PBO placebo, SE standard error
Changes from Baseline in Fatigue and Spinal Pain at Night
At week 16, a significant improvement in Fatigue NRS was reported by patients receiving ixekizumab Q4W compared to placebo (p = 0.024; Fig. 2a, Table 2). Improvements in fatigue were not statistically significant with either ixekizumab dosing regimen at other time points (Fig. 2a, b, Table 2). Similarly, improvements in BASDAI fatigue were numerically greater but not statistically significant with both ixekizumab dosing regimens at weeks 16 and 52 (Fig. 2b, Table 2). Significant improvements in spinal pain at night were reported by patients treated with ixekizumab Q4W and Q2W as early as week 1 (p = 0.029 and p = 0.013, respectively) and at weeks 16 and 52 with ixekizumab Q2W as well (p = 0.004 and p = 0.005, respectively) (Fig. 2c, Table 2).
Fig. 2.

Changes from baseline in fatigue as measured by Fatigue NRS (a) and BASDAI fatigue (b), as well as spinal pain at night through week 52 (c). Values are LSM from MMRM. Fatigue NRS—week 8: PBO, Nx = 101; IXE Q4W, Nx = 95; IXE Q2W, Nx = 101. Week 16: PBO, Nx = 99; IXE Q4W, Nx = 96; IXE Q2W, Nx = 98. Week 36: PBO, N = 39; IXE Q4W, Nx = 56; IXE Q2W, Nx = 58. Week 52: PBO, Nx = 34; IXE Q4W, Nx = 53; IXE Q2W; Nx = 52. BASDAI Fatigue and Spinal Pain at Night—week 1: PBO, Nx = 103; IXE Q4W, Nx = 95; IXE Q2W, Nx = 99. Week 2: PBO, Nx = 102; IXE Q4W, Nx = 96; IXE Q2W, Nx = 102. Weeks 4 and 8: PBO, Nx = 101; IXE Q4W, Nx = 96; IXE Q2W, Nx = 101. Weeks 12 and 16: PBO, Nx = 99; IXE Q4W, Nx = 96; IXE Q2W, Nx = 98. Week 20: PBO, Nx = 55; IXE Q4W, Nx = 68; IXE Q2W, Nx = 73. Week 24: PBO, Nx = 50; IXE Q4W, Nx = 64; IXE Q2W, Nx = 64. Week 28: PBO, Nx = 43; IXE Q4W, Nx = 63; IXE Q2W, Nx = 61. Week 32: PBO, Nx = 43; IXE Q4W, Nx = 59; IXE Q2W, Nx = 60. Week 36: PBO, Nx = 39; IXE Q4W, Nx = 56; IXE Q2W, Nx = 58. Week 44: PBO, Nx = 36; IXE Q4W, Nx = 54; IXE Q2W, Nx = 56. Week 52: PBO, Nx = 34; IXE Q4W, Nx = 53; IXE Q2W, Nx = 52. P values were from MMRM (treatment vs. placebo). *p < 0.05, ‡p < 0.01, †p < 0.001. BASDAI Bath Ankylosing Spondylitis Disease Activity Index, IXE Q2W 80-mg ixekizumab every 2 weeks, IXE Q4W 80-mg ixekizumab every 4 weeks, LSM least squares mean, MMRM mixed-effect model for repeated measures, NRS numeric rating scale, Nx number of patients with non-missing values, PBO placebo
Association of ASAS Responses with Improvements in Patient Global Disease Activity, Spinal Pain, Function, and Stiffness
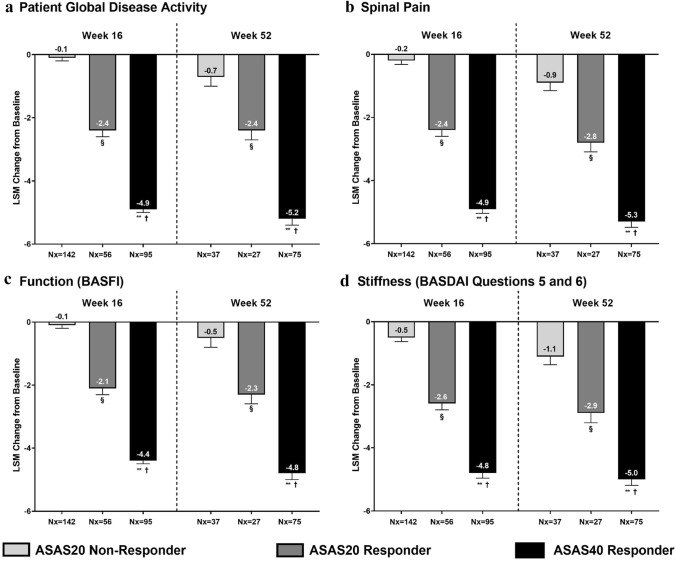
To examine how improvements in individual PROs relate to ASAS responses, improvement in the ASAS response domains PtGA, spinal pain, function, and stiffness (as well as the additional outcomes fatigue and spinal pain at night) were compared among three ASAS response levels pooled across treatment groups: ASAS20 non-responders, ASAS20 responders (but not ASAS40 responders), and ASAS40 responders. ASAS40 responders reported the greatest improvements in all PROs evaluated (Figs. 3 and S1). ASAS40 responders reported significantly greater improvements in PtGA, spinal pain, function, and stiffness at weeks 16 and 52 than ASAS20 non-responders (Fig. 3). ASAS40 responders at week 16 reported a 48.0-fold improvement in PtGA, 23.5-fold improvement in spinal pain, 43.0-fold improvement in function, and 8.6-fold improvement in stiffness compared to ASAS20 non-responders. The magnitudes of improvement among ASAS40 responders compared to ASAS20 non-responders at week 52 were 6.4-fold in PtGA, 4.9-fold in spinal pain, 8.6-fold in function, and 3.5-fold in stiffness. ASAS20 responders also reported significant improvements in PtGA, spinal pain, function, and stiffness at weeks 16 and 52 (Fig. 3).
Fig. 3.
Association between ASAS response and changes from baseline in measures of patient global disease activity (a), spinal pain (b), function (c), and stiffness (d) (BASDAI questions 5 and 6) at weeks 16 and 52. Values are LSM (SE) from ANCOVA. Only observed data were used in this analysis. P values were from ANCOVA after correcting for baseline value, age, gender, and ASAS response category. §p < 0.001, ASAS20 responder vs. ASAS20 non-responder; **p < 0.001, ASAS40 responder vs. ASAS20 non-responder; †p < 0.001, ASAS40 responder vs. ASAS20 responder. ANCOVA analysis of covariance, ASAS Assessment of Spondyloarthritis International Society, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, LSM least squares mean, Nx number of non-missing observations, SE standard error
Association of ASAS Responses with Improvements in Fatigue and Spinal Pain at Night
ASAS40 responders reported significantly greater improvements in fatigue and spinal pain at night at weeks 16 and 52 compared with ASAS20 non-responders (Fig. S1). ASAS40 responders at week 16 reported an 11.7-fold improvement in fatigue and an 11.8-fold improvement in spinal pain at night compared to ASAS20 non-responders. The magnitudes of improvement among ASAS40 responders compared to ASAS20 non-responders at week 52 were 6.2-fold in fatigue and 3.5-fold in spinal pain at night. ASAS20 responders also reported significant improvements in fatigue and spinal pain at night at weeks 16 and 52 (Fig. S1).
Discussion
ASAS40 responses at weeks 16 and 52 were the primary endpoints of the COAST-X clinical trial, a high bar that is challenging to meet since it necessitates considerable improvement in patients' perceptions of symptoms [5, 15]. Because high placebo responses have been observed in AS studies, ASAS40 responses help better differentiate treatment effects [19].
At week 16 in the COAST-X trial, 35% of patients treated with ixekizumab Q4W and 40% of patients treated with ixekizumab Q2W were ASAS40 responders (compared with 19% receiving placebo). At week 52, 30% and 31% of patients receiving ixekizumab Q4W and ixekizumab Q2W, respectively, were ASAS40 responders (compared with 13% receiving placebo) [5]. ASAS40 responders reported significantly greater improvements in all ASAS components compared with ASAS20 responders and ASAS20 non-responders. In this study, ASAS40 responders reported a 48.0-fold improvement in PtGA and a 43.0-fold improvement in function compared to ASAS20 non-responders at week 16. With such notable improvements, PtGA and function appear to be major drivers in ASAS40 response for patients with nr-axSpA.
PtGA, spinal pain, stiffness, function, fatigue, and spinal pain at night reflect different burdens of disease that interact with each other and play an important part in overall health outcomes. In axSpA, severe spinal pain, impaired work productivity, and worse HRQoL have been linked [20]. Fatigue has been associated with greater disease activity and worse scores on the Ankylosing Spondylitis Quality of Life questionnaire [21]. It is now well established that the burden of disease is similar in both axSpA subtypes, nr-axSpA and AS [6, 22–24]. While widespread use of bDMARDs in nr-axSpA remains limited due to regulatory constraints, use of bDMARDs can improve patient outcomes [8, 11, 24].
In a previous study, patients with r-axSpA who were treated with ixekizumab and enrolled in the COAST-V and COAST-W trials reported greater improvements in patient-reported outcomes compared to placebo, and ASAS40 responders had greater improvements in PROs than ASAS20 non-responders [15]. In the current analysis of data from the COAST-X clinical trial of patients with nr-axSpA, we demonstrate that ixekizumab is effective at improving spinal PROs in patients with nr-axSpA. This analysis from COAST-X along with the previously reported results from COAST-V and COAST-W in r-axSpA indicate that treatment with ixekizumab is effective in improving the most impactful symptoms across the axSpA disease spectrum (r-axSpA and nr-axSpA) [15]. As previously reported in the r-axSpA trials assessing ixekizumab (COAST-V and COAST-W), here we also show that ASAS40 responders report greater improvements in PtGA, spinal pain, function, and stiffness, fatigue, and spinal pain at night compared with ASAS20 responders, and ASAS20 responders also report greater improvements in PROs than ASAS20 non-responders.
This study was limited due to the decrease in sample sizes after week 16 when patients who required changes in treatment were identified and switched either to ixekizumab Q2W or subsequent TNFi at the discretion of the investigator. The small sample sizes after week 16 affected analyses measuring significance in changes from baseline in PROs. The lack of specific pre-defined switch criteria to guide changes in study treatment may have impacted treatment effect size. Because of the decrease in sample size due to patients switched to ixekizumab Q2W after week 16, non-responder imputation was not applicable to the intent-to-treat sample. As a result, only observed data were used for post hoc association analysis between ASAS responses and PROs, potentially limiting the generalizability of results to the intent-to-treat population. The different recall periods of the Fatigue NRS and BASDAI questionnaires may have contributed to the significance of Fatigue NRS results and the non-significance of BASDAI fatigue results.
Strengths of this COAST-X RCT include a patient population representative of multiple global regions and inclusion criteria that required objective signs of inflammation (screening MRI/CRP status). Patients also had mean duration of symptoms over 10 years consistent with nr-axSpA and did not “convert” to r-axSpA. In addition, this analysis used recognized and validated instruments (ASAS response criteria, BASDAI, BASFI, and Fatigue NRS) to assess PROs.
Conclusions
Patients with nr-axSpA treated with ixekizumab reported significant improvements in PtGA, spinal pain, function, and stiffness compared with placebo at week 16, as early as week 1 and sustained in patients treated with ixekizumab Q2W through week 52. Greater improvements in fatigue were also reported by patients treated with either ixekizumab dosing regimen. ASAS40 responders reported significantly greater improvements in all response domains and in PROs than ASAS20 responders; ASAS20 responders also reported significantly greater PRO improvements than ASAS20 non-responders. ASAS40 responders reported 3.5- to 48.0-fold greater improvements in PtGA, spinal pain, function, stiffness, fatigue, and spinal pain at night compared to ASAS20 non-responders. Improvements in PtGA and function appear to be the major drivers in ASAS responses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The views expressed in this work are those of the author and not necessarily those of the (UK) National Health Service (NHS), the NIHR, or the (UK) Department of Health.
Funding
Eli Lilly and Company, Indianapolis, IN, USA provided funding for the sponsorship for this study and the journal’s Rapid Service Fee. All authors had complete access to the data used in this study and take full responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Nicole Lipitz of Syneos Health provided writing and editorial assistance with funding provided by Eli Lilly and Company.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship of this manuscript, take responsibility for the integrity of the whole work, and have given approval for the publication of this version of the manuscript.
Prior Presentation
Preliminary results from this analysis were presented at the European Congress of Rheumatology 2020 and the American College of Rheumatology 2020 Annual Meeting.
Disclosures
Atul Deodhar has received grants from AbbVie, Eli Lilly and Company, GlaxoSmithKline, Novartis, Pfizer, and UCB as well as honoraria/consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galapagos, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB. Philip Mease has received research grants, consulting, and/or speaker fees from AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly and Company, Galapagos, Gilead, GlaxoSmithKline, Novartis, Pfizer, SUN Pharma, and UCB. Proton Rahman has received grants from Janssen and Novartis and honoraria/consultancy fees from Abbott, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, and Pfizer. Victoria Navarro-Compán has received research grants from AbbVie, Merck, Novartis, Pfizer, and ASAS; consulting fees from AbbVie, Lilly, Novartis, Pfizer, and UCB. Helena Marzo-Ortega has received grants from Janssen and Novartis; and honoraria/consultancy fees from AbbVie, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Takeda, and UCB. Helen Marzo-Ortega is also supported by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre (LBRC). Theresa Hunter, David Sandoval, Andris Kronbergs, Luis Leon, and Mingyang Shan are employees and shareholders of Eli Lilly and Company. Ann Leung is an employee of Syneos Health. Kurt de Vlam has received grants from Celgene and honoraria/consulting fees from AbbVie, Affibody, Amgen, Eli Lilly and Company, Galapagos, GlaxoSmithKline, Novartis, Pfizer, and UCB. Vibeke Strand has received consulting fees from AbbVie, Amgen, Arena, AstraZeneca, Bayer, Bioventus, Bristol-Myers Squibb, Boehringer Ingelheim, Celltrion, CORRONA, Crescendo/Myriad, EMD Serono, Equillium, Flexion, Genentech/Roche, Glenmark, GlaxoSmithKline, Horizon, Inmedix, Janssen, Kypha, Eli Lilly and Company, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., Samsung, Samumed, Sandoz, Sanofi, Servier, Setpoint, TwoXAR, and UCB.
Compliance with Ethics Guidelines
Ethical review boards approved COAST-X at each site before the trial began. Procedures involving human participants were performed within the ethical standards of the institutional and national research committees at all sites. COAST-X was conducted in accordance with the standards of the Declaration of Helsinki. All patients gave written informed consent before undergoing procedures related to the trials. The master ethics committee was Schulman Associates IRB, Cincinnati, OH, USA; complete listings of sites and investigators are available in the supplements of previously published results from COAST-X [5].
Data Availability
The datasets analyzed in this study are available from the corresponding author upon reasonable request.
References
- 1.Deodhar A, Reveille JD, van den Bosch F, Braun J, Burgos-Vargas R, Caplan L, et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis International Society in response to the US Food and Drug Administration's comments and concerns. Arthritis Rheumatol. 2014;66(10):2649–2656. doi: 10.1002/art.38776. [DOI] [PubMed] [Google Scholar]
- 2.Sieper J, Holbrook T, Black CM, Wood R, Hu X, Kachroo S. Burden of illness associated with non-radiographic axial spondyloarthritis: a multiperspective European cross-sectional observational study. Clin Exp Rheumatol. 2016;34(6):975–983. [PubMed] [Google Scholar]
- 3.Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 4.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 5.Deodhar A, van der Heijde D, Gensler LS, Kim T-H, Maksymowych WP, Østergaard M, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020;395(10217):53–64. doi: 10.1016/S0140-6736(19)32971-X. [DOI] [PubMed] [Google Scholar]
- 6.Boonen A, Sieper J, van der Heijde D, Dougados M, Bukowski JF, Valluri S, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum. 2015;44(5):556–562. doi: 10.1016/j.semarthrit.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Kiltz U, Essers I, Hiligsmann M, Braun J, Maksymowych WP, Taylor WJ, et al. Which aspects of health are most important for patients with spondyloarthritis? A Best Worst Scaling based on the ASAS Health Index. Rheumatology (Oxford) 2016;55(10):1771–1776. doi: 10.1093/rheumatology/kew238. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh N, Ruderman EM. Nonradiographic axial spondyloarthritis: clinical and therapeutic relevance. Arthritis Res Ther. 2017;19(1):286. doi: 10.1186/s13075-017-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American college of rheumatology/spondylitis association of America/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599–1613. doi: 10.1002/art.41042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson PC, Bird P, Lim I, Saad N, Schachna L, Taylor AL, et al. Consensus statement on the investigation and management of non-radiographic axial spondyloarthritis (nr-axSpA) Int J Rheum Dis. 2014;17(5):548–556. doi: 10.1111/1756-185X.12358. [DOI] [PubMed] [Google Scholar]
- 11.Robinson PC, Sengupta R, Siebert S. Non-radiographic axial spondyloarthritis (nr-axSpA): advances in classification, imaging and therapy. Rheumatol Ther. 2019;6(2):165–177. doi: 10.1007/s40744-019-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deodhar A, Poddubnyy D, Pacheco-Teno C, Salvarani C, Lespessailles E, Rahman P, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 2019;71(4):599–611. doi: 10.1002/art.40753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16-week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392(10163):2441–2451. doi: 10.1016/S0140-6736(18)31946-9. [DOI] [PubMed] [Google Scholar]
- 14.Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. (2009) The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 68(Suppl 2):ii1–44. [DOI] [PubMed]
- 15.Mease P, Walsh JA, Baraliakos X, Inman R, de Vlam K, Cheng-Chung Wei J, et al. Translating improvements with ixekizumab in clinical trial outcomes into clinical practice: ASAS40, pain, fatigue, and sleep in ankylosing spondylitis. Rheumatol Ther. 2019;6(3):435–450. doi: 10.1007/s40744-019-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68(10):1520–1527. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 17.Naegeli AN, Flood E, Tucker J, Devlen J, Edson-Heredia E. The patient experience with fatigue and content validity of a measure to assess fatigue severity: qualitative research in patients with ankylosing spondylitis (AS) Health Qual Life Outcomes. 2013;11:192. doi: 10.1186/1477-7525-11-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheffé H. A method for judging all contrasts in the analysis of variance*. Biometrika. 1953;40(1–2):87–110. [Google Scholar]
- 19.van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Rheumatol Ther. 2018;392(10163):2441–2451. doi: 10.1016/S0140-6736(18)31946-9. [DOI] [PubMed] [Google Scholar]
- 20.van Lunteren M, Scharloo M, Ez-Zaitouni Z, de Koning A, Landewé R, Fongen C, et al. The impact of illness perceptions and coping on the association between back pain and health outcomes in patients suspected of having axial spondyloarthritis: data from the SPondyloArthritis caught early cohort. Arthritis Care Res (Hoboken) 2018;70(12):1829–1839. doi: 10.1002/acr.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stebbings SM, Treharne GJ, Jenks K, Highton J. Fatigue in patients with spondyloarthritis associates with disease activity, quality of life and inflammatory bowel symptoms. Clin Rheumatol. 2014;33(10):1467–1474. doi: 10.1007/s10067-013-2445-6. [DOI] [PubMed] [Google Scholar]
- 22.Sieper J, Hu X, Black CM, Grootscholten K, van den Broek RWM, Kachroo S. Systematic review of clinical, humanistic, and economic outcome comparisons between radiographic and non-radiographic axial spondyloarthritis. Semin Arthritis Rheum. 2017;46(6):746–753. doi: 10.1016/j.semarthrit.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol. 2012;8(5):262–268. doi: 10.1038/nrrheum.2012.39. [DOI] [PubMed] [Google Scholar]
- 24.Lockwood MM, Gensler LS. Nonradiographic axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2017;31(6):816–829. doi: 10.1016/j.berh.2018.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are available from the corresponding author upon reasonable request.