Abstract
The transcription factor NF-κB promotes immunity by controlling the expression of genes involved in inflammation. Cytokines and pathogen-associated molecular patterns stimulate cell surface receptors, including toll-like receptors, to initiate a signalling cascade resulting in the activation of NF-κB. NF-κB drives the expression of target genes that mediate cell proliferation and release antimicrobial molecules and cytokines to activate an immune response. Filariasis is one of the most complex infections of humans. The actual causes of the heterogeneity in infection are not well understood. However, they have been attributed to differences in inflammatory processes that are immune-mediated, secondary bacterial infections, and host immune-genetics. Elevated production of angiogenic molecules (VEGFs, CEACAM and MMPs) in filarial pathology has been shown to be dependent on phosphorylation and intracellular activation of NF-κB. This review examines the role of NF-κB in filarial pathology and its potential therapeutic options for individuals with the disease.
Keywords: Lymphatic filariasis, Inflammation, Cytokines, NF-κB
General overview of lymphatic filariasis
Lymphatic filariasis (LF) is a neglected tropical disease (NTD) that has a direct impact on social and public health issues (WHO 2013). LF infections may result in pathologies, such as hydrocele and lymphedema. These pathologies are usually accompanied by chronic pain, hindering affected individuals from being productive and isolating them from family and the community as a whole (Litt et al. 2012). Globally, LF is endemic in 73 countries in Africa, Asia, and the Americas. Approximately 1.1 billion people are exposed to the infection, and more than one-third of these individuals are in sub-Saharan Africa (WHO 2013). About 120 million individuals are affected globally, with approximately 40 million individuals disabled owing to filariasis-related morbidity (WHO 2013).
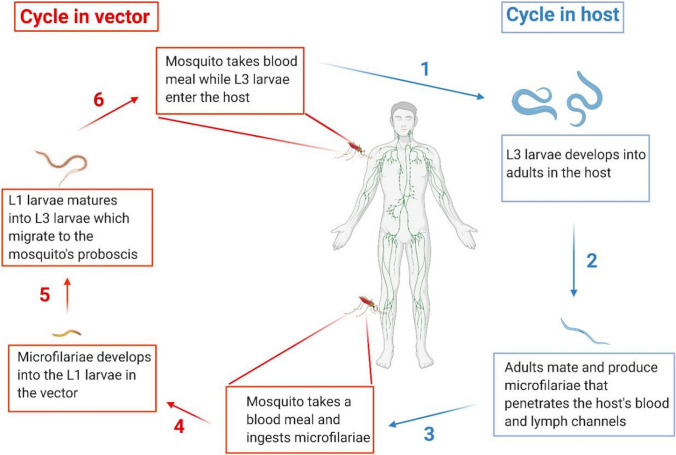
Blood nematodes cause LF infection in the host’s lymphatic vessel. There are three species of these filarial worms: Wuchereria bancrofti, which accounts for almost 90 % of the cases, with the remaining 10 % caused by Brugia malayi and Brugia timori. Adult filarial parasites are located in the lymph vessels and are sexually dimorphic, producing thousands of first-stage larvae (microfilaria) for up to 8 years (Pfarr et al. 2009). Mosquito vectors of the genera Aedes, Anopheles, Culex or Mansonia are required to develop the larvae into the human infective stage and for transmission to the human hosts. The vectors ingest microfilaria during blood meals. In the insect, the larvae develop into infective larvae (L3), which are deposited on the skin of the host during subsequent blood meals. The larvae enter the body through the wound made by the insect and undergo maturation into adult worms, completing the cycle (Fig. 1).
Fig. 1.
Life-cycle of filarial parasites, demonstrated with W. bacncrofti. Both a vector and a mammalian host are required for the development of the nematode. (1) The infected vector transmits the third-stage larvae (L3) into the human host during a blood meal. (2) The L3 mature into adult worms. (3) The parasites develop into adults and then produce microfilariae (MF), which migrate to the lymphatics and blood for circulation. (4) The vector once again ingests the microfilariae during a blood meal on an infected host. (5) The microfilariae develop into the L1 stage. (6) The L1 larvae matures into L3 larvae which migrate to the vector’s proboscis via the hemocel. (1) The infected vector transmits the infective-stage larvae into the human host during blood meal. Created with Biorender.com
Clinically, LF can be classified into two categories; individuals with detectable microfilaraemia but without any overt disease manifestation and individuals with pathological manifestations such as elephantiasis, lymphedema, hydrocele, chyluria, damage to the kidneys and other organs (Pfarr et al. 2009). LF pathologies (hydrocele, lymphedema, and elephantiasis) are usually linked to acute episodes such as adenolymphangitis and filarial fever depending on the stage of the disease (Litt et al. 2012; Pfarr et al. 2009). In vitro studies have shown that individuals with LF pathologies have relatively higher immune-reactivity to parasite antigen, while asymptomatic microfilaraemic individuals manifest immunological hypo-responsiveness to the filarial antigen (Mahanty et al. 1994; Maizels et al. 1995; McSorley and Maizels 2012). The prevalence of overt clinical manifestations among adult residents in many endemic areas is approximately 7% for lymphedema and 30–50% for hydrocele (Maizels et al. 1995). The high prevalence rates of hydrocele indicate that the disease’s pathological manifestations disproportionately affect men (McSorley and Maizels 2012).
The Global Programme to Eliminate Lymphatic Filariasis (GPELF) was initiated to address LF with two main goals. The first was to use annual mass drug administration (MDA) of two-drug combinations given to communities at risk of infection. In most endemic countries, a combination of diethylcarbamazine (DEC) and albendazole is recommended. On the contrary, due to the possibilities of serious adverse reactions seen in communities exposed to Onchocerca volvulus in Africa, a combination of ivermectin and albendazole is used in countries which are endemic with LF and onchocerciasis (Simonsen et al. 2010; WHO 2013). The second goal was to manage morbidity and prevent disability by providing supportive care.
Filariasis is one of the most complex infections of humans, the actual causes of the heterogeneity in infection are not well understood. Nonetheless, these have been attributed to differences in inflammatory processes that are immune-mediated, secondary bacterial infections, and host immune-genetics (Partono 1987; King and Nutman 1991; Wammes et al. 2012). NF-κB is a transcription factor implicated in inflammatory processes during disease conditions such as cancer, asthma, and diabetes (Liu et al. 2017; Hayden and Ghosh 2008). Improved angiogenic molecule production in filarial pathology is dependent on phosphorylation and intracellular activation of nuclear factor kappa B (NF-κB) (Dreyer et al. 2000; Babu et al. 2003; Babu et al. 2011; Babu et al. 2012). In this review, we discuss the role of NF-κB in filarial pathology and its potential therapeutic options.
Current treatment and preventive strategies of LF
The current treatment for LF infection is to interrupt, terminate or counter the development of pathology by using diethylcarbamazine (DEC) and albendazole or ivermectin and albendazole in African countries co-endemic with onchocerciasis (Carlingford et al. 2019; Simonsen et al. 2010; WHO 2013). Currently, morbidity management for lymphedema by the World Health Organization standard include maintaining hygiene, caring for affected limbs, exercising and lifting the limb above the body when resting.
Apart from the established MDA regimens, other drugs such as tetracyclines (doxycycline) are useful for controlling filarial infections (Debrah et al. 2006, 2009). However, no unique drug is efficient against all symptomatic and asymptomatic manifestations of the disease.
The MDA programs are usually integrated with vector control strategies to control filarial infections. This is competent in interrupting the transmission of LF and other infections transmitted by the vectors. Based on the parasite-vector species, dimensions such as treating nets with insecticide, indoor residual spraying, or personal protection procedures could help protect individuals from infection.
Pathogenesis of lymphatic filariasis
Despite the global filariasis elimination program’s efforts by the World Health Organization (WHO), LF is still serving as the world’s second leading cause of long-term disability (Hotez and Kamath 2009). This could be due to the paucity of knowledge in understanding the pathway of filarial pathogenesis. The onset of filarial pathology comprises multiple origins, including reactions from the adult worm’s life or death (releases filarial antigens, Wolbachia antigens, etc.). Although there are challenges in establishing a straightforward pathway for the onset and progression of lymphedema and hydrocele, the worms’ presence in the lymphatic vessels has been implicated as a fundamental trigger for pathogenesis (Witt and Ottessen 2001; Figueredo-Silva et al. 2002; Taylor et al. 2010). Immuno-histology has reported that no or few reactions are seen around the live adult worms, suggesting subclinical pathologic dilation of the lymph vessels due to live worms (Figueredo-Silva et al. 2002). It has also been reported that elevated inflammatory responses after the adult worm’s death could be a player in the pathogenesis (King and Nutman 1991; McSorley et al. 2012; Wammes et al. 2012). Distinct from the live worms, which are found entirely free in the lumen of the lymphatic vessels, it has been suggested that dead worms sometimes appear connected to the vascular wall by strands of fibrin-like material (Dreyer et al. 2000).
After the release of filarial antigens from dead or dying worms, the phagocytic immune response is believed to trigger the release of pro-inflammatory cytokines and molecules that favor angiogenesis (Babu et al. 2011; Babu et al. 2012). Apart from antigens from the worm itself, antigens of the intracellular Wolbachia also contribute to LF pathological outcomes such as hydrocele and lymphedema development (Brattig et al. 2004). Higher filarial and Wolbachia antigens have been suggested to induce an elevated amount of vascular endothelial growth factors (VEGFs) (Babu et al. 2012). VEGFs and their receptors are linked to the pathogenesis of LF. Doxycycline treatment has been shown to reduce VEGFs levels, leading to improved pathologies in patients (Debrah et al. 2006, 2007). Therefore, the role of lymphangiogenesis-promoting factors is an area gaining recognition in the research of LF. Studies have shown that the intracellular transcriptional factor, NF-κB, regulates the release of VEGFs (Kiriakidis et al. 2003; Babu et al. 2012). Single nucleotide polymorphisms in the vascular endothelial growth factor receptor 3 (VEGFR-3), matrix metalloprotease 2 (MMP-2), and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1) are reported to influence filarial pathology development (Debrah et al. 2007, 2017; Gu et al. 2009).
CEACAM-1, a type 1 transmembrane protein that regulates cell-to-cell adhesion, is an effective activator of VEGFs-mediated angiogenesis (Oliveira-Ferrer et al. 2004; Ergun et al. 2000). It also stimulates microvascular endothelial cell growth in the presence of VEGFs (Gu et al. 2009). Experiments have demonstrated that the inactivation of NF-κB by specific inhibitory peptide blocks the expression of CEACAM-1 (Muenzner et al. 2002). Hence, the down-regulation of CEACAM-1 could affect the activation and production of VEGFs.
Furthermore, MMP2, which is also known to have angiogenic properties, plays a crucial role in the breakdown of extracellular matrix in physiological processes such as embryonic development, reproduction, and tissue remodeling (Shigemori et al. 2006; Kelly et al. 2006; Yang et al. 2007). It has been hypothesized that a higher amount of MMP-2 alters and remodels the extracellular matrix around the vessels that contribute to edema development, and could disrupt tight junctions (Shigemori et al. 2006; Yang et al. 2007). A similar phenomenon is seen in lymphedema development in which the lymph vessels dilate, reducing lymph flow (Benuru et al. 2009). Although earlier studies have shown that doxycycline improves LF pathology despite active filarial infection, the mechanism of action remains elucidated. In another study, the effect of doxycycline treatment was attributed to reduction of MMP-2 and thus explained the amelioration observed among individuals presenting with LF pathology, even though most of these patients do not have active infections (Debrah et al. 2006; Alexander-Savino et al. 2016). Studies have recently shown that doxycycline acts as an NF-κB inhibitor in CTCL cell lines (Alexander-Savino et al. 2016). These associations indicate that angiogenic/lymphangiogenic pathways are important in LF pathology, further confirming the contribution of NF-κB in filarial pathology.
Structural composition and pathways of NF-κB
NF-κB is ubiquitously expressed and plays a fundamental role in regulating the expression of many genes involved in immune, inflammatory, and apoptotic processes (Hayden et al. 2006; Hayden and Ghosh 2008). The NF-κB/Rel proteins include five proteins; p50, p52, p65 or RelA, RelB, and c-Rel, and they can exist as homo- and heterodimers (Ghosh et al. 1998). Studies in knockout T. muris-infected mice have shown that there are differential requirements of the NF-κB family proteins, resulting in the regulation of Th2 cytokine-mediated responses to helminth infection NF-κB1(p50/p105), inducing critical protective Th2 cytokine responses (Artis et al. 2002). NF-κB is mainly sequestered in the cytoplasm by its association with proteins belonging to the IκB inhibitor family (Karin and Ben-Neriah 2000).
During an infection, toll-like receptors (TLRs) trigger the rapid phosphorylation of specific serine residues of IκB proteins by a multi-protein complex termed the IKK (inhibitor kappa B kinase) complex, which consists of two catalytic components, IKKα and IKKβ, and a regulatory component, NEMO (NF-κB essential modifier, also known as IKKγ). Phosphorylated IκB proteins are subsequently poly-ubiquitinated and degraded by the 26S proteasome, allowing NF-κB to move into the nucleus to target transcription. This pathway is called the ‘canonical pathway’ and is responsible for TLR-mediated induction of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-6 (Artis et al. 1999). Studies have shown that TNF-alpha plays a mediatory role in acute filariasis (Das et al. 1996; Artis et al. 1999). Furthermore, the TNFR-II gene is documented to predispose some individuals in filarial-endemic communities to elephantiasis (Panda et al. 2011).
TNF-TNFR is a potent activator of NF-κB in T. muris infection (Artis et al. 2002; Das et al. 1996), and has been reported in animal models of asthma (Poynter et al. 2003). Genome study has also revealed that IKKβ, rather than IKKα, plays an essential role in the activation of NF-κB in TLR signalling (Anderson 2000; Hacker and Karin 2006). Alternatively, the non-canonical pathway is triggered by particular members of the TNFR superfamily (O’Neil 1998).
Toll‐like receptor/NF-κB axis in filarial pathology
During an infection, especially in LF, the first line of defense mounted by the host against the invading filarial/Wolbachia antigen is the innate immunity, followed by the specific immunity. Toll-like receptors (TLRs), which are pathogen recognition receptors (PRRs), acts as primary sensors that detect a wide variety of microbial components and elicit innate immune responses (Kawai et al. 2007). All TLRs signalling pathways culminate in activation of the transcription factor nuclear factor-kappa B (NF-κB). Upon activation, TLRs most likely form homodimers, resulting in a conformational change in the cytoplasmic Toll interleukin receptor (TIR) domain and subsequent recruitment of an adapter named MyD88 (Hise et al. 2007; Semnani et al. 2004; Babu et al. 2012). MyD88 associates with the TLR via a homophilic interaction using the TIR domains. The death domain of MyD88 then recruits downstream IL-1 receptor–associated kinase (IRAK) to the receptor complex (Semnani et al. 2004). IRAK is then auto-phosphorylated and dissociated from the receptor complex and recruits TNF receptor–associated factor 6 (TRAF6) which activates downstream kinases. Several such kinases have been found to be involved in TLR/NF-κB signalling pathways, including NF-κB–inducing kinase (NIK) and mitogen-activated protein kinase/ERK kinase 1 (MEKK1) (Zhang and Ghosh 2001).
The intracellular nature of NF-κB requires an upstream activation via cytokines and pathogen-associated molecular patterns (PAMPs), stimulating cell surface receptors, including toll-like receptors, to initiate a signalling cascade. Toll-like receptors play a vital role in innate immunity and have been implicated in filarial infection. Studies with animal models of filarial infection and in vitro studies of humans have suggested that TLR-mediated responses in filarial infections are key inducers of pro-inflammatory cytokines, which contribute either directly or indirectly to the development of lymphatic pathology (Babu et al. 2012; Mukherjee et al. 2016). Moreover, this inflammatory response to Wolbachia has been shown to be mediated primarily through TLR2, TLR4, and TLR6.
Studies have shown that lysophosphatidic acid (LPA) upregulates expression levels of TLR4, thus promoting NF-κB activation and nuclear translocation for target genes (Plastira et al. 2020; Yang et al. 2016). Furthermore, LPA could also promote the secretion of the inflammatory factor TNF-a which reduces significantly when the TLR4 signal transduction pathway is blocked by the TLR4 antibody (Yang et al. 2016). This suggests that increased LPA levels in LF pathology individuals elevate TLR4 expression and consequently increase NF-κB activation.
In addition, with a mouse model of onchocerciasis, it was demonstrated that Wolbachia interaction with the host innate immune system resulted in inflammatory keratitis, a characteristic feature of human onchocercal eye disease (Saint Andre et al. 2002).
Downregulation of TLR on antigen-presenting cells and T cells has been shown to be a possible mechanism by which deleterious pathology in clinically asymptomatic filarial infections can be circumvented (Takeda and Akira 2004). Babu et al.. have demonstrated that pro-inflammatory cytokines are indeed upregulated in individuals presenting with filarial lymphatic pathology compared with infected or endemic normals in response to specific TLR ligands (Babu et al. 2009). Furthermore, TLR2-mediated enhancement of angiogenic growth factor production in individuals with pathology has been shown to be dependent on NF-κB (Tabruyn and Griffioen 2008; Mukherjee et al. 2016). Regulating the activity of NF-κB is key to controlling several inflammatory-related diseases such as filarial pathologies.
Mechanism of NF-κB in filarial pathology
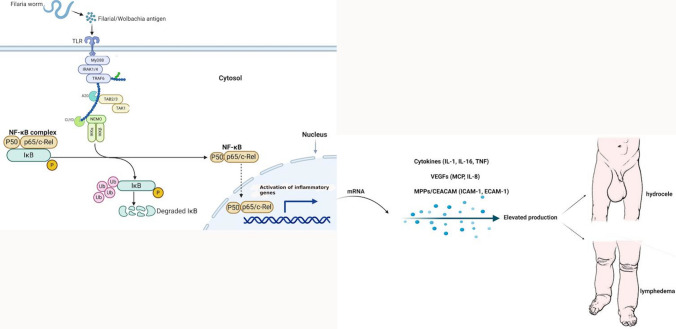
The mechanistic insights on the regulation of immune cell functions in filarial patients that shift host immunity from ‘protection’ to ‘pathology’ are still unclear. Figure 2 illustrates the current understanding of the involvement of the NF-κB pathway in filarial pathology. Recent studies have demonstrated that filarial sheath antigen (FSAg) of W. bancrofti acts as a ligand for TLR4 on macrophages and promotes pro-inflammatory responses activating TLR4-NF-κB signalling cascade (Mukherjee et al. 2017; Mukherjee et al. 2019). Transcription of pro-inflammatory cytokines is primarily governed by NF-кB (Babu and Nutman 2003; Liu et al. 2017). Human studies suggest that Th2 cytokines dominate the immune responses seen in chronic, longstanding helminth infections (Babu et al. 2006; Babu et al. 2009). In filarial infection, Th2 cells produce both anti-and pro-inflammatory cytokines that together instruct B cells to proliferate and differentiate into antibody-secreting plasma cells and activate the NF-κB pathway via TLRs (Zhang and Ghosh 2001; Babu and Nutman 2003; Mukherjee et al. 2016). TLR2 has been shown to be significantly activated in individuals with chronic filarial pathology (Babu et al. 2012). For some time with chronic infections, the feedbacks mentioned above are augmented by adaptive and regulatory T cells, preferably activated macrophages (McSorley and Maizels 2012). However, inflammatory damage in LF results from numerous factors, with endogenous parasite products, Wolbachia, and host immune responses actively performing vital roles. The biomolecules of Wolbachia were proposed to be principal activators of pro-inflammatory cytokines (Saint Andre et al. 2002; Brattig et al. 2004). Studies have shown that microfilarial protein (MfP) acts as a novel ligand of TLR4 to drive NF-κB activation to induce macrophage inflammation, resulting in the recruitment of several downstream signalling molecules such as MyD88 and pTAK1 (Mukherjee et al. 2017). Existing data also suggest that the release of pro-inflammatory cytokines from T cells of individuals encountering L3 larvae for the first time (or perhaps even subsequent times) could mediate the clinical spectrum of acute filariasis (Babu and Nutman 2003).
Fig. 2.
Role of NF-κB in lymphatic filariasis pathology. Toll-like signalling and cytokine receptors signalling by filaria antigens results in phosphorylation of IKK and activation of the IKK complex kinase activity. NF-κB is constitutively bound to IκB molecules which confine its localization to the cytosol. IKK starts phosphorylation of serine residues on IκB and promotes its polyubiquitination and degradation, thereby freeing NF-κB to enter the nucleus and activate transcription of target genes. Inflammation triggers expression of pro-inflammatory genes through IKK and NF-κB activation. Created with Biorender.com
Under normal conditions, NF-κB is rapidly activated upon microbial invasion, and this activation usually correlates with the resistance of the host to infection (Zhang and Ghosh 2001). However, persistent activation of NF-κB may lead to excessive amounts of pro-inflammatory mediators, resulting in tissue damage, organ failure, and even death of the host, as seen in bacterial infection-induced septic shock. It is interesting to note that in order to survive in the host, certain pathogens, such as Bordetella and Yersinia, have evolved mechanisms to counteract or escape the host immune system by inhibiting NF-κB activation (Yuk et al. 2000; Ruckdeschel et al. 1998). Some viruses, including HIV-1, CMV, and SV-40, have established escape mechanisms involving the NF-κB (Zhang and Ghosh 2001). Given the complexity and diversity of LF and the possibilities that each stage could elicit a specific response that could play a crucial role in NF-κB activation, the need for further studies to clarify the role of each of the stage of the filarial parasite in the NF-κB pathway activation is imperative.
Could the NF-κB pathway be explored for LF pathology therapy?
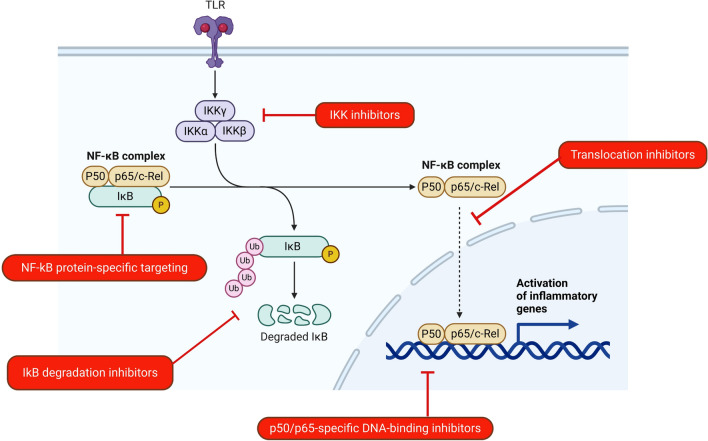
Targeting the NF-κB signalling pathway could represent an attractive approach toward advancement in therapy for individuals with LF pathology. The NF-κB signalling pathway has offered a promising therapeutic avenue for cancer management, as extensively discussed elsewhere (Pramanik et al. 2018; Durand and Balwin 2017; Yu et al. 2017). In view of this, we speculate that the NF-κB signalling pathway could be targeted as a therapeutic option for inflammatory pathologies resulting from filarial infections. However, specific targeting would have to be considered for this approach. The possible targets in the NF-kB signalling pathway to manage inflammatory pathologies in filarial patients are illustrated in Fig. 3. Several categories of inhibitors have been developed to block different NF-κB signalling steps, including IKK inhibitors such as aspirin and salicylate (Hacker and Karim 2006). Tetracyclines (doxycycline) have been shown to have anti-inflammatory and inhibitory properties towards NF-κB activation.Thus, its treatment in LF pathology results in improved conditions such as reduced filarial pains and attacks and reduced mossy lesions observed in human field trials in Ghana (Alexander-Savino et al. 2016; Debrah et al. 2006, 2009).
Fig. 3.
Potential targets in the NF-κB pathway. NF-κB pathway is implicated in filarial pathologies, thus the inhibition of particular proteins/processes in the pathway offer a potential avenue for reducing the inflammation associated. Created by Biorender.com
Additionally, the p50 ubiquitination pathway can be selectively targeted to control harmful inflammatory diseases (Carmody et al. 2007). Further, in exploring LF therapies, the microfilaria protein (Mfp) represents a potential target, considering its role in NF-κB activation (Mukherjee et al. 2017). In the future, Mfp could be a practical choice for the pre-stimulation of host macrophages for re-establishing Th1-biased immunity to challenge an existing or upcoming infection. Similarly, targeting the Mfp may reduce inflammatory damages in patients with chronic filarial infection.
Conclusions
Unlike those with active states of filarial infection, individuals living with pathologies experience unabated excruciating pains resulting from uncontrolled filarial attacks. These attacks drive the pathology through the NF-κB-mediated release of inflammatory factors such as VEGFs, CEACAM-1, and MMP-2. Thus, understanding the mechanisms underlying the pathological activation of NF-κB in individuals presenting with filarial pathologies is critical. Therefore, identifying new or adapting existing therapeutic agents to complement existing strategies could provide relief for LF pathology patients in filarial-endemic communities.
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflict of interest to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alexander-Savino CV, Hayden MS, Richardson C, Zhao J, Poligone B. Doxycycline is an NF-κB inhibitor that induces apoptotic cell death in malignant T-cells. Oncotarget. 2016;7(46):75954–75967. doi: 10.18632/oncotarget.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KV. Toll signalling pathways in the innate immune response. Curr Opin Immunol. 2000;12(1):13–19. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- Artis D, Humphreys NE, Bancroft AJ, Rothwell NJ, Potten CS, Grencis RK. Tumor necrosis factor α Is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during Helminth Infection. J Exp Med. 1999;190(7):953–962. doi: 10.1084/jem.190.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Shapira S, Mason N, Speirs KM, Goldschmidt M, Caamaño J, Liou H-C, Hunter CA, Scott P. Differential requirement for NF-κB family members in control of Helminth infection and intestinal inflammation. J Immunol. 2002;169(8):4481–4487. doi: 10.4049/jimmunol.169.8.4481. [DOI] [PubMed] [Google Scholar]
- Babu S, Nutman TB. Proinflammatory cytokines dominate the early immune response to filarial parasites. J Immunol. 2003;171(12):6723–6732. doi: 10.4049/jimmunol.171.12.6723. [DOI] [PubMed] [Google Scholar]
- Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176(5):3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- Babu S, Bhat SQ, Pavan Kumar N, Lipira AB, Kumar S, Karthik C, Kumaraswami V, Nutman TB. Filarial lymphedema is characterized by antigen-specific Th1 and th17 proinflammatory responses and a lack of regulatory T cells. PLoS Neglect Trop Dis. 2009;3(4):e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu S, Anuradha R, Kumar NP, George PJ, Kumaraswami V, Nutman TB. Filarial lymphatic pathology reflects augmented toll-like receptor-mediated, mitogen-activated protein kinase-mediated proinflammatory cytokine production. Infect Immun. 2011;79(11):4600–4608. doi: 10.1128/IAI.05419-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu S, Anuradha R, Kumar NP, George PJ, Kumaraswami V, Nutman TB. Toll-like receptor- and filarial antigen-mediated, mitogen-activated protein kinase- and NF-κB-dependent regulation of angiogenic growth factors in filarial lymphatic pathology. Infect Immun. 2012;80(7):2509–2518. doi: 10.1128/IAI.06179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennuru S, Nutman TB. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLOS Pathogens. 2009;5(12):1–12. doi: 10.1371/journal.ppat.1000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattig NW, Bazzocchi C, Kirschning CJ, Reiling N, Büttner DW, Ceciliani F, Geisinger F, Hochrein H, Ernst M, Wagner H, Bandi C, Hoerauf A. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J Immunol. 2004;173(1):437–445. doi: 10.4049/jimmunol.173.1.437. [DOI] [PubMed] [Google Scholar]
- Carlingford CN, Melrose W, Mokoia G, Graves PM, Ichimori K, Capuano C, Kim SH, Aratchige P, Nosa M. Elimination of lymphatic filariasis as a public health problem in Niue under PacELF, 1999–2016. Trop Med Health. 2019;47:20. doi: 10.1186/s41182-019-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signalling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317(5838):675–678. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- Das BK, Sahoo PK, Ravindran B. A role for tumour necrosis factor-α in acute lymphatic filariasis. Parasite Immunol. 1996;18(8):421–424. doi: 10.1046/j.1365-3024.1996.d01-126.x. [DOI] [PubMed] [Google Scholar]
- Debrah AY, Mand S, Specht S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Larbi J, Lawson B, Taylor M, Adjei O, Hoerauf A. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006;2(9):e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrah AY, Mand S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Buttner M, Adjei O, Buttner D, Hoerauf A. Macrofilaricidal effect of 4 weeks of treatment with doxycycline on Wuchereria bancrofti. Trop Med Intl Health. 2007;12:1433–1441. doi: 10.1111/j.1365-3156.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- Debrah AY, Mand S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Lawson B, Taylor M, Adjei O, Hoerauf A. Reduction in levels of plasma vascular endothelial growth factor-A and improvement in hydrocele patients by targeting endosymbiotic Wolbachia sp. in Wuchereria bancrofti with doxycycline. Am J Trop Med Hyg. 2009;80(6):956–963. doi: 10.4269/ajtmh.2009.80.956. [DOI] [PubMed] [Google Scholar]
- Debrah LB, Albers A, Debrah AY, Brockschmidt FF, Becker T, Herold C, Hofmann A, Osei-Mensah J, Mubarik Y, Fröhlich H, Hoerauf A, Pfarr K. Single nucleotide polymorphisms in the angiogenic and lymphangiogenic pathways are associated with lymphedema caused by Wuchereria bancrofti. Hum Genomics. 2017;11(1):26. doi: 10.1186/s40246-017-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deribe K, Meribo K, Gebre T, Hailu A, Ali A, Aseffa A, Davey G. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasites Vectors. 2012;5(1):240. doi: 10.1186/1756-3305-5-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G, Norões J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000;16(12):544–548. doi: 10.1016/s0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- Durand JK, Baldwin AS. Targeting IKK and NF-κB for therapy. Adv Protein Chem Struct Biol. 2017;107:77–115. doi: 10.1016/bs.apcsb.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Ergün S, Kilic N, Ziegeler G, Hansen A, Nollau P, Götze J, Wurmbach J-H, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell Adhesion molecule 1. Mol Cell. 2000;5(2):311–320. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- Figueredo-Silva J, Norões J, Cedenho A, Dreyer G. The histopathology of bancroftian filariasis revisited: the role of the adult worm in the lymphatic-vessel disease. Ann Trop Med Parasitol. 2002;96(6):531–541. doi: 10.1179/000349802125001348. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gu A, Tsark W, Holmes KV, Shively JE. Role of Ceacam1 in VEGF induced vasculogenesis of murine embryonic stem cell-derived embryoid bodies in 3D culture. Exp Cell Res. 2009;315(10):1668–1682. doi: 10.1016/j.yexcr.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Programme to Eliminate Lymphatic Filariasis: Progress Report (2016) (2017). Releve epidemiologique hebdomadaire, 92(40):594–607 [PubMed]
- Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Science’s STKE Signal Trans Knowl Environ. 2006;2006(357):re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signalling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25(51):6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- Hise AG, Daehnel K, Gillette-Ferguson I, Cho E, McGarry HF, Taylor MJ, Golenbock DT, Fitzgerald KA, Kazura JW, Pearlman E. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J Immunol. 2007;178(2):1068–1076. doi: 10.4049/jimmunol.178.2.1068. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Neglect Trop Dis. 2009;3(8):e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signalling to NF-κB by toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp Neurol. 2006;200(1):38–49. doi: 10.1016/j.expneurol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- King CL, Nutman TB. Regulation of the immune response in lymphatic filariasis and onchocerciasis. Immunol Today. 1991;12(3):A54–A58. doi: 10.1016/S0167-5699(05)80016-7. [DOI] [PubMed] [Google Scholar]
- Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-κB-dependent: studies using adenoviruses expressing the endogenous NF-κB inhibitor IκBα and a kinase-defective form of the IκB kinase 2. J Cell Sci. 2003;116(4):665–674. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- Litt E, Baker MC, Molyneux D. Neglected tropical diseases and mental health: a perspective on comorbidity. Trends Parasitol. 2012;28(5):195–201. doi: 10.1016/j.pt.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun S-C. NF-κB signalling in inflammation. Signal Trans Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S, Day KP, Alpers MP, Kazura JW. Antifilarial IgG4 antibodies in children from filaria-endemic areas correlate with duration of infection and are dissociated from antifilarial IgE antibodies. J Infect Dis. 1994;170(5):1339–1343. doi: 10.1093/infdis/170.5.1339. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Sartono E, Kurniawan A, Partono F, Selkirk ME, Yazdanbakhsh M. T-cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today. 1995;11(2):50–56. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19(2):80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25(4):585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzner P, Billker O, Meyer TF, Naumann M. Nuclear factor-kappa B directs carcinoembryonic antigen-related cellular adhesion molecule 1 receptor expression in Neisseria gonorrhoeae-infected epithelial cells. J Biol Chem. 2002;277(9):7438–7446. doi: 10.1074/jbc.M108135200. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Karmakar S, Babu SPS. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. 2016;20(2):193–204. doi: 10.1016/j.bjid.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Mukherjee S, Maiti TK, Bhattacharya S, Sinha Babu SP. A novel ligand of toll-like receptor 4 from the sheath of Wuchereria bancrofti microfilaria induces proinflammatory response in macrophages. J Infect Dis. 2017;215(6):954–965. doi: 10.1093/infdis/jix067. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Karnam A, Das M, Babu SPS, Bayry J. Wuchereria bancrofti filaria activates human dendritic cells and polarizes T helper 1 and regulatory T cells via toll-like receptor 4. Commun Biol. 2019;2(1):169. doi: 10.1038/s42003-019-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofanoa R, Ofa T, Padmasiri EA, Kapa DR. Elimination of lymphatic filariasis as a public health problem from Tonga. Trop Med Health. 2019;47(1):2–3. doi: 10.1186/s41182-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, Kilic E, Huland H, Friedrich M, Ergün S. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Can Res. 2004;64(24):8932–8938. doi: 10.1158/0008-5472.CAN-04-0505. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signalling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63(6):650–657. doi: 10.1002/jlb.63.6.650. [DOI] [PubMed] [Google Scholar]
- Panda AK, Sahoo PK, Kerketta AS, Kar SK, Ravindran B, Satapathy AK. Human lymphatic filariasis: genetic polymorphism of endothelin-1 and tumor necrosis factor receptor II correlates with development of chronic disease. J Infect Dis. 2011;204(2):315–322. doi: 10.1093/infdis/jir258. [DOI] [PubMed] [Google Scholar]
- Partono F. The spectrum of disease in lymphatic filariasis. Ciba Found Symp. 1987;127:15–31. doi: 10.1002/9780470513446.ch3. [DOI] [PubMed] [Google Scholar]
- Pfarr KM, Debrah AY, Specht S, Hoerauf A. Filariasis and lymphoedema. Parasite Immunol. 2009;31(11):664–672. doi: 10.1111/j.1365-3024.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastira I, Bernhart E, Joshi L, Koyani CN, Strohmaier H, Reicher H, Malle E, Sattler W. MAPK signaling determineslysophosphatidic acid (LPA)-induced inflammation in microglia. J neuroinflamm. 2020;17(1):127. doi: 10.1186/s12974-020-01809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter ME, Irvin CG, Janssen-Heininger YMW. A prominent role for airway epithelial NF-κB activation in lipopolysaccharide-induced airway Inflammation. J Immunol. 2003;170(12):6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- Pramanik KC, Makena MR, Bhowmick K, Pandey MK. Advancement of NF-κB signalling pathway: a novel target in pancreatic cancer. Int J Mol Sci. 2018 doi: 10.3390/ijms19123890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaram S, Anuradha R, Manokaran G, Bethunaickan R. An overview of lymphatic filariasis lymphedema. Lymphology. 2017;50(4):164–182. [PubMed] [Google Scholar]
- Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Köhler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J Exp Med. 1998;187(7):1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint André A, Blackwell NM, Hall LR, Hoerauf A, Brattig NW, Volkmann L, Taylor MJ, Ford L, Hise AG, Lass JH, Diaconu E, Pearlman E. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295(5561):1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- Semnani RT, Nutman TB. Toward an understanding of the interaction between filarial parasites and host antigen-presenting cells. Immunol Rev. 2004;201:127–138. doi: 10.1111/j.0105-2896.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochirurgica Suppl. 2006;96:130–133. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- Simonsen PE, Pedersen EM, Rwegoshora RT, Malecela MN, Derua YA, Magesa SM. Lymphatic filariasis control in Tanzania: effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission. PLOS Neglect Trop Dis. 2010;4(6):1–10. doi: 10.1371/journal.pntd.0000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabruyn SP, Griffioen AW. NF-kappa B: a new player in angiostatic therapy. Angiogenesis. 2008;11(1):101–106. doi: 10.1007/s10456-008-9094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376(9747):1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- Wammes LJ, Hamid F, Wiria AE, Wibowo H, Sartono E, Maizels RM, Smits HH, Supali T, Yazdanbakhsh M. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLOS Neglect Trop Dis. 2012;6(5):1–9. doi: 10.1371/journal.pntd.0001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt C, Ottesen EA. Lymphatic filariasis: an infection of childhood. Trop Med Int Health. 2001;6(8):582–606. doi: 10.1046/j.1365-3156.2001.00765.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2000) Operational guidelines for rapid mapping of Bancroftian filariasis in Africa (p. Revised during an inter-country workshop held in Ouagadougou, 8–12 March 2000). World Health Organization
- World Health Organization (2013) Lymphatic filariasis (Elephantiasis)---Global. https://www.who.int/health-topics/lymphatic-filariasis#tab=tab_1
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cerebral Blood Flow Metab Off J Int Soc Cerebral Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhou Z, Li X, Niu J. The effect of lysophosphatidic acid on toll-like receptor 4 expression and the nuclear factor-ΚB signaling pathway in THP-1 cells. Mol Cell Biochem. 2016;422(1–2):41–49. doi: 10.1007/s11010-016-2804-0. [DOI] [PubMed] [Google Scholar]
- Yu L, Li L, Medeiros LJ, Young KH. NF-κB signalling pathway and its potential as a target for therapy in lymphoid neoplasms. Blood Rev. 2017;31(2):77–92. doi: 10.1016/j.blre.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk MH, Harvill ET, Cotter PA, Miller JF. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol Microbiol. 2000;35(5):991–1004. doi: 10.1046/j.1365-2958.2000.01785.x. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Investig. 2001;107(1):13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]