Abstract
Introduction
We assessed patient-reported outcomes from a multicenter, randomized, double-blind, placebo-controlled study of repository corticotropin injection (RCI; Acthar® Gel) in patients with persistently active systemic lupus erythematosus (SLE) despite treatment with moderate-dose glucocorticoids.
Methods
The trial enrolled adults with active SLE and moderate-to-severe rash and/or arthritis despite use of stable glucocorticoids (7.5 mg/day to 30 mg/day prednisone equivalent), antimalarials, and nonsteroidal anti-inflammatory drugs for ≥ 4 weeks and/or immunosuppressants for ≥ 8 weeks before screening. Patients were randomly assigned to 80 U of RCI or placebo subcutaneously every other day through week 4, then twice weekly through week 24. Primary analyses evaluated the change from baseline to week 24 in the Lupus Quality of Life (QoL) and Work Productivity and Activity Impairment (WPAI)-Lupus questionnaires. Post hoc analyses stratified results by baseline disease activity (SLE Disease Activity Index-2000 [SLEDAI-2K] < 10 or ≥ 10; Cutaneous Lupus Erythematosus Disease Area and Severity Index [CLASI]-Activity < 11 or ≥ 11; and British Isles Lupus Assessment Group [BILAG]-2004 < 20 or ≥ 20) and by BILAG-based Combined Lupus Assessment (BICLA) response at weeks 20 and 24.
Results
RCI treatment resulted in greater improvement in the LupusQoL pain domain at week 16 and planning domain at week 24 compared with placebo. Post hoc analyses demonstrated greater improvements with RCI in the pain, planning, and fatigue domains than with placebo at multiple time points in patients with higher disease activity by baseline SLEDAI-2K ≥ 10, CLASI-Activity ≥ 11, and BILAG-2004 ≥ 20 and/or in BICLA responders. Compared with placebo, RCI also resulted in greater improvements in percentage work time missed at week 24 in patients with baseline CLASI-Activity < 11 and in percentage impairment while working at week 16 in BICLA responders.
Conclusions
RCI may improve QoL and work productivity in patients who have persistently active SLE despite treatment with standard SLE therapy.
Trial Registration
ClinicalTrials.gov identifier NCT02953821.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-021-00294-z.
Keywords: Acthar Gel, Autoimmune disease, Glucocorticoid, Patient-reported outcomes, Repository corticotropin injection, Systemic lupus erythematosus
Key Summary Points
| Why carry out this study? |
| The objective of our 24-week multicenter, randomized, double-blind, placebo-controlled study was to determine the safety and efficacy of repository corticotropin injection (RCI; Acthar® Gel) in patients with persistency active systemic lupus erythematosus (SLE). |
| The primary results from this study showed that RCI was associated with improvements in the 28 Swollen and Tender Joint Count and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)-Activity scores. Post hoc analyses found a greater proportion of British Isles Lupus Assessment Group-based Combined Lupus Assessment (BICLA) responders for RCI than for placebo and a greater SLE Responder Index-4 response in RCI-treated patients with baseline SLE Disease Activity Index-2000 (SLEDAI-2K) ≥ 10, CLASI-Activity ≥ 11, and British Isles Lupus Assessment Group (BILAG)-2004 ≥ 20 than in patients with lower disease activity. |
| Here we report the patient-reported outcome results from this study and further characterize the effects of RCI on quality of life (QoL) and work productivity as they relate to baseline disease activity. |
| What was learned from this study? |
| Treatment with RCI was associated with greater improvements in the LupusQoL pain, planning, and fatigue domains compared to placebo treatment in patients with baseline SLEDAI-2K ≥ 10, CLASI-Activity ≥ 11, and BILAG-2004 ≥ 20 and/or in BICLA responders. Treatment with RCI was also associated with greater improvements in the Work Productivity and Activity Impairment (WPAI)-Lupus absenteeism (percentage work time missed) and presenteeism (percentage impairment while working) domains compared to placebo treatment in patients with baseline CLASI-Activity < 11 and in BICLA responders, respectively. |
| The improvements in QoL and work productivity observed in this study further support the efficacy of RCI for the treatment of persistently active SLE despite use of moderate-dose glucocorticoids. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14054816
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by widespread inflammation and damage to organs and tissues [1]. The worldwide prevalence of SLE varies geographically, with estimated rates between 3.2 and 517.5 cases per 100,000 individuals [2]. Its unpredictable disease course can have devastating socioeconomic implications for affected individuals and their families [2, 3]. SLE is associated with considerable health care costs and reduced quality of life (QoL) due to work disability and difficulty performing daily tasks [2]. SLE symptoms, manifestations, and comorbidities impose a high disease burden that is not fully captured by disease activity assessments [2, 4, 5]. Thus, collection of patient-reported outcomes (PROs) as part of routine clinical care and clinical trials is important for gaining a more complete understanding of disease progression and evaluating treatment effectiveness [6].
Repository corticotropin injection (RCI; Acthar® Gel) is US Food and Drug Administration approved for use during an exacerbation or as maintenance therapy in select cases of SLE [7]. RCI is a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides that is thought to exhibit steroid-dependent and steroid-independent anti-inflammatory effects by engaging all five melanocortin receptors [8–12]. RCI is effective in the treatment of persistently active SLE that is not responsive to standard-of-care therapies [13–17]. An open-label study [13] and a small pilot study [15] demonstrated significant improvements in PROs after treatment with RCI. These results prompted the evaluation of RCI-dependent effects on QoL in a larger SLE trial.
We conducted a 24-week multicenter, randomized, double-blind, placebo-controlled study in patients with persistently active SLE despite use of moderate-dose glucocorticoids [17]. RCI-treated patients showed improvements from baseline in the 28 Swollen and Tender Joint Count and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)-Activity scores compared with placebo-treated patients. Greater benefits of RCI vs. placebo were noted in post hoc analyses of SLE Responder Index (SRI)-4 responses in patients with higher disease activity. The RCI-treated group had a greater proportion of British Isles Lupus Assessment Group (BILAG)-based Combined Lupus Assessment (BICLA) responders compared with the placebo group. Additionally, we evaluated data from the LupusQoL and Work Productivity and Activity Impairment (WPAI)-Lupus questionnaires in RCI-treated and placebo-treated patients.
The objective of the current publication was to analyze the PRO results from the study and report the post hoc analyses that further characterize the RCI-dependent effects on QoL and work productivity in patients with higher disease activity.
Methods
The methods and procedures for the study have been described in more detail previously [17, 18].
Ethics and Compliance
The study protocol was approved by the local ethics committees and institutional review boards at the individual study sites and by the Western Institutional Review Board centrally. Patients were required to provide written informed consent. The study was conducted in agreement with requirements of registered clinical trials (ClinicalTrials.gov identifier NCT02953821) and with the Declaration of Helsinki.
Patients
The study was conducted at 54 study sites in five countries (supplemental Table S1). Investigators enrolled adults aged ≥ 18 years with active SLE. SLE was defined as meeting ≥ 4 of 11 American College of Rheumatology criteria [19]. Active SLE required an SLE Disease Activity Index-2000 (SLEDAI-2K) [20] score ≥ 6 at screening with a clinical SLEDAI-2K (excluding laboratory results) score ≥ 4 at both screening and randomization and moderate-to-severe rash and/or arthritis on the basis of BILAG-2004 [21] A or B scores in the mucocutaneous or musculoskeletal domains at both screening and randomization. Patients could enroll if they were treated with glucocorticoids for at least 8 weeks prior to screening and were receiving stable doses of 7.5–30 mg of daily prednisone equivalent for at least 4 weeks prior to screening. Additionally, patients were required to be on stable doses of antimalarials or nonsteroidal anti-inflammatory drugs (NSAIDs) for ≥ 4 weeks and/or immunosuppressants for ≥ 8 weeks before screening. Patients with severe active lupus nephritis (serum creatinine > 2.5 mg/dL, proteinuria > 1.5 g/g, or required hemodialysis) or active central nervous system lupus within 3 months before screening were excluded.
Procedures
Patients were randomly assigned (1:1) to receive 80 U RCI or placebo subcutaneously every other day for 4 weeks and then twice per week through week 24. Randomization was stratified by study site location (US or outside US) and glucocorticoid dose (prednisone or equivalent of ≤ 20 mg/day and > 20 mg/day). Glucocorticoid doses remained stable until week 16. If clinically indicated, glucocorticoid taper was permitted between week 16 and week 24 at the discretion of the investigator. Doses of antimalarials, NSAIDs, and immunosuppressants remained stable throughout the study.
Outcomes
The planned exploratory PRO analyses evaluated the change from baseline to week 24 in LupusQoL [22] and WPAI-Lupus [23] domains. The eight domains of the LupusQoL are body image, burden to others, emotional health, fatigue, intimate relationships, pain, physical health, and planning. Patients score their experiences in the 4 weeks prior to the evaluation on a scale of 0–100; lower scores are indicative of poorer health-related QoL. The four domains of the WPAI-Lupus are percentage work time missed due to SLE, percentage impairment while working due to SLE, percentage overall work impairment due to SLE, and percentage activity impairment due to SLE. Each domain scores patients’ self-reported experiences in the 7 days prior on a scale of 0–100%; higher scores are indicative of greater impairment.
In post hoc analyses, LupusQoL and WPAI-Lupus results were evaluated in subgroups stratified by baseline SLEDAI-2K score (< 10 and ≥ 10), baseline CLASI-Activity [24] score (< 11 and ≥ 11), baseline BILAG-2004 score (< 20 and ≥ 20), and BICLA [25] responders and non-responders—BICLA responders had persistent BICLA responses at both week 20 and week 24.
Statistical Analyses
Analyses were performed in the modified intention-to-treat (mITT) population (patients who received at least one dose of study drug and contributed any postbaseline efficacy data). Endpoints were analyzed using analysis of covariance models with the change from baseline as the dependent variable, treatment as the factor, and baseline value of the corresponding endpoint as the covariate and were stratified for location (US and outside the US) and baseline prednisone or equivalent glucocorticoid dose (≤ 20 mg/day and > 20 mg/day). Because the primary endpoint (proportion of patients who achieved an SRI-4 response at week 16) was not significantly different (p < 0.05) between RCI and placebo, all p values presented here are nominal and are for information purposes only.
Results
Demographics and baseline disease characteristics for patients in each treatment group have been previously described [17, 18]. Briefly, the mITT population (N = 169; RCI, n = 84; placebo, n = 85) was predominantly female (91.7%) with a mean age of 39.7 years and a mean baseline SLEDAI-2K score of 9.9. Most were of Hispanic or Latino ethnicity (80.5%) and located outside of the US (66.9%). The mean baseline prednisone or equivalent glucocorticoid dose was 11.1 mg, and most patients (95.3%) were receiving ≤ 20 mg daily. A list of prior SLE medications and the percentage of patients using each drug are provided in supplemental Table S2.
LupusQoL
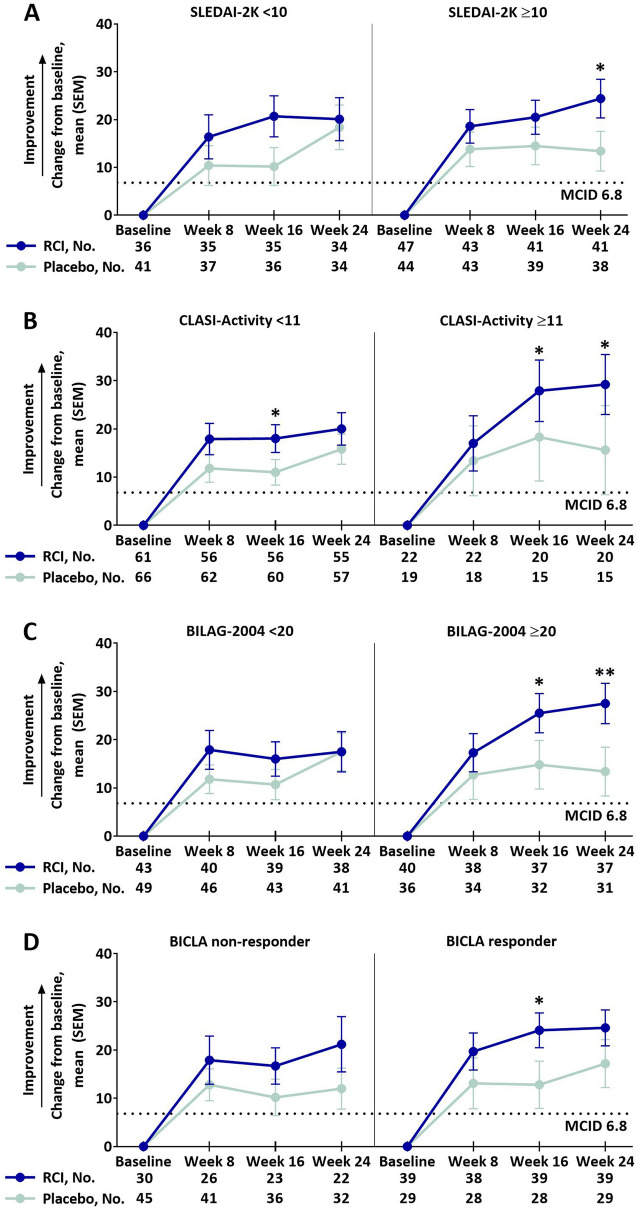
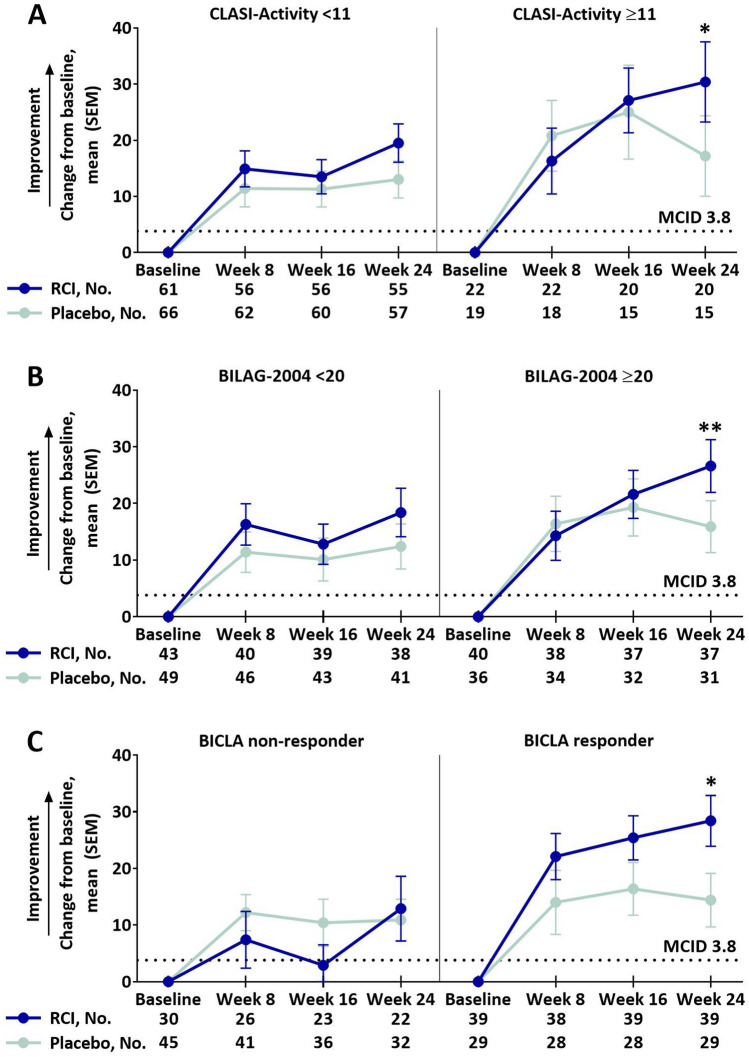
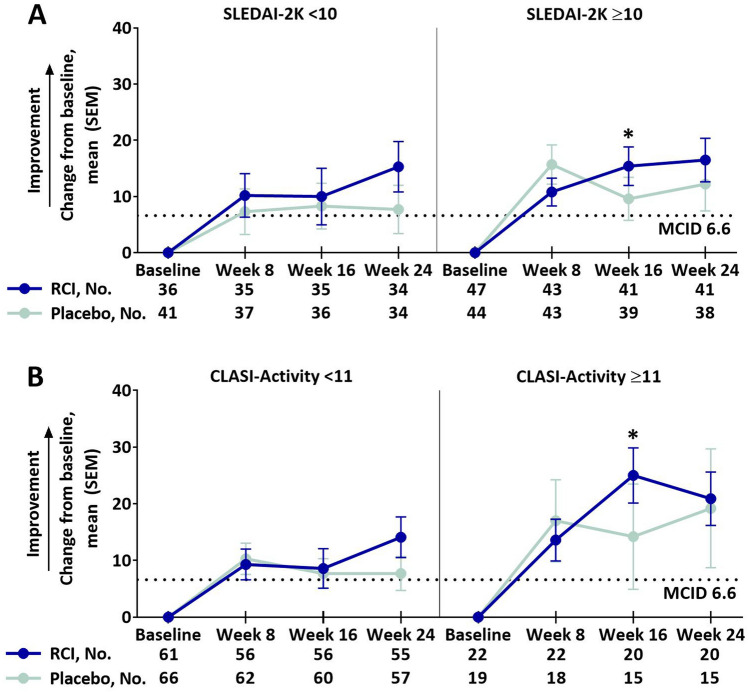
Of the eight LupusQoL domains, RCI treatment resulted in greater improvements from baseline compared to placebo in the pain domain at week 16 and planning domain at week 24 (supplemental Table S3). Post hoc analyses showed that RCI-treated patients had greater improvements from baseline compared with placebo-treated patients in the pain domain at several time points in patients with higher activity scores as follows: at week 24 in the SLEDAI-2K ≥ 10 subgroup (Fig. 1a), at week 16 and week 24 in the CLASI-Activity ≥ 11 (Fig. 1b) and BILAG-2004 ≥ 20 (Fig. 1c) subgroups and at week 16 in BICLA responders (Fig. 1d). Compared with placebo, greater improvements from baseline were also observed for the RCI group in the LupusQoL planning domain at week 24 in the CLASI-Activity ≥ 11, BILAG-2004 ≥ 20, and BICLA responder subgroups (Fig. 2) and in the fatigue domain at week 16 in the SLEDAI-2K ≥ 10 and CLASI-Activity ≥ 11 subgroups (Fig. 3). Differences exceeded the minimal clinically important difference (MCID) at all time points in the planned analysis and at almost all time points in the post hoc analyses [26].
Fig. 1.
Change from baseline in the LupusQoL pain domain by baseline SLEDAI-2K (a), baseline CLASI-Activity (b), baseline BILAG-2004 (c), and BICLA response (d), mITT populationa. aPatients who received ≥ 1 dose of study drug and contributed any postbaseline efficacy data. MCIDs are based on the definitions proposed by McElhone et al. [26]. *p < 0.05 (nominal); **p < 0.01 (nominal) for the LS mean difference using ANCOVA models with the change from baseline as the dependent variable, treatments as the factor, and baseline values of corresponding endpoints as the covariate, with stratification for location (US and outside the US) and baseline prednisone or equivalent glucocorticoid dose (≤ 20 mg/day and > 20 mg/day). ANCOVA analysis of covariance, BICLA British Isles Lupus Assessment Group-based Combined Lupus Assessment, BILAG-2004 British Isles Lupus Assessment Group-2004, CLASI-Activity Cutaneous Lupus Erythematosus Disease Severity Index-Activity, LS least squares, LupusQoL Lupus Quality of Life, MCID minimal clinically important difference, mITT modified intention-to-treat, RCI repository corticotropin injection, SEM standard error of the mean, SLEDAI-2K Systemic Lupus Erythematosus Disease Activity Index-2000
Fig. 2.
Change from baseline in the LupusQoL planning domain by baseline CLASI-activity (a), baseline BILAG-2004 (b), and BICLA response (c), mITT populationa. aPatients who received ≥ 1 dose of study drug and contributed any postbaseline efficacy data. MCIDs are based on the definitions proposed by McElhone et al. [26]. *p < 0.05 (nominal); **p < 0.01 (nominal) for the LS mean difference using ANCOVA models with the change from baseline as the dependent variable, treatments as the factor, and baseline values of corresponding endpoints as the covariate, with stratification for location (US and outside the US) and baseline prednisone or equivalent glucocorticoid dose (≤ 20 mg/day and > 20 mg/day). ANCOVA analysis of covariance, BICLA British Isles Lupus Assessment Group-based Combined Lupus Assessment, BILAG-2004 British Isles Lupus Assessment Group-2004, CLASI-Activity Cutaneous Lupus Erythematosus Disease Severity Index-Activity, LS least squares, LupusQoL Lupus Quality of Life, MCID minimal clinically important difference, mITT modified intention-to-treat, RCI repository corticotropin injection, SEM standard error of the mean
Fig. 3.
Change from baseline in the LupusQoL fatigue domain by baseline SLEDAI-2K (a) and baseline CLASI-activity (b), mITT populationa. aPatients who received ≥ 1 dose of study drug and contributed any postbaseline efficacy data. MCIDs are based on the definitions proposed by McElhone et al. [26]. *p < 0.05 (nominal) for the LS mean difference using ANCOVA models with the change from baseline as the dependent variable, treatments as the factor, and baseline values of corresponding endpoints as the covariate, with stratification for location (US and outside the US) and baseline prednisone or equivalent glucocorticoid dose (≤ 20 mg/day and > 20 mg/day). ANCOVA analysis of covariance, CLASI-Activity Cutaneous Lupus Erythematosus Disease Severity Index-Activity, LS least squares, LupusQoL Lupus Quality of Life, MCID minimal clinically important difference, mITT modified intention-to-treat, RCI repository corticotropin injection, SEM standard error of the mean, SLEDAI-2K Systemic Lupus Erythematosus Disease Activity Index-2000
WPAI-Lupus
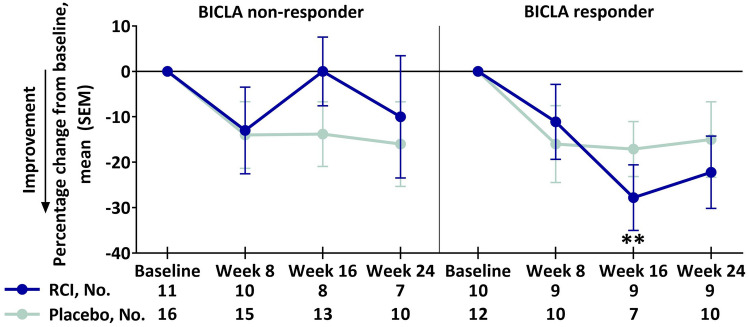
The planned analysis and the post hoc analyses of baseline SLEDAI-2K or BILAG-2004 subgroup scores showed no differences between the RCI and placebo groups for any four WPAI-Lupus domains in the change from baseline through week 24 (data not shown). However, compared with placebo, there were greater improvements in percentage work time missed at week 24 after RCI therapy in patients with baseline CLASI-Activity < 11 (RCI mean change, − 8.4 [SD, 25.1]; placebo mean change, 7.8 [SD, 21.9]; p = 0.0182 for least squares mean difference). Also compared with placebo, RCI therapy led to greater improvements from baseline in percentage impairment while working at week 16 in BICLA responders (Fig. 4).
Fig. 4.
Percentage change from baseline in the WPAI-Lupus percentage impairment while working domain by BICLA response, mITT populationa. aPatients who received ≥ 1 dose of study drug and contributed any postbaseline efficacy data. **p < 0.01 (nominal) for the LS mean difference using ANCOVA models with the change from baseline as the dependent variable, treatments as the factor, and baseline values of corresponding endpoints as the covariate, with stratification for location (US and outside the US) and baseline prednisone or equivalent glucocorticoid dose (≤ 20 mg/day and > 20 mg/day). ANCOVA analysis of covariance, BICLA British Isles Lupus Assessment Group-based Combined Lupus Assessment, LS least squares, mITT modified intention-to-treat, RCI repository corticotropin injection, SEM standard error of the mean, WPAI-Lupus Working Productivity and Activity Impairment-Lupus
Discussion
In this 24-week randomized, double-blind, placebo-controlled study, RCI treatment for persistently active SLE despite treatment with moderate-dose glucocorticoids resulted in improvements in validated SLE-specific PRO measures. Our study showed greater improvements from baseline, over the MCID thresholds, in LupusQoL scores for the pain, planning, and fatigue domains in patients treated with RCI who had higher disease activity. The pain domain assessed how often SLE pain interfered with daily activities, sleep quality, and mobility; planning assessed how often SLE interfered with the ability to plan for future events, organize life efficiently, and commit to social engagements; and fatigue assessed how often SLE prevented long periods of concentration, caused feelings of sluggishness or exhaustion, or contributed to the need for an early bedtime [22]. These data suggest that the effects of RCI on QoL measures may be more pronounced in patients with higher disease activity at baseline (measured by SLEDAI-2K, CLASI-Activity, and BILAG-2004 scores) and in BICLA responders. Our study also showed greater improvements from baseline in the absenteeism (percentage work time missed) and presenteeism (percentage impairment while working) WPAI-Lupus domains for RCI-treated patients compared to placebo-treated patients. As seen for the LupusQoL, differences from placebo were more apparent in patients with baseline CLASI-Activity < 11 and in BICLA responders. For patients with higher disease activity, treatment with RCI may reduce the amount of work time missed due to SLE and may increase the amount or type of work that patients with SLE are able to complete.
Few randomized clinical trials for SLE have reported LupusQoL or WPAI-Lupus results [27, 28]. This is the first randomized study to report LupusQoL or WPAI-Lupus results after treatment with RCI. A previous open-label study of RCI showed significant improvements from baseline to day 28 in a 16-item adapted QoL scale and from baseline to day 14 and day 28 in the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale [13]. A randomized, placebo-controlled pilot study showed greater improvements in the Medical Outcomes Survey Short Form (SF)-36 for RCI-treated patients than for those receiving placebo after 4 weeks of treatment [15]. LupusQoL and SF-36 data are comparable in evaluating QoL improvements for patients with SLE [29]. However, LupusQoL is an SLE disease-specific measure of QoL. Similar to our study, both the open-label study and the pilot study included patients who had persistently active SLE despite receiving standard-of-care treatments [13, 15]. These data suggest that RCI may improve SLE-related QoL and impairments beyond the improvements provided by background glucocorticoid and other standard-of-care treatments. Notably, previous studies have demonstrated that RCI induces an endogenous cortisol response that is comparable to low-to-moderate daily glucocorticoid doses [30]; thus, the use of RCI instead of increasing glucocorticoid doses in persistently active disease may be advantageous for reducing steroid exposure while still providing clinical benefit. Additionally, these data suggest that RCI may have greater benefit in patients with higher disease activity.
Limitations of the primary study have been described previously [17, 18]. As a post hoc analysis, this study is inherently subject to subgroup selection bias. However, baseline disease measurements in the primary study were balanced across RCI and placebo groups [17]. Still, these post hoc results should be interpreted with caution and warrant investigation in a randomized clinical trial in which disease activity subgroups have been determined a priori.
Conclusions
RCI treatment was associated with improvements in LupusQoL pain, planning, and fatigue domains and in the WPAI-Lupus percentage work time missed and percentage impairment while working domains compared with placebo. These results suggest that RCI may improve QoL and work productivity in patients with persistently active SLE despite treatment with moderate-dose glucocorticoids, particularly in those with higher disease activity.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the participants of this study.
Funding
Sponsorship for this study and the Rapid Service Fee were funded by Mallinckrodt Pharmaceuticals (Hampton, NJ, USA).
Medical Writing, Editorial, and Other Assistance
Professional writing and editorial support were provided by Amber Watson, PharmD, of MedLogix Communications, LLC, Itasca, Illinois, under the direction of the authors and was funded by Mallinckrodt Pharmaceuticals.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
This manuscript is based on work previously presented at the Congress of Clinical Rheumatology-West Virtual Congress (October 8–11, 2020) and at the American College of Rheumatology Convergence Virtual Congress (November 5–9, 2020).
Disclosures
Anca D. Askanase and Richard A. Furie have received grant/research support from Mallinckrodt Pharmaceuticals. George J. Wan, Enxu Zhao, and Roman Bilyk are current employees of Mallinckrodt Pharmaceuticals. Mary P. Panaccio is a paid consultant of Mallinckrodt Pharmaceuticals. Julie Zhu is a former employee of Mallinckrodt Pharmaceuticals and is a current employee of Galderma. Enxu Zhao and Julie Zhu are current shareholders of Mallinckrodt Pharmaceuticals. Richard A. Furie has received consultation fees from Mallinckrodt Pharmaceuticals.
Compliance with Ethics Guidelines
The study protocol was approved by the local ethics committees and institutional review boards at the individual study sites and by the Western Institutional Review Board centrally. Patients were required to provide written informed consent. The study was conducted in agreement with requirements of registered clinical trials (ClinicalTrials.gov identifier NCT02953821) and with the Declaration of Helsinki.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available. Individual patient data may be requested if allowed per informed consent and appropriately anonymized. Requests should be sent to Mallinckrodt Pharmaceuticals’ department for Clinical Trial Disclosure and Transparency at clinicaltrials@mnk.com.
References
- 1.Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12(10):605–620. doi: 10.1038/nrrheum.2016.137. [DOI] [PubMed] [Google Scholar]
- 3.Barber MRW, Clarke AE. Socioeconomic consequences of systemic lupus erythematosus. Curr Opin Rheumatol. 2017;29(5):480–485. doi: 10.1097/BOR.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 4.Mahieu M, Yount S, Ramsey-Goldman R. Patient-reported outcomes in systemic lupus erythematosus. Rheum Dis Clin N Am. 2016;42(2):253–263. doi: 10.1016/j.rdc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolly M, Utset TO. Can disease specific measures for systemic lupus erythematosus predict patients’ health-related quality of life? Lupus. 2004;13(12):924–926. doi: 10.1191/0961203304lu2034oa. [DOI] [PubMed] [Google Scholar]
- 6.Azizoddin DR, Jolly M, Arora S, Yelin E, Katz P. Patient-reported outcomes predict mortality in lupus. Arthritis Care Res (Hoboken) 2019;71(8):1028–1035. doi: 10.1002/acr.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acthar Gel. Package insert. Mallinckrodt Pharmaceuticals; 2019.
- 8.Huang YJ, Galen K, Zweifel B, Brooks LR, Wright AD. Distinct binding and signaling activity of Acthar Gel compared to other melanocortin receptor agonists. J Recept Signal Transduct. 2020:1–9. [DOI] [PubMed]
- 9.Olsen NJ, Decker DA, Higgins P, Becker PM, McAloose CA, Benko AL, et al. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300. doi: 10.1186/s13075-015-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healy LM, Jang JH, Lin YH, Rao V, Antel JP, Wright D. Melanocortin receptor mediated anti-inflammatory effect of repository corticotropin injection on human monocyte-derived macrophages [ECTRIMS-ACTRIMS abstract EP1481] Mult Scler J. 2017;23(suppl 3):777. [Google Scholar]
- 11.Decker DA, Grant C, Oh L, Becker PM, Young D, Jordan S. Immunomodulatory effects of H.P. Acthar Gel on B cell development in the NZB/W F1 mouse model of systemic lupus erythematosus. Lupus. 2014;23(8):802–812. doi: 10.1177/0961203314531840. [DOI] [PubMed] [Google Scholar]
- 12.Wright D, Zweifel B, Prabha S, Galen K, Fitch R. Reduced steroidogenic activity of repository corticotropin injection (RCI) induces a distinct cytokine response following T cell activation [EULAR abstract AB0082] Ann Rheum Dis. 2019;78(suppl 2):1504. [Google Scholar]
- 13.Fiechtner JJ, Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus. 2014;23(9):905–912. doi: 10.1177/0961203314532562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiechtner J, Montroy T. Six months’ treatment of moderately to severely active systemic lupus erythematosus with repository corticotropin injection: an extension of a single-site, open-label trial. J Immunol Clin Res. 2016;3(1):1025–1030. [Google Scholar]
- 15.Furie R, Mitrane M, Zhao E, Das M, Li D, Becker PM. Efficacy and tolerability of repository corticotropin injection in patients with persistently active SLE: results of a phase 4, randomised, controlled pilot study. Lupus Sci Med. 2016;3(1):e000180. doi: 10.1136/lupus-2016-000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furie RA, Mitrane M, Zhao E, Becker PM. Repository corticotropin injection in patients with persistently active SLE requiring corticosteroids: post hoc analysis of results from a two-part, 52-week pilot study. Lupus Sci Med. 2017;4(1):e000240. doi: 10.1136/lupus-2017-000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askanase AD, Zhao E, Zhu J, Bilyk R, Furie RA. Repository corticotropin injection for persistently active systemic lupus erythematosus: results from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial. Rheumatol Ther. 2020;7(4):893–908. doi: 10.1007/s40744-020-00236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Askanase AD, Zhao E, Zhu J, Connolly-Strong E, Furie RA. Acthar Gel (repository corticotropin injection) for persistently active SLE: study design and baseline characteristics from a multicentre, randomised, double-blind, placebo-controlled trial. Lupus Sci Med. 2020;7(1):e000383. doi: 10.1136/lupus-2020-000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–291. [PubMed] [Google Scholar]
- 21.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44(7):902–906. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 22.McElhone K, Abbott J, Shelmerdine J, Bruce IN, Ahmad Y, Gordon C, et al. Development and validation of a disease-specific health-related quality-of-life measure, the LupusQoL, for adults with systemic lupus erythematosus. Arthritis Rheum. 2007;57(6):972–979. doi: 10.1002/art.22881. [DOI] [PubMed] [Google Scholar]
- 23.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht J, Taylor L, Berlin JA, Dulay S, Ang G, Fakharzadeh S, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Investig Dermatol. 2005;125(5):889–894. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DJ, Strand V, Furie R, Petri M, Kalunian K, Pike M, et al. Evaluation of treatment success in systemic lupus erythematosus clinical trials: development of the British Isles Lupus Assessment Group-based Composite Lupus Assessment Endpoint. Poster session presented at: American College of Rheumatology; 2011 Nov 5–9; Chicago.
- 26.McElhone K, Abbott J, Sutton C, Mullen M, Lanyon P, Rahman A, et al. Sensitivity to change and minimal important differences of the LupusQoL in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2016;68(10):1505–1513. doi: 10.1002/acr.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strand V, Simon LS, Meara AS, Touma Z. Measurement properties of selected patient-reported outcome measures for use in randomised controlled trials in patients with systemic lupus erythematosus: a systematic review. Lupus Sci Med. 2020;7(1):e000373. doi: 10.1136/lupus-2019-000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clowse ME, Wallace DJ, Furie RA, Petri MA, Pike MC, Leszczynski P, et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol. 2017;69(2):362–375. doi: 10.1002/art.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touma Z, Gladman DD, Ibanez D, Urowitz MB. Is there an advantage over SF-36 with a quality of life measure that is specific to systemic lupus erythematosus? J Rheumatol. 2011;38(9):1898–1905. doi: 10.3899/jrheum.110007. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Pham L, Poola N, Brooks LR, Due B. Comparison of steroidogenic exposure following the administration of repository corticotropin injection with a synthetic ACTH1–24 depot and methylprednisolone in healthy subjects. Clin Pharmacol Drug Dev. 2020 doi: 10.1002/cpdd.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available. Individual patient data may be requested if allowed per informed consent and appropriately anonymized. Requests should be sent to Mallinckrodt Pharmaceuticals’ department for Clinical Trial Disclosure and Transparency at clinicaltrials@mnk.com.