Abstract
Rationale
There are no validated measures of disease activity in COPD. Since “active” disease is expected to have worse outcomes (e.g. mortality), we explored potential markers of disease activity in patients enrolled in the ECLIPSE cohort in relation to 8-year all-cause mortality.
Methods
We investigated 1) how changes in relevant clinical variables over time (1 or 3 years) relate to 8-year mortality; 2) whether these variables inter-relate; and 3) if any clinical, imaging and/or biological marker measured cross-sectionally at baseline relates to any activity component.
Results
Results showed that 1) after 1 year, hospitalisation for COPD, exacerbation frequency, worsening of body mass index, airflow obstruction, dyspnoea and exercise (BODE) index or health status (St George's Respiratory Questionnaire (SGRQ)) and persistence of systemic inflammation were significantly associated with 8-year mortality; 2) at 3 years, the same markers, plus forced expiratory volume in 1 s (FEV1) decline and to a lesser degree computed tomography (CT) emphysema, showed association, thus qualifying as markers of disease activity; 3) changes in FEV1, inflammatory cytokines and CT emphysema were not inter-related, while the multidimensional indices (BODE and SGRQ) showed modest correlations; and 4) changes in these markers could not be predicted by any baseline cross-sectional measure.
Conclusions
In COPD, 1- and 3-year changes in exacerbation frequency, systemic inflammation, BODE and SGRQ scores and FEV1 decline are independent markers of disease activity associated with 8-year all-cause mortality. These disease activity markers are generally independent and not predictable from baseline measurements.
Short abstract
In patients with COPD, 1- and 3-year changes in exacerbation frequency, systemic inflammation, BODE and SGRQ scores, and FEV1 decline, are independent markers of disease activity associated with 8-year all-cause mortality https://bit.ly/2CyifcN
Introduction
COPD is a complex, heterogeneous disease that is a major cause of morbidity and mortality worldwide [1]. To address the complexity and heterogeneity of COPD in clinical practice, a strategy based on “treatable traits” was proposed [2].
Disease severity and disease activity are distinct attributes that are not necessarily correlated [3]. In COPD, severity has traditionally been expressed by forced expiratory volume in 1 s (FEV1) as a proportion of its predicted value, because FEV1 is a good predictor of exacerbations [4], healthcare cost [5] and mortality [6]. Several observational studies have defined prognostic measures that predict mortality in COPD better than FEV1. In this context, the body mass index, obstruction, dyspnoea and exercise (BODE) index has been accepted as a valid multidimensional index to determine COPD severity [7]. By contrast, there are no validated measures in COPD for disease activity as have been developed in other diseases such as Crohn's disease [8], where the tools utilised are composite indices combining symptoms with laboratory values and/or functional limitation. Currently, the only reliable measures of disease activity in COPD are changes in subjective and/or objective measurements of disease severity. No biomarkers for cross-sectional measurement of disease activity have yet been established, although these would be useful [9–12].
ECLIPSE (NCT00292552) [13] was an observational, longitudinal study in which COPD patients were evaluated regularly for 3 years. In addition, survival status at 8 years after recruitment was obtained in an ECLIPSE subpopulation. ECLIPSE had multiple longitudinal assessments of variables related to disease severity, thereby offering a unique opportunity to investigate relationships between changes in those measures of disease and explore them as surrogate markers of disease activity in relation to a critical outcome, such as mortality [14].
We sought to investigate how changes in relevant variables over time (1 or 3 years) related to 8-year mortality, as a way to identify potential surrogate markers of disease activity; whether these components were inter-related, to help define the value of each of these components; and if any clinical, imaging and/or biological marker measured at baseline related to any identified activity component.
Methods
ECLIPSE study design and ethics
The ECLIPSE study design and entry criteria have been published previously [13]. Briefly, participants with Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades II–IV COPD, plus smoking and non-smoking controls, were evaluated regularly for 3 years during the initial study period, and survival status was recorded for consenting participants at 8 years. ECLIPSE complied with the Declaration of Helsinki and was approved by ethics committees of participating centres. All participants provided written informed consent.
This study included only participants from the ECLIPSE study with COPD.
Measurements
Methods for ECLIPSE have been described previously [15–17]. Briefly, modified questionnaires of the American Thoracic Society, Medical Research Council and the St George's Respiratory Questionnaire (SGRQ) recorded patient-reported comorbidities, breathlessness and health-related quality of life. Moderate and severe exacerbations in the year before the study or during the 3-year study were also recorded.
Statistical analysis
Demographic characteristics are summarised as mean±sd or as percentages. The relationships between disease activity variables and mortality at 8 years were determined by categorising change from baseline to year 1, and to year 3 for variables known to relate to mortality. This included the following variables: FEV1 decline; emphysema measured by quantitative computed tomography (CT) using the lowest 15th percentile density method (PD15); BODE index; frequent exacerbations (defined as two or more exacerbations per year); SGRQ total score; and the selected systemic biomarkers. Survival was evaluated to 8 years. Kaplan–Meier curves of individual clinical risk factors and biomarkers were analysed. Cox proportional hazards regression models were used to compare hazard ratios between change categories based on these clinical phenotypes, adjusting for baseline BODE, age and previous COPD hospitalisation. In addition, hazard ratios and 95% confidence intervals for survival to year 8 based on change are reported. Diagnostics for optimising model fit and predictive capability used Akaike's information criterion and the adequacy index, respectively [18]. Potential correlations between disease activity components (and with potential baseline markers) were explored using Spearman's ρ. All tests performed (SAS version 9.2; SAS Institute, Cary, NC, USA) were two-sided with a 0.05 significance level. All p-values were nominal; no adjustment was made for multiple comparisons, as the analyses were considered exploratory.
Results
Characteristics and survival of participants
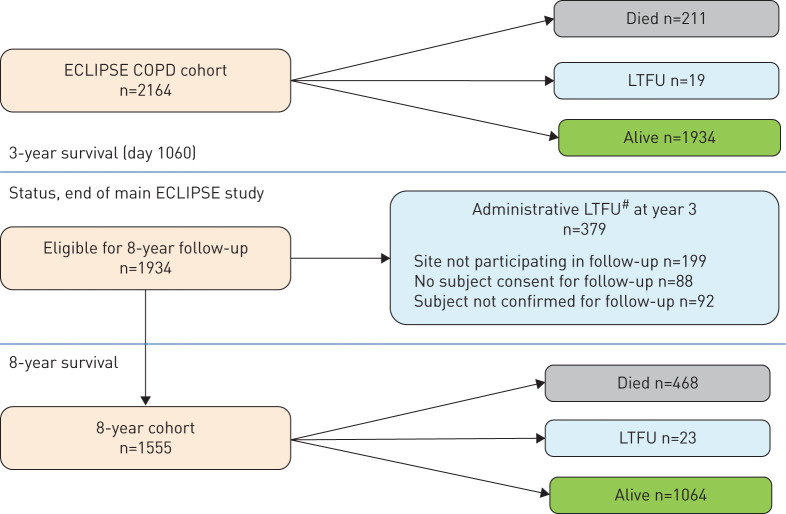
Out of the 2164 original ECLIPSE COPD participants, 1934 (89%) were alive at end of the 3-year follow-up and potentially available for inclusion in the 8-year follow-up phase. A total of 211 participants had died and 19 were lost to follow-up (LTFU) at year 3. For the 8-year survival assessment, 1555 (80.4%) of the eligible subjects consented to and participated in the 8-year follow-up. Of these, 468 subjects died, 23 were LTFU at year 8 and 1064 were confirmed alive. Clinical and physiological characteristics of participants LTFU either due to administrative reasons (mainly due to a late protocol amendment, n=379) or unable to be accounted for at 8 years (n=42), were in between those of subjects alive and those dead at 8 years' follow-up (figure 1). Table 1 shows the baseline characteristics and clinical characteristics at year 3 for the 1555 participants who consented to participate in the 8-year follow-up, split by survival status at 8 years. Nonsurvivors were older, had more severe airflow limitation, reported more respiratory symptoms and worse health status, had a lower 6-min walk distance, more emphysema, a higher BODE score, more comorbidities and were more likely to have a COPD exacerbation history compared to survivors. Supplementary table E1 shows that patients that did not participate in the follow-up extension of ECLIPSE had baseline clinical characteristics that were in between that of those patients who died and those who survived in the 8-year study. A total of 679 COPD patients in ECLIPSE died over the 8-year period. The Kaplan–Meier estimate of probability of death over this period was 37%.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram of all subjects in the ECLIPSE COPD study. #: subjects or sites who stopped study participation or did not consent to 8-year survival status. LTFU: lost to follow-up.
TABLE 1.
Demographic and clinical characteristics of participants with COPD in the ECLIPSE study, based on 8-year vital status
| Alive at year 8 | Died by year 8 | LTFU by year 8 | |
| Subjects n | 1064 | 468 | 23 |
| Baseline | |||
| Age years | 62±7 | 65±6 | 63±8 |
| Male | 62 | 72 | 43 |
| Post-BD FEV1 L | 1.50±0.53 | 1.18±0.44 | 1.24±0.46 |
| Post-BD FEV1 % predicted | 53±15 | 43±14 | 49±16 |
| FEV1/FVC ratio | 0.47±0.11 | 0.40±0.10 | 0.42±0.09 |
| GOLD II/III/IV % | 56/37/7 | 30/50/20 | 35/61/4 |
| GOLD 2017 A/B/C/D % | 32/14/26/28 | 13/8/24/55 | 9/14/23/55 |
| 6MWD m | 411±112 | 346±117 | 350±94 |
| Emphysema PD15 g·L−1 | 57±27 | 44±24 | 32±25 |
| BODE index | 2.4±1.8 | 3.9±2.1 | 3.0±1.8 |
| SGRQ total score | 44±18 | 51±17 | 51±19 |
| COPD hospitalisation history | 11 | 21 | 22 |
| Frequent exacerbation history | 18 | 25 | 17 |
| Cardiovascular history | 30 | 39 | 9 |
| Depression symptoms | 22 | 25 | 30 |
| Asthma history | 23 | 22 | 26 |
| Diabetes history | 9 | 13 | 13 |
| At year 3 | |||
| Post-BD FEV1 L | 1.42±0.55 | 1.07±0.44 | 1.11±0.37 |
| Post-BD FEV1 % predicted | 50±16 | 39±14 | 44±14 |
| FEV1 decline during main study mL·year−1 | 32±45 | 42±40 | 45±40 |
| 6MWD m | 405±122 | 327±129 | 321±109 |
| Emphysema PD15 g·L−1 | 55±28 | 40±23 | 38±28 |
| BODE index | 2.6±2.0 | 4.4±2.2 | 3.8±1.8 |
| SGRQ total score | 43±19 | 54±19 | 55±20 |
| COPD hospitalisation during main study | 23 | 51 | 22 |
| Frequent exacerbations, all 3 years of main study | 8 | 20 | 13 |
Data are presented as mean±sd or %, unless otherwise stated. All comparisons between those alive and those who died by year 8 were statistically significant (p<0.05), excepting baseline depression and asthma history. LTFU: lost to follow-up; BD: bronchodilator; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; 6MWD: 6-min walk distance; PD15: lowest 15th percentile of lung density; BODE: body mass index, obstruction, dyspnoea and exercise; SGRQ: St George's Respiratory Questionnaire.
Markers of disease severity and 8-year mortality
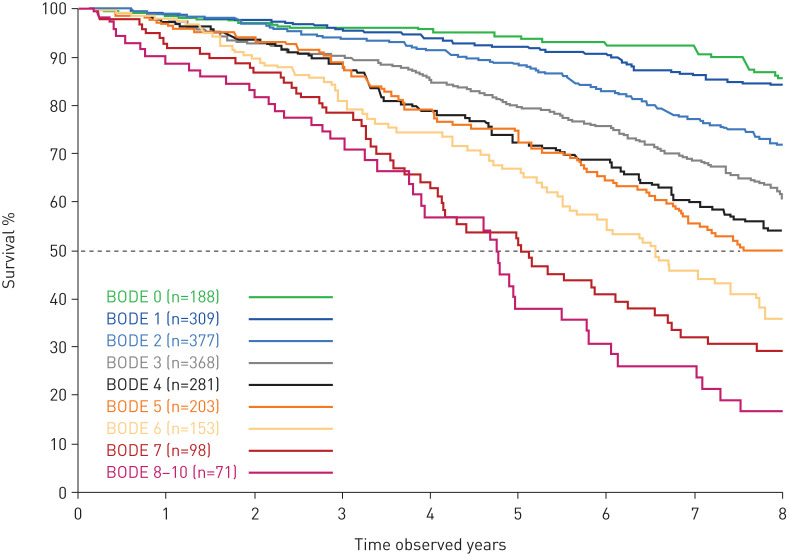
The baseline BODE index, a well-established marker of disease severity, was a good predictor of 8-year mortality risk (figure 2). We evaluated a panel of systemic biomarkers for their additive predictive ability for 8-year mortality risk by adding them to a base model with known markers of disease severity (BODE, age and COPD hospitalisation). Interleukin (IL)-6, neutrophils and surfactant protein D (SP-D) together improved the predictive capability of the model to predict 8-year mortality (optimising Akaike's information criteria among biomarker covariates). The base model of clinical covariates (BODE, age and COPD hospitalisation) provided 87% of the prognostic value of the full base plus biomarkers model (adequacy index=0.87).
FIGURE 2.
Kaplan–Meier curves for mortality at 8 years' follow-up, split by body mass index, obstruction, dyspnoea and exercise (BODE) index at baseline.
Markers of disease activity and 8-year mortality
Table 2 presents the hazard ratios and 95% confidence intervals for risk of death for the variables of disease activity studied. Determined as change from baseline to the first year, hospitalisation for COPD, two or more exacerbations, ≥1-unit worsening of BODE index or ≥4-unit worsening of SGRQ total score and persistent elevation of inflammatory cytokines were all associated with increased risk of death. When determined over 3 years, the same variables, as well as FEV1 decline, had a stronger association with risk of death at 8 years' follow-up. Change or decline in CT emphysema (PD15) showed weak association with risk of death at 1 year, which became nominally significant at 3 years.
TABLE 2.
Hazard ratios (HRs) for survival to year 8 based on markers of disease activity measured as change from baseline at the first year and the third year, respectively
| 1-year change/event (n=2097) | 3-year change/event (n=1555) | |||||
| Subjects# n | HR (95% CI) | p-value | Subjects# n | HR (95% CI) | p-value | |
| COPD hospitalisation | ||||||
| No | 1770 | Reference group | 1064 | Reference group | ||
| Yes | 308 | 1.65 (1.34–2.02) | <0.001 | 487 | 1.95 (1.59–2.39) | <0.001 |
| Frequent exacerbations¶ | ||||||
| No frequent exacerbations all years | 1460 | Reference group | 1320 | Reference group | ||
| Frequent exacerbations all years | 618 | 1.30 (1.09–1.54) | 0.003 | 175 | 1.63 (1.27–2.10) | <0.001 |
| Health status (SGRQ) | ||||||
| ≥4 units improvement | 675 | 0.94 (0.76–1.17) | 0.606 | 444 | 0.96 (0.72–1.28) | 0.796 |
| −4–4 units change | 630 | Reference group | 405 | Reference group | ||
| ≥4 units worse | 526 | 1.37 (1.11–1.69) | 0.004 | 538 | 1.58 (1.23–2.03) | <0.001 |
| FEV1 decline+ mL·year−1 | ||||||
| Per 20 mL·year−1 increment | 1916 | 1.01 (1.00–1.02) | 0.044 | 1555 | 1.14 (1.09–1.19) | <0.001 |
| BODE index | ||||||
| Improved | 520 | 0.84 (0.67–1.07) | 0.157 | 244 | 0.76 (0.54–1.08) | 0.126 |
| No change | 623 | Reference group | 409 | Reference group | ||
| Worse | 576 | 1.27 (1.02–1.58) | 0.032 | 565 | 1.52 (1.17–1.97) | 0.002 |
| Persistent inflammation | ||||||
| No markers | 431 | Reference group | § | § | ||
| One marker | 768 | 1.32 (1.03–1.71) | 0.030 | |||
| ≥2 markers | 220 | 1.48 (1.08–2.04) | 0.016 | |||
| Emphysema PD15 decline+ g·L−1·year−1 | ||||||
| Per 1 g·L−1·year−1 increment | 1556 | 1.00 (0.99–1.01) | 0.078 | 1380 | 1.12 (1.00–1.24) | 0.046 |
All models were adjusted for baseline age, baseline body mass index, obstruction, dyspnoea and exercise (BODE) index, and prior COPD hospitalisation. Year 1 change analyses reflect the original ECLIPSE cohort who remained in the study after year 1. Data for the 379 participants who did not consent for 8-year follow-up were censored at year 3, corresponding with the end of their original study participation. SGRQ: St George's Respiratory Questionnaire; FEV1: forced expiratory volume in 1 s; PD15: lowest 15th percentile density method. #: number of participants with available data for the given parameter at the specified time point; ¶: ≥2 exacerbations per year; +: change from baseline used for year 1 analysis, rate of decline used for year 3 analysis; §: not all inflammatory markers were measured at year 3.
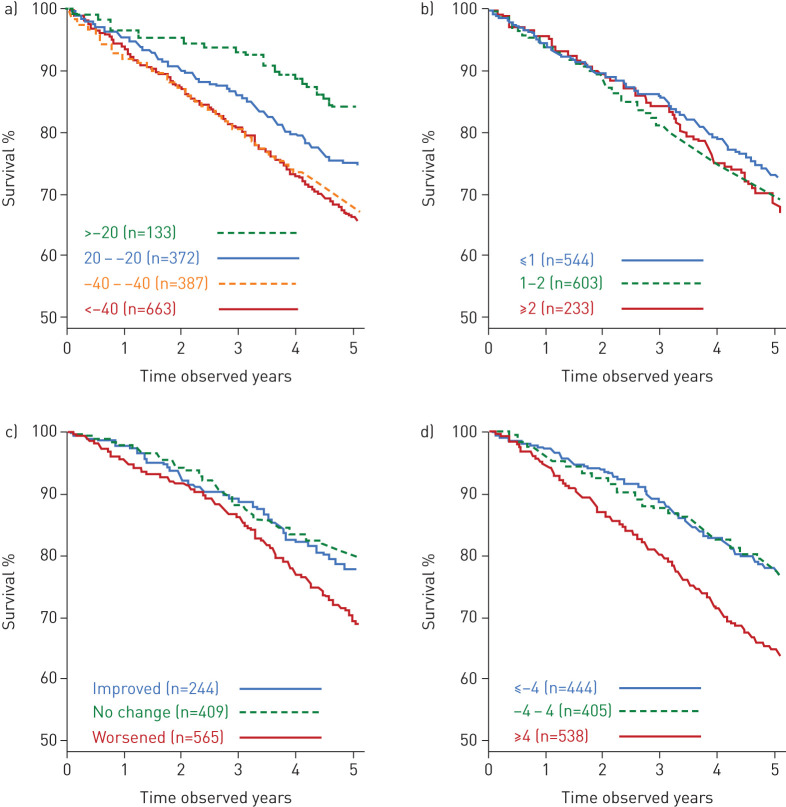
Figures 3 and 4 show Kaplan–Meier estimates of survival at 8 years based on change in each of the variables determined from baseline to year 3 (table 2). Thresholds of 20 mL·year−1 and 40 mL·year−1 for FEV1 decline were selected based on mean decline values for healthy never-smokers and smokers from the Framingham Offspring Cohort [19]; thresholds provided are not intended to suggest optimal decline thresholds. Probability of survival related to FEV1 change was 84% for improvers (>20 mL·year−1 gain); 75% for the stable group (decline ±20 mL·year−1); 67% for modest decliners (decline of 20–40 mL·year−1); and 65% for substantial decliners (decline of >40 mL·year−1).
FIGURE 3.
Kaplan–Meier curves for mortality from year 3 to year 8 for a) forced expiratory volume in 1 s decline (mL·year−1); b) computed tomography decline (lowest 15th percentile density method) (g·L−1·year−1); c) body mass index, obstruction, dyspnoea, and exercise (BODE) index change; d) St George's Respiratory Questionnaire total score change.
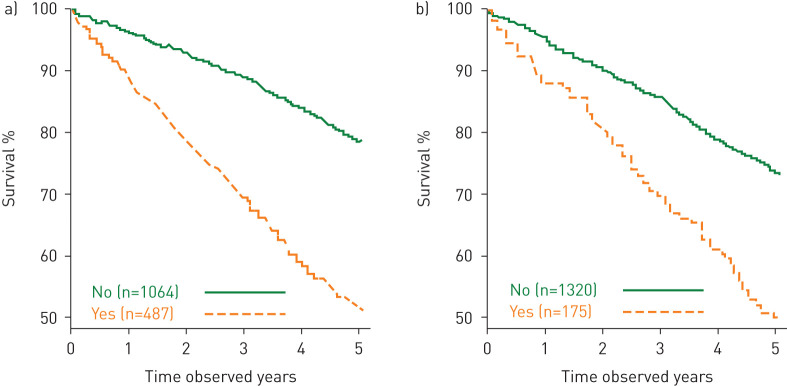
FIGURE 4.
Kaplan–Meier curves for mortality from year 3 to year 8 for a) any COPD hospitalisation over 3 years; b) frequent exacerbations each year over 3 years.
Table 3 shows that changes in disease activity were independent of lung function severity, as the same variables were significant across GOLD severity grades (II/III). In patients with GOLD grade IV, only rate of FEV1 decline and hospitalisations reached statistical significance at 8 years, but the number of patients in this stage was relatively small (n=173).
TABLE 3.
Clinical characteristics and disease activity profile over 3 years classified according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric severity stage (II/III/IV)
| Alive at year 8 | Died by year 8 | p-value, alive versus died | |
| GOLD II at baseline | |||
| Subjects | 594 | 139 | |
| FEV1 decline mL·year−1 | −33±48 | −52±45 | <0.001 |
| Emphysema (PD15) decline g·L−1·year−1 | −1.1±1.1 | −1.1±1.1 | 0.861 |
| SGRQ total score change to year 3 | −0.6±12.4 | 2.7±13.8 | 0.011 |
| 6MWD change to year 3 | 0±85 | −12±89 | 0.221 |
| BODE index change to year 3 | 0.5±1.2 | 0.9±1.4 | 0.001 |
| Subjects with COPD hospitalisation % | 16 | 40 | <0.001 |
| GOLD III at baseline | |||
| Subjects | 393 | 233 | |
| FEV1 decline mL·year−1 | −32±41 | −41±39 | 0.014 |
| Emphysema (PD15) decline g·L−1·year−1 | −1.2±0.9 | −1.3±0.9 | 0.143 |
| SGRQ total score change to year 3 | 0.5±12.4 | 5.2±14.4 | <0.001 |
| 6MWD change to year 3 | −19±102 | −54±101 | <0.001 |
| BODE index change to year 3 | 0.3±1.5 | 0.9±1.7 | <0.001 |
| Subjects with COPD hospitalisation % | 30 | 53 | <0.001 |
| GOLD IV at baseline | |||
| Subjects | 77 | 96 | |
| FEV1 decline mL·year−1 | −16±42 | −33±31 | 0.003 |
| Emphysema (PD15) decline g·L−1·year−1 | −1.2±0.9 | −1.3±0.8 | 0.492 |
| SGRQ total score change to year 3 | −0.7±14.0 | 2.3±10.1 | 0.128 |
| 6MWD change to year 3 | −37±102 | −48±112 | 0.576 |
| BODE index change to year 3 | 0.4±1.6 | 0.5±1.4 | 0.556 |
| Subjects with COPD hospitalisation % | 43 | 62 | 0.012 |
Data are presented as n or mean±sd, unless otherwise stated. Participants are divided by survival status 5 years later (year 8 of observation). FEV1: forced expiratory volume in 1 s; PD15: lowest 15th percentile density method; SGRQ: St George's Respiratory Questionnaire; 6MWD: 6-min walk distance; BODE: body mass index, obstruction, dyspnoea and exercise.
Table 4 presents a matrix correlation (Spearman's ρ-values) between the disease activity markers identified in table 2. Changes in single variables (FEV1, exacerbations and hospitalisations, inflammatory biomarkers and CT (PD15)) were not related to each other. There were modest and expected relations between changes in some of these individual variables and multidimensional indices (BODE and SGRQ) that integrate some of them.
TABLE 4.
Correlations (Spearman's ρ) between different disease activity components
| Description of variable | FEV1-1y | FEV1-Decl | PD15-1y | PD15-Decl | BODE-1y | BODE-3y | HOSP-1y | HOSP-3y | FE-1y | FE-3y | SGRQ-1y | SGRQ-3y | |
| FEV1-1y | FEV1 change, baseline to year 1 | 1.00 | |||||||||||
| FEV1-Decl | FEV1 decline, baseline to year 3 | 0.39 | 1.00 | ||||||||||
| PD15-1y | Emphysema (PD15) change, baseline to year 1 | −0.02 | 0.03 | 1.00 | |||||||||
| PD15-Decl | Emphysema (PD15) decline, baseline to year 3 | 0.04 | 0.08 | 0.37 | 1.00 | ||||||||
| BODE-1y | BODE change, baseline to year 1 | −0.38 | −0.15 | 0.02 | −0.01 | 1.00 | |||||||
| BODE-3y | BODE change, baseline to year 3 | −0.27 | −0.43 | −0.02 | −0.11 | 0.47 | 1.00 | ||||||
| HOSP-1y | COPD hospitalisation, year 1 | −0.01 | −0.06 | −0.06 | −0.04 | 0.06 | 0.08 | 1.00 | |||||
| HOSP-3y | COPD hospitalisation, first 3 years | −0.08 | −0.09 | −0.08 | −0.06 | 0.06 | 0.13 | 0.63 | 1.00 | ||||
| FE-1y | Frequent exacerbations, year 1 | −0.06 | −0.03 | −0.01 | −0.05 | 0.07 | 0.06 | 0.39 | 0.36 | 1.00 | |||
| FE-3y | Frequent exacerbations, year 1, year 2 and year 3 | −0.04 | −0.03 | 0.04 | −0.04 | 0.08 | 0.05 | 0.28 | 0.30 | 0.58 | 1.00 | ||
| SGRQ-1y | SGRQ change, baseline to year 1 | −0.16 | −0.08 | 0.04 | −0.02 | 0.23 | 0.11 | 0.06 | 0.08 | 0.06 | 0.02 | 1.00 | |
| SGRQ-3y | SGRQ change, baseline to year 3 | −0.10 | −0.26 | 0.04 | −0.08 | 0.13 | 0.32 | 0.06 | 0.13 | 0.04 | 0.07 | 0.46 | 1.00 |
| SysInfl | ≥2 inflammation markers, year 1 | −0.01 | 0.00 | 0.01 | 0.07 | −0.03 | 0.01 | 0.19 | 0.19 | 0.15 | 0.09 | 0.04 | −0.02 |
(Absolute) correlations >0.25 are presented in bold. FEV1: forced expiratory volume in 1 s; PD15: lowest 15th percentile density method; BODE: body mass index, obstruction, dyspnoea and exercise; SGRQ: St George's Respiratory Questionnaire.
Supplementary table E2 shows that these disease activity components were mostly unrelated to any baseline, cross-sectional measure.
Discussion
This long-term analysis of the ECLIPSE COPD cohort had three main novel observations. Change over time (1 or 3 years) of a number of variables (including FEV1 decline, COPD exacerbations, BODE index, SGRQ score and presence of persistent inflammation) significantly predicted risk of death at 8 years' follow-up, suggesting that these variables should be considered as surrogate markers of disease activity. Changes in these variables were, generally, independent of each other, indicating that the construct of disease activity (like the disease itself) is heterogeneous and complex. Finally, change over time cannot yet be predicted accurately from any cross-sectional measurement obtained at baseline. Collectively, these findings could help to inform clinical directions for sequential measurements of the variables explored to develop therapies targeting disease progression.
Previous studies
Markers of disease severity in COPD in relation to their capacity to predict clinically relevant outcomes, such as mortality, have been widely studied [20–23]. Our results confirm the robustness of the observations made in the original BODE 3-year report [7], as the assessment of disease severity provided by the BODE index remains a good predictor of longer-term mortality even among those who survived for an initial 3-year observation period. The prognostic value of BODE is improved by complementing the information with the presence of older age and of hospitalisation, easily determined using electronic medical records. As was true at 3 years' follow-up [24], the systemic levels of neutrophils, IL-6 and SP-D were associated with increased risk of death at 8 years. The levels of these biomarkers provided 13% of the prognostic ability of the model when added to the base model of BODE index, age and COPD hospitalisation to predict risk of death at 8 years' follow-up. To our knowledge, the current study is the first to investigate the relationship of multiple candidates for surrogate markers of disease activity in a large, well-characterised cohort of COPD patients with varying degrees of disease severity followed long-term (8 years) and to validate them in relation to an important clinical outcome (all-cause mortality).
Interpretation of novel findings
This study provides three novel observations. First, changes from baseline to 1 year in frequency of COPD exacerbations, BODE index, SGRQ score, and presence of persistent inflammation related significantly to 8-year mortality. The relationship becomes even stronger when these changes are determined at 3 years. FEV1 decline, and to a lesser degree CT emphysema decline, also show a relationship with the outcome. Collectively, these observations provide evidence that changes in these variables are surrogate markers of disease activity. It is important to note here that these longitudinal measurements (i.e. changes over time) of disease activity are different from their baseline values, which may or may not be markers of disease severity.
To our knowledge, although previous studies have associated FEV1 decline to mortality with measurements made between 2- and 6-year intervals [25, 26], only one previous study [27] has determined how long it may take to characterise the value of the FEV1 decline in shorter time spans and relate it to impact on outcome. The results of that study, along with this study, suggest a time span between 1 and 3 years. Furthermore, FEV1 decline (and COPD hospitalisation) over 3 years were the only predictors in the limited number of patients with GOLD grade IV. These results are important because, with increased use of electronic medical records, it is feasible to identify these patients in daily clinical practice. Monitoring of lung function can help identify individuals at risk and further explore the mechanisms associated with decline to support the development of modifying therapies aimed at ameliorating disease progression.
For the first year of observation, the presence of exacerbations leading to hospitalisations was a significant predictor, extending the original 3-year findings in ECLIPSE [28] and in other studies [29] to 8-year follow-up; development of two or more moderate exacerbations over the same time frame also related to increased mortality. When disease activity measures were determined by changes from baseline to 3-year follow-up, results confirmed that hospitalisations for COPD and at least two exacerbations per year were important predictors of mortality, and hence a marker of disease activity. This establishes a practical timeline for targeted evaluation of therapies aimed at preventing these events [30].
Additionally, worsening of the BODE index and SGRQ total score after 1 year were predictors of 8-year risk of death. These findings are aligned with previous studies. Casanova et al. [27] demonstrated that 1-year changes in the BODE index were less variable and a better predictor of survival than changes in FEV1 in 740 COPD patients followed over 5 years. In the National Emphysema Treatment Trial, changes in the BODE index at 1 year were significant predictors of 2- and 5-year survival in that cohort of patients with COPD [31]. Additionally, the SGRQ score is known to relate to mortality, with a 4-unit change representing its minimum clinically important difference [32, 33]. In the current study, worsening in the SGRQ of ≥4 units at 1 year was associated with increased risk of death over the subsequent 7 years. When changes were considered over 3 years, worsening of the BODE index or SGRQ score became even stronger predictors of risk of death at 8 years' follow-up.
The association between persistent systemic inflammation (i.e. high levels of two or more inflammatory biomarkers in blood samples obtained in two sequential years) and increased risk of death in the ECLIPSE study has already been published [34], and is supported by results from our 8-year mortality analyses. However, we only observed a weak relationship between 1- and 3-year worsening of CT emphysema (PD15) with all-cause mortality. These observations do not suggest a lack of association between CT-detected degree of emphysema (i.e. an assessment of disease severity) and risk of death, as previously documented [35, 36], but rather that the changes detected using the methods implemented in ECLIPSE are not as strong as other markers of disease activity. It is possible too that the impact of progression via quantitative CT is more informative in earlier COPD (GOLD I) or those at risk of COPD (former GOLD 0) rather than in patients who already have a significant amount of emphysema.
The other two novel observations of this study relate to the lack of correlation between most of the different variables identified here and 8-year mortality, and the inability of cross-sectional measurements determined at baseline to predict disease activity. The former indicates that the variables studies here are heterogeneous and complex, reflecting the construct of COPD itself. Results of this study show that many important and disease-relevant individual measurements need to be considered independently to assess disease activity. In clinical practice, these observations support a management strategy based on the proposed “treatable traits” approach [2]. The multidimensional BODE and SGRQ indices, as well as the frequency and severity of exacerbations, are more sensitive to reflect activity, but lack the specificity needed to provide personalised therapeutic approaches. As with different trajectories of lung function throughout life [37, 38], there may be different trajectories for the multiple dimensions affected by COPD reflected in different biomarkers. However, none of our multiple cross-sectional measurements determined at baseline were significantly related to any of the disease activity components we determined, subsequently highlighting the pressing need to identify and validate other biomarkers of disease activity.
Strengths and limitations
Important strengths of the study are that the ECLIPSE cohort was followed in a prospective clinical setting with well-characterised COPD patients suffering from a wide range of airflow limitation. Moreover, markers of disease severity and of disease activity (as determined by their dynamic change over time) were separately assessed as predictors of all-cause mortality.
The main limitation of this study is that the 8-year survival status assessment was implemented as a late protocol amendment, and as consent for participation was not available from all sites, 19.6% of eligible subjects required censoring at the end of year 3, primarily due to administrative reasons. However, the baseline characteristics of the patients censored in this group were intermediate between that of survivors and nonsurvivors, suggesting that they were representative of the patients included in the overall study. The baseline and year 3 characteristics of the 1555 participants who consented to the 8-year follow-up versus the 398 participants who were alive but did not consent to participate in the 8-year follow-up are provided in supplementary table E1. Overall, the nonparticipants had worse values in most clinical variables than those who participated in the study. However, the fact that change in those parameters that related to mortality were evident in patients with slightly milder disease further support the relevance of our findings. Another limitation of the data was the lack of adjudication of the cause of death, as performed in several prospective interventional trials [39–41]. A limited number of serum and blood biomarkers were evaluated and did not include recently described markers such as collagen and elastin turnover biomarkers [42], which could be of use in assessing different components of COPD disease activity. Finally, the ECLIPSE population was recruited by specialists, often at academic centres with expertise in clinical trials, and does not represents a population-based sample.
Conclusions
This 8-year follow-up of patients with COPD enrolled in the ECLIPSE study shows that changes over time (1 or 3 years) in FEV1 decline, exacerbation frequency, BODE and SGRQ scores and systemic inflammation could be used as surrogate markers of disease activity that relate significantly to all-cause mortality after 8-years' follow-up. Different variables of disease components are independent of each other and cannot currently be predicted by cross-sectional measures.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01339-2020.SUPPLEMENT (225.2KB, pdf)
Shareable PDF
Acknowledgements
J. Vestbo is supported by the National Institute for Health Research, Manchester Biomedical Research Centre. D.A. Lomas is a National Institute for Health Research Senior Investigator and supported by the University College London Hospital National Institute for Health Research Biomedical Research Centre.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.02933-2020
This article has supplementary material available from erj.ersjournals.com
The ECLIPSE study is registered as a clinical trial at www.clinicaltrials.gov with identifier number NCT00292552. Information on GlaxoSmithKline's data sharing commitments and requesting access to anonymised individual participant data and associated documents can be found at www.clinicalstudydatarequest.com
Author contributions: All authors made substantial contributions to the conception or design of the work reported. N. Locantore and J.C. Yates participated in the acquisition of reported data. All authors participated in the analysis and in the interpretation of reported data. All authors reviewed and/or critically revised the manuscript for important intellectual content and provided final approval of the version to be published.
Conflict of interest: B. Celli reports grants and personal fees for scientific committee work from GlaxoSmithKline, during the conduct of the study; grants and provision of research facilities from AstraZeneca, personal fees for consultancy and scientific committee work from GlaxoSmithKline, personal fees for consultancy from Boehringer Ingelheim, Sanofi-Aventis, Menarini, Chiesi and Pulmonx, outside the submitted work.
Conflict of interest: N. Locantore is an employee and shareholder of GSK.
Conflict of interest: J.C. Yates is an employee of and owns shares in GSK.
Conflict of interest: P. Bakke reports personal fees for advisory board work and lectures from GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim, personal fees for advisory board work from Chiesi, outside the submitted work.
Conflict of interest: P.M.A. Calverley reports personal fees from GSK, Boehringer Ingelheim, Novartis, Zambon, Respironics and Recipharm, outside the submitted work.
Conflict of interest: C. Crim is an employee and shareholder of GlaxoSmithKline.
Conflict of interest: H.O. Coxson reports grants and personal fees for scientific committee work from GSK, during the conduct of the study.
Conflict of interest: D.A. Lomas reports grants and personal fees from GlaxoSmithKline, during the conduct of the study; grants and personal fees for advisory board work and lectures from GlaxoSmithKline, personal fees for advisory board work from Griffols, outside the submitted work.
Conflict of interest: W. MacNee reports personal fees for scientific committee work from GSK, during the conduct of the study; personal fees from GSK and AstraZeneca, grants and personal fees from Pfizer, outside the submitted work.
Conflict of interest: B.E. Miller is an employee and shareholder of GSK.
Conflict of interest: H. Mullerova is a former employee of GlaxoSmithKline.
Conflict of interest: S.I. Rennard is a former employee of AstraZeneca, has provided consultancy to GSK, Verona, Bergenbio and NovoVentures, and currently holds shares in AstraZeneca, outside of the submitted work. Prior to 2007, S.I. Rennard received funding from the tobacco industry for studies relating to harm reduction and to the impact of tobacco smoke on stem cells, and also consulted with RJ Reynolds (without personal fee) on the topic of harm reduction: funding from RJ Reynolds to evaluate the effect of a harm reduction product in normal smokers (1996) and in subjects with chronic bronchitis (1999) and to assess the effect of smoking cessation on lower respiratory tract inflammation (2000); participation in a Philip Morris multicentre study to assess biomarkers of smoke exposure (2002); funding for a clinical trial from the Institute for Science and Health (2005), which receives support from the tobacco industry, to evaluate biomarkers in exhaled breath associated with smoking cessation and reduction (this study was supplemented with funding from Lorillard and RJ Reynolds); grants from the Philip Morris External Research Program (2005) to assess the impact of cigarette smoking on circulating stem cells in the mouse; consultancy for RJ Reynolds on the topic of harm reduction until 2007 (no personal remuneration). There are no active tobacco-industry funded projects. All ties with tobacco industry companies and entities supported by tobacco companies were terminated in 2007.
Conflict of interest: E.K. Silverman reports grants, personal fees and travel expenses from GlaxoSmithKline, grants from NIH, during the conduct of the study.
Conflict of interest: E. Wouters reports personal fees for advisory board work from Nycomed and Boehringer Ingelheim BV, grants and personal fees for lectures from AstraZeneca and GSK, personal fees for lectures from Novartis and Chiesi, outside the submitted work.
Conflict of interest: R. Tal-Singer reports is an employee and shareholder of GlaxoSmithKline.
Conflict of interest: A. Agusti reports personal fees for scientific committee work from GSK, during the conduct of the study; personal fees from AstraZeneca, Chiesi and Nuvaira, grants and personal fees from Menarini and GSK, outside the submitted work.
Conflict of interest: J. Vestbo reports personal fees for steering committee work from GSK, during the conduct of the study; personal fees for lectures and consultancy from AstraZeneca, Chiesi and Novartis, grants and personal fees for lectures and consultancy from Boehringer Ingelheim, grants and personal fees for consultancy from GSK, outside the submitted work; and has a family member employed by Chiesi (Denmark).
Support statement: The study was sponsored by GlaxoSmithKline plc. A steering committee and a scientific committee comprising academic and sponsor representatives developed the original study design, the plan for analyses, and had full access to the data. The study sponsor did not place any restrictions regarding statements made in the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.GBD Cause of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 3.Vestbo J, Rennard S. Chronic obstructive pulmonary disease biomarker(s) for disease activity needed – urgently. Am J Respir Crit Care Med 2010; 182: 863–864. doi: 10.1164/rccm.201004-0602ED [DOI] [PubMed] [Google Scholar]
- 4.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 5.Ke X, Marvel J, Yu TC, et al. Impact of lung function on exacerbations, health care utilization, and costs among patients with COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 1689–1703. doi: 10.2147/COPD.S108967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomason MJ, Strachan DP. Which spirometric indices best predict subsequent death from chronic obstructive pulmonary disease? Thorax 2000; 55: 785–788. doi: 10.1136/thorax.55.9.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 8.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index: National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70: 439–444. doi: 10.1016/S0016-5085(76)80163-1 [DOI] [PubMed] [Google Scholar]
- 9.Bowler RP, Wendt CH, Fessler MB, et al. New strategies and challenges in lung proteomics and metabolomics. An official American Thoracic Society workshop report. Ann Am Thorac Soc 2017; 14: 1721–1743. doi: 10.1513/AnnalsATS.201710-770WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto-Plata V, Toso J, Lee K, et al. Profiling serum biomarkers in patients with COPD: associations with clinical parameters. Thorax 2007; 62: 595–601. doi: 10.1136/thx.2006.064428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockley RA, Halpin DMG, Celli BR, et al. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med 2019; 199: 1195–1204. doi: 10.1164/rccm.201810-1860SO [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ, Chowdhury B, Kharitonov SA, et al. Pulmonary biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 174: 6–14. doi: 10.1164/rccm.200510-1659PP [DOI] [PubMed] [Google Scholar]
- 13.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 2008; 31: 869–873. doi: 10.1183/09031936.00111707 [DOI] [PubMed] [Google Scholar]
- 14.Celli BR, Decramer M, Wedzicha JA, et al. An Official American Thoracic Society/European Respiratory Society Statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: e4–e27. doi: 10.1164/rccm.201501-0044ST [DOI] [PubMed] [Google Scholar]
- 15.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11: 122. doi: 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickens JA, Miller BE, Edwards LD, et al. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res 2011; 12: 146. doi: 10.1186/1465-9921-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rennard SI, Locantore N, Delafont B, et al. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc 2015; 12: 303–312. doi: 10.1513/AnnalsATS.201403-125OC [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE. Regression Modeling Strategies. 2nd Edn. New York, Springer, 2015. [Google Scholar]
- 19.Kohansal R, Martinez-Camblor P, Agustí A, et al. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 2009; 180: 3–10. doi: 10.1164/rccm.200901-0047OC [DOI] [PubMed] [Google Scholar]
- 20.Marin JM, Alfageme I, Almagro P, et al. Multicomponent indices to predict survival in COPD: The COCOMICS study. Eur Respir J 2013; 42: 323–332. doi: 10.1183/09031936.00121012 [DOI] [PubMed] [Google Scholar]
- 21.Almagro P, Soriano JB, Cabrera FJ, et al. Short- and medium-term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest 2014; 145: 972–980. doi: 10.1378/chest.13-1328 [DOI] [PubMed] [Google Scholar]
- 22.Soler-Cataluña JJ, Martínez-García MA, Sánchez LS, et al. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med 2009; 103: 692–699. doi: 10.1016/j.rmed.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 23.Celli BR. Predictors of mortality in COPD. Respir Med 2010; 104: 773–779. doi: 10.1016/j.rmed.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 24.Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 1065–1072. doi: 10.1164/rccm.201110-1792OC [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez BL, Masaki K, Burchfiel C, et al. Pulmonary function decline and 17-year total mortality: the Honolulu Heart Program. Am J Epidemiol 1994; 140: 398–408. doi: 10.1093/oxfordjournals.aje.a117262 [DOI] [PubMed] [Google Scholar]
- 26.Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med 2006; 173: 985–990. doi: 10.1164/rccm.200508-1344OC [DOI] [PubMed] [Google Scholar]
- 27.Casanova C, Agruirre-Jaíme A, de Torres J, et al. Longitudinal assessment in COPD patients: multidimensional variability and outcomes. Eur Respir J 2014; 43: 745–753. doi: 10.1183/09031936.00096913 [DOI] [PubMed] [Google Scholar]
- 28.Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest 2015; 147: 999–1007. doi: 10.1378/chest.14-0655 [DOI] [PubMed] [Google Scholar]
- 29.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60: 925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calverley PM, Anzueto AR, Dusser D, et al. Treatment of exacerbations as a predictor of subsequent outcomes in patients with COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 1297–1308. doi: 10.2147/COPD.S153631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez FJ, Han MK, Andrei AC, et al. Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med 2008; 178: 491–499. doi: 10.1164/rccm.200709-1383OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonelli-Incalzi R, Pedone C, Scarlata S, et al. Correlates of mortality in elderly COPD patients: focus on health-related quality of life. Respirology 2009; 14: 98–104. doi: 10.1111/j.1440-1843.2008.01441.x [DOI] [PubMed] [Google Scholar]
- 33.Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2: 75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 34.Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012; 7: e37483. doi: 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han MK, Tayob N, Murray S, et al. Association between emphysema and chronic obstructive pulmonary disease outcomes in the COPDGene and SPIROMICS cohorts: a post hoc analysis of two clinical trials. Am J Respir Crit Care Med 2018; 198: 265–267. doi: 10.1164/rccm.201801-0051LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest 2012; 141: 1216–1223. doi: 10.1378/chest.11-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med 2015; 373: 111–122. doi: 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 38.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019; 7: 358–364. doi: 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
- 39.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789. doi: 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 40.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. doi: 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 41.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016; 387: 1817–1826. doi: 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 42.Rønnow SR, Langholm LL, Sand JMB, et al. Specific elastin degradation products are associated with poor outcome in the ECLIPSE COPD cohort. Sci Rep 2019; 9: 4064. doi: 10.1038/s41598-019-40785-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01339-2020.SUPPLEMENT (225.2KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01339-2020.Shareable (481.3KB, pdf)