Abstract
Toxoplasma gondii and Plasmodium falciparum parasites both extrude l-lactate, a byproduct of glycolysis. The P. falciparum Formate Nitrite Transporter, PfFNT, mediates l-lactate transport across the plasma membrane of P. falciparum parasites and has been validated as a drug target. The T. gondii genome encodes three FNTs that have been shown to transport l-lactate, and which are proposed to be the targets of several inhibitors of T. gondii proliferation. Here, we show that each of the TgFNTs localize to the T. gondii plasma membrane and are capable of transporting l-lactate across it, with TgFNT1 making the primary contribution to l-lactate transport during the disease-causing lytic cycle of the parasite. We use the Xenopus oocyte expression system to provide direct measurements of l-lactate transport via TgFNT1. We undertake a genetic analysis of the importance of the tgfnt genes for parasite proliferation, and demonstrate that all three tgfnt genes can be disrupted individually and together without affecting the lytic cycle under in vitro culture conditions. Together, our experiments identify the major lactate transporter in the disease causing stage of T. gondii, and reveal that this transporter is not required for parasite proliferation, indicating that TgFNTs are unlikely to be targets for anti-Toxoplasma drugs.
Subject terms: Parasite biology, Membrane proteins
Introduction
Toxoplasma gondii and Plasmodium falciparum are unicellular protozoan parasites that belong to the phylum Apicomplexa. P. falciparum is the most deadly of the Plasmodium species that cause malaria in humans1. T. gondii infects a large proportion of the world’s population and can cause severe disease in immunocompromised individuals2. T. gondii can also have devastating effects on the development of foetuses when it infects women during pregnancy2. New medicines are needed urgently against both parasites.
The disease-causing tachyzoite stage of T. gondii parasites utilizes both glucose and glutamine as energy sources for the generation of ATP3,4. These parasites acquire glucose through a plasma-membrane localized Glucose Transporter (TgGT1)3. Glucose is metabolized via glycolysis to generate pyruvate. Pyruvate has multiple possible fates in the parasite5. It can be transported into the mitochondrion, where it is further metabolized by the TCA cycle to generate ATP by oxidative phosphorylation6. Alternatively, pyruvate can be metabolized to form l-lactate, which is then exported from the parasite7,8.
Lactate synthesis is catalyzed by lactate dehydrogenase (LDH). The T. gondii genome encodes two LDH enzymes (TgLDH1 and TgLDH2), neither of which is required for proliferation of the disease-causing tachyzoite stage under standard in vitro culture conditions9,10. T. gondii tachyzoites that are rendered defective in glucose import or some aspects of glycolysis can still grow in vitro and in vivo, catabolizing glutamine via the TCA cycle to produce ATP and generating essential glycolytic intermediates via gluconeogenesis4,7,11–13. When glucose and glutamine are both available, both are used for ATP generation7.
By contrast, the disease-causing blood stages of P. falciparum parasites rely almost exclusively on glycolysis to generate ATP14. Here, l-lactate is the major product of glycolysis and must be exported from the parasite in symport with H+ by the P. falciparum Formate Nitrite Transporter (PfFNT) present on the parasite’s plasma membrane15,16. Members of the FNT family transport a variety of monocarboxylates and other anions17–19. They are found in numerous prokaryotic and eukaryotic microorganisms, but are not present in mammalian cells15. Compounds that kill P. falciparum parasites via inhibition of PfFNT have recently been identified, thereby validating PfFNT as a novel antimalarial drug target20,21.
A recent study provided evidence that the three homologues of PfFNT encoded in the T. gondii genome are l-lactate transporters that localize to the plasma membrane when expressed from a non-native promoter22. Compounds found to inhibit the activity of the transporters showed some inhibition of tachyzoite proliferation at micromolar concentrations, leading to the suggestion that the TgFNTs might be essential during the T. gondii lytic cycle and ‘druggable’22. The possibility that the compounds inhibited parasite growth via alternate means (‘off-target effects’) was not excluded. Although T. gondii tachyzoites can survive without glucose, it is possible that in the presence of glucose, the lack of a l-lactate efflux mechanism would cause l-lactate to accumulate intracellularly to concentrations sufficient to jeopardize pH regulation and/or osmotic stability. However, a genome-wide screen has provided evidence that each of the TgFNTs are dispensable during the lytic cycle23, and a TgFNT1 knockout parasite line was recently generated and found to grow normally despite secreting less lactate into the medium than wild-type parasites24. Here, we employed genetic and physiological approaches to investigate the roles of the three TgFNTs in situ and to determine whether the activity of one or more TgFNTs is required for the proliferation of T. gondii tachyzoites.
Results
TgFNTs localize to the parasite plasma membrane
The T. gondii genome encodes three members of the FNT family, termed TgFNT1 (TGGT1_209800), TgFNT2 (TGGT1_292110) and TgFNT3 (TGGT1_229170)22. The TgFNTs share considerable sequence similarity with each other, with PfFNT, and with the well-characterized E. coli FNT family member FocA (Supplementary Fig. S1).
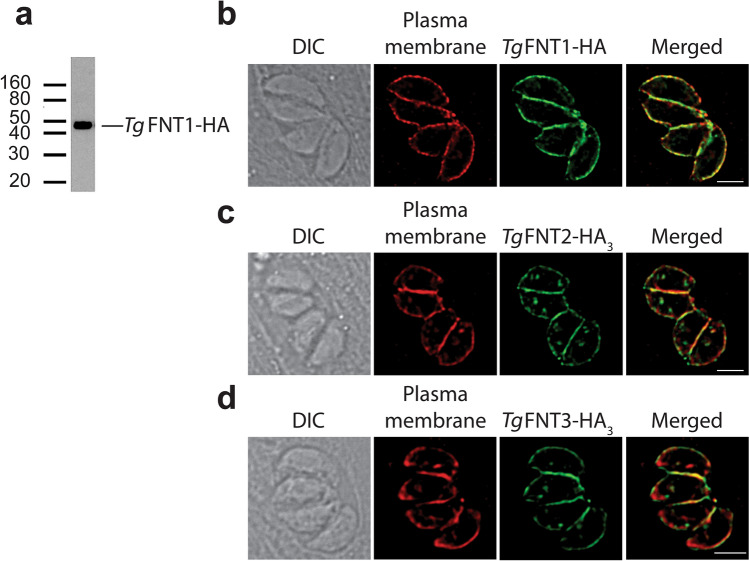
To investigate the expression and localization of each TgFNT, we attempted to generate parasite lines in which a single hemagglutinin (HA) tag was fused to the 3′ end of the open reading frame of the encoding gene. This approach was successful for TgFNT1 (Supplementary Fig. S2). Western blotting with an anti-HA antibody revealed that the resultant TgFNT1-HA protein is expressed in tachyzoites and that it had the expected mass of approximately 45 kDa (Fig. 1a). Immunofluorescence assays revealed that TgFNT1-HA localizes to the T. gondii plasma membrane (Fig. 1b).
Figure 1.
TgFNT1 and ectopically-expressed TgFNT2 and TgFNT3 localize to the plasma membrane of T. gondii. (a) Western blot of TgFNT1-HA-expressing parasites performed using an anti-HA antibody. The uncropped image is shown in Supplementary Fig. S3. (b–d) Immunofluorescence assay reveals co-localization of TgFNT1-HA (b; green), TgFNT2-HA3 (c; green) and TgFNT3-HA3 (d; green) with the plasma membrane marker P3039 (red). The scale bars represent 2 µm.
Our attempts to generate parasites expressing detectable levels of TgFNT2-HA or TgFNT3-HA with this approach were not successful. An alternate approach was therefore used to localize TgFNT2 and TgFNT3. Parasites were transiently transfected with a vector containing the open reading frame of the encoding gene fused at the 3′ end to a 3 × HA tag, under the regulation of the constitutive α-tubulin promoter. Consistent with the findings of Erler et al.22, the ectopically expressed TgFNT2-HA3 and TgFNT3-HA3 proteins both localized to the T. gondii plasma membrane (Fig. 1c,d).
TgFNTs are not required for tachyzoite proliferation in vitro
To determine whether any of the TgFNTs are important for parasite proliferation, we used CRISPR-Cas9 targeted genome editing to generate parasites in which the open reading frame of the tgfnt1, tgfnt2 or tgfnt3 gene was disrupted by frameshift mutations. For tgfnt1, we selected a clone (‘Δtgfnt1’) with a single nucleotide insertion at position 176 from the start codon of the gene. For tgfnt2, we selected a clone (‘Δtgfnt2’) in which nucleotide 126 was deleted, and for tgfnt3 (‘Δtgfnt3’) we selected a clone with an insertion of five nucleotides from position 123 of the gene. In all cases, the resulting frameshifts led to the introduction of premature stop codons and truncation of the encoded proteins (Supplementary Fig. S4), which are likely to render the resulting proteins non-functional. We also generated a parasite line in which all three tgfnt genes were disrupted. This was performed by first targeting the tgfnt1 gene in Δtgfnt3 parasites. We selected a clone (‘Δtgfnt1/3’) that had a single nucleotide insertion at position 176 of the tgfnt1 gene. Next, we targeted the tgfnt2 gene in Δtgfnt1/3 parasites. We selected a clone (‘Δtgfnt1/2/3’) with a single nucleotide insertion at position 127 of the tgfnt2 gene. Each of the tgfnt genes in Δtgfnt1/2/3 parasites has a premature stop codon (Supplementary Fig. S4).
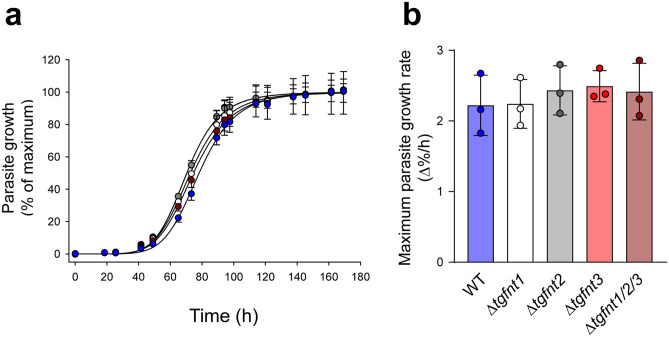
We tested whether any of the TgFNT isoforms were important for parasite proliferation. We first assessed this using plaque assays. Plaques (zones of clearance) in a host cell monolayer result from multiple cycles of host cell invasion, replication and lysis, with the size of the plaques providing an indication of parasite growth rate. Δtgfnt1, Δtgfnt2, Δtgfnt3, and Δtgfnt1/2/3 parasites all produced plaques comparable in size to those of their parents (Supplementary Fig. S5), indicating no significant growth defect in any of the four knockout lines.
We also compared the growth rates of the different parasites using a fluorescence assay (described previously25), taking advantage of the fact that all the parasites express a tandem dimeric Tomato (tdTomato) red fluorescent protein. In each experiment, a sigmoidal growth curve was generated for each parasite line, with a lag phase, exponential growth phase and plateau (Fig. 2a). Using the maximum slope of the growth curve as a read-out for parasite proliferation, we found no differences in growth rates among Δtgfnt1, Δtgfnt2, Δtgfnt3, Δtgfnt1/2/3, and wild-type parental parasites (Fig. 2b). We conclude that none of the TgFNT isoforms, individually or collectively, are required for the normal proliferation of tachyzoites under in vitro culture conditions.
Figure 2.
TgFNTs are not required for tachyzoite proliferation in vitro. (a) Data from a single representative experiment showing the growth of the different parasite lines (wild-type, blue; Δtgfnt1, white; Δtgfnt2, grey; Δtgfnt3, red; Δtgfnt1/2/3, dark red). Parasite growth was normalized to the maximum level reached for each parasite line after subtraction of the background fluorescence. The mean and SD from triplicate measurements shown. Where not shown, error bars fall within the symbols. For clarity, only positive or negative error bars are shown for the data for certain parasite lines. (b) The maximum growth rate for each parasite line. The bars show the mean ± SD obtained from three independent experiments for each line, and the symbols show the results obtained in individual experiments. There was no significant difference in the maximum growth rate between any of the parasite lines (P = 0.8; one-way ANOVA).
TgFNTs transport l-lactate, with TgFNT1 making the primary contribution to l-lactate transport across the plasma membrane in extracellular tachyzoites
We used the tgfnt knockout parasites to investigate whether one or more of the TgFNTs provide a route for l-lactate transport across the plasma membrane in T. gondii. The parasite experiments described in this section were all performed with extracellular tachyzoites that had either recently emerged from their host cells or that had been manually released from their host cells by passage of the cell culture through a 26G needle. First, we investigated the effect on parasite cytosolic pH of adding a high concentration of l-lactate to the extracellular solution. This approach has been used previously with P. falciparum parasites to investigate PfFNT, which transports l-lactate in symport with H+ ions15,16. Although the physiological role of PfFNT is to remove l-lactate from the parasite cytosol, it is bidirectional. Thus, when a high concentration of l-lactate is added to the extracellular solution to impose an inward l-lactate concentration gradient, a decrease in cytosolic pH resulting from l-lactate:H+ entry can be detected15,21.
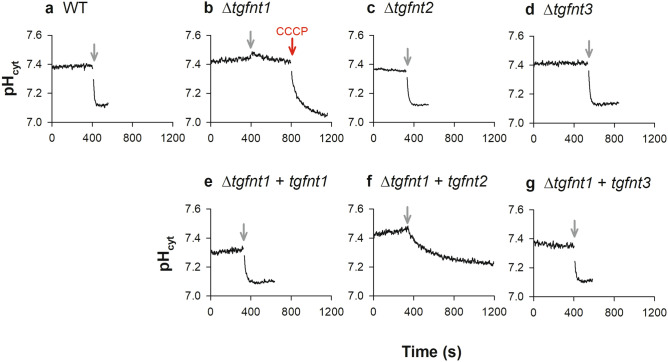
We measured cytosolic pH in extracellular T. gondii tachyzoites by loading them with the pH-sensitive dye BCECF. The measurements were performed at 4 °C to reduce the rate at which pH-regulatory mechanism(s) (including the H+-extruding V-type H+ ATPase26,27) counteract the l-lactate-mediated pH change. When the wild-type parental parasites suspended in a physiological saline solution were exposed to 10 mM l-lactate, we observed a rapid decrease in the pH of the parasite cytosol (Fig. 3a). An abrupt l-lactate-induced decrease in cytosolic pH was also seen in Δtgfnt2 (Fig. 3c) and Δtgfnt3 (Fig. 3d) parasites. In contrast, the addition of 10 mM l-lactate to Δtgfnt1 parasites had little effect on the pH of the cytosol (Fig. 3b), consistent with parasites lacking TgFNT1 having a greatly reduced capacity for l-lactate:H+ symport across the plasma membrane. The subsequent addition of the protonophore CCCP (10 µM) dissipated the H+ gradient across the plasma membrane, with the cytosolic pH decreasing to a value close to the extracellular pH (Fig. 3b), demonstrating the responsiveness of the pH-detection system in these cells.
Figure 3.
Measurements of the effect of l-lactate on cytosolic pH (pHcyt) reveal a role for TgFNT1 in lactate transport across the parasite plasma membrane. Extracellular tachyzoites were loaded with BCECF and suspended in Saline Solution at 4 °C. The grey arrows denote the addition of 10 mM l-lactate. The red arrow denotes the addition of the protonophore CCCP (10 µM). The top panels show data for Δtgfnt1 (b), Δtgfnt2 (c), Δtgfnt3 (d) parasites and their wild-type parental parasites (a). The bottom panels show data for Δtgfnt1 parasites complemented with tgfnt1 (e), tgfnt2 (f) or tgfnt3 (g). The traces are from a single experiment for each parasite line, and are representative of those obtained in at least three independent experiments. The time taken to reach a minimum pHcyt value after the addition of l-lactate was > 400 s in all experiments for Δtgfnt1 parasites complemented with tgfnt2, compared to < 150 s in all experiments for WT parasites, Δtgfnt2 parasites, Δtgfnt3 parasites, and Δtgfnt1 parasites complemented with tgfnt1 or tgfnt3.
To confirm that the reduction in l-lactate:H+ transport in Δtgfnt1 parasites was a consequence of the absence of TgFNT1 expression, we complemented Δtgfnt1 parasites with an ectopically-expressed copy of tgfnt1 (under the control of the constitutive α-tubulin promoter). In these parasites, the addition of 10 mM l-lactate to the extracellular solution caused a pronounced decrease in cytosolic pH (Fig. 3e). These data are consistent with TgFNT1 serving as the primary l-lactate:H+ transporter on the plasma membrane in extracellular tachyzoites.
We also investigated whether TgFNT2 or TgFNT3 could restore l-lactate:H+ transport in Δtgfnt1 parasites when constitutively expressed in these parasites. We found that Δtgfnt1 parasites complemented with either tgfnt2 or tgfnt3 under the control of the α-tubulin promoter underwent a decrease in cytosolic pH on addition of 10 mM l-lactate to the extracellular solution (Fig. 3f,g). The rate of the l-lactate -induced cytosolic pH decrease was lower in Δtgfnt1 parasites complemented with tgfnt2 (Fig. 3f) than in parasites complemented with tgfnt1 (Fig. 3e) or tgfnt3 (Fig. 3g). The initial rates (ΔpH/min, mean ± SEM, n = 3; determined by fitting lines to the initial linear portions of the traces) were 0.82 ± 0.19, 0.18 ± 0.09, and 0.57 ± 0.10 for Δtgfnt1 parasites complemented with tgfnt1, tgfnt2 and tgfnt3, respectively. There was a significant difference between the rates of pH decrease for Δtgfnt1 parasites complemented with tgfnt2 and Δtgfnt1 parasites complemented with either tgfnt1 or tgfnt3 (P < 0.05, unpaired t-tests). This could result from a difference between TgFNT2 and the other TgFNTs with respect to transporter function, regulation, and/or plasma membrane expression. Together, our data show that knockout of TgFNT1 prevents l-lactate -induced pH changes in the parasite cytosol, which can be rescued by ectopic expression of TgFNT1, TgFNT2 and TgFNT3. Our data also indicate that TgFNT2 and TgFNT3 make minimal contribution to l-lactate:H+ transport in wild-type tachyzoites. It is possible that the native tgfnt2 and tgfnt3 genes are not expressed at significant levels during this stage of the parasite life cycle.
Our pH data implicate TgFNT1 as the primary l-lactate transporter in tachyzoites. Erler et al.22 provided evidence for l-lactate transport by TgFNT1-3 expressed in yeast, however truncation of the proteins was required to achieve their functional expression. To test whether full-length TgFNT1 is a l-lactate transporter, we expressed it in Xenopus oocytes. We have previously expressed PfFNT in this system and shown that it transports l-[14C]lactate15 and is inhibited by the antiplasmodial compound MMV00783921. We measured the uptake of l-[14C]lactate into Xenopus oocytes expressing full-length TgFNT1 (with a C-terminal HA tag) at 27.5 °C over 10 min at pH 6.4. The uptake of l-[14C]lactate by oocytes injected with 30 ng of cRNA encoding TgFNT1 was higher than that of non-injected oocytes and of oocytes injected with 30 ng of cRNA encoding PfFNT (P < 0.001; one-way ANOVA; Fig. 4). Consistent with previous results21, l-[14C]lactate uptake by PfFNT-expressing oocytes was inhibited by MMV007839 (4 µM; Fig. 4). In contrast, 4 µM MMV007839 had no effect on the uptake of l-[14C]lactate by TgFNT1-expressing oocytes (Fig. 4). These data provide direct evidence that TgFNT1 is a l-lactate transporter. The finding that TgFNT1 is less sensitive than PfFNT to inhibition by MMV007839 is consistent with the results of Erler et al.22, who found that 29 µM MMV007839 was required for half-maximal inhibition of l-lactate transport by a C-terminally truncated form of TgFNT1, whereas half-maximal inhibition of l-lactate transport by PfFNT is achieved at sub-micromolar concentrations20,21.
Figure 4.
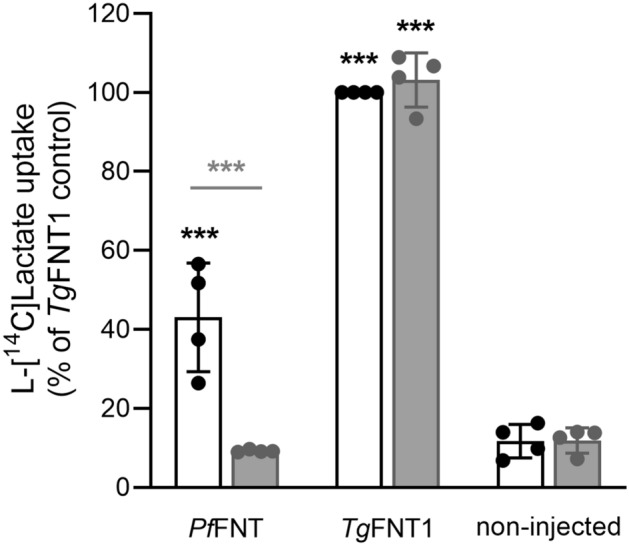
TgFNT1 transports l-lactate in Xenopus oocytes. l-[14C]lactate uptake into non-injected oocytes and oocytes expressing PfFNT or TgFNT1-HA was measured in the absence of compound (0.008% v/v DMSO; solvent control; white bars) and in the presence of the PfFNT inhibitor MMV007839 (4 µM; grey bars). The uptake of l-[14C]lactate is expressed as a percentage of that determined for TgFNT1-HA in the absence of MMV007839. The data are averaged from four independent experiments (using oocytes from different frogs), within which measurements were made from 7 to 10 oocytes per treatment. The symbols show the data from each experiment and the bars show the mean ± SD. Where not shown, error bars fall within the symbols. The data were tested for statistical difference from the non-injected control (black asterisks). The data obtained in the presence or absence of MMV007839 were also compared for each oocyte type (grey asterisks). ***P < 0.001 (one-way ANOVA with post hoc Tukey test); other comparisons did not show significant differences.
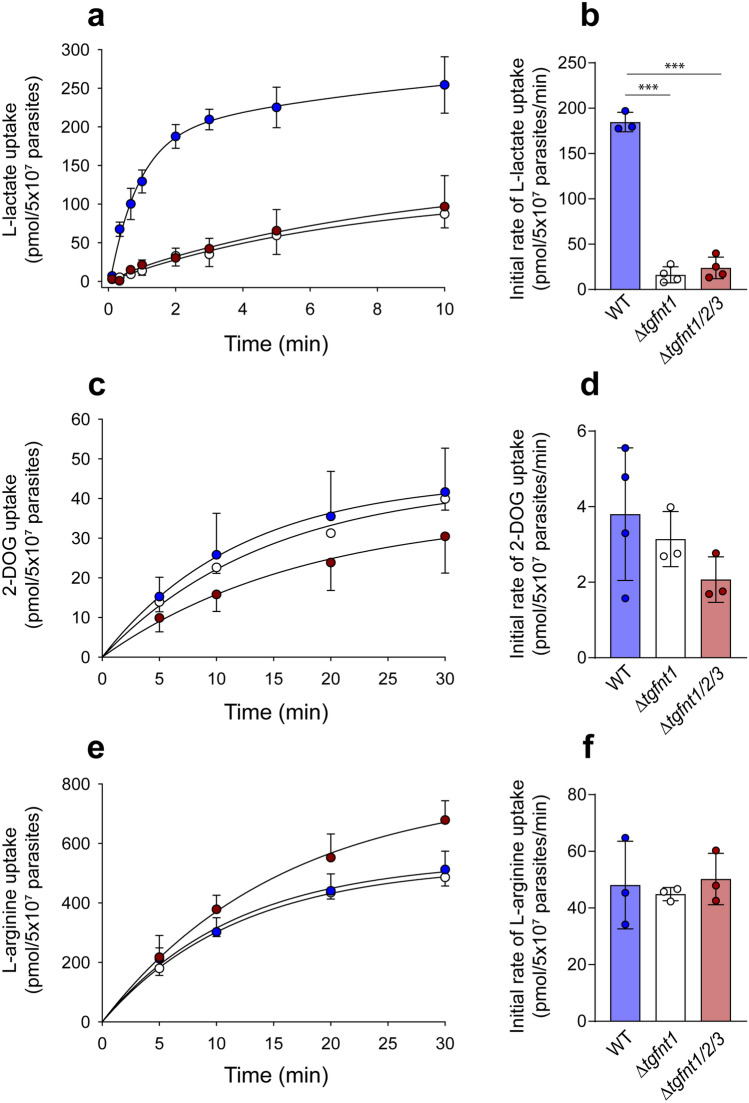
As a final test of the role of TgFNT1 in l-lactate transport across the plasma membrane in extracellular tachyzoites, we measured the uptake of l-[14C]lactate by extracellular Δtgfnt1, Δtgfnt1/2/3, and wild-type parental parasites. As noted above, FNTs mediate bidirectional transport, and measuring the movement of l-[14C]lactate into parasites provides a convenient means of assessing transport activity. Similar to previous experiments with P. falciparum15,21, the experiments with T. gondii presented here were conducted at a low pH (6.1) to increase (H+-coupled) l-lactate uptake by the parasites, and at a low temperature (4 °C) to slow the transport process. We found that the uptake of lactate, as measured over 10 min, was greatly reduced in Δtgfnt1 and Δtgfnt1/2/3 parasites relative to wild-type parental parasites, with little difference observed between Δtgfnt1 and Δtgfnt1/2/3 parasites (Fig. 5a). We estimated the initial rate of l-lactate uptake for each parasite line. The initial rate of l-lactate uptake was > 7.5-fold greater in wild-type parental parasites than in Δtgfnt1 or Δtgfnt1/2/3 parasites, and there was no significant difference in the initial rate between Δtgfnt1 and Δtgfnt1/2/3 parasites (Fig. 5b). Consistent with the pH data, these data indicate that TgFNT1 plays the primary role in l-lactate transport across the plasma membrane in wild-type extracellular parasites.
Figure 5.
TgFNT1 is critical for l-lactate transport across the parasite plasma membrane. (a,c,e) Time courses for the uptake of l-lactate (a), 2-DOG (c) and L-arginine (e) by wild-type parasites (blue symbols), Δtgfnt1 parasites (white symbols) and Δtgfnt1/2/3 parasites (dark red symbols). The data shown are the mean ± SD from three to four independent experiments for each line. For clarity, only positive or negative error bars are shown for the data for certain parasite lines. Where not shown, error bars fall within the symbols. (b,d,f) The initial rates of uptake for l-lactate (b), 2-DOG (d), and L-arginine (f) estimated from the same set of experiments. The bars show the mean ± SD obtained from three or four independent experiments for each line, and the symbols show the results obtained in individual experiments. For panels b,d and f, the data for each parasite line were compared with the data for every other parasite line (one-way ANOVA, followed by post hoc Tukey tests where significant differences were present). ***P < 0.001; other comparisons (those in d and f) did not reveal significant differences.
To investigate the possibility that disruption of the tgfnt1 gene could give rise to a general defect in solute uptake, we measured the uptake of two additional radiolabelled compounds: the glucose analogue [14C]2-deoxyglucose ([14C]2-DOG) and the amino acid l-[14C]arginine. The time courses for the uptake of 2-DOG and L-arginine were similar in wild-type, Δtgfnt1 and Δtgfnt1/2/3 parasites (Fig. 5c,e), and there were no significant differences in the initial rates of uptake for either of these compounds between any of the parasite lines (Fig. 5d,f). These data indicate that Δtgfnt1 and Δtgfnt1/2/3 parasites do not have a general defect in solute uptake, but rather have a specific defect in the uptake of FNT substrates including l-lactate.
Discussion
In this study we pinpoint TgFNT1 as the primary contributor to l-lactate transport across the plasma membrane of extracellular T. gondii tachyzoites. Tagging the 3′ end of the native tgfnt1 gene revealed that the encoded protein localizes to the parasite plasma membrane. Experiments in which TgFNT1 was studied in isolation in Xenopus oocytes provided direct evidence that full-length TgFNT1 mediates the transport of l-[14C]lactate. Using genetic and physiological approaches we confirmed that TgFNT1 functions as a plasma membrane l-lactate transporter in situ. We measured the rate of l-lactate transport across the parasite plasma membrane, and found that it was greatly reduced in Δtgfnt1 tachyzoites compared to wild-type parasites. We also investigated the effect on cytosolic pH of adding 10 mM l-lactate to the external medium. The decrease in cytosolic pH normally induced by a large inward l-lactate concentration gradient was not observed in Δtgfnt1 parasites, consistent with these parasites having a defect in l-lactate:H+ transport. Complementing Δtgfnt1 parasites with an ectopic copy of the tgfnt1 gene restored the l-lactate-induced pH change.
Consistent with Erler et al.22, we found that TgFNT2 and TgFNT3 localize to the plasma membrane when expressed ectopically under the control of a non-native promoter. Furthermore, in line with previous evidence that truncated forms of TgFNT1-3 transport l-lactate in yeast22, our data suggest that (full-length) TgFNT2 and TgFNT3 can transport l-lactate. Complementing Δtgfnt1 parasites with constitutively-expressed tgfnt2 or tgfnt3 resulted in the restoration of l-lactate:H+ transport, as observed in measurements of cytosolic pH.
Our data indicate that, despite being capable of transporting l-lactate, TgFNT2 and TgFNT3 make little contribution to l-lactate transport across the plasma membrane in wild-type extracellular tachyzoites. Disrupting tgfnt1 alone was sufficient to abolish the l-lactate-induced acidification observed in parental parasites. Furthermore, there was no difference in the rate of l-[14C]lactate transport across the plasma membrane in Δtgfnt1 parasites and Δtgfnt1/2/3 parasites. Thus, expression of the endogenous tgfnt2 or tgfnt3 genes in Δtgfnt1 parasites does not appear to compensate for the l-lactate transport defect observed when TgFNT1 is not expressed. It is possible that TgFNT2 and TgFNT3 are not expressed at appreciable levels during the lytic cycle in the T. gondii strain used in this study. This would also explain our inability to localize these proteins by tagging the 3′ ends of the endogenous genes. Our data are partially supported by a recent proteome-wide localization study of tachyzoites, which predicted that TgFNT1 localizes to the plasma membrane whereas TgFNT3 was not detectable in the proteome28. This study predicted that TgFNT2 may localize to micronemes, which is inconsistent with our localization studies.
A key finding in our study was that the lack of expression of all three TgFNT proteins does not affect the proliferation of T. gondii tachyzoites under standard in vitro culture conditions. This contrasts with the situation in P. falciparum, where PfFNT has been validated as a drug target and must therefore be important for parasite proliferation20,21. However, it is consistent with previous studies highlighting the metabolic flexibility of T. gondii parasites, including recent evidence that the LDH enzymes required to convert pyruvate to lactate are not essential for T. gondii proliferation under standard in vitro culture conditions9,10. Our data are also consistent with a genome-wide screen that provided evidence that each of the TgFNTs are dispensable during the lytic cycle (the ‘phenotype scores’ for TgFNT1, TgFNT2 and TgFNT3 were 1.10, 1.31 and -0.23, respectively23), and with a very recent study in which TgFNT1 was knocked out24. Kloehn et al.24 found that TgFNT1 knockout parasites grew normally in vitro, but that they secreted less lactate into the medium than wild-type parasites. Our finding that all three TgFNTs can be knocked out simultaneously call into question the view that the TgFNTs are suitable drug targets, and suggest that the compounds reported to inhibit the activity of TgFNT proteins by Erler et al.22 are unlikely to exert their effects on T. gondii growth via inhibition of any of the three transporters.
It should be noted that the disruption of certain glycolytic enzymes that are not essential for the in vitro proliferation of T. gondii parasites has been associated with virulence defects in mice and/or reductions in the formation of bradyzoite cysts in the brain. Parasites lacking hexokinase had a greatly reduced ability to form mature bradyzoite cysts in the brain in mice compared to parental parasites13. Furthermore, while tachyzoites in which the genes encoding TgLDH1 and TgLDH2 were simultaneously disrupted proliferated normally under standard conditions9,10, their growth was impaired in low-oxygen conditions in vitro10 and in mice9,10, and the numbers of bradyzoite cysts formed in the brain were greatly reduced9,10. Thus, it remains possible that the expression of one or more TgFNT proteins is important in vivo and/or in different stages of the T. gondii life cycle.
Methods
Ethics statement
Ethical approval of the work performed with the adult female Xenopus laevis frogs was obtained from the Australian National University (ANU) Animal Experimentation Ethics Committee (Animal Ethics Protocol Number A2013/13) in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Host cell and parasite culture
T. gondii parasites were cultured in human foreskin fibroblasts (a gift from Holger Schlüter, Peter MacCallum Cancer Centre) at 37 °C in a humidified 5% CO2 incubator. The culture medium was Dulbecco’s modified Eagle’s medium (DMEM) containing 25 mM glucose, and supplemented with 1% v/v fetal calf serum, 200 µM L-glutamine, 50 U/mL penicillin, 50 µg/mL streptomycin, 10 µg/mL gentamicin, and 0.25 µg/mL amphotericin B. TATi/Tomato parasites29 were used as the parental wild-type strain for the ∆tgfnt parasite strains that we generated in this study. TATi∆ku80 parasites30 were used as the parental wild-type strain for the 3′ replacement strains generated in this study.
Generation of genetically modified T. gondii lines for localization studies
To generate a 3′ HA tag replacement in the tgfnt1 locus, we amplified a 1.1 kb region of the 3′ end of the tgfnt1 open reading frame using primers p4 and p5 (Supplementary Table S1). The PCR product was digested with BglII and AvrII and ligated into the BglII and AvrII sites of the vector pgCH(ΔPstI), a variant of pgCH31 wherein the PstI site has been filled in. The resulting vector was linearized with SphI and transfected into TATi/Δku80 parasites using standard procedures32, with successfully transfected parasites selected with chloramphenicol.
TgFNT2 and TgFNT3 were localized by expressing C-terminally 3 × HA tagged forms of the proteins under the control of the constitutive α-tubulin promoter. The tgfnt2 open reading frame was amplified from T. gondii cDNA using primers p6 and p7 (Supplementary Table S1). The PCR product was digested with AvrII and BclI and ligated into the AvrII and BglII sites of the pUgCTH3 plasmid31. The tgfnt3 open reading frame was amplified from cDNA using primers p8 and p9 (Supplementary Table S1). The PCR product was digested with AvrII and BglII and ligated into the AvrII and BglII sites of pUgCTH3. In both cases the vectors were transfected into TATi/tomato parasites29.
Generation and complementation of tgfnt knockout parasites
We used a CRISPR/Cas9-based genome editing approach to generate parasite lines in which the open reading frames of tgfnt genes were disrupted. Using the Q5 Site-Directed Mutagenesis kit (New England Biolabs), we generated modified versions of the pSAG1::Cas9-U6::sgUPRT vector (Addgene plasmid # 5446733) to express guide RNAs targeting each tgfnt gene using a Q5 mutagenesis approach, as described by the manufacturer (New England Biolabs). The primers used for the Q5 mutagenesis reactions were p10 and p11 for tgfnt1, p12 and p11 for tgfnt2, and p13 and p11 for tgfnt3 (Supplementary Table S1). The resulting vectors co-express the guide RNA targeting the appropriate tgfnt gene and Cas9-GFP. They were transfected into TATi/tomato parasites, generating Δtgfnt1, Δtgfnt2, and Δtgfnt3 parasites (Supplementary Fig. S4). Parasites in which two and three tgfnt genes were disrupted were then made by targeting the tgfnt1 gene in Δtgfnt3 parasites then the tgfnt2 gene in Δtgfnt1/3 parasites (Supplementary Fig. S4).
For complementation studies, Δtgfnt1 parasites were transfected with vectors containing the tgfnt1, tgfnt2 or tgfnt3 gene fused at the 3′ end to a 3 × HA tag, under the control of the constitutive α-tubulin promoter. For TgFNT2 and TgFNT3, the vectors used were those described above for the localization of the ectopically expressed proteins. The complementation vector for TgFNT1 was made by amplifying the tgfnt1 open reading frame from genomic DNA using primers p14 and p5 (Supplementary Table S1). The PCR product was digested with AvrII and BglII and ligated into the AvrII and BglII sites of pUgCTH3.
Immunofluorescence assays and western blotting
Immunofluorescence assays (IFAs) and western blots were carried out as described previously25. The primary antibodies used were rat anti-HA (Roche clone 3F10; 1:100 – 1:1000 dilution; used for IFAs and western blots) and mouse anti-P30 (Abcam clone TP3; 1:500 dilution; used for IFAs). The secondary antibodies used for IFAs (all from Life Technologies) were goat anti-rat AlexaFluor 488 (1:100 – 1:250 dilution), goat anti-mouse AlexaFluor 546 (1:500 dilution), and goat anti-mouse AlexaFluor 647 (1:500 dilution). The microscopy was performed as described previously26. For western blots the secondary antibody was horse radish peroxidase-conjugated goat anti-rat (Santa Cruz Biotechnology; 1:5000 dilution).
Parasite growth assays
Parasite growth was measured in the culture medium described above, which contains 25 mM glucose. Plaque assays were performed essentially as described previously32, with 400 parasites added per 25 cm2 flask. Fluorescence growth assays were performed in 96-well plates25,34, with 4000 parasites added to each well and the fluorescence from tdTomato (excitation: 540 nm; emission: 590 nm) used to monitor parasite growth. Prior to estimating parasite growth rates, a value corresponding to the background fluorescence (averaged from wells to which parasites had not been added) was subtracted from all other data. The fluorescence intensity corresponding to maximum growth was then determined for each parasite line by fitting a sigmoidal curve to the data: y = a/[1 + (t/t1/2)b], where y is fluorescence intensity, a is the maximum fluorescence intensity, t is time and t1/2 is the time at which half-maximal growth is reached. The data for each parasite line were then expressed as a % of the maximum growth for that line. For each parasite line, the maximum growth rate was determined from the slope of the sigmoidal curve in a 4 h window surrounding the t1/2 (estimated by fitting the sigmoidal curve described above).
pH measurements in extracellular tachyzoites
Parasites were harvested by passage of cultures through a 26G needle. The parasites were then passed through a 3 µm filter to remove host cell debris and centrifuged for 20 min at 1500×g. The supernatant media was removed and the parasites were suspended in ‘BCF’ (bicarbonate-free RPMI supplemented with 25 mM HEPES, 11 mM additional glucose, 0.2 mM hypoxanthine and pH-adjusted to 7.1) then centrifuged at 12,000 × g for 1 min. The supernatant media was removed and the parasites suspended again in BCF, to which the acetoxymethyl ester (AM) form of the pH-sensitive dye BCECF (Molecular Probes) was added at a final concentration of 5 µM. The parasites were incubated in the BCECF-containing media for 14 min before being centrifuged (12,000 × g, 1 min), resuspended in BCF and maintained at 37 °C. Directly before their use in fluorescence measurements, parasites were centrifuged (12,000 × g, 1 min) and resuspended in pH 7.1 Saline Solution (125 mM NaCl, 5 mM KCl, 25 mM HEPES, 20 mM glucose and 1 mM MgCl2). Fluorescence measurements were performed at 4 °C in a Cary Eclipse Fluorescence Spectrophotometer (Agilent). The excitation wavelengths were 440 nm and 495 nm and the emission wavelength was 520 nm. The ratio of fluorescence (495 nm/440 nm) was used as an indicator of cytosolic pH. The relationship between Fluorescence Ratio and pH was determined by suspending parasites in calibration salines of known pH35 containing 16 µM nigericin (to equilibrate H+ across the plasma membrane) and measuring the Fluorescence Ratio after it stabilized.
Measurements of the uptake of radiolabelled compounds by parasites
The uptake of radiolabelled compounds was measured in extracellular tachyzoites suspended in pH 6.1 Saline Solution (for l-[14C]lactate and l-[14C]arginine experiments) or Glucose-free Saline Solution (for [14C]2-DOG experiments; 125 mM NaCl, 5 mM KCl, 25 mM HEPES, 1 mM MgCl2; pH 6.1). The reactions for l-lactate experiments contained 200 µM unlabelled l-lactate and 0.2 µCi/mL l-[14C]lactate, those for the 2-DOG experiments contained 25 µM unlabelled 2-DOG and 0.2 µCi/mL [14C]2-DOG, and those for the L-arginine experiments contained 50 µM unlabelled L-arginine and 0.1 µCi/mL l-[14C]arginine.
The l-[14C]lactate and [14C]2-DOG experiments were conducted on ice, with centrifugation steps performed at 4 °C, to slow the rate of uptake such that initial rates could be determined. For the l-[14C]arginine experiments, parasites were incubated on ice for 30 min prior to commencing the experiments to mimic exposure conditions for the l-[14C]lactate experiments, but the experiments themselves were performed at 37 °C.
Uptake assays were performed as described previously36. To commence an experiment, a volume of parasite suspension was added to an equal volume of a saline solution containing the radiolabelled compound. The uptake of radiolabelled compounds was stopped at pre-determined time points (in duplicate 200 µL samples) by centrifuging the samples in tubes containing 250 µL oil mix (84% Clerco PM-125 silicone fluid, 16% light mineral oil), thereby sedimenting the parasites below the oil layer. Aliquots (10 µL) of the supernatant solution were taken from above the oil layer to enable the extracellular concentrations of the radiolabelled compounds to be determined. For each tube, the remaining supernatant solution was removed, the tubes washed twice in running water, the oil aspirated, and the parasite pellet lysed with 500 µL 0.1% v/v Triton X-100. The lysate was transferred into a scintillation vial containing 1.5 mL scintillation fluid (Ultima Gold; Perkin Elmer), and the radioactivity measured using a scintillation counter.
To account for the radioactivity present in the extracellular fluid trapped around the cell pellet under the oil layer, we subtracted the amount of radioactivity present at time zero from the remaining data. Where there was significant uptake of radioactivity early in the time course (l-[14C]lactate and l-[14C]arginine experiments), we fit a curve to the time course data for each experiment [y = y0 + a(1 – e(-bx))] to determine the amount of radioactivity present at time zero (y0). For [14C]2-DOG experiments the radioactivity measured at the first time point (taken within 30 s of exposure of parasites to [14C]2-DOG) was subtracted directly. A curve [y = a(1 − e(-bx))] was fitted to the data for intracellular radioactivity versus time and the initial rates were determined from the first derivative.
Experiments with Xenopus laevis oocytes
The frogs were purchased from Nasco (Cat# LM00535M) and were housed in the Xenopus Frog Facility of the ANU Research School of Biology in compliance with the relevant institutional and Australian Government regulations.
The oocyte expression vector containing pffnt was made previously15. The oocyte expression vector containing tgfnt1 was made by amplifying the tgfnt1 open reading frame from gDNA using primers p14 and p5 (Supplementary Table S1). The PCR product was digested with AvrII and XmaI and ligated into the AvrII and XmaI sites of pGHJ-HA31.
Oocytes were harvested from X. laevis frogs and prepared as outlined previously37. cRNA encoding PfFNT and TgFNT1-HA was made using the mMessage mMachine T7 transcription kit and the MEGAclear kit (Ambion) and 30 ng was microinjected into oocytes, as described elsewhere37. The uptake of l-[14C]lactic acid (Na+ salt; 150.6 mCi/mmol; Perkin Elmer) was measured 3–4 days post-injection at 27.5 °C in ND96 buffer (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 10 mM MES and 10 mM Tris-base; pH 6.4). Within each experiment measurements were made from 7 to 10 oocytes per condition. The influx of l-[14C]lactate was terminated by removing the reaction buffer and washing the oocytes twice in 3.5 mL of ice-cold ND96 buffer. The oocytes were then lysed and the radioactivity measured as described elsewhere38.
Supplementary Information
Acknowledgements
This work was supported by an Australian Research Council (ARC) Discovery Early Career Researcher Award (DE160101035 to A.M.L.), ARC Discovery Grants (DP150102883 and DP200100483 to K.K. and G.G.v.D.), an ARC Future Fellowship (1053082 to R.E.M.), and the Australian Department of Education (Australian Postgraduate Awards to S.V.H. and S.H.S.).
Author contributions
J.M.Z., K.K., G.G.v.D. and A.M.L. designed the study. J.M.Z, S.V.H. and S.H.S. performed experiments. J.M.Z., S.V.H., S.H.S., R.E.M., G.G.v.D. and A.M.L. analysed data. R.E.M., K.K., G.G.v.D. and A.M.L. supervised the study and provided resources. J.M.Z., G.G.v.D. and A.M.L. wrote the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Giel G. van Dooren and Adele M. Lehane.
Contributor Information
Giel G. van Dooren, Email: giel.vandooren@anu.edu.au
Adele M. Lehane, Email: adele.lehane@anu.edu.au
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86204-3.
References
- 1.W.H.O. World malaria report. (2018).
- 2.Blader IJ, Coleman BI, Chen CT, Gubbels MJ. Lytic cycle of Toxoplasma gondii: 15 years later. Annu. Rev. Microbiol. 2015;69:463–485. doi: 10.1146/annurev-micro-091014-104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blume M, Rodriguez-Contreras D, Landfear S, Fleige T, Soldati-Favre D, et al. Host-derived glucose and its transporter in the obligate intracellular pathogen Toxoplasma gondii are dispensable by glutaminolysis. Proc. Natl Acad. Sci. USA. 2009;106:12998–13003. doi: 10.1073/pnas.0903831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitzsche R, Zagoriy V, Lucius R, Gupta N. Metabolic cooperation of glucose and glutamine is essential for the lytic cycle of obligate intracellular parasite Toxoplasma gondii. J. Biol. Chem. 2016;291:126–141. doi: 10.1074/jbc.M114.624619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia N, Ye S, Liang X, Chen P, Zhou Y, et al. Pyruvate homeostasis as a determinant of parasite growth and metabolic plasticity in Toxoplasma gondii. mBio. 2019;10:e00898-19. doi: 10.1128/mBio.00898-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayward JA, van Dooren GG. Same same, but different: Uncovering unique features of the mitochondrial respiratory chain of apicomplexans. Mol. Biochem. Parasitol. 2019;232:111204. doi: 10.1016/j.molbiopara.2019.111204. [DOI] [PubMed] [Google Scholar]
- 7.MacRae JI, Sheiner L, Nahid A, Tonkin C, Striepen B, et al. Mitochondrial metabolism of glucose and glutamine is required for intracellular growth of Toxoplasma gondii. Cell Host Microbe. 2012;12:682–692. doi: 10.1016/j.chom.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohsaka A, Yoshikawa K, Hagiwara T. 1H-NMR spectroscopic study of aerobic glucose metabolism in Toxoplasma gondii harvested from the peritoneal exudate of experimentally infected mice. Physiol. Chem. Phys. 1982;14:381–384. [PubMed] [Google Scholar]
- 9.Abdelbaset AE, Fox BA, Karram MH, Abd Ellah MR, Bzik DJ, et al. Lactate dehydrogenase in Toxoplasma gondii controls virulence, bradyzoite differentiation, and chronic infection. PLoS ONE. 2017;12:e0173745. doi: 10.1371/journal.pone.0173745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia N, Yang J, Ye S, Zhang L, Zhou Y, et al. Functional analysis of Toxoplasma lactate dehydrogenases suggests critical roles of lactate fermentation for parasite growth in vivo. Cell. Microbiol. 2018;20:e12794. doi: 10.1111/cmi.12794. [DOI] [PubMed] [Google Scholar]
- 11.Nitzsche R, Gunay-Esiyok O, Tischer M, Zagoriy V, Gupta N. A plant/fungal-type phosphoenolpyruvate carboxykinase located in the parasite mitochondrion ensures glucose-independent survival of Toxoplasma gondii. J. Biol. Chem. 2017;292:15225–15239. doi: 10.1074/jbc.M117.802702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen B, Sibley LD. Toxoplasma aldolase is required for metabolism but dispensable for host-cell invasion. Proc. Natl Acad. Sci. USA. 2014;111:3567–3572. doi: 10.1073/pnas.1315156111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukla A, Olszewski KL, Llinas M, Rommereim LM, Fox BA, et al. Glycolysis is important for optimal asexual growth and formation of mature tissue cysts by Toxoplasma gondii. Int. J. Parasitol. 2018;48:955–968. doi: 10.1016/j.ijpara.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 14.MacRae JI, Dixon MW, Dearnley MK, Chua HH, Chambers JM, et al. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013;11:67. doi: 10.1186/1741-7007-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti RV, et al. A lactate and formate transporter in the intraerythrocytic malaria parasite, Plasmodium falciparum. Nat. Commun. 2015;6:6721. doi: 10.1038/ncomms7721. [DOI] [PubMed] [Google Scholar]
- 16.Wu B, Rambow J, Bock S, Holm-Bertelsen J, Wiechert M, et al. Identity of a Plasmodium lactate/H+ symporter structurally unrelated to human transporters. Nat. Commun. 2015;6:6284. doi: 10.1038/ncomms7284. [DOI] [PubMed] [Google Scholar]
- 17.Czyzewski BK, Wang DN. Identification and characterization of a bacterial hydrosulphide ion channel. Nature. 2012;483:494–497. doi: 10.1038/nature10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia W, Tovell N, Clegg S, Trimmer M, Cole J. A single channel for nitrate uptake, nitrite export and nitrite uptake by Escherichia coli NarU and a role for NirC in nitrite export and uptake. Biochem. J. 2009;417:297–304. doi: 10.1042/BJ20080746. [DOI] [PubMed] [Google Scholar]
- 19.Suppmann B, Sawers G. Isolation and characterization of hypophosphite-resistant mutants of Escherichia coli: Identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol. Microbiol. 1994;11:965–982. doi: 10.1111/j.1365-2958.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 20.Golldack A, Henke B, Bergmann B, Wiechert M, Erler H, et al. Substrate-analogous inhibitors exert antimalarial action by targeting the Plasmodium lactate transporter PfFNT at nanomolar scale. PLoS Pathog. 2017;13:e1006172. doi: 10.1371/journal.ppat.1006172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hapuarachchi SV, Cobbold SA, Shafik SH, Dennis AS, McConville MJ, et al. The malaria parasite's lactate transporter PfFNT is the target of antiplasmodial compounds identified in whole cell phenotypic screens. PLoS Pathog. 2017;13:e1006180. doi: 10.1371/journal.ppat.1006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erler H, Ren B, Gupta N, Beitz E. The intracellular parasite Toxoplasma gondii harbors three druggable FNT-type formate and l-lactate transporters in the plasma membrane. J. Biol. Chem. 2018;293:17622–17630. doi: 10.1074/jbc.RA118.003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidik SM, Huet D, Ganesan SM, Huynh MH, Wang T, et al. A genome-wide CRISPR screen in Toxoplasma identifies essential apicomplexan genes. Cell. 2016;166:1423–1435. doi: 10.1016/j.cell.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloehn J, Oppenheim RD, Siddiqui G, De Bock PJ, Kumar Dogga S, et al. Multi-omics analysis delineates the distinct functions of sub-cellular acetyl-CoA pools in Toxoplasma gondii. BMC Biol. 2020;18:67. doi: 10.1186/s12915-020-00791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dooren GG, Tomova C, Agrawal S, Humbel BM, Striepen B. Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc. Natl Acad. Sci. USA. 2008;105:13574–13579. doi: 10.1073/pnas.0803862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehane AM, Dennis ASM, Bray KO, Li D, Rajendran E, et al. Characterization of the ATP4 ion pump in Toxoplasma gondii. J. Biol. Chem. 2019;294:5720–5734. doi: 10.1074/jbc.RA118.006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno SN, Zhong L, Lu HG, Souza WD, Benchimol M. Vacuolar-type H+-ATPase regulates cytoplasmic pH in Toxoplasma gondii tachyzoites. Biochem. J. 1998;330:853–860. doi: 10.1042/bj3300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barylyuk K, et al. A comprehensive subcellular atlas of the Toxoplasma proteome via hyperLOPIT provides spatial context for protein functions. Cell Host Microbe. 2020;28:752–766.e759. doi: 10.1016/j.chom.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker KER, Fairweather SJ, Rajendran E, Blume M, McConville MJ, et al. The tyrosine transporter of Toxoplasma gondii is a member of the newly defined apicomplexan amino acid transporter (ApiAT) family. PLoS Pathog. 2019;15:e1007577. doi: 10.1371/journal.ppat.1007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheiner L, Demerly JL, Poulsen N, Beatty WL, Lucas O, et al. A systematic screen to discover and analyze apicoplast proteins identifies a conserved and essential protein import factor. PLoS Pathog. 2011;7:e1002392. doi: 10.1371/journal.ppat.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajendran E, Hapuarachchi SV, Miller CM, Fairweather SJ, Cai Y, et al. Cationic amino acid transporters play key roles in the survival and transmission of apicomplexan parasites. Nat. Commun. 2017;8:14455. doi: 10.1038/ncomms14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Striepen, B. & Soldati, D. Genetic manipulation of Toxoplasma gondii. In Toxoplasma gondii: The Model Apicomplexan - Perspectives and Methods. (Weiss, L. D., and Kim, K. eds.) 391–415 (Elsevier, 2007).
- 33.Shen B, Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. mBio. 2014;5:e01114–14. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubbels MJ, Li C, Striepen B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chemother. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saliba KJ, Kirk K. pH regulation in the intracellular malaria parasite, Plasmodium falciparum. H+ extrusion via a V-type H+-ATPase. J. Biol. Chem. 1999;274:33213–33219. doi: 10.1074/jbc.274.47.33213. [DOI] [PubMed] [Google Scholar]
- 36.Rajendran E, Kirk K, van Dooren GG. Measuring solute transport in Toxoplasma gondii parasites. Methods Mol. Biol. 2020;2071:245–268. doi: 10.1007/978-1-4939-9857-9_14. [DOI] [PubMed] [Google Scholar]
- 37.van Schalkwyk DA, Nash MN, Shafik SH, Summers RL, Lehane AM, et al. Verapamil-sensitive transport of quinacrine and methylene blue via the Plasmodium falciparum Chloroquine Resistance Transporter reduces the parasite's susceptibility to these tricyclic drugs. J. Infect. Dis. 2016;213:800–810. doi: 10.1093/infdis/jiv509. [DOI] [PubMed] [Google Scholar]
- 38.Richards SN, Nash MN, Baker ES, Webster MW, Lehane AM, et al. Molecular mechanisms for drug hypersensitivity induced by the malaria parasite's Chloroquine Resistance Transporter. PLoS Pathog. 2016;12:e1005725. doi: 10.1371/journal.ppat.1005725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibley LD, Krahenbuhl JL, Adams GM, Weidner E. Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. J. Cell Biol. 1986;103:867–874. doi: 10.1083/jcb.103.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.