Abstract
In the first case, a 60-year-old man was referred to our hospital for a sudden stomachache. A computed tomography scan revealed a thrombus at ascending aorta with acute mesenteric ischemia. In the second case, a 62-year old man developed a hypoglycemic attack with unbalanced diet. A computed tomography showed a thrombus at ascending aorta without thromboembolism. Laboratory data of both cases showed elevated platelet and a loss of antithrombin III. We administered a resection of thrombus to prevent a systemic embolism. We suggested that the risk of ascending aorta thrombus was elevated platelet and a loss of antithrombin III.
Keywords: thrombus, ascending aorta, platelet
Introduction
A localized thrombus involving the ascending aorta rarely occurs in the absence of an underlying cause, such as chest trauma, atherosclerosis, a hypercoagulable state, or instrumentation.1) Conversely, the cause of thrombus was not often revealed. A high elevated platelet was suggested risk of an arterial thrombus.2) We reported the two cases of a protruding thrombus at the ascending aorta.
Case.1
A 60-year-old man was referred to our hospital for a sudden stomachache. He presented with no previous history of thrombosis; he received an endoscopic mucosal resection for colon cancer two weeks prior. The pathological diagnosis was tubular adenocarcinoma, a well-differentiated type with submucosal layer and no vascular invasion, with a negative specimen stump. A computed tomography scan simultaneously revealed a protruding tumor at ascending aorta and an embolism in the superior mesenteric artery (Figs. 1A and 1B). An electrocardiogram showed sinus rhythm. Laboratory data showed elevated platelet, a loss of antithrombin III (63%), and mild liver dysfunction. No tumor was present in the heart, and the cardiac function was normal based on the echocardiography results. He was diagnosed was acute superior mesenteric artery occlusion and ascending aortic thrombus.
Fig. 1 (A, B) A contrast computed tomography showed thrombosis in ascending aorta (arrows). (C) In operative findings, the thrombus was size of 25×40 mm, fragile and high mobile (double arrows).
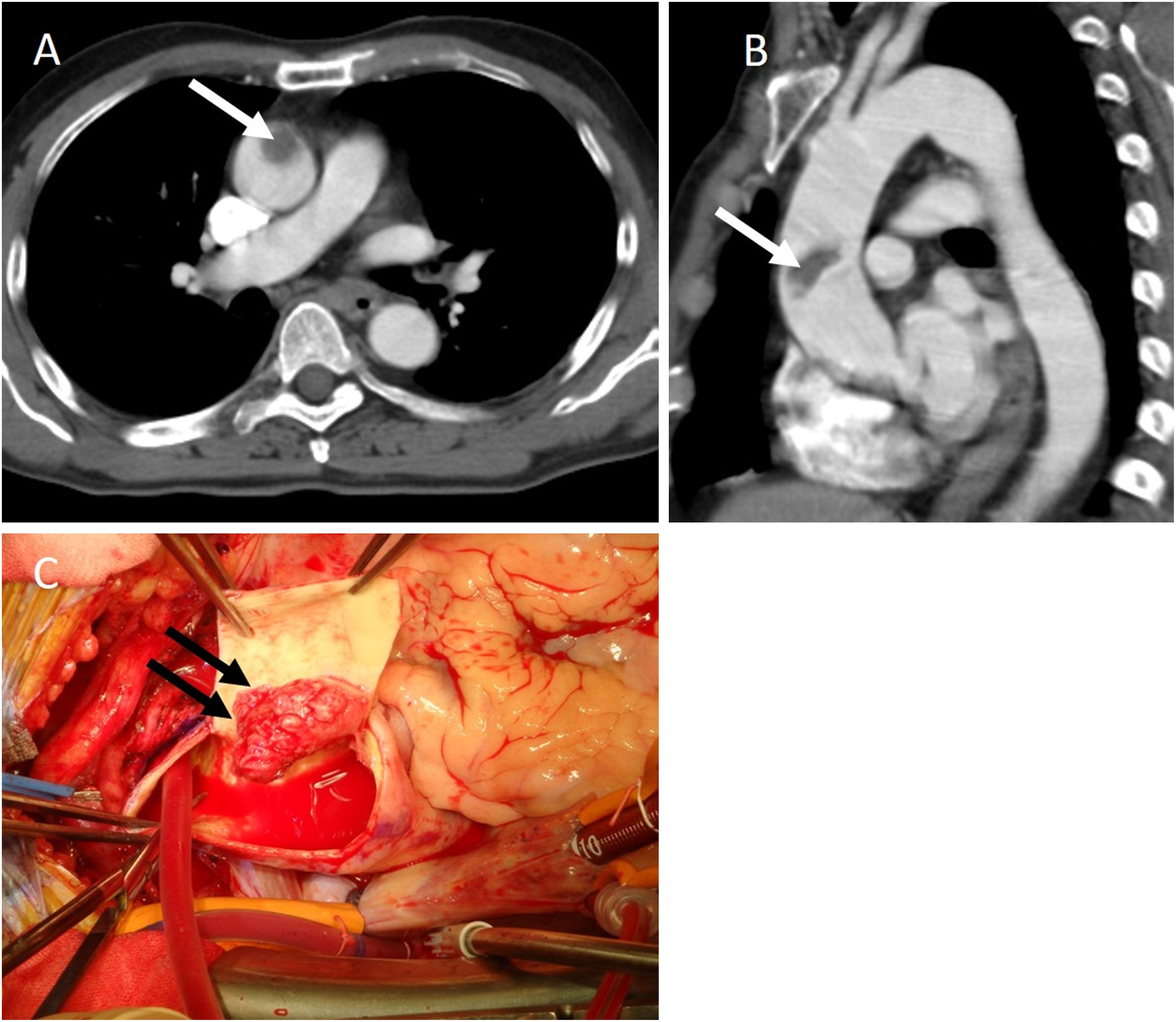
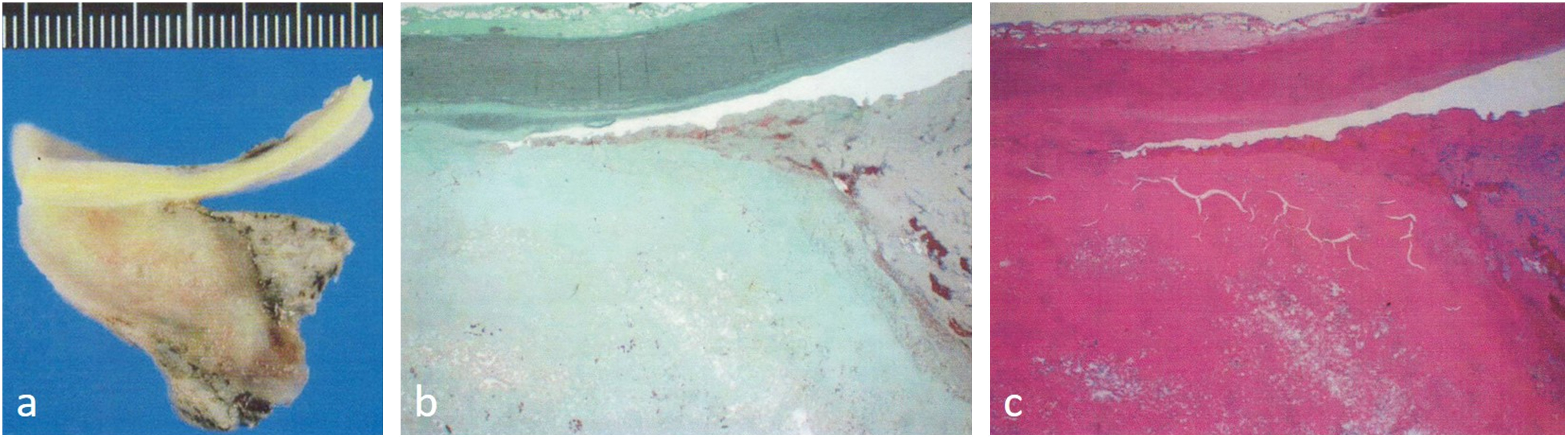
We surgically resected the thrombus at ascending aorta after bowel resection. Intraoperative transesophageal echocardiography revealed the protruding thrombus (22.5×15.7 mm in size) to be mobile with a smooth surface. With supported cardiopulmonary bypass, the tumor and ascending aorta was resected under circulation arrest and selective antegrade cerebral perfusion. The thrombus was size of 25×40 mm, fragile and high mobile (Fig. 1C). The ascending aortic wall was a smooth surface and less arteriosclerotic change. A replacement of the ascending aorta was performed using a prosthesis graft [J Graft SHIELD NEO, 26 mm (Japan Lifeline Co., Ltd., Tokyo, Japan)]. Postoperative laboratory data showed a decline of antithrombin III (67%) and normal platelet. The patient survived for 2 years postoperatively. The pathological findings indicated a thrombus originating from intimal laminae. The joint surface of the aortic wall and thrombus was clear, and the ascending aorta showed no plaque or malignancy (Fig. 2). Postoperatively, warfarin was administered.
Fig. 2 The pathological findings showed thrombus originating from intimal laminae. The joint surface with aortic wall and thrombus was clear, and the ascending aorta showed no plaque and no malignancy.
Case.2
A 62-year old man was referred to our hospital for a hypoglycemic attack with unbalanced diet. Laboratory data showed high elevated platelet and a loss of antithrombin III (72%). In additional examination, a computed tomography showed a thrombus as a 10×11 mm at ascending aorta without thromboembolism (Fig. 3A). We decided to perform a resection of thrombus to prevent a systemic embolism. Under the cardiopulmonary bypass, the ascending aorta was clamped proximal to the brachiocephalic artery because the thrombus was confirmed by an intraoperative direct echocardiography (movie). The thrombus with ascending aorta was resected. The thrombus exhibited a size of 10×11 mm, high mobile and fragile (Fig. 3B). The aortic wall was smooth surface and less arteriosclerotic change. A replacement of the ascending aorta was performed using a prosthesis graft [Triplex, 24 mm (Terumo Co., Tokyo, Japan)]. Postoperative laboratory data showed a decline of antithrombin III (70%) and normal platelet. The patient was in good condition for 12 months postoperatively. The pathological findings showed a thrombus without malignancy. Warfarin was administered for 3 months after the operation.
Fig. 3 (A) A contrast computed tomography showed thrombus in ascending aorta (arrow). (B) In operative findings, protruding thrombosis (16×10 mm: double arrows) in ascending aorta.

Discussion
In both cases, the differential diagnosis was arrested thrombus, vegetation, malignant tumor, etc. The vegetation was excluded because of no infectious symptoms. The characteristics of the malignant tumor were a wide basis and hard adhesions.
A previous study reported that the cause of an ascending aorta thrombus could be chest trauma, atherosclerosis, a hypercoagulable state, or instrumentation.1) The etiology of a thrombus in the aorta is complex. Aortic thrombi can be caused by blood disorders (e.g., protein S or protein C deficiency and anti-phospholipid antibody syndrome), tumors, aortitis, collagen disease, aortic structural abnormalities (e.g., aortic aneurysms), intra-aortic atheroma, hormone therapy, steroid use, and atrial fibrillation.3) In both cases, electrocardiogram showed sinus rhythm. No previous trauma or a supported device was found. Laparche et al. reported that risk factors of mobile thrombus in the aortic arch included heavy smoking, hypercholesterolemia, and elevated fibrinogen (> 400 mg/dl).4) In addition, the hypercoagulable state related with malignant tumor has been referred to as Trousseau’s syndrome, one of the paraneoplastic syndromes.5,6) One case suggested that it might be related with a malignant tumor. In both cases, laboratory data showed high elevated platelet and a loss of antithrombin III (Supplemental). Because disease was not obvious, blood agglutination by dehydration can cause thrombus.2)
Because a surgical indication of ascending aorta thrombus was risk of embolism, these cases were performed in emergency operation. The previous study reported that endovascular therapy may be a useful option for descending thoracic aortic thrombus.7) In addition, few case reports of endovascular treatment for ascending aortic thrombus exist.8)
Our strategy of procedure was resection of all thrombus and aortic wall all together. In the previous report, a floating thrombus in the ascending aorta was suggested to be associated with atherosclerosis of the aortic wall. We replaced ascending aorta with prosthesis graft in order to prevent recurrence and systemic embolism.3,9) One case was clamp and another case was non-clamp. The difference in these cases was the site of thrombus. If the thrombus was near brachiocephalic artery, a non-clamp procedure was selected. We were able to avoid recurrent embolism. The pathological findings revealed that the thrombus originating from intimal laminae and the joint surface was clear, and the ascending aorta showed no plaque or malignancy.
Conclusion
We reported an extremely rare two cases where surgical repair was performed for an ascending aorta thrombus. We proved that the thrombus originated in the intimal laminae with edge clarity by pathological diagnosis. Lastly, we suggested that the risk of ascending aorta thrombus was elevated platelet and a loss of antithrombin III.
Disclosure Statement
There are no conflicts of interest to declare.
Additional Note
Because this study followed the ethical standards of the institution, this work does not require ethical considerations.
Author Contributions
Study conception: HM, TY
Data collection: KY, NS
Analysis: NA
Investigation: NA, KY, NS
Writing: NA
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Supplementary Information
Supplementary movie is available at the online article site on J-STAGE and PMC.
Supplementary Data
References
- 1).Mosquera VX, Cuenca JJ, Pazos P, et al. Subclinical thrombosis of the ascending aorta: a possible paraneoplastic syndrome. Ann Thorac Surg 2009; 88: 263-5. [DOI] [PubMed] [Google Scholar]
- 2).van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol 2019; 16: 166-79. [DOI] [PubMed] [Google Scholar]
- 3).Yamase Y, Kuwabara F, Watanabe K, et al. A case of floating thrombus in the ascending aorta that caused recurrent peripheral arterial embolic events. J Cardiol Cases 2015; 12: 57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Laperche T, Laurian C, Roudaut R, et al. Mobile thromboses of the aortic arch without aortic debris. A transesophageal echocardiographic finding associated with unexplained arterial embolism. Circulation 1997; 96: 288-94. [DOI] [PubMed] [Google Scholar]
- 5).Trousseau A. Phlegmasia alba dolens. Clinique Medicale de l’Hotel-Dieu de Paris, 654-712, (In French). Translated by the New Sydenham Society, London as ‘Lectures on Clinical Medicine delivered at the Hotel Dieu de Paris,’ 281-95, 1872.
- 6).Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Semin Thromb Hemost 1999; 25: 173-82. [DOI] [PubMed] [Google Scholar]
- 7).Kahlberg A, Montorfano M, Cambiaghi T, et al. Endovascular stent-grafting of the ascending aorta for symptomatic parietal thrombus. J Endovasc Ther 2016; 23: 969-72. [DOI] [PubMed] [Google Scholar]
- 8).Meyermann K, Trani J, Caputo FJ. Descending thoracic aortic mural thrombus presentation and treatment strategies. J Vasc Surg 2017; 66: 931-6. [DOI] [PubMed] [Google Scholar]
- 9).Wang B, Ma D, Cao D, et al. Huge thrombus in the ascending aorta: a case report and literature review. J Cardiothorac Surg 2019; 14: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


