Abstract
Objective: To determine the prognostic value of regional tissue oxygenation saturation (rSO2) for ulcer healing after endovascular treatment (EVT) of peripheral arterial disease (PAD).
Materials and Methods: Among PAD patients, 34 patients with chronic limb-threatening ischemia underwent EVT for limb salvage. We retrospectively analyzed the cutoff rSO2 values on postoperative day 1 to predict ulcer healing and patient prognosis. Skin perfusion pressure (SPP) and transcutaneous oxygen pressure (TcPO2) were also used to assess wound healing.
Results: A finger-mounted tissue oximeter can easily measure rSO2 on the dorsal foot. Among the 34 patients, the ulcer healed in 25, and no changes were observed in 2 patients at 1 month after EVT. However, 7 patients needed major amputation at the same time. Wound healing was achieved in all patients with rSO2≥50%. With this cutoff, the sensitivity and specificity of the new device for wound healing were 100% and 64%, respectively. In all the wound healing cases, SPP was ≥45 mmHg, and TcPO2 was ≥40 mmHg.
Conclusion: To assess limb ischemia, rSO2 can be measured quickly and easily using this device. We suggest that an rSO2>50% shows good prognosis for ulcer healing.
Keywords: pilot study, oximetry, near-infrared spectroscopy, ischemia, wound healing
Introduction
The prevalence of peripheral arterial disease (PAD) has been reported to be 3%–10%, increasing to 15%–20% in people aged >70 years, and 0.1% of the population experience chronic limb-threatening ischemia (CLTI).1–3) In Japan, the prevalence of PAD has been reported to be 1%–3%, and the incidence is increasing.4,5) CLTI represents the most advanced form of PAD and is associated with high rates of mortality and amputation.1) Several guidelines on PAD have been published. The Society for Vascular Surgery Wound, Ischemia and foot Infection (WIfI) classification system has been proposed to predict 1-year amputation risk and potential benefits from revascularization.6) Assessing the blood flow of lower limbs is important in this context.
Various non-invasive methods have been used to diagnose PAD, including ankle brachial index (ABI), toe brachial index (TBI), skin perfusion pressure (SPP),7) and transcutaneous oxygen pressure (TcPO2). However, these methods have some limitations. ABI is the current gold standard for evaluating PAD worldwide, and values≤0.9 have a specificity of ≥98% to detect PAD. However, ABI values above 1.3 are commonly observed in patients with diabetes or chronic kidney disease, probably due to the calcification of arteries of the lower extremities,8) such that false-negative ABI results occur in 17%–24% of patients with diabetes and on hemodialysis.9) ABI measurements are painful for patients because of the compression exerted by the cuff. Moreover, the measurement site is limited to the ankle.10) The American College of Cardiology Foundation/American Heart Association 2005 guidelines similarly defined TBI<0.7 as a diagnostic for PAD; however, none of the studies cited in support of this cutoff value actually advocated the use of TBI<0.7 as a diagnostic tool. Moreover, TBI can diagnose PAD, but fails to give an indication of its severity.11) SPP is a non-invasive method that uses laser Doppler measurement to measure arterial microcirculatory pressure at the skin level and slowly reduces the inflation cuff pressure at the measurement site to detect red blood cell movement. Although SPP is believed to help assess the severity of lower limb ischemia, patients often experience intolerable pain caused by cuff pressure.7)
TcPO2 was developed to measure skin tissue oxygenation. When carbon dioxide (PCO2) is released from the tissue, it diffuses through the membrane into the electrolyte, where it reacts with carbonated water. The signal detected at the electrodes is digitized and reconverted to pressure of oxygen (PO2) and PCO2 expressed in mmHg. Measuring TcPO2 requires a warm-up period of 15 min for the sensor electrodes, a flat skin surface on which to place the sensors, and a stable body position.12,13)
It is difficult to use the traditional diagnostic methods in patients with rest pain and skin ulcers. Therefore, we focused on a novel, quick, simple, accurate, and painless method that can be used on any skin area; near-infrared spectroscopy (NIRS) is used to assess tissue oxygenation. In NIRS, the light-emitting diodes emit light at two different wavelengths, which penetrate the tissue. The reflection wavelengths obtained from oxygenated hemoglobin and deoxygenated hemoglobin are used to calculate the regional tissue oxygenation saturation (rSO2).13,14) The method was developed more than 20 years ago and has been used for limb blood flow evaluation in muscles with a depth of 30 mm or more; it is useful in PAD patients with intermittent claudication. However, its usefulness in skin blood flow and wound healing has not been shown.15,16) Although available, these previously described tests are unsuitable for predicting prognosis after postoperative revascularization. Currently, no test can be used as an index for improving blood circulation in the early postoperative period of endovascular treatment (EVT).
A finger-mounted tissue oximeter using the NIRS technique (Toccare: Astem Co., Ltd., Kawasaki, Japan) was developed, enabling skin measurements at a 5-mm depth.14) In obstetrics and gynecology, this device is used transvaginally to touch the scalp of the fetus and evaluate fetal acidosis.17,18) Assessment of lower limb blood flow using this device has already been performed in patients with PAD and in healthy volunteers, and its correlation with conventional devices has been shown.19) In this study, a newly developed finger-mounted tissue oximeter was used to measure tissue oxygenation before and after EVT, with the purpose of assessing lower limb blood flow to predict wound healing and treatment efficacy.
Materials and Methods
Participants
In this single-center observational study, we retrospectively analyzed the database of patients with CLTI who underwent EVT in the Hamamatsu University School of Medicine Hospital from January 2016 to April 2019 and consented to follow-up. When treating patients with CLTI, we first assess whether bypass surgery is possible or not based on the patient’s condition, and if the patient’s condition is poor, we consider EVT. All patients had non-healing ulcerations or gangrene (Fontaine stage IV). Lower limb echo-color Doppler was performed preoperatively on all patients to confirm the absence of varicose veins or deep vein thrombosis in order to exclude venous ulcers. Patients were included in the study if they had successful revascularization of at least one artery among the iliac, superficial femoral, popliteal, tibioperoneal trunk, anterior tibial, posterior tibial, or peroneal arteries at the final EVT angiography. Patients with rest pain but no ulceration or gangrene (Rutherford class 4), with EVT failure, and with infection based on the WIfI classification criteria for foot infection were excluded (Fig. 1).
Fig. 1 Patient selection and classification scheme.
EVT: endovascular treatment

Patients were divided into two groups: those who achieved wound healing without major amputation 1 month after surgery were assigned to the wound healing group, and those who did not were assigned to the non-wound healing group.
Study approval
The study protocol was approved by the Ethical Review Board of Hamamatsu University School of Medicine (approval number 16-057). The study protocol was registered in the UMIN Clinical Trials Registry (UMIN-CTR; ID: UMIN000025021). Written informed consent was obtained from all participants.
Procedure
A team of 2 vascular surgeons and 1 vascular technician performed the tests in our institution. Before EVT, we first measured rSO2 of the foot dorsum with the new device. Next, we measured TcPO2 of the foot dorsum, then ABI, and finally SPP. We allowed 5 min of rest between ABI and SPP measurements to eliminate the side effects of cuff pressure. On the first day after EVT, we measured rSO2, TcPO2, ABI, and SPP of the foot dorsum in the same manner.
The instruments used for each measurement were as follows: ABI: BP-203, Omron, Kyoto, Japan; SPP: SensiLase PAD 3000, Vasamed, Eden Prairie, MN, USA; TcPO2: TCM 4 series, Radiometer, Copenhagen, Denmark; and rSO2: Toccare: Astem Co., Ltd., Kawasaki, Japan. All procedures and measurements were performed with patients in the supine position. The temperature of the laboratory was maintained at 25°C.
All EVT procedures were performed with the patients under local anesthesia, and no oxygen was given to the patients before or after treatment. Our vascular surgery unit has a wound management team with plastic surgeons, rehabilitation doctors, and nutritionists, which manages the patients’ wounds after EVT.
Clinical outcomes
The endpoint of this study was wound healing without major amputation (above or below the knee) or treatment re-intervention. All patients were followed up at 1 week, 1 month, and 3 months after treatment, with vascular surgeons determining the extent of wound healing.
Statistical analysis
The unpaired t-test was used to compare continuous variables, and the chi-square test was used to compare categorical variables between the wound healing and non-healing groups. Data are shown as mean±standard deviation. To determine whether rSO2, TcPO2, and SPP measurements can function as predictors of wound healing, we calculated the threshold values for rSO2, TcPO2, and SPP for predicting wound healing in patients with CLTI. Receiver operating characteristic (ROC) analysis was performed. p values <0.05 were considered to be statistically significant. Data analyses were performed using SPSS 24.0 software (IBM Corporation, Armonk, NY, USA).
Results
Between August 2016 and July 2019, 89 EVT procedures were performed in our department, of which 40 were performed for patients with Fontaine stage IV (Fig. 1). Four cases of foot infections and 2 cases with technical failure were excluded. The remaining 34 patients were included in this study. Patients’ demographics, stratified by groups, are shown in Table 1. Among the 9 wound non-healing patients, 4 underwent repeat EVT, and 2 underwent major amputation within 1 month after surgery. When background factors were compared between the two groups, there was a tendency for the wound healing group to be slightly depleted in dialyzed cases compared with the wound non-healing group, but this was not statistically significant. Next, the condition of the lower extremity was evaluated, but there were no significant differences between the wound healing group and the non-healing group in preoperative ABI, SPP, TcPO2, and rSO2 (Table 1). Regarding ulcer location, most ulcers were located on the toes, and patients with arterial lesions limited to the region below the knee tended to be more frequent in the non-healing group than in the wound healing group (Table 1).
Table 1 Baseline characteristics of the patients and lower limbs.
| All (n=34) | Wound healing (n=25) | Wound non-healing (n=9) | p Value | |
|---|---|---|---|---|
| Age, years | 74.7±9.7 | 74.2±9.8 | 76.3±9.2 | 0.572 |
| Male sex | 25 (73.5%) | 18 (72.0%) | 7 (77.8%) | 0.917 |
| Hypertension | 18 (52.9%) | 14 (56.0%) | 4 (44.4%) | 0.707 |
| Diabetes mellitus | 17 (50.0%) | 13 (52.0%) | 4 (44.4%) | 0.719 |
| Dyslipidemia | 16 (47.1%) | 12 (48.0%) | 4 (44.4%) | 0.837 |
| Hemodialysis | 20 (58.8%) | 12 (48.0%) | 8 (88.9%) | 0.050 |
| Smoking | 23 (67.6%) | 15 (60.0%) | 8 (88.9%) | 0.214 |
| Coronary artery disease | 22 (64.7%) | 14 (56.0%) | 8 (88.9%) | 0.113 |
| Cerebrovascular disease | 5 (14.7%) | 5 (20.0%) | 0 (0%) | 0.293 |
| Total protein level, mg/dL | 6.8±0.5 | 6.8±0.4 | 6.9±0.8 | 0.727 |
| Albumin level, mg/dL | 3.5±0.5 | 3.5±0.5 | 3.5±0.5 | 1.000 |
| Hemoglobin level, mg/dL | 10.7±1.9 | 10.7±2.0 | 10.8±1.4 | 1.000 |
| HbA1c level, % | 6.3±1.0 | 6.4±1.1 | 6.1±0.8 | 0.396 |
| Total cholesterol level, mg/dL | 157.2±30.1 | 154.7±31.9 | 164.1±25.6 | 0.390 |
| Triglyceride level, mg/dL | 103.8±42.7 | 100.0±37.5 | 114.9±52.1 | 0.447 |
| Rutherford category 5 | 33 (97.1%) | 24 (96.0%) | 9 (100%) | |
| Rutherford category 6 | 1 (2.9%) | 1 (4.0%) | 0 (0%) | 0.543 |
| WIfI stage 1 | 5 (14.7%) | 5 (20.0%) | 0 (0%) | |
| WIfI stage 2 | 4 (11.8%) | 4 (16.0%) | 0 (0%) | |
| WIfI stage 3 | 18 (52.9%) | 11 (44.0%) | 7 (77.8%) | |
| WIfI stage 4 | 7 (20.6%) | 5 (20.0%) | 2 (22.2%) | 0.111 |
| Location of the ulcer | ||||
| Toe | 28 (82.3%) | 20 (80.0%) | 8 (88.9%) | |
| Dorsum | 3 (8.8%) | 3 (12.0%) | 0 (0%) | |
| Heel | 2 (5.9%) | 1 (4.0%) | 1 (11.1%) | |
| Ankle | 1 (2.9%) | 1 (4.0%) | 0 (0%) | |
| ABI before EVT | 0.56±0.17 (n=31, 91.2%) | 0.56±0.20 (n=23, 92.0%) | 0.56±0.08 (n=8, 89.9%) | 1.000 |
| SPP before EVT | 30.8±12.7 (n=24, 70.6%) | 30.1±12.4 (n=18, 72.0%) | 32.8±13.3 (n=6, 66.7%) | 0.654 |
| TcPO2 before EVT | 23.4±20.6 (n=29, 85.3%) | 24.1±21.1 (n=22, 88.0%) | 21.3±18.7 (n=7, 77.8%) | 0.756 |
| rSO2 before EVT | 46.3±4.5 (n=34, 100%) | 46.5±4.5 (n=25, 100%) | 45.9±4.4 (n=9, 100%) | 0.732 |
| Treated lesion | ||||
| Aortoiliac | 5 (14.7%) | 4 (16.0%) | 1 (11.1%) | 0.846 |
| Femoropopliteal | 23 (67.6%) | 19 (68.0%) | 4 (44.4%) | 0.187 |
| Infrapopliteal | 16 (47.1%) | 8 (32.0%) | 8 (88.9%) | 0.006 |
| EVT procedure | ||||
| A-I | 2 (5.9%) | 2 (8.0%) | 0 (0.0%) | 0.382 |
| A-I+Fem-Pop | 2 (5.9%) | 2 (8.0%) | 0 (0.0%) | 0.382 |
| A-I+Fem-Pop+tibial | 1 (2.9%) | 0 (0.0%) | 1 (11.1%) | 0.091 |
| Fem-Pop | 14 (41.2%) | 13 (52.0%) | 1 (11.1%) | 0.033 |
| Fem-Pop+tibial | 6 (17.6%) | 4 (16.0%) | 2 (22.2%) | 0.675 |
| Tibial | 9 (26.25%) | 4 (16.0%) | 5 (55.6%) | 0.021 |
Data are presented as mean±standard deviation or n (%). HbA1c: glycated hemoglobin; WIfI: Society for Vascular Surgery Wound, Ischemia and foot Infection; ABI: ankle brachial index; EVT: endovascular treatment; SPP: skin perfusion pressure; TcPO2: transcutaneous oxygen pressure; rSO2: regional tissue oxygenation saturation; A-I: iliac artery; Fem-Pop: superficial femoral artery and popliteal artery
With regard to the EVT procedures, there were 34 technically successful EVT procedures. The distributions of the focal arterial target lesions for revascularization are shown in Table 1. Comparison of the two groups identified that EVT of a solitary tibial artery was most likely to be associated with non-wound healing.
ABI, SPP, TcPO2, and rSO2 measurements were performed before and after EVT, but some patients could not be tested for ABI and SPP because of excessive pain caused by cuff pressure (3 and 5 patients for ABI, 10 and 8 for SPP, before and after surgery, respectively). The TcPO2 measurement requires at least 15 min of rest but could not be performed because of involuntary movements and pain in 5 and 6 patients before and after the operation, respectively. The rSO2 measurement was performed in all patients without difficulty (Supplemental Table 1).
On postoperative day (POD) 1, ABI values were 0.77±0.19 in the wound healing group and 0.65±0.06 in the non-healing group. SPP values were 44.5±10.6 in the wound healing group and 27.9±12.1 in the non-healing group. TcPO2 values were 34.3±23.3 in the wound healing group and 14.6±15.0 in the non-healing group. rSO2 values were 50.1±4.8 in the healing group and 46.4±2.0 in the non-healing group (Fig. 2). ABI was elevated compared with the preoperative values, but without statistical significance. All the other tests demonstrated significant changes after EVT.
Fig. 2 Comparison of postoperative blood flow assessments between the wound healing and non-wound healing groups.
EVT: endovascular treatment

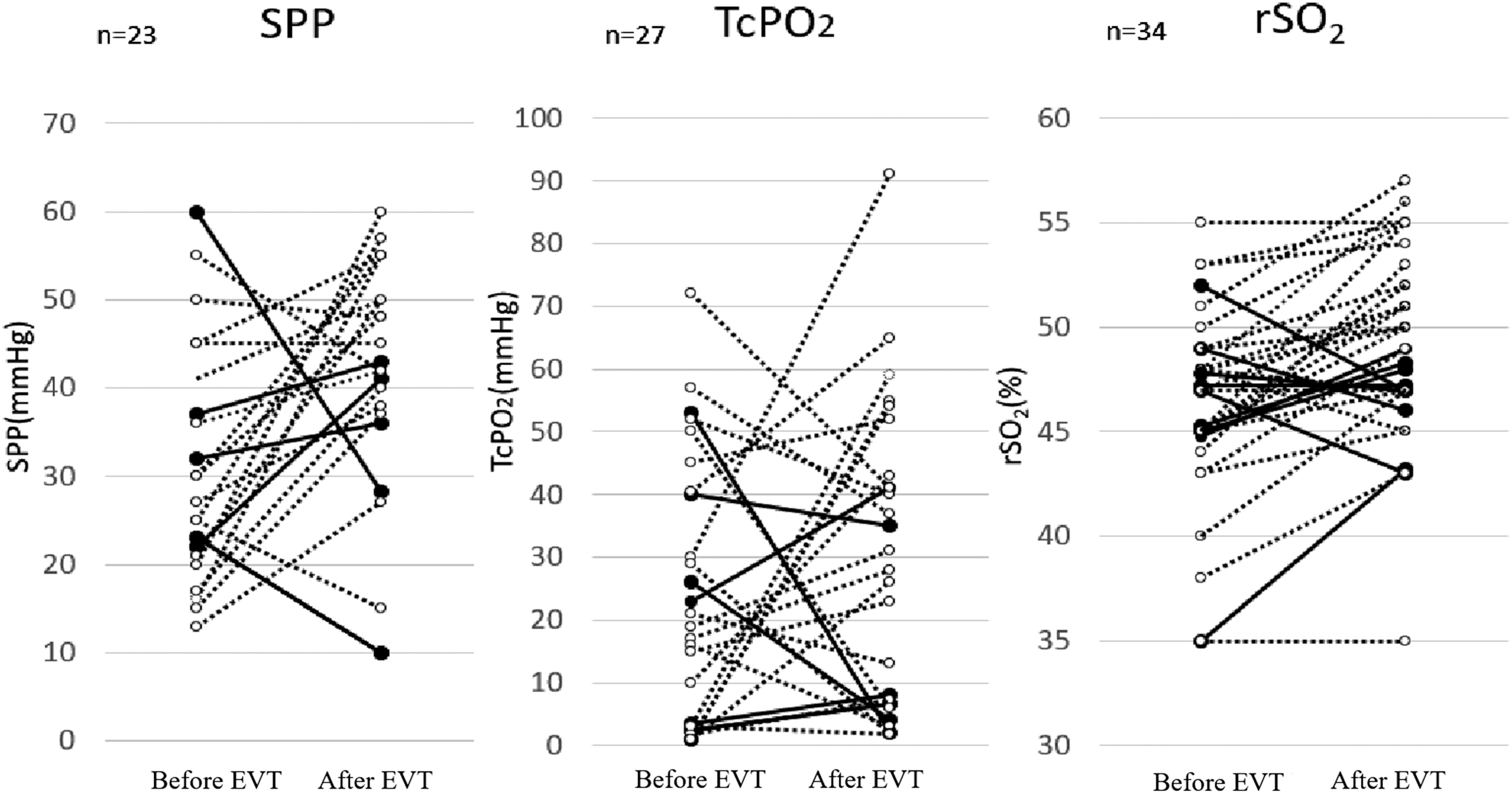
Figure 3 shows the transition of perfusion parameters of SPP, TcPO2, and rSO2 from pre-EVT to POD 1 in each case. Some cases had values that worsened even after successful EVT procedures, and they were associated with wound non-healing. There were cases of wound non-healing even though the patients’ SPP or TcPO2 values were >40 mmHg. However, wound healing was achieved in all patients with rSO2 values of 50% or more after EVT (Fig. 3).
Fig. 3 Changes in blood flow measurements before and after EVT. Each line represents a change in the value for each case. Dotted and solid lines represent the wound healing group and the non-wound healing group, respectively.
EVT: endovascular treatment; SPP: skin perfusion pressure; TcPO2: transcutaneous oxygen pressure; rSO2: regional tissue oxygenation saturation
In the ROC analysis of SPP, the area under the curve (AUC) was 0.846 (95% confidence interval [CI] 0.683–1.000, p<0.01). The optimal cutoff value for predicting wound healing was 36.5 mmHg with a sensitivity of 89.5% and a specificity of 71.4%. For TcPO2, the AUC was 0.725 (95%CI 0.523–0.927, p=0.081). The optimal cutoff value for predicting wound healing was 10.5 mmHg with a sensitivity of 75.0% and a specificity of 71.4%. ROC analysis of rSO2 showed an AUC of 0.780 (95%CI 0.629–0.931, p=0.014). The optimal cutoff value for predicting wound healing was 49.5 mmHg, with 100% sensitivity and 64% specificity (Fig. 4).
Fig. 4 ROC curves for predicting wound healing. ROC curves for the estimation of cutoff values for predicting wound healing using SPP, TcPO2, and rSO2.
ROC: receiver operating characteristic; SPP: skin perfusion pressure; TcPO2: transcutaneous oxygen pressure; rSO2: regional tissue oxygenation saturation

Discussion and Conclusion
CLTI occurs in association with chronic ischemia, which often leads to prolonged ulcer healing or necrosis. The ischemia causes tissue hypoxia and deterioration of one’s nutritional status, which hinders wound healing. The appropriate assessment and management of wounds is essential in reducing amputation risk. There has been considerable progress in the field of wound care over the last three decades. Management strategies include debridement, wound dressing, pressure off-loading, and good glycemic control. In our institution, a multidisciplinary approach is adopted before and after EVT to prepare for wound care. In close cooperation with plastic surgeons and dermatologists, effective and efficient EVT should be sought for proper revascularization.
Our results indicate that measuring rSO2 is useful for evaluating lower limb blood flow, as are the existing lower limb blood flow tests, such as SPP and TcPO2, and also for predicting wound healing after EVT. In this study, EVT was performed in patients with poor limb blood flow and Fontaine stage IV to improve blood flow and heal the ulcers. Overall, the preoperative values indicated poor blood flow with all the methods. After EVT, the blood flow values often improved compared with the preoperative values.
Traditional diagnostic modalities, namely, ABI, SPP, and TcPO2, are limited by long measurement times, pain during measurement, measurable skin lesions, or difficulty maintaining the patient’s position during the measurement. In our study, we were unable to measure ABI or SPP in more than 10% of the patients because of intolerable pain during the measurements with a cuff pressure, or to measure TcPO2 of the ankle in more than 10% of them because of the difficulty in maintaining a stable patient position during the measurement. Conversely, measuring rSO2 using a finger-mounted tissue oximetry device was successful with no difficulty in all ischemic limbs of the participants in this study. Even in patients with rest pain or skin ulcers, the rSO2 measurement could be performed at the bedside quickly, simply, painlessly, and repeatedly, in any skin area.
We considered postoperative blood flow evaluations and their relationships with wound healing. SPP, TcPO2, and rSO2 showed significantly lower values on the first day after EVT in the non-healing group than in the healing group, indicating that wounds are difficult to heal if postoperative values are poor and that measurement of rSO2 and existing tests may also reflect this hemodynamic status.
A non-invasive lower limb blood flow evaluation device is important. Pressure measurements using ABI are widely used as objective indicators. For patients with CLTI who had rest pain and those with dialysis and gangrene, the ankle systolic pressures were ≤40 mmHg and ≤60 mmHg, respectively. However, patients with CLTI, including several dialysis patients, often have abnormally high pressure due to calcification, and thus are not accurately evaluated.20) SPP measurements are useful for the diagnosis of CLTI. An SPP value of ≤30 mmHg is a strong predictor of CLTI, with good sensitivity and specificity.7,21) However, SPP examinations require both time and a rest period, and the measurement is often poorly reproducible and varies depending on the patient’s posture. TcPO2 values were also used as a predictor of successful wound healing. Reliable wound healing can be expected at values >30 mmHg.21,22) One limitation of this method is its relatively long measuring time. A heating phase of approximately 20 min is required before the measurement can be taken. The resting TcPO2 level is defined as the level that changes by less than 2 mmHg in the 2 min following a 10-min response time.7,23) TcPO2 cannot be measured intraoperatively and has not been used as an indicator of the endpoint of EVT.
On the basis of these results, we then estimated the cutoff values for wound healing. From the ROC curves, the wound healing cutoff values were 36.5 mmHg for SPP, 11.5 mmHg for TcPO2, and 49.5% for rSO2. It is generally believed that for wound healing, SPP should be ≥30 mmHg and TcPO2 should be ≥40 mmHg.24,25) Although the TcPO2 values were slightly different from those reported in the literature, the SPP values obtained in this study were similar to those in the previous studies. The ROC curve of rSO2 suggested that rSO2>50% is the optimal cutoff value for wound healing with 100% sensitivity for wound healing outcome and should be considered as a guide for treatment. However, in the assessment of SPP for wound prediction, Utsunomiya et al. reported that the optimal cutoff point for predicting wound healing is 30 mmHg, with a sensitivity of 81.4% and a specificity of 69.2%.25) Thus, we believe that rSO2 values may be most useful for predicting postoperative wound healing, with values of 50% strongly predicting wound healing.
When focusing on individual cases, we noted that, in several patients, preoperative blood flow was poor and improved after EVT, and wounds were healed. However, some cases had post-EVT values that worsened compared with the preoperative values even with successful EVT procedures. In such cases, the wounds did not heal. Successful EVT does not necessarily improve blood flow and heal ulcers. EVT may sometimes cause damage to blood vessels that are blocking collateral flow and perturbing tissue perfusion. However, there were cases in which both preoperative and postoperative rSO2 levels were low, suggesting that we should have planned another revascularization procedure immediately after the initial EVT. At that time, if revascularization is difficult either technically or because of a patient’s general condition, amputation should be considered as a surgical option.
Contrary to conventional devices, the new device may be useful for monitoring tissue oxygenation during EVT, conferring a huge advantage. The device has the potential to determine the need for additional treatment and to help surgeons make decisions during EVT. Targeting rSO2 levels in excess of 50% at the ischemic region may become an endpoint of EVT for wound healing.26)
Our study has some limitations, in particular because of its single-center, retrospective design and the limited sample size. Moreover, in some cases, ABI, SPP, and TcPO2 could not be examined. This particular limitation can, however, be interpreted as an advantage of the new device, which can measure blood flow in more severe cases unsuitable for existing tests. In all cases, the measurement site was limited to the dorsal foot. If a new device that measures rSO2 with multiple probes is developed, simultaneous measurement of rSO2 at not only the dorsal foot but also the wound site could help improve prediction of wound healing. Finally, only cases of successful EVT without infection were enrolled in this study.
In summary, we evaluated hemodynamic changes after EVT with a new oximeter and compared the device with conventional methods. We identified that the device has advantages of quickness, portability, and less invasiveness in measuring rSO2 values at ischemic sites. Retrospective assessment of wound healing indicated that the optimal rSO2 value for predicting wound healing is 50%. When performing EVT, rSO2>50% may be an endpoint to achieve.
Acknowledgments
This work was supported by the project “Development of Medical Devices and Systems for Advanced Medical Services” from the Division of Medical Device Research of the Japan Agency for Medical Research and Development, AMED (awarded to N.U.).
Funding
This work was supported by the project “Development of Medical Devices and Systems for Advanced Medical Services” from the Division of Medical Device Research of the Japan Agency for Medical Research and Development, AMED (awarded to N.U.).
Disclosure Statement
All authors report no conflicts of interest.
Author Contributions
Study conception: TK, MS, KI, NU
Data collection: TK, MS, KI, KK, TY, YY, EN
Analysis: TK, MS, KI, NY, HT
Writing: TK, MS, NU
Funding acquisition: NU
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Supplementary Materials
Supplementary materials are available at the online article sites on J-STAGE and PMC.
Supplementary Data
References
- 1).Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007; 33 Suppl 1: S1-75. [DOI] [PubMed] [Google Scholar]
- 2).Conte MS, Pomposelli FB, Clair DG, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg 2015; 61 Suppl: 2S-41S, 41S.e1. [DOI] [PubMed] [Google Scholar]
- 3).Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017; 135: e686-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Ohnishi H, Sawayama Y, Furusyo N, et al. Risk factors for and the prevalence of peripheral arterial disease and its relationship to carotid atherosclerosis: the Kyushu and Okinawa Population Study (KOPS). J Atheroscler Thromb 2010; 17: 751-8. [DOI] [PubMed] [Google Scholar]
- 5).Usui T, Ninomiya T, Nagata M, et al. Albuminuria as a risk factor for peripheral arterial disease in a general population: the Hisayama study. J Atheroscler Thromb 2011; 18: 705-12. [DOI] [PubMed] [Google Scholar]
- 6).Darling JD, McCallum JC, Soden PA, et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system after first-time lower extremity revascularizations. J Vasc Surg 2017; 65: 695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Castronuovo JJ Jr, Adera HM, Smiell JM, et al. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg 1997; 26: 629-37. [DOI] [PubMed] [Google Scholar]
- 8).Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113: e463-654. [DOI] [PubMed] [Google Scholar]
- 9).Carter SA. Ankle and toe systolic pressures comparison of value and limitations in arterial occlusive disease. Int Angiol 1992; 11: 289-97. [PubMed] [Google Scholar]
- 10).Cao P, Eckstein HH, De Rango P, et al. Chapter II: diagnostic methods. Eur J Vasc Endovasc Surg 2011; 42 Suppl 2: S13-32. [DOI] [PubMed] [Google Scholar]
- 11).Høyer C, Sandermann J, Petersen LJ. The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg 2013; 58: 231-8. [DOI] [PubMed] [Google Scholar]
- 12).Kalani M, Brismar K, Fagrell B, et al. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999; 22: 147-51. [DOI] [PubMed] [Google Scholar]
- 13).Boezeman RP, Becx BP, van den Heuvel DA, et al. Monitoring of foot oxygenation with near-infrared spectroscopy in patients with critical limb ischemia undergoing percutaneous transluminal angioplasty: a pilot study. Eur J Vasc Endovasc Surg 2016; 52: 650-6. [DOI] [PubMed] [Google Scholar]
- 14).Uchida T, Kanayama N, Kawai K, et al. Craniofacial tissue oxygen saturation is associated with blood pH using an examiner’s finger-mounted tissue oximetry in mice. J Biomed Opt 2016; 21: 040502. [DOI] [PubMed] [Google Scholar]
- 15).Watanabe T, Matsushita M, Nishikimi N, et al. Near-infrared spectroscopy with treadmill exercise to assess lower limb ischemia in patients with atherosclerotic occlusive disease. Surg Today 2004; 34: 849-54. [DOI] [PubMed] [Google Scholar]
- 16).Ubbink DT, Koopman B. Near-infrared spectroscopy in the routine diagnostic work-up of patients with leg ischaemia. Eur J Vasc Endovasc Surg 2006; 31: 394-400. [DOI] [PubMed] [Google Scholar]
- 17).Kanayama N, Niwayama M. Examiner’s finger-mounted fetal tissue oximetry. J Biomed Opt 2014; 19: 067008. [DOI] [PubMed] [Google Scholar]
- 18).Mukai M, Uchida T, Itoh H, et al. Tissue oxygen saturation levels from fetus to neonate. J Obstet Gynaecol Res 2017; 43: 855-9. [DOI] [PubMed] [Google Scholar]
- 19).Yata T, Sano M, Kayama T, et al. Utility of a finger-mounted tissue oximeter with near-infrared spectroscopy to evaluate limb ischemia in patients with peripheral arterial disease. Ann Vasc Dis 2019; 12: 36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26: 517-38. [DOI] [PubMed] [Google Scholar]
- 21).Lo T, Sample R, Moore P, et al. Prediction of wound healing outcome using skin perfusion pressure and transcutaneous oximetry: a single-center experience in 100 patients. Wounds 2009; 21: 310-6. [PubMed] [Google Scholar]
- 22).Göthgen I, Jacobsen E. Transcutaneous oxygen tension measurement I. Age variation and reproducibility. Acta Anaesthesiol Scand Suppl 1978; 22: 66-70. [PubMed] [Google Scholar]
- 23).Quigley FG, Faris IB. Transcutaneous oxygen tension measurements in the assessment of limb ischaemia. Clin Physiol 1991; 11: 315-20. [DOI] [PubMed] [Google Scholar]
- 24).Ballard JL, Eke CC, Bunt TJ, et al. A prospective evaluation of transcutaneous oxygen measurements in the management of diabetic foot problems. J Vasc Surg 1995; 22: 485-90; discussion, 490-2. [DOI] [PubMed] [Google Scholar]
- 25).Utsunomiya M, Nakamura M, Nagashima Y, et al. Predictive value of skin perfusion pressure after endovascular therapy for wound healing in critical limb ischemia. J Endovasc Ther 2014; 21: 662-70. [DOI] [PubMed] [Google Scholar]
- 26).Unno N, Inuzuka K, Sano M, et al. Target region oxygenation-based endovascular treatment in a chronic limb-threatening ischemia patient with multifocal arterial diseases. J Vasc Surg Cases Innov Tech 2020; 6: 228-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


