Abstract
Recent data suggest that cystic fibrosis transmembrane conductance regulator (CFTR) gene alterations negatively impact male fertility beyond obstruction. We sought to compare gene alterations, sperm retrieval rates, and intracytoplasmic sperm injection (ICSI) outcomes among men with cystic fibrosis (CF) disease and congenital bilateral absence of the vas deferens (CBAVD) only. We retrospectively evaluated all men who underwent surgical sperm retrieval at two academic, high-volume andrology centers from 2010 to 2018. Only men with documented CFTR alterations and obstructive azoospermia from either CBAVD or CF were included. Differences between groups for CFTR abnormality, sperm retrieval, and ICSI outcomes were statistically analyzed. Overall,39 patients were included with 10 in the CF and 29 in the CBAVD groups. Surgical sperm retrieval rates were significantly lower in the CF group for sperm concentration (14.8 × 106 ml-1 vs 61.4 × 106 m-1, P = 0.02) and total motile sperm count (2.9 million vs 11.4 million, P = 0.01). This difference was only predicted by homozygous delta F508 CFTR mutations (P < 0.05). The CF group also demonstrated a significantly higher rate of rescue testicular sperm extraction (70.0% vs 27.6%, P < 0.03) and lower fertilization rate with ICSI (32.5% vs 68.9%, P < 0.01). In conclusion, those with CF demonstrated lower sperm quality, greater difficulty with sperm retrieval, and worse ICSI outcomes compared with CBAVD-only patients. Homozygous delta F508 CFTR mutations appear to significantly impair spermatogenesis and sperm function.
Keywords: congenital bilateral absence of the vas deferens, cystic fibrosis, intracytoplasmic sperm injection outcomes, male infertility, obstructive azoospermia
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive disease, affecting up to 1 in 1600 individuals of Northern European descent.1 Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene underlie the multiorgan system disease, with nearly 2000 distinct mutations in CFTR identified. Any CFTR alteration may result in congenital bilateral absence of the vas deferens (CBAVD), but only those homozygous mutations exhibit CF disease.2
CBAVD is a rare cause of male factor infertility, but represents the most common etiology of obstructive azoospermia (OA).3 CBAVD patients may exhibit epididymal atresia, seminal vesicle hypoplasia, and acidic ejaculate (pH <7.2).4 Nonetheless, reliable success at achieving pregnancy for these patients is possible by performing surgical sperm retrieval, microsurgical epididymal sperm aspiration (MESA), in conjunction with intracytoplasmic sperm injection (ICSI).5,6,7 Importantly, the combination of ICSI with preimplantation genetic testing can prevent the passage of CFTR mutations to offspring.8,9
CFTR mutations may also result in pathophysiologic changes beyond OA. Recent data suggest that CFTR plays an important role in sperm function. Sperm from men with CBAVD is associated with an increased risk of miscarriage and stillbirth after ICSI compared to men without CBAVD or any CFTR mutation.10 Indeed, animal and human data have shown downregulation of CFTR in males with nonobstructive azoospermia (NOA) and reduced sperm motility and fertilizing capacity in men harboring sperm CFTR mutations.11,12 These data and others suggest that CFTR plays a larger role in male fertility beyond CBAVD.13,14,15,16 Yet, data evaluating the influence of CFTR mutations on ICSI outcomes have been inconclusive.17,18,19
Data comparing ICSI outcomes in men with either CF disease or CBAVD only stratified by CFTR abnormality are lacking and none have directly compared sperm retrieval or ICSI outcomes. Therefore, we sought to compare CF- and CBAVD-only patients to determine differences in underlying CFTR abnormalities, sperm retrieval, and ICSI outcomes.
PATIENTS AND METHODS
With institutional review board approval from the Baylor College of Medicine in Houston, TX, USA (protocol H-37282), we retrospectively evaluated all men who underwent surgical sperm retrieval at two academic, high-volume andrology centers from January 2010 to February 2018. The two participating centers were the Center for Reproductive Medicine and Surgery at Baylor College of Medicine in Houston, TX, USA and University of North Carolina at Chapel Hill (UNC) Fertility center in Raleigh, NC, USA. Only men with documented CFTR alterations and obstructive azoospermia from either CBAVD or CF were included. At both institutions, CFTR testing is routinely initiated on the male partner who has low-volume azoospermia in the setting of clinical OA, when any vasal anomaly including absence of one or both vasa deferentia is noted on physical examination, if there is a family history of cystic fibrosis, or if the female partner has cystic fibrosis or tested positive as a heterozygous carrier. Any patient undergoing surgical sperm retrieval and unknown CFTR abnormality status was excluded. Given the rarity of these disorders, we retrospectively reviewed to the limit of available records and did not target a specific number of patients.
All patients underwent a complete clinical evaluation by a fellowship-trained reproductive urologist (LIL and RMC). We recorded patient age at the time of surgery, CFTR integrity, and the preoperative levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), total testosterone (TT), free testosterone (FT), estradiol, and serum hormone binding globulin (SHBG). CFTR integrity was determined by either a CFTR mutation panel or gene sequence analysis. Data recorded from sperm retrieval procedures included aspirate volume, sperm concentration, motility, total sperm count (TSC), and total motile sperm count (TMSC). Among those undergoing ICSI, the ICSI cycle outcomes were recorded, including partner age at the time of ICSI, number of ICSI cycles performed, and per cycle data including the number of mature eggs retrieved, fertilization rate, number of embryos generated, and number of embryos transferred, pregnancies, and live births.
All patients from both institutions underwent a similar approach for surgical sperm retrieval, which were performed by two experienced microsurgeons (LIL and RMC). A standard obliterative microsurgical epididymal sperm aspiration (MESA) was performed at the Center for Reproductive Medicine,20 whereas a modified MESA using loupe magnification and a keyhole incision was performed at UNC Fertility.21 For each case, a single epididymis was examined under magnification with the patient under general or monitored anesthesia care. Multiple epididymotomies were made, and epididymal fluid was drawn into an angiocatheter primed with sperm wash medium. Epididymal sperm aspiration began at the distal most level of dilated tubules and proceeded proximally toward the caput epididymis until copious whole motile sperm were identified. The presence of sperm in each epididymal aspirate was determined intraoperatively in real time by performing wet mount compound microscopy at ×40 magnification (Olympus CX41, Tokyo, Japan).
An attempt to extract all of the available epididymal sperm from the respective testicular unit was made in the fashion of an obliterative MESA. All of the extracted epididymal fluid was flushed into a vial of approximately 3 ml of sperm wash medium. If insufficient or no sperm were retrieved with MESA, a rescue testicular sperm extraction (TESE) was performed. The technique for TESE was identical at each institution, with an incisional biopsy of approximately 250 g of seminiferous tubules.21
For patients treated at the Center for Reproductive Medicine that subsequently underwent ICSI, outside providers and embryology labs performed all ICSI cycles. Therefore, the integrity of the ICSI outcome data from this center is limited to the information provided by the outside providers. In each case, surgically retrieved sperm were either processed and cryopreserved on-site at the Center for Reproductive Medicine andrology lab or transported by courier service the day of surgery for processing and cryopreservation off-site for later ICSI use. All patients with sperm retrieved at UNC Fertility had the sample processed and cryopreserved by the on-site embryology lab, and the couple later underwent ICSI using a cryopreserved sample. Inclusion of female partner variables and preimplantation genetic testing results were not included in our analysis due to the heterogeneity of available data from various outside clinics where ICSI was performed using sperm retrieved at the Center for Reproductive Medicine.
Medians and ranges were calculated for all recorded variables. Comparisons between the CF and CBAVD groups were made using the Mann–Whitney U test to determine statistical significance (P < 0.05). CFTR abnormalities were correlated with surgically retrieved sperm concentration and TMSC using a whisker plot with ANOVA analysis to determine any significant predictors of MESA outcome based on CFTR gene mutation status.
RESULTS
Overall, 39 patients met study inclusion criteria with 10 in the CF and 29 in the CBAVD groups (Table 1). There were no significant differences between groups based on patient or partner age, patient preoperative laboratory data, or rate of use of MESA or ICSI. However, the rate of required concurrent rescue TESEd with MESA was significantly higher in the CF group (70.0% vs 27.6%, P = 0.03).
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Cystic fibrosis | CBAVD | P |
|---|---|---|---|
| Couples included (n) | 10 | 29 | NA |
| Male age (year), median (IQR) | 32.5 (27.5–36.2) | 32.3 (29.3–35.5) | 0.96 |
| Female age (year), median (IQR) | 34 (30–35) | 30.5 (27.3–33.0) | 0.21 |
| FSH (IU l−1), median (IQR) | 3 (2.1–4.3) | 3.5 (3.0–5.3) | 0.4 |
| LH (IU l−1), median (IQR) | 4 (2.8–5.5) | 3 (2.0–3.7) | 0.19 |
| Total testosterone (ng dl−1), median (IQR) | 324 (232–473) | 360 (283–396) | 0.89 |
| Free testosterone (ng dl−1), median (IQR) | 6.1 (5.8–9.4) | 7.5 (5.5–8.0) | 0.87 |
| MESA, n/total (%) | 10/10 (100) | 29/29 (100) | 0.72 |
| Rescue TESE, n/total (%) | 7/10 (70.0) | 8/29 (27.6) | 0.03 |
| ICSI (total cycles per group), n/total (%) | 6/10 (60.0) | 26/29 (89.7) | 0.06 |
CBAVD: congenital bilateral absence of the vas deferens; MESA: microsurgical epididymal sperm aspiration; TESE: testicular sperm extraction; IQR: interquartile range; ICSI: intracytoplasmic sperm injection; FSH: follicle-stimulating hormone; LH: luteinizing hormone; NA: not applicable
There were similarities in the distribution of CFTR abnormalities between the CF and CBAVD groups (Table 2). The most common CFTR mutation was the delta F508 deletion, which was homozygous positive in 80.0% of CF patients and heterozygous positive in 48.3% of CBAVD patients. Other homozygous and heterozygous CFTR abnormalities comprised 20.0% and 51.7% of the CF and CBAVD groups, respectively.
Table 2.
Observed cystic fibrosis transmembrane conductance regulator mutations in cystic fibrosis and congenital bilateral absence of the vas deferens groups
| CFTR mutation | Cystic fibrosis (%) | CBAVD (%) |
|---|---|---|
| Included men | 10 | 29 |
| Delta F508 (heterozygous) | 0 | 14/29 (48.3) |
| Delta F508 (homozygous) | 8/10 (80.0) | 0 |
| 5T allele | 0 | 7/29 (24.1) |
| d551 (homozygous) | 1/10 (10.0) | 0 |
| r117a (heterozygous) | 0 | 1/29 (3.5) |
| r117c (heterozygous) | 0 | 1/29 (3.5) |
| r117h (homozygous) | 1/10 (10.0) | 1/29 (3.5) |
| c.601 G>A (heterozygous) | 0 | 1/29 (3.5) |
| c.3528 del (heterozygous) | 0 | 1/29 (3.5) |
| w.1282x (heterozygous) | 0 | 1/29 (3.5) |
| c.3232 T>C (heterozygous) | 0 | 1/29 (3.5) |
| n.1301 del (heterozygous) | 0 | 1/29 (3.5) |
Data are shown as numerator/denominator (%). CFTR: cystic fibrosis transmembrane conductance regulator; CBAVD: congenital bilateral absence of the vas deferens
Analysis of surgical sperm retrieval data demonstrated a significantly lower median sperm concentration (14.8 × 106 ml-1 vs 61.4 × 106 ml−1, P = 0.02), TSC (22.4 million vs 77.8 million, P = 0.02), and TMSC (2.9 million vs 11.4 million, P = 0.01) during MESA in the CF versus CBAVD groups (Table 3). For those cases requiring a rescue TESE, there were no statistically significant differences between groups. ANOVA analysis demonstrated that only the homozygous delta F508-mutation group, which is only observed in CF disease patients, predicted a lower median sperm concentration during MESA (P < 0.05; Figure 1). A noticeably lower median TMSC during MESA was also observed in the homozygous delta F508 mutation group, but the difference was not statistically significant (P = 0.09; Figure 2).
Table 3.
Sperm retrieval and intracytoplasmic sperm injection outcomes
| Cystic fibrosis | CBAVD | P | |
|---|---|---|---|
| MESA | |||
| Volume (ml), median (IQR) | 0.8 (0.5–1.8) | 0.6 (0.5–1.2) | 0.74 |
| Sperm concentration (×106 ml−1), median (IQR) | 14.8 (4.9–30.0) | 61.4 (21.3–110) | 0.02 |
| Total sperm count (million), median (IQR) | 22.4 (4.0–38.9) | 77.8 (35.4–145) | 0.02 |
| Total motile sperm count (million), median (IQR) | 2.9 (0.007–14.5) | 11.4 (0.41–59.9) | 0.01 |
| TESE | |||
| Successful sperm recovery, n/total (%) | 5/7 (71.4) | 8/8 (100) | 0.23 |
| ICSI | |||
| Couples attempting (n) | 5 | 13 | NA |
| Total cycle (n) | 8 | 30 | NA |
| Total mature eggs (n) | 77 | 216 | NA |
| Mature eggs per cycle (n), median (IQR) | 11 (8–18) | 16 (10–17) | 0.26 |
| Fertilization rate, n/total (%) | 25/77 (32.5) | 149/216 (68.9) | <0.01 |
| Blastocyst/embryo total (n) | 16 | 113 | NA |
| Embryos transferred per cycle (n), median (IQR) | 1 (1–3) | 1 (1–2) | 0.43 |
| Pregnancy total (n) | 4 | 18 | NA |
| Pregnancy rate per cycle, n/total (%) | 4/8 (50.0) | 18/30 (60.0) | 0.70 |
| Live birth total (n) | 5 | 16 | 0.95 |
| Live birth rate per cycle, n/total (%) | 5/8 (62.5) | 16/30 (53.3) | 1.00 |
CBAVD: congenital bilateral absence of the vas deferens; MESA: microsurgical epididymal sperm aspiration; TESE: testicular sperm extraction; IQR: interquartile range; ICSI: intracytoplasmic sperm injection; NA: not analyzed
Figure 1.
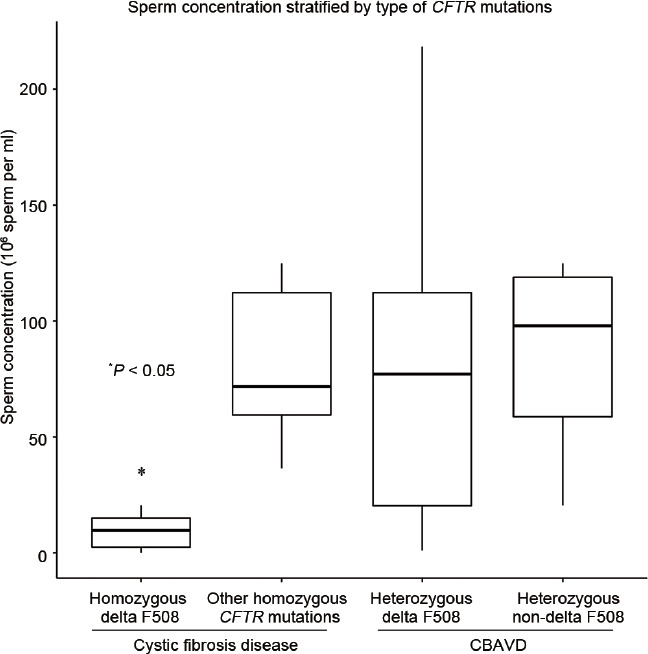
Whisker plot analysis comparing sperm concentration after surgical sperm retrieval for men with various CFTR mutations. Outliers not shown.*P < 0.05 defines the relationship between homozygous delta F508 CFTR mutation and sperm concentration (sperm per ml). CBAVD: congenital bilateral absence of the vas deferens; CF: cystic fibrosis; CFTR: cystic fibrosis transmembrane conductance regulator.
Figure 2.
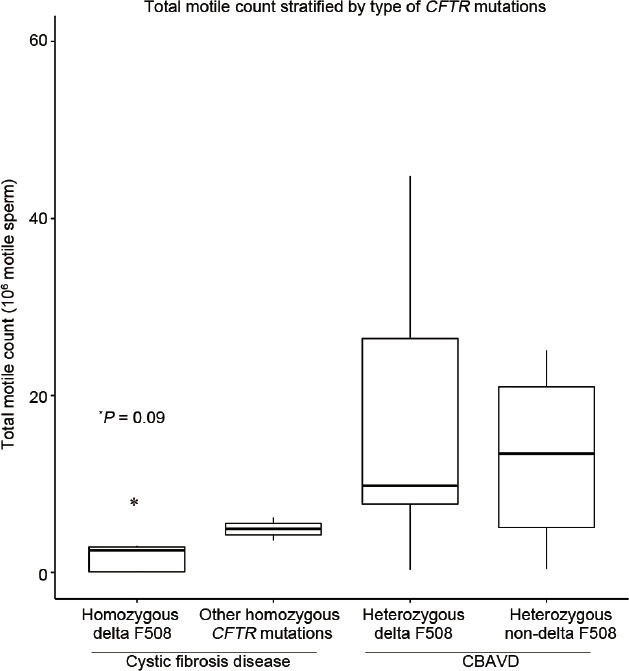
Whisker plot analysis comparing sperm concentration after surgical sperm retrieval for men with various CFTR mutations. Outliers not shown.*P = 0.09 defines the relationship between homozygous delta F508 CFTR mutation and TMSC. CBAVD: congenital bilateral absence of the vas deferens; CF: cystic fibrosis; CFTR: cystic fibrosis transmembrane conductance regulator; TMSC: total motile sperm count.
After undergoing surgical sperm retrieval, 5 (50.0%) in the CF and 13 (44.8%) in the CBAVD groups proceeded with ICSI (Table 3). Three patients in the CF group (30.0%) died from complications of their CF disease and did not undergo ICSI. In those completing ICSI, a lower fertilization rate in the CF cohort (32.5% vs 68.9%, P < 0.01) was the only statistically significant difference between groups. Overall, 5 live births from 4 pregnancies (1 set of twins) and 16 live births from 18 pregnancies (1 set of twins) were observed in the CF and CBAVD groups, respectively, resulting in a similar live birth rate per ICSI cycle (62.5% vs 53.3%). For this analysis, the CF group contained men with either homozygous delta F508 CFTR mutations or other homozygous CFTR abnormalities.
To better determine the impact of homozygous delta F508 CFTR mutations on MESA and ICSI outcomes, the CF group was substratified into those with homozygous delta F508 mutations compared to nondelta F508 homozygous CFTR mutations (Table 4). While the number of patients in each group was insufficient to determine statistical significance, a lower fertilization rate (25.0% vs 48.0%), lower pregnancy rate per ICSI cycle (33.3% vs 100%), and lower live birth rate per ICSI cycle (33.3% vs 100%) were observed in the homozygous delta F508 CFTR mutation group.
Table 4.
Cystic fibrosis patient outcomes stratified by cystic fibrosis transmembrane conductance regulator mutation
| Patient outcome | Homozygous d508 | Other homozygous | P |
|---|---|---|---|
| MESA (n=10) | 7 | 3 | NA |
| Successful sperm recovery, n/total (%) | 7/7 (100) | 2/3 (66.6) | 0.3 |
| Volume (ml), median (IQR) | 0.9 (0.7–2.1) | 1.4 (1.2–1.5) | NA |
| Sperm concentration (×106 ml−1), median (IQR) | 14.4 (3.3–17.9) | 30 (30–31) | 0.69 |
| Total sperm count (million), median (IQR) | 13.8 (0.7–41.5) | 41 (36–46) | NA |
| Total motile sperm count (million), median (IQR) | 2.5 (0.07–2.9) | 4.9 (4.3–5.6) | 0.71 |
| TESE (n=7) | 7 | 0 | NA |
| Successful sperm recovery, n/total (%) | 5/7 (71.4) | 0 | NA |
| ICSI (n=5) | 3 | 2 | NA |
| Total cycles (n) | 6 | 2 | NA |
| Fertilization rate, n/total (%) | 13/52 (25.0) | 12/25 (48.0) | 0.8 |
| Blast/embryo total (n) | 12 | 4 | NA |
| Embryos transferred per ICSI cycle (n), median (IQR) | 2 (1–3) | 1.5 (1–2) | 0.6 |
| Pregnancy total (n) | 2 | 2 | NA |
| Pregnancy rate per ICSI cycle, n/total (%) | 2/6 (33.3) | 2/2 (100) | NA |
| Live birth total (n) | 2 | 3 | NA |
| Live birth rate per ICSI cycle, n/total (%) | 2/6 (33.3) | 2/2 (100) | NA |
MESA: microsurgical epididymal sperm aspiration; TESE: testicular sperm extraction; ICSI: intracytoplasmic sperm injection; IQR: interquartile range; NA: not analyzed
DISCUSSION
This is the first known comparison of CFTR mutations, surgical sperm retrieval, and ICSI outcomes between CF- and CBAVD-only patients with OA. We found the delta F508 CFTR mutation to be the most prevalent among CF and CBAVD patients, which, when homozygously present, predicted worse sperm retrieval and ICSI outcomes. Also, CF patients demonstrated a significantly lower sperm quantity and quality with MESA and experienced a worse fertilization rate with ICSI.
Only two other published series have evaluated the use of MESA and ICSI in CF patients. Nearly 20 years ago, McCallum et al.6 reported 13 CF patients with 69% possessing homozygous delta F508 mutations. Eight underwent MESA and ICSI, resulting in a mean sperm concentration of 41.5 × 106 ml−1, fertilization rate of 60%, and pregnancy rate of 62.5%, with 50% of couples achieving a live birth (75% twins). In 2006, Hubert et al.5 reviewed 25 CF patients with 19 undergoing ICSI. A 61% fertilization rate and 40% pregnancy rate per cycle (63% of couples) was observed with 47% of couples achieving live birth (22% twins). However, only 48% and 28% of patients harbored homozygous and heterozygous delta F508 mutations, respectively. The reported sperm concentration with MESA by McCallum et al.6 is higher than our series; however, those authors did not distinguish total motile count. Likewise, both series reported higher fertilization and pregnancy rates than ours, but similar to both prior series, 50% of couples in this series achieved a live birth with a similar or lower twin rate (25% twins in this series). The lower pregnancy and twin rates in our series may be explained by the practice of transferring fewer embryos per frozen embryo transfer cycle in the years since the publication of prior data.22 Also, the higher proportion of homozygous delta F508 mutations in our CF cohort (80.0%) may underlie the lower observed sperm retrieval and fertilization rates compared to CBAVD-only patients.
Over the past several decades, controversy has developed over whether CFTR abnormalities have an independently negative effect on spermatogenesis or sperm function or not. Among data evaluating men with OA secondary to CBAVD undergoing ICSI, one series found significantly worse fertilization and pregnancy rates in those with CBAVD from heterozygous CFTR mutations versus those with CBAVD without CFTR mutations. Interestingly, delta F508 mutations (91.3%) predicted worse ICSI outcomes.18 Yet, a similarly designed study did not detect a significant difference in ICSI outcomes between groups of heterozygous CFTR mutation and non-CFTR mutation-related CBAVD; however, delta F508 mutations only comprised 18% of the CBAVD group with heterozygous CFTR mutations.19
A similar situation exists when evaluating the effect of CFTR alterations on spermatogenesis in men without CBAVD or OA. CFTR mutations have been reported in up to 9.5% of men with NOA and 8.9% of all infertile males, most commonly delta F508.23 Yet, a subsequent series of NOA patients found no association between CFTR mutations and NOA, but only one delta F508 mutation was reported.17 A similar report of NOA and normal fertile patients also found no association between CFTR mutations and NOA status, but the CFTR assay used was limited, potentially missing CFTR mutations in both groups.14 In contrast, a more recent, large cohort of NOA, severe oligospermia, and mild oligospermia patients detected CFTR mutations in 12.9%, 11.1%, and 8.1% of patients, respectively, compared with 4% of normal controls.13 Similar series have shown the presence of CFTR mutations to range from 7.3% to 11.7% in NOA patients and 5.4%–6.8% in oligospermia patients, compared with 4%–4.4% of normal controls.15,24,25
While the data are few, there does appear to be a physiologic relationship between normal CFTR function and normal spermatogenesis. Normal sperm capacitation and acrosome reaction have both been shown to be significantly impaired in those with CFTR mutations when compared to normal, fertile controls.16 Also, CFTR function is known to contribute to normal release of extracellular ATP into the epididymal lumen to support sperm fertilizing capacity, which is reduced in those with CFTR mutations.26 Such a relationship has led some to advocate for CFTR mutation testing in all cases of azoospermia and select cases of severe oligospermia in the absence of other genetic etiologies.24,25 While these authors do not currently advocate for CFTR mutation testing in all azoospermic patients, we would consider the findings of our study to be consistent with the aforementioned data, collectively supporting a link between CFTR mutations and abnormal spermatogenesis.
There are several limitations to our study that must be acknowledged. First, the retrospective nature of these data contains inherent bias related to the two andrology centers and multiple embryology labs involved that cannot be accounted for in our statistical analysis. Second, the number of patients in our analysis is small; however, the frequency with which CF patients present for treatment, even at high-volume centers, is low. Third, our ICSI outcome data were comprised from multiple academic and private centers and available records are limited to what each site was able to provide. Therefore, more detailed information regarding the female partners and use of preimplantation genetic testing were not available for inclusion in our analysis. In addition, information about the specific CFTR test was not consistently available because all patients with CF were previously diagnosed with a CFTR mutation and were not retested, and patients with CBAVD often presented as referrals from outside institutions having already undergone CFTR testing. Finally, CF patients possess a higher degree of medical comorbidity and worse overall mortality inherent to their disease, and many of these factors, including frequent febrile illnesses and medication side effects, cannot be accounted for in retrospective analysis. Despite these limitations, the results herein provide valuable insight to the sperm retrieval and ICSI outcomes, as well as underlying CFTR mutations, among men with CF and CBAVD only.
CONCLUSION
This novel comparison of CFTR mutations and fertility outcomes of patients with either CF or CBAVD alone found that CF men were more likely to exhibit lower sperm quality, greater difficulty with sperm retrieval, and worse ICSI outcomes compared with CBAVD-only patients. In this cohort, homozygous delta F508 CFTR mutations were also a significant predictor of worse sperm retrieval outcomes. These data support previous work linking CFTR mutations, impaired spermatogenesis, and worse functional outcomes. These findings should guide future research efforts and inform treatment expectations for patients and clinicians.
AUTHOR CONTRIBUTIONS
JAM and RMC performed study design, data review, manuscript authorship, submission, and revision. TPK performed statistical analysis and prepared figures. DJM performed manuscript review. LIL performed study design and manuscript review. All authors read and approved the final manuscript.
COMPETING INTEREST
All authors declared no competing interests.
ACKNOWLEDGEMENTS
The authors would like to thank Ryan C Owen of UNC Chapel Hill, Jabez Gondokusomo and Sukhkamal Campbell of Baylor College of Medicine for their assistance in data collection.
REFERENCES
- 1.Hotaling J, Carrell DT. Clinical genetic testing for male factor infertility: current applications and future directions. Andrology. 2014;2:339–50. doi: 10.1111/j.2047-2927.2014.00200.x. [DOI] [PubMed] [Google Scholar]
- 2.Castellani C, Cuppens H, Macek M, Jr, Cassiman JJ, Kerem E, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibrosis. 2008;7:179–96. doi: 10.1016/j.jcf.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb DJ. Genetic aspects of infertility. In: Lipshultz IL, Howards SS, Niederberger SC, editors. Infertility in the Male. 4th ed. New York: Cambridge University Press; 2009. pp. 251–76. [Google Scholar]
- 4.Attardo T, Vicari E, Mollica F, Grazioso C, Burrello N, et al. Genetic, andrological and clinical characteristics of patients with congenital bilateral absence of the vas deferens. Int J Androl. 2001;24:73–9. doi: 10.1046/j.1365-2605.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 5.Hubert D, Patrat C, Guibert J, Thiounn N, Bienvenu T, et al. Results of assisted reproductive technique in men with cystic fibrosis. Hum Reprod. 2006;21:1232–6. doi: 10.1093/humrep/dei453. [DOI] [PubMed] [Google Scholar]
- 6.McCallum TJ, Milunsky JM, Cunningham DL, Harris DH, Maher TA, et al. Fertility in men with cystic fibrosis: an update on current surgical practices and outcomes. Chest. 2000;118:1059–62. doi: 10.1378/chest.118.4.1059. [DOI] [PubMed] [Google Scholar]
- 7.Oates RD, Honig S, Berger MJ, Harris D. Microscopic epididymal sperm aspiration (MESA): a new option for treatment of the obstructive azoospermia associated with cystic fibrosis. J Assist Reprod Genet. 1992;9:36–40. doi: 10.1007/BF01204112. [DOI] [PubMed] [Google Scholar]
- 8.Girardet A, Ishmukhametova A, Willems M, Coubes C, Hamamah S, et al. Preimplantation genetic diagnosis for cystic fibrosis: the Montpellier center's 10-year experience. Clin Genet. 2015;87:124–32. doi: 10.1111/cge.12411. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Lissens W, Silber SJ, Devroey P, Liebaers I, et al. Birth after preimplantation diagnosis of the cystic fibrosis delta F508 mutation by polymerase chain reaction in human embryos resulting from intracytoplasmic sperm injection with epididymal sperm. JAMA. 1994;272:1858–60. [PubMed] [Google Scholar]
- 10.Lu S, Cui Y, Li X, Zhang H, Liu J, et al. Association of cystic fibrosis transmembrane-conductance regulator gene mutation with negative outcome of intracytoplasmic sperm injection pregnancy in cases of congenital bilateral absence of vas deferens. Fertil Steril. 2014;101:1255–60. doi: 10.1016/j.fertnstert.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Diao R, Fok KL, Zhao L, Chen H, Tang H, et al. Decreased expression of cystic fibrosis transmembrane conductance regulator impairs sperm quality in aged men. Reproduction. 2013;146:637–45. doi: 10.1530/REP-13-0146. [DOI] [PubMed] [Google Scholar]
- 12.Xu WM, Chen J, Chen H, Diao RY, Fok KL, et al. Defective CFTR-dependent CREB activation results in impaired spermatogenesis and azoospermia. PLoS One. 2011;6:e19120. doi: 10.1371/journal.pone.0019120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elia J, Mazzilli R, Delfino M, Piane M, Bozzao C, et al. Impact of cystic fibrosis transmembrane regulator (CFTR) gene mutations on male infertility. Arch Ital Urol Androl. 2014;86:171–4. doi: 10.4081/aiua.2014.3.171. [DOI] [PubMed] [Google Scholar]
- 14.Heidari S, Hojati Z, Motovali-Bashi M. Screening of two neighboring CFTR mutations in iranian infertile men with non-obstructive azoospermia. Int J Fertil Steril. 2017;10:390–4. doi: 10.22074/ijfs.2016.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L, Jin J, Wang S, Zhang F, Dai Y, et al. CFTR gene mutations and polymorphism are associated with non-obstructive azoospermia: from case-control study. Gene. 2017;626:282–9. doi: 10.1016/j.gene.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Li CY, Jiang LY, Chen WY, Li K, Sheng HQ, et al. CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum Reprod. 2010;25:317–27. doi: 10.1093/humrep/dep406. [DOI] [PubMed] [Google Scholar]
- 17.Mak V, Zielenski J, Tsui LC, Durie P, Zini A, et al. Cystic fibrosis gene mutations and infertile men with primary testicular failure. Hum Reprod. 2000;15:436–9. doi: 10.1093/humrep/15.2.436. [DOI] [PubMed] [Google Scholar]
- 18.Patrizio P, Ord T, Silber SJ, Asch RH. Cystic fibrosis mutations impair the fertilization rate of epididymal sperm from men with congenital absence of the vas deferens. Hum Reprod. 1993;8:1259–63. doi: 10.1093/oxfordjournals.humrep.a138237. [DOI] [PubMed] [Google Scholar]
- 19.Schlegel PN, Cohen J, Goldstein M, Alikani M, Adler A, et al. Cystic fibrosis gene mutations do not affect sperm function during in vitro fertilization with micromanipulation for men with bilateral congenital absence of vas deferens. Fertil Steril. 1995;64:421–6. doi: 10.1016/s0015-0282(16)57745-0. [DOI] [PubMed] [Google Scholar]
- 20.Karpman E, Williams IV HD. Techniques of sperm retrieval. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the Male. 4th ed. New York: Cambridge University Press; 2009. pp. 407–20. [Google Scholar]
- 21.Coward RM, Mills JN. A step-by-step guide to office-based sperm retrieval for obstructive azoospermia. Transl Androl Urol. 2017;6:730–44. doi: 10.21037/tau.2017.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. 2017;15:6. doi: 10.1186/s12958-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Ven K, Messer L, van der Ven H, Jeyendran RS, Ober C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod. 1996;11:513–7. doi: 10.1093/humrep/11.3.513. [DOI] [PubMed] [Google Scholar]
- 24.Mocanu E, Shattock R, Barton D, Rogers M, Conroy R, et al. All azoospermic males should be screened for cystic fibrosis mutations before intracytoplasmic sperm injection. Fertil Steril. 2010;94:2448–50. doi: 10.1016/j.fertnstert.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Sharma H, Mavuduru RS, Singh SK, Prasad R. Increased frequency of CFTR gene mutations identified in Indian infertile men with non-CBAVD obstructive azoospermia and spermatogenic failure. Gene. 2014;548:43–7. doi: 10.1016/j.gene.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Ruan YC, Shum WW, Belleannee C, Da Silva N, Breton S. ATP secretion in the male reproductive tract: essential role of CFTR. J Physiol. 2012;590:4209–22. doi: 10.1113/jphysiol.2012.230581. [DOI] [PMC free article] [PubMed] [Google Scholar]


