Abstract
Wheat is among the ten top and most widely grown crops in the world. Several diseases cause losses in wheat production in different parts of the world. Bipolaris sorokiniana (teleomorph, Cochliobolus sativus) is one of the wheat pathogens that can attack all wheat parts, including seeds, roots, shoots, and leaves. Black point, root rot, crown rot and spot blotch are the main diseases caused by B. sorokiniana in wheat. Seed infection by B. sorokiniana can result in black point disease, reducing seed quality and seed germination and is considered a main source of inoculum for diseases such as common root rot and spot blotch. Root rot and crown rot diseases, which result from soil-borne or seed-borne inoculum, can result in yield losses in wheat. Spot blotch disease affects wheat in different parts of the world and cause significant losses in grain yield. This review paper summarizes the latest findings on B. sorokiniana, with a specific emphasis on management using genetic, chemical, cultural, and biological control measures.
Keywords: Triticum aestivum, Helminthosporium, Cochliobolus sativus, Drechslera, control
Introduction
Wheat (Triticum aestivum) is among the most widely cultivated crops in the world. Wheat production exceeded 734 million tons in 2018 from 214 million ha of land (FAO, 2021). China, India, Russia, USA, and France were the largest producers of wheat in the world in 2018, accounting for more than 50% of the world’s production (FAO, 2021).
Wheat production is limited by several biotic stresses, with diseases being a major limiting factor to wheat production worldwide. The total number of wheat diseases exceeds 200, but 50 diseases cause economic losses and are widely distributed (Wiese, 1987; Al-Sadi, 2016; Jarroudi et al., 2017; Lalic et al., 2017; Riaz et al., 2017; Sharma et al., 2017). Each year about 20% of wheat is lost due to diseases. Some of the major wheat diseases are rusts, spot blotch, common root rot, smut, tan spot, Septoria blotch, powdery mildew, fusarium head blight, blast and a number of viral, nematode, and bacterial diseases (Wiese, 1987; Chowdhury et al., 2013; Fetch et al., 2015; Zhu et al., 2015; Al-Sadi, 2017; Abdullah et al., 2020; Aboukhaddour et al., 2020; Gultyaeva et al., 2020). They can reduce yield or result in mortality of the infected plants. The focus of this review will be on the etiology and management of B. sorokiniana diseases in wheat.
The genus Helminthosporium is a large group of the class Hyphomycetes that includes many species pathogenic to plants and animals. This genus has been split into three genera: Exserohilum, Bipolaris, and Drechslera, on the basis of conidial ontogeny and morphology (Alcorn, 1988).
Bipolaris sorokiniana
B. sorokiniana (Sacc.) Shoemaker, (syn. Helminthosporium sativum Pammel, King & Bakke, H. sorokinianum Sacc. in Sorokin, and Drechslera sorokiniana (Sacc.) Subramanian & Jain, causes diseases on a number of cereals, including wheat (Tunali et al., 2008; Devi et al., 2018; Gultyaeva et al., 2018; Gupta et al., 2018b; Jamil et al., 2018; Singh et al., 2019; Villa-Rodríguez et al., 2019; Li et al., 2020). The teleomorph for this fungus is Cochliobolus sativus (Ito & Kuribayashi) Drechs. ex Dastur, which is the sexual (perfect) state. C. sativus was not reported in nature, except in Zambia (Raemaekers, 1991). However, sexual reproduction of C. sativus has been rarely reported (Sultana et al., 2018). On the other hand, most of the reproduction of B. sorokiniana occurs through the production of asexual conidia (Gupta et al., 2018a).
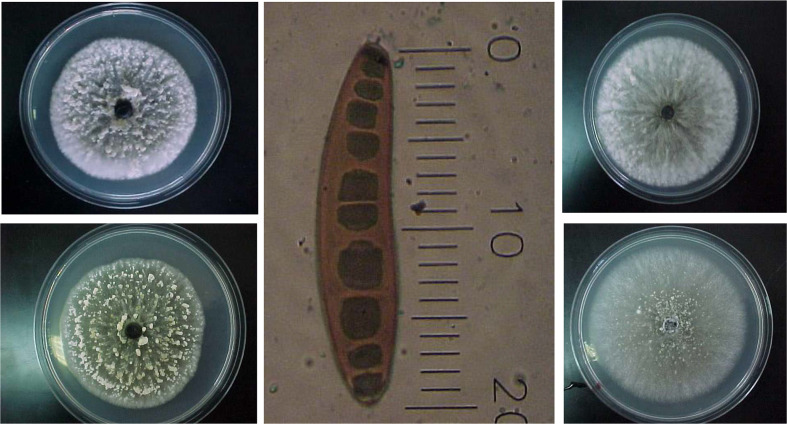
The genus Bipolaris has brown conidiophores, mostly simple, producing conidia through the apical pore. The conidia are brown, several-celled (phragmosporous), elliptical, straight, or curved, germinating by one germ tube at each end (Barnett and Hunter, 1998; Navathe et al., 2020) ( Figure 1 ). B. sorokiniana has olive-brown, ovate conidia, with tapered ends and a prominent basal scar. The conidia are 15-28 X 40-120 µm and have 3- to 10- septa (Wiese, 1987) ( Figure 1 ).
Figure 1.
Morphology of Bipolaris sorokiniana culture and spores (1 scale is equivalent to 5 µm) grown on potato dextrose agar. The mycelial growth of the four isolates shows mixed color (white and black) as explanied by Navathe et al. (2020), with varying intensities of the black color among the isolates.
Diseases Caused by B. sorokiniana
Bipolaris sorokiniana attacks different cereals, including wheat, and causes common root rot, spot blotch, and black point diseases. Root rot is one of the most widespread diseases of wheat and it occurs in all areas where wheat is grown. Losses in wheat due to common root rot and seedling blight vary. Canada lost approx. 5.7% of wheat during 1969–1971 due to common root rot, which is equivalent to $42 million (Ledingham et al., 1973). Smiley et al. (2005) estimated 35% loss in wheat yield due to crown rot in the Pacific Northwest. Spot blotch is found wherever wheat is grown, and it can cause significant losses (15-25%) in warm areas (Gupta et al., 2018a). Seed infection by B. sorokiniana can result in black point disease, which may result in root rot and seedling blight (Wiese, 1987; Al-Sadi and Deadman, 2010; Li et al., 2019b).
B. sorokiniana attacks several host plants from different genera and families (Wiese, 1987; Farr et al., 1989). The major plant hosts (listed by the genera name) that attacked by B. sorokiniana are Agrohordeum, Agropyron, Agrostis, Ammophila, Andropogon, Arthraxon, Avena, Bouteloua, Bromus, Buchloe, Calamagrostis, Calamovilfa, Cenchrus, Chloris, Cynodon, Dactylis, Dendrobium, Dichanthelium, Digitaria, Echinochloa, Elymus, Eragrostis, Eremopyrum, Festuca, Hordeum, Hystrix, Koeleria, Linum, Lolium, Medicago, Muhlenbergia, Oryzopsis, Panium, Phalaris, Phleum, Poa, Secale, Setaria, Sorghum, Stipa, Trifolium, Triticum, Vulpia, Zea, and Zizania species (Farr et al., 1989). Disease symptoms and yield losses in these hosts are variable. B. sorokiniana do not have host specialization (forma speciales). However, isolates have been found to differ in their aggressiveness on wheat and barley (Al-Sadi, 2016).
Black Point
Importance and Etiology of Black Point
Black point is a disease of cereal seeds, exhibiting a brown to black tip at the embryo end of the grain. The affected kernels usually become heavier than normal. The disease can result in lowering quality and market value of grains, production of fungal toxins in the seeds that may become harmful to livestock, and causing seedling blight, root rot and different diseases. In addition, it can reduce seed germination, seedling emergence, total photosynthetic area, and normal growth of plants (Al-Sadi and Deadman, 2010; Neupane et al., 2010; Ghosh et al., 2018; Li et al., 2019b; Somani et al., 2019).
The disease is caused by B. sorokiniana (Sisterna and Sarandon, 1996; Xu et al., 2018b; Li et al., 2019b; Somani et al., 2019; Li et al., 2020). In addition, some reports indicated the association of Alternaria alternata, Fusarium spp., and Penicillium spp. with wheat seeds developing black point symptoms (Al-Sadi and Deadman, 2010; Gannibal, 2018; Ghosh et al., 2018; Xu et al., 2018b; Li et al., 2019b; Li et al., 2020). Black point has been reported in different parts of the world, including China, Argentina, Oman, Australia, India, and Bangladesh (Rashid et al., 1992; Sisterna and Sarandon, 2005; Al-Sadi and Deadman, 2010; Poole et al., 2015; Xu et al., 2018b).
Bipolaris sorokiniana has been reported in the embryo (Weniger, 1923) and in the endosperm of wheat seeds (Rashid et al., 1997). Seed infection in wheat increases after flowering (Andersen, 1952). It can also be affected by the genotype, location (especially in warm and humid climates) and the management practices (Zhang et al., 1990). Penetration into the seed is achieved through the ovary wall and seed coat (Han et al., 2010; Ansari et al., 2017). B. sorokiniana was reported to remain viable for 10 years in wheat seeds (Machacek and Wallace, 1952). The fungus can also survive as a resting mycelium for 5 years (Mead, 1942). B. sorokiniana is very frequently isolated from the seeds of wheat, reaching as high as 80%–90.5%, and with a common level of infection of about 9%–22%, depending on the cultivar and the prevailing conditions (Rashid et al., 1992; Rashid et al., 1997; Rashid, 1998; Li et al., 2019b).
The incidence of black point disease is affected by many factors, most importantly temperature and humidity. Higher humidity (especially above 90%), rain and relatively lower temperatures (<30 oC) after heading usually increase the disease incidence (Cromey and Mulholland, 1988; Li et al., 2019a; Li et al., 2019b).
Management of Black Point
Control of B. sorokiniana in the seeds of wheat could be achieved through the use of resistant cultivars, fungicides, seed treatment, or biocontrol agents. Cultivars can react differently to seed infection due to several factors such as non-compatibility to infection, restricted pathogen invasion of the seed parts due to inhibitors, or reduced testa permeability (Gannibal, 2018; Singh et al., 2019). In a study by Li et al. (2014) on 403 wheat genotypes in the North China Plain, 62.5% of the genotypes were classified as susceptible, while 37.5% were resistant to black point disease. In another study, considerable variation was found among wheat cultivars in their resistance to black point disease, with no relationship between the earliness of ripening and resistance (Cromey and Mulholland, 1988). Connert and Davidson (1988) showed that wheat cultivar resistance to black point disease can be affected by the causal agent, with some cultivars having more resistance to B. sorokiniana than to A. alternata.
A study in Pakistan revealed that tebuconazole + imidacloprid and difenoconazole + cyproconazole were the most effective chemicals for the management of black point disease of wheat (Shahbaz et al., 2018). Triazole fungicides (e.g., propiconazole and tebuconazole) inhibit the synthesis of sterols, which are building blocks of the membranes of fungal cells. This makes them ideal chemicals for the management of Bipolaris and other fungal pathogens (Ansari et al., 2017; Somani et al., 2019). Treating seeds with fungicides helps protect wheat seeds from infection. In addition, it helps manage diseases associated with seed infection, including root and crown rot. Some of the common fungicides used in seed treatment include fludioxonil and difenoconazole (Wei et al., 2021) and Vitavax-200 (Carboxin 37.5% + Thiram 37.5%) and Homai-80WP (Thiophanate methyl 50% + Thiram 30%) (Malaker and Mian, 2009).
The use of biocontrol agents has been effective in reducing black point disease. Bacillus amyloliquefaciens, B. megaterium, Trichoderma harzianum, and Epicoccum sp. were found antagonistic against the causal agents of black point disease (El-Gremi et al., 2017). The isolates also improved germination and seedling growth of wheat, with B. amyloliquefaciens being the most efficacious, as it was as effective as the fungicide diniconazole in increasing the weight of kennels. In another study, the antifungal compounds produced by B. vallismortis were effective in inhibiting black point fungi (Kaur et al., 2015; Kaur et al., 2017). A study by Mónaco et al. (2004) showed that T. harzianum and T. koningii significantly inhibited the growth and caused mycelial abnormalities in Bipolaris sorokiniana and A. alternata.
Spot Blotch
Importance and Etiology of Spot Blotch
Spot blotch is a common disease on wheat in all continents (Duveiller et al., 1998; Al-Saadi et al., 2002; Neupane et al., 2010; Al-Sadi, 2016; Devi et al., 2018; Gultyaeva et al., 2018; Gupta et al., 2018a; Gupta et al., 2018b). Losses due to spot blotch are high, especially in warmer areas of the world. They have been reported to reach 16%–43% (Sharma and Dubin, 1996; Duveiller and Sharma, 2009; Ayana et al., 2018; Devi et al., 2018). In addition, the hotspot for spot blotch disease is in South Asia (Van Ginkel and Rajaram, 1998; Joshi et al., 2007; Sharma et al., 2018; Sultana et al., 2018).
Spot blotch symptoms appear as brown lesions with yellow halos, which enlarge with time to cover larger areas of the leaf. Lesions can turn olive brown in color, especially under humid conditions that promote sporulation of the fungus (Al-Sadi, 2016; Gupta et al., 2018a; Gupta et al., 2018b). Bipolaris sorokiniana is the pathogen responsible for spot blotch disease in wheat (Devi et al., 2018; Gupta et al., 2018a; Gupta et al., 2018b; Tembo et al., 2018; Aggarwal et al., 2019). The symptoms of Pyrenophora tritici-repentis-induced tan spot, and Alternaria leaf blight resemble those of spot blotch. One difference is that tan spot is characterized by the appearance of dark fruiting structures, called pseudothecia, on wheat straw, which is not the case for spot blotch (Carmona et al., 2006). Spot blotch differs from Alternaria blight by the development of dark spot areas, which represent masses of conidia that are produced at later infection stages (Neupane et al., 2010; Viani et al., 2017). The spot blotch symptoms elongate and coalesce (Chand et al., 2010).
Leaf infection by B. sorokiniana could come from seeds, root or air. If the pathogen is in the soil, then infection could occur through stomata on the hypocotyl, from where the fungus progresses to the root, shoot and coleoptile (Sprague, 1950). Spore germination can occur within 4–6 h and penetration by B. sorokiniana occurs through stomata and epidermis (Raguchander et al., 1988).
Studies in India and Brazil have shown that spot blotch is usually favored by warm weather (Chaurasia et al., 2000; Kumar et al., 2002; Acharya et al., 2011; Singh, 2017). Also, high humidity is an important factor in enhancing symptom development (Viani et al., 2017). Infection usually starts on the older leaves (Gupta et al., 2018a). In addition, water stress and terminal heat stress have negative effects on the resistance of wheat to B. sorokiniana (Duveiller and Sharma, 2009).
Management of Spot Blotch
No complete resistance to spot blotch has been reported in wheat, but wheat cultivars have been reported to differ in resistance to the disease ( Table 1 ) (Ahirwar et al., 2018; Ayana et al., 2018; Gurjar et al., 2018; Jamil et al., 2018; Singh et al., 2018; Tembo et al., 2018). Therefore, breeding and selecting resistant cultivars is the best option for managing spot blotch in the long term (Gupta et al., 2018a). Among 150 wheat genotypes screened in Zambia, the genotypes 19HRWSN6, 19HRWSN7, and 19HRWSN15 were found resistant (Tembo et al., 2018). In addition, a study on 60 wheat genotypes in Nepal indicated that the genotype NL750 had a high level of resistance to spot blotch, while the tolerant genotype BL1473 is able to produce good yields despite the high disease levels (Sharma et al., 2004; Rosyara et al., 2007).
Table 1.
Examples of wheat genotypes having less susceptibility to spot blotch.
| Country | Wheat genotypes/cultivars | References |
|---|---|---|
| Afganistan | PAMIR‐94 | (Bainsla et al., 2020) |
| Brazil | BH 1146 | (Singh et al., 2016; Singh et al., 2018) |
| China | Ning 9415, Ning 8201 | (Schlegel, 1997; Bainsla et al., 2020) |
| India | Chirya 7, Chirya 3, Ning 8139, Suzhou, Milan-3, HD 2888, HD 2967, WR 95, IC529962 and IC443652 | (Gurjar et al., 2018; Kumari et al., 2018; Choudhary et al., 2019) |
| Mexico | BARTAI, WUYA | (Singh et al., 2018) |
| Nepal | NL750 | (Sharma et al., 2004; Rosyara et al., 2007) |
| Zambia | 19HRWSN6, 19HRWSN7 and 19HRWSN15 | (Tembo et al., 2018) |
Resistance can be induced using some microorganisms and compounds. The combined application of Trichoderma harzianum and methyl jasmonate was found to enhance the activities of defense related enzymes, including catalase, ascorbate peroxidase, phenylalanine lyase, and peroxidase (Singh et al., 2019). In addition, methyl jasmonate is known to inhibit spore germination in B. sorokiniana. In another study, wheat was found to strongly elicit salicylic acid signaling, followed by an enhanced expression of phenylpropanoid pathway genes, which leads to the accumulation of phenolics that play a role in the resistance against spot blotch (Sahu et al., 2016). Also (Sharma et al., 2018) showed that salicylic acid and syringic acid negatively correlated with spot blotch severity, indicating their role in disease defense.
In a study on the efficacy of 195 bacterial strains in suppressing B. sorokiniana, Bacillus subtilis TE3 strain proved to be the most efficacious in suppressing the disease (Villa-Rodríguez et al., 2019). The mechanisms of actions of the antagonistic bacterial strain were through colonizing the wheat phyllosphere and the antimicrobial compounds produced by the bacterium. Additionally, B. safensis and Ochrobactrum pseudogrignonense have been reported to promote resistance to spot blotch in wheat (Sarkar et al., 2018). However, the efficacy of biocontrol agents is usually limited by environmental factors and growing conditions.
Several fungicides have been developed and used for the management of spot botch. The yield increase in fungicide treated plots suffering from leaf diseases compared to untreated plots was 10% in Sweden (Djurle et al., 2018) and 30% in Argentina (Castro et al., 2018). The fungicides carbendazim (Yadav et al., 2013), difenoconazole (Ishikawa et al., 2012), propiconazole (Singh and Singh, 2007; Gupta et al., 2017), and Azoxistrobin (Navathe et al., 2019) were efficacious in managing spot blotch. In addition, Mishra et al. (2014) showed that silver nanoparticles act as a fungicide against spot blotch. The use of silicon was also found to improve resistance of wheat leaves to B. sorokiniana infection (Domiciano et al., 2010). In addition to these management strategies, balanced nutrition and crop rotation should form a part of the integrated management strategies in managing spot blotch in wheat (Sharma et al., 2005; Sharma et al., 2006; Yadav et al., 2013; Mazzilli et al., 2016; Bankina et al., 2018; Sˇvarta and Bimsˇteine, 2019). The application of nitrogen alone without phosphorus and potassium is known to increase the severity of spot blotch (Singh et al., 2012).
Common Root Rot and Crown Rot Diseases
Importance and Symptoms
Common root rot and crown rot of wheat are important diseases in most wheat-growing countries, including China, Australia, Middle East, and Europe (Fedel-Moen and Harris, 1987; Tunali et al., 2008; Al-Sadi and Deadman, 2010; Poole et al., 2015; Gupta et al., 2018b; Xu et al., 2018a). They are characterized by the development of necrotic lesions on the roots, subcrown, and crown. The lesions are dark brown to black in color. Development of symptoms on the root is usually followed by symptoms on wheat crowns (Al-Sadi and Deadman, 2010; Qostal et al., 2019).
The disease is caused by B. sorokiniana (Tunali et al., 2008; Xu et al., 2018a; Yue et al., 2018), which is also associated with other fungi including Fusarium pseudograminearum, F. culmorum, Microdochium nivale, Pythium spp., and Rhizoctonia cerealis (Moya-Elizondo et al., 2011; Saremi and Saremi, 2013; Kazan and Gardiner, 2018; Xu et al., 2018a; White et al., 2019).
Yield and quality of wheat could be reduced by common root rot and crown rot. Common root rot was reported to result in yield losses of 6%–24% (Wildermuth et al., 1992). Yield reduction due to crown rot has been estimated to range from 0 to 89% in New South Wales, Australia (Klein et al., 1991). In Queensland (Australia), crown rot caused up to 26% yield loss in some fields, with an overall reduction by 5% for the whole state (Burgess et al., 1981), while a reduction by up to 35% was reported in the Pacific Northwest, North America (Smiley et al., 2005). Reduction in yield is usually because of the effect of common root rot and crown rot diseases on the number of tillers and on the number and size of kernels (Duczek and Jones-Flory, 1993).
Common root rot is a disease of dry and warm areas. Disease severity and incidence is affected by soil moisture, soil temperature, cultural practices, pathogen population in the soil, and time of infection. Disease severity increases when the plant is under stress or grown in warm soil and less moisture (Mathieson et al., 1990; Acharya et al., 2011). In addition, the incidence of common root rot was found to be affected by the soil populations of B. sorokiniana at the time of planting (Boer et al., 1991). Propagules of B. sorokiniana can go to a depth of 40 cm in the soil, but the population of the fungus is highest in the top 10 cm (Mathieson et al., 1990).
Management of Common Root Rot and Crown Rot
Different methods have been used in the control of common root rot and crown rot of wheat. The use of the endophytic bacterium Pseudomonas mediterranea resulted in a significant reduction in root and crown rot of wheat in Pakistan (Ullah et al., 2020). Disease severity index of wheat common root rot decreased from 90.8% to 27.7% following the use of the actinobacterium Nocardiopsis dassonvillei as a biocontrol agent, which was attributed to the ability of this isolate to produce siderophores and hydrogen cyanide (Allali et al., 2019). The actinobacterium was also found to enhance growth of wheat through the production of indole-3-acetic acid. In another study, the bacterial strain Lysobacter enzymogenes C3 and the fungal strain Rhizoctonia BNR-8-2 were found to result in a significant reduction in the common root rot of wheat, which was attributed to the production of chitinases, β-1,3-glucanases and antibiotics, especially by L. enzymogenes C3 (Eken and Yuen, 2014). Yue et al. (2018) showed that the biocontrol fungus Chaetomium globosum is effective in inhibiting B. sorokiniana associated with wheat common root rot, which is attributed to the production of secondary metabolites by C. globosum.
Cultural practices are important for the management of plant diseases. Crop rotation of wheat with Brassica carinata was found to result in a significant reduction in common root rot and crown rot diseases (Campanella et al., 2020). In Iran, soil solarization was found effective in reducing wheat root rot (Saremi and Saremi, 2013). The use of organic agriculture helped reduce populations of Fusarium populations associated with crown rot of wheat in Canada (Fernandez et al., 2011), while zero tillage was found to increase wheat yields and reduce the incidence of wheat root rot in Mexico (Govaerts et al., 2006).
Different cultivars of wheat were reported to differ in resistance to common root rot (Al-Sadi and Deadman, 2010; Manghwar et al., 2018). In addition, the production of GmPGIP3 transgenic wheat plants enhanced the resistance of wheat to Bipolaris sorokiniana-induced common root rot as well as Gaeumannomyces graminis var. tritici-induced take-all diseases in wheat (Wang et al., 2015). Fungicides are not a good choice for the management of wheat root and crown diseases (Fernandez et al., 2010).
Conclusion
Bipolaris sorokiniana is a serious pathogen, not only because it results in significant yield losses, but also because it can attack most wheat organs, including roots, crown area, stems, leaves and kernels. This means that management strategies should not only focus on limiting the presence of the fungus in the aerial parts of the plants, but attention should be given to B. sorokiniana inoculum present in soil. In addition, it is important to develop an integrated disease management program for managing B. sorokiniana using cultural practices, biological control and chemical fungicides. Since the search for biocontrol agents has been given more attention during recent years, it is important to find antagonistic strains that can complement cultural and chemical practices in the field. The search for new sources for resistance should consider finding less susceptible cultivars to all diseases caused by B. sorokiniana, instead of focusing on one disease.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
Thanks to the financial support from Sultan Qaboos University, VALE Oman and Oman Animal and Plant Genetic Resources Center.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abdullah A. S., Gibberd M. R., Hamblin J. (2020). Co-infection of wheat by Pyrenophora tritici - Repentis and Parastagonospora nodorum in the wheatbelt of Western Australia. Crop Pasture Sci. 71, 119–127. 10.1071/CP19412 [DOI] [Google Scholar]
- Aboukhaddour R., Fetch T., McCallum B. D., Harding M. W., Beres B. L., Graf R. J. (2020). Wheat diseases on the prairies: A Canadian story. Plant Pathol. 69, 418–432. 10.1111/ppa.13147 [DOI] [Google Scholar]
- Acharya K., Dutta A. K., Pradhan P. (2011). Bipolaris sorokiniana (Sacc.) Shoem.: The most destructive wheat fungal pathogen in the warmer areas. Aust. J. Crop Sci. 5, 1064–1071. [Google Scholar]
- Aggarwal R., Sharma S., Singh K., Gurjar M. S., Saharan M. S., Gupta S., et al. (2019). First draft genome sequence of wheat spot blotch pathogen Bipolaris sorokiniana BS_112 from India, obtained using hybrid assembly. Microbiol. Resour. Announc. 8, e00308–e00319. 10.1128/MRA.00308-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahirwar R. N., Mishra V. K., Chand R., Budhlakoti N., Mishra D. C., Kumar S., et al. (2018). Genome-wide association mapping of spot blotch resistance in wheat association mapping initiative (WAMI) panel of spring wheat (Triticum aestivum L.). PloS One 13, e0208196. 10.1371/journal.pone.0208196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn J. L. (1988). The taxonomy of “Helminthosporium“ species. Annu. Rev. Phytopathol. 26, 37–56. 10.1146/annurev.py.26.090188.000345 [DOI] [Google Scholar]
- Allali K., Goudjal Y., Zamoum M., Bouznada K., Sabaou N., Zitouni A. (2019). Nocardiopsis dassonvillei strain MB22 from the Algerian Sahara promotes wheat seedlings growth and potentially controls the common root rot pathogen Bipolaris sorokiniana. J. Plant Pathol. 101, 1115–1125. 10.1007/s42161-019-00347-x [DOI] [Google Scholar]
- Al-Saadi A. M., Deadman M. L., Al-Maskari A. Y. (2002). Screening wheat and barley varieties for resistance to spot blotch disease. Tests Agrochemicals Cultivars, 23, 16–17. [Google Scholar]
- Al-Sadi A. M., Deadman M. L. (2010). Influence of seed-borne Cochliobolus sativus (Anamorph Bipolaris sorokiniana) on crown rot and root rot of barley and wheat. J. Phytopathol. 158, 683–690. 10.1111/j.1439-0434.2010.01684.x [DOI] [Google Scholar]
- Al-Sadi A. M. (2016). Variation in resistance to spot blotch and the aggressiveness of Bipolaris sorokiniana on barley and wheat cultivars. J. Plant Pathol. 98, 97–103. 10.4454/JPP.V98I1.029 [DOI] [Google Scholar]
- Al-Sadi A. M. (2017). “Epidemiology and management of fungal diseases in dry environments,” in Innovations in Dryland Agriculture (Cham, Switzerland: Springer International Publishing; ), 187–209. [Google Scholar]
- Andersen A. L. (1952). Development of wheat headblight by Helmithosporium sativum . Phytopathology 42, 453–456. [Google Scholar]
- Ansari M. S. Q., Pandey A., Mishra V. K., Joshi A. K., Chand R. (2017). “Black point of wheat caused by Bipolaris sorokiniana and its management,” in Management of Wheat and Barley Diseases (Florida, USA: Apple Academic Press; ), 239–255. [Google Scholar]
- Ayana G. T., Ali S., Sidhu J. S., Gonzalez Hernandez J. L., Turnipseed B., Sehgal S. K. (2018). Genome-wide association study for spot blotch resistance in hard winter wheat. Front. Plant Sci. 9, 926. 10.3389/fpls.2018.00926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainsla N. K., Phuke R. M., He X., Gupta V., Bishnoi S. K., Sharma R. K., et al. (2020). Genome-wide association study for spot blotch resistance in Afghan wheat germplasm. Plant Pathol. 69, 1161–1171. 10.1111/ppa.13191 [DOI] [Google Scholar]
- Bankina B., Bimšteine G., Arhipova I., Kaņeps J., Stanka T. (2018). Importance of agronomic practice on the control of wheat leaf diseases. Agriculture (Switzerland) 8 56. 10.3390/agriculture8040056 [DOI] [Google Scholar]
- Barnett H. L., Hunter B. B. (1998). Illustrated Genera of Imperfect Fungi (USA: APS Press; ). [Google Scholar]
- Boer R., Kollmorgen J. F., Macauley B. J., Franz P. R., Boer R. F. D. (1991). Effects of cultivation on Rhizoctonia root rot, cereal cyst nematode, common root rot and yield of wheat in the Victorian Mallee. Aust. J. Exp. Agric. 31, 367–372. 10.1071/EA9910367 [DOI] [Google Scholar]
- Burgess L. W., Dodman R. L., Pont W., Mayers P. (1981). “Fusarium diseases of wheat, maize and grain sorghum in eastern Australia,” in Fusarium: Diseases, Biology and Taxonomy. Eds. Nelson P. E., Toussoun T. A., Cook R. J. (Pennsylvania, USA: The Pennsylvania State University Press; ), 64–76. [Google Scholar]
- Campanella V., Mandalà C., Angileri V., Miceli C. (2020). Management of common root rot and Fusarium foot rot of wheat using Brassica carinata break crop green manure. Crop Prot. 130, 105073. 10.1016/j.cropro.2019.105073 [DOI] [Google Scholar]
- Carmona M. A., Ferrazini M., Barreto D. E. (2006). Tan spot of wheat caused by Drechslera tritici-repentis: Detection, transmission, and control in wheat seed. Cereal Res. Commun. 34, 1043–1049. 10.1556/CRC.34.2006.2-3.236 [DOI] [Google Scholar]
- Castro A. C., Fleitas M. C., Schierenbeck M., Gerard G. S., Simón M. R. (2018). Evaluation of different fungicides and nitrogen rates on grain yield and bread-making quality in wheat affected by Septoria tritici blotch and yellow spot. J. Cereal Sci. 83, 49–57. 10.1016/j.jcs.2018.07.014 [DOI] [Google Scholar]
- Chand R., Pradhan P. K., Prasad L. C., Kumar D., Verma R. P. S., Singh D. P., et al. (2010). Diversity and association of isolates and symptoms of spot blotch caused by Bipolaris sorokiniana of barley (Hordeum vulgare L.). Indian Phytopathol. 63, 154–157. [Google Scholar]
- Chaurasia S., Chand R., Joshi A. K. (2000). Relative dominance of Alternaria triticina Pras. et Prab. and Bipolaris sorokiniana (Sacc.) Shoemaker in different growth stages of wheat (T. aestivum L.). J. Plant Dis. Prot. 107, 176–181. [Google Scholar]
- Choudhary D. K., Aggarwal R., Bashyal B. M., Shanmugam V., Padaria J. C. (2019). Phenotyping and marker based identification of resistant lines in wheat (Triticum aestivum) against spot blotch pathogen (Cochliobolus sativus). Indian J. Agric. Sci. 89, 1885–1889. [Google Scholar]
- Chowdhury A. K., Singh G., Tyagi B. S., Ojha A., Dhar T., Bhattacharya P. M. (2013). Spot blotch disease of wheat – a new thrust area for sustaining productivity. J. Wheat Res. 5, 1–11. [Google Scholar]
- Connert R. L., Davidson J. C. N. (1988). Resistance in wheat to black point caused by Alternarie alternata and Cochliobolus sativus . Can. J. Plant Sci. 68, 351–359. 10.4141/cjps88-046 [DOI] [Google Scholar]
- Cromey M. G., Mulholland R. I. (1988). Blackpoint of wheat: fungal associations, cultivar susceptibility, and effects on grain weight and germination. New Zeal. J. Agr. Res. 31, 51–56. 10.1080/00288233.1988.10421363 [DOI] [Google Scholar]
- Devi H. M., Mahapatra S., Das S. (2018). Assessment of yield loss of wheat caused by spot blotch using regression model. Indian Phytopathol. 71, 291–294. 10.1007/s42360-018-0036-9 [DOI] [Google Scholar]
- Djurle A., Twengström E., Andersson B. (2018). Fungicide treatments in winter wheat: The probability of profitability. Crop Prot. 106, 182–189. 10.1016/j.cropro.2017.12.018 [DOI] [Google Scholar]
- Domiciano G. P., Rodrigues F. A., Moreira W. R., de Oliveira H. V., do Vale F. X. R., Filha M. S. X. (2010). Silicon on the progress of spot blotch on wheat leaf fag. Trop. Plant Pathol. 35, 186–189. [Google Scholar]
- Duczek L. J., Jones-Flory L. L. (1993). Relationships between common root rot, tillering, and yield loss in spring wheat and barley. Can. J. Plant Pathol. 15, 153–158. 10.1080/07060669309500816 [DOI] [Google Scholar]
- Duveiller E. M., Sharma R. C. (2009). Genetic improvement and crop management strategies to minimize yield losses in warm non-traditional wheat growing areas due to spot blotch pathogen Cochliobolus sativus . J. Phytopathol. 157, 521–534. 10.1111/j.1439-0434.2008.01534.x [DOI] [Google Scholar]
- Duveiller E., Dubin H. J., Reeves J., McNab A. (Eds.) (1998). Helminthosophism diseases of wheat: spot blotch and tan spot (Mexico: CIMMYT; ). [Google Scholar]
- Eken C., Yuen G. (2014). Biocontrol of common root rot of wheat with Lysobacter enzymogenes and binucleate Rhizoctonia . Rom Agric. Res. 31, 309–314. [Google Scholar]
- El-Gremi S. M., Draz I. S., Youssef W. A. E. (2017). Biological control of pathogens associated with kernel black point disease of wheat. Crop Prot. 91, 13–19. 10.1016/j.cropro.2016.08.034 [DOI] [Google Scholar]
- FAO (2021). FAOSTAT (FAO; ). Available at: http://www.fao.org/faostat/en/#data/QC/visualize (Accessed 5/1/2021). [Google Scholar]
- Farr D. F., Bills G. F., Chamuris G. P., Rossman A. Y. (1989). Fungi on plants and plant products in the United States (St. Paul, MN, USA: The American Phytopathological Society Press; ), 1252. [Google Scholar]
- Fedel-Moen R., Harris J. R. (1987). Stratified distribution of Fusarium and Bipolaris on wheat and barley with dryland root rot in South Australia. Plant Pathol. 36, 447–454. 10.1111/j.1365-3059.1987.tb02261.x [DOI] [Google Scholar]
- Fernandez M. R., May W. E., Lafond G. P. (2010). Effect of fungicide seed treatments on root pathogens of cereal crops under field conditions. Can. J. Plant Sci. 90, 905–917. 10.4141/CJPS09172 [DOI] [Google Scholar]
- Fernandez M. R., Ulrich D., Brandt S. A., Zentner R. P., Wang H., Thomas A. G., et al. (2011). Crop management effects on root and crown rot of wheat in West-Central Saskatchewan, Canada. Agron. J. 103, 756–765. 10.2134/agronj2010.0190 [DOI] [Google Scholar]
- Fetch T., Mitchell Fetch J., Xue A. (2015). Races of Puccinia graminis on barley, oat, and wheat in Canada in 2007 and 2008. Can. J. Plant Pathol. 37, 331–341. 10.1080/07060661.2015.1066865 [DOI] [Google Scholar]
- Gannibal P. B. (2018). Factors affecting Alternaria appearance in grains in European Russia. Sel’skokhozyaistvennaya Biol. 53, 605–615. 10.15389/agrobiology.2018.3.605eng [DOI] [Google Scholar]
- Ghosh T., Biswas M. K., Guin C., Roy P., Aikat K. (2018). A review on seed borne mycoflora associated with different cereal crop seeds and their management. Plant Cell Biotechnol. Mol. Biol. 19, 107–117. [Google Scholar]
- Govaerts B., Mezzalama M., Sayre K. D., Crossa J., Nicol J. M., Deckers J. (2006). Long-term consequences of tillage, residue management, and crop rotation on maize/wheat root rot and nematode populations in subtropical highlands. Appl. Soil Ecol. 32, 305–315. 10.1016/j.apsoil.2005.07.010 [DOI] [Google Scholar]
- Gultyaeva E. I., Kovalenko N. M., Shamanin V. P., Tyunin V. A., Shreyder E. R., Shaydayuk E. L., et al. (2018). Population structure of leaf pathogens of common spring wheat in the West Asian regions of Russia and North Kazakhstan in 2017. Vavilovskii Zhurnal Genet. Selektsii 22, 363–369. 10.18699/VJ18.372 [DOI] [Google Scholar]
- Gultyaeva E., Yusov V., Rosova M., Mal’chikov P., Shaydayuk E., Kovalenko N., et al. (2020). Evaluation of resistance of spring durum wheat germplasm from Russia and Kazakhstan to fungal foliar pathogens. Cereal Res. Commun. 48, 71–79. 10.1007/s42976-019-00009-9 [DOI] [Google Scholar]
- Gupta A. K., Raj R., Choudhary R., Singh S. P., Solanki I. S. (2017). Effect of mineral nutrients, seed treatment and foliar fungicides on spot blotch (Bipolaris sorokiniana) severity and agronomic performance of wheat (Triticum aestivum) in hot-humid environment of eastern Gangetic plain in India. Indian J. Agric. Sci. 87, 505–511. [Google Scholar]
- Gupta P. K., Chand R., Vasistha N. K., Pandey S. P., Kumar U., Mishra V. K., et al. (2018. a). Spot blotch disease of wheat: the current status of research on genetics and breeding. Plant Pathol. 67, 508–531. 10.1111/ppa.12781 [DOI] [Google Scholar]
- Gupta P. K., Vasistha N. K., Aggarwal R., Joshi A. K. (2018. b). Biology of B. sorokiniana (syn. Cochliobolus sativus) in genomics era. J. Plant Biochem. Biotechnol. 27, 123–138. 10.1007/s13562-017-0426-6 [DOI] [Google Scholar]
- Gurjar M. S., Bhardwaj N. R., Jain S., Sharma S., Gupta S., Aggarwal R. (2018). Identification of resistance sources and expression level of defense-related genes in wheat against Bipolaris sorokiniana . Indian Phytopathol. 71, 127–134. 10.1007/s42360-018-0020-4 [DOI] [Google Scholar]
- Han Q., Huang L., Buchenauer H., Wang C., Kang Z. (2010). Cytological study of wheat spike infection by bipolaris sorokiniana. J. Phytopathol. 158, 22–29. 10.1111/j.1439-0434.2009.01570.x [DOI] [Google Scholar]
- Ishikawa M. S., de Batista Fonseca I. C., Igarashi S. (2012). Chemical treatment in seeds on the development of the spot blotch in wheat plants. Cienc. Rural 42, 1341–1346. 10.1590/S0103-84782012000800002 [DOI] [Google Scholar]
- Jamil M., Ali A., Gul A., Ghafoor A., Ibrahim A. M. H., Mujeeb-Kazi A. (2018). Genome-wide association studies for spot blotch (Cochliobolus sativus) resistance in bread wheat using genotyping-by-sequencing. Phytopathology 108, 1307–1314. 10.1094/PHYTO-02-18-0047-R [DOI] [PubMed] [Google Scholar]
- Jarroudi M. E., Kouadio L., Bock C. H., Junk J., Pasquali M., Maraite H., et al. (2017). A threshold-based weather model for predicting stripe rust infection in winter wheat. Plant Dis. 101, 693–703. 10.1094/pdis-12-16-1766-re [DOI] [PubMed] [Google Scholar]
- Joshi A. K., Mishra B., Chatrath R., Ferrara G. O., Singh R. P. (2007). Wheat imporvement in India: present status, emerging challenges and future prospects. Euphytica 157, 431–446. 10.1007/s10681-007-9385-7 [DOI] [Google Scholar]
- Kaur P. K., Kaur J., Saini H. S. (2015). Antifungal potential of Bacillus vallismortis R2 against different phytopathogenic fungi. Span. J. Agric. Res. 13, 1–11. 10.5424/sjar/2015132-6620 [DOI] [Google Scholar]
- Kaur P. K., Joshi N., Singh I. P., Saini H. S. (2017). Identification of cyclic lipopeptides produced by Bacillus vallismortis R2 and their antifungal activity against Alternaria alternata . J. Appl. Microbiol. 122, 139–152. 10.1111/jam.13303 [DOI] [PubMed] [Google Scholar]
- Kazan K., Gardiner D. M. (2018). Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: recent progress and future prospects. Mol. Plant Pathol. 19, 1547–1562. 10.1111/mpp.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. A., Burgess L. W., Ellison F. W. (1991). The incidence and spatial patterns of wheat plants infected by Fusarium graminearum group 1 and the effect of crown rot on yield. Aust. J. Agric. Res. 42, 399–407. 10.1071/AR9910399 [DOI] [Google Scholar]
- Kumar J., Schafer P., Huckelhoven R., Langen G., Baltruschat H., Stein E., et al. (2002). Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol. Plant Pathol. 3, 185–195. 10.1046/j.1364-3703.2002.00120.x [DOI] [PubMed] [Google Scholar]
- Kumari J., Kumar S., Singh N., Vaish S. S., Das S., Gupta A., et al. (2018). Identification of new donors for spot blotch resistance in cultivated wheat germplasm. Cereal Res. Commun. 46, 467–469. 10.1556/0806.46.2018.028 [DOI] [Google Scholar]
- Lalic B., Jankovic D., Dekic L., Eitzinger J., Sremac A. F. (2017). Testing efficacy of monthly forecast application in agrometeorology: Winter wheat phenology dynamic. IOP Conf. Ser.: Earth Environ. Sci. 57, 12002. 10.1088/1755-1315/57/1/012002 [DOI] [Google Scholar]
- Ledingham R. J., Atkinson T. G., Horricks J. S., Mills J. T., Piening L. J., Tinline R. D. (1973). Wheat losses due to common root rot in the Praire Provinces of Canada 1969-1971. Can. Plant Dis. Surv. 53, 113–122. [Google Scholar]
- Li Q. Y., Qin Z., Jiang Y. M., Shen C. C., Duan Z. B., Niu J. S. (2014). Screening wheat genotypes for resistance to black point and the effects of diseased kernels on seed germination. J. Plant Dis. Prot. 121, 79–88. 10.1007/BF03356495 [DOI] [Google Scholar]
- Li Q. Y., Wang S. Y., Chang S. W., Xu K. G., Li M. Y., Xu Q. Q., et al. (2019. a). Key periods and effects of meteorological factors affecting incidence of wheat black point in the Yellow and Huai wheat area of China. Crop Prot. 125, 104882. 10.1016/j.cropro.2019.104882 [DOI] [Google Scholar]
- Li Q. Y., Xu Q. Q., Jiang Y. M., Niu J. S., Xu K. G., He R. S. (2019. b). The correlation between wheat black point and agronomic traits in the North China Plain. Crop Prot. 119, 17–23. 10.1016/j.cropro.2019.01.004 [DOI] [Google Scholar]
- Li Q., Niu H., Xu K., Xu Q., Wang S., Liang X., et al. (2020). GWAS for resistance against black point caused by Bipolaris sorokiniana in wheat. J. Cereal Sci. 91:102859. 10.1016/j.jcs.2019.102859 [DOI] [Google Scholar]
- Machacek J. E., Wallace H. A. H. (1952). Longevity of some fungi in cereal seed. Can. J. Bot. 30, 164. 10.1139/b52-015 [DOI] [Google Scholar]
- Malaker P., Mian I. (2009). Effect of seed treatment and foliar spray with fungicides in controlling black point disease of wheat. Bangladesh J. Agric. Res. 34, 425–434. 10.3329/bjar.v34i3.3968 [DOI] [Google Scholar]
- Manghwar H., Hussain A., Ullah A., Gul S., Shaban M., Khan A. H., et al. (2018). Expression analysis of defense related genes in wheat and maize against Bipolaris sorokiniana . Physiol. Mol. Plant Pathol. 103, 36–46. 10.1016/j.pmpp.2018.04.002 [DOI] [Google Scholar]
- Mathieson J. T., Rush C. M., Bordovsky D., Clark L. E., O.R J. (1990). Effects of tillage on common root rot of wheat in Texas. Plant Dis. 74, 1006–1008. 10.1094/PD-74-1006 [DOI] [Google Scholar]
- Mazzilli S. R., Ernst O. R., de Mello V. P., Pérez C. A. (2016). Yield losses on wheat crops associated to the previous winter crop: Impact of agronomic practices based on on-farm analysis. Eur. J. Agron. 75, 99–104. 10.1016/j.eja.2016.01.007 [DOI] [Google Scholar]
- Mead H. W. (1942). Host-parasite relationships in a seed-borne disease of barley caused by Helminthosporium sativum Pammel, King and Bakke. Can. J. Res. Sect. C Bot. Sci. 20, 501–523. 10.1139/cjr42c-042 [DOI] [Google Scholar]
- Mishra S., Singh B. R., Singh A., Keswani C., Naqvi A. H., Singh H. B. (2014). Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PloS One 9, e97881. 10.1371/journal.pone.0097881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mónaco C., Sisterna M., Perelló A., Bello G. D. (2004). Preliminary studies on biological control of the blackpoint complex of wheat in Argentina. World J. Microbiol. Biotechnol. 20, 285–290. 10.1023/B:WIBI.0000023835.05701.10 [DOI] [Google Scholar]
- Moya-Elizondo E. A., Rew L. J., Jacobsen B. J., Hogg A. C., Dyer A. T. (2011). Distribution and prevalence of fusarium crown rot and common root rot pathogens of wheat in Montana. Plant Dis. 95, 1099–1108. 10.1094/PDIS-11-10-0795 [DOI] [PubMed] [Google Scholar]
- Navathe S., Chand R., Mishra V. K., Pandey S. P., Kumar U., Joshi A. K. (2019). Management of spot blotch and heat stress in spring wheat through Azoxystrobin-mediated redox balance. Agric. Res. 9, 169–178. 10.1007/s40003-019-00417-7 [DOI] [Google Scholar]
- Navathe S., Yadav P. S., Chand R., Mishra V. K., Vasistha N. K., Meher P. K., et al. (2020). ToxA-TSN1 interaction for spot blotch susceptibility in Indian wheat: An example of inverse gene-for-gene relationship. Plant Dis. 104, 71–81. 10.1094/PDIS-05-19-1066-RE [DOI] [PubMed] [Google Scholar]
- Neupane A., Sharma R., Duveiller E., Shrestha S. (2010). Sources of Cochliobolus sativus inoculum causing spot blotch under warm wheat growing conditions in South Asia. Cereal Res. Commun. 38, 541–549. 10.1556/crc.38.2010.4.11 [DOI] [Google Scholar]
- Poole G. J., Harries M., Hüberli D., Miyan S., MacLeod W. J., Lawes R., et al. (2015). Predicting cereal root disease in Western Australia using soil DNA and environmental parameters. Phytopathology 105, 1069–1079. 10.1094/phyto-07-14-0203-r [DOI] [PubMed] [Google Scholar]
- Qostal S., Kribel S., Chliyeh M., Serghat S., KarimaSelmaoui A. O. T., Zaarati H., et al. (2019). Study of the fungal complex responsible for root rot of wheat and barley in the north-west of Morocco. Plant Arch. 19, 2143–2157. [Google Scholar]
- Raemaekers R. H. (1991). “First occurrence in nature of Cochliobolus sativus, the teleomorph of Bipolaris sorokiniana,” in Contribution to the epidemiology of Bipolaris sorokiniana diseases and the development of rainfed wheat, a new crop in Zambia (Belgium: Katholieke Universiteit te Leuven; ), 70–85. [Google Scholar]
- Raguchander T., Srikant K., Hedge P. K., S K. (1988). Studies on leaf blight caused by Bipolaris sorokiniana (Sacc.) Shoem. anamorph of Cochliobolus sativus (Ito and Kurib.) Drechsler ex Dastur. Plant Pathol. Newslett. 6, 45. [Google Scholar]
- Rashid A. Q. M. B., Meah M. B., Fakir G. A. (1992). Importance and distribution of seedborne Bipolaris sorokiniana in wheat in Bangladesh. Bangladesh J. Plant Pathol. 8, 5–7. [Google Scholar]
- Rashid A. Q. M. B., Fakir G. A., Hossain I., Kulshrestha D. D. (1997). Association of Bipolaris sorokiniana with wheat seeds and its transmission from seed to plant. Bangladesh J. Plant Pathol. 13, 17–20. [Google Scholar]
- Rashid A. Q. M. B. (1998). Effect of seed transmitted Bipolaris sorokiniana on growth and survival of wheat seedlings. Indian Phytopathol. 51, 329–333. [Google Scholar]
- Riaz A., Athiyannan N., Periyannan S., Afanasenko O., Mitrofanova O., Aitken E. A. B., et al. (2017). Mining vavilov’s treasure chest of wheat diversity for adult plant resistance to Puccinia triticina . Plant Dis. 101, 317–323. 10.1094/PDIS-05-16-0614-RE [DOI] [PubMed] [Google Scholar]
- Rosyara U. R., Duveiller E., Pant K., Sharma R. C. (2007). Variation in chlorophyll content, anatomical traits and agronomic performance of wheat genotypes differing in spot blotch resistance under natural epiphytotic conditions. Australas. Plant Pathol. 36, 245–251. 10.1071/AP07014 [DOI] [Google Scholar]
- Sahu R., Sharaff M., Pradhan M., Sethi A., Bandyopadhyay T., Mishra V. K., et al. (2016). Elucidation of defense-related signaling responses to spot blotch infection in bread wheat (Triticum aestivum L.). Plant J. 86, 35–49. 10.1111/tpj.13149 [DOI] [PubMed] [Google Scholar]
- Saremi H., Saremi H. (2013). Isolation of the most common Fusarium species and the effect of soil solarisation on main pathogenic species in different climatic zones of Iran. Eur. J. Plant Pathol. 137, 585–596. 10.1007/s10658-013-0272-x [DOI] [Google Scholar]
- Sarkar J., Chakraborty U., Chakraborty B. N. (2018). Induced defense response in wheat plants against Bipolaris sorokiniana following application of Bacillus safensis and Ochrobactrum pseudogrignonense . Indian Phytopathol. 71, 49–58. 10.1007/s42360-018-0006-2 [DOI] [Google Scholar]
- Schlegel R. (1997). Current list of wheats with rye introgressions of homoeologous group 1. 2nd update. Wheat Inf. Serv. 84, 64–69. [Google Scholar]
- Sˇvarta A., Bimsˇteine G. (2019). “Winter wheat leaf diseases and several steps included in their integreated control: A review,” in Research for Rural Development. Eds. Treija S., Skujeniece S.(Jelgava: Latvia University of Agriculture; ). [Google Scholar]
- Shahbaz M., Riaz M., Ali S., Ahmad F., Hussain A., Nabi G., et al. (2018). Effect of seed dressing chemicals on emergence, yield and against soil & seed born diseases of wheat. Pakistan J. Phytopathol. 30, 183–189. 10.33866/phytopathol.030.02.0461 [DOI] [Google Scholar]
- Sharma R. C., Dubin H. J. (1996). Effect of wheat cultivar mixtures on spot blotch (Bipolaris sorokiniana) and grain yield. Field Crop Res. 48, 95–101. 10.1016/S0378-4290(96)01031-3 [DOI] [Google Scholar]
- Sharma R., Duveiller E., Gyawali S., Shrestha S., Chaudhary N., Bhatta M. (2004). Resistance to Helminthosporium leaf blight and agronomic performance of spring wheat genotypes of diverse origins. Euphytica 139, 33–44. 10.1007/s10681-004-2292-2 [DOI] [Google Scholar]
- Sharma S., Duveiller E., Basnet R., Karki C. B., Sharma R. C. (2005). Effect of potash fertilization on Helminthosporium leaf blight severity in wheat, and associated increases in grain yield and kernel weight. Field Crops Res. 93, 142–150. 10.1016/j.fcr.2004.09.016 [DOI] [Google Scholar]
- Sharma P., Duveiller E., Sharma R. C. (2006). Effect of mineral nutrients on spot blotch severity in wheat, and associated increases in grain yield. Field Crops Res. 95, 426–430. 10.1016/j.fcr.2005.04.015 [DOI] [Google Scholar]
- Sharma V. K., Niwas R., Karwasra S. S., Saharan M. S. (2017). Progression of powdery mildew on different varieties of wheat and triticale in relation to environmental conditions. J. Agrometeorol. 19, 84–87. [Google Scholar]
- Sharma S., Sahu R., Navathe S., Mishra V. K., Chand R., Singh P. K., et al. (2018). Natural variation in elicitation of defense-signaling associates to field resistance against the spot blotch disease in bread wheat (Triticum aestivum L.). Front. Plant Sci. 9:636. 10.3389/fpls.2018.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Singh R. N. (2007). Management of spot blotch of wheat (Triticum aestivum) caused by Bipolaris sorokiniana . Indian J. Agric. Sci. 77, 323–326. [Google Scholar]
- Singh S. K., Gupta V., Razdan V. K., Singh V. B., Vikas G. (2012). Effect of fertilizers on severity of spot blotch caused by Bipolaris sorokiniana and yield of Wheat. Ann. Plant Prot. Sci. 20, 411–413. [Google Scholar]
- Singh V., Singh G., Chaudhury A., Ojha A., Tyagi B. S., Chowdhary A. K., et al. (2016). Phenotyping at hot spots and tagging of QTLs conferring spot blotch resistance in bread wheat. Mol. Biol. Rep. 43, 1293–1303. 10.1007/s11033-016-4066-z [DOI] [PubMed] [Google Scholar]
- Singh P. K., He X., Sansaloni C. P., Juliana P., Dreisigacker S., Duveiller E., et al. (2018). Resistance to spot blotch in two mapping populations of common wheat is controlled by multiple QTL of minor effects. Int. J. Mol. Sci. 19, 4054. 10.3390/ijms19124054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U. B., Malviya D., Singh S., Kumar M., Sahu P. K., Singh H. V., et al. (2019). Trichoderma harzianum-and methyl jasmonate-induced resistance to Bipolaris sorokiniana through enhanced phenylpropanoid activities in bread wheat (Triticum aestivum L.). Front. Microbiol. 10, 1697. 10.3389/fmicb.2019.01697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. P. (2017). “Host resistance to spot blotch (Bipolaris sorokiniana) in wheat and barley,” in Management of Wheat and Barley Diseases (Florida, USA: Apple Academic Press; ), 327–339. [Google Scholar]
- Sisterna M. N., Sarandon S. J. (1996). Black point of wheat (Bipolaris sorokiniana (Sacc.) Shoem.) influenced by N fertilization under no till and conventional tillage. Cereal Res. Commun. 24, 217–221. [Google Scholar]
- Sisterna M. N., Sarandon S. J. (2005). Preliminary studies on the natural incidence of wheat black point under different fertilization levels and tillage systems in Argentina. Plant Pathol. J. 4, 26–28. 10.3923/ppj.2005.26.28 [DOI] [Google Scholar]
- Smiley R. W., Gourlie J. A., Easley S. A., Patterson L. M., Whittaker R. G. (2005). Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis. 89, 595–604. 10.1094/PD-89-0595 [DOI] [PubMed] [Google Scholar]
- Somani D., Adhav R., Prashant R., Kadoo N. Y. (2019). Transcriptomics analysis of propiconazole-treated Cochliobolus sativus reveals new putative azole targets in the plant pathogen. Funct. Integr. Genomics 19, 453–465. 10.1007/s10142-019-00660-9 [DOI] [PubMed] [Google Scholar]
- Sprague R. (1950). Diseases of Cereals and Grasses in North America (New York: Ronald Press; ). [Google Scholar]
- Sultana S., Adhikary S. K., Rahman S. M. M., Islam M. M. (2018). Sexuality and compatibility of Bipolaris sorokiniana and segregation pattern in teleomorph (Cochliobolus sativus): geographic origin and segregation ratio. Indian Phytopathol. 71, 365–375. 10.1007/s42360-018-0066-3 [DOI] [Google Scholar]
- Tembo B., Sibiya J., Tongoona P. (2018). Genetic variability among wheat (Triticum aestivum L.) germplasm for resistance to spot blotch disease. J. Agric. Rural Dev. Trop. Subtropics 119, 85–93. [Google Scholar]
- Tunali B., Nicol J. M., Hodson D., Uçkun Z., Büyük O., Erdurmuş D., et al. (2008). Root and crown rot fungi associated with spring, facultative, and winter wheat in Turkey. Plant Dis. 92, 1299–1306. 10.1094/pdis-92-9-1299 [DOI] [PubMed] [Google Scholar]
- Ullah H., Yasmin H., Mumtaz S., Jabeen Z., Naz R., Nosheen A., et al. (2020). Multitrait Pseudomonas spp. isolated from monocropped wheat (Triticum aestivum) suppress Fusarium root and crown rot. Phytopathology 110, 582–592. 10.1094/PHYTO-10-19-0383-R [DOI] [PubMed] [Google Scholar]
- Van Ginkel M., Rajaram S. (1998). “Breeding for resistance to spot blotch in wheat: global perspective,” in Helminthosophism diseases of wheat: spot blotch and tan spot. Eds. Duveiller E., Dubin H., Reeves J., McNab A. (Mexico: CIMMYT; ), 162–170. [Google Scholar]
- Viani A., Sinha P., Sharma T., Bhar L. M. (2017). A model for forecasting spot blotch disease in wheat. Australas. Plant Pathol. 46, 601–609. 10.1007/s13313-017-0514-z [DOI] [Google Scholar]
- Villa-Rodríguez E., Parra-Cota F., Castro-Longoria E., López-Cervantes J., de los Santos-Villalobos S. (2019). Bacillus subtilis TE3: A promising biological control agent against Bipolaris sorokiniana, the causal agent of spot blotch in wheat (Triticum turgidum L. subsp. durum). Biol. Contr. 132, 135–143. 10.1016/j.biocontrol.2019.02.012 [DOI] [Google Scholar]
- Wang A., Wei X., Rong W., Dang L., Du L. P., Qi L., et al. (2015). GmPGIP3 enhanced resistance to both take-all and common root rot diseases in transgenic wheat. Funct. Integr. Genomics 15, 375–381. 10.1007/s10142-014-0428-6 [DOI] [PubMed] [Google Scholar]
- Wei X., Xu Z., Zhang N., Yang W., Liu D., Ma L. (2021). Synergistic action of commercially available fungicides for protecting wheat from common root rot caused by Bipolaris sorokiniana in China. Plant Dis. 105. 10.1094/PDIS-03-20-0627-RE [DOI] [PubMed] [Google Scholar]
- Weniger W. (1923). Pathological morphology of drum wheat grains affected with black point. Phytopathology 13, 48. [Google Scholar]
- White D. J., Chen W., Schroeder K. L. (2019). Assessing the contribution of ethaboxam in seed treatment cocktails for the management of metalaxyl-resistant Pythium ultimum var. ultimum in Pacific Northwest spring wheat production. Crop Prot. 115, 7–12. 10.1016/j.cropro.2018.08.026 [DOI] [Google Scholar]
- Wiese M. V. (1987). Compendium of Wheat Diseases (St. Paul, MN: APS Press; ). [Google Scholar]
- Wildermuth G. B., Tinline R. D., McNamara R. B. (1992). Assessment of yield loss caused by common root rot in wheat cultivars in Queensland. Aust. J. Agric. Res. 43, 43–58. 10.1071/AR9920043 [DOI] [Google Scholar]
- Xu F., Yang G., Wang J., Song Y., Liu L., Zhao K., et al. (2018. a). Spatial distribution of root and crown rot fungi associated with winter wheat in the North China Plain and its relationship with climate variables. Front. Microbiol. 9, 1054. 10.3389/fmicb.2018.01054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K. G., Jiang Y. M., Li Y. K., Xu Q. Q., Niu J. S., Zhu X. X., et al. (2018. b). Identification and pathogenicity of fungal pathogens causing black point in wheat on the North China plain. Indian J. Microbiol. 58, 159–164. 10.1007/s12088-018-0709-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav B., Singh R., Kumar A. (2013). Effect of micronutrients and fungicides on spot blotch of wheat. Vegetos 26, 212–219. 10.5958/j.2229-4473.26.2.077 [DOI] [Google Scholar]
- Yue H. M., Wang M., Gong W. F., Zhang L. Q. (2018). The screening and identification of the biological control fungi Chaetomium spp. against wheat common root rot. FEMS Microbiol. Lett. 365, fny242. 10.1093/femsle/fny242 [DOI] [PubMed] [Google Scholar]
- Zhang T. V., Wang H. L., Xu F. L. (1990). Effects of grain black point disease of wheat and the pathogenic fungi. Acta-Phytophylacica Sin. 4, 313–316. [Google Scholar]
- Zhu Z., Bonnett D., Ellis M., Singh P., Heslot N., Dreisigacker S., et al. (2015). Mapping resistance to spot blotch in a CIMMYT synthetic-derived bread wheat. Mol. Breed. 34, 1215–1228. 10.1007/s11032-014-0111-6 [DOI] [Google Scholar]