Abstract
Introduction
The benefit of three-dimensional (3D) visualization for liver disease is uncertain.
Aim
To evaluate the effectiveness and safety of 3D versus two-dimensional (2D) video-assisted hepatectomy for LD.
Material and methods
We searched PubMed, Embase, Cochrane Library, Medline, and Web of Science for studies addressing 3D versus 2D for 2D until 30 February 2020. Study-specific effect sizes and their 95% confidence intervals (CIs) were combined to calculate the pooled value using a fixed-effects or random-effects model.
Results
Nine studies with 808 patients were included. The 3D group had shorter operative time (mean difference (MD) = 34.39; 95% CI = 59.50, 9.28), experienced less intraoperative blood loss (MD = 106.55; 95% CI = 183.76, 29.34), and a smaller blood transfusion volume (MD = 88.25; 95% CI = 141.26, 35.24). The 3D group had a smaller difference between the predicted volume and the actual resected volume (MD = 103.25; 95% CI = 173.24, 33.26) and a lower rate of postoperative complications (odds ratio (OR) = 0.57; 95% CI: 0.35, 0.91).
Conclusions
During surgery, 3D video-assisted hepatectomy could effectively reduce operative time, intraoperative bleeding, and blood transfusion volume, and had a smaller difference between the predicted volume and the actual resected volume and a lower rate of postoperative complications. More high-quality randomized controlled trials are required to verify the reliability and validity of our conclusion.
Keywords: three dimensional, two dimensional, video-assisted hepatectomy, meta-analysis
Introduction
Although the frequency of hepatic resection is increasing, hepatectomy remains one of the most difficult operative procedures due to anatomical complexity and hepatic vascular variability [1]. Preoperative understanding of individual liver anatomy and selection of appropriate surgical methods can ensure safety and effectiveness of surgery. Clavien et al. stated that, for patients without cirrhosis, to effectively prevent the occurrence of postoperative liver failure, the postoperative residual liver volume should exceed 30%. For patients with cirrhosis, the residual volume of the resected liver should exceed 50% [2]. To predict this, one needs to rely on the experience and imagination of a clinician, which will in turn impart a certain extent of subjectivity and inconsistency [3]. Therefore, it is necessary to adopt a more intuitive and reliable technique in the clinic to make up for such deficiencies of the traditional techniques.
At present, two-dimensional imaging technologies such as ultrasound scan, computed tomography (CT scan), and magnetic resonance imaging are usually used in the clinical evaluation of the liver prior to surgery. However, using conventional two-dimensional displays, surgeons rely on indirect visual cues in the form of relative and expected motion, shadows, textures, and relative color differences to extract indirect three-dimensional (3D) information. This may lead to the loss of depth perception and consequently exert more cognitive workload on surgeons [4]. Recently, with the gradual application of 3D visualization technology in clinical practice, more and more authors have discussed the value of this technology in liver surgery [5–7]. Most of them believe that 3D visualization technology can produce intuitive and clear 3D images which will enable them to perform virtual surgery, calculate liver volume, and significantly guide them through the clinical surgery [8].
However, application of three-dimensional technology in liver resection is still in the developmental stage, and is lacking high-quality evidence to show that this technique can improve the effectiveness of a hepatectomy. Therefore, the short-term outcomes and safety of 3D images remain controversial. In the current study, we aimed to perform a meta-analysis to generate valid data on the short-term outcomes and safety of three-dimensional video-assisted hepatectomy for liver disease.
Aim
This meta-analysis was conducted to evaluate the effectiveness and safety of three-dimensional versus two-dimensional video-assisted hepatectomy for liver disease.
Material and methods
Search strategies
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. Systematic computerized searches were performed using PubMed, Embase, Scopus, Cochrane Library, Web of Science, and Science Direct electronic databases, as well as major scientific websites for studies published up to 30 February 2020. The following keywords and subject terms were used in the search: three-dimensional visualization, liver neoplasms, and hepatectomy. All reference lists from the studies selected by electronic searching were scanned to further identify relevant studies.
Study selections
The studies were selected for further analysis if they met the following inclusion criteria: the studies should be either randomized controlled trials (RCTs) or observational studies (case–control or cohort studies); in each study, all patients should undergo a hepatectomy due to liver disease (LD); and the most recent or complete studies were included if they were based on overlapping patients. The studies were excluded if they had no comparison between three dimensional and two dimensional video-assisted hepatectomy; case reports, abstracts, conference reports, or experiments; and papers that did not report any relevant data for analysis.
Data extraction
Search and screening of retrieved records at the title and abstract level were independently performed by two reviewers. The same two reviewers assessed the full-text eligibility of the identified trials, and discrepancies were resolved by consensus under the supervision of the other two investigators. Two authors independently reviewed all included studies and assigned a judgment of “high”, “low”, or “unclear” based on the risk of bias considering the following parameters: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting [10]. Data from original reports were collected into specific electronic spreadsheets. Data were carefully extracted from these studies using a standardized data collection method. The following data were extracted from each study: first author, year, country, study design, tumor size (cm), age, lesion type, sample size, and outcomes. The outcomes for analyses included operative time, intraoperative blood loss, blood transfusion volume, the difference between the predicted volume and the actual resected volume, hospital stay and postoperative complications. For quantitative data without means or standard deviations (SDs), if the missing information was unavailable from the authors, an alternate method [11] was used to estimate the mean and SD based on the median, range, and sample size.
Quality assessment
The quality evaluation of RCTs was based on the Jadad scale, where randomness (0–2 points), blinding (0–2 points), and extraction (0–1 points) were used. The study was defined as high quality when the mass fraction was greater than 3 points. The quality evaluation of observational studies was evaluated by the Newcastle-Ottawa scale (NOS) [12, 13], which is made up of three factors: patient selections, comparability of the study groups, and assessment of outcomes. The total score of each observational study was 9 stars, and a high-quality study was defined as a study with a quality score of ≥ 7 stars. Disagreements were resolved by common consensus among the researchers.
Statistical analysis
The study-specific odds ratio (OR) for categorical variables, the mean difference (MD) for continuous variation, and the 95% confidence intervals (CIs) were combined to calculate the pooled value of each study using Cochrane Review Manager software (RevMan; version 5.3). Cochran’s chi-square test and I2 were used to examine the heterogeneity among the effect estimates. Statistical heterogeneity among studies was defined as I2 statistic > 50% [14]. The fixed-effects model was preferred to the random-effects model when there was no statistically significant heterogeneity and vice versa when there was significant heterogeneity [15]. If the number of studies was > 10, the study bias was detected using the methods of the funnel plot and Begg’s and Egger’s test [16]. Sensitivity analysis was performed by excluding the studies with the lowest quality score. When possible, subgroup analyses of lesion type (malignant lesions (ML) or benign lesions (BL)) were performed to determine whether these factors affected the conclusion. Statistical significance was set at p < 0.05.
Results
Eligible studies and quality assessment and risk of bias
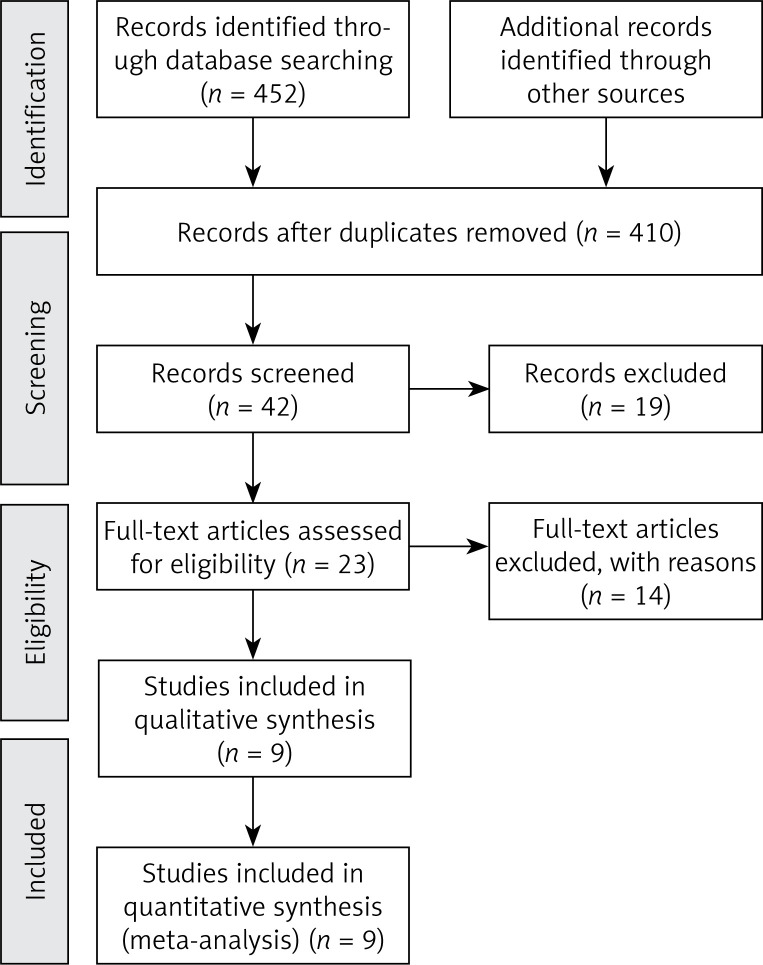
Based on the pre- designed strategy, nine retrospective cohort studies (RCSs) [17–25] with a total of 808 patients for final analyses were selected (Figure 1). Table I lists the main data extracted from these studies. The baseline data of the patients in each study were comparable or well matched. Table I lists the quality assessments of these studies. Nine retrospective cohort studies were evaluated by NOS. Quality evaluation of the retrospective cohort studies indicated a low risk of bias since the studies were ranked as studies with high quality.
Figure 1.
Flow diagram of the literature selection. PubMed, Embase, Cochrane Library, Medline and Web of Science were searched for the literature with designed searching terms. After screening the ps, abstracts, and then the full text for relevance step by step, nine studies were considered suitable to conduct the said meta-analysis in the end
Table I.
Characteristics of included studies
| References | Year | Country | Study design | Tumor size [cm]a | Age [years]a | Lesion type | Sample size | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 3D | 2D | ||||||||
| Yamanaka et al. | 2007 | Japan | RCS | NA | NA | ML | 226 | 113 | 113 | 7 |
| Pianka et al. | 2011 | Germany | RCS | 9.5 ±3.6/ 10.4 ±3.8 |
50 ±10.6/ 48 ±10.0 |
ML | 26 | 13 | 13 | 7 |
| Fang et al. | 2013 | China | RCS | NA | 50.6 ±11.5/ 53.5 ±12.6 |
BL | 98 | 56 | 42 | 9 |
| Begin et al. | 2014 | Canada | RCS | NA | 61.6 ±12.9/ 64.8 ±16.8 |
ML | 72 | 36 | 36 | 7 |
| Fang et al. | 2015 | China | RCS | 7.6 ±2.8/ 7.4 ±2.6 |
47.5 ±13.8/ 46.5 ±13.3 |
ML | 116 | 60 | 56 | 8 |
| He et al. | 2015 | China | RCS | NA | 41.4 ±13.1/ 42.5 ±13.2 |
BL | 106 | 59 | 47 | 9 |
| Wei et al. | 2015 | China | RCS | 9.5 ±3.6/ 10.4 ±3.8 |
50 ±10.6/ 48 ±10.0 |
ML | 74 | 31 | 43 | 8 |
| Su et al. | 2016 | China | RCS | NA | NA | ML | 26 | 16 | 10 | 8 |
| Zhang et al. | 2019 | China | RCS | NA | 55.7 ±11.2/ 52.5 ±12.1 |
ML | 64 | 30 | 34 | 9 |
Experimental group/control group.
ML – malignant lesions, BL – benign lesions, NOS – Newcastle-Ottawa Scale, NA – not available.
Meta-analysis of primary outcomes
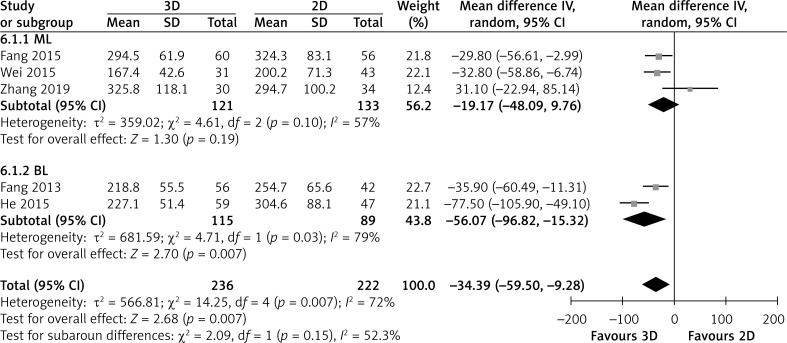
Five studies directly compared operative time. Patients showed significantly shorter operative time in the 3D group than in the 2D group (MD = -34.39; 95% CI: -59.50, -9.28; p = 0.007; I2 = 72%, Figure 2). In subgroup analysis, two BL showed that the 3D group had significantly shorter operative time than the 2D group (MD = -56.07; 95% CI: -96.82, -15.32; p = 0.007; I2 = 79%), but three ML showed that the operative time did not differ between the two groups, Figure 2.
Figure 2.
Forest plot of MD of operating time
Six studies reported detailed data about intraoperative blood loss. Patients in the 3D group had significantly less intraoperative blood loss than the 2D group (MD = -106.55; 95% CI: -183.76, -29.34; p = 0.007; I2 = 71%, Figure 3). In subgroup analysis, two BL showed that the 3D group had significantly less intraoperative blood loss than the 2D group (MD = -107.56; 95% CI: -192.60, -22.51; p = 0.01; I2 = 72%), but four ML showed that the intraoperative blood loss did not differ between the two groups (MD = -107.61; 95% CI: -256.59, 41.38; p = 0.16; I2 = 78%, Figure 3).
Figure 3.
Forest plot of MD of intraoperative blood loss
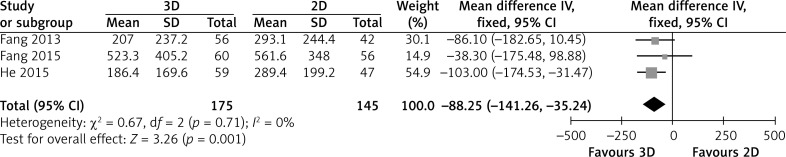
Three studies reported detailed data about blood transfusion volume. Patients in the 3D group had a significantly smaller blood transfusion volume than the 2D group (MD = -88.25; 95% CI: -141.26, -35.24; p = 0.001; I2 = 0%, Figure 4).
Figure 4.
Forest plot of MD of blood transfusion volume
Meta-analysis of secondary outcome
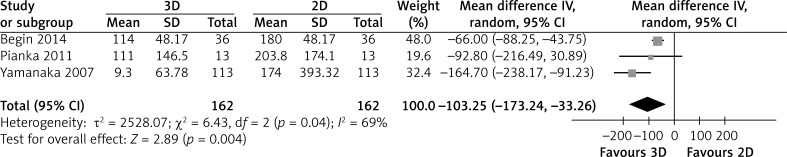
Three studies reported detailed data about the difference between the predicted volume and the actual resected volume. The 3D group had a significantly smaller difference between the predicted volume and the actual resected volume than the 2D group (MD = -103.25; 95% CI: -173.24, -33.26; p = 0.004; I2 = 69%, Figure 5).
Figure 5.
Forest plot of MD of difference between predicted volume and actual resected volume
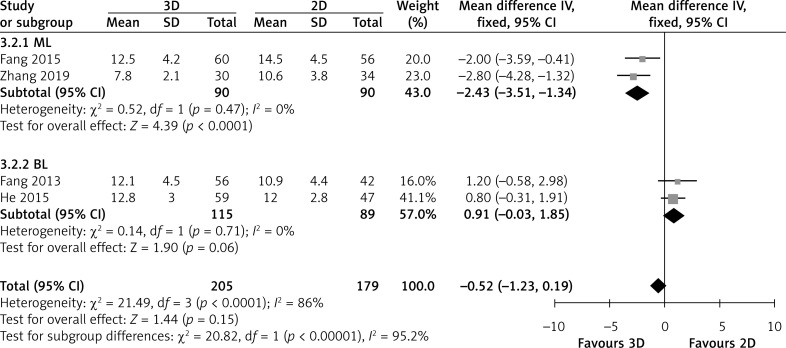
Four studies directly compared hospital stay. Overall analysis showed that the hospital stay did not differ between the two groups (MD = -0.52; 95% CI: -1.23, 0.19; p = 0.15; I2 = 86%, Figure 6). In subgroup analysis, two ML showed that the 3D group had a significantly shorter hospital stay than the 2D group (MD = -2.43; 95% CI: -3.51, -1.34; p < 0.0001; I2 = 0%), but two BL showed that the hospital stay did not differ between the two groups (MD = 0.91; 95% CI: -0.03, 1.85; p = 0.06; I2 = 0%, Figure 6).
Figure 6.
Forest plot of MD of hospital stay
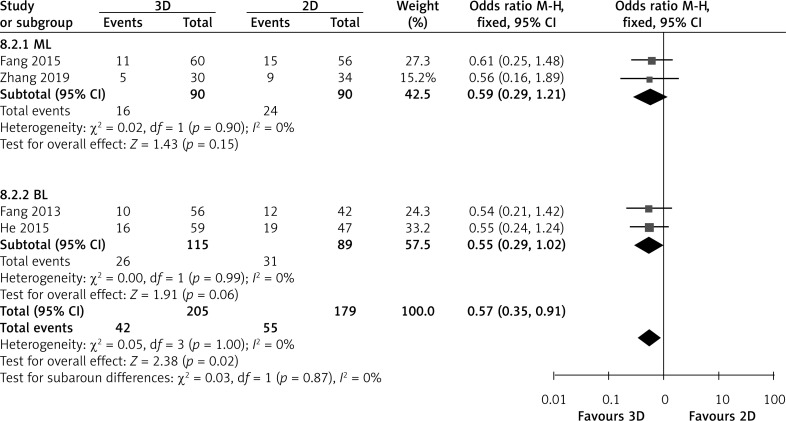
The rate of postoperative complications was obtained from four studies. Overall analysis of postoperative complications showed that 3D video-assisted technology achieved a significantly lower rate of postoperative complications than 2D video-assisted technology (OR = 0.57; 95% CI: 0.35, 0.91; p = 0.02; I2 = 0%, Figure 7). However, in subgroup analysis, two BL and two ML showed that the hospital stay did not differ between the two groups (BL: OR = 0.55; 95% CI: 0.29, 1.02; p = 0.06; I2 = 0% and ML: OR = 0.59; 95% CI = 0.29, 1.21; p = 0.15; I2 = 0%, Figure 7).
Figure 7.
Forest plot of MD of postoperative complications
Sensitivity analysis and publication bias
To evaluate the consistency of overall results, sensitivity analysis was performed by sequential removal of each study. Sensitivity analysis showed that the outcomes of primary overall analysis were not changed. The number of the included studies with each outcome was < 10; thus, the funnel plot was not used to evaluate the publication bias.
Discussion
At present, surgery is one of the best choices to treat LD [26]. However, due to the complex anatomical structure and many anatomical variations of the liver vasculature, it is difficult to obtain visual information and diagrams of the fine liver anatomy [27], especially in laparoscopic special segmental hepatectomy [28]. Therefore, obtaining accurate anatomical images of the liver prior to surgery for the accurate resection of liver lesions has become a hot topic of current research. 3D visualization is a tool used to display, describe, and interpret the 3D anatomical and morphological features of the liver. 3D images are used to describe and interpret the shape and spatial distribution of liver and target [29]. Three-dimensional reconstruction models facilitate the acquisition of intuitionistic and omnidirectional information about the hepatic parenchyma, bile duct system, and tumors in detail, and furthermore, they eliminate concern about the vagueness and instability of CT and MRI images [30]. Computer-aided 3D reconstruction of the liver was applied first for the planning of liver resection by Oldhafer et al. [31] in 1999. Until now, the technology has been used widely in the diagnosis of LD and planning of liver surgery [32–34]. For the latter, the strategy involves surgical assessment of benign/malignant liver tumors, assessment of the safety of living-donor liver transplantation, and evaluation of the anatomical complexity of hepatolithiasis [35]. However, the technology is still in the development stage, and most of the published clinical studies are single-center small-sample studies, and there is a lack of multicenter large-sample RCTs. Hence it is difficult to make accurate conclusions on the clinical efficacy of preoperative three-dimensional reconstruction. Therefore, the present meta-analysis was conducted to systematically review the published literature and evaluate the effectiveness and safety of three-dimensional versus two-dimensional video-assisted hepatectomy for LD.
This is probably the first systematic review and meta-analysis comparing 3D versus 2D for LD. In this meta-analysis, only nine studies involving 808 patients were included; to date, this possibly represents the best information available to compare 3D versus 2D for LD. According to the NOS used for assessing the quality of the studies, articles included in this meta-analysis were graded with a score of 9 [19, 22, 25], 8 [21, 23, 24], or 7 [17, 18, 20] representing high quality concerning selection of patients, comparability, and exposure measurements. Our analysis reflects the latest surgical results. Overall, significantly shorter operating time, less blood loss, and smaller blood transfusion volume were found in the 3D display group. Meanwhile, the difference between the predicted volume of 3D was smaller than 2D groups. Compared with the 2D system, the 3D video assisted system can provide three-dimensional vision that allows simpler presentation of anatomical structures and a better sense of depth to facilitate precise operation and shorten the operation time [21, 23]. At the same time, the high-definition 3D vision also allows surgeons to quickly improve surgical skills and shorten the learning curve in simulated settings. In terms of intraoperative blood loss, some studies have shown that intraoperative bleeding in hepatectomy is significantly correlated with the prognosis of patients, and for liver malignancies, postoperative recurrence is closely correlated with intraoperative bleeding. Therefore, reducing the amount of blood loss during the operation can promote the good prognosis of patients [36]. Therefore, reducing the bleeding of patients with a good prognosis in the process of the operation has a promoting effect, and our results show that the preoperative 3D reconstruction is helpful to reduce the intraoperative blood loss. Combined with the research and analysis, the main reason lies in the preoperative 3D reconstruction technology, which can accurately measure the blood supply of liver lesions and local anatomy, be aided by the virtual surgery resection, design the best surgical approach, help the operator to perform the operation in the best way, and avoid intraoperative unnecessary damage to normal liver tissue and the vascular system, so that the intraoperative blood loss in the 3D reconstruction group was significantly lower than that in the traditional two-dimensional group. There was also a corresponding decrease in blood transfusions [37].
Moreover, the significant advantage of 3D reconstruction technology is that it can accurately estimate the remaining liver volume after hepatectomy before surgery, determine the necessary functional liver volume and the minimum range that should be retained after surgery, and ensure good liver reserve function after surgery. Radtke et al. [38] compared the preoperative planning and surgical strategy of 3D reconstruction with that of conventional CT. It is believed that 3D reconstruction changed the preoperative program determined by traditional CT by 33%. Our analysis also suggested that the difference between the predicted volume and the actual resected volume of the preoperative 3D reconstruction technique was smaller than that of the two-dimensional group, which was consistent with the previous research results. Cai et al. [39] pointed out that the 3D reconstruction technology can accurately estimate the amount of intraoperative resection and the volume of remaining liver after operation through preoperative surgical rehearsal and 3D tumor model display, which can minimize the risk of liver failure and effectively improve the safety of liver surgery. Our analysis also showed a lower incidence of postoperative complications in the 3D imaging group. The use of 3D reconstruction can reduce the damage to the vascular and bile duct system, avoid the occurrence of necrosis and infection caused by the large suture, and facilitate the recovery of liver function after hepatectomy, to minimize the incidence of liver function injury and liver failure after the surgery, and significantly improve the safety of hepatectomy. However, in the comparison of hospital stay, the analysis found that it was not statistically significant.
Our study found that the operating time, intraoperative blood loss, and hospital stay indicators have higher heterogeneity. We tried to find the sources of heterogeneity by subgroup analysis and sensitivity analysis. However, the sources of heterogeneity were not found, due to there being many sources of heterogeneity in this analysis. And heterogeneity could not be effectively reduced by excluding a single influencing factor, due to the uneven quality of the included studies, the surgical methods adopted by various researchers, and the differences in inclusion criteria of the included cases, and all of them may affect the operating time, amount of intraoperative blood loss, and the length of hospital stay of the patients, and ultimately lead to the generation of heterogeneity. Moreover, this systematic review had some limitations. First, the analytical capacity may have been affected by the limited number of trials and the small sample size. Second, significant heterogeneity was found in some outcome indicators at the time of combination, which may affect the reliability of the results owing to the lack of other subgroup analysis, such as surgical approach and lesion size. Third, many confounding factors may have influenced the results.
Conclusions
This systematic review showed that 3D video-assisted hepatectomy could effectively reduced operative time, intraoperative bleeding, blood transfusion rates, and postoperative complications. More high-quality RCTs are required to verify the reliability and validity of our conclusion.
Acknowledgments
Shumao Zhang and Zhanwen Huang contributed equally to this work.
This work was supported by grants from the National Natural Science Foundation of China (no. 81671721) and the nuclear medicine and molecular imaging key laboratory of Sichuan province open project (no. HYX18028).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–51. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 2.Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 3.Maluccio M, Covey A. Recent progress in understanding,diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–9. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 4.Hanna GB, Shimi SM, Cuschieri A. Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet. 1998;351:248–51. doi: 10.1016/S0140-6736(97)08005-7. [DOI] [PubMed] [Google Scholar]
- 5.Frauenfelder T, Tutic M, Weder W, et al. Volumetry: an alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J. 2011;38:162–8. doi: 10.1183/09031936.00146110. [DOI] [PubMed] [Google Scholar]
- 6.Endo I, Shimada H, Sugita M, et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery. 2007;142:666–75. doi: 10.1016/j.surg.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Saito S, Yamanaka J, Miura K, et al. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005;41:1297–304. doi: 10.1002/hep.20684. [DOI] [PubMed] [Google Scholar]
- 8.Wigmore SJ, Redhead DN, Yan XJ, et al. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221–6. doi: 10.1097/00000658-200102000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaf J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions 5.1.0 [updated March 2011] www.cochranehandbook.org.
- 11.Hou XW, Shi JP, Chen X. How to estimate the mean and standard deviation based on the median, range and sample size when conducting meta-analysis. Chin J Evid Based Med. 2015;15:484–7. [Google Scholar]
- 12.Hozo SP, Djubegovic B, Hozo I. Estimating the mean and variance from the median, range and the size of a simple. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:e603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Seagroatt V, Stratton I. Bias in meta-analysis detected by a simple, graphical test. Test had 10% false positive rate. BMJ. 1998;316:470–1. [PMC free article] [PubMed] [Google Scholar]
- 17.Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249–55. doi: 10.1007/s00268-007-9020-8. [DOI] [PubMed] [Google Scholar]
- 18.Pianka F, Baumhauer M, Stein D, et al. Liver tissue sparing resection using a novel planning tool. Langenbecks Arch Surg. 2011;396:201–8. doi: 10.1007/s00423-010-0734-y. [DOI] [PubMed] [Google Scholar]
- 19.Fang CH, Liu J, Fan YF, et al. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg. 2013;217:280–8. doi: 10.1016/j.jamcollsurg.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Begin A, Martel G, Lapointe R, et al. Accuracy of preoperative automatic measurement of the liver volume by CT-scan combined to a 3D virtual surgical planning software (3DVSP) Surg Endosc. 2014;28:3408–12. doi: 10.1007/s00464-014-3611-x. [DOI] [PubMed] [Google Scholar]
- 21.Fang CH, Tao HS, Yang J, et al. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J Am Coll Surg. 2015;220:28–37. doi: 10.1016/j.jamcollsurg.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 22.He YB, Bai L, Aji T, et al. Application of 3D reconstruction for surgical treatment of hepatic alveolar echinococcosis. World J Gastroenterol. 2015;21:10200–7. doi: 10.3748/wjg.v21.i35.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei XB, Xu J, Li N, et al. The role of three-dimensional imaging in optimizing diagnosis, classification and surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. HPB. 2016;18:287–95. doi: 10.1016/j.hpb.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su L, Dong Q, Zhang H, et al. Clinical application of a threedimensional imaging technique in infants and young children with complex liver tumors. Pediatr Surg Int. 2016;32:387–95. doi: 10.1007/s00383-016-3864-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, Luo H, Zhu W, et al. Real-time navigation for laparoscopic hepatectomy using image fusion of preoperative 3D surgical plan and intraoperative indocyanine green fuorescence imaging. Surg Endosc. 2020;34:3449–59. doi: 10.1007/s00464-019-07121-1. [DOI] [PubMed] [Google Scholar]
- 26.Mcglynn KA, Petrick JL, London WT, et al. Global epidemiology: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223–38. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama K, Oshiro Y, Miyamoto R, et al. The effect of three-dimensional preoperative simulation on liver surgery. World J Surg. 2017;41:1840–7. doi: 10.1007/s00268-017-3933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubinkiewicz M, Mizera M, Małczak P, et al. Laparoscopic vs. open liver resections of posterolateral liver segments-a systematic review and meta-analysis. Videosurgery Miniinv. 2020;15:395–402. doi: 10.5114/wiitm.2020.94268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang H, Radtke A, Liu C, et al. Extended left hepatectomymodified operation planning based on three-dimensional visualization of liver anatomy. Langenbecks Arch Surg. 2004;389:306e310. doi: 10.1007/s00423-003-0441-z. [DOI] [PubMed] [Google Scholar]
- 30.Yeo CT, Macdonald A, Ungi T, et al. Utility of 3D reconstruction of 2D liver computed tomography/magnetic resonance images as a surgical planning tool for residents in liver resection surgery. J Surg Education. 2017;75:792–7. doi: 10.1016/j.jsurg.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Oldhafer KJ, Högemann D, Stamm G, et al. 3-dimensional (3-D) visualization of the liver for planning extensive liver resections. Chirurg. 1999;70:233–8. doi: 10.1007/s001040050636. [DOI] [PubMed] [Google Scholar]
- 32.Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int. 2014;13:361–70. doi: 10.1016/s1499-3872(14)60267-8. [DOI] [PubMed] [Google Scholar]
- 33.Clavien PA, Emond J, Vauthey JN, et al. Protection of the liver during hepatic surgery. J Gastrointest Surg. 2004;8:313–27. doi: 10.1016/j.gassur.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Radtke A, Nadalin S, Sotiropoulos GC, et al. Computer-assisted operative planning in adult living donor liver transplantation: a new way to resolve the dilemma of the middle hepatic vein. World J Surg. 2007;31:175–85. doi: 10.1007/s00268-005-0718-1. [DOI] [PubMed] [Google Scholar]
- 35.Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19:1304–10. doi: 10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 36.Masato N, Junichi K, Toshiyuki A, et al. “Anatomic” right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243:28–32. doi: 10.1097/01.sla.0000193604.72436.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: from diagnosis to treatment. World J Hepatol. 2015;7:74–85. doi: 10.4254/wjh.v7.i8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radtke A, Sotiropoulos GC, Molmenti EP, et al. Computer-assisted surgery planning for complex liver resections: when is it helpful? A single-center experience over an 8-year period. Ann Surg. 2010;252:876–83. doi: 10.1097/SLA.0b013e3181fdd012. [DOI] [PubMed] [Google Scholar]
- 39.Cai W, Fan Y, Hu H, et al. Postoperative liver volume was accurately predicted by a medical image three dimensional visualization system in hepatectomy for liver cancer. Surg Oncol. 2017;26:188–94. doi: 10.1016/j.suronc.2017.03.006. [DOI] [PubMed] [Google Scholar]