Abstract
Purpose
The purpose of this study was to describe the face mask (FM)-related ocular surface changes using clinical tests, in vivo confocal microscopy (IVCM) and impression cytology (IC), and to investigate the Dry Eye-related Quality of life Score (DEQS).
Methods
Sixty-six patients with dry eye disease (DED) and 62 healthy subjects (group 2) using FM were enrolled. Groups were divided into: groups 1A and 2A: < 3 hours of FM wear; groups 1B and 2B: 3 to 6 hours; and groups 1C and 2C: > 6 hours. Patients underwent DEQS questionnaire, break-up time (BUT), Schirmer test I (STI), fluorescein and lissamine staining (FS and LS), IVCM to determine corneal dendritic cell density (DCD) and goblet cell density (GCD), and IC to measure HLA-DR, at baseline and after 3 months.
Results
FM use duration before enrollment was 27 ± 2.3 and 30 ± 4.1 (days ± SD) for groups 1 and 2 (P > 0.05). After 3 months, DEQS worsened in groups 1B and 1C, STI in groups 1A to 1C, FS and LS in group 1C (P < 0.05); in controls, BUT and FS worsened only in group 2C (P < 0.05). DCD significantly increased in groups 1A to 1C and HLA-DR in groups 1B and 1C (P < 0.05), whereas GCD did not significantly change. DCD and HLA-DR increased only in group 2C (P < 0.05). DEQS significantly correlated with DCD (P = 0.05, r = 0.698; P < 0.001, r = 0.832) and HLA-DR (P = 0.043, r = −0.687; P < 0.001, r = 0.861) at baseline and 3 months.
Conclusions
Use of FM increases ocular surface inflammation and negatively impacts the quality of life in patients with DED.
Translational Relevance
The study of the prolonged use of FM effects may be relevant to managing DED.
Keywords: face mask, coronavirus disease 2019 (COVID-19) pandemic, ocular surface, dry eye, in vivo confocal microscopy, impression cytology, quality of life
Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic changed many aspects of the human life, most of them because of the measures taken to limit the virus (severe acute respiratory syndrome-coronavirus 2 [SARS-CoV-2]) spread. In fact, because there is no vaccine or validated therapy yet, unprecedented public health measures have been adopted. Besides physical distancing, hand hygiene, and contact tracing, the use of personal protective equipment (PPE), including face masks (FMs), represents critical strategies to limit the diffusion of virus.1–3 SARS-CoV-2 is in fact spread primarily via respiratory droplets during close face-to-face contact, or in particular conditions by aerosol.3 Although the real utility of the FM wear is still a matter of debate, the recommendations of regulatory agencies led to a rapid increase in their utilization, especially in crowded places or confined environments where physical distancing cannot be adequately met.4,5
Nevertheless, the prolonged use of FMs may lead to several side effects, such as increasing respiratory resistance, pain and pressure on the nose and ears, temporomandibular joint changes, itch, and ocular discomfort.6–9 Emerging evidences have in fact indicated an increased prevalence of ocular symptoms during the COVID-19 pandemic, with the prolonged use of FMs probably representing one of the contributing factors.10,11
To analyze the prevalence and risk factors leading to ocular discomfort during the COVID-19 pandemic, Giannaccare et al. administered an ad hoc survey to university students.10
The authors hypothesized that the increased use of visual display terminals and the prolonged FM wearing were the two main causes involved in the ocular discomfort development.10 When focusing on FMs, it has been suggested that the air blowing upward during breathing out, or the limited excursion of the lower eyelid promote an accelerated evaporation of tears and, thus, the onset or worsening of dry eye disease (DED)-related symptoms.10,11 However, to date, studies that objectively supported at cellular or molecular levels the hypothesized detrimental effects of the FM use on the ocular surface, or their impact on patient quality of life (QOL), are not available.
The aims of the present study were to prospectively evaluate whether the prolonged use of a FM modifies the clinical indicators of the ocular surface, the conjunctival goblet cell density (CGCD), and corneal dendritic cell density (DCD) at in vivo confocal microscopy (IVCM), the HLA-DR expression at impression cytology (IC), and whether these modifications correlate with the Dry Eye-related Quality of life Score (DEQS), after 3 months of continuous use of FMs.
Materials and Methods
Patient's Enrollment
This was a prospective study conducted at the Ophthalmic Clinic of the G. d'Annunzio University of Chieti-Pescara, Italy, during the COVID-19 pandemic. The protocol used was approved by our Institutional Review Board (Department of Medicine and Ageing Science, G. d'Annunzio University of Chieti-Pescara, Chieti, Italy), and the study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all the participants before enrollment after explanation of the nature and possible consequences of the study.
Sixty-six eyes of 66 patients with DED (group 1) and 62 eyes of 62 healthy subjects (group 2) with history of a continuative use of FM have been consecutively enrolled from April to June 2020. Only subjects using a triple layer surgical FM were enrolled.
Patients with DED were enrolled during routine ambulatory visits, whereas healthy subjects were enrolled among healthcare professionals of the clinic or subjects referring to general ambulatory for a routine visit. Both patients with DED and controls underwent a complete ophthalmological examination, including best-corrected visual acuity and intra-ocular pressure determination, slit lamp assessment of the ocular anterior segment, and fundus evaluation.
Inclusion criteria for DED corresponded to those required by Dry Eye Workshop (DEWS) guidelines,12 whereas for the exclusion criteria we adopted those proposed by the American-European Consensus Group.13
Healthy subjects had to show a completely normal ophthalmological assessment. Exclusion criteria for healthy subjects were: history of systemic or intra-ocular inflammatory diseases, systemic or topical therapies in the last 6 months that could have modified ocular surface, and smart working activities requiring continuous use of video-terminals (VDTs). At the moment of enrollment, patients with DED were treated with preservative-free solution of sodium hyaluronate (0.15%) for up to 6 times a day, and did not receive topical steroids during the last 2 months.
According to the number of daily hours of FM use before enrollment, the 2 groups were further divided into 3 subgroups: groups 1A and 2A: less than 3 hours; groups 1B and 2B: 3 to 6 hours; and groups 1C and 2C: more than 6 hours. After enrollment, patients were asked to maintain the same number of hours of FM wear during the day; to motivate patients to respect this strategy, we recommended them to report in a daily diary the time slots of FM wear.
In addition, all the subjects were asked to provide information regarding their exposure to other additional risk factors for dry eye, especially the use of computers and the exposure to air conditioning.
QOL Questionnaire and Clinical Examinations
All subjects were evaluated at baseline and after 90 days of continuous use of FMs, and underwent the DEQS questionnaire to assess QOL, ocular surface clinical tests, and finally IVCM and IC of the conjunctiva.
DEQS is a survey composed of 15 questions that scores the frequency and the degree of dry eye-related symptoms using an overall summary scale.14 The final score, which ranges from 0 to 100 (with higher values indicating greater discomfort and disability), is considered a quantitative index of dry eye symptoms.
The DEQS questionnaire was completed before clinical tests, which comprised tear film break-up time (T-BUT), Schirmer test I (STI) with topical preservative-free oxybuprocaine, corneal fluorescein staining (FS), and lissamine green staining (LS). According to the DEWS guidelines, BUT, FS, LS, and STI (30 minutes after T-BUT) were consecutively performed.12 BUT was recorded as the average of three consecutive measurements. FS was evaluated with 1% sodium fluorescein and scored 0 to 3 according to the Oxford grading scale, whereas LS was graded according to the classification proposed by Sullivan and coworkers.15,16
The Oxford grading is a method to quantify the epithelial surface damage in patients with dry eye disease. This system is based on a chart formed by a series of panels, A to E, in each chart, staining is represented by punctate dots. The number of dots increases by 1 log unit between panel A and B and by 0.5 log units among other panels from B to E. To grade staining from 0 to 5, the operator compares the panels with the appearance of staining on the ocular surface of the patient.16
In Vivo Confocal Microscopy of the Cornea and Conjunctiva
Two hours after completing clinical tests, patients underwent IVCM (HRT III Rostock Cornea Module (Heidelberg Engineering, Heidelberg, Germany) of the cornea and bulbar conjunctiva to evaluate dendritic cells (DCs) and goblet cells (GCs).
The image acquisition procedure for the corneal and conjunctival analysis were performed as already described.17,18
For the corneal analysis, at least 30 images of the central cornea were acquired at the level of the subepithelial layer. In each frame, the presence and the density of DCs were evaluated. DC features had to be consistent with literature, presenting as bright cells with a branching dendritic morphology, located at the level of basal membrane of the corneal epithelium, at a depth of 40 to 50 µm.17 DCD was calculated on 15 randomly selected high-quality images, using the analysis software provided by the instrument, by averaging numbers of cells counted manually within a region of interest of standardized dimensions (250 × 250 µm). DCD was given as cells/mm2.
For the conjunctival analysis, 30 images were acquired for each eye, and 14 high quality images were selected from the superior and temporal sectors (7 images per sector) to calculate the GCD (using the Cell Count Software of the device, in manual mode). The morphological features of GCs had to be consistent with those reported in literature, as oval-shaped and hyper-reflective cells 2/3 times larger than the epithelial cells, dispersed or crowded in groups, recognizable at a depth of 15 to 30 µm.18
Impression Cytology of the Conjunctiva
IC was performed to evaluate the HLA-DR expression at the temporal bulbar conjunctiva, and was conducted 24 hours after the IVCM assessment to avoid misinterpretation due to the technical execution of the confocal microscopy. Samples were collected using Millicell-CM 0.4 µm (Millipore, Bedford, MA); the specimen acquiring procedure was performed as previously described.18,19 Zeiss Confocal LSM 510 (Carl Zeiss MicroImaging GmbH, Vertrieb, Germany) was used to visualize cells. Five different fields for each sample were evaluated. Positive (red nucleus and green cytoplasm) and negative (red nucleus) cells were counted. Minor irregularities that may have been present after cutting the membranes were normalized by reporting the percentage of positive cells for HLA-DR.
All evaluations of impression cytology samples were performed by two independent observers (authors L.B. and R.D.A.). IVCM (author M.L.) and IC (authors L.B. and R.D.A.) operators were masked for grouping and subject history of patients. Both eyes for patients were evaluated in the study, but one eye per subject was randomly chosen (using a computer-generated random number list) for the statistical analysis.
Outcomes of the Study
The main outcomes of the study were baseline and 3 months DCD and GCD at IVCM, HLA-DR positivity at IC, and DEQS score. Correlations among clinical, confocal, and IC parameters with the DEQS score were also investigated.
Statistical Analysis
The sample size calculation was established calculating a difference among groups of at least 10%, a type I error rate (α) of 5%, and a power of 80%. Analysis was performed using SPSS Advanced Statistical version 25.0 Software (Chicago, IL, 2017). Shapiro-Wilk's test was performed to evaluate the normality of distribution for each variable. Student's t-test and χ2 test were used to evaluate age and gender differences between healthy subjects and patients with DED. The Mann-Whitney U test was used to determine differences among groups, and ANOVA repeated measures was used to calculate the differences between baseline and 3 months’ follow-up values. Spearman's nonparametric correlation analysis was used to investigate relations among clinical, IVCM, and IC parameters. Any P values less than 0.05 were considered statistically significant.
Results
Demographic Data
There were no significant differences among groups on the duration of FM use before enrollment: 27 ± 2.3 and 30 ± 4.1 (days ± SD) for groups 1 and 2, respectively (P > 0.05). Similarly, there were no significant differences for gender and age: male/female was 40/26 and 35/27, and mean age 49.2 ± 9.1 and 45.5 ± 8.3 (years ± SD), for groups 1 and 2, respectively (P > 0.05).
All enrolled subjects concluded the study and, according to their self-reported diary, did not modify the number of hours of daily use of FM during the 3 months of follow-up.
The mean time of computer working did not significantly differ between groups, with values of 1.2 ± 0.5 and 1.4 ± 0.6 hours per day in groups 1 and 2, respectively (P < 0.05).
DEQS Questionnaire and Tear Film Function Tests
Overall, DEQS score, TBUT, STI, FS, and LS were significantly different between groups 1 and 2, both at baseline and at the 3 months’ follow-up (P < 0.01), with all values 4 times worse (Table 1). Significant differences were also found between DED and healthy subgroups. In DED, at baseline, group 1C presented significant worse values of DEQS score compared with groups 1A and 1B, and worse values of T-BUT and FS compared to group 1A (P < 0.05); at 3 months, DEQS values were significantly worse in group 1C compared to group 1A (P < 0.001) and group 1B (P < 0.05), whereas the other parameters were significantly different only between group 1C and group 1A (P < 0.001). When comparing baseline and 3 months’ data, DEQS significantly worsened in groups 1B and 1C, STI in all the 3 subgroups, whereas FS and LS worsened only in group 1C (P < 0.05).
Table 1.
Baseline and Follow-Up QOL and Tear Film Data
| Baseline | 3 months Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DEQS | T-BUT | FS | LS | STI | DEQS | T-BUT | FS | LS | STI | |
| Group 1 | 48.0 ± 8.9 a | 4.1 ± 1.5 a | 2.4 ± 1.6 a | 2.6 ± 1.7 a | 5.3 ± 1.2 a | 50.7 ± 5.2 a | 3.8 ± 1.1 a | 2.8 ± 1.9 a | 2.9 ± 1.5 a | 4.3 ± 0.8 a |
| Group 1A | 45.7 ± 7.9b,d | 4.6 ± 1.6b,c | 2.2 ± 1.2b,c | 2.5 ± 1.6b | 5.3 ± 1.3c | 46.3 ± 4.8b,d,f | 4.3 ± 1.7b,d | 2.6 ± 3.2b,d | 2.7 ± 1.9b,d | 4.8 ± 1.5b,c,d |
| Group 1B | 48.2 ± 8.1b,c | 4.2 ± 1.9b | 2.5 ± 1.5b | 2.6 ± 1.7b | 5.5 ± 1.1b | 51.8 ± 5.9b,c,d,f | 4.1 ± 1.9b,c | 2.7 ± 2.1b,c | 2.8 ± 1.4b | 4.4 ± 2.1b,d |
| Group 1C | 50.1 ± 6.7b | 3.6 ± 1.5b | 2.6 ± 1.7b | 2.7 ± 1.3b | 5.1 ± 0.9b | 54.1 ± 6.4b,e,f | 3.1 ± 2.2b,e | 3.1 ± 2.2b,e | 3.4 ± 1.7b,e | 3.7 ± 1.2b,e |
| Group 2 | 11.7 ± 3.5 | 13.2 ± 0.8 | 0.7 ± 0.3 | 1.0 ± 0.5 | 18.4 ± 3.6 | 12.7 ± 1.5 | 13.0 ± 1.4 | 0.9 ± 0.4 | 1.1 ± 0.5 | 17.9 ± 3.3 |
| Group 2A | 11.3 ± 4.8f | 13.5 ± 2.2 | 0.6 ± 0.2 | 0.9 ± 0.7 | 19.2 ± 3.5 | 11.9 ± 2.1g,h | 13.7 ± 2.4g | 0.7 ± 1.1g | 0.9 ± 0.9g,h | 18.4 ± 4.8 |
| Group 2B | 11.1 ± 3.2 | 13.2 ± 1.9 | 0.7 ± 0.5 | 0.8 ± 1.1 | 18.9 ± 4.9 | 12.3 ± 2.8g | 13.1 ± 1.8g | 0.9 ± 0.7 | 1.1 ± 0.5g | 18.2 ± 5.2 |
| Group 2C | 12.6 ± 3.7 | 13.1 ± 2.3 | 0.9 ± 1.2 | 1.2 ± 0.6 | 17.1 ± 3.2 | 13.9 ± 3.2 | 12.2 ± 2.1e | 1.2 ± 0.9e | 1.4 ± 1.1e | 17.2 ± 4.3 |
DEQS, Dry Eye-Related Quality-of-Life Score Summary Score; T-BUT, tear film break-up time in seconds; FS, corneal staining with fluorescein stain, points; LS, conjunctival staining with green lissamine, points; STI, Schirmer I Test; mm.
P < 0.01 vs. group 2.
P < 0.01 vs. groups 2A, 2B, and 2C.
P < 0.05 vs. group 1C.
P < 0.001 vs. group 1C.
P < 0.05 vs. baseline.
P < 0.001 vs. groups 2A, 2B, and 2C.
P < 0.05 vs. group 2C.
P < 0.01 vs. group 2C.
In healthy controls, baseline DEQS score was significantly worse in group 2C compared to group 2A and group 2B; at 3 months follow-up, DEQS score, T-BUT, FS, and LS (Fig. 1) were significantly worse in group 2C (P < 0.05). When comparing baseline and 3 months’ data, group 2C showed a significant worsening of T-BUT, FS, and LS values (P < 0.05). DEQS scores did not change in any of the three subgroups.
Figure 1.
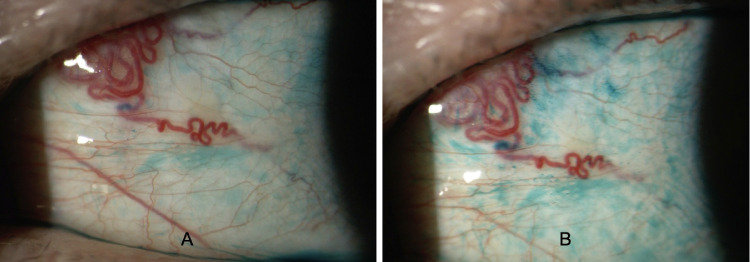
Representative images of lissamine green staining of bulbar conjunctiva in a patient affected by dry eye at baseline (A) and after 3 months of daily use of face mask > 6 hours (B). At the end of follow-up, an increased staining of conjunctival epithelium is evident, indicating a progression of ocular surface damage.
In Vivo Confocal Microscopy and Impression Cytology
IVCM and IC data are summarized in Table 2. Overall, DCD and HLA-DR positivity were significantly higher, whereas GCD lower in DED compared with healthy controls at both baseline and at 3 months’ follow-up. At the third month follow-up, DCD and HLA-DR positivity significantly increased only in group 1 (P < 0.01).
Table 2.
In Vivo Confocal Microscopy and Impression Cytology Data
| Baseline | 3 Months Follow-Up | |||||
|---|---|---|---|---|---|---|
| DCD, Cells/mm2 | GCD, Cells/mm2 | HLA-DR +, % | DCD, Cells/mm2 | GCD, Cells/mm2 | HLA-DR +, % | |
| Group 1 | 58.70 ± 19.91a | 120.37 ± 12.15 a | 40.26 ± 20.87 a | 62.01 ± 17.65 a,b | 118.09 ± 16.13 a | 43.23 ± 13.05 a , b |
| Group 1A | 56.32 ± 16.54c,e | 120.34 ± 13.11c | 39.51 ± 11.43c | 59.21 ± 13.23c,e,g | 119.26 ± 12.37c | 39.87 ± 12.34c,e |
| Group 1B | 58.65 ± 14.37c,d | 119.18 ± 10.31c | 38.65 ± 14.51c | 61.72 ± 12.11c,d,g | 118.76 ± 13.46c | 42.21 ± 13.67c,e,g |
| Group 1C | 61.0 ± 12.75c | 121.61 ± 12.24c | 42.62 ± 12.75c | 65.12 ± 14.54b.c | 116.25 ± 11.69c | 47.63 ± 11.52b,c |
| Group 2 | 19.70 ± 6.97 | 239.47 ± 19.41 | 5.19 ± 2.76 | 22.19 ± 8.54 | 237.70 ± 17.93 | 6.06 ± 2.19 |
| Group 2A | 18.23 ± 9.07 | 241.71 ± 23.58 | 4.73 ± 1.47f | 20.13 ± 8.76b,c | 239.27 ± 20.39 | 5.47 ± 2.13f |
| Group 2B | 19.76 ± 7.21 | 238.22 ± 21.33 | 4.93 ± 2.36 | 21.34 ± 7.54 | 237.13 ± 19.81 | 5.28 ± 1.74 |
| Group 2C | 21.12 ± 6.05 | 238.49 ± 19.71 | 5.91 ± 1.95 | 25.12 ± 8.42h | 236.72 ± 22.35 | 7.43 ± 1.98h |
DCD, dendritic cell density; cells/mm2. GCD, goblet cell density; cells/mm2.
P < 0.01 vs. group 2.
P < 0.01 vs. baseline.
P < 0.01 vs. groups 2A, 2B, and 2C.
P < 0.05 vs. group 1C.
P < 0.001 vs. group 1C.
P < 0.05 vs. group 2C.
P < 0.05 vs. baseline.
P < 0.01 vs. group 2C.
In DED subgroups, baseline DCD, GCD, and HLA-DR were significantly worse compared to healthy controls (P < 0.01), with higher DCD values in group 1C compared to group 1B (P < 0.01) and 1A (P < 0.001). At the third month follow-up, the use of FMs significantly increased DCD in all DED subgroups (P < 0.05), and HLA-DR positivity in groups 1B and 1C (P < 0.05), whereas GCD did not significantly change.
In healthy controls subgroups, baseline HLA-DR in group 2C was significantly higher compared to group 2A (P < 0.05). At the third month follow-up, DCD and HLA-DR positivity significantly increased only in group 2C (P < 0.05).
Figure 2 and Figure 3 show baseline and 3 months DCs and GCS at IVCM, whereas Figure 4 shows the HLA-DR positivity at IC, in the DED and healthy controls subgroups.
Figure 2.
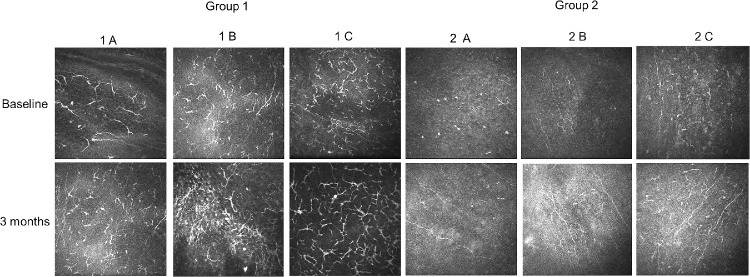
Representative images of dendritic cells (DCs) visualized at the level of the subepithelial basal lamina by means of in vivo confocal microscopy. After 3 months of follow-up, DCs density showed a significant increasing increased in all patients with dry eye (groups 1A, 1B, and 1C) and in healthy subjects wearing a face mask for more than 6 hours a day.
Figure 3.
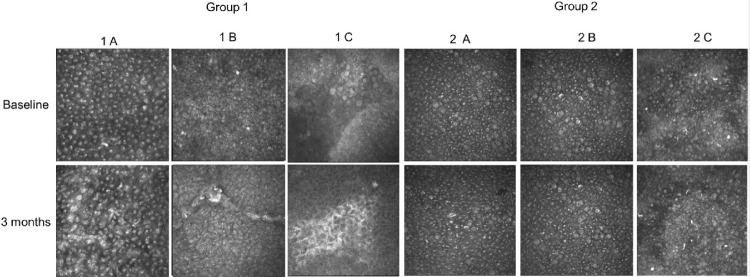
Representative images of goblet cells (GCs) visualized at the level of bulbar conjunctival epithelium by means of in vivo confocal microscopy. At baseline GCs density was significantly lower in patients with dry eye compared with healthy controls. No modification of GCs density was observed after 3 months of follow-up in all subgroups analyzed.
Figure 4.
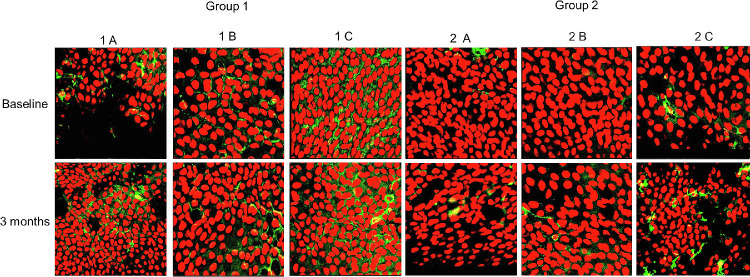
Expression of HLA-DR in bulbar conjunctival epithelium in patients with dry eye and healthy subjects. HLA-DR positivity at baseline was significantly higher in group 1 and after 3 months showed a significant increase in all dry eye subgroups (groups 1A, 1B, and 1C) and in healthy controls wearing a face mask for more than 6 hours a day.
Correlations
At baseline, DCD correlated positively with DEQS score and FS (P = 0.05, r =0 .698; P = 0.034, r = 0.653) and negatively with T-BUT and STI (P < 0.001 r = −0.651; P =0 .042 r = −0.540); no significant correlations were found between DCD and LS. At 3 months, the correlation between DCD and DEQS was stronger compared to that of baseline (P < 0.001, r = 0.832). At baseline, HLA-DR positivity negatively correlated with STI (P =0 .043, r = −0.687) and positively with DEQS score (P = 0.05, r = 0.754). At 3 months, as for DCD, the correlation between HLA-DR positivity and DEQS score was stronger than at baseline (P < 0.001, r = 0.861).
Discussion
Even though the role of FMs in limiting the SARS-CoV-2 transmission is still a matter of debate, different evidence suggests that their use, particularly in confined environments, may reduce the virus from spreading.
Preliminary studies, however, reported that the use of FMs may harm the ocular surface.10,11
In a comprehensive interpretation of the results of our study, we found that the continuative use of FMs for 3 months worsened clinical indicators of ocular surface disease (OSD), such as T-BUT and ocular surface staining, and increased cellular and molecular markers of inflammation, such as DCD and HLA-DR, especially in subjects using FMs for more than 6 hours per day. These changes, seen in healthy subjects, were markedly greater in patients with dry eye, where clinical, cellular and molecular markers of OSD were altered even in case of less than 6 hours per day of FM wear.
Thus, the continuative use of FMs may represent a potential risk factor for dry eye in normal cases, whereas is a significant risk factor for ocular surface worsening in patients with DED.
These evidences represent the first described during the COVID-19 pandemic and, thus, cannot be compared with other studies. Nevertheless, when considering the preliminary findings reported by Giannaccare et al., our results seem to confirm the suspect of a potential causative role of FMs in symptoms reported by students during the pandemic.10 In fact, the Ocular Surface Disease Index (OSDI) score was abnormal in more than half of the cases.
In our study, clinical and imaging data were in line between them for the most part. In fact, the FS and LS increase, which may be considered as potential clinical markers of inflammation, were consistent with the DCD and HLA-DR positivity increase, which represent inflammatory markers at IVCM and IC, respectively.17,19 On the other hand, the unmodified values of GCD after 3 months of FM use was not in line with the T-BUT reduction, which indicates a reduction of the tear film stability as a consequence of mucin loss.
This could depend on two reasons: the first one is that inflammatory modifications of the ocular surface may appear prior to that of GCs in the presence of an OSD form, as previously reported in patients with glaucoma19; the second is that IVCM, although currently represents the most diffuse to way to study GCs, could not timely recognize the GCD reduction in the presence of a trigger stimulus. Therefore, further studies using IC, which represents the gold standard method to analyze GCs within the conjunctiva, are required.
A crucial point that emerged from our study is the impact of the FM-related changes on the patients’ QOL. As for other parameters, we cannot state whether baseline intergroups differences of DEQS were related to the initial use of FMs. When analyzing the 3 months’ data, the QOL score worsened in patients with DED wearing FMs for at least 3 hours per day, whereas it did not change in healthy subjects. Moreover, DEQS showed strong correlations with DCD and HLA-DR positivity, indicating that the QOL worsening is probably related to the increased ocular surface inflammation.
To our knowledge, there were no previous studies that investigated the relationship between the use of FMs and modifications of the QOL. However, in a recent study that evaluated the effects of surgical and FFP2/N95 FMs on the cardiopulmonary exercise capacity in healthy subjects, the authors concluded that medical masks significantly impair the QOL of wearers.20
These aspects should be strongly kept in consideration, because patients with DED already have an impaired QOL as a result of the underlying disease. Therefore, medical and behavioral strategies aimed at counterbalance of the detrimental effects of FMs should considered when symptoms and signs of dry eye begin to worse, or prior to their worsening in the presence of a severe form of OSD.
A limited aspect of our study is that we did not consider the impact of other concomitant risk factors for dry eye on the described ocular surface changes, especially the use of video terminals. As known, the lockdown period forced a significant proportion of people to convert their job in smart working, with an increase of the time spent in front of computers.
When considering the pathophysiological mechanisms underlying the ocular surface changes, as hypothesized, the airflow reaching the ocular surface during expiration probably represents the trigger factor initiating the cascade of structural and molecular changes of the tear film (TF).10,11
In fact, the exposure of the ocular surface to high air velocity causes evaporation of water from the precorneal TF by eliminating the boundary of air adjacent to the TF in conditions of stagnant ambient air. This hypothesis was confirmed in previous studies that reported that exposure of the TF to high velocity airflow (1.0–1.4 m/s) on normal eyes significantly decreases BUT, and increases blink frequency as a result of the changes on the ocular surface.21,22 These findings were more recently confirmed in an anterior segment-optical coherence tomography study, which found that the exposure of the ocular surface to high speed air flow (1.5 m/s) reduces the lower tear meniscus height and area and increases the blink frequency.23 Therefore, airflow reduces the TF stability and the tear volume, inducing dry eye-like alterations; these findings are in line with the reduced T-BUT and STI values we observed especially in subjects with DED.
HLA-DR is a marker of inflammatory activity on the ocular surface, both at the corneal and conjunctival level, as it reflects the expression of DCs. It is widely demonstrated that in case of DED, there is a pro-inflammatory cellular activation with increased DC density.17,24,25 HLA-DR was found increased in DED, Sjögren syndrome, and meibomian gland disease. In addition, the HLA-DR marker was described in literature to be useful for monitoring anti-inflammatory effects of treatments in DED.24
The present study suffers from some limitations. First, we did not consider control groups of patients with DED and healthy subjects not using FMs, because in our country the entire population is strongly invited to wear FMs. Therefore, one cannot ascertain whether observed modifications in DED are induced by the use of FMs or represent a normal worsening of the ocular surface related to the concomitant disease. However, the DCD and HLA-DR increases also in healthy subjects, which do not have an underlying OSD, and the worse values observed in subjects using FMs for more than 6 hours/day seem to highlight the potential causative role of the FM use in the ocular surface worsening. Second, we exclusively investigated the impact of surgical FMs on ocular surface, because the largest part of the population wears this type of PPE; further prospective studies are required to evaluate the impact of most protective FMs, such as FFP2 or FFP3, because they could differently harm the ocular surface. Third, this a short-term prospective study, which, for this reason, cannot unravel the ocular surface changes in full; in fact, it is at least hypothesizable that long-term studies may reveal also FM-related GCD changes. In addition, prospective studies with a longer follow-up and a period of FM wear discontinuation could also unravel whether these changes are reversible at the suspension of FMs, and in which time the ocular surface may recover its initial status. Finally, to avoid potential biases in the final results, we did not enroll patients on VDT-related smart working; considering that the use of PPE and the remodulation of job activities will still continue for several months, further studies analyzing the impact of VDT activities and FM use in the ocular surface modifications are mandatory.
In conclusion, our study found that: (i) the regular daily use of FMs harms the ocular surface in the presence of dry eye, inducing a significant worsening of several clinical and molecular parameters when the use is prolonged during the day; (ii) the ocular surface worsening has a significant negative impact on the patient quality of life; (iii) more limited is the detrimental effects of FMs in the presence of healthy ocular surface, even though they tend to become significant when the number of daily hours increases; and (iv) the increase of inflammation appears as the main molecular mechanisms underlying all these aspects. Therefore, FM users should pay attention in the presence of a concomitant ocular surface disorder, such as dry eye. In this case, a modification of environmental factors along with modulations of drug strategies should be strongly considered to increase the FM tolerability and limit the OSD worsening.
Acknowledgments
Disclosure: L. Mastropasqua, (N); M. Lanzini, (N); L. Brescia, (N); R. D'Aloisio, (N); M. Nubile, (N); M. Ciancaglini, (N); C. D'Amario, (N); L. Agnifili, (N); R. Mastropasqua, (N)
References
- 1. Kratzel A, Todt D, V'kovski P, et al.. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020; 26(7): 1592–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang J, Pan L, Tang S, Ji JS, Shi X.. Mask use during COVID-19: a risk adjusted strategy. Environ Pollut. 2020; 266(Pt 1): 115099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sommerstein R, Fux CA, Vuichard-Gysin D, et al.. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020; 9(1): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control. How to protect yourself & others. April 4, 2020, https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
- 5. Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L.. Face masks for the public during the covid-19 crisis. BMJ. 2020; 369: m1435. [DOI] [PubMed] [Google Scholar]
- 6. Kyung SY, Kim Y, Hwang H, Park JW, Jeong SH.. Risks of N95 face mask use in subjects with COPD. Respir Care. 2020; 65(5): 658–664. [DOI] [PubMed] [Google Scholar]
- 7. Kainth GS. Novel tip to prevent ear irritation with surgical face masks (FRSM) during the coronavirus (COVID-19) pandemic. Ann R Coll Surg Engl. 2020; 102(6): 470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szepietowski JC, Matusiak L, Szepietowska M, Krajewski PK, Białynicki-Birula R. Face mask-induced itch: a self-questionnaire study of 2,315 responders during the COVID-19 pandemic. Acta Derm Venereol. 2020; 100(10): adv00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xinqi H, Cen X, Liu J. Effect of protraction facemask on the temporomandibular joint: a systematic review. BMC Oral Health. 2018; 18: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giannaccare G, Vaccaro S, Mancini A, Scorcia V.. Dry eye in the COVID-19 era: how the measures for controlling pandemic might harm ocular surface. Graefes Arch Clin Exp Ophthalmol. 2020; 19: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moshirfar M, West WB Jr, DP Marx. Face mask-associated ocular irritation and dryness. Ophthalmol Ther. 2020; 15: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007; 5: 75–92. [DOI] [PubMed] [Google Scholar]
- 13. Vitali C, Bombardieri S, Jonsson R, et al.. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakane Y, Yamaguchi M, Yokoi N, et al.. Development and validation of the Dry Eye-Related Quality-of-Life Score questionnaire. JAMA Ophthalmol. 2013; 131(10): 1331–1338. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan BD, Crews LA, Messmer EM, et al.. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmologica. 2014; 92(2): 161–166. [DOI] [PubMed] [Google Scholar]
- 16. Bron AJ, Evans VE, Smith JA.. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22: 640–649. [DOI] [PubMed] [Google Scholar]
- 17. Mastropasqua L, Nubile M, Lanzini M, et al.. Epithelial dendritic cell distribution in normal and inflamed human cornea: in vivo confocal microscopy study. Am J Ophthalmol. 2006; 142(5): 736–744. [DOI] [PubMed] [Google Scholar]
- 18. Agnifili L, Fasanella V, Mastropasqua R, et al.. In vivo goblet cell density as a potential indicator of glaucoma filtration surgery outcome. Invest Ophthalmol Vis Sci. 2016; 57(7): 2928–2935. [DOI] [PubMed] [Google Scholar]
- 19. Agnifili L, Brescia L, Oddone F, et al.. The ocular surface after successful glaucoma filtration surgery: a clinical, in vivo confocal microscopy, and immune-cytology study. Sci Rep . 2019; 9(1): 11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fikenzer S, Uhe T, Lavall D, et al.. Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin Res Cardiol. 2020; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wyon NM, Wyon DP. Measurement of acute response to draught in the eye. Acta Ophthalmologica. 1987; 65,385–392. [DOI] [PubMed] [Google Scholar]
- 22. Nakamori K, Odawara M, Nakajima T, Mizutani T, Tsubota K.. Blinking is controlled primarily by ocular surface conditions. Am J Ophthalmol. 1997; 457(1): 24–30. [DOI] [PubMed] [Google Scholar]
- 23. Koh S, Tung C, Kottaiyan R, Zavislan J, Yoon G, Aquavella J.. Effect of airflow exposure on the tear meniscus. J Ophthalmol. 2012; 2012: 983182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brignole-Baudouin F, Riancho L, Ismail D, Deniaud M, Amrane M, Baudouin C.. Correlation between the inflammatory marker HLA-DR and signs and symptoms in moderate to severe dry eye disease. Invest Ophthalmol Vis Sci . 2017; 58(4): 2438–2448. [DOI] [PubMed] [Google Scholar]
- 25. Mastropasqua R, Agnifili L, Fasanella V, et al.. Corneoscleral limbus in glaucoma patients: in vivo confocal microscopy and immunocytological study. Invest. Ophthalmol. Vis. Sci. 2015; 56: 2050–2058. [DOI] [PubMed] [Google Scholar]