Abstract
Purpose
To determine possible impacts on retinal microvasculature in healthy young adults during exercise with a face mask, using optical coherence tomography angiography (OCTA).
Methods
Twenty-three healthy participants (23 eyes, 17 women and 6 men) performed the incremental continuous running test (ICRT) with different masks. OCTA of the macula and optic nerve head were performed before and after ICRT to detect changes in retinal vessel density (VD). All participants were in groups A, B, and C (before ICRT) and groups A′, B′, and C′ (after ICRT), which comprised data from volunteers without a mask, with a surgical mask, and with an N95 mask, respectively.
Results
Before ICRT, group C showed significantly reduced VD in the superficial plexus (SP), except foveal VD, compared with group A (P < 0.05). After ICRT, groups B′ and C′ showed significantly shorter maximum running time, lower oxygen saturation, and lower perifoveal VD of SP compared with group A′ (P < 0.05).
Conclusions
Use of an N95 mask reduced VD in SP even under quiescent conditions, which might have clinical implications for protecting healthy workers and indoor manual labor workers from potential risks of retinal damage due to long-term mask use. Moreover, mask use while exercising might lead to attenuated exercise ability and lower VD in SP, which should be investigated in additional studies.
Translational Relevance
Retina vascular perfusion dynamics could be monitored in vivo by OCTA, which would be valuable to study physiologic retinal blood flow redistribution and potential impacts on retinal vascular perfusion during exercise with face masks.
Keywords: retinal vessel density, face mask, exercise, incremental continuous running test
Introduction
In late December 2019, a new acute respiratory syndrome coronavirus was first reported in Wuhan, China; it causes coronavirus disease 2019 (COVID-19), a disease that has rapidly spread internationally.1 This disease exhibits human-to-human transmission among close contacts, potentially by droplet transmission.2,3 Many countries and regions have required or advised their citizens to wear masks in public places to reduce such transmission; masks are considered a useful adjunct to social distancing and an irreplaceable method to prevent the spread of the virus during the COVID-19 pandemic.4–6 Under such situations, people should wear masks more frequently and on more occasions to protect themselves. For example, it was rare in the past to wear a mask during aerobic exercise or manual labor indoors, but it has become more common nowadays. Therefore, it is important to investigate whether mask wearing influences activities of daily living, especially with respect to aerobic exercise.
The vascular morphologic and physiologic properties of the retina are similar to those of the heart and brain, such that the retina can offer a unique surrogate for assessment of changes in cardiac or cerebral vasculature.7,8 Recently, several studies have shown that retina vessel perfusion in healthy adults decreases after physical activity; these studies used optical coherence tomography angiography (OCTA), which constitutes a rapid, sensitive, and noninvasive tool to monitor retinal vessel density (VD) changes and predict the effects of physical exercise on cardiac vasculature.9–11 Furthermore, use of a surgical mask while walking has been shown to aggravate clinical dyspnea.12 Although there have been some studies of postexercise OCTA findings, it remains unclear whether the use of a mask aggravates reduction of retinal VD. Therefore, this OCTA study was performed to evaluate macular and Optic nerve head (ONH) VD changes in mask-wearing healthy adults after aerobic exercise.
Methods
This study was approved by the institutional review board of Wuhan University and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each volunteer before OCTA imaging.
Subjects
In total, 23 healthy volunteers (6 female and 17 male), aged 18 to 30 years, were recruited for this study at the Eye Center, Renmin Hospital of Wuhan University between July 2020 and August 2020.
Each volunteer underwent a COVID-19 reverse transcriptase polymerase chain reaction test (within 5 days), slit-lamp fundus examination, intraocular pressure (IOP) measurement, and collection of basic clinic data. Exclusion criteria were as follows: (1) IOP >21 mm Hg; (2) refractive error >±3 diopters; (3) obvious media opacity; (4) history of ophthalmologic disease and existing uveitis, glaucoma, retinal diseases, and/or choroidal diseases; (5) history of cerebral disease; (6) history of respiratory disease; (7) coronary artery disease, stroke, atrial fibrillation, and/or history of cardiac disease; (8) history of cardiac disease–related medication (e.g., angiotensin-converting enzyme inhibitors, β-blockers, and/or calcium channel blockers); (9) history of arterial hypertension and diabetes mellitus; and (10) a positive COVID-19 reverse transcriptase polymerase chain reaction test.
All participants were in six groups: groups A, B, and C comprised data from 23 volunteers without a mask, with a surgical mask, and with an N95 mask, respectively, before the incremental continuous running test (ICRT); groups A′, B′, and C′ comprised data from 23 volunteers without a mask, with a surgical mask, and with an N95 mask, respectively, after the ICRT.
Exercise Test Procedures
All participants performed a standardized ICRT, as described previously.13 The test was performed indoors at an ambient temperature (18–22°C) and in a well-ventilated environment on a treadmill (Treadmill HOME T5; LifeFitness, Chicago, Illinois, USA). The test began at 8.0 km/h and increased by 2.0 km/h at 3-minute intervals until the heart rate reached 190 beats per minute. The maximum running speed and maximum running time were recorded automatically by software within the treadmill. Blood pressure was recorded before and immediately after ICRT by an electronic sphygmomanometer (Electronic Sphygmomanometer YE660CR; Yuwell, Suzhou, China). Oxygen saturation and heart rate were monitored by a pulse oximetry sensor placed on the right index fingertip (oximeter 303; Yuwell); these measurements were recorded at 3-minute intervals throughout the test and upon completion of exercise. IOP was measured immediately after OCTA imaging.
In accordance with cardiologic stress protocols,14 the ICRT was terminated when individuals reached the maximum theoretical heart rate of approximately 190 beats (220 – age). Any individuals who could not run for ≥9 minutes without a mask were excluded. Furthermore, the ICRT was discontinued in the event of intense headache, intense dyspnea, or any uncomfortable symptoms; any affected individuals were excluded from the study.
OCTA Imaging
OCTA imaging was used to investigate retinal vascular changes by prototype AngioVue software within the AngioVue device (RTVue XR Avanti with AngioVue; Optovue, Fremont, CA, USA), which generated OCTA images by using split-spectrum amplitude-decorrelation angiography.15 In this study, we used 6.0 × 6.0-mm macula scans and 4.5 × 4.5-mm ONH scans to extract VD of the ONH and macular areas; these data were analyzed automatically by OCTA devices. The superficial plexus (SP) was defined as a layer from 3 µm beneath the internal limiting membrane to 9 µm below the inner plexiform layer; the deep plexus (DP) was defined as a layer from 9 µm below the inner plexiform layer to 9 µm above the outer plexiform layer. All layers were segmented automatically by AngioVue software. An integrated eye-tracking system and a follow-up system in the OCTA device (DualTrac Motion Correction; Optovue) were respectively used to improve the imaging quality by removing motion artifacts and to ensure the OCTA image position remained consistent with that of previous images.
OCTA measurements were taken over 3 days; each day, they were performed in the morning from 8 to 11 am. Only one eye of each participant was randomly included and was not dilated in the study. On the first day, volunteers were asked to rest without wearing a mask for 60 minutes; OCTA images were then acquired under quiescent conditions directly after rest. ICRT began directly after quiescent OCTA images were obtained. All exercise OCTA images were recorded within 3 minutes after ICRT. On the second and third days, the protocol was similar to that of the first day; however, all subjects were asked to wear a surgical mask (Medical Surgical Mask 20162640493; HAINUO, Qingdao, China) and an N95 mask (N95 1860; 3M, St. Paul, Minnesota, USA), respectively. Additionally, all volunteers were asked to refrain from physical activity for the remaining portions of the first and second days. For quality control, all images were included if their quality was better than 8/10 and they had fewer than two motion artifacts. All OCTA images were obtained by a trained ophthalmic photographer under consistent conditions.
Statistics
Data management was performed using GraphPad Prism version 8 (GraphPad Prism, La Jolla, CA, USA). Comparisons of demographic data and OCTA measurements before and after ICRT (AvsA′, BvsB′, and CvsC′) were performed using t-tests for paired data; nonnormally distributed variables were compared using the Wilcoxon signed rank test. Groups A, B, and C were compared using repeated-measures one-way analysis of variance with the Geisser-Greenhouse correction; groups A′, B′, and C′ were compared in a similar manner, and nonnormally distributed variables were compared using the Kruskal-Wallis test. All data are presented as means ± standard deviations. P values <0.05 were considered statistically significant.
Results
In total, 23 volunteers (17 males and 6 females) were included in our study; their demographic information is listed in Table 1. Group C showed a significantly higher heart rate (P = 0.001) and lower oxygen saturation (P = 0.006) compared with group A. All other parameters did not significantly differ among the three groups (all P > 0.05) (Table 1).
Table 1.
Demographics and Pre-ICRT Clinical Characteristics
| Characteristic | Group A: No Mask (n = 23) | Group B: Surgical Mask (n = 23) | Group C: N95 Mask (n = 23) |
|---|---|---|---|
| Age, y | 26.9 ± 3.72 | 26.9 ± 3.72 | 26.9 ± 3.72 |
| Sex (male/female), No. | 17/6 | 17/6 | 17/6 |
| IOP, mm Hg | 16.1 ± 2.13 | 16.2 ± 1.92 | 16.6 ± 2.04 |
| Systolic blood pressure, mm Hg | 121.6 ± 9.37 | 122.3 ± 9.84 | 123.3 ± 8.72 |
| Diastolic blood pressure, mm Hg | 76.9 ± 6.46 | 78.5 ± 6.22 | 78.8 ± 5.42 |
| Heart rate, beats per minute | 79.5 ± 5.18 | 80.8 ± 4.32 | 83.4 ± 5.69* |
| Oxygen saturation, % | 97.4 ± 0.78 | 96.8 ± 1.07 | 96.4 ± 1.41* |
All data are shown as mean ± standard deviation unless otherwise indicated.
Statistically significant results (p < 0.05).
Groups B′ and C′ showed significantly shorter maximum running time and lower speed compared to group A′ (all P < 0.001). Oxygen saturation after ICRT was significantly decreased in groups B′ and C′ compared to group A′ (P = 0.003; P < 0.001). All other parameters did not significantly differ among the three groups (all P > 0.05) (Table 2).
Table 2.
Demographics and Post-ICRT Clinical Characteristics
| Characteristic | Group A′: No Mask (n = 23) | Group B′: Surgical Mask (n = 23) | Group C′: N95 mask (n = 23) |
|---|---|---|---|
| Systolic blood pressure, mm Hg | 154.7 ± 7.73 | 155.4 ± 8.57 | 156.3 ± 7.94 |
| Diastolic blood pressure, mm Hg | 90.2 ± 6.80 | 92.9 ± 7.40 | 90.0 ± 6.63 |
| Oxygen saturation at completion of ICRT, % | 96.4 ± 0.89 | 95.2 ± 1.13* | 94.5 ± 1.34* |
| Maximum running time, min | 10.1 ± 1.21 | 7.5 ± 1.37* | 6.6 ± 0.92* |
| Maximum running speed, km/h | 14.4 ± 0.78 | 12.0 ± 0.85* | 11.7 ± 0.98* |
| IOP, mm Hg | 16.1 ± 1.79 | 16.5 ± 1.73 | 16.7 ± 1.64 |
All data are shown as mean ± standard deviation.
Statistically significant results (p < 0.05).
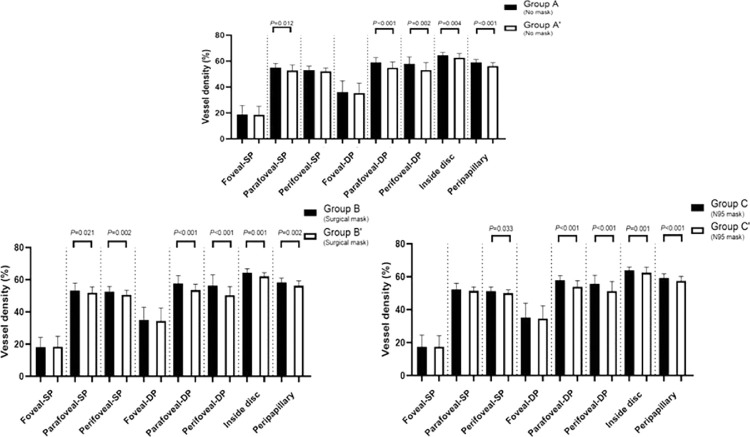
The OCTA parameters of participants are listed in Table 3. Comparisons of groups A and A′, groups B and B′, and groups C and C′ revealed that most measured retinal regions exhibited significant reductions of VD (all P < 0.05), except the foveal region (all P > 0.05). Furthermore, the choroidal flow area and IOP did not significantly differ between before and after ICRT in groups (all P > 0.05) (Table 3, Fig. 1).
Table 3.
Parameters of Pre- and Post-ICRT Measurements (6.0 × 6.0-mm Macula Scan and 4.5 × 4.5-mm ONH Scan)
| Chacteristic | Group A: No Mask (n = 23) | Group A′: No Mask (n = 23) | Group B: Surgical Mask (n = 23) | Group B′: Surgical Mask (n = 23) | Group C: N95 Mask (n = 23) | Group C′: N95 Mask (n = 23) |
|---|---|---|---|---|---|---|
| Superficial plexus | ||||||
| Vessel density (macular area), % | ||||||
| Foveal | 18.9 ± 6.95 | 18.5 ± 6.62 | 18.1 ± 6.16 | 18.5 ± 6.44 | 17.5 ± 7.13 | 17.5 ± 6.74 |
| Parafoveal | 55.0 ± 3.27 | 52.8 ± 4.39* | 53.4 ± 4.63 | 52.0 ± 3.52* | 52.5 ± 3.59 | 51.4 ± 2.35 |
| Perifoveal | 53.2 ± 2.99 | 52.2 ± 2.63 | 52.7 ± 3.11 | 50.7 ± 2.81* | 51.3 ± 2.50 | 50.1 ± 2.10* |
| Deep plexus | ||||||
| Vessel density (macular area), % | ||||||
| Foveal | 36.2 ± 8.52 | 35.4 ± 7.71 | 35.2 ± 7.71 | 34.4 ± 8.11 | 35.3 ± 8.66 | 34.7 ± 7.64 |
| Parafoveal | 59.0 ± 3.83 | 54.9 ± 4.48* | 57.6 ± 5.01 | 53.7 ± 3.62* | 57.9 ± 2.83 | 54.1 ± 3.66* |
| Perifoveal | 58.0 ± 5.32 | 53.2 ± 5.76* | 56.4 ± 6.65 | 50.5 ± 5.27* | 55.8 ± 5.22 | 51.3 ± 5.87* |
| Vessel density (ONH area), % | ||||||
| Inside disc | 64.7 ± 2.06 | 62.7 ± 3.29* | 64.3 ± 2.53 | 62.1 ± 2.30* | 63.9 ± 2.09 | 62.5 ± 3.27* |
| Peripapillary | 59.1 ± 2.22 | 56.3 ± 2.60* | 58.3 ± 2.86 | 56.3 ± 3.05* | 59.2 ± 2.70 | 57.5 ± 2.81* |
| Choriocapillaris flow area (3.144 mm2) | ||||||
| Flow area | 2.16 ± 0.128 | 2.17 ± 0.143 | 2.16 ± 0.199 | 2.19 ± 0.114 | 2.17 ± 0.135 | 2.20 ± 0.121 |
All data are shown as mean ± standard deviation.
Statistically significant results (p < 0.05).
Figure 1.
Comparisons of groups A and A′, B and B′, and C and C′ revealed that most measured retinal regions exhibited significant reductions of VD (all P < 0.05), except the foveal region (all P > 0.05).
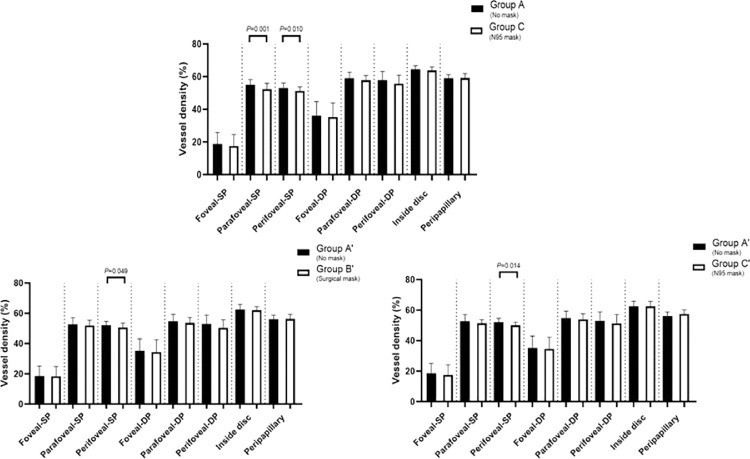
Before the ICRT, only group C showed a significant reduction in VD in SP (except the foveal area) compared to group A (all P < 0.05). After the ICRT, the perifoveal VD in SP was significantly reduced in groups B′ and C′ compared to group A′ (all P < 0.05) (Figs. 2, 3).
Figure 2.
Before the ICRT, only group C showed a significant reduction in VD in SP (except the foveal area), compared with group A (all P < 0.05). After the ICRT, the perifoveal VD in SP was significantly reduced in groups B′ and C′ compared with group A′ (all P < 0.05).
Figure 3.
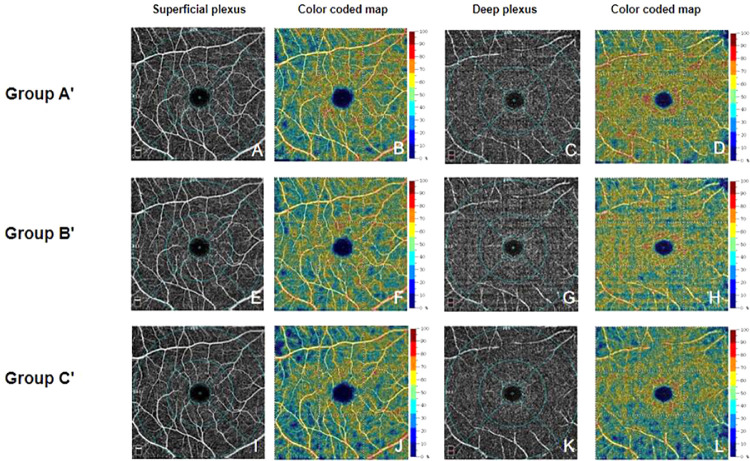
Representative OCTA macula images of each group after ICRT. (A–D) Images of volunteers without a mask. (E–H) Images of volunteers with a surgical mask. (I–L) Images of volunteers with an N95 mask. A, E, and I show the superficial plexus layer; C, G, and H show the deep plexus layer. B, F, J, D, H, and L show calculated blood flow, from low to high (indicated by blue to red). From A, the foveal region is defined as a circle centered on the macula, surrounded by a 1-mm diameter blue circle; the parafoveal region is defined as an annulus centered on the fovea, with an outer diameter of 3 mm and an inner diameter of 1 mm (indicated by blue circles); the perifoveal region is defined as an annulus centered on the fovea, with an outer diameter of 6 mm and an inner diameter of 3 mm (indicated by blue circles). All parameters were measured automatically.
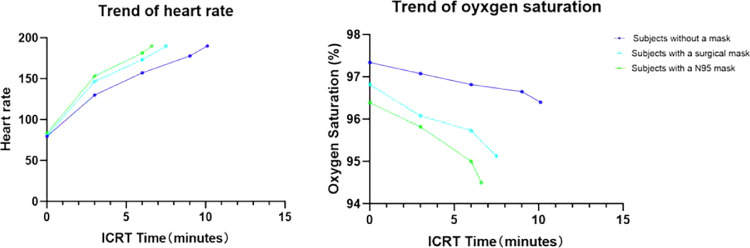
The mean heart rates at 3 minutes and at 6 minutes in group C′ were significantly higher than groups A′ and B′ (all P < 0.05). The mean heart rates at 3 minutes and at 6 minutes in group B′ were significantly higher compared to group A′ (P < 0.05). The mean oxygen saturation levels at 3 minutes in group C′ were significantly lower compared to groups A′ and B′ (all P < 0.05). The mean oxygen saturation levels at 6 minutes showed significant differences among groups A′, B′, and C′ (all P < 0.05) (Fig. 4).
Figure 4.
Trends of heart rate and oxygen saturation changes in volunteers during ICRT without a mask, with a surgical mask, and with an N95 mask. The mean heart rates at 3 minutes in groups A′, B′, and C′ were 130.0 ± 7.67, 146.9 ± 10.00, and 153.1 ± 8.05 beats per minute, respectively (all P < 0.05). The mean heart rates at 6 minutes in groups A′, B′, and C′ were 157.2 ± 7.24, 173.3 ± 10.07, and 181.4 ± 6.89 beats per minute, respectively (all P < 0.05). The mean oxygen saturation levels at 3 minutes in groups A′, B′, and C′ were 97.1% ± 0.90%, 96.1% ± 1.04%, and 95.8% ± 1.27%, respectively (groups A′ and B′ vs C′, P < 0.05). The mean oxygen saturation levels at 6 minutes in groups A′, B′, and C′ were 96.8% ± 0.83%, 95.7% ± 0.86%, and 95.0% ± 1.21%, respectively (all P < 0.05).
Discussion
To the best of our knowledge, this is the first study regarding variations in retinal VD in mask-wearing healthy young volunteers, before and after physical activity, as determined by OCTA. We found that parafoveal and perifoveal VD in SP were significantly reduced with use of an N95 mask under quiescent conditions. Furthermore, perifoveal VD in SP showed significant reduction in mask-wearing groups after ICRT; the maximum theoretical heart rate (190 beats) was reached significantly earlier and oxygen saturation was significantly lower upon completion of ICRT.
In a previous study, retinal vasodilation and enhanced retinal blood flow were found to compensate for hypoxia, although there was a limit to the scope of such vascular adjustment; for example, there were no significant changes in retinal vascular diameter and blood flow from 98% to 90% oxygen saturation.16 In our study, parafoveal and perifoveal VD in SP were reduced by use of an N95 mask under quiescent conditions. Furthermore, significant heart rate enhancement and oxygen saturation reduction were observed under those conditions, which might be similar to light exercise. Reductions of parafoveal and perifoveal VD may be explained in the same manner as in previous studies: physiological blood supply redistribution via diversion to other organs9,11 (e.g., heart and lungs). In addition, there were no significant VD differences in DP during use of an N95 mask under quiescent conditions. This is presumably because DP contains mainly capillary vessels, while SP consists of arterioles, venules, and capillaries. Because of the myogenic constriction of arterioles, SP blood flow appears to be more affected by perfusion pressure, blood gasses, and IOP compared to DP blood flow.17 For safety reasons, only young volunteers (18–30 years old) were recruited for this study; they showed a significant reduction in VD in SP during 1 hour of N95 mask use, which might suggest that long-term N95 mask use reduces retina vascular perfusion. Furthermore, additional attention is needed regarding front-line health workers and indoor manual labor workers who must continuously wear an N95 mask for extended periods (up to the entire working day) during the pandemic; they might have reduction of retina vascular perfusion and have a potential risk of retinal damage.18–21 However, face mask use has been necessary and irreplaceable and can limit the transmission of COVID-19 in crowded public places during the pandemic.6 Our results have implications for wearing masks that should provide additional scientific and health-related guidance. Further prospective studies are needed to elucidate the safe durations of mask use and associated breaks to protect against COVID-19 while avoiding potential risk of retina damage among individuals who perform different activities.
In this study, VD was reduced in most measured retinal regions after ICRT, which is consistent with the results of previous studies.9,11 The results of those previous studies indicated that the reductions of ONH and macular VD after exercise could be related to enhancement of IOP and reduction of ocular perfusion pressure due to blood supply redistribution.22–24 However, changes in IOP after exercise seem to be controversial; these might depend on exercise intensity and type, as well as whether the volunteer performs regular exercise outside of the study.25,26 Our results indicated no significant differences in IOP after ICRT. This finding was presumably because IOP measurements were taken after OCTA images, which might have reduced the differences in IOP among postexercise groups because of the 3-minute delay in measurements.
A notable finding was that, although only perifoveal VD in SP showed significant reductions in mask-wearing groups after ICRT, heart rate reached 190 beats per minute significantly earlier and oxygen saturation was significantly lower upon completion of ICRT during the use of surgical or N95 masks. A previous study showed that surgical mask use significantly aggravated clinical dyspnea during the 6-minute walk test compared to the absence of a surgical mask during the test.12 Moreover, N95 and surgical masks might perform airflow restriction comparable to that of an elevation training mask, which has been used to simulate a hypoxic environment (similar to altitude training) for athletes.27 Mask use has been shown to cause some adverse effects, including delayed cardiac-autonomic recovery from exercise, attenuated exercise ability, reduced awareness of sports injury, and hypoxemia.27,28 Our results indicate that the use of an N95 or surgical mask also might lead to a decline in exercise ability and earlier and stronger reductions of retinal VD in young adults. Therefore, some danger may be involved in exercising with a face mask; without obvious symptoms, individuals may follow standard training protocols and exhibit reduced awareness of when to discontinue training before maximum exercise ability is reached, which might lead to retinal damage or severe physical damage (e.g., myocardial infarction and stroke). Physical activity is regarded as an important component in reducing the risk of chronic diseases and improving personal fitness29; therefore, exercise safety when using a mask should receive greater attention, and the need to limit physical activities should be conveyed to the public. Our results indicate that it might be safe for people to reduce a part of exercise in each training bout with a mask, in combination with prolonged break time. Notably, the duration of safe exercise with a mask remains unclear, which merits further prospective studies.
Importantly, measurement of blood flow in choroidal circulation is limited because vessels in Sattler's and Haller's layers are sheltered by the retinal pigment epithelium.30 Therefore, we could measure only the perfusion area of the choriocapillaris layer by OCTA; the results showed no significant differences before and after ICRT, regardless of mask use. Unlike retinal circulation, the choroidal circulation might be more sensitive to parasympathetic efferent and sympathetic efferent nerve control; it shows only mild alteration following changes in perfusion pressure and oxygen saturation, except in Partial Pressure of Carbon Dioxide (pCO2).17 Sympathetic efferent nerves regulate reductions in choroidal blood flow by releasing noradrenaline31; in turn, enhancements of choroidal blood flow are controlled by parasympathetic efferent nerves via Nitric Oxide (NO) signaling.32 In our study, choroidal blood flow might have initially decreased during ICRT because of sympathetic efferent activation. Following activation of parasympathetic efferent nerves, weakening of sympathetic nerves, and enhancement of pCO2 after the ICRT, choroidal blood flow might have increased and maintained a level similar to that of the normal condition due to the modulating effects of those various factors. These aspects might explain why we found no significant differences between before and after ICRT.
There were several limitations in our study. First, our sample size was small, and there were no older individuals in the study; therefore, the results of this study may not be generalizable to older individuals. Second, despite use of the eye-tracking system, some motion artifacts may have been present in some OCTA images because of rapid breathing immediately after ICRT, which might have influenced the data. Third, we always measured macula scans prior to ONH scans, which might have influenced the data regarding ONH VD; it also might have reduced the differences among postexercise groups because of additional time for blood flow recovery. Finally, dynamic changes in retinal VD could not be continuously monitored by OCTA during the ICRT.
Despite the above limitations, there were some merits in this study. The OCTA findings revealed that parafoveal and perifoveal VD in SP significantly decreased in young volunteers with an N95 mask under quiescent conditions. In addition, use of a mask negatively influenced exercise activities and caused greater reductions in retinal VD in SP after ICRT. Further studies are needed to overcome the above limitations by considering some of the following factors: age, systemic disease, safe durations of mask use, and dynamic changes in retinal VD.
In conclusion, we found that use of an N95 mask reduced VD in SP even under quiescent conditions, which might have clinical implications for healthy workers and indoor manual labor workers from potential risks of retinal damage due to long-term mask use. Moreover, mask use while exercising might lead to attenuated exercise ability and lower VD in SP, which should be investigated in additional studies.
Acknowledgments
The authors thank all study participants for their support of the current work.
Supported by the China Scholarship Council (Grant 201806270215 to Dihao Hua and Grant 201706270193 to Yishuang Xu).
Disclosure: D. Hua, None; Y. Xu, None; P. Heiduschka, None; W. Zhang, None; X. Zhang, None; X. Zeng, None; X. Zhu, None; T. He, None; H. Zheng, None; X. Xiao, None; Y. Xing, None; Z. Chen, None; C. Chen, None
References
- 1. Guan WJ, Ni ZY, Hu Y, et al.. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18): 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al.. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020; 382(13): 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li H, Liu Z, Ge J. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. J Cell Mol Med. 2020; 24(12): 6558–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee HK. South Korea takes new measures to have enough face masks domestically amid coronavirus. ABC News. April 27, 2020. Available at: https://abcnews.go.com/International/south-korea-takes-measures-face-masks-domestically-amid/story?id=69254114. Accessed April 27, 2020.
- 5. Government of Canada. Considerations in the use of homemade masks to protect against COVID-19. Notice to general public and healthcare professionals. 2020. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/activities/announcements/covid19-notice-home-made-masks.html. Accessed June 26, 2020.
- 6. Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity. Lancet. 2020:S0140-6736(20)30918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong TY, Klein R, Sharrett AR, et al.. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002; 287(9): 1153–1159. [DOI] [PubMed] [Google Scholar]
- 8. Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005; 206(4): 319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alnawaiseh M, Lahme L, Treder M, Rosentreter A, Eter N.. Short-term effects of exercise on optic nerve and macular perfusion measured by optical coherence tomography angiography. Retina. 2017; 37(9): 1642–1646. [DOI] [PubMed] [Google Scholar]
- 10. Schmitz B, Nelis P, Rolfes F, et al.. Effects of high-intensity interval training on optic nerve head and macular perfusion using optical coherence tomography angiography in healthy adults. Atherosclerosis. 2018; 274: 8–15. [DOI] [PubMed] [Google Scholar]
- 11. Vo Kim S, Semoun O, Pedinielli A, Jung C, Miere A, Souied EH. Optical coherence tomography angiography quantitative assessment of exercise-induced variations in retinal vascular plexa of healthy subjects. Invest Ophthalmol Vis Sci. 2019; 60(5): 1412–1419. [DOI] [PubMed] [Google Scholar]
- 12. Person E, Lemercier C, Royer A, Reychler G. Effect of a surgical mask on six minute walking distance. Rev Mal Respir. 2018; 35(3): 264–268. [DOI] [PubMed] [Google Scholar]
- 13. Schmitz B, Klose A, Schelleckes K, Jekat CM, Krüger M, Brand SM. Yo-Yo IR1 vs incremental continuous running test for prediction of 3000m performance. J Sports Med Phys Fitness. 2017; 57(11): 1391–1398. [DOI] [PubMed] [Google Scholar]
- 14. Fletcher GF, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards: a statement for health professionals from the American Heart Association. Circulation. 1990; 82(6): 2286–2322. [DOI] [PubMed] [Google Scholar]
- 15. Jia Y, Tan O, Tokayer J, et al.. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012; 20(4): 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng RW, Yusof F, Tsui E, et al.. Relationship between retinal blood flow and arterial oxygen. J Physiol. 2016; 594(3): 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012; 31(5): 377–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al.. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology. 2017; 124(5): 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gür Güngör S, Sarıgül Sezenöz A, Öztürk C, Gökgöz G, Akman A. Peripapillary and macular vessel density measurement with optical coherence angiography in exfoliation syndrome. J Glaucoma. 2020; 29(5): 381–385.32079991 [Google Scholar]
- 20. Zeng Y, Cao D, Yu H, et al.. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 2019; 103(12): 1747–1752. [DOI] [PubMed] [Google Scholar]
- 21. You QS, Wang J, Guo Y, et al.. Detection of reduced retinal vessel density in eyes with geographic atrophy secondary to age-related macular degeneration using projection-resolved optical coherence tomography angiography. Am J Ophthalmol. 2020; 209: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jasien JV, Jonas JB, de Moraes CG, Ritch R. Intraocular pressure rise in subjects with and without glaucoma during four common yoga positions. PLoS One. 2015; 10(12): e0144505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vieira GM, Oliveira HB, de Andrade DT, Bottaro M, Ritch R. Intraocular pressure variation during weight lifting. Arch Ophthalmol. 2006; 124(9): 1251–1254. [DOI] [PubMed] [Google Scholar]
- 24. Lovasik JV, Kergoat H, Riva CE, Petrig BL, Geiser M.. Choroidal blood flow during exercise-induced changes in the ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2003; 44(5): 2126–2132. [DOI] [PubMed] [Google Scholar]
- 25. Karabatakis VE, Natsis KI, Chatzibalis TE, et al.. Correlating intraocular pressure, blood pressure, and heart rate changes after jogging. Eur J Ophthalmol. 2004; 14(2): 117–122. [DOI] [PubMed] [Google Scholar]
- 26. Passo MS, Goldberg L, Elliot DL, Van Buskirk EM.. Exercise conditioning and intraocular pressure. Am J Ophthalmol. 1987; 103(6): 754–757. [DOI] [PubMed] [Google Scholar]
- 27. Jagim AR, Dominy TA, Camic CL, et al.. Acute effects of the elevation training mask on strength performance in recreational weight lifters. J Strength Cond Res. 2018; 32(2): 482–489. [DOI] [PubMed] [Google Scholar]
- 28. Jung HC, Lee NH, John SD, Lee S. The elevation training mask induces modest hypoxaemia but does not affect heart rate variability during cycling in healthy adults. Biol Sport. 2019; 36(2): 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haskell WL, Lee IM, Pate RR, et al.. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007; 116(9): 1081–1093. [DOI] [PubMed] [Google Scholar]
- 30. Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018; 67: 30–55. [DOI] [PubMed] [Google Scholar]
- 31. Kawarai M, Koss MC. Sympathetic vasoconstriction in the rat anterior choroid is mediated by a1-adrenoceptors. Eur J Pharmacol. 1998; 363: 35–40. [DOI] [PubMed] [Google Scholar]
- 32. Nilsson SF. Nitric oxide as a mediator of parasympathetic vasodilation in ocular and extraocular tissues in the rabbit. Invest Ophthalmol Vis Sci. 1996; 37: 2110–2119 [PubMed] [Google Scholar]