Key Points
Questions
Is the risk of suicide attempt increased in patients diagnosed with mild cognitive impairment (MCI) or dementia, and does the recency of diagnosis matter?
Findings
In this propensity-matched cohort study of 147 595 older adults, the risk of suicide attempt was higher in patients with recently diagnosed MCI and those with recently diagnosed dementia compared with those without MCI or dementia, after adjusting for demographic characteristics and medical and psychiatric comorbidities.
Meaning
The finding of increased risk of suicide attempt in individuals with recent diagnosis of MCI or dementia suggests the importance of supportive services soon after diagnosis.
This cohort study examines the association between diagnoses of mild cognitive impairment or dementia and suicide attempt, explores potential psychiatric moderators, and assesses whether the association differs based on recency of diagnosis in 5 US databases.
Abstract
Importance
Little is known about the association between mild cognitive impairment (MCI) and suicide. Most studies have focused on dementia and suicidal behavior, with inconsistent results.
Objectives
To examine the association between diagnoses of MCI and dementia and suicide attempt and explore potential psychiatric moderators and to assess whether the association differs based on recency of diagnosis.
Design, Setting, and Participants
This nationwide cohort study integrated 5 national databases from the Department of Veterans Affairs (VA) and Centers for Medicare & Medicaid Services and included all VA medical centers in the US. US veterans 50 years or older with MCI diagnoses at baseline (October 1, 2011, to September 30, 2013) or earlier (October 1, 2007, to September 30, 2011) were propensity matched 1:3 with (1) patients with dementia diagnoses and (2) patients without either diagnosis based on demographic characteristics and the Charlson Comorbidity Index. Diagnoses of MCI or dementia were defined as recent if there were no diagnosis codes before baseline. Data were analyzed from March 16, 2020, to January 15, 2021.
Main Outcomes and Measures
Information on suicide attempts through December 31, 2016, provided by the National Suicide Prevention Applications Network (nonfatal) and Mortality Data Repository (fatal).
Results
The study population of 147 595 participants included 21 085 patients with MCI, 63 255 with dementia, and 63 255 in the propensity-matched comparison group. Participants had a mean (SD) age of 74.7 (10.3) years, 143 353 (97.1%) were men, 4242 (2.9%) were women, and 127 065 (86.1%) were non-Hispanic White. A total of 138 patients with MCI (0.7%) and 400 patients with dementia (0.6%) attempted suicide during follow-up, compared with 253 patients without MCI or dementia (0.4%). Exploratory analyses revealed that no psychiatric comorbidity moderated the association between MCI or dementia and suicide attempt. After adjustment for demographic details and medical and psychiatric comorbidities, risk of suicide attempt was consistently highest for patients with a recent MCI or dementia diagnosis, with adjusted hazard ratios (HRs) of 1.73 (95% CI, 1.34-2.22; P < .001) for recent MCI and 1.44 (95% CI, 1.17-1.77; P = .001) for recent dementia. Risk associated with prior diagnosis was not significant (HR for prior MCI, 1.03 [95% CI, 0.78-1.36; P = .84]; HR for prior dementia, 1.14 [95% CI, 0.95-1.36; P = .15]).
Conclusions and Relevance
This study found that older adults with recent MCI or dementia diagnoses were at increased risk of attempting suicide. These findings suggest that involvement of supportive services at the time of or soon after diagnoses of MCI or dementia may help mitigate risk of suicide attempts.
Introduction
Suicide in late life is a significant public health concern, with greater lethality among older adults compared with younger groups.1,2,3 Although many potential risk factors have been examined,4 it remains unclear what role cognitive impairment, such as mild cognitive impairment (MCI) and dementia, may play in increasing risk of attempting and dying by suicide.
Mild cognitive impairment, a relatively new clinical construct for which criteria were first introduced in 1999,5 has been less studied as a potential risk factor for suicide in late life. As a heterogeneous diagnosis of cognitive well-being that often includes psychiatric symptoms, MCI could be a unique indicator of underlying distress6,7,8,9,10 that may be highly associated with suicide risk. To our knowledge, the association between MCI and suicide has not been studied in a national sample. In addition, the association between dementia and suicidal behavior remains poorly understood. Some studies have found no association11,12,13 or even a lower risk of suicide in those with dementia,14,15 whereas other studies15,16,17,18 have found an increased risk of suicide, particularly in patients recently diagnosed with dementia. Prior literature reviews19,20 concluded that risk of suicide is primarily increased in those who have dementia combined with depression, but other psychiatric comorbidities have not been examined as potential effect modifiers, despite evidence suggesting high occurrence in patients with MCI or dementia.21,22,23
The purpose of our study was to use national data from the Veterans Affairs (VA) Health Care System to examine (1) the association between MCI and dementia and the risk of suicide attempt, including conducting exploratory analyses examining common psychiatric disorders as potential moderators, and (2) whether recency of MCI or dementia diagnoses affects the association with suicide risk. In addition, we investigated whether respective associations hold when exclusively examining death by suicide as an outcome.
Methods
Data and Participants
We conducted a longitudinal cohort study with baseline from October 1, 2011, to September 30, 2013, and follow-up through December 31, 2016, integrating 5 national databases: (1) the VA’s National Patient Care Database, containing inpatient and outpatient records; (2) Centers for Medicare & Medicaid Services data, including medical claims and diagnoses; (3) the National Suicide Prevention Applications Network, including information about nonfatal suicide attempts24,25,26; (4) the Mortality Data Repository, containing cause-specific mortality data27,28; and (5) the VA’s Pharmacy Managerial Cost Accounting National Data Extract,29,30 providing information on medications prescribed. Demographic information including age, sex, and race/ethnicity was extracted from the National Patient Care Database and/or Centers for Medicare & Medicaid Services database records. Levels of education and income were extrapolated from 2012 census data based on residential zip code. The institutional review board of the University of California, San Francisco, and the Research and Development Committee of the San Francisco VA Health Care System approved this study. Informed consent was waived because the data were already collected medical record data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The initial cohort included all patients 50 years or older who used VA health care services at least once during fiscal years 2012 to 2013 (baseline) and at least once prior during fiscal years from October 1, 2007, to September 30, 2011. Then, we extracted all patients who had been diagnosed with MCI during or before baseline and performed 1:3 propensity matching using demographic characteristics (ie, age, sex, race/ethnicity, income, and educational level) and the Charlson Comorbidity Index (CCI)31 to create comparison samples of patients diagnosed with dementia and patients without either diagnosis during this time period. Propensity score matching with no replacement was conducted using nearest-neighbor caliper matching with caliper width of 0.2 SDs of the logit of the propensity score using Stata/MP, version 16.1 (StataCorp LLC).
For patients who were diagnosed with MCI or dementia during baseline, the index date was defined as date of first diagnosis. For patients who were diagnosed with MCI or dementia before baseline and patients without MCI or dementia diagnoses, the index date was defined as the first date they came to a VA facility during the baseline period.
Measures
MCI and Dementia
Mild cognitive impairment and dementia were determined using codes from the International Classification of Diseases, Ninth Revision (ICD-9), in the National Patient Care Database and/or Centers for Medicare & Medicaid Services databases (MCI, 331.83; any dementia, including the following: Alzheimer disease, 331.0; vascular dementia, 290.4; frontotemporal dementia, 331.1; Lewy body dementia, 331.82; dementia not otherwise specified, 294.2; senile dementia, 290.0-290.3; alcohol- and drug-induced dementia, 291.2 and 292.82, respectively; dementia in conditions classified elsewhere, 294.1; Creutzfeldt-Jakob disease, 046.1; progressive multifocal leukoencephalopathy, 046.3; and other and unspecified prion disease of central nervous system, 046.79). To increase specificity, we required participants to have ICD-9 dementia-related codes on at least 2 different occasions or a single dementia diagnosis code in combination with prescription of dementia medications (ie, donepezil, memantine, rivastigmine, and galantamine). Participants who were diagnosed with both MCI and dementia were assigned to the dementia group. We classified MCI or dementia diagnoses only during baseline (fiscal years 2012-2013) as recent and diagnoses from fiscal years 2008 to 2011 as prior. Patients who received both prior and recent diagnoses were assigned as having prior diagnosis. Patients who had not received an MCI or a dementia diagnosis or been prescribed dementia medication from fiscal years 2008 to 2013 were eligible for the propensity-matched comparison control group.
Fatal and Nonfatal Suicide Attempt
Data on suicide attempts were provided by the national Mortality Data Repository (fatal attempt) and National Suicide Prevention Applications Network (nonfatal attempt) databases. We used codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, to determine death by suicide (ie, fatal attempt), including X60 to X84 and Y87.0. The National Suicide Prevention Applications Network is the VA’s internal suicide event case management and tracking system, recording standardized information on serious suicidal behavior. Suicide prevention coordinators and clinicians compile individual-level case records for suicide events based on reports in VA medical facilities24,25,26,28 using the Centers for Disease Control and Prevention’s Self-directed Violence Classification System.32 Participants who had a documented nonfatal suicide attempt and subsequently died by suicide were assigned to the latter.
Medical and Psychiatric Diagnoses
We used ICD-9 codes included in the National Patient Care Database and/or Centers for Medicare & Medicaid Services database records to identify common and major medical and psychiatric disorders diagnosed within 2 years before the index date. We used the CCI to assess medical comorbidities, which includes 17 chronic medical disorders weighted depending on associated mortality risk.31 We excluded dementia, one of the factors, from the CCI score. Psychiatric disorders included depression, posttraumatic stress disorder, generalized anxiety disorder, substance use disorders (ie, alcohol abuse and drug abuse), and sleep disorder. A history of suicide ideation and attempt was determined using ICD-9 codes (V62.84 and E950-E959) as well as the National Suicide Prevention Applications Network database.
Statistical Analysis
Data were analyzed from March 16, 2020, to January 15, 2021. We examined cumulative incidence of any suicide attempt (ie, fatal and nonfatal combined) and of death by suicide as a function of follow-up time graphically and used Fine-Gray proportional hazards regression to estimate time to any suicide attempt while accounting for competing risk of death with follow-up time as the timescale and follow-up censored at December 31, 2016.33 We performed exploratory moderation analyses to assess whether associations between MCI or dementia and suicide attempt differed for participants with depression, posttraumatic stress disorder, generalized anxiety disorder, substance abuse disorders, or sleep disorder by including interaction terms in Fine-Gray models (considering any psychiatric disorder and each psychiatric diagnosis individually as potential moderators).
We then estimated hazard ratios (HRs) for the overall association between MCI and dementia and suicide attempt and differentiated between recent and prior diagnoses. Analyses were unadjusted and then adjusted for covariates in steps for demographic characteristics and CCI (model 1) and for demographic characteristics, CCI, and any psychiatric disorder (model 2).
Analyses were repeated to investigate associations between MCI and dementia and death by suicide. In addition, we conducted sensitivity analyses adjusting for an ordinal (instead of binary) variable of count of psychiatric disorders (0, 1, 2, and ≥3) to assess whether multimorbidity of psychiatric disorders meaningfully affected the associations. Fine-Gray assumptions were tested statistically and graphically and were met for all models. SAS, version 9.4 (SAS Institute Inc) and Stata/MP, version 16.1, were used for the analyses, with statistical tests using a 2-tailed P < .05 to indicate statistical significance.
Results
Sample Characteristics
The final cohort included 147 595 patients (21 085 with MCI; 63 255 with dementia; and 63 255 without MCI or dementia). Patients with and without MCI or dementia were well matched with respect to measured characteristics (Table 1). Study participants had a mean (SD) age of 74.7 (10.3) years at their index date; 143 353 (97.1%) were men and 4242 (2.9%) were women. The race/ethnicity distribution was 127 065 (86.1%) non-Hispanic White, 15 852 (10.7%) non-Hispanic Black, 806 (0.5%) Hispanic, and 3872 (2.6%) other or unknown. Most participants (117 226 [79.4%]) had a score of at least 1 on the CCI, with a mean (SD) score of 2.7 (2.4). Median follow-up time was 4.2 (range, 0.04-5.3) years.
Table 1. Baseline Characteristics by MCI and Dementia Statusa.
| Characteristic | Patient group | ||
|---|---|---|---|
| No MCI or dementia (n = 63 255) | MCI (n = 21 085) | Dementia (n = 63 255) | |
| Age, mean (SD), y | 74.7 (10.3) | 74.7 (10.4) | 74.7 (10.3) |
| Age group, y | |||
| 50-64 | 12 692 (20.1) | 4228 (20.1) | 12 627 (20.0) |
| 65-79 | 26 483 (41.9) | 8825 (41.9) | 26 494 (41.9) |
| ≥80 | 24 080 (38.1) | 8032 (38.1) | 24 134 (38.2) |
| Female | 1961 (3.1) | 660 (3.1) | 1621 (2.6) |
| Race/ethnicity | |||
| Non-Hispanic White | 54 493 (86.1) | 18 152 (86.1) | 54 420 (86.0) |
| Non-Hispanic Black | 6646 (10.5) | 2222 (10.5) | 6984 (11.0) |
| Hispanic | 385 (0.6) | 134 (0.6) | 287 (0.5) |
| Other or unknown | 1731 (2.7) | 577 (2.7) | 1564 (2.5) |
| Live in area with >25% college educational level | 29 954 (47.4) | 9973 (47.3) | 30 386 (48.0) |
| Median annual income | |||
| <$41 721 | 18 230 (28.8) | 6089 (28.9) | 18 285 (28.9) |
| $41 721-$54 784 | 20 694 (32.7) | 6897 (32.7) | 20 731 (32.8) |
| >$54 784 | 24 331 (38.5) | 8099 (38.4) | 24 239 (38.3) |
| Charlson Comorbidity Index | |||
| Mean (SD) | 2.7 (2.5) | 2.7 (2.4) | 2.7 (2.4) |
| 0 | 13 056 (20.6) | 4355 (20.7) | 12 958 (20.5) |
| 1 | 11 514 (18.2) | 3834 (18.2) | 11 493 (18.2) |
| 2 | 11 403 (18.0) | 3795 (18.0) | 11 305 (17.9) |
| 3 | 8230 (13.0) | 2743 (13.0) | 8422 (13.3) |
| 4 | 6307 (10.0) | 2103 (10.0) | 6446 (10.2) |
| 5 | 4225 (6.7) | 1413 (6.7) | 4255 (6.7) |
| ≥6 | 8520 (13.5) | 2842 (13.5) | 8376 (13.2) |
Abbreviation: MCI, mild cognitive impairment.
Includes 147 595 patients. Patients with and without MCI and dementia were matched using propensity scores on demographic characteristics (age, sex, race/ethnicity, education, income) and Charlson Comorbidity Index. Differences between groups are not clinically meaningful. Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages have been rounded and may not total 100.
Even after propensity matching for demographic details and CCI, we observed large differences in the prevalence of psychiatric comorbidities across study groups. Prevalence of psychiatric conditions was higher in both the MCI (10 807 [51.3%] and dementia (34 289 [54.2%]) groups compared with the group without dementia or MCI (18 988 [30.0%]) and consistently highest in patients with dementia (Table 2).
Table 2. Psychiatric Comorbidities at Baseline by MCI and Dementia Statusa.
| Psychiatric comorbidity | Patient group | ||
|---|---|---|---|
| No MCI or dementia (n = 63 255) | MCI (n = 21 085) | Dementia (n = 63 255) | |
| Any | 18 988 (30.0) | 10 807 (51.3) | 34 289 (54.2) |
| Depression | 10 483 (16.6) | 7433 (35.3) | 25 451 (40.2) |
| Posttraumatic stress disorder | 5064 (8.0) | 3158 (15.0) | 8340 (13.2) |
| Generalized anxiety disorder | 1208 (1.9) | 949 (4.5) | 2744 (4.3) |
| Alcohol or drug abuse | 4790 (7.6) | 2217 (10.5) | 9201 (14.5) |
| Alcohol abuse | 3886 (6.1) | 1816 (8.6) | 7775 (12.3) |
| Drug abuse | 1882 (3.0) | 936 (4.4) | 3623 (5.7) |
| Sleep disturbances/disorders | 5711 (9.0) | 3509 (16.6) | 7871 (12.4) |
| History of suicidal ideation | 581 (0.9) | 495 (2.3) | 2699 (4.3) |
| History of suicide attempt | 51 (0.1) | 65 (0.3) | 234 (0.4) |
Abbreviation: MCI, mild cognitive impairment.
Includes 147 595 patients. Comorbidities were assessed 2 years before index date; P values for differences among no MCI or dementia, dementia, and MCI are all significant at P < .05 based on χ2 tests.
Dementia, MCI, and Suicide Attempt
A total of 138 patients with MCI (0.7%) and 400 patients with dementia (0.6%) attempted suicide during follow-up, compared with 253 patients without MCI or dementia (0.4%). The moderation analyses revealed that neither individual psychiatric comorbidities nor having any psychiatric disorder were statistically significant moderators in the association between MCI or dementia and suicide attempt. In the fully adjusted model, the risk of suicide attempt was approximately 1.2 to 1.3 times higher in patients with MCI (HR, 1.34; 95% CI, 1.09-1.65; P = .005) or dementia diagnoses (HR, 1.23; 95% CI, 1.05-1.44; P = .01).
However, the associations between MCI and dementia diagnoses and suicide attempt differed strongly based on the recency of the diagnosis (Figure). After adjusting for demographic details, CCI, and any psychiatric comorbidity, the risk of suicide attempt was 73% and 44% greater in patients with a recent diagnosis in the MCI and dementia groups, respectively (HR for MCI, 1.73; 95% CI, 1.34-2.22; P < .001; HR for dementia, 1.44; 95% CI, 1.17-1.77; P = .001), but not in those with a prior diagnosis (HR for MCI, 1.03; 95% CI, 0.78-1.36; P = .84; HR for dementia, 1.14; 95% CI, 0.95-1.36; P = .15) relative to the comparison group (Table 3).
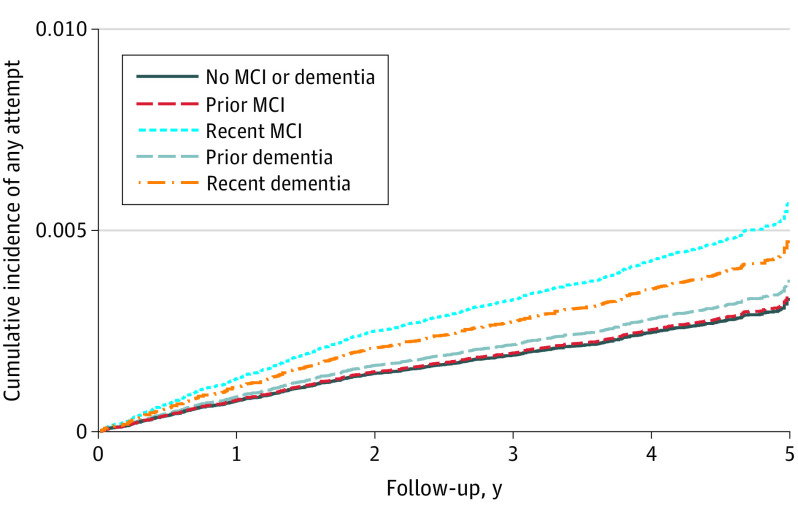
Figure. Adjusted Cumulative Incidence of Any Suicide Attempt by Recency of Mild Cognitive Impairment (MCI) or Dementia Diagnosis.
The adjusted cumulative incidence of suicide attempt (fatal and nonfatal) based on follow-up time is shown as a function of MCI or dementia diagnosis. After adjustment for demographic characteristics, the Charlson Comorbidity Index, and comorbid psychiatric disorders, suicide attempt rates were consistently higher for patients with a recent diagnosis of MCI or dementia with hazard ratios of 1.73 (95% CI, 1.34-2.22; P < .001 [n = 11 117]) for recent MCI and 1.03 (95% CI, 0.78-1.36; P = .84 [n = 9968]) for prior MCI diagnosis, 1.44 (95% CI, 1.17-1.77, P = .001 [n = 22 270]) for recent dementia, and 1.14 (95% CI, 0.95-1.36; P = .15 [n = 40 985]) for prior dementia diagnosis compared with no MCI or dementia (n = 63 255).
Table 3. Unadjusted and Adjusted Risk of Any Suicide Attempt and Death by Suicide by Recency of MCI/Dementia Diagnosisa.
| Model | HR (95% CI) | ||
|---|---|---|---|
| Unadjusted model | Model 1b | Model 2c | |
| Recency of diagnosis associated with any suicide attempt (fatal or nonfatal) | |||
| No MCI or dementia (n = 63 255) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Prior MCI (n = 9968) | 1.44 (1.09-1.89)d | 1.34 (1.02-1.78)d | 1.03 (0.78-1.36) |
| Recent MCI (n = 11 117) | 2.00 (1.56-2.57)e | 2.10 (1.64-2.70)e | 1.73 (1.34-2.22)e |
| Prior dementia (n = 40 985) | 1.52 (1.28-1.81)e | 1.50 (1.26-1.79)e | 1.14 (0.95-1.36) |
| Recent dementia (n = 22 270) | 1.66 (1.35-2.04)e | 1.72 (1.40-2.12)e | 1.44 (1.17-1.77)d |
| Recency of diagnosis associated with death by suicide | |||
| No MCI or dementia (n = 63 255) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Prior MCI (n = 9968) | 1.11 (0.69-1.81) | 1.10 (0.68-1.79) | 0.92 (0.57-1.51) |
| Recent MCI (n = 11 117) | 1.10 (0.68-1.79) | 1.12 (0.69-1.82) | 0.99 (0.61-1.61) |
| Prior dementia (n = 40 985) | 0.78 (0.57-1.08) | 0.78 (0.56-1.07) | 0.64 (0.46-0.89)d |
| Recent dementia (n = 22 270) | 0.93 (0.63-1.37) | 0.95 (0.64-1.39) | 0.85 (0.57-1.25) |
Abbreviations: HR, hazard ratio; MCI, mild cognitive impairment.
Includes 147 595 patients.
Adjusted for demographic characteristics (age, sex, race/ethnicity, income, and educational level) and Charlson Comorbidity Index.
Adjusted for demographic characteristics, Charlson Comorbidity Index, and any psychiatric disorder (depression, posttraumatic stress disorder, generalized anxiety disorder, alcohol abuse, drug abuse, and sleep disorder).
P < .05 compared with reference group.
P < .001 compared with reference group.
Dementia, MCI, and Death by Suicide
Death by suicide occurred in 38 patients with MCI (0.2%), 88 patients with dementia (0.1%), and 110 patients of the matched comparison sample (0.2%). Firearms were the most commonly used method for death by suicide across the 3 study groups (MCI group, 26 [68.4%]; dementia group, 66 [75.0%]; comparison group, 89 [80.9%]; P = .26).
Although patients with recent diagnoses of MCI or dementia were more likely to attempt suicide, they were not more likely to die by suicide. The fully adjusted risk of death by suicide in the MCI group was not significantly different from the comparison sample (HR, 0.96; 95% CI, 0.66-1.39; P = .82), and the dementia group had a 29% lower risk of death by suicide (HR, 0.71; 95% CI, 0.53-0.94; P = .02). The reduced risk of death by suicide in patients with dementia (36%) was restricted to patients with a prior diagnosis (HR, 0.64; 95% CI, 0.46-0.89; P = .007), and adjusted HRs for death by suicide in the other groups were not statistically different from the comparison group (Table 3). In the sensitivity analyses, although the risk of suicide attempt was generally reduced after adjusting for psychiatric multimorbidity, results did not meaningfully change with regard to recency of MCI or dementia diagnoses and risk of suicide attempt or death by suicide.
Discussion
In a cohort of 147 595 older veterans, risk of suicide attempt was 73% higher in patients recently diagnosed with MCI and 44% higher in those recently diagnosed with dementia, compared with those without MCI or dementia diagnoses, independent of demographic characteristics and medical and psychiatric comorbidities. In contrast, there was no association for prior diagnoses of MCI or dementia with suicide attempts. In addition, those with a dementia diagnosis had a statistically significant 29% reduced risk of death by suicide. This finding was likely driven by recency of diagnoses, because patients with prior dementia diagnoses had a 36% lower risk of dying by suicide. To our knowledge, this study is the first to investigate these associations in a national cohort.
Prior studies that have examined MCI as a risk factor for suicide attempts have been preliminary and inconsistent. A case-control study including 214 older psychiatric inpatients34 found that cognitive impairment or mild dementia (defined using the Mini-Mental State Examination) were frequently present among individuals who attempted suicide. Another case-control study with a smaller sample,35 however, found no association between cognitive impairment (assessed with the Mini-Mental State Examination, Cognitive Abilities Screening Instrument, and a diagnostic interview) and suicide attempt. Our findings are consistent with a recent cohort study11 that found risk of death by suicide was not significantly increased among 4260 outpatients clinically diagnosed with MCI.
Our finding that especially recent dementia diagnosis is associated with elevated risk of suicide attempt is in line with previous studies.15,16,17,18 In a large prospective cohort study including nearly 2.5 million individuals, Erlangsen and colleagues16 found that risk of death by suicide was significantly elevated during the first 6 months after a hospital diagnosis of dementia compared with a longer diagnosis time. Similarly, in a recent Danish nationwide retrospective cohort study,15 risk of death by suicide was 3 times higher within the first month after dementia diagnosis but reduced in those ever diagnosed with dementia, compared with those without a neurological disorder.
Several potential mechanisms may explain the observed associations. First, patients who were recently diagnosed with MCI or dementia may have preserved insight into what such a diagnosis entails, anticipating progressive cognitive and functional decline, fearing loss of autonomy, and worrying that they become a burden to significant others.36,37 This is in line with current prevailing interpersonal theory of suicide, in which perceived burdensomeness on others is 1 of 2 crucial interpersonal constructs assumed to influence desire to die by suicide,38 possibly by eroding one’s sense of meaning in life.39 Such insight and perception might be especially maintained in those with MCI, potentially explaining the slightly greater risk of suicide attempt in this group.
Second, although cognition may allow patients to plan and implement a suicide attempt at the early stages of MCI and dementia, functioning may decline with the progression of MCI to dementia.37,40,41 Our findings suggest that a prior diagnosis of dementia, representing the most severe cognitive and functional decline of all 4 groups (ie, recent and prior MCI and dementia diagnosis), may protect an individual from death by suicide.
Third, one reason for significantly increased risk of suicide attempts but not death by suicide in newly diagnosed MCI or dementia may lie in access to or use of lethal means. Although differences in firearm use across study groups were nonsignificant, the comparison group appeared to use firearms particularly often. Patients with prior diagnoses of MCI or dementia may not only be less likely to prepare and carry out a suicide plan but also choose less lethal means. In addition, caregivers may remove or monitor access to firearms, or patients with dementia may live in residential homes where guns are not allowed.42
Last, increased prevalence of psychiatric comorbidities may be linked to elevated risk of attempting suicide in patients with MCI or dementia. Although we did not find significant moderators, power may have been insufficient to detect significant interactions. Nevertheless, prevalence rates of psychiatric comorbidities were noticeably greater in the MCI and dementia groups relative to the matched comparison group. In addition, risk for either outcome was reduced when adjusting for common psychiatric comorbidities. Mild cognitive impairment may or may not progress to dementia. Some individuals with MCI have preclinical dementia (ie, prodrome of dementia), whereas others remain stable or even revert to a status of normal cognition.6,8 Thus, as a prognostic entity for suicide risk, MCI is likely important in and of itself.
Implications
Our findings have important public health and policy implications. Being diagnosed with MCI or dementia often is a profound life-changing event for which no disease-modifying treatment exists.43 It may be helpful to provide continuous individualized postdiagnostic support tailored to the patient’s needs, including education and access to supportive services (eg, social events to reduce isolation or advance care planning).44,45 In addition, patients with dementia often have poor access to advance care planning, potentially owing to public and self-stigmatization, lack of skills, and delegation of responsibility among health care providers.45,46,47 Such services may help individuals affected to come to terms with their diagnosis sooner rather than later. In addition, our findings suggest that screening, informing, and possibly restricting access to firearms for individuals with dementia should occur early and at the time of diagnosis, not only when cognitive impairment has progressed or crossed a particular threshold.
The findings indicate that psychological distress and neuropsychiatric symptoms, including depression and anxiety, should be considered important factors in age-related cognitive decline.48 Early detection, treatment, and management of these symptoms through mental health services may help to reduce the risk of attempting suicide in patients with MCI or dementia.17 A recent meta-analysis found a positive and salient effect of psychological treatments (eg, cognitive-behavioral therapy) on improving depression and clinician-rated anxiety in people with dementia, suggesting the potential to enhance well-being.49 To date, a diagnosis of dementia may negate receipt of such mental health support, because providers may erroneously believe the patient’s mental health conditions will not improve.50
Strengths and Limitations
Our study has several important strengths. We used a large, nationally based sample of older adults with clinical diagnoses of MCI, dementia, and medical and psychiatric comorbidities, enabling us to examine potential moderators and adjust for important confounders. We used propensity matching to further minimize potential confounding; we examined clinically documented suicide attempts as well as death by suicide, including event dates even for nonfatal attempts; and we considered the recency of MCI and dementia diagnoses.
There are limitations to this study. As with all observational studies, causation cannot be inferred. Importantly, immortal time bias51,52,53,54 may limit interpretability of the nonrecent MCI and dementia categories, given they were defined before baseline. This would, for example, exclude those who died by suicide before baseline. This limitation should be considered when interpreting our results for death by suicide and suicide attempt, including biases in either direction. Moreover, we want to emphasize that our study investigated timing of diagnosis, not course or stage of illness itself, and one does not necessarily reflect the other. The sample included mainly male veterans, and it is not clear whether results are generalizable to the general population and especially women. We used administrative data and cannot be sure that consistent definitions and diagnostic criteria were used across clinical staff. Especially for MCI, diagnostic criteria have been controversial and changed over time.6,8 We cannot exclude the possibility that MCI diagnoses were secondary symptoms to other conditions. However, we controlled for psychiatric comorbidities. Precise timing of onset of MCI, dementia, or psychiatric comorbidities could not be ensured. In addition, through propensity matching, counts of outcomes were reduced, possibly resulting in insufficient power to detect moderators. Although there may be a potential limitation in interpreting the comparison of the MCI and dementia groups given similar ages at diagnoses imbedded in the propensity matching, we additionally adjusted for age in the multivariable models to help overcome this. The present study did not include other potential risk factors for suicide that may have direct, confounding, or moderating effects on suicide attempt, such as social isolation, including loneliness,38 or assessment of brain injury.55,56
Conclusions
The findings in this cohort study suggest that patients who have recently been diagnosed with MCI or dementia should be viewed as a high-risk group for suicide attempt. Additional supportive services in the care of patients with MCI or dementia are imperative, especially around the time of initial diagnosis. Future studies are needed to confirm our findings, to identify reasons underlying the associated suicide risk in patients with MCI and dementia, and to investigate interventions that will effectively reduce this risk.
References
- 1.McKeown RE, Cuffe SP, Schulz RM. US suicide rates by age group, 1970-2002: an examination of recent trends. Am J Public Health. 2006;96(10):1744-1751. doi: 10.2105/AJPH.2005.066951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drapeau CW, McIntosh JL. USA suicide: 2018 official final data. Obtained February 12, 2020. Accessed May 23, 2020. https://suicidology.org/wp-content/uploads/2020/02/2018datapgsv2_Final.pdf
- 3.Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999-2018. NCHS Data Brief. 2020;(362):1-8. [PubMed] [Google Scholar]
- 4.Conwell Y, Duberstein PR, Caine ED. Risk factors for suicide in later life. Biol Psychiatry. 2002;52(3):193-204. doi: 10.1016/S0006-3223(02)01347-1 [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303-308. doi: 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 6.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240-246. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. doi: 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126-135. doi: 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753-772. doi: 10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447-1455. doi: 10.1001/archneurol.2009.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An JH, Lee KE, Jeon HJ, Son SJ, Kim SY, Hong JP. Risk of suicide and accidental deaths among elderly patients with cognitive impairment. Alzheimers Res Ther. 2019;11(1):32. doi: 10.1186/s13195-019-0488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiktorsson S, Runeson B, Skoog I, Östling S, Waern M. Attempted suicide in the elderly: characteristics of suicide attempters 70 years and older and a general population comparison group. Am J Geriatr Psychiatry. 2010;18(1):57-67. doi: 10.1097/JGP.0b013e3181bd1c13 [DOI] [PubMed] [Google Scholar]
- 13.Borges G, Acosta I, Sosa AL. Suicide ideation, dementia and mental disorders among a community sample of older people in Mexico. Int J Geriatr Psychiatry. 2015;30(3):247-255. doi: 10.1002/gps.4134 [DOI] [PubMed] [Google Scholar]
- 14.Erlangsen A, Zarit SH, Tu X, Conwell Y. Suicide among older psychiatric inpatients: an evidence-based study of a high-risk group. Am J Geriatr Psychiatry. 2006;14(9):734-741. doi: 10.1097/01.JGP.0000225084.16636.ec [DOI] [PubMed] [Google Scholar]
- 15.Erlangsen A, Stenager E, Conwell Y, et al. Association between neurological disorders and death by suicide in Denmark. JAMA. 2020;323(5):444-454. doi: 10.1001/jama.2019.21834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erlangsen A, Zarit SH, Conwell Y. Hospital-diagnosed dementia and suicide: a longitudinal study using prospective, nationwide register data. Am J Geriatr Psychiatry. 2008;16(3):220-228. doi: 10.1097/01.JGP.0000302930.75387.7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annor FB, Bayakly RA, Morrison RA, et al. Suicide among persons with dementia, Georgia, 2013 to 2016. J Geriatr Psychiatry Neurol. 2019;32(1):31-39. doi: 10.1177/0891988718814363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyfried LS, Kales HC, Ignacio RV, Conwell Y, Valenstein M. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi: 10.1016/j.jalz.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haw C, Harwood D, Hawton K. Dementia and suicidal behavior: a review of the literature. Int Psychogeriatr. 2009;21(3):440-453. doi: 10.1017/S1041610209009065 [DOI] [PubMed] [Google Scholar]
- 20.Diehl-Schmid J, Jox R, Gauthier S, et al. Suicide and assisted dying in dementia: what we know and what we need to know: a narrative literature review. Int Psychogeriatr. 2017;29(8):1247-1259. doi: 10.1017/S1041610217000679 [DOI] [PubMed] [Google Scholar]
- 21.Mirza SS, Ikram MA, Bos D, Mihaescu R, Hofman A, Tiemeier H. Mild cognitive impairment and risk of depression and anxiety: a population-based study. Alzheimers Dement. 2017;13(2):130-139. doi: 10.1016/j.jalz.2016.06.2361 [DOI] [PubMed] [Google Scholar]
- 22.Lai AX, Kaup AR, Yaffe K, Byers AL. High occurrence of psychiatric disorders and suicidal behavior across dementia subtypes. Am J Geriatr Psychiatry. 2018;26(12):1191-1201. doi: 10.1016/j.jagp.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 23.Rozzini L, Chilovi BV, Peli M, et al. Anxiety symptoms in mild cognitive impairment. Int J Geriatr Psychiatry. 2009;24(3):300-305. doi: 10.1002/gps.2106 [DOI] [PubMed] [Google Scholar]
- 24.Hoffmire C, Stephens B, Morley S, Thompson C, Kemp J, Bossarte RM. VA suicide prevention applications network: a national health care system–based suicide event tracking system. Public Health Rep. 2016;131(6):816-821. doi: 10.1177/0033354916670133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemp J, Bossarte R. Suicide data report, 2012. In: Veteran Suicide: Data, Analysis, and VHA Suicide Prevention Efforts. Published May 2013. Accessed March 20, 2020. https://www.va.gov/opa/docs/suicide-data-report-2012-final.pdf
- 26.Kemp J, Bossarte RM. Surveillance of suicide and suicide attempts among veterans: addressing a national imperative. Am J Public Health. 2012;102(suppl 1):e4-e5. doi: 10.2105/AJPH.2012.300652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Defense Suicide Prevention Office (DSPO) . Suicide Data Repository (SDR) fact sheet. Accessed March 20, 2020. https://www.dspo.mil/Portals/113/Documents/SDR%20Fact%20Sheet.pdf
- 28.United States Department of Veterans Affairs, Office of Mental Health and Suicide Prevention . Suicide among veterans and other Americans 2001-2014. Updated August 3, 2016. Accessed March 20, 2020. https://www.sprc.org/sites/default/files/resource-program/2016suicidedatareport.pdf
- 29.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):92S-123S. doi: 10.1177/1077558703256726 [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Veteran Affairs . Pharmacy data. Updated February 5, 2021. Accessed March 31, 2020. https://www.herc.research.va.gov/include/page.asp?id=pharmacy
- 31.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 32.Crosby AE, Ortega L, Melanson C. Self-directed violence surveillance: uniform definitions and recommended data elements, Version 1.0. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Published February 2011. Accessed March 20, 2020. https://stacks.cdc.gov/view/cdc/11997
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 34.Osvath P, Kovacs A, Voros V, Fekete S. Risk factors of attempted suicide in the elderly: the role of cognitive impairment. Int J Psychiatry Clin Pract. 2005;9(3):221-225. doi: 10.1080/13651500510029020 [DOI] [PubMed] [Google Scholar]
- 35.Liu IC, Chiu CH. Case-control study of suicide attempts in the elderly. Int Psychogeriatr. 2009;21(5):896-902. doi: 10.1017/S1041610209990056 [DOI] [PubMed] [Google Scholar]
- 36.Cipriani G, Vedovello M, Lucetti C, Di Fiorino A, Nuti A. Dementia and suicidal behavior. Aggress Violent Behav. 2013;18(6):656-659. doi: 10.1016/j.avb.2013.07.016 [DOI] [Google Scholar]
- 37.Conejero I, Navucet S, Keller J, Olié E, Courtet P, Gabelle A. A complex relationship between suicide, dementia, and amyloid: a narrative review. Front Neurosci. 2018;12:371. doi: 10.3389/fnins.2018.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE Jr. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575-600. doi: 10.1037/a0018697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Orden KA, Bamonti PM, King DA, Duberstein PR. Does perceived burdensomeness erode meaning in life among older adults? Aging Ment Health. 2012;16(7):855-860. doi: 10.1080/13607863.2012.657156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Draper B, Peisah C, Snowdon J, Brodaty H. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement. 2010;6(1):75-82. doi: 10.1016/j.jalz.2009.04.1229 [DOI] [PubMed] [Google Scholar]
- 41.Bélanger S, Belleville S, Gauthier S. Inhibition impairments in Alzheimer’s disease, mild cognitive impairment and healthy aging: effect of congruency proportion in a Stroop task. Neuropsychologia. 2010;48(2):581-590. doi: 10.1016/j.neuropsychologia.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 42.Betz ME, Azrael D, Johnson RL, et al. Views on firearm safety among caregivers of people with Alzheimer disease and related dementias. JAMA Netw Open. 2020;3(7):e207756. doi: 10.1001/jamanetworkopen.2020.7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 44.Kelly F, Innes A. Facilitating independence: the benefits of a post-diagnostic support project for people with dementia. Dementia (London). 2016;15(2):162-180. doi: 10.1177/1471301214520780 [DOI] [PubMed] [Google Scholar]
- 45.Sampson EL. Palliative care for people with dementia. Br Med Bull. 2010;96:159-174. doi: 10.1093/bmb/ldq024 [DOI] [PubMed] [Google Scholar]
- 46.Robinson L, Dickinson C, Bamford C, Clark A, Hughes J, Exley C. A qualitative study: professionals’ experiences of advance care planning in dementia and palliative care, ‘a good idea in theory but ...’. Palliat Med. 2013;27(5):401-408. doi: 10.1177/0269216312465651 [DOI] [PubMed] [Google Scholar]
- 47.Mukadam N, Livingston G. Reducing the stigma associated with dementia: approaches and goals. Aging Health. 2012;8(4):377-386. doi: 10.2217/ahe.12.42 [DOI] [Google Scholar]
- 48.Simard M, Hudon C, van Reekum R. Psychological distress and risk for dementia. Curr Psychiatry Rep. 2009;11(1):41-47. doi: 10.1007/s11920-009-0007-z [DOI] [PubMed] [Google Scholar]
- 49.Orgeta V, Qazi A, Spector A, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298. doi: 10.1192/bjp.bp.114.148130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dick-Muehlke C. Mental health services for Californians with Alzheimer’s disease. Published 2016. Accessed October 10, 2020. https://chhs-data-prod.s3.us-west-2.amazonaws.com/uploads/2019/09/Mental-Health-Access-for-Persons-with-Dementia.pdf
- 51.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 52.Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19(5):841-843. doi: 10.1681/ASN.2007121354 [DOI] [PubMed] [Google Scholar]
- 53.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31(2):125-130. doi: 10.1111/tri.13081 [DOI] [PubMed] [Google Scholar]
- 54.Catalogue of Bias Collaboration . Lee H, Nunan D. Immortal time bias. In: Catalogue of Bias 2020. Accessed January 17, 2021. https://catalogofbias.org/biases/immortal-time-bias/#cite
- 55.Brenner LA, Ignacio RV, Blow FC. Suicide and traumatic brain injury among individuals seeking Veterans Health Administration services. J Head Trauma Rehabil. 2011;26(4):257-264. doi: 10.1097/HTR.0b013e31821fdb6e [DOI] [PubMed] [Google Scholar]
- 56.Fonda JR, Fredman L, Brogly SB, McGlinchey RE, Milberg WP, Gradus JL. Traumatic brain injury and attempted suicide among veterans of the wars in Iraq and Afghanistan. Am J Epidemiol. 2017;186(2):220-226. doi: 10.1093/aje/kwx044 [DOI] [PubMed] [Google Scholar]