Key Points
Question
In routine US clinical practice, how does the New York Heart Association (NYHA) class compare with patient-reported outcomes for serial assessment of health status among patients with heart failure with reduced ejection fraction?
Findings
During outpatient follow-up of 12 months in this cohort study, 75% of 2872 patients had a clinically meaningful change in Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OS) of 5 or more points, whereas 35% had a change of 1 class or more in NYHA class. Improvement in KCCQ-OS of 5 or more points was independently associated with decreased mortality, whereas improvement in NYHA class was not.
Meaning
Compared with the clinician-assigned NYHA class, the patient-reported KCCQ-OS is more likely to detect meaningful change in health status over time, and changes in KCCQ-OS may have more prognostic value than changes in NYHA class.
Abstract
Importance
It is unclear how New York Heart Association (NYHA) functional class compares with patient-reported outcomes among patients with heart failure (HF) in contemporary US clinical practice.
Objective
To characterize longitudinal changes and concordance between NYHA class and the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OS), and their associations with clinical outcomes.
Design, Setting, and Participants
This cohort study included 2872 US outpatients with chronic HF with reduced ejection fraction across 145 practices enrolled in the CHAMP-HF registry between December 2015 and October 2017. All patients had complete NYHA class and KCCQ-OS data at baseline and 12 months. Longitudinal changes and correlations between the 2 measure were examined. Multivariable models landmarked at 12 months evaluated associations between improvement in NYHA and KCCQ-OS from baseline to 12 months with clinical outcomes occurring from months 12 through 24. Statistical analyses were performed from March to August 2020.
Exposure
Change in health status, as defined by 12-month change in NYHA class or KCCQ-OS.
Main Outcomes and Measures
All-cause mortality, HF hospitalization, and mortality or HF hospitalization.
Results
In total, 2872 patients were included in this analysis (median [interquartile range] age, 68 [59-75] years; 872 [30.4%] were women; and 2156 [75.1%] were of White race). At baseline, 312 patients (10.9%) were NYHA class I, 1710 patients (59.5%) were class II, 804 patients (28.0%) were class III, and 46 patients (1.6%) were class IV. For KCCQ-OS, 1131 patients (39.4%) scored 75 to 100 (best health status), 967 patients (33.7%) scored 50 to 74, 612 patients (21.3%) scored 25 to 49, and 162 patients (5.6%) scored 0 to 24 (worst health status). At 12 months, 1002 patients (34.9%) had a change in NYHA class (599 [20.9%] with improvement; 403 [14.0%] with worsening) and 2158 patients (75.1%) had a change of 5 or more points in KCCQ-OS (1388 [48.3%] with improvement; 770 [26.8%] with worsening). The most common trajectory for NYHA class was no change (1870 [65.1%]), and the most common trajectory for KCCQ-OS was an improvement of at least 10 points (1047 [36.5%]). After adjustment, improvement in NYHA class was not associated with subsequent clinical outcomes, whereas an improvement of 5 or more points in KCCQ-OS was independently associated with decreased mortality (hazard ratio, 0.59; 95% CI, 0.44-0.80; P < .001) and mortality or HF hospitalization (hazard ratio, 0.73; 95% CI, 0.59-0.89; P = .002).
Conclusions and Relevance
Findings of this cohort study suggest that, in contemporary US clinical practice, compared with NYHA class, KCCQ-OS is more sensitive to clinically meaningful changes in health status over time. Changes in KCCQ-OS may have more prognostic value than changes in NYHA class.
This registry-based cohort study compares the capture of clinically meaningful health status changes between physician-assigned New York Heart Association functional class and patient-reported outcome in Kansas City Cardiomyopathy Questionnaire Overall Summary Score for patients with chronic heart failure with reduced ejection fraction.
Introduction
The New York Heart Association (NYHA) functional classification system has been a cornerstone nomenclature for quantifying the health status of patients with heart failure (HF) for almost a century, and remains foundational to eligibility criteria for contemporary HF trials and application of clinical guidelines.1 However, despite widespread use, the NYHA functional classification may carry considerable limitations.2,3,4 For example, it assesses patients’ functional class through the lens of clinicians, who may vary in their rigor of acquiring this information, resulting in marked variability across clinicians.4
Since the initial development of the NYHA classification, patient-reported outcomes (PROs) have evolved substantially.2 Given the increasing availability of validated HF-specific PRO instruments, such as the Kansas City Cardiomyopathy Questionnaire (KCCQ), the appropriate role and relative importance of clinician-assessed NYHA class, as compared with directly assessing health status from patients in clinical practice, is unclear. The CHAMP-HF (Change the Management of Patients With Heart Failure) registry represents a novel opportunity to compare the role and clinical value of NYHA class and PROs in contemporary US clinical practice. This cohort study aimed to (1) assess longitudinal changes and level of agreement between NYHA functional class and PROs for quality of life and symptoms, (2) identify factors associated with discordance between NYHA class and PROs, and (3) compare the prognostic value associated with changes in HF status as defined by changes in NYHA class vs KCCQ.
Methods
Study Design
The design of the CHAMP-HF registry has been previously described.5 In brief, CHAMP-HF was a prospective, observational, noninterventional study that enrolled adult outpatients with HF with reduced ejection fraction (HFrEF) in the US between December 2015 and October 2017. Eligible patients had received a diagnosis of chronic HF, had a left ventricular ejection fraction of 40% or lower on most recent imaging within 12 months of enrollment, and were receiving at least 1 oral medication for HF at study enrollment. The registry was conducted in accordance with the Declaration of Helsinki6 and with institutional review board or ethics committee approval at all sites. All patients provided written informed consent that was obtained in a manner consistent with the Declaration of Helsinki. No one received compensation or was offered any incentive for participating in this study.
NYHA Class and Patient-Reported Outcomes
The NYHA class consists of a 4-tier schema of class I through class IV, with class IV denoting worst functional status (ie, HF symptoms at rest).7 Four PROs were evaluated in the current study as follows: (1) KCCQ Overall Summary Score (KCCQ-OS), a 12-item form composed of 4 domains (ie, physical limitation, symptom frequency, quality of life score, and social limitation) with the overall score and each individual domain scored 100 to 0, with 100 indicating the best health status and 0 indicating the worst; (2) EuroQoL 5-dimensions (EQ-5D) utility index, which reflects the degree of patient difficulty with mobility, self-care, usual activity, pain or discomfort, and anxiety or depression, with a maximal score of 1 designating perfect health, 0 equivalent to death, and lower than 0 worse than death; (3) EQ-5D visual analog scale (VAS), a global assessment of health status ranging from 100 to 0, with 0 indicating worst health status; and (4) number of HF symptoms, that is, the number of symptoms reported by patients among the following 5 options: dyspnea at rest, dyspnea with exertion, orthopnea, edema, and exercise intolerance. In addition, 3 KCCQ subscores were evaluated: (1) KCCQ Clinical Summary Score, which is equal to the mean of the scores from the physical limitation and symptom frequency domains; (2) KCCQ Physical Limitation Score, that is, the score from the physical limitation domain of the KCCQ-OS; and (3) KCCQ Symptom Frequency Score, the score from the symptom frequency domain of the KCCQ-OS.
Discordance Between NYHA and Patient-Reported Outcomes
There are no established ranges for KCCQ or other PRO scores that map to each of the 4 NYHA classes. Thus, discordance among the NYHA class, KCCQ-OS, and EQ-5D scores was determined at baseline and 12 months using a prespecified framework. To define discordance, each scale was organized into 4 levels from “best” to “worst,” as outlined in eFigure 1 in the Supplement. Prespecified cut points for the KCCQ-OS and EQ-5D scores were chosen for consistency with prior publications and ease of communication, and reflected the investigators’ best judgment.8,9 For each pair of measures, concordance was defined as each scale registering the same level. Mild discordance was defined when the pair of measures differed by 1 level, and moderate or severe discordance was defined as measures differing by 2 to 3 levels. Furthermore, recognizing potential bias based on cut points selected in categorical analyses of discordance, complementary analyses of correlation between continuous NYHA and PROs were performed, as described in the Statistical Analysis.
Clinical Outcomes
Clinical outcomes included all-cause mortality, HF hospitalization, and the composite of all-cause mortality or HF hospitalization. Outcomes were determined by review of the medical record.
Statistical Analysis
Patient characteristics were compared by degree of discordance between NYHA and KCCQ-OS at baseline, with patients assigned to 1 of 3 groups (concordance, mild discordance [differed by 1 level], and moderate to severe discordance [differed by 2-3 levels]). Recognizing that discordance can occur in 2 directions (eg, NYHA worse than KCCQ-OS or NYHA better than KCCQ-OS), for each pair of measures, we determined the proportions of patients differing in 1, 2, or 3 levels in both directions.
Correlations between NYHA class, KCCQ scores, EQ-5D scores, and number of symptoms were assessed at baseline and 12 months. Specifically, correlations between NYHA class and KCCQ-OS were calculated, and correlations were calculated for each of these core measures with remaining PROs. Spearman rank correlation, a nonparametric test appropriate for both ordinal data (NYHA class, number of symptoms) and continuous but nonnormal data (KCCQ scores, EQ-5D scores), was used.
Changes in NYHA class and KCCQ-OS scores between baseline and 12 months were summarized in categorical and continuous analyses. For NYHA class, change categories included worsening of 2 or more classes, worsening of 1 class, no change, improvement of 1 class, and improvement of 2 or more classes. Recognizing that a change of 5 or more points in KCCQ-OS is considered clinically significant, change categories for KCCQ-OS included worsening of 10 or more points, worsening of 5 to 9 points, no significant change (<5-point change), improvement of 5 to 9 points, and improvement of 10 or more points.10 For each change category of NYHA class and KCCQ-OS, median (interquartile range) 12-month change in other measures were calculated.
Multivariable models were constructed to determine whether certain patient characteristics were associated with greater likelihood of discordance between clinician-assessed and patient-reported assessments. These analyses included the NYHA class, KCCQ-OS, and EQ-5D VAS. For each pair of measures, any level of discordance (ie, ≥1 level) was considered a binary outcome, with concordance considered a nonevent. Logistic regression models were used, with generalized estimating equations to account for clustering within sites. Separate models were constructed for baseline and 12-month time points. Values for candidate variables were taken from baseline for baseline models and taken from 12 months (or most recent available) for the 12-month models. Model selection was based on backward elimination. Variables with a P ≥ .05 were removed, with the highest P value removed first, and subsequent assessment continuing with remaining variables. Model selection continued until all remaining variables were P < .05.
Unadjusted and adjusted Cox proportional hazards models were used to separately evaluate associations between improvements in NYHA class and KCCQ-OS and clinical outcomes. Models were landmarked at 12 months so that change in each measure between baseline and 12 months could be associated with clinical outcomes occurring between months 12 through 24. Models were adjusted for 20 prespecified covariates, including age, sex, race/ethnicity, total household income, body mass index (BMI), systolic blood pressure, heart rate, ejection fraction, glomerular filtration rate, atrial fibrillation, coronary artery disease, diabetes, chronic obstructive pulmonary disease, HF hospitalization in the prior 12 months, cardiac resynchronization therapy, implantable cardioverter-defibrillator, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, sacubitril/valsartan, evidence-based β-blocker, and mineralocorticoid receptor antagonist. Time-updated covariate values collected at 12 months (or most recent available) were used. Robust sandwich variance estimators were used to account for clustering within site. Hazard ratios and P values were calculated. Statistical analyses were performed from March to August 2020 using SAS, version 9.4 (SAS Institute Inc). A 2-tailed P < .05 was considered statistically significant.
Results
Patient Cohort and Baseline NYHA Class and KCCQ-OS
From the total sample of 5009 patients, the present analysis included 2872 patients with complete NYHA and KCCQ-OS data at baseline and 12 months (eFigure 2 in the Supplement). Overall, median age was 68 years (interquartile range [IQR], 59-75 years), 872 (30.4%) were women, 2156 (75.1%) were of White race, and the median EF was 30% (IQR, 23%-35%) (eTable 1 in the Supplement).
At baseline, 312 patients (10.9%) were NYHA class I, 1710 patients (59.5%) were NYHA class II, 804 patients were NYHA class III (28.0%), and 46 patients (1.6%) were NYHA class IV. For KCCQ-OS, 1131 patients (39.4%) scored 75 to 100, 967 patients (33.7%) scored 50 to 74, 612 patients (21.3%) scored 25 to 49, and 162 patients (5.6%) scored 0 to 24 (eTables 2 and 3 in the Supplement).
Discordance Between NYHA Class and Patient-Reported Outcomes
At baseline, 1085 patients (37.8%) showed concordance between NYHA class and KCCQ-OS, whereas 1494 patients (52.0%) had mild discordance by 1 level and 293 patients (10.2%) had moderate to severe discordance by 2 to 3 levels (Table 1). Patient characteristics were generally similar across discordant groups, with few exceptions.
Table 1. Baseline Demographic, Clinical, and Socioeconomic Characteristics by Degree of Baseline Discordance Between NYHA and KCCQ-OS.
| Characteristic | No. (%) of participants | P value | ||
|---|---|---|---|---|
| Concordance (n = 1085) | Discordance | |||
| Mild (n = 1494) | Moderate-severe (n = 293) | |||
| Age, median (IQR), y | 67 (58-75) | 68 (59-75) | 67 (58-77) | .53 |
| Women | 350 (32.3) | 434 (29.0) | 88 (30.0) | .22 |
| Race | ||||
| White | 813 (74.9) | 1138 (76.2) | 205 (70.0) | .13 |
| Black/African American | 181 (16.7) | 239 (16.0) | 65 (22.2) | |
| Othera | 91 (8.4) | 117 (7.8) | 23 (7.8) | |
| Hispanic ethnicity | 228 (21.0) | 270 (18.1) | 42 (14.3) | .02 |
| Ejection fraction, median (IQR), % | 30 (23-36) | 30 (24-35) | 30 (23-34) | .009 |
| NYHA class | ||||
| I | 219 (20.2) | 67 (4.5) | 26 (8.9) | <.001 |
| II | 604 (55.7) | 1047 (70.1) | 59 (20.1) | |
| III | 249 (22.9) | 366 (24.5) | 189 (64.5) | |
| IV | 13 (1.2) | 14 (0.9) | 19 (6.5) | |
| KCCQ-OS, median (IQR) | 63 (50-72) | 72 (47-88) | 79 (41-89) | <.001 |
| Vital signs and laboratory findings, median (IQR)b | ||||
| Systolic blood pressure, mm Hg | 120 (110-131) | 120 (110-130) | 120 (110-130) | .68 |
| Heart rate, bpm | 72 (65-82) | 72 (65-80) | 72 (66-82) | .20 |
| BMI | 29.6 (26.1-34.5) | 29.5 (25.8-33.9) | 29.4 (25.8-34.1) | .12 |
| Hemoglobin, g/dL | 13.3 (12.0-14.5) | 13.4 (12.0-14.5) | 12.8 (11.5-14.1) | .005 |
| Serum sodium, mEq/L | 139 (137-141) | 140 (138-141) | 139 (137-141) | .19 |
| BUN, mg/dL | 20 (15-27) | 20 (16-27) | 21 (16-29) | .21 |
| eGFR, mL/min/1.73 m2 | ||||
| <30 | 38 (5.3) | 43 (4.3) | 11 (5.3) | .49 |
| 30-44 | 103 (14.4) | 132 (13.3) | 36 (17.3) | |
| 45-59 | 151 (21.2) | 221 (22.2) | 51 (24.5) | |
| ≥60 | 421 (59.0) | 598 (60.2) | 110 (52.9) | |
| NT-proBNP, median (IQR), pg/mL | 1621 (591-3930) | 1755 (804-4227) | 1542 (751-4037) | .53 |
| Hemoglobin A1c, median (IQR), % | 6.4 (5.9-7.4) | 6.6 (5.9-7.9) | 6.5 (5.9-7.7) | .51 |
| Medical history | ||||
| HF hospitalization within past 12 mo | 389 (35.9) | 512 (34.3) | 126 (43.0) | .02 |
| Coronary artery disease | 712 (65.6) | 939 (62.9) | 189 (64.5) | .35 |
| Hypertension | 919 (84.7) | 1248 (83.5) | 257 (87.7) | .19 |
| Hyperlipidemia | 862 (79.4) | 1213 (81.2) | 230 (78.5) | .40 |
| Diabetes | 463 (42.7) | 635 (42.5) | 125 (42.7) | .99 |
| Atrial fibrillation | 366 (33.7) | 564 (37.8) | 106 (36.2) | .11 |
| Chronic renal insufficiency | 221 (20.4) | 305 (20.4) | 63 (21.5) | .91 |
| Asthma or COPD | 367 (33.8) | 426 (28.5) | 98 (33.4) | .01 |
| History of ventricular tachycardia or fibrillation | 207 (19.1) | 325 (21.8) | 64 (21.8) | .23 |
| Depression | 318 (29.3) | 350 (23.4) | 68 (23.2) | .002 |
| Active cigarette smoking | 225 (20.7) | 282 (18.9) | 53 (18.1) | .41 |
| Medical therapy | ||||
| ACEI or ARB | 727 (68.1) | 983 (67.2) | 174 (60.6) | .05 |
| Sacubitril/valsartan | 169 (15.7) | 243 (16.3) | 68 (23.2) | .007 |
| Evidence-based β-blocker | 876 (82.3) | 1172 (80.0) | 237 (82.6) | .27 |
| MRA | 405 (37.5) | 526 (35.4) | 114 (39.9) | .28 |
| Heart failure device therapy | ||||
| Implantable cardioverter-defibrillator | 463 (42.7) | 632 (42.3) | 146 (49.8) | .05 |
| Cardiac resynchronization therapy | 83 (7.6) | 135 (9.0) | 24 (8.2) | .45 |
| Socioeconomic characteristics | ||||
| Insurance status | ||||
| Private insurance or managed care (HMO, PPO) | 298 (27.5) | 404 (27.0) | 69 (23.5) | .22 |
| Medicare | 612 (56.4) | 850 (56.9) | 169 (57.7) | |
| Medicaid | 113 (10.4) | 131 (8.8) | 28 (9.6) | |
| Other | 51 (4.7) | 81 (5.4) | 23 (7.8) | |
| Uninsured | 11 (1.0) | 28 (1.9) | 4 (1.4) | |
| Highest level of education | ||||
| <High school | 122 (11.2) | 162 (10.8) | 37 (12.6) | .07 |
| High school/GED | 387 (35.7) | 491 (32.9) | 100 (34.1) | |
| Some college | 341 (31.4) | 494 (33.1) | 86 (29.4) | |
| 4-y College (bachelor’s degree) | 145 (13.4) | 208 (13.9) | 29 (9.9) | |
| Graduate or other professional degree | 90 (8.3) | 139 (9.3) | 41 (14.0) | |
| Total annual household income, $ | ||||
| <25 000 | 355 (40.3) | 498 (42.2) | 87 (40.8) | .19 |
| 25 000-49 999 | 214 (24.3) | 308 (26.1) | 66 (31.0) | |
| 50 000-99 999 | 229 (26.0) | 260 (22.0) | 45 (21.1) | |
| ≥100 000 | 82 (9.3) | 114 (9.7) | 15 (7.0) | |
| Employment status | ||||
| Full-time employee (≥35 h/wk) | 143 (13.2) | 215 (14.4) | 38 (13.0) | .35 |
| Part-time employee (<35 h/wk) | 76 (7.0) | 116 (7.8) | 29 (9.9) | |
| Disability for medical reasons | 302 (27.8) | 385 (25.8) | 88 (30.0) | |
| Not employed for other reasons (eg, retired, student, or unemployed) | 564 (52.0) | 778 (52.1) | 138 (47.1) | |
| Outpatient clinic characteristics | ||||
| Investigator specialty | ||||
| Family or general medicine | 68 (6.3) | 126 (8.4) | 17 (5.8) | .004 |
| Internal medicine | 134 (12.4) | 132 (8.8) | 20 (6.8) | |
| Heart failure cardiologist | 314 (28.9) | 393 (26.3) | 83 (28.3) | |
| Cardiologist (non–heart failure) | 548 (50.5) | 822 (55.0) | 166 (56.7) | |
| Other | 21 (1.9) | 21 (1.4) | 7 (2.4) | |
| Practice setting | ||||
| Primary university | 15 (1.4) | 30 (2.0) | 5 (1.7) | .79 |
| Teaching university | 199 (18.3) | 262 (17.5) | 54 (18.4) | |
| Nonteaching, nonuniversity | 871 (80.3) | 1202 (80.5) | 234 (79.9) | |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); bpm, beats per minute; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GED, General Educational Development; HF, heart failure; HMO, health maintenance organization; IQR, interquartile range; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; PPO, preferred provider organization.
SI conversion factors: To convert hemoglobin to grams per liter, multiply by 10; sodium to millimoles per liter, by 1.0; urea nitrogen to millimoles per liter, by 0.357; hemoglobin A1c to proportion of total hemoglobin, by 0.01.
Defined as Asian, American Indian, Alaska Native, Native Hawaiian, Pacific Islander, multiracial, or other.
Data were available for hemoglobin level for 1696 patients, serum sodium level for 2139 patients, BUN level for 2106 patients, eGFR for 1915 patients, NT-proBNP concentration for 348 patients, and hemoglobin A1c level for 669 patients.
For NYHA class, KCCQ-OS, and other PROs, the proportions of patients with varying directionality and severity of discordance are given in eTables 4 and 5 in the Supplement. At baseline, among patients with discordance between NYHA class and KCCQ-OS, most had worse NYHA class (1222 of 1787 [68.4%]) rather than worse KCCQ-OS (565 of 1787 [31.6%]). Discordance with the EQ-5D index and EQ-5D VAS was more common for NYHA class (EQ-5D index, 2160 [75.2%] and EQ-5D VAS, 1854 [64.6%]) than for KCCQ-OS (EQ-5D index, 1500 [52.2%] and EQ-5D VAS, 1364 [47.5%]). For both NYHA class and KCCQ-OS, discordance with EQ-5D scales was mostly owing to better EQ-5D status. Discordance with the number of symptoms was comparable for NYHA class (1850 [64.4%]) and KCCQ-OS (2042 [71.1%]), and in both cases was more frequently due to worse number of symptoms. For all measures, the degree and direction of discordance was generally similar at both baseline and 12 months.
In assessment of correlation coefficients between NYHA class, KCCQ-OS, and other PROs, all measures showed statistically significant correlations with each other at baseline and 12 months (eTable 6 in the Supplement). The magnitude of correlation between NYHA class and KCCQ-OS at baseline was modest (ρ = 0.33; P < .001). Likewise, correlations for the NYHA class with other KCCQ subscales and PROs were modest (ρ ranging from 0.22 to 0.35 at baseline), whereas correlations between KCCQ-OS and other PROs were stronger (ρ ranging from 0.35 to 0.66 at baseline).
Factors Associated With Discordance Between NYHA Class and Patient-Reported Outcomes
At baseline, older patients had a higher likelihood of NYHA class being worse than KCCQ-OS (eTable 7 in the Supplement). By contrast, being a woman or of Hispanic ethnicity and having higher BMI, chronic obstructive pulmonary disease, and coronary artery disease were each associated with a lower likelihood of worse NYHA class than KCCQ-OS. At 12 months, age and Hispanic ethnicity continued to be associated with the likelihood of NYHA class being worse than KCCQ-OS, with addition of higher left ventricular ejection fraction associated with lower likelihood of NYHA class–KCCQ-OS category discordance.
In terms of KCCQ-OS being worse than NYHA class, lower household income and higher heart rate up to 65 beats per minute were associated with higher risk of discordance at baseline, whereas older age up to 75 years was associated with lower risk of discordance. At 12 months, lower household income, chronic obstructive pulmonary disease, diabetes, and being a woman were associated with higher risk of discordance, and no factors were associated with lower risk of discordance. Factors associated with discordance between NYHA class and EQ-5D VAS and discordance between KCCQ-OS and EQ-5D VAS are presented in eTables 8 and 9 in the Supplement.
Change in NYHA and KCCQ-OS During 12 Months
In comparing status at baseline and 12 months, 1002 patients (34.9%) had a change in NYHA class, whereas 2158 (75.1%) had a meaningful change in KCCQ-OS (Figure 1). For NYHA class, 599 patients (20.9%) had any improvement, and 403 (14.0%) had any worsening, with the large majority of changes representing a 1-class change. For KCCQ-OS, 1388 patients (48.3) had at least a 5-point improvement, and 770 (26.8%) had at least a 5-point worsening. Overall, the most common scenario for NYHA class trajectory was no change (1870 [65.1%]), and the most common scenario for KCCQ-OS trajectory was at least a 10-point improvement (1047 [36.5%]). Patterns of longitudinal change in NYHA class and KCCQ-OS were similar among patients enrolled from sites with cardiologist investigators vs noncardiologist investigators (ie, family medicine, internal medicine, and other) (eFigure 3 in the Supplement).
Figure 1. Change From Baseline to 12-Month Follow-up in New York Heart Association (NYHA) Class and Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OS).
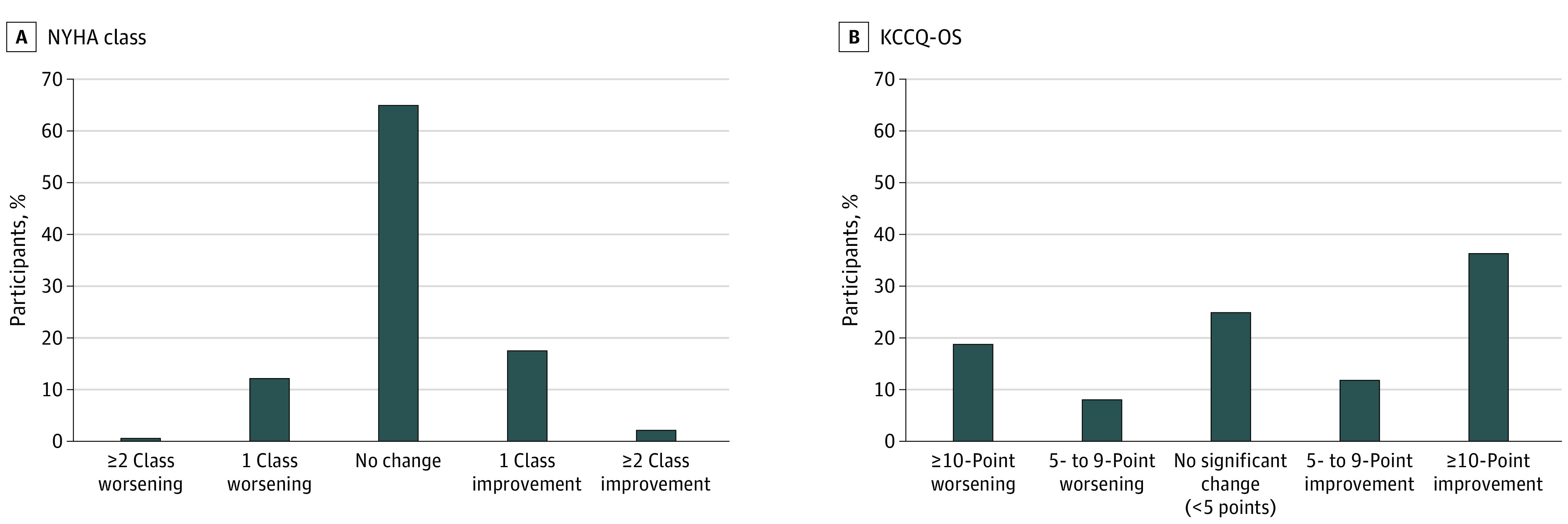
Data reflect the proportion of patients with a change in NYHA class or meaningful change in KCCQ-OS of 5 or more points from baseline to 12-month follow-up.
Of patients with no change in NYHA class, the median change in KCCQ-OS was 3 (IQR, −6 to 16) (Table 2). For patients who worsened by 2 or more NYHA classes, the median change in KCCQ-OS was 2 points (IQR, −24 to 9 points), and the median change in EQ-5D VAS was −4 points (IQR, −11 to 8 points). For patients who improved by 2 or more NYHA classes, the median change in KCCQ-OS was 11 points (IQR, 0 to 31 points), and the median change in EQ-5D VAS was 0 points (IQR, −9 to 11 points).
Table 2. Change in Scores From Baseline to 12 Months by Change in NYHA Class.
| Change | Worsening of ≥2 classes (n = 34) | Worsening of 1 class (n = 369) | No change (n = 1870) | Improvement of 1 class (n = 520) | Improvement of ≥2 classes (n = 79) |
|---|---|---|---|---|---|
| KCCQ, median (IQR) | |||||
| OS | 2 (−24 to 9) | 1 (−11 to 11) | 3 (−6 to 16) | 8 (−1 to 19) | 11 (0 to 31) |
| CSS | −5 (−22 to 9) | 0 (−13 to 11) | 3 (−7 to 16) | 6 (−2 to 21) | 8 (−1 to 27) |
| PLS | −8 (−17 to 8) | 0 (−17 to 17) | 0 (−8 to 17) | 8 (0 to 25) | 17 (0 to 42) |
| SFS | −8 (−25 to 10) | 0 (−17 to 12) | 0 (−8 to 15) | 6 (−4 to 21) | 8 (−4 to 25) |
| EQ-5D, median (IQR) | |||||
| VAS | −4 (−11 to 8) | 0 (−11 to 10) | 0 (−10 to 10) | 3 (−6 to 14) | 0 (−9 to 11) |
| Index | −0.05 (−0.13 to 0.04) | −0.01 (−0.12 to 0.04) | 0.00 (−0.06 to 0.09) | 0.01 (−0.03 to 0.13) | 0.03 (−0.01 to 0.14) |
Abbreviations: EQ-5D, EuroQoL 5-dimension; IQR, interquartile range; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score; KCCQ-PLS, Kansas City Cardiomyopathy Questionnaire Physical Limitation Score; KCCQ-SFS, Kansas City Cardiomyopathy Questionnaire Symptom Frequency Score; NYHA, New York Heart Association; VAS, visual analog scale.
For all categories of KCCQ-OS change ranging from worsening of 10 or more points to improvement of 10 or more points, the median change in NYHA class was 0 (IQR, 0-0 for all categories, except 0-1 for improvement of 10 or more) (Table 3). Changes in KCCQ subscores were consistent with changes in KCCQ-OS. Changes in EQ-5D VAS scores ranged from a median of −9 points (IQR, −20 to 2 points) for patients with worsening of 10 or more points in KCCQ-OS to 6 points (IQR, −3 to 20 points) for patients with improvement of 10 or more points in KCCQ-OS.
Table 3. Change in Scores From Baseline to 12 Months by Change in KCCQ-OS.
| Change | Worsening of ≥10 points (n = 536) | Worsening of 5-9 points (n = 234) | No change (n = 714) | Improvement of 5-9 points (n = 341) | Improvement of ≥10 points (n = 1047) |
|---|---|---|---|---|---|
| NYHA class, median (IQR) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 1) |
| KCCQ, median (IQR) | |||||
| CSS | −19 (−29 to −10) | −7 (−13 to −2) | 0 (−4 to 4) | 6 (0 to 13) | 21 (13 to 33) |
| PLS | −21 (−33 to −8) | −8 (−17 to 0) | 0 (−8 to 8) | 8 (0 to 17) | 25 (8 to 40) |
| SFS | −17 (−33 to −6) | −6 (−15 to 0) | 0 (−8 to 6) | 4 (−4 to 13) | 19 (8 to 33) |
| EQ-5D, median (IQR) | |||||
| VAS | −9 (−20 to 2) | −3 (−15 to 5) | 0 (−8 to 9) | 1 (−8 to 11) | 6 (−3 to 20) |
| Index | −0.06 (−0.17 to 0.00) | −0.02 (−0.13 to 0.01) | 0.00 (−0.06 to 0.05) | 0.00 (−0.02 to 0.11) | 0.05 (−0.01 to 0.15) |
Abbreviations: EQ-5D, EuroQoL 5-dimension; IQR, interquartile range; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score; KCCQ-PLS, Kansas City Cardiomyopathy Questionnaire Physical Limitation Score; KCCQ-SFS, Kansas City Cardiomyopathy Questionnaire Symptom Frequency Score; NYHA, New York Heart Association; VAS, visual analog scale.
Change in NYHA Class and KCCQ-OS and Clinical Outcomes
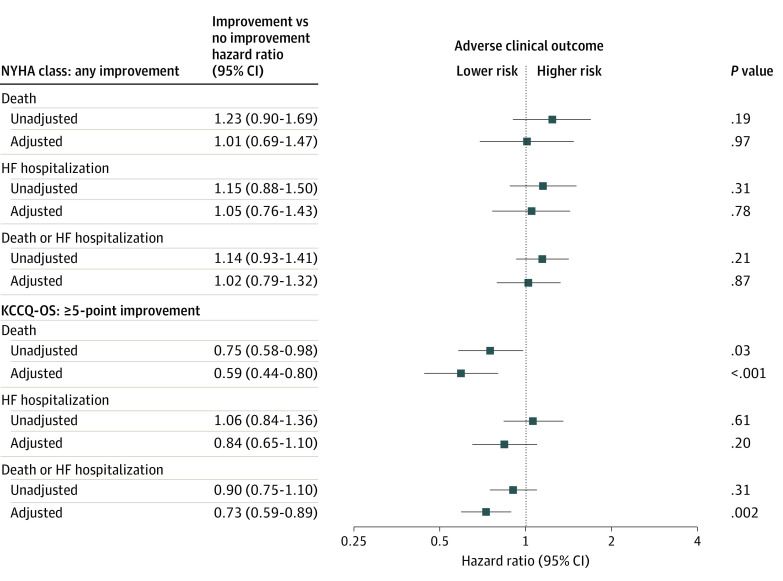
In landmark analysis at the 12-month time point, there were 206 (7.2%) deaths, 231 (8.0%) HF hospitalizations, and 398 (13.9%) deaths or HF hospitalizations between months 12 and 24. In unadjusted and adjusted analyses, there were no significant associations between improvement in NYHA class and subsequent all-cause death, HF hospitalization, or the composite of death or HF hospitalization during 1 year of follow-up (Figure 2). By contrast, after adjustment, improvement in KCCQ-OS of 5 points or more was associated with decreased risk of all-cause mortality (hazard ratio, 0.59; 95% CI, 0.44-0.80; P < .001) and the composite of all-cause death or HF hospitalization (hazard ratio, 0.73; 95% CI, 0.59-0.89; P = .002). There was no significant association between improvement in KCCQ-OS and HF hospitalization.
Figure 2. Associations Between Change in New York Heart Association (NYHA) Class and Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OS) With Clinical Outcomes Among Patients With Heart Failure (HF) With Reduced Ejection in Contemporary US Outpatient Practice.
Unadjusted and adjusted hazard ratios indicating the risk of adverse clinical outcomes associated with improvement in NYHA class and improvement in KCCQ-OS. Adjusted models include age, sex, race/ethnicity, total household income, body mass index, systolic blood pressure, heart rate, ejection fraction, glomerular filtration rate, atrial fibrillation, coronary artery disease, diabetes, chronic obstructive pulmonary disease, prior heart failure hospitalization, cardiac resynchronization therapy, implantable cardioverter-defibrillator, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, sacubitril/valsartan, evidence-based β-blocker, and mineralocorticoid receptor antagonist.
Discussion
In this large contemporary US registry of outpatients with HFrEF, there was discordance in health status between the clinician-assigned NYHA class and the patient-reported KCCQ-OS. This discordance was moderate to severe in more than 10% of patients and was mostly due to assigned NYHA classes being disproportionately worse than KCCQ-OS. In multivariate analysis, multiple patient factors were independently associated with higher risk of discordance, including age, sex, household income, and comorbidities. During 12 months of follow-up, the majority of patients had meaningful changes in KCCQ-OS, yet most patients had no change in NYHA class. Improvement in NYHA class was not associated with subsequent clinical outcomes, whereas improvement in KCCQ-OS was independently associated with lower risks of death and the composite of death and HF hospitalization, but not with HF hospitalization alone.
To our knowledge, we present the first comprehensive direct comparison of NYHA class and PROs in contemporary US clinical practice. Prior large studies have assessed NYHA class and KCCQ in clinical trial cohorts, but data from large clinical practice-based patient populations have not been available.11,12,13 These data from the CHAMP-HF registry suggest relative advantages of measuring health status via serial KCCQ assessments, as compared with NYHA class, in the longitudinal care of patients with HFrEF. Although numerous reliable clinical risk markers for HF already exist, the sheer magnitude of the 41% lower risk of mortality independently associated with improvement in KCCQ-OS in the present study is notable. The relative strength of this association is further magnified by the lack of a corresponding association between clinical outcomes and NYHA class. A similarly clear distinction between KCCQ-OS and NYHA class was observed when comparing changes over time. Although cross-sectional analyses showed statistically significant correlations between the 2 measures, more than twice as many patients had meaningful changes in KCCQ-OS compared with NYHA class during 12 months, and meaningful changes in KCCQ were not reliably reflected in the NYHA class. Even among patients with improvement or worsening of 10 points or more in KCCQ-OS, there was generally no corresponding change in NYHA class. Although the exact reasons for such dissociations are unclear, limited change in clinician assessment of NYHA class would be consistent with other examples of clinician inertia in ambulatory HF care.14 In contrast to NYHA class, KCCQ-OS was not only more sensitive for detecting change in health status, but such patient-reported changes were further validated by strong prognostic value.
To date, the uptake of PRO measures within HF care has been slow and varied, even though they have been recommended as measures of health care quality.15,16,17 Barriers have been proposed, including concerns regarding logistics of data collection, results interpretation, and perceived utility and value of PROs by clinicians.15 This may stand in contrast with NYHA class, which can conceivably be assigned in seconds by a clinician familiar with the patient. Nonetheless, routine collection of PROs within the HF clinic has proven feasible at some centers, with mean PRO assessment times of less than 7 minutes and computerized scoring within the electronic health record.18 Emphasizing clinical education, automated data collection and scoring, and easily interpretable presentation may be critical elements for more widespread adoption of PRO measures.15
Aside from prognostic implications, these data highlight the potential for clinician and patient perspectives to differ regarding health status, with discordance occurring in both directions (ie, clinician more favorable than patient and vice versa). Such dissociations were more likely in certain demographic subsets based on age, sex, race/ethnicity, and household income, raising the question of clinician bias in assigning the NYHA class and the potential contribution to disparities in care.19 Likewise, these findings could reflect challenges in patient-clinician communication in the care of particular patient subsets. Certain comorbidities may also increase risk for discordance. For example, it may be difficult for clinicians to reliably distinguish pulmonary vs HF contributions to health status among patients with HF and chronic obstructive pulmonary disease, and the NYHA class assessment may be limited to the perceived HF symptoms. Nonetheless, given the weight NYHA class carries in application of guideline recommendations and patient eligibility for clinical trials, any potential for bias in clinician assignment of NYHA class and dissociation from PROs is highly relevant. By comparison, despite the clear association with clinical outcomes observed here, little to no guidance is available for using PROs to inform HF management decisions. To best ensure patient-centered care, future studies may consider novel KCCQ-based eligibility criteria.
Limitations
Limitations of this study should be acknowledged. First, there is no clear range of KCCQ scores that matches the 4 NYHA classes, in part because of the variability in assigning NYHA class. Thus, we selected, a priori, 4 equally sized 25-point ranges of the PROs, which are consistent with prior literature and may facilitate ease of interpretation.9 Moreover, recognizing potential bias with arbitrary cut points, we assessed correlation between continuous NYHA and PROs scales in complementary analyses. Second, although study sites included a broad set of HF, cardiology, and primary care practices, these data reflect sites and patients who elected to participate in the registry, and thus may not be generalizable to patients receiving care at all US HF clinics. Although exact concordance between NYHA class and PROs may not be expected, the magnitude of difference found here is noteworthy and supports the conclusion that clinicians and patients rate patient health status differently. Third, this analysis should be interpreted in the context of including patients with complete NYHA class and PRO data at baseline and 12 months, thus excluding patients with interval death or with missing data. Survival bias may have contributed to NYHA class and KCCQ-OS generally showing more improvement than worsening over time.
Conclusions
In this contemporary US outpatient HFrEF registry, as compared with NYHA class, KCCQ-OS was more likely to detect meaningful changes in health status during 12 months of follow-up, and even patients with large changes in KCCQ-OS generally had no change in NYHA class. Improvement in KCCQ-OS was independently associated with lower risk of subsequent mortality and the composite of mortality and HF hospitalization, whereas improvement in NYHA class was not associated with clinical outcomes.
eTable 1. Characteristics of Patients Included and Excluded from the Current Study
eTable 2. Patient Characteristics by Baseline NYHA Class
eTable 3. Patient Characteristics by Baseline KCCQ-OS
eTable 4. Agreement Between NYHA Class and Other Measures at Baseline and 12 Months
eTable 5. Agreement Between KCCQ-OS and Other Measures at Baseline and 12 Months
eTable 6. Correlations Among NYHA Class and Patient-Reported Outcomes
eTable 7. Factors Associated With Discordance Between NYHA Class and KCCQ-OS Categories
eTable 8. Factors Associated With Discordance Between NYHA Class and EQ-5D VAS Categories
eTable 9. Factors Associated With Discordance Between KCCQ-OS and EQ-5D VAS Categories
eFigure 1. Study Measures and Defining Discordance
eFigure 2. Selection of the Analytic Cohort
eFigure 3. Change from Baseline to 12-month Follow-up in NYHA Class and KCCQ-OS by Site Investigator Type
References
- 1.White PD, Myers MM. The classification of cardiac diagnosis. JAMA. 1921;77(18):1414-1415. doi: 10.1001/jama.1921.02630440034013 [DOI] [Google Scholar]
- 2.Kelkar AA, Spertus J, Pang P, et al. Utility of patient-reported outcome instruments in heart failure. JACC Heart Fail. 2016;4(3):165-175. doi: 10.1016/j.jchf.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 3.Patel RB, Vaduganathan M, Greene SJ, Butler J. Nomenclature in heart failure: a call for objective, reproducible, and biologically-driven terminology. Eur J Heart Fail. 2018;20(10):1379-1381. doi: 10.1002/ejhf.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raphael C, Briscoe C, Davies J, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93(4):476-482. doi: 10.1136/hrt.2006.089656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVore AD, Thomas L, Albert NM, et al. Change the management of patients with heart failure: rationale and design of the CHAMP-HF registry. Am Heart J. 2017;189:177-183. doi: 10.1016/j.ahj.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 6.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 8.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 9.Joseph SM, Novak E, Arnold SV, et al. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6(6):1139-1146. doi: 10.1161/CIRCHEARTFAILURE.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spertus J, Peterson E, Conard MW, et al. ; Cardiovascular Outcomes Research Consortium . Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707-715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Caraballo C, Desai NR, Mulder H, et al. Clinical implications of the New York Heart Association classification. J Am Heart Assoc. 2019;8(23):e014240. doi: 10.1161/JAHA.119.014240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosiborod M, Soto GE, Jones PG, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115(15):1975-1981. doi: 10.1161/CIRCULATIONAHA.106.670901 [DOI] [PubMed] [Google Scholar]
- 13.Pokharel Y, Khariton Y, Tang Y, et al. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2017;2(12):1315-1321. doi: 10.1001/jamacardio.2017.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365-2383. doi: 10.1016/j.jacc.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wohlfahrt P, Zickmund SL, Slager S, et al. Provider perspectives on the feasibility and utility of routine patient-reported outcomes assessment in heart failure: a qualitative analysis. J Am Heart Assoc. 2020;9(2):e013047. doi: 10.1161/JAHA.119.013047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns DJP, Arora J, Okunade O, et al. International Consortium for Health Outcomes Measurement (ICHOM): standardized patient-centered outcomes measurement set for heart failure patients. JACC Heart Fail. 2020;8(3):212-222. doi: 10.1016/j.jchf.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene SJ, Adusumalli S, Albert NM, et al. ; Heart Failure Society of America Quality of Care Committee . Building a heart failure clinic: a practical guide from the Heart Failure Society of America. J Card Fail. 2021;27(1):2-19. doi: 10.1016/j.cardfail.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 18.Stehlik J, Rodriguez-Correa C, Spertus JA, et al. Implementation of real-time assessment of patient-reported outcomes in a heart failure clinic: a feasibility study. J Card Fail. 2017;23(11):813-816. doi: 10.1016/j.cardfail.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 19.Khariton Y, Nassif ME, Thomas L, et al. Health status disparities by sex, race/ethnicity, and socioeconomic status in outpatients with heart failure. JACC Heart Fail. 2018;6(6):465-473. doi: 10.1016/j.jchf.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Patients Included and Excluded from the Current Study
eTable 2. Patient Characteristics by Baseline NYHA Class
eTable 3. Patient Characteristics by Baseline KCCQ-OS
eTable 4. Agreement Between NYHA Class and Other Measures at Baseline and 12 Months
eTable 5. Agreement Between KCCQ-OS and Other Measures at Baseline and 12 Months
eTable 6. Correlations Among NYHA Class and Patient-Reported Outcomes
eTable 7. Factors Associated With Discordance Between NYHA Class and KCCQ-OS Categories
eTable 8. Factors Associated With Discordance Between NYHA Class and EQ-5D VAS Categories
eTable 9. Factors Associated With Discordance Between KCCQ-OS and EQ-5D VAS Categories
eFigure 1. Study Measures and Defining Discordance
eFigure 2. Selection of the Analytic Cohort
eFigure 3. Change from Baseline to 12-month Follow-up in NYHA Class and KCCQ-OS by Site Investigator Type