BACKGROUND
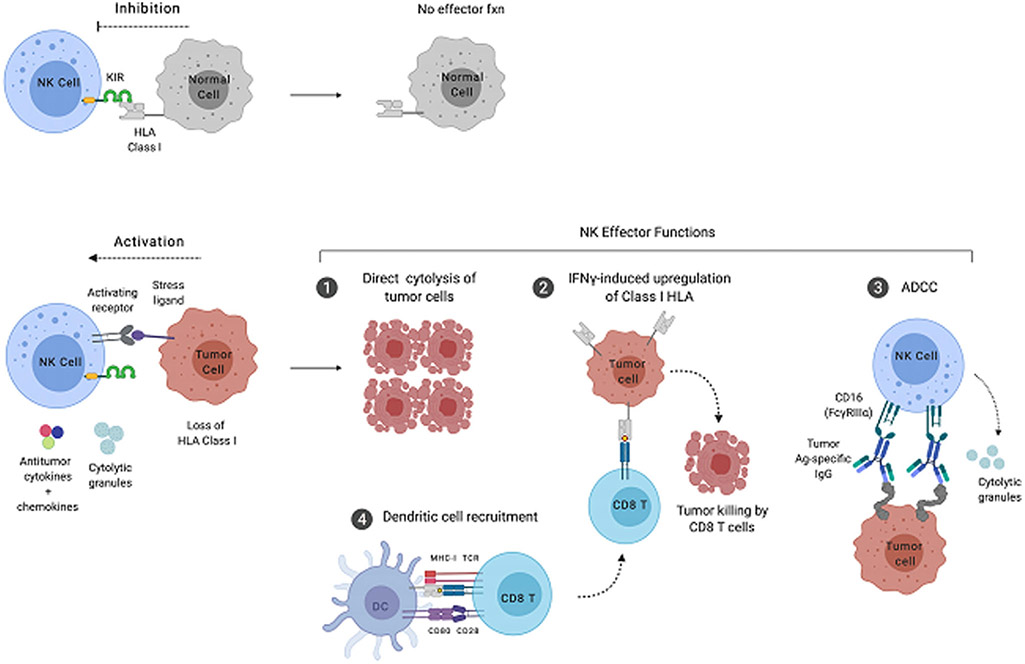
Natural killer (NK cells) are cytotoxic lymphocytes that are members of the innate immune system. As such, they lack the antigen specificity of T and B cells but recognize cells that have downregulated human leukocyte antigen (HLA) class I and upregulated markers of cell stress, such as MICA, MICB, ULBP-1, and the polio virus receptor. Because loss of class I and expression of these stress ligands often occurs during viral infection and cancer, NK cells are important components of both antiviral defense and tumor immunosurveillance. NK-cell recognition of potential target cells relies on the ability to detect missing self, as initially proposed by Klause Kärre.1 During surveillance in the peripheral blood, secondary lymphoid organs, and tissue, killer immunoglobulinlike receptors (KIRs) on NK cells recognize HLA-A, HLA-B, and HLA-C expressed by putative targets. Binding of the KIR to its cognate class I HLA molecule delivers an inhibitory signal to the NK cell via phosphorylation of ITIM (immunoreceptor tyrosine-based inhibitory motif) motifs in the KIR’s cytosolic domain (Fig. 1, top). Subsequent recruitment of the SHP-1 tyrosine phosphatase results in suppression of NK-cell effector function and prevents the NK cell from killing the target. However, when an NK cell encounters a virally infected cell or tumor cell that has downregulated class I HLA, the inhibitory KIR-HLA signal is not delivered. If there is a concurrent, activating signal delivered through interaction of a cell stress ligand with its cognate, activating receptor on the NK cell, then NK effector functions can proceed (see Fig. 1, bottom).
Fig. 1.
Target recognition and effector functions of NK cells. (Top) In the absence of viral infection and cancer, NK cells receive inhibitory signals through class I HLA-KIR interactions. (Bottom) Cells that have lost class I HLA expression and upregulated molecules associated with cell stress deliver stimulatory signals to NK cells, resulting in the execution of effector function. The major antitumor functions of NK cells include production of tumoricidal cytokines, direct cytolytic activity, recruitment of dendritic cells (DCs), and antibody-dependent cellular cytotoxicity (ADCC). Ag, antigen; CD, cluster of differentiation; fxn, function; IFN, interferon; Ig, immunoglobulin; MHC, major histocompatibility complex; TCR, time to castration resistance.
NK cells perform 4 major effector functions after successful recognition of target cells (see Fig. 1, bottom). The first is direct cytolytic activity resulting in specific killing of the target through the induction of apoptosis. NK cells, unlike naive CD8+ T cell, contain preformed, cytolytic granules that are released into the synapse between the NK cell and target cell as the NK cell degranulates. These granules contain perforin, a protein that creates holes in the target cell membrane, and various granzymes: serine proteases that cleave caspases in the target cell, thus initiating an apoptotic cascade. The second NK-cell effector function is the release of cytokines with both tumoricidal and chemoattractant properties. Two of the best-characterized NK-cell cytokines are interferon (IFN-γ) and tumor necrosis factor (TNF-α) IFN-γ promotes upregulation of class I HLA by target cells. Although this generally renders the targets less susceptible to NK cell–mediated killing, it is important for the concurrent or subsequent CD8+ T cell response by facilitating presentation of peptides loaded on class I HLA to specific CD8+ T cell clones. TNF-α has direct tumoricidal effects on binding TNF receptor 1 (TNFR1), and also plays important roles in monocyte/macrophage function, and destabilization of regulatory T cells.2 More recently, a third NK-cell effector function has been identified pertaining to the recruitment of dendritic cells (DCs). NK cells have been shown to produce the DC chemoattractant cytokines XCL1, XCL2, and CCL5, as well as the DC growth factor FLT3 ligand.3 These cytokines can recruit DC to tumor-draining lymph nodes and tumor tissue itself, thereby improving antigen presentation to tumor-reactive T cells. In addition, a fourth NK-cell effector function is antibody-dependent cellular cytotoxicity (ADCC). During ADCC, CD16, a receptor that binds the Fc region of immunoglobulin G1, is cross-linked by antibodies bound to extracellular proteins on tumor cells. This cross-linking induces a conformational change in the signaling domains of CD16 leading to phosphorylation of its associated ITAM (immunoreceptor tyrosine-based activating motif), and a subsequent signaling cascade that results in NK-cell degranulation, and lysis of the antibody-coated target cell.4
Two major subpopulations of NK cells have been identified in humans: CD56bright and CD56dimCD16+. CD56bright NK cells are considered developmentally less mature but secrete most of the cytokines discussed earlier. CD56dimCD16+ NK cells represent more mature NK cells, and possess more potent cytolytic capability compared with the CD56bright subset. Approximately 10% of healthy peripheral blood mononuclear cells (PBMCs) are NK cells, and, of these ~5% to 10% are CD56bright with the remaining cells belonging to the CD56dimCD16+ subpopulation. However, patients with prostate cancer, and non–muscle-invasive (NMI) bladder cancer (BlCa), have increased frequencies of circulating NK cells,5,6 suggesting that genitourinary tumors can elicit an immune response in the periphery. Furthermore, infiltration of CD56bright NK cells has been associated with improved survival in both BlCa and renal cell carcinoma (RCC).7-9
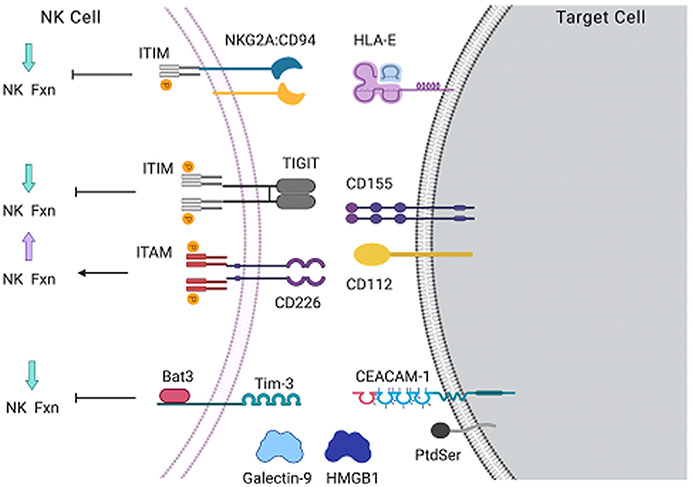
As mentioned earlier, unlike T cells, NK cells do not recognize specific peptide antigens, and instead detect the presence or absence of class I HLA. However, loss of HLA in the presence of additional inhibitory signals elicits a suboptimal effector response, whereas HLA expression in the context of strong activating signals can elicit a strong response.10 The magnitude of NK-cell functional responses is calibrated by integrating stimulatory and inhibitory signals delivered through a diverse array of accessory receptors. Three such inhibitory receptors, NKG2A, Tim-3, and TIGIT (T-cell immunoreceptor with immunoglobulin and ITIM domains), are expressed by NK cells in solid tumors (Fig. 2), with Tim-3 expression reported as anticorrelated with survival in patients with BlCa.11-14 When NKG2A binds its ligand, the noncanonical class I molecule HLA-E expressed on tumor cells, phosphorylation of its cytosolic ITIM occurs, followed by recruitment of the SHP-1 phosphatase. SHP-1 can then prevent phosphorylation of the stimulatory motifs associated with neighboring activating receptors.15 This process ultimately results in suppression of NK-cell effector functions. Tim-3 binds multiple ligands expressed on both tumor cells and immune cells, including soluble galectin-9 and HMGB1, phosphatidylserine on apoptotic cells, and CEACAM-1.16 Unlike many other inhibitory receptors, Tim-3 does not contain an ITIM motif but uses the adaptor protein Bat3 to mediate suppression of NK-cell function. In addition, TIGIT mediates NK-cell inhibition via canonical ITIM signaling like NKG2A; however, it shares the ligands CD112 and CD155 with the activating receptor DNAM-1 (CD226). This competition to bind shared ligands provides another layer of control in tuning the NK-cell response. The inhibitory receptor programmed cell death protein 1 (PD-1), although highly relevant in the context of immune checkpoint blockade (ICB) therapy, is predominantly expressed by tumor-resident T cells, with minimal expression by NK cells.
Fig. 2.
Inhibitory receptors tune the magnitude of NK cell effector function. Expression of inhibitory receptors expressed by NK cells in solid tumors. The ligands for each receptor are shown on the right, and the effect on NK cell effector function shown on the left. ITAM, immunoreceptor tyrosine-based activating motif.
NATURAL KILLER CELLS IN BLADDER CANCER
Natural Killer Cells Infiltrate Bladder Tumors
Most studies of tumor-infiltrating lymphocytes (TILs) in BlCa have focused on T cells, with much recent attention appropriately focused on the role of the PD-1–programmed death-ligand 1 inhibitory axis, and ICB approaches to ameliorate it.17-19 However, a recent study profiling 50 NMI and muscle-invasive (MI) bladder tumors suggests that ~25% of the immune infiltrate is made up of NK cells, making them the most frequent lineage examined.7 In addition, despite this small cohort, a statistically significant correlation was found between the frequency of CD56bright NK cells and overall survival (OS).7 Importantly, in healthy bladder,20,21 and in noninvolved bladder tissue from cystectomy specimens (Farkas, 2020; unpublished observations), NK cells represent a much smaller component of the resident immune cells. This finding suggests that NK cells can specifically infiltrate and conduct immunosurveillance of bladder tumors, making them rational targets for immunotherapeutic modulation.
Natural Killer Cell Function in the Tumor and Peripheral Blood of Patients with Bladder Cancer
Immune exhaustion occurs during chronic infection as well as cancer, and refers to defects in effector functions that result from prolonged stimulation and suppressive factors in the tumor microenvironment (TME).22 Few studies have examined the functional potential of tumor-resident NK cells, in part because of challenges associated with establishing a pipeline in which a sufficient quantity of freshly resected tumor tissue is processed and analyzed in the research laboratory. Similarly, there are few data comparing differences in NK-cell function between patient-matched peripheral blood and tumor. An early study found no defect in the ability of peripheral blood NK cells from patients with BlCa to degranulate in response to HLA-deficient target cells compared with healthy donors. However, there was a substantial defect in degranulation observed in NK cells isolated from tumor and lymph node.5 In contrast, subsequent work showed that the peripheral blood NK cells from patients with NMI BlCa had no cytolytic defect, whereas those from patients with MI disease did.23 In addition, a recent study found that tumor-resident CD56bright NK cells produced more IFN-γ than CD56dimCD16+ cells, but did not compare these cells with NK cells in healthy or patient blood.7
The Role of Natural Killer Cells in Bladder Cancer Treatments
Bacillus Calmette-Guérin (BCG) was the first immunotherapy widely used for cancer treatment, but its precise mechanism of efficacy is not defined. Several studies have pointed toward a role for NK cells in BCG responders. A study of healthy volunteers receiving BCG vaccination showed that production of interleukin (IL)-1b, IL-6, and TNF-α by NK cells was enhanced during ex vivo restimulation after vaccination, suggesting that NK cells from BCG-experienced individuals develop a form of memory.24 In addition, NK cells cultured in the presence of BCG for 1 week gained improved cytolytic function against bladder tumor cell lines.25
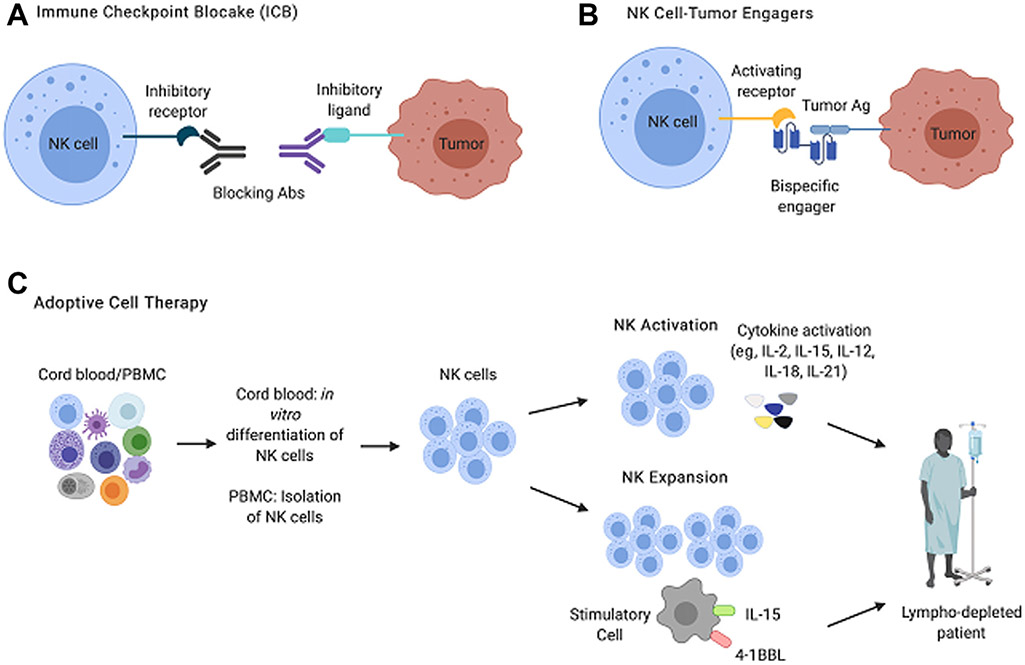
Novel, preclinical studies have also focused on improving NK-cell surveillance against bladder tumors. Combination chemotherapy–epigenetic therapy using cisplatin and an EZH2 inhibitor showed efficacy against MI BlCa, both in direct tumor killing and in improving the NK-cell response against surviving tumor clones.26 NK cells can be differentiated in vitro from cord blood–derived progenitors, and it is possible to activate NK cells isolated from adult peripheral blood with cytokines such as IL-2, IL-15, IL-12, IL-18, and IL-21, all of which improve effector functionality, induce proliferation, and improve NK-cell survival27 (Fig. 3). Both of these approaches can be used as sources of NK cells for adoptive cell transfer therapy. For example, adoptive transfer of IL-2/IL-15-activated NK cells from healthy donors into immunodeficient mice bearing orthotopic, chemoresistant bladder tumors resulted in tumor regression. However, transfer of activated NK cells isolated from patients with high-grade NMI BlCa was less effective, suggesting that peripheral blood NK cells from patients with BlCa are exhausted.28 In addition, preclinical ICB to enhance NK-cell tumor surveillance in murine models and primary human cells ex vivo, although not exclusively in BlCa, have shown that blockade of Tim-3,29,30 TIGIT,13 and NKG2A11 all improve NK function.
Fig. 3.
Major approaches to NK cell immunotherapy: 3 broad immunotherapeutic strategies to improve NK cell tumor surveillance currently used preclinically and in clinical trials. (A) ICB relies on the administration of monoclonal antibodies that prevent signaling through inhibitory receptors. (B) NK-tumor engagers are biologics with double or triple specificities. These molecules confer specificity to NK-tumor interactions by binding a protein antigen expressed by tumor cells and simultaneously delivering a stimulatory signal to the NK cell through an activating or cytokine receptor. (C) Adoptive cell therapy approaches infuse NK cells isolated from PBMCs, or differentiated in vitro from cord blood progenitors, into autologous, haploidentical, or allogeneic recipients. The transferred NK cells can be preactivated with cytokines to enhance NK cell effector functions or can be expanded using irradiated stimulatory cells engineered to express cytokines and stimulatory ligands. Ab, antibody.
Clinical Trials Targeting Natural Killer Cells in Bladder Cancer
Many ongoing and recently completed trials of NK cell–centric immunotherapy involve adoptive transfer of in vitro differentiated NK cells, infusion of preactivated adult NK cells, or transfer of NK cells stably transduced with chimeric antigen receptor (CAR) T cell-–like receptors that confer tumor-antigen specificity (see Fig. 3). Most of these studies use haploidentical donors for source material, and are infused into patients with hematologic malignancies such as chronic myeloid leukemia, acute myeloid leukemia, chronic lymphocytic leukemia, and non-Hodgkin lymphoma.27,31 However, there are also NK cell–centric trials for patients with metastatic, locally advanced, and/or cisplatin-ineligible BlCa. For example, there is a phase I/II dose-escalation study of DF1001, a novel biologic that simultaneously targets Her2+ tumors and activates NK cells (NCT04143711). This study will enroll 220 patients with solid tumors, including those with metastatic and locally advanced BlCa, and includes a DF1001+X-PD-1 arm. Several trials will also determine the effects of cytokine activation on NK-cell efficacy in BlCa, such as a phase II study of 205 patients with cisplatin-ineligible MI BlCa who will receive bempegaldesleukin, a PEGylated IL-2, alone or in combination with X-PD-1 (NCT03785925). Two trials being conducted in China are testing the efficacy of so-called cytokine-induced killer (CIK) cells; autologous PBMCs expanded and activated to enhance NK and T cell function. One of these will examine infusion of CIK cells or CIK cells plus chemotherapy in individuals with MI BlCa (NCT02489890). The second will test infusions of autologous PBMC after culture conditions intended to increase the number and activity of NK and T cells, as well as dendritic cells (D-CIK) using IL-2, IFN-γ, IL-1α, X-CD3, and X-PD-1 (NCT02886897), In addition, a phase Ib neoadjuvant trial in patients with MI BlCa before scheduled, radical cystectomy will compare X-PD-1 treatment with a combination of X-PD-1 and lirilumab (NCT03532451). Lirilumab is monoclonal antibody that blocks the interaction of 3 inhibitory KIRs (KIR2DL1/2/3) with various HLA-C alleles, with the goal of decreasing the inhibitory interactions that might preclude optimal NK-cell function.
NATURAL KILLER CELLS IN KIDNEY CANCER
In RCC, NK cells represent a significant portion of TILs.32 In a rat model of RCC, injection of an NK cell–depleting antibody significantly increased the tumor growth rate, suggesting that NK cells are important in antitumor defense.33 Eckl and colleagues8 showed that, after stratifying patients into 2 groups based on the percentage of their TILs that were NK cells, patients with more NK cells had significantly longer cancer-specific survival. Another study found that, as the tumor T stage increased, the percentage of infiltrating NK cells decreased significantly.34
Natural Killer Cell Function in the Tumor and Peripheral Blood of Patients with Renal Cell Carcinoma
Studies have identified pathways by which the TME inhibits NK-cell function. Prinz and colleagues35 found that a significantly lower number of NK cells in TIL expressed perforin or granzyme B than NK cells from the nontumor kidney. In addition, TIL NK cells had low levels of phosphorylated ERK1/2 (extracellular signal-regulated kinase) and JNK (Jun kinase), which are required to initiate lytic granule exocytosis. ERK activation depends on diacylglycerol, which is metabolized by diacylglycerol kinase (DGK). DGK levels were higher in TIL NK cells than in normal renal tissue, leading to less ERK activation and poor NK-cell degranulation. In addition, DGK inhibition led to improved NK-cell cytotoxicity. These findings suggest that DGK and suppression of the ERK pathway may be a way for RCC cells to escape NK cell–mediated destruction.35 Xia and colleagues36 showed that exosomes from RCC tumor cells inhibited NK-cell activity in a dose-dependent manner. They then showed that the exosomes of patients with RCC expressed increased levels of transforming growth factor (TGF) β-1 and that inhibiting TGFβ-1 improved NK-cell cytotoxic acitivity.36
The Role of Natural Killer Cells in Renal Cell Carcinoma Treatments
There are several medications approved for the treatment of RCC, many of which affect NK-cell activity. IL-2 was the first widely used treatment of advanced RCC and is the only medical treatment that has resulted in a cure. In the 1980s, scientists showed that IL-2 increases NK-cell cytotoxicity.37 In addition, in patients with metastatic RCC treated with IL-2 plus or minus IFNα and histamine, low intratumoral CD57+ NK-cell count was an independent poor prognostic factor (<50 cells/mm2 tumor tissue; hazard ratio, 2.1; P = .01). These findings show that at least some of the antitumor activity of IL-2 is through NK-cell cytotoxicity. Sunitinib and sorafenib are multikinase inhibitors with antiangiogenic effects. Studies have shown that sorafenib but not sunitinib significantly reduced NK-cell activity, possibly through suppressing the ERK pathway.38,39 Axinitib has been shown to exert its antitumor effects at least partially through increasing RCC tumor susceptibility to NK cell–mediated degranulation.40
Clinical Trials Targeting Natural Killer Cells in Renal Cell Carcinoma
Although NK cells do not express PD-1 to the extent that T cells do, there is an active clinical trial to determine the effects of nivolumab on NK-cell function and cytotoxicity in both the blood and tumor tissue in patients with metastatic RCC (NCT03891485). Most other clinical trials, as in BlCa, involve adoptive transfer of in vitro differentiated NK cells or infusion of preactivated adult NK cells. For example, there is currently a trial underway to determine whether there are any differences in progression-free survival (PFS) between patients treated with the PD-1 inhibitor camrelizumab alone or in combination with CIK in patients with metastatic RCC who have progressed on tyrosine kinase inhibitors (NCT03987698). There are several trials that include incubating CIK cells with DCs. Coculture of DCs and CIKs (D-CIKs) improves CIK cell antitumor activity through cell-to-cell contact by increasing NK-cell proliferation and cytotoxicity. One phase II trial is assessing the effect of a PD-1 inhibitor and D-CIK on PFS (NCT02886897) and another is assessing the effect of axitinib in combination with D-CIKs and the PD-1 inhibitor pembrolizumab on PFS (NCT03736330). Alternatively, DCs can be pulsed with tumor lysates or tumor-associated antigens to create a DC vaccine. A study is underway to compare outcomes of DC vaccines and CIKs compared with IL-2/IFNα in patients with RCC (NCT00862303).
NATURAL KILLER CELLS IN PROSTATE CANCER
Although, compared with bladder and kidney cancer, prostate cancer is considered less immunogenic, NK cells have been identified in prostate cancer tumors.41 In both tumor and healthy prostatic tissue, infiltrating NK cells expressed activation markers but had poor degranulation capabilities compared with circulating NK cells. When comparing NK cells found in tumor with those in healthy tissue, expression of the activating receptors NKp46 and NKG2D was significantly decreased and the inhibitory receptor ILT2 was significantly increased. In addition, decreased expression of NKp46 and NKG2D and increased expression of ILT2 were more pronounced in NK cells from metastatic tumors than from localized or locoregional tumors (ie, tumor with extraprostatic extension, seminal vesicle invasion, or local lymph node invasion).42
NK-cell activity has been correlated with prostate cancer outcomes. Increased concentrations of infiltrating NK cells have been associated with a lower risk of cancer progression.43 When examining circulating NK cells, low levels of NK activity have been associated with an increased likelihood of having a positive prostate biopsy.41,44,45 Koo and colleagues46 found that patients with prostate cancer had a significantly higher CD56dim/CD56bright cell ratio compared with controls (41.8 vs 30.3; P<.001) and that the ratio gradually increased as disease stage progressed (P for trend = .001). They also showed that levels of NK-cell activity were significantly lower in patients with prostate cancer than in controls, and patients with higher-stage disease had a greater reduction of activity.46 Another study found that, among patients with metastatic prostate cancer, blood levels of the activating receptors NKp30 and NKp46 were predictive of OS and time to castration resistance (TCR) (OS, P = .0018 and .0009; TCR, P = .007 and P<.0001 respectively).42 There is currently a clinical trial underway to prospectively validate these findings (NCT02963155).
Several studies have also examined how the prostate cancer TME inhibits or evades NK cells. TGFβ has been identified in the prostate cancer microenvironment and is known to inhibit NK-cell function. In addition, in coculture experiments, prostate cancer cells promoted the expression of the inhibitory receptor ILT2 and suppressed the expression of activating receptors NKp46, NKG2D, and CD16, preventing NK-cell activity against tumor cells.47 As in BlCa, exosomes play a critical role in prostate cancer’s ability to invade the immune response. Lundholm and colleagues48 showed that prostate cancer cells secrete exosomes, which downregulate NKG2D expression, leading to impaired cytotoxicity in vitro. As expected from these results, patients with castration-resistant prostate cancer had a significant decrease in the expression of NKG2D on circulating NK cells compared with controls.48
The Role of Natural Killer Cells in Prostate Cancer Treatments
The effects of current prostate cancer therapies on NK cells are not well defined and research on the issue is limited. Studies to determine whether androgen deprivation leads to an increase in NK-cell tumor infiltration have mixed results.43,49 At present, sipuleucel-T is the only immunotherapy approved to treat prostate cancer. Sipuleucel-T is generated by culturing autologous blood mononuclear cells with a fusion protein composed of prostatic acid phosphatase and granulocyte-macrophage colony-stimulating factor. The final product is composed primary of T cells but also contains NK cells.50 To better understand the effects of sipuleucel-T on the TME, a trial was performed in which patients with localized prostate cancer were treated with sipuleucel-T as a neoadjuvant. After radical prostatectomy (RP), TILs in the specimen were assessed and compared with the infiltrating immune cells in the pretreatment prostate biopsy specimens. NK-cell levels were not higher in RP specimens, indicating that NK cells do not play a significant role in sipuleucel-T activity.51
Clinical Trials Targeting Natural Killer Cells in Prostate Cancer
Immunotherapy as a treatment of prostate cancer has not been as well explored as in renal cancer and BlCa. Therefore, there are currently several studies underway to evaluating the effects of various treatments on NK-cell activity. For example, there is a phase 1 clinical trial assessing the effects of intraprostatic injection of mobilan, which is an adenovirus carrying TLR5 (toll-like receptor 5) and a TLR5 activator, on circulating immune-cell levels in patients with prostate cancer, including NK-cell counts (NCT02654938). At Johns Hopkins, a clinical trial is underway to assess the effect of neoadjuvant enoblituzumab, an antibody directed against cancer stem cells, on the intraprostatic immune response, including mean NK-cell density, after RP (NCT02923180). At Henry Ford, a study of intraprostatic injections of an adenovirus carrying IL-12 in patients with recurrence after brachytherapy is currently underway. Outcomes of interest include the association with disease-specific outcomes, such as prostate-specific antigen response and disease-free survival with serum NK-cell cytolytic activity (NCT02555397). Roswell Park Cancer Institute is currently performing a study to determine whether radiation therapy potentiates the effects of sipuleucel-T in patients with bone metastasis. One of the primary end points will be the quantification of circulating NK cells (NCT01833208). There is also a trial that involves transfer of autologous NK cells and the protease inhibitor bortezomib, which has been shown to increase the sensitivity of cancer cells to NK-cell activity52 in patients with metastatic prostate cancer (NCT00720785).
SUMMARY
NK cells recognize target cells that have downregulated HLA class I and upregulated markers of cell stress. These changes often occur during viral infections and cancer and therefore NK cells play an important role in the body’s defense against these disease processes. The magnitude of NK cell’s functional response is determined by integrating stimulatory and inhibitory signals delivered though an array of receptors on the NK cell. NK cells infiltrate bladder, kidney, and prostate tumors. In all 3 malignancies, the frequency of NK cells in tumor tissue has been correlated with survival. There are currently several trials designed to increase NK-cell activity to improve cancer outcomes.
CLINICAL CARE POINTS.
- When an NK cell encounters a potential target cell there are 2 possible outcomes.
- Inhibition: KIRs on NK cells recognize HLA class 1. Binding of the KIR to its cognate class I HLA molecule delivers an inhibitory signal to the NK cell and prevents the cell from killing its target.
- Activation: when an NK cell encounters a tumor cell that has downregulated class I HLA, an inhibitory signal is not delivered.
Magnitude of NK-cell activity depends on the stimulation of several accessory receptors found on NK cells.
NK cells have been found in bladder, kidney, and prostate tumor tissue.
In all 3 malignancies, higher NK-cell levels are associated with better outcomes.
Blockade of inhibitory NK-cell receptors (such as Tim-3) have been shown, in preclinical models and in ex vivo human cells, to improve NK-cell function and may serve as future drug targets.
NK cells likely play a greater role in cancer immunotherapies than previously realized. For example, IL-2, which was the first immunotherapy for RCC and the only one that has led to durable cures, is a potent NK-cell activator.
Several trials are underway to determine how to best activate NK cells.
At present there are many clinical trials that involve adoptive transfer of preactivated NK cells.
KEY POINTS.
Recent studies show that urologic tumors are infiltrated by natural killer (NK) cells and that these NK cells are often dysfunctional.
Strategies interfering with inhibitory axes have significant potential to alleviate this dysfunction.
Preclinical studies show that NK-cell antitumor functions can be enhanced.
Diverse, NK cell-centric clinical trials are ongoing for patients with genitourinary cancers.
Acknowledgments
DISCLOSURE
This work was funded by a Translational Team Science Award from the Department of Defense (CA181008) to N. Bhardwaj and J.P. Sfakianos, and National Institutes of Health R01 CA201189 to N. Bhardwaj. N. Bhardwaj is an advisory board member for Neon, Tempest, CPS Companion Diagnostics, Curevac, Primevax, Novartis, Array BioPharma, Roche, Avidea, Boehringer Ingelheim, Rome Therapeutics, and Roswell Park. N. Bhardwaj is an extramural member of the Parker Institute for Cancer Immunotherapy, and has received research support from Celldex, Genentech, Oncovir, and Regeneron. A.M. Farkas, Z. Gul, and J.P. Sfakianos have no disclosures to report.
REFERENCES
- 1.Kärre K NK cells, MHC class I molecules and the missing self. Scand J Immunol 2002;55(3):221–8. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F Tumour necrosis factor and cancer. Nat Rev Cancer 2009;9(5):361–71. [DOI] [PubMed] [Google Scholar]
- 3.Barry Kevin C., Hsu Joy, Broz Miranda L., et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nature Medicine volume 24, p:1178–1191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez Román VR, Murray JC, Weiner LM. Chapter 1 - antibody-dependent cellular cytotoxicity (ADCC). In: Ackerman ME, Nimmerjahn F, editors. Antibody Fc. Boston: Academic Press; 2014. p. 1–27. [Google Scholar]
- 5.Tsujihashi H, Matsuda H, Uejima S, et al. Role of natural killer cells in bladder tumor. Eur Urol 1989;16(6): 444–9. [DOI] [PubMed] [Google Scholar]
- 6.Audenet F, Farkas AM, Anastos H, et al. Immune phenotype of peripheral blood mononuclear cells in patients with high-risk non-muscle invasive bladder cancer. World J Urol 2018;36(11):1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee N, Ji N, Hurez V, et al. Intratumoral CD56(bright) natural killer cells are associated with improved survival in bladder cancer. Oncotarget 2018;9(92):36492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckl J, Buchner A, Prinz PU, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med (Berl) 2012;90(1):55–66. [DOI] [PubMed] [Google Scholar]
- 9.Schleypen JS, Baur N, Kammerer R, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res 2006;12(3 Pt 1):718–25. [DOI] [PubMed] [Google Scholar]
- 10.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2016;16(1):7–19. [DOI] [PubMed] [Google Scholar]
- 11.Andre P, Denis C, Soulas C, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both t and nk cells. Cell 2018; 175(7):1731–43.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Yu Q, Liu J, et al. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: clinicopathologic correlations and association with survival. J Surg Oncol 2015;112(4):430–5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018;19(7):723–32. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Cai P, Liang T, et al. TIM-3 is a potential prognostic marker for patients with solid tumors: A systematic review and meta-analysis. Oncotarget 2017;8(19):31705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998;391(6669):795–9. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016; 44(5):989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Gong Y, Saci A, et al. Fibroblast growth factor receptor 3 alterations and response to PD-1/PD-L1 blockade in patients with metastatic urothelial cancer. Eur Urol 2019;76(5):599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18(3): 312–22. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z, Liao J, Chen Y, et al. Single-Cell Transcriptomic Map Of The Human And Mouse Bladders. J Am Soc Nephrol 2019;30(11):2159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christmas TJ. Lymphocyte sub-populations in the bladder wall in normal bladder, bacterial cystitis and interstitial cystitis. Br J Urol 1994;73(5):508–15. [DOI] [PubMed] [Google Scholar]
- 22.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15(8):486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carballido J, Alvarez-Mon M, Solovera OJ, et al. Clinical significance of natural killer activity in patients with transitional cell carcinoma of the bladder. J Urol 1990;143(1):29–33. [DOI] [PubMed] [Google Scholar]
- 24.Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol 2014; 155(2):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Cuesta EM, Lopez-Cobo S, Alvarez-Maestro M, et al. NKG2D is a Key receptor for recognition of bladder cancer cells by IL-2-activated NK Cells and BCG Promotes NK Cell Activation. Front Immunol 2015;6:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramakrishnan S, Granger V, Rak M, et al. Inhibition of EZH2 induces NK cell-mediated differentiation and death in muscle-invasive bladder cancer. Cell Death Differ 2019;26(10):2100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov 2020; 19(3):200–18. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira-Teixeira M, Paiva-Oliveira D, Parada B, et al. Natural killer cell-based adoptive immunotherapy eradicates and drives differentiation of chemoresistant bladder cancer stem-like cells. BMC Med 2016; 14(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silva IP, Gallois A, Jimenez-Baranda S, et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res 2014;2:410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ndhlovu LC, Lopez-Verges S, Barbour JD, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012; 119:3734–43. American Society of Hematology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020;382(6): 545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schleypen JS, Von Geldern M, Weiss EH, et al. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer 2003;106(6):905–12. [DOI] [PubMed] [Google Scholar]
- 33.Winter BK, Wu S, Nelson AC, et al. Renal cell carcinoma and natural killer cells: studies in a novel rat model in vitro and in vivo. Cancer Res 1992;52(22):6279–86. [PubMed] [Google Scholar]
- 34.Cozar JM, Canton J, Tallada M, et al. Analysis of NK cells and chemokine receptors in tumor infiltrating CD4 T lymphocytes in human renal carcinomas. Cancer Immunol Immunother 2005;54(9):858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prinz PU, Mendler AN, Brech D, et al. NK-cell dysfunction in human renal carcinoma reveals diacylglycerol kinase as key regulator and target for therapeutic intervention. Int J Cancer 2014;135(8): 1832–41. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y, Zhang Q, Zhen Q, et al. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget 2017;8(23):37783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henney CS, Kuribayashi K, Kern DE, et al. Interleukin-2 augments natural killer cell activity. Nature 1981;291(5813):335–8. [DOI] [PubMed] [Google Scholar]
- 38.Krusch M, Salih J, Schlicke M, et al. The kinase inhibitors sunitinib and sorafenib differentially affect NK cell antitumor reactivity in vitro. J Immunol 2009;183(12):8286–94. [DOI] [PubMed] [Google Scholar]
- 39.Manuela S, Matthias K, Baessler T, et al. The kinase inhibitors sunitinib (Sutent®) and Sorafenib (Nexavar®) Differentially Affect Reactivity of NK cells against renal cell cancer. Blood 2007;110(11):4182. [Google Scholar]
- 40.Morelli MB, Amantini C, Santoni M, et al. Axitinib induces DNA damage response leading to senescence, mitotic catastrophe, and increased NK cell recognition in human renal carcinoma cells. Oncotarget 2015;6(34):36245–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barkin J, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and prostate cancer: a pilot study. Can J Urol 2017;24(2):8708–13. [PubMed] [Google Scholar]
- 42.Pasero C, Gravis G, Granjeaud S, et al. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget 2015;6(16):14360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gannon PO, Poisson AO, Delvoye N, et al. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods 2009;348(1–2):9–17. [DOI] [PubMed] [Google Scholar]
- 44.Tae BS, Jeon BJ, Lee YH, et al. Can natural killer cell activity help screen patients requiring a biopsy for the diagnosis of prostate cancer? Int Braz J Urol 2020;46:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vidal AC, Howard LE, Wiggins E, et al. Natural killer cell activity and prostate cancer risk in veteran men undergoing prostate biopsy. Cancer Epidemiol 2019;62:101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koo KC, Shim DH, Yang CM, et al. Reduction of the CD16(−)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS One 2013;8(11):e78049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasero C, Gravis G, Guerin M, et al. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res 2016;76(8):2153–65. [DOI] [PubMed] [Google Scholar]
- 48.Lundholm M, Schroder M, Nagaeva O, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One 2014;9(9):e108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen YC, Ghasemzadeh A, Kochel CM, et al. Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the Myc-CaP model. Prostate Cancer Prostatic Dis 2018;21(1):113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother 2013;62(1):137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014; 106(11). dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellom ST Jr, Dudimah DF, Thounaojam MC, et al. Modulatory effects of bortezomib on host immune cell functions. Immunotherapy 2015;7(9):1011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]