Abstract
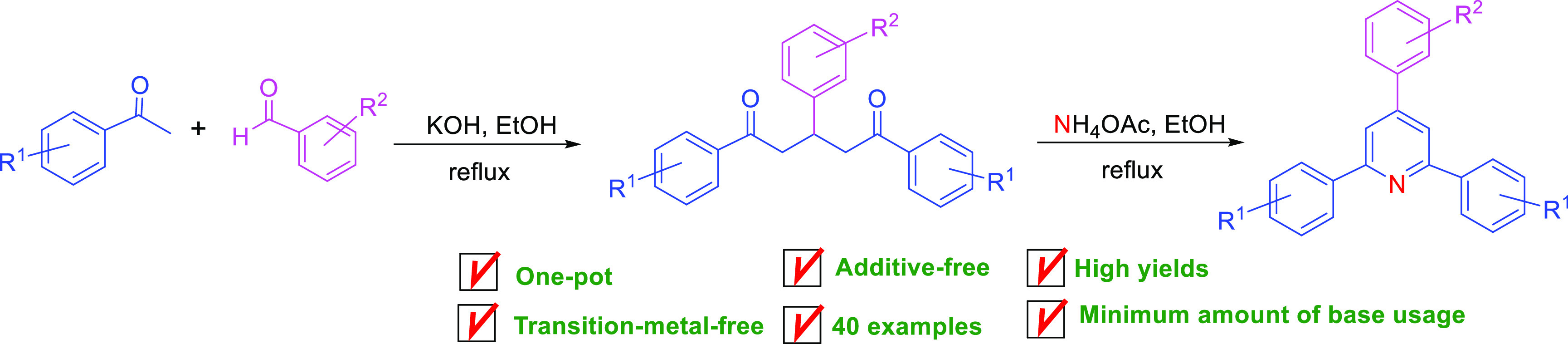
We developed a facile and green one-pot synthetic method for substituted 1,3,5-triaryl-1,5-diketones by Claisen–Schmidt condensation following Michael addition reaction of aryl ketones and aryl aldehydes under a transition-metal-free condition. This convenient one-pot synthetic strategy has several advantages, including being transition-metal-free, having no extra additives or reagents, having a broad substrate scope, having a high isolated yield, having a minimum amount of base employment, having a shorter reaction time, use of cheap starting materials, cost-effectiveness, and being environment friendly. Some of the chemical structures of 1,5-diketones were confirmed by X-ray single-crystal diffraction analysis. The application of 1,5-diketones was demonstrated in the preparation of 2,4,6-triaryl pyridine derivatives under a catalyst-free system using ammonium acetate as a nitrogen source.
Introduction
In the construction of natural products, medicinal compounds, functionalized five- or six-membered-ring heterocycles, and polycyclic aromatic compounds, 1,5-diketones have been used as important building blocks.1−3 In particular, 1,5-diones are key intermediates in the synthesis of various heterocyclic compounds such as substituted pyridines,1,4−9 quinolines,10,11 pyrylium,12 pyrylogen,13,14 and 2-aroyl-3,5-diarylthiophenes.15 These heterocycles have important applications in modern drug design and in the chemistry of functional materials.6 Consequently, much effort has been devoted to the chemical synthesis of 1,5-diketones.8,9,16 The most common classical methods of 1,5-diones synthesis are (i) Claisen–Schmidt condensation following Michael addition reaction of aryl methyl ketones and benzaldehyde derivatives under a basic condition17−25 and using silica vanadic acid as a heterogeneous catalyst26 (Scheme 1a), (ii) catalyzed Michael reactions of chalcones (α,β-unsaturated ketones) with aryl methyl ketones,27 silyl enol ethers,28,29 and diphenacyl sulfides30 (Scheme 1b), (iii) from α,β-unsaturated ketones via base-induced retro-aldol and Michael addition using copper and cobalt as catalysts,31,32 (Scheme 1c), (iv) via nickel on silica-alumina-catalyzed direct α-alkylation of aryl methyl ketones with benzyl alcohol33 (Scheme 1d), and (v) silver- and rhodium-catalyzed reactions of aromatic and aliphatic aldehydes with cyclopropanol and cyclobutanol derivatives.1,34 However, these synthetic methods use expensive catalysts and additives, toxic organic solvents, and air- and moisture-sensitive materials. Additionally, the yields of 1,5-diketones through these reactions were low either due to by-product formation such as aromatic aldehydes31,32 or no product formation at all under base-mediated condensation.22 Hence, the search for an efficient synthetic method for 1,5-diketones based on inexpensive starting materials should be given high attention in organic chemistry.
Scheme 1. Synthetic Methods of 1,5-Diketones.
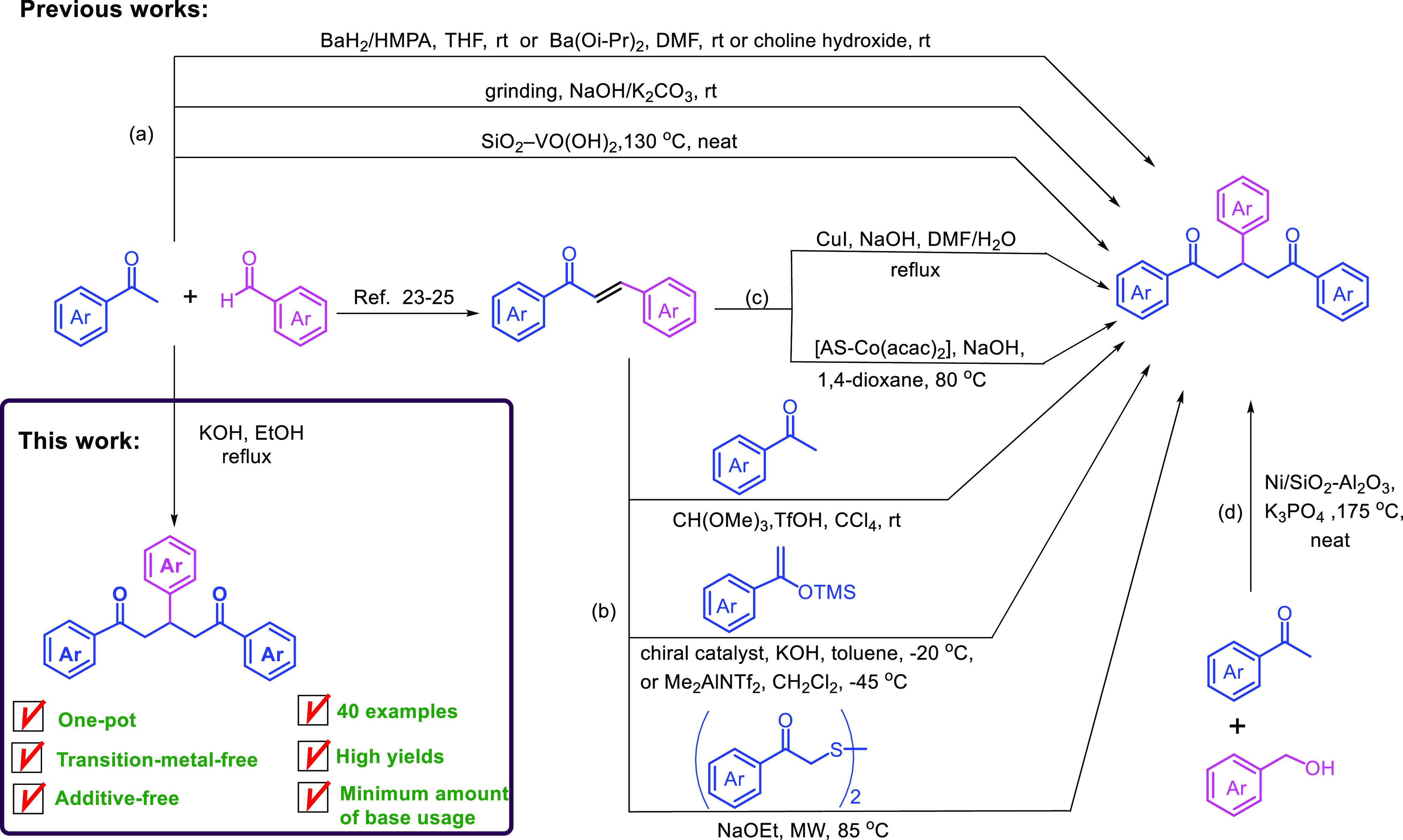
Over the past decades, one-pot or multi-component reactions have been widely employed in organic synthesis because of their synthetic efficiency relative to the step-wise reactions.5,35−37 Moreover, these synthetic strategies have several advantages, including cost-effectiveness, high product yield, intrinsic atom economy, short reaction time, simple experimental procedure, and minimum wastage. Although there have been significant developments in the one-pot synthetic method, the previous approaches for 1,5-diketone synthesis were conducted in the presence of additives or ligands as well as expensive and toxic catalysts (Scheme 1a,d). To overcome such limitations, the development of a facile, inexpensive, expedient, and efficient synthetic method is yet to be explored.
In continuation of our efforts on the development of efficient synthetic methods for the generation of heterocyclic compounds,38−40 we report here a facile and green one-pot synthetic method for substituted 1,3,5-triaryl-1,5-diketones through aldol condensation of aryl ketones and aryl aldehydes with subsequent Michael addition reaction under the transition-metal-free condition.
Results and Discussion
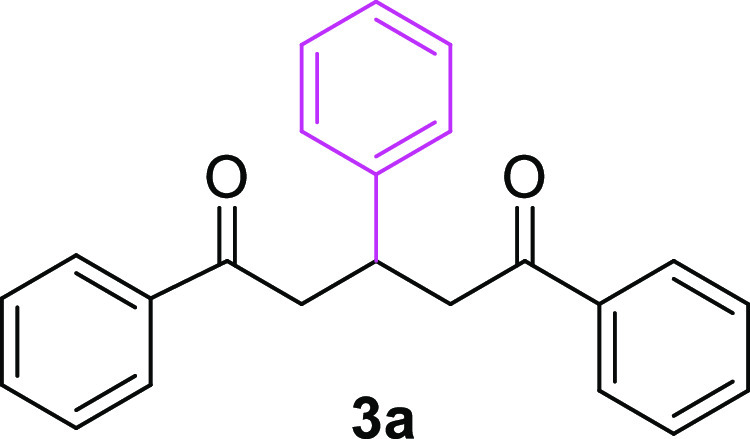
Initially, we selected relatively low-cost acetophenone (1a) and benzaldehyde (2a) as model substrates for the synthesis of 1,5-dione 3a in one pot. Accordingly, 2 equiv of compound 1a were dissolved in anhydrous ethanol in the presence of 1 equiv of aqueous KOH and the reaction mixture was stirred for 0.25 h at 0 °C. After the enol formation was ensured, 1 equiv of benzaldehyde (2a) was added and the reaction temperature was raised to room temperature (rt) to afford the well-known 1,5-diketone 3a(31) at a yield of 37% over 18 h (Table 1, entry 1). The yield of 1,5-dione 3a was slightly improved to 40% upon employing 2 equiv of KOH under the same temperature and reaction time (Table 1, entry 2); however, the yield declined to 36% with a shorter reaction time (Table 1, entry 3).
Table 1. Optimization of the Reaction Conditions for Synthesis of 3aa in One Pot.
| entry | base (equiv) | T (°C) | solvent | time (h) | yield (%)b |
|---|---|---|---|---|---|
| 1 | KOH (1.0) | Rt | EtOH | 18 | 37 |
| 2 | KOH (2.0) | Rt | EtOH | 18 | 40 |
| 3 | KOH (2.0) | Rt | EtOH | 12 | 36 |
| 4 | KOH (1.0) | reflux | EtOH | 3 | 62 |
| 5 | KOH (2.0) | reflux | EtOH | 3 | 49 |
| 6 | KOH (1.0) | reflux | MeOH | 3 | 49 |
| 7 | KOH (1.0) | reflux | MeCN | 3 | 60 |
| 8 | NaOH (1.0) | reflux | EtOH | 3 | 55 |
| 9 | NaOH (1.0) | reflux | MeOH | 3 | 47 |
| 10 | NaOH (2.0) | reflux | EtOH | 3 | 52 |
Reaction conditions: 1a (1.88 mmol, 2.0 equiv), 2a (0.94 mmol, 1.0 equiv).
Isolated yields.
To further prepare 1,5-diketone 3a in a high yield, we refluxed the reaction mixture under a reduced reaction time. Gratifyingly, 62% yield of 3a was produced using 1 equiv of KOH under refluxing for 3 h (Table 1, entry 4). However, the reaction yield decreased to 49% with an increased amount of KOH at the same reaction time. We also evaluated the effect of solvent types on the product yield under the same length of reaction time (Table 1, entries 6–10). In line with this, acetonitrile (MeCN) gave better efficiency (60%) than methanol (49%) with the same quantity of KOH (1 equiv). Changing bases such as NaOH did not significantly improve the yield of 3a in the different solvents (Table 1, entries 8–10) with the exception of a relatively better yield in ethanol than in methanol.
Therefore, the optimized reaction conditions for the one-pot synthesis of 3a were taken to be 1 equiv of KOH as base, ethanol as solvent, and refluxing (Table 1, entry 4). The one-pot synthetic strategy produced compound 3a in line with the principles of green chemistry.41 The 1H NMR, 13C NMR, and mass spectrometry data of 3a are in good agreement with the reported data.34
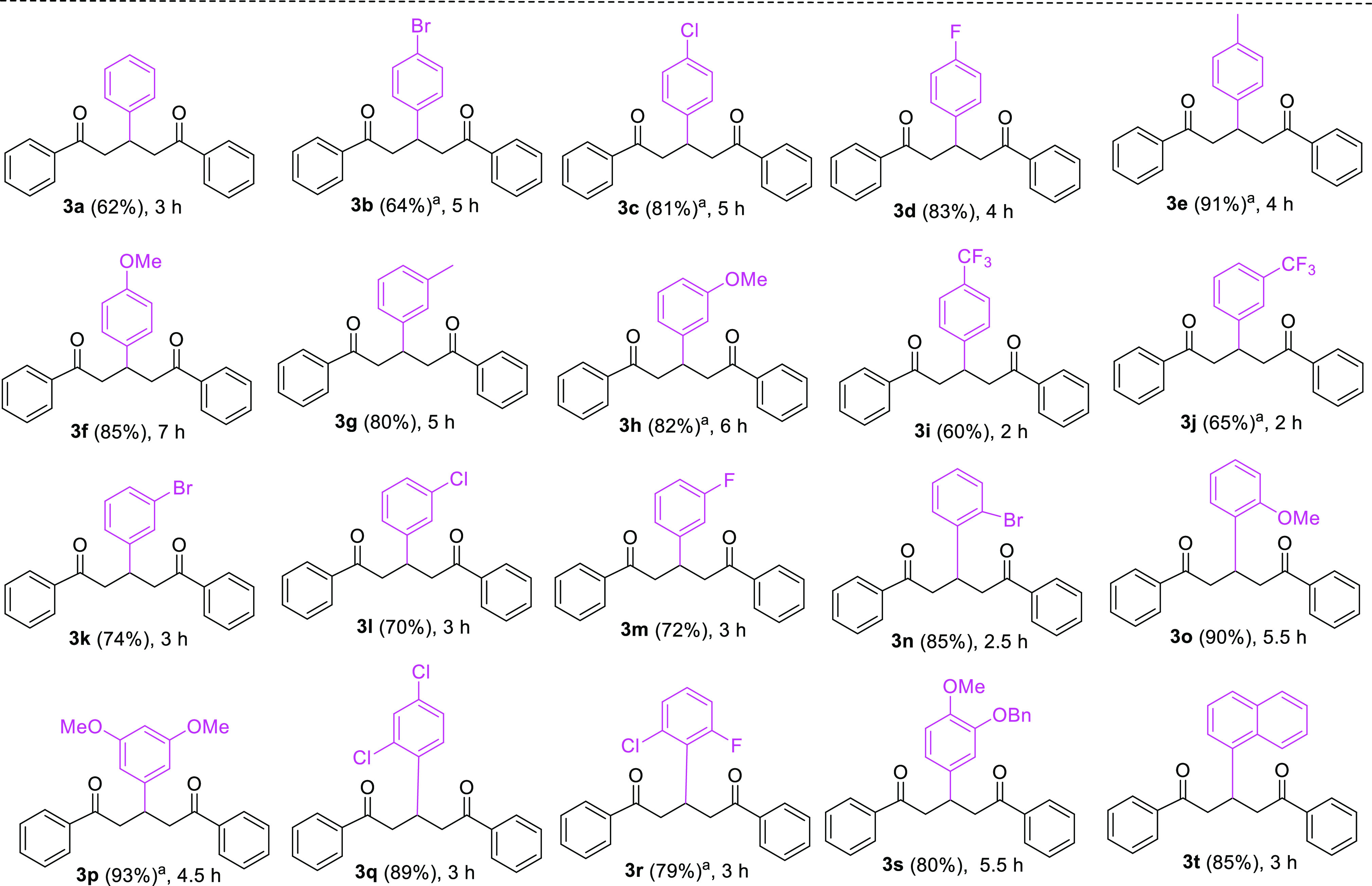
After having established the optimized reaction conditions for the one-pot synthesis of 3a, we studied the substrate scope of KOH-mediated reactions using several substituted aryl aldehydes (2b-t) and acetophenone (1a), and the results are summarized in Table 2. Thus, the Claisen–Schmidt condensation-Michael addition reaction of aryl methyl ketone (1a) with aryl aldehydes (2c–h and 2n–s), which bear different mono- or di-substituents such as bromide at the ortho-position and chlorides, fluorides, methyl, and methoxy at different positions on their benzene ring reacted smoothly to produce the corresponding 1,5-diketones 3c–h and 3n–s in high yields ranging from 79 to 93% under the same reaction condition.
Table 2. Substrate Scope with Respect to Aryl Aldehydes 2.
Dichloromethane (0.5 mL) was added to dissolve the aldehydes 2b, 2c, 2e, 2h, 2j, 2p, and 2r.
Similarly, benzaldehyde derivatives containing trifluoromethyl functionality at both para- and meta-positions (2i and 2j), chloro and fluoro substituents at the meta-position (2l and 2m), and bromo groups at both para- and meta-positions (2b and 2k) offered good yields (60–74%) of the respective products (3b, 3i–m). Similarly, the yield of 1,5-dicarbonyl 3t was 85% through the aldol reaction of 1a with the aldehyde bearing two fused benzene rings (2t) followed by a Michael reaction. The reaction time of aryl ketone 1a with benzaldehyde derivatives 2 possessing an electron-withdrawing group was observed to be shorter (2–3 h) than those aldehydes which contained electron-donating substituents (4–7 h). This observation was also supported by Kamble and Shankarling.22
Next, we evaluated the aldol condensation reaction of 4-methoxybenzaldehyde (2f) with numerous aryl methyl ketones (1b–t) having electron-donating and electron-withdrawing substituents on the aromatic rings under the established optimized condition (Table 3).
Table 3. Substrate Scope of Substituted Aryl Ketones 1.
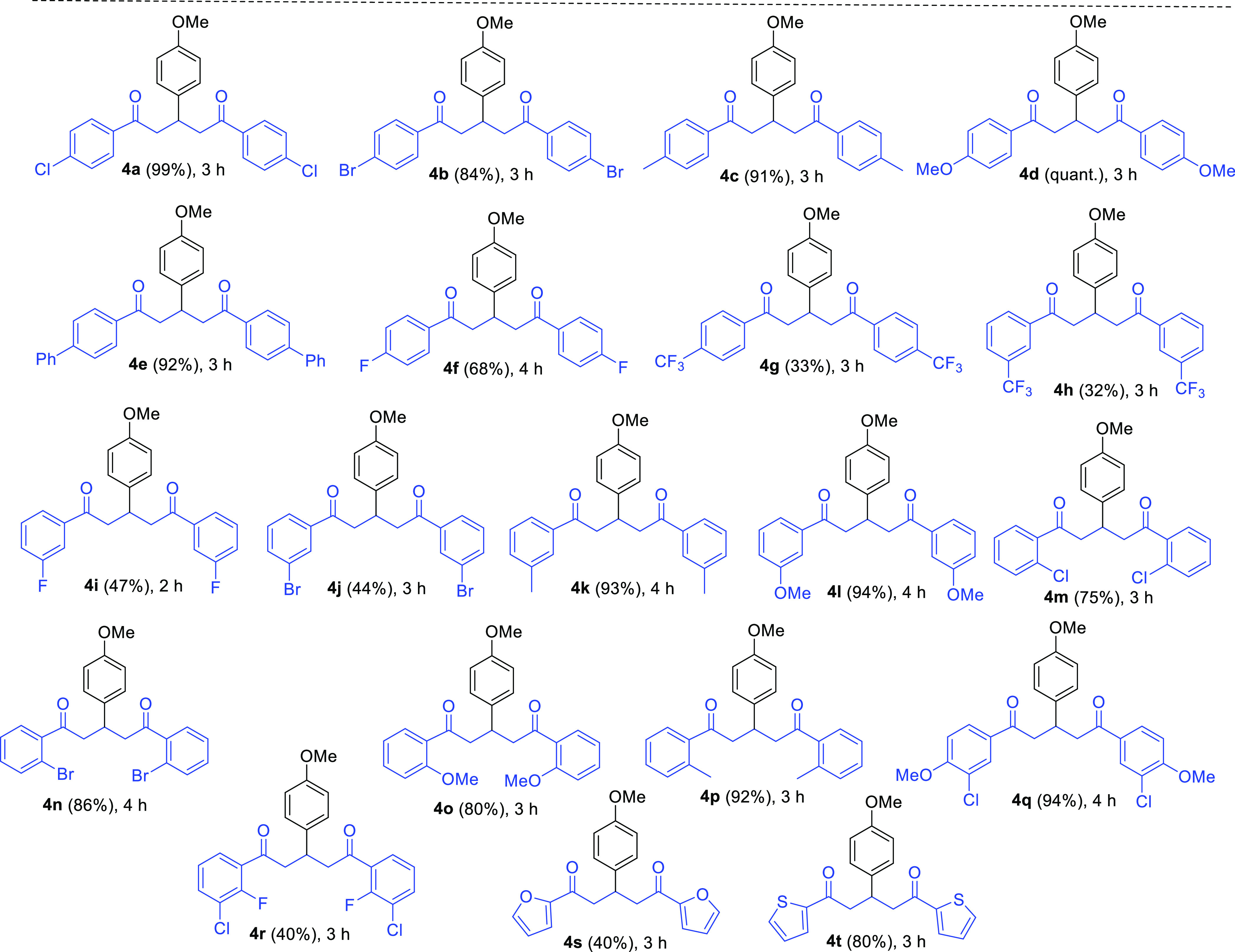
The reaction of aryl methyl ketones with methyl, methoxy, phenyl, bromo, and chloro groups present on the aryl rings occurred efficiently with the aldehyde 2f to produce the corresponding 1,5-diketones 4a–e, 4k, 4l, and 4n–q in very good to excellent yields (80% to quantitative) regardless of the presence of their substituents at the para-, meta-, and ortho-positions. On the other hand, the sequential Claisen–Schmidt condensation-Michael addition reaction of 2f with substituted acetophenones such as 4′-fluoroacetophenone (1f), 4′-(trifluoromethyl)acetophenone (1g), 3′-(trifluoromethyl)acetophenone (1h), 3′-fluoroacetophenone (1i), 3′-bromoacetophenone (1j), and 3′-chloro-2′-fluoroacetophenone (1r) produced poor to moderate yields (32–68%) of 1,5-dicarbonyls 4f–j and 4r. The poor yields of the products such as 4g, 4h, and 4r may be attributed to the high electron-withdrawing nature of the substituents on the aryl substrates. The scope of the reaction was further examined on simple heterocyclic aromatic compounds. Therefore, the condensation reaction of 2f with 2-acetylthiophene (1t) offered a very good yield (80%) of 4t, whereas the reaction of 2-acetylfuran (1s) with 2f produced 1,5-dione 4s in unsatisfactory yield (40%).
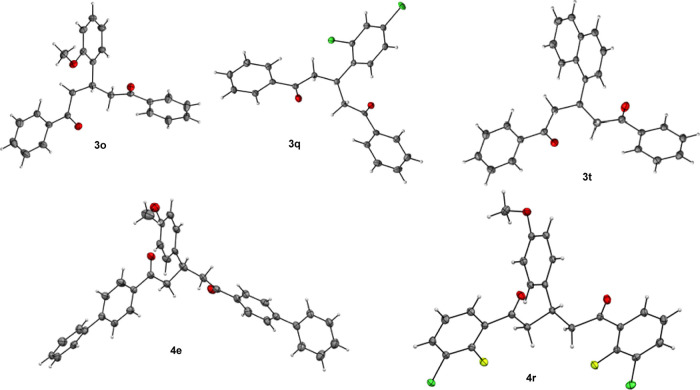
The chemical structures of 1,5-dicarbonyls 3o, 3q, 3t, 4e, and 4r were confirmed by single X-ray crystallography (Figure 1).42
Figure 1.
Single X-ray crystal structure of 1, 5-dicarbonyls 3o, 3q, 3t, 4e, and 4r.
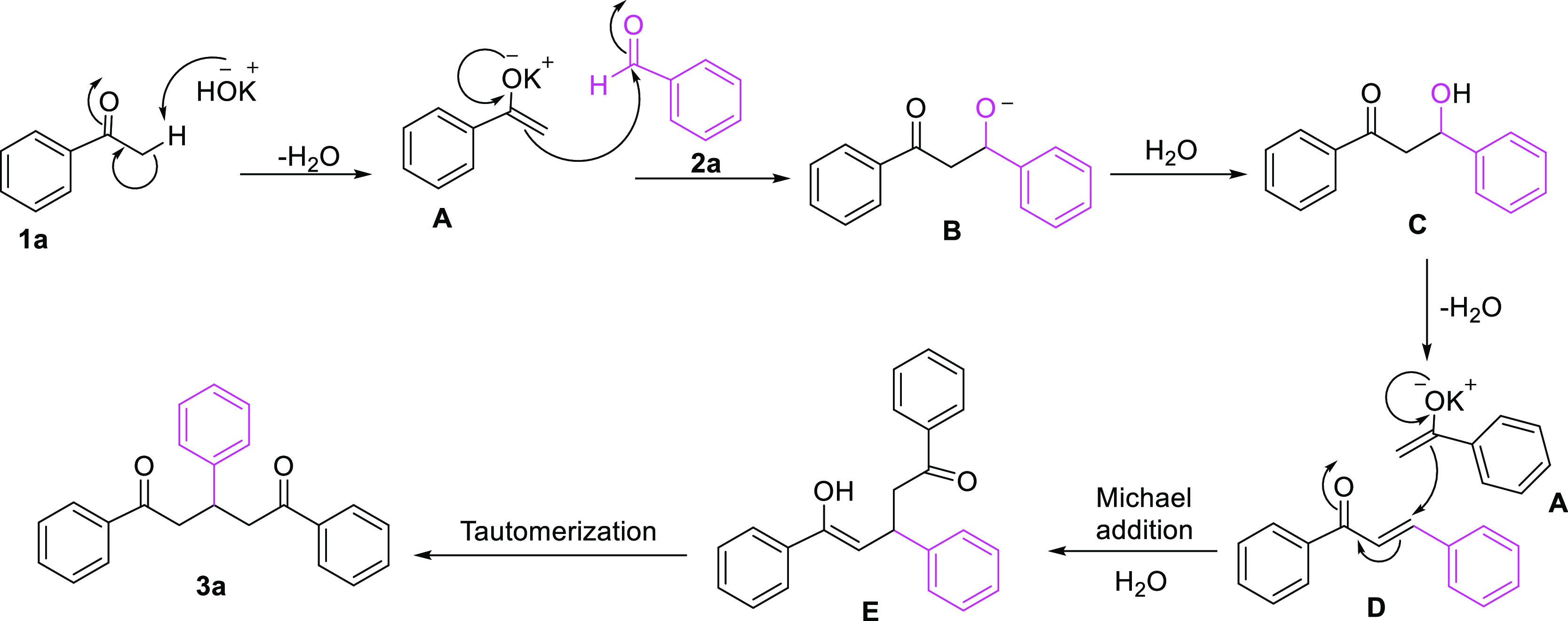
Upon comparison with the previous reports,19,22,27 we propose a possible reaction mechanism for the KOH-promoted synthesis of 1,5-diketones in one pot (Scheme 2). Initially, KOH abstracts the α-proton of 1a to give the enolate specie A. Next, nucleophilic addition of enol A to the carbonyl carbon of 2a affords the oxyanion B, which is then protonated from water to provide the aldol addition product C. The β-hydroxy-ketone C is then subjected to dehydration reaction under heating to generate the chalcone D. Subsequently, a second nucleophile A attacks the β-position of the α,β-unsaturated ketone D through Michael addition and hydrolysis reaction to generate the enol E. Finally, tautomerization of E affords the desired 1,5-diketone 3a.
Scheme 2. Plausible Mechanism for the One-Pot Synthesis of 1, 5-Diketone 3a.
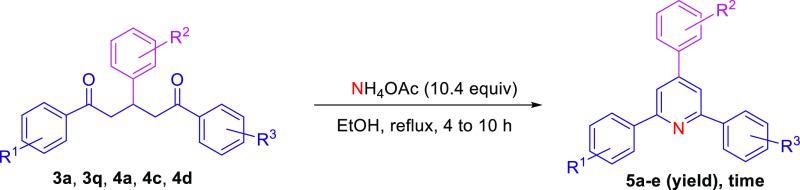
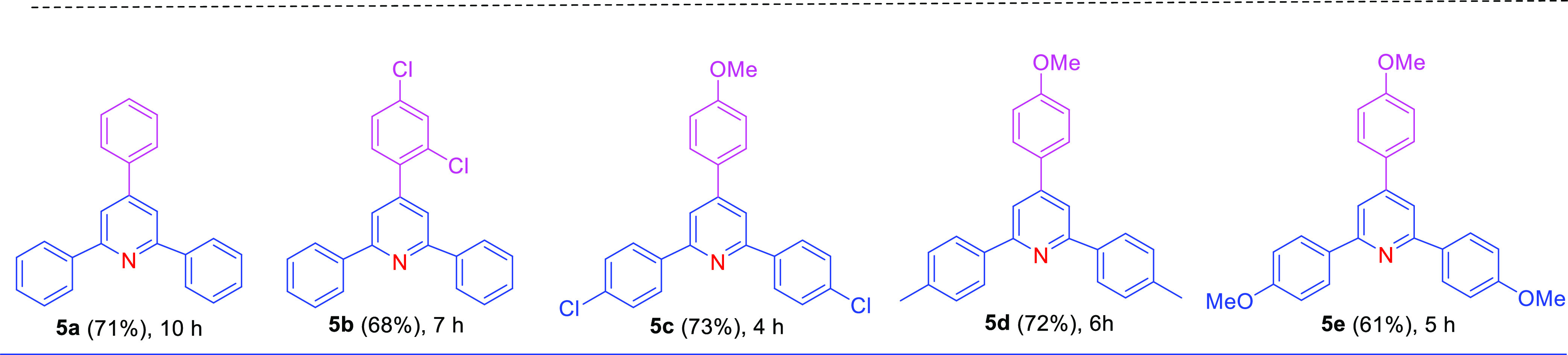
As stated above, 1,5-diketones are versatile intermediates for the synthesis of heterocyclic compounds that have significant applications in modern drug design and in functional material chemistry. Functionalized pyridines are among the most common heterocyclic compounds that have been used in therapeutic drugs,5,43 chemosensors,44 photosensitizers,45 as ligands for catalysis,46 and as starting materials in the synthesis of agricultural chemicals such as insecticides, herbicides, and surfactants.47 Thus, several synthetic methods have been established to produce 2,4,6-triaryl pyridine derivatives.48−56 The most common approach for the synthesis of substituted pyridine is ammonium acetate (NH4OAc)-mediated cyclization of 1,5-diketones.54,57−62 Accordingly, the treatment of 1,5-diketones 3 and 4 with NH4OAc as a nitrogen source successfully produced good yields of the reported pyridine-based molecules 5a–e(53,55,63,64) within a moderate reaction time under a catalyst-free system (Table 4). Our finding reveals that 1,5-diones with weak electron-donating groups, such as methyl and chloro groups, on the aromatic ring could provide relatively higher yields of the corresponding 2,4,6-triaryl-substituted pyridines (5a–d) than those substrates with strong electron-donating groups, such as the methoxy group (5e). These results are in accordance with those reported by Chen et al., who synthesized numerous polysubstituted pyridines using a one-pot protocol with Cu(OTf)2 as the catalyst.53−55
Table 4. Catalyst-Free Cyclization of 1,5-Diketones into 2,4,6-Triaryl Pyridine Derivatives.
Conclusions
In conclusion, we report here a highly efficient, facile, and green one-pot synthesis of highly substituted 1,5-diketones via Claisen–Schmidt condensation-Michael addition reaction of numerous aryl methyl ketones with diversified aryl aldehydes in the absence of transition-metal catalysts. This one-pot synthetic strategy has several advantages, including additives or transition-metal-free condition, minimum amount of base associated to promote the reaction, wide substrate scope, short reaction time, good to excellent yields, use of inexpensive starting materials, eco-friendliness, and simple workup and reaction procedure. Moreover, the application of the synthesized 1,3,5-triaryl-1,5-diketones was demonstrated in the generation of 2,4,6-triaryl pyridines in good yields using NH4OAc as the nitrogen source.
Experimental Section
General Information
All reactions were conducted in flame-dried glassware under a nitrogen atmosphere. All reagents obtained from commercial sources were used without purification unless otherwise mentioned. Flash column chromatography was carried out by Silica Gel Geduran Si 60 (0.040–0.063 mm, E. Merck). Thin-layer chromatography (TLC) was performed on pre-coated glass plates of Silica Gel 60 F254 (0.25 mm, E. Merck); detection was executed by spraying with a solution of Ce(SO4)2, (NH4)2MoO4, and H2SO4 in water and subsequently heating on a hot plate. UV light for TLC analysis was a UVGL-25 compact UV lamp (4 W/254 nm). Melting points were determined with a MP-2D melting apparatus. 1H NMR, 13C NMR, and DEPT spectra were recorded by Bruker DRX500 and AVIII 500. Chemical shifts are in ppm from Me4Si, generated from the CDCl3 lock signal at δ 7.24 and 77.16 ppm for 1H and 13C NMR, respectively. Multiplicities are reports by using the following abbreviations: s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, m = multiplet, br = broad, dd = doublet of doublets, dt = doublet of triplets, td = triplet of doublets; J = coupling constant values in Hertz. High-resolution mass spectrometry (HR-MS) was performed on a Waters Premier XE instrument with an ESI source. Structural assignments were made with additional information from selective 1D-TOCSY, 2D-HSQC, 2D-HMBC, and X-ray experiments.
General Procedure for the One-Pot Synthesis of 1,5-Diketones17,22−25,65,66
To a stirred solution of acetophenone derivatives 1a–t (0.540–0.832 mmol, 1 equiv) in ethanol (EtOH) (1.5 mL), 60% aqueous solution of KOH (0.54–0.832 mmol, 1 equiv) was added and the mixture was stirred at 0 °C for 0.25 h. After the enol is formed, the aldehydes 2a–t (0.54–0.832 mmol, 1 equiv) were added to the reaction solution at the same temperature. Less amount of dichloromethane (0.5 mL) was added to dissolve for those aldehydes such as 2b, 2c, 2e, 2h, 2j, 2p, and 2r, which were not soluble in EtOH. Upon raising the reaction temperature to rt, the resulting mixture was stirred for 1 h till complete consumption of the starting materials. After TLC showed the formation of the corresponding chalcone intermediates via Claisen–Schmidt condensation, other acetophenone derivatives 1a–t (1.08–1.664 mmol, 2 equiv) were added for the preparation of the desired 1,5-diketones 3a–t and 4a–t through Michael addition reaction and the resulting reaction mixture was stirred at rt over 1–2 h. Those Michael addition reactions in which their starting materials could not be consumed at rt were refluxed at 100 °C from 2 to 7 h depending on the reactivity of the ketone and aldehyde functionality. The progress of the reaction was monitored using TLC analysis under UV light. Then, EtOH was removed using a rotary vacuum evaporator and the resulting crude products were dissolved in ethyl acetate and cooled to 0 °C using ice. Next, 1 N HCl solution was added drop-wise till the pH of the solution became 4, which was then neutralized again by the addition of distilled water. The required products were extracted from the aqueous phases with ethyl acetate and the combined organic phases were washed with saturated brine solution. Next, the organic layers were separated and dried over anhydrous MgSO4, filtered, and concentrated in rotary vacuum to give the crude products. Purification of the crude products by flash column chromatography using 3–15% ethyl acetate in hexane afforded the corresponding pure 1,5-diketones 3a–t and 4a–t.
1,3,5-Triphenylpentane-1,5-dione (3a)31
 Colorless
syrup (192.0 mg, 62% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (d, J = 7.9 Hz, 4H, ArH), 7.53 (t, J = 7.4 Hz, 2H, ArH),
7.42 (t, J = 7.4 Hz, 4H, ArH), 7.28–7.26 (m,
4H, ArH), 7.17–7.15 (m, 1H, ArH), 4.05 (p, J = 7.1 Hz, 1H, CH), 3.48 (dd, J = 7.1, 16.7 Hz,
2H, CH2), 3.34 (dd, J = 7.0, 16.6 Hz,
2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.7 (CO), 143.9 (C), 137.0 (C), 133.2 (C), 128.8
(CH), 128.7 (CH), 128.3 (CH), 127.6 (CH), 126.8 (CH), 45.0 (CH2), 37.3 (CH); HRMS (ESI) m/z: calcd for C23H20O2Na [M + Na]+, 351.1356; found, 351.1346.
Colorless
syrup (192.0 mg, 62% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (d, J = 7.9 Hz, 4H, ArH), 7.53 (t, J = 7.4 Hz, 2H, ArH),
7.42 (t, J = 7.4 Hz, 4H, ArH), 7.28–7.26 (m,
4H, ArH), 7.17–7.15 (m, 1H, ArH), 4.05 (p, J = 7.1 Hz, 1H, CH), 3.48 (dd, J = 7.1, 16.7 Hz,
2H, CH2), 3.34 (dd, J = 7.0, 16.6 Hz,
2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.7 (CO), 143.9 (C), 137.0 (C), 133.2 (C), 128.8
(CH), 128.7 (CH), 128.3 (CH), 127.6 (CH), 126.8 (CH), 45.0 (CH2), 37.3 (CH); HRMS (ESI) m/z: calcd for C23H20O2Na [M + Na]+, 351.1356; found, 351.1346.
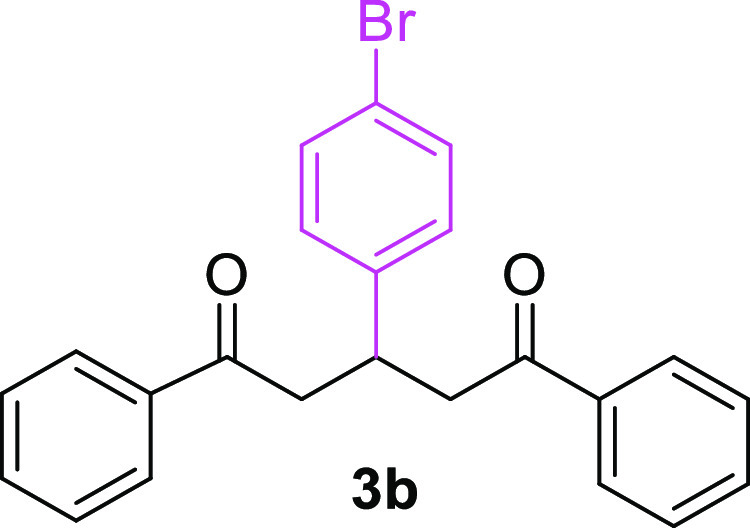
3-(4-Bromophenyl)-1,5-diphenylpentane-1,5-dione (3b)31
 White solid (142 mg, 64% yield); mp 110–112 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 1.3, 8.5 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH),
7.44–7.41 (m, 4H, ArH), 7.38–7.36 (m, 2H, ArH), 7.17–7.14
(m, 2H ArH), 4.03 (p, J = 7.0 Hz, 1H, CH), 3.46 (dd, J = 6.7, 16.8 Hz, 2H, CH2), 3.31 (dd, J = 7.3, 16.8 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 198.3 (CO), 143.0 (C),
136.9 (C), 133.4 (CH), 131.8 (CH), 129.4 (CH), 128.8 (CH), 128.2 (CH),
120.5 (C), 44.8 (CH2), 36.6 (CH); HRMS (EI) m/z: calcd for C23H19BrO2Na [M+], 406.0572; found, 406.0568.
White solid (142 mg, 64% yield); mp 110–112 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 1.3, 8.5 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH),
7.44–7.41 (m, 4H, ArH), 7.38–7.36 (m, 2H, ArH), 7.17–7.14
(m, 2H ArH), 4.03 (p, J = 7.0 Hz, 1H, CH), 3.46 (dd, J = 6.7, 16.8 Hz, 2H, CH2), 3.31 (dd, J = 7.3, 16.8 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 198.3 (CO), 143.0 (C),
136.9 (C), 133.4 (CH), 131.8 (CH), 129.4 (CH), 128.8 (CH), 128.2 (CH),
120.5 (C), 44.8 (CH2), 36.6 (CH); HRMS (EI) m/z: calcd for C23H19BrO2Na [M+], 406.0572; found, 406.0568.
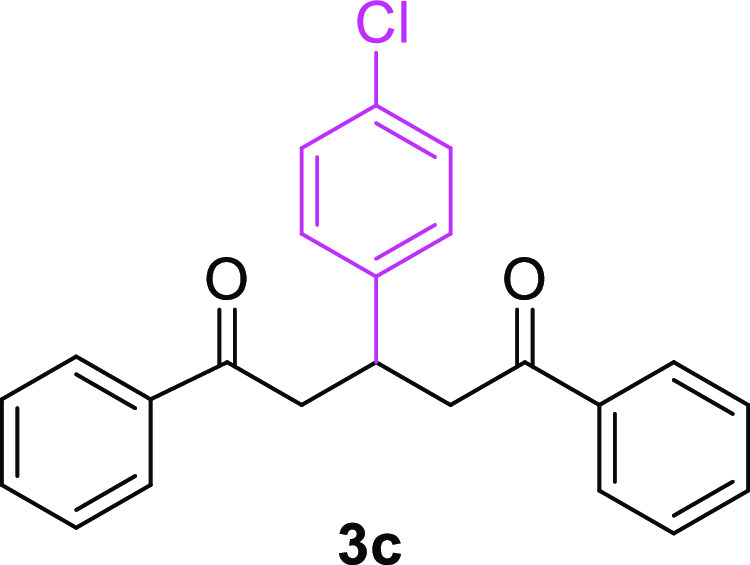
3-(4-Chlorophenyl)-1,5-diphenylpentane-1,5-dione (3c)23,31
 White
solid (207.9 mg, 81% yield); mp 100–102 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 0.8, 8.0 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH),
7.44–7.41 (m, 4H, ArH), 7.23–7.20 (m, 4H, ArH), 4.04
(p, J = 7.0 Hz, 1H, CH), 3.46 (dd, J = 6.7, 16.7 Hz, 2H, CH2), 3.31 (dd, J = 7.2, 16.7 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 198.4 (CO), 142.4 (C), 136.9
(C), 133.4 (CH), 132.5 (C), 129.0 (CH), 128.9 (CH), 128.8 (CH), 128.2
(CH), 44.9 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C23H19ClO2Na [M + Na]+, 385.0966; found, 385.0958.
White
solid (207.9 mg, 81% yield); mp 100–102 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 0.8, 8.0 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH),
7.44–7.41 (m, 4H, ArH), 7.23–7.20 (m, 4H, ArH), 4.04
(p, J = 7.0 Hz, 1H, CH), 3.46 (dd, J = 6.7, 16.7 Hz, 2H, CH2), 3.31 (dd, J = 7.2, 16.7 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 198.4 (CO), 142.4 (C), 136.9
(C), 133.4 (CH), 132.5 (C), 129.0 (CH), 128.9 (CH), 128.8 (CH), 128.2
(CH), 44.9 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C23H19ClO2Na [M + Na]+, 385.0966; found, 385.0958.
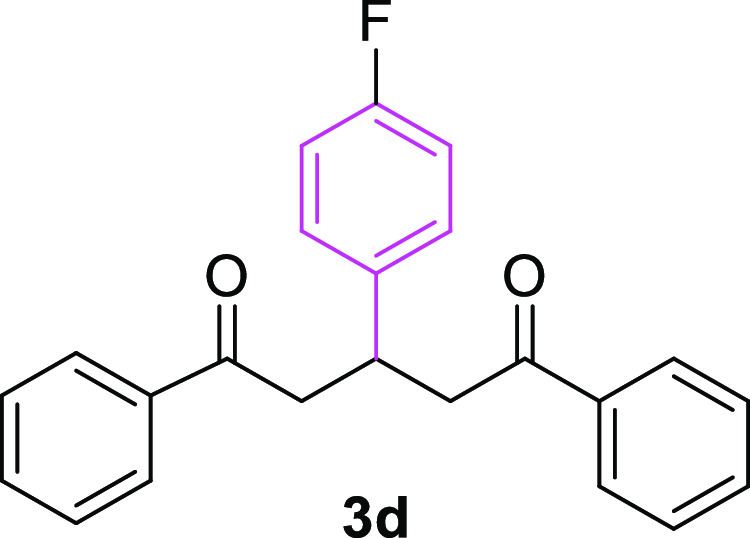
3-(4-Flurophenyl)-1,5-diphenylpentane-1,5-dione (3d)19
 White
powder (213.6 mg, 83% yield); mp 88–90 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.86 (dd, J = 1.0, 8.3 Hz, 4H, ArH), 7.47 (t, J =
7.4 Hz, 2H, ArH), 7.37 (t, J = 7.5 Hz, 4H, ArH),
7.18–7.16 (m, 2H, ArH), 6.87 (t, J = 8.7 Hz,
2H, ArH), 3.99 (p, J = 7.1 Hz, 1H, CH), 3.40 (dd, J = 6.8, 16.7 Hz, 2H, CH2), 3.25 (dd, J = 7.3, 16.7 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 198.5 (CO), 162.6 (C),
160.7 (C), 139.6 (C), 139.6 (C), 137.0 (C), 133.3 (CH), 129.1 (CH),
129.1 (CH), 128.8 (CH), 128.2 (CH), 115.6 (CH), 115.4 (CH), 45.1 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C23H19FO2Na [M+], 346.1369; found, 346.1369.
White
powder (213.6 mg, 83% yield); mp 88–90 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.86 (dd, J = 1.0, 8.3 Hz, 4H, ArH), 7.47 (t, J =
7.4 Hz, 2H, ArH), 7.37 (t, J = 7.5 Hz, 4H, ArH),
7.18–7.16 (m, 2H, ArH), 6.87 (t, J = 8.7 Hz,
2H, ArH), 3.99 (p, J = 7.1 Hz, 1H, CH), 3.40 (dd, J = 6.8, 16.7 Hz, 2H, CH2), 3.25 (dd, J = 7.3, 16.7 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 198.5 (CO), 162.6 (C),
160.7 (C), 139.6 (C), 139.6 (C), 137.0 (C), 133.3 (CH), 129.1 (CH),
129.1 (CH), 128.8 (CH), 128.2 (CH), 115.6 (CH), 115.4 (CH), 45.1 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C23H19FO2Na [M+], 346.1369; found, 346.1369.
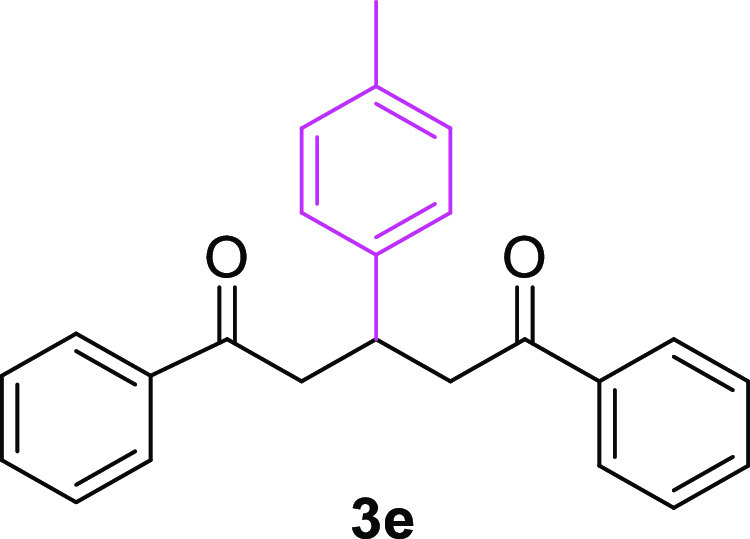
3-(4-Methylphenyl)-1,5-diphenylpentane-1,5-dione (3e)31
 Colorless syrup (260.2 mg, 91% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.87 (dd, J = 1.2, 8.5 Hz, 4H, ArH), 7.48–7.45 (m, 2H, ArH), 7.38–7.35
(m, 4H, ArH), 7.10–7.08 (m, 2H, ArH), 7.01–7.00 (m,
2H, ArH), 3.95 (p, J = 7.0 Hz, 1H, CH), 3.40 (dd, J = 7.0, 16.6 Hz, 2H, CH2), 3.25 (dd, J = 7.1, 16.6 Hz, 2H, CH2), 2.21 (s, 3H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 198.8 (CO), 140.9 (C), 137.1 (C), 136.3 (C), 133.2 (CH), 129.4
(CH), 128.7 (CH), 128.3 (CH), 127.4 (CH), 45.2 (CH2), 37.0
(CH), 21.1 (CH3); HRMS (ESI) m/z: calcd for C24H22O2Na
[M + Na]+, 365.1512; found, 365.1506.
Colorless syrup (260.2 mg, 91% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.87 (dd, J = 1.2, 8.5 Hz, 4H, ArH), 7.48–7.45 (m, 2H, ArH), 7.38–7.35
(m, 4H, ArH), 7.10–7.08 (m, 2H, ArH), 7.01–7.00 (m,
2H, ArH), 3.95 (p, J = 7.0 Hz, 1H, CH), 3.40 (dd, J = 7.0, 16.6 Hz, 2H, CH2), 3.25 (dd, J = 7.1, 16.6 Hz, 2H, CH2), 2.21 (s, 3H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 198.8 (CO), 140.9 (C), 137.1 (C), 136.3 (C), 133.2 (CH), 129.4
(CH), 128.7 (CH), 128.3 (CH), 127.4 (CH), 45.2 (CH2), 37.0
(CH), 21.1 (CH3); HRMS (ESI) m/z: calcd for C24H22O2Na
[M + Na]+, 365.1512; found, 365.1506.
3-(4-Methoxyphenyl)-1,5-diphenylpentane-1,5-dione (3f)23,31
 Colorless
syrup (223.8 mg, 85% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 1.0, 8.2 Hz, 4H, ArH), 7.54–7.51 (m, 2H, ArH), 7.44–7.41
(m, 4H, ArH), 7.18–7.16 (m, 2H, ArH), 6.80–6.77 (m,
2H, ArH), 4.00 (p, J = 7.1 Hz, 1H, CH), 3.74 (s,
3H, OCH3), 3.45 (dd, J = 6.8, 16.5 Hz,
2H, CH2), 3.30 (dd, J = 7.2, 16.5 Hz,
2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.9 (CO), 158.4 (C), 137.1 (C), 135.9 (C), 133.2
(CH), 128.7 (CH), 128.5 (CH), 128.3 (CH), 114.1 (CH), 55.3 (OCH3), 45.3 (CH2), 36.7 (CH); HRMS (ESI) m/z: calcd for C24H22O3Na [M + Na]+, 381.1461; found, 381.1452.
Colorless
syrup (223.8 mg, 85% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 1.0, 8.2 Hz, 4H, ArH), 7.54–7.51 (m, 2H, ArH), 7.44–7.41
(m, 4H, ArH), 7.18–7.16 (m, 2H, ArH), 6.80–6.77 (m,
2H, ArH), 4.00 (p, J = 7.1 Hz, 1H, CH), 3.74 (s,
3H, OCH3), 3.45 (dd, J = 6.8, 16.5 Hz,
2H, CH2), 3.30 (dd, J = 7.2, 16.5 Hz,
2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.9 (CO), 158.4 (C), 137.1 (C), 135.9 (C), 133.2
(CH), 128.7 (CH), 128.5 (CH), 128.3 (CH), 114.1 (CH), 55.3 (OCH3), 45.3 (CH2), 36.7 (CH); HRMS (ESI) m/z: calcd for C24H22O3Na [M + Na]+, 381.1461; found, 381.1452.
3-(3-Methylphenyl)-1,5-diphenylpentane-1,5-dione (3g)
 Colorless syrup (229.3 mg, 91% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 0.9, 8.1 Hz, 4H, ArH), 7.54–7.51 (m, 2H, ArH), 7.44–7.41
(m, 4H, ArH), 7.15 (t, J = 7.52 Hz, 1H, ArH), 7.06
(d, J = 8.6 Hz, 2H, ArH), 7.00 (d, J = 7.6 Hz, 1H, ArH), 4.01 (p, J = 7.0 Hz, 1H, CH),
3.46 (dd, J = 7.0, 16.5 Hz, 2H, CH2),
3.33 (dd, J = 7.0, 16.7 Hz, 2H, CH2),
2.29 (s, 3H, CH3); 13C {H} NMR (125 MHz, CDCl3): δ (ppm) 198.8 (CO), 143.9 (C), 138.3 (C), 137.1 (C),
133.2 (CH), 128.7 (CH), 128.6 (CH), 128.4 (CH), 128.3 (CH), 127.6
(CH), 45.1 (CH2), 37.2 (CH), 21.6 (CH3); HRMS
(ESI) m/z: calcd for C24H22O2Na [M + Na]+, 365.1512; found,
365.1503.
Colorless syrup (229.3 mg, 91% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 0.9, 8.1 Hz, 4H, ArH), 7.54–7.51 (m, 2H, ArH), 7.44–7.41
(m, 4H, ArH), 7.15 (t, J = 7.52 Hz, 1H, ArH), 7.06
(d, J = 8.6 Hz, 2H, ArH), 7.00 (d, J = 7.6 Hz, 1H, ArH), 4.01 (p, J = 7.0 Hz, 1H, CH),
3.46 (dd, J = 7.0, 16.5 Hz, 2H, CH2),
3.33 (dd, J = 7.0, 16.7 Hz, 2H, CH2),
2.29 (s, 3H, CH3); 13C {H} NMR (125 MHz, CDCl3): δ (ppm) 198.8 (CO), 143.9 (C), 138.3 (C), 137.1 (C),
133.2 (CH), 128.7 (CH), 128.6 (CH), 128.4 (CH), 128.3 (CH), 127.6
(CH), 45.1 (CH2), 37.2 (CH), 21.6 (CH3); HRMS
(ESI) m/z: calcd for C24H22O2Na [M + Na]+, 365.1512; found,
365.1503.
3-(3-Methoxyphenyl)-1,5-diphenylpentane-1,5-dione (3h)
 Colorless
syrup (215.0 mg, 82% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 1.2, 7.2 Hz, 4H, ArH), 7.54–7.51 (m, 2H, ArH), 7.42 (t, J = 7.9 Hz, 4H, ArH), 7.18 (t, J = 7.8
Hz, 1H, ArH), 6.86 (d, J = 7.6 Hz, 1H 2H, ArH), 6.80
(t, J = 2.1 Hz, 1H, ArH), 6.70 (dd, J = 2.4, 8.2 Hz, 1H, ArH), 4.03 (p, J = 7.0 Hz, 1H,
CH), 3.74 (s, 3H, OCH3), 3.45 (dd, J =
7.0, 16.6 Hz, 2H, CH2), 3.33 (dd, J =
7.0, 16.6 Hz, 2H, CH2); 13C{H} NMR (125 MHz,
CDCl3): δ (ppm) 198.7 (CO), 159.8 (C), 145.7 (C),
137.1 (C), 133.2 (CH), 129.8 (CH), 128.7 (CH), 128.3 (CH), 119.9 (CH),
113.7 (CH), 111.9 (CH), 55.3 (OCH3), 45.0 (CH2), 37.3 (CH); HRMS (ESI) m/z: calcd
for C24H22O3Na [M + Na]+, 381.1461; found, 381.1452.
Colorless
syrup (215.0 mg, 82% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 1.2, 7.2 Hz, 4H, ArH), 7.54–7.51 (m, 2H, ArH), 7.42 (t, J = 7.9 Hz, 4H, ArH), 7.18 (t, J = 7.8
Hz, 1H, ArH), 6.86 (d, J = 7.6 Hz, 1H 2H, ArH), 6.80
(t, J = 2.1 Hz, 1H, ArH), 6.70 (dd, J = 2.4, 8.2 Hz, 1H, ArH), 4.03 (p, J = 7.0 Hz, 1H,
CH), 3.74 (s, 3H, OCH3), 3.45 (dd, J =
7.0, 16.6 Hz, 2H, CH2), 3.33 (dd, J =
7.0, 16.6 Hz, 2H, CH2); 13C{H} NMR (125 MHz,
CDCl3): δ (ppm) 198.7 (CO), 159.8 (C), 145.7 (C),
137.1 (C), 133.2 (CH), 129.8 (CH), 128.7 (CH), 128.3 (CH), 119.9 (CH),
113.7 (CH), 111.9 (CH), 55.3 (OCH3), 45.0 (CH2), 37.3 (CH); HRMS (ESI) m/z: calcd
for C24H22O3Na [M + Na]+, 381.1461; found, 381.1452.
3-(4-Trifluoromethylphenyl)-1,5-diphenylpentane-1,5-dione (3i)23
 White solid (136.6 mg, 60% yield); mp 127–129 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 1.2, 8.4 Hz, 4H, ArH), 7.56–7.51 (m, 4H, ArH),
7.45–7.40 (m, 6H, ArH), 4.14 (p, J = 7.0 Hz,
1H, CH), 3.50 (dd, J = 6.8, 17.0, Hz, 2H, CH2), 3.36 (dd, J = 7.3, 17.0, Hz, 2H, CH2); 13C {H} NMR (125 MHz, CDCl3): δ
(ppm) 198.1 (CO), 148.2 (C), 136.8 (C), 133.4 (CH), 128.8 (CH), 128.2
(CH), 128.1 (CH), 125.7 (CH), 125.7 (CH), 44.6 (CH2), 36.9
(CH); HRMS (ESI) m/z: calcd for
C24H19F3O2Na [M + Na]+, 419.1229; found, 419.1222.
White solid (136.6 mg, 60% yield); mp 127–129 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 1.2, 8.4 Hz, 4H, ArH), 7.56–7.51 (m, 4H, ArH),
7.45–7.40 (m, 6H, ArH), 4.14 (p, J = 7.0 Hz,
1H, CH), 3.50 (dd, J = 6.8, 17.0, Hz, 2H, CH2), 3.36 (dd, J = 7.3, 17.0, Hz, 2H, CH2); 13C {H} NMR (125 MHz, CDCl3): δ
(ppm) 198.1 (CO), 148.2 (C), 136.8 (C), 133.4 (CH), 128.8 (CH), 128.2
(CH), 128.1 (CH), 125.7 (CH), 125.7 (CH), 44.6 (CH2), 36.9
(CH); HRMS (ESI) m/z: calcd for
C24H19F3O2Na [M + Na]+, 419.1229; found, 419.1222.
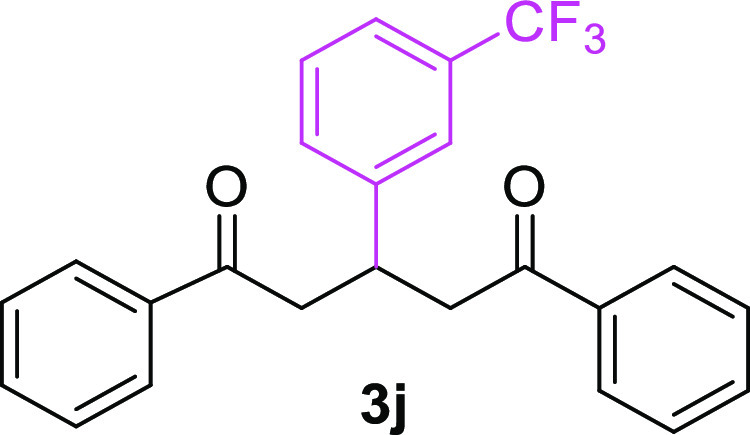
3-(3-Trifluoromethylphenyl)-1,5-diphenylpentane-1,5-dione (3j)
 Colorless
syrup (148.9 mg, 65% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93–7.91 (m, 4H,
ArH), 7.56–7.49 (m, 4H, ArH), 7.45–7.42 (m, 5H, ArH),
7.40–7.36 (m, 1H, ArH), 4.14 (p, J = 7.0 Hz,
1H, CH), 3.51 (dd, J = 6.7, 16.9, Hz, 2H, CH2), 3.35 (dd, J = 7.1, 16.9, Hz, 2H, CH2); 13C {H} NMR (125 MHz, CDCl3): δ
(ppm) 198.2 (CO), 145.0 (C), 136.9 (C), 133.4 (CH), 131.5 (CH), 129.2
(CH), 128.8 (CH), 128.2 (CH), 124.3 (CH), 124.2 (CH), 123.8 (CH),
123.7 (CH), 123.7 (CH), 44.7 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C24H19F3O2Na [M + Na]+, 419.1229; found,
419.1234.
Colorless
syrup (148.9 mg, 65% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93–7.91 (m, 4H,
ArH), 7.56–7.49 (m, 4H, ArH), 7.45–7.42 (m, 5H, ArH),
7.40–7.36 (m, 1H, ArH), 4.14 (p, J = 7.0 Hz,
1H, CH), 3.51 (dd, J = 6.7, 16.9, Hz, 2H, CH2), 3.35 (dd, J = 7.1, 16.9, Hz, 2H, CH2); 13C {H} NMR (125 MHz, CDCl3): δ
(ppm) 198.2 (CO), 145.0 (C), 136.9 (C), 133.4 (CH), 131.5 (CH), 129.2
(CH), 128.8 (CH), 128.2 (CH), 124.3 (CH), 124.2 (CH), 123.8 (CH),
123.7 (CH), 123.7 (CH), 44.7 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C24H19F3O2Na [M + Na]+, 419.1229; found,
419.1234.
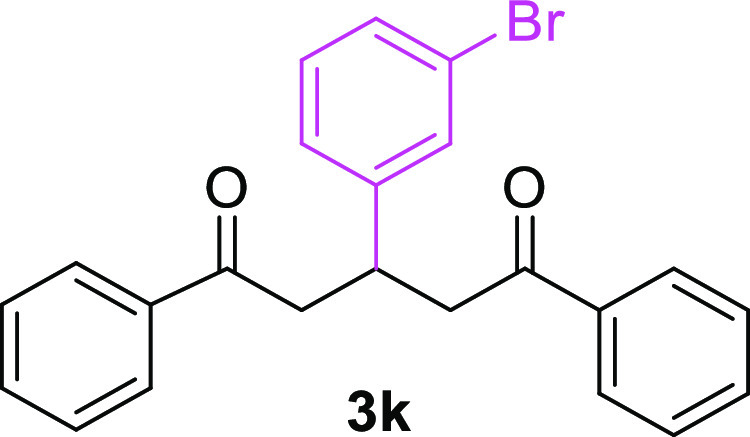
3-(3-Bromophenyl)-1,5-diphenylpentane-1,5-dione (3k)
 Colorless syrup (162.1 mg, 74% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 1.4, 8.6 Hz, 4H, ArH), 7.56–7.52 (m, 2H, ArH), 7.45–7.42
(m, 5H, ArH), 7.30–7.28 (m, 1H, ArH), 7.23–7.22 (m,
1H ArH), 7.12 (t, J = 7.8 Hz, 1H ArH), 4.03 (p, J = 7.0 Hz, 1H, CH), 3.46 (dd, J = 6.8,
16.9 Hz, 2H, CH2), 3.32 (dd, J = 7.1,
16.8 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.2 (CO), 146.5 (C), 136.9 (C), 133.4 (CH),
130.6 (CH), 130.3 (CH), 130.0 (CH), 128.8 (CH), 128.3 (CH), 126.6
(CH), 122.8 (C), 44.7 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C23H19BrO2Na [M + Na]+, 429.0461; found, 429.0456.
Colorless syrup (162.1 mg, 74% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.92 (dd, J = 1.4, 8.6 Hz, 4H, ArH), 7.56–7.52 (m, 2H, ArH), 7.45–7.42
(m, 5H, ArH), 7.30–7.28 (m, 1H, ArH), 7.23–7.22 (m,
1H ArH), 7.12 (t, J = 7.8 Hz, 1H ArH), 4.03 (p, J = 7.0 Hz, 1H, CH), 3.46 (dd, J = 6.8,
16.9 Hz, 2H, CH2), 3.32 (dd, J = 7.1,
16.8 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.2 (CO), 146.5 (C), 136.9 (C), 133.4 (CH),
130.6 (CH), 130.3 (CH), 130.0 (CH), 128.8 (CH), 128.3 (CH), 126.6
(CH), 122.8 (C), 44.7 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C23H19BrO2Na [M + Na]+, 429.0461; found, 429.0456.
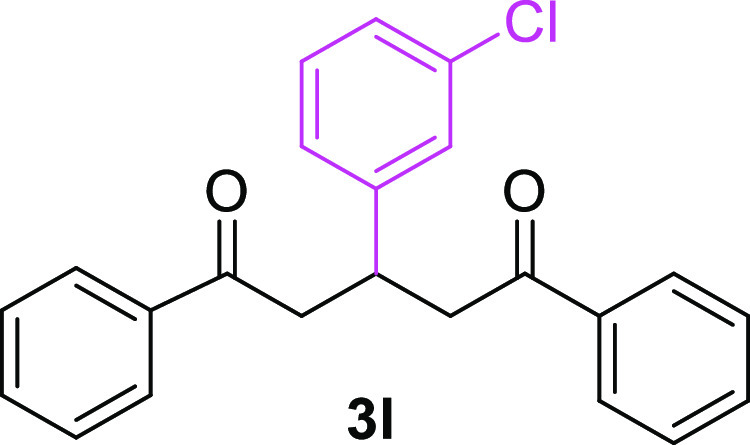
3-(3-Chlorophenyl)-1,5-diphenylpentane-1,5-dione (3l)23
 Colorless
syrup (181.5 mg, 70% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93–7.91 (m, 4H,
ArH), 7.55–7.52 (m, 2H, ArH), 7.43 (t, J =
7.9 Hz, 4H, ArH), 7.26 (br s, 1H, ArH), 7.19–7.17 (m, 2H, ArH),
7.15–7.13 (m, 1H, ArH), 4.05 (p, J = 7.0 Hz,
1H, CH), 3.47 (dd, J = 6.8, 16.9 Hz, 2H, CH2), 3.32 (dd, J = 7.1, 16.8 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
198.2 (CO), 146.2 (C), 136.9 (C), 134.5 (C), 133.4 (CH), 130.0 (CH),
128.8 (CH), 128.2 (CH), 127.7 (CH), 127.0 (CH), 126.1 (CH), 44.7 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C23H19ClO2Na [M + Na]+, 385.0966; found, 385.0958.
Colorless
syrup (181.5 mg, 70% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93–7.91 (m, 4H,
ArH), 7.55–7.52 (m, 2H, ArH), 7.43 (t, J =
7.9 Hz, 4H, ArH), 7.26 (br s, 1H, ArH), 7.19–7.17 (m, 2H, ArH),
7.15–7.13 (m, 1H, ArH), 4.05 (p, J = 7.0 Hz,
1H, CH), 3.47 (dd, J = 6.8, 16.9 Hz, 2H, CH2), 3.32 (dd, J = 7.1, 16.8 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
198.2 (CO), 146.2 (C), 136.9 (C), 134.5 (C), 133.4 (CH), 130.0 (CH),
128.8 (CH), 128.2 (CH), 127.7 (CH), 127.0 (CH), 126.1 (CH), 44.7 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C23H19ClO2Na [M + Na]+, 385.0966; found, 385.0958.
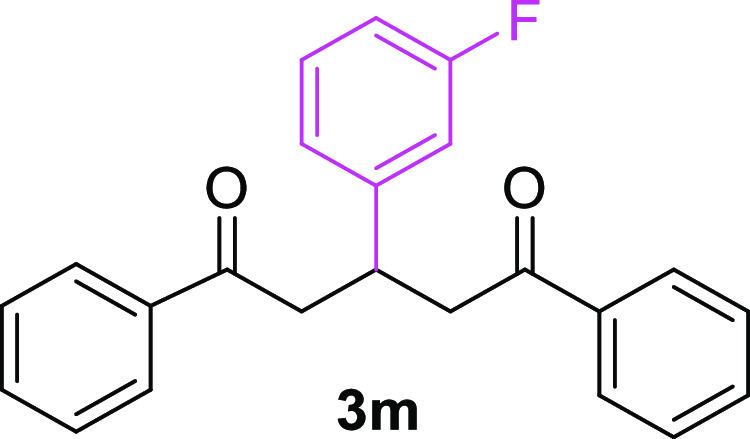
3-(3-Fluorophenyl)-1,5-diphenylpentane-1,5-dione (3m)
 Colorless syrup (201.1 mg, 72% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.94–7.91 (m, 4H,
ArH), 7.55–7.52 (m, 2H, ArH), 7.45–7.42 (m, 4H, ArH),
7.24–7.19 (m, 1H, ArH), 7.06 (d, J = 7.7 Hz,
1H, ArH), 6.98 (dt, J = 2.2, 10.1 Hz, 1H, ArH), 6.87–6.83
(m, 1H, ArH), 4.07 (p, J = 7.0 Hz, 1H, CH), 3.46
(dd, J = 6.8, 16.9 Hz, 2H, CH2), 3.33
(dd, J = 7.2, 16.9 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.3
(CO), 164.0 (C), 162.1 (C), 146.7 (C), 146.6 (C), 136.9 (C), 133.3
(CH), 130.2 (CH), 130.2 (CH), 128.8 (CH), 128.2 (CH), 123.4 (CH),
123.4 (CH), 114.6 (CH), 114.4 (CH), 113.8 (CH), 113.6 (CH), 44.8 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C23H19FO2Na [M + Na]+, 369.1261; found, 369.1253.
Colorless syrup (201.1 mg, 72% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.94–7.91 (m, 4H,
ArH), 7.55–7.52 (m, 2H, ArH), 7.45–7.42 (m, 4H, ArH),
7.24–7.19 (m, 1H, ArH), 7.06 (d, J = 7.7 Hz,
1H, ArH), 6.98 (dt, J = 2.2, 10.1 Hz, 1H, ArH), 6.87–6.83
(m, 1H, ArH), 4.07 (p, J = 7.0 Hz, 1H, CH), 3.46
(dd, J = 6.8, 16.9 Hz, 2H, CH2), 3.33
(dd, J = 7.2, 16.9 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.3
(CO), 164.0 (C), 162.1 (C), 146.7 (C), 146.6 (C), 136.9 (C), 133.3
(CH), 130.2 (CH), 130.2 (CH), 128.8 (CH), 128.2 (CH), 123.4 (CH),
123.4 (CH), 114.6 (CH), 114.4 (CH), 113.8 (CH), 113.6 (CH), 44.8 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C23H19FO2Na [M + Na]+, 369.1261; found, 369.1253.
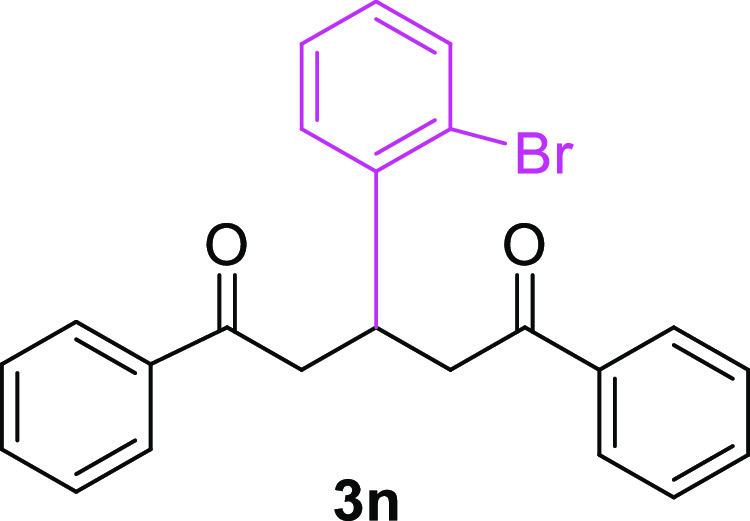
3-(2-Bromophenyl)-1,5-diphenylpentane-1,5-dione (3n)23
 White solid (186.9 mg, 85% yield); mp 120–122 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.95 (dd, J = 1.2, 8.5 Hz, 4H, ArH), 7.55–7.51 (m, 3H, ArH),
7.42 (t, J = 7.9 Hz, 4H, ArH), 7.31 (dd, J = 1.5, 7.8 Hz, 1H, ArH), 7.23–7.20 (m, 1H ArH),
7.04–7.01 (m, 1H ArH), 4.52 (p, J = 6.9 Hz,
1H, CH), 3.51 (dd, J = 7.2, 16.9 Hz, 2H, CH2), 3.42 (dd, J = 6.7, 16.9 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
198.5 (CO), 142.6 (C), 136.9 (C), 133.5 (CH), 133.3 (CH), 128.7 (CH),
128.3 (CH), 128.2 (CH), 127.8 (CH), 124.6 (C), 43.3 (CH2), 36.4 (CH); HRMS (ESI) m/z: calcd
for C23H19BrO2Na [M + Na]+, 429.0461; found, 429.0455.
White solid (186.9 mg, 85% yield); mp 120–122 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.95 (dd, J = 1.2, 8.5 Hz, 4H, ArH), 7.55–7.51 (m, 3H, ArH),
7.42 (t, J = 7.9 Hz, 4H, ArH), 7.31 (dd, J = 1.5, 7.8 Hz, 1H, ArH), 7.23–7.20 (m, 1H ArH),
7.04–7.01 (m, 1H ArH), 4.52 (p, J = 6.9 Hz,
1H, CH), 3.51 (dd, J = 7.2, 16.9 Hz, 2H, CH2), 3.42 (dd, J = 6.7, 16.9 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
198.5 (CO), 142.6 (C), 136.9 (C), 133.5 (CH), 133.3 (CH), 128.7 (CH),
128.3 (CH), 128.2 (CH), 127.8 (CH), 124.6 (C), 43.3 (CH2), 36.4 (CH); HRMS (ESI) m/z: calcd
for C23H19BrO2Na [M + Na]+, 429.0461; found, 429.0455.
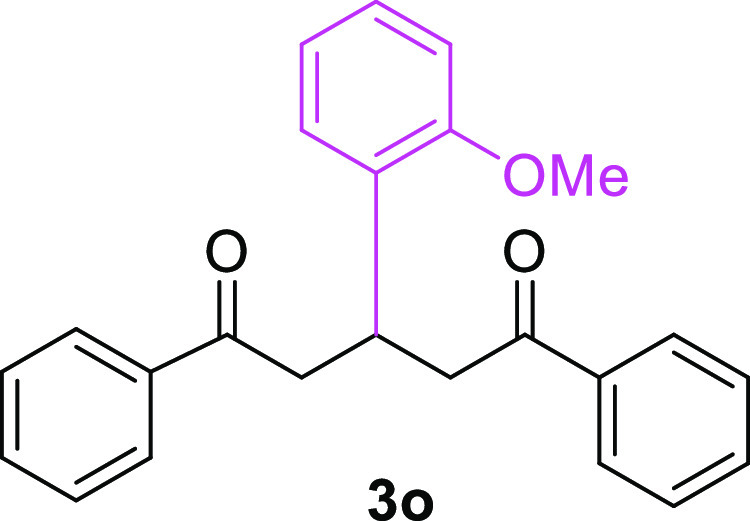
3-(2-Methoxyphenyl)-1,5-diphenylpentane-1,5-dione (3o)23
 White solid (236.6 mg, 90% yield); mp 116–1118 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.97 (dd, J = 1.3, 8.4 Hz, 4H, ArH), 7.54–7.50 (m, 2H, ArH),
7.42 (t, J = 7.9 Hz, 4H, ArH), 7.20 (dd, J = 1.4, 7.5 Hz, 1H, ArH), 7.15 (td, J =
1.6, 8.2 Hz, 1H, ArH), 6.87–6.81 (m, 2H, ArH), (4.33 (p, J = 7.0 Hz, 1H, CH), 3.76 (s, 3H, OCH3), 3.48
(dd, J = 7.2, 16.5 Hz, 2H, CH2), 3.42
(dd, J = 6.9, 16.4 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 199.4
(CO), 157.2 (C), 137.2 (C), 133.0 (CH), 131.5 (C), 128.6 (CH), 128.3
(CH), 127.8 (CH), 120.8 (CH), 111.0 (CH), 55.3 (OCH3),
43.2 (CH2), 33.0 (CH); HRMS (ESI) m/z: calcd for C24H22O3Na
[M + Na]+, 381.1461; found, 381.1454.
White solid (236.6 mg, 90% yield); mp 116–1118 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.97 (dd, J = 1.3, 8.4 Hz, 4H, ArH), 7.54–7.50 (m, 2H, ArH),
7.42 (t, J = 7.9 Hz, 4H, ArH), 7.20 (dd, J = 1.4, 7.5 Hz, 1H, ArH), 7.15 (td, J =
1.6, 8.2 Hz, 1H, ArH), 6.87–6.81 (m, 2H, ArH), (4.33 (p, J = 7.0 Hz, 1H, CH), 3.76 (s, 3H, OCH3), 3.48
(dd, J = 7.2, 16.5 Hz, 2H, CH2), 3.42
(dd, J = 6.9, 16.4 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 199.4
(CO), 157.2 (C), 137.2 (C), 133.0 (CH), 131.5 (C), 128.6 (CH), 128.3
(CH), 127.8 (CH), 120.8 (CH), 111.0 (CH), 55.3 (OCH3),
43.2 (CH2), 33.0 (CH); HRMS (ESI) m/z: calcd for C24H22O3Na
[M + Na]+, 381.1461; found, 381.1454.
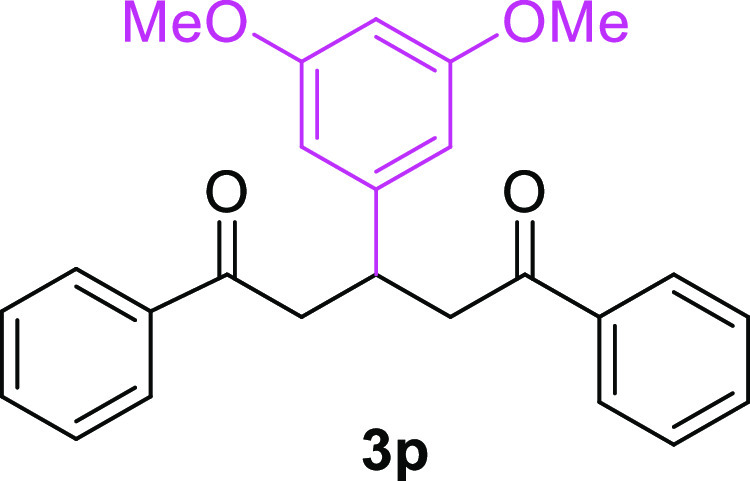
3-(3,3-Dimethoxyphenyl)-1,5-diphenylpentane-1,5-dione (3p)
 Colorless
syrup (217.2 mg, 93% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 1.3, 7.1 Hz, 4H, ArH), 7.55–7.51 (m, 2H, ArH), 7.42 (t, J = 8.0 Hz, 4H, ArH), 6.41 (d, J = 2.2
Hz, 2H, ArH), 6.26 (t, J = 2.2 Hz, 1H, ArH), 4.00
(p, J = 7.0 Hz, 1H, CH), 3.72 (s, 6H, OCH3), 3.42 (dd, J = 7.1, 16.7 Hz, 2H, CH2), 3.32 (dd, J = 7.0, 16.7 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
198.7 (CO), 161.0 (C), 146.5 (C), 137.1 (C), 133.2 (CH), 128.7 (CH),
128.3 (CH), 105.8 (CH), 98.5 (CH), 55.4 (OCH3), 44.9 (CH2), 37.5 (CH); HRMS (ESI) m/z: calcd for C25H24O4Na [M + Na]+, 411.1567; found, 411.1563.
Colorless
syrup (217.2 mg, 93% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.93 (dd, J = 1.3, 7.1 Hz, 4H, ArH), 7.55–7.51 (m, 2H, ArH), 7.42 (t, J = 8.0 Hz, 4H, ArH), 6.41 (d, J = 2.2
Hz, 2H, ArH), 6.26 (t, J = 2.2 Hz, 1H, ArH), 4.00
(p, J = 7.0 Hz, 1H, CH), 3.72 (s, 6H, OCH3), 3.42 (dd, J = 7.1, 16.7 Hz, 2H, CH2), 3.32 (dd, J = 7.0, 16.7 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
198.7 (CO), 161.0 (C), 146.5 (C), 137.1 (C), 133.2 (CH), 128.7 (CH),
128.3 (CH), 105.8 (CH), 98.5 (CH), 55.4 (OCH3), 44.9 (CH2), 37.5 (CH); HRMS (ESI) m/z: calcd for C25H24O4Na [M + Na]+, 411.1567; found, 411.1563.
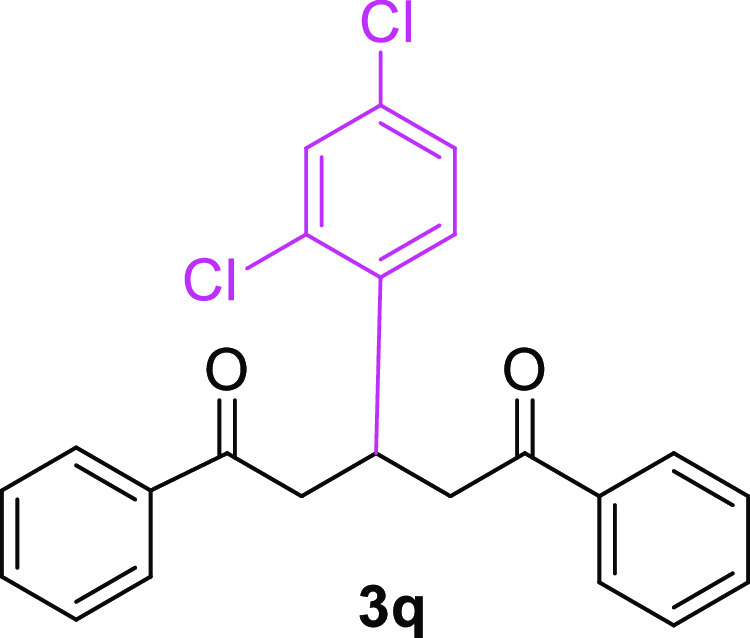
3-(2,4-Dichlorophenyl)-1,5-diphenylpentane-1,5-dione (3q)23
 White
solid (201.2 mg, 81% yield); mp 115–117 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.94 (dd, J = 1.3, 8.5 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH),
7.43 (t, J = 7.9 Hz, 4H, ArH), 7.35 (d, J = 2.2 Hz, 1H, ArH), 7.27 (d, J = 8.4 Hz, 1H, ArH),
7.15 (dd, J = 2.1, 8.4 Hz, 1H, ArH), 4.47 (p, J = 6.9 Hz, 1H, CH), 3.51 (dd, J = 7.0,
17.0 Hz, 2H, CH2), 3.41 (dd, J = 6.8,
17.0 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.2 (CO), 139.6 (C), 136.8 (C), 134.5 (C),
133.4 (CH), 132.9 (C), 129.9 (CH), 129.5 (CH), 128.8 (CH), 128.3 (CH),
127.4 (CH), 42.9 (CH2), 33.6 (CH); HRMS (ESI) m/z: calcd for C23H18Cl2O2Na [M + Na]+, 419.0576; found, 419.0572.
White
solid (201.2 mg, 81% yield); mp 115–117 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.94 (dd, J = 1.3, 8.5 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH),
7.43 (t, J = 7.9 Hz, 4H, ArH), 7.35 (d, J = 2.2 Hz, 1H, ArH), 7.27 (d, J = 8.4 Hz, 1H, ArH),
7.15 (dd, J = 2.1, 8.4 Hz, 1H, ArH), 4.47 (p, J = 6.9 Hz, 1H, CH), 3.51 (dd, J = 7.0,
17.0 Hz, 2H, CH2), 3.41 (dd, J = 6.8,
17.0 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.2 (CO), 139.6 (C), 136.8 (C), 134.5 (C),
133.4 (CH), 132.9 (C), 129.9 (CH), 129.5 (CH), 128.8 (CH), 128.3 (CH),
127.4 (CH), 42.9 (CH2), 33.6 (CH); HRMS (ESI) m/z: calcd for C23H18Cl2O2Na [M + Na]+, 419.0576; found, 419.0572.
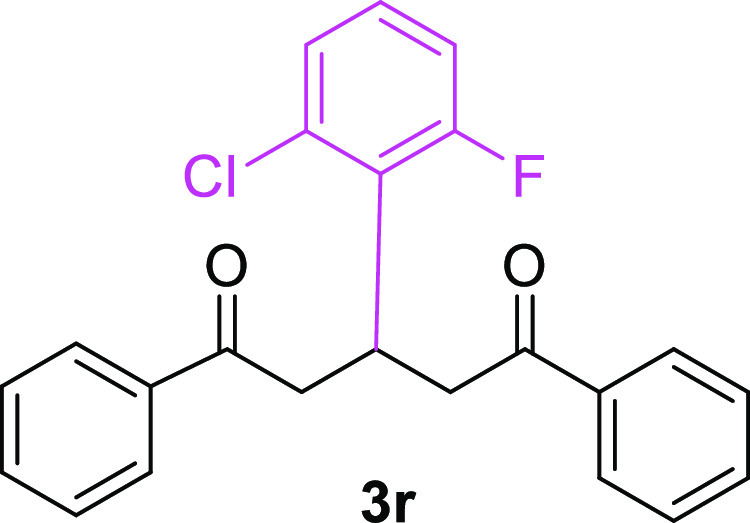
3-(2-Chloro-6-fluorophenyl)-1,5-diphenylpentane-1,5-dione (3r)
 Colorless syrup (190.0 mg, 79% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.96 (dd, J = 1.4, 8.5 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH), 7.44–7.41
(m, 4H, ArH), 7.14 (d, J = 8.0 Hz, 1H, ArH), 7.10–7.06
(m, 1H, ArH), 6.93–6.89 (m, 1H, ArH), 4.70 (p, J = 7.2 Hz, 1H, CH), 3.61 (dd, J = 7.1, 16.9 Hz,
2H, CH2), 3.49 (dd, J = 7.2, 16.9 Hz,
2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.4 (CO), 163.2 (C), 161.3 (C), 136.8 (C), 133.3
(CH), 129.1 (C), 129.0 (C), 128.7 (CH), 128.5 (CH), 128.4 (CH), 128.3
(CH), 126.1 (CH), 126.1 (CH), 115.0 (CH), 114.8 (CH), 42.1 (CH2), 42.1 (CH2), 31.4 (CH); HRMS (ESI) m/z: calcd for C23H18ClFO2Na [M + Na]+, 403.0872; found, 403.0863.
Colorless syrup (190.0 mg, 79% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.96 (dd, J = 1.4, 8.5 Hz, 4H, ArH), 7.55–7.52 (m, 2H, ArH), 7.44–7.41
(m, 4H, ArH), 7.14 (d, J = 8.0 Hz, 1H, ArH), 7.10–7.06
(m, 1H, ArH), 6.93–6.89 (m, 1H, ArH), 4.70 (p, J = 7.2 Hz, 1H, CH), 3.61 (dd, J = 7.1, 16.9 Hz,
2H, CH2), 3.49 (dd, J = 7.2, 16.9 Hz,
2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.4 (CO), 163.2 (C), 161.3 (C), 136.8 (C), 133.3
(CH), 129.1 (C), 129.0 (C), 128.7 (CH), 128.5 (CH), 128.4 (CH), 128.3
(CH), 126.1 (CH), 126.1 (CH), 115.0 (CH), 114.8 (CH), 42.1 (CH2), 42.1 (CH2), 31.4 (CH); HRMS (ESI) m/z: calcd for C23H18ClFO2Na [M + Na]+, 403.0872; found, 403.0863.
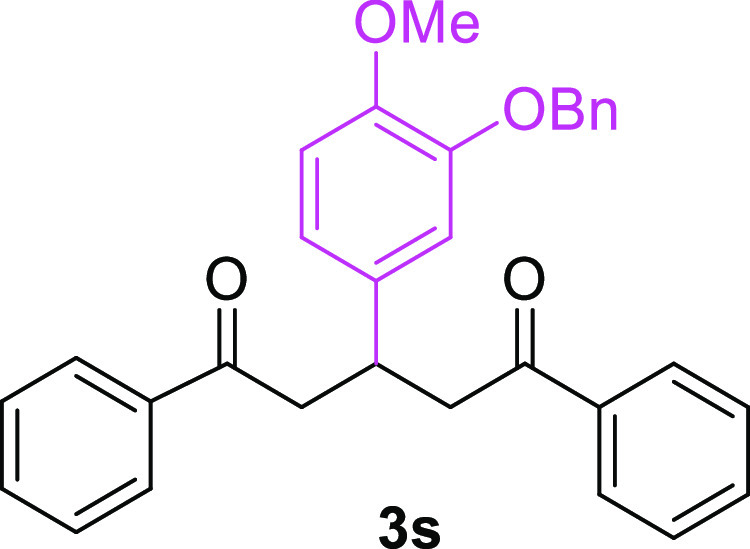
3-(3-Benzyloxy-4-methoxyphenyl)-1,5-diphenylpentane-1,5-dione (3s)
 Colorless syrup (152.7 mg, 80% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.89 (dd, J = 0.8, 8.2 Hz, 4H, ArH), 7.53 (t, J = 7.3 Hz, 2H,
ArH), 7.44–7.37 (m, 6H, ArH), 7.30 (t, J =
7.3 Hz, 2H, ArH), 7.25–7.22 (m, 1H, ArH), 6.81 (d, J = 8.3 Hz, 1H, ArH), 6.78–6.76 (m, 2H, ArH), 5.06
(s, 2H, OCH2), 3.94 (p, J = 6.9 Hz, 1H,
CH), 3.81 (s, 3H, OCH3), 3.37 (dd, J =
6.9, 16.5 Hz, 2H, CH2), 3.22 (dd, J =
6.9, 16.5 Hz, 2H, CH2); 13C{H} NMR (125 MHz,
CDCl3): δ (ppm) 198.8 (CO), 148.6 (C), 148.1 (C),
137.4 (C), 137.1 (C), 136.4 (C), 133.2 (CH), 128.7 (CH), 128.6 (CH),
128.3 (CH), 127.9 (CH), 127.6 (CH), 120.3 (CH), 114.3 (CH), 112.1
(CH), 71.3 (OCH2), 56.1 (OCH3), 45.1 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C31H28O4Na [M + Na]+, 487.1880; found, 487.1876.
Colorless syrup (152.7 mg, 80% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.89 (dd, J = 0.8, 8.2 Hz, 4H, ArH), 7.53 (t, J = 7.3 Hz, 2H,
ArH), 7.44–7.37 (m, 6H, ArH), 7.30 (t, J =
7.3 Hz, 2H, ArH), 7.25–7.22 (m, 1H, ArH), 6.81 (d, J = 8.3 Hz, 1H, ArH), 6.78–6.76 (m, 2H, ArH), 5.06
(s, 2H, OCH2), 3.94 (p, J = 6.9 Hz, 1H,
CH), 3.81 (s, 3H, OCH3), 3.37 (dd, J =
6.9, 16.5 Hz, 2H, CH2), 3.22 (dd, J =
6.9, 16.5 Hz, 2H, CH2); 13C{H} NMR (125 MHz,
CDCl3): δ (ppm) 198.8 (CO), 148.6 (C), 148.1 (C),
137.4 (C), 137.1 (C), 136.4 (C), 133.2 (CH), 128.7 (CH), 128.6 (CH),
128.3 (CH), 127.9 (CH), 127.6 (CH), 120.3 (CH), 114.3 (CH), 112.1
(CH), 71.3 (OCH2), 56.1 (OCH3), 45.1 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C31H28O4Na [M + Na]+, 487.1880; found, 487.1876.
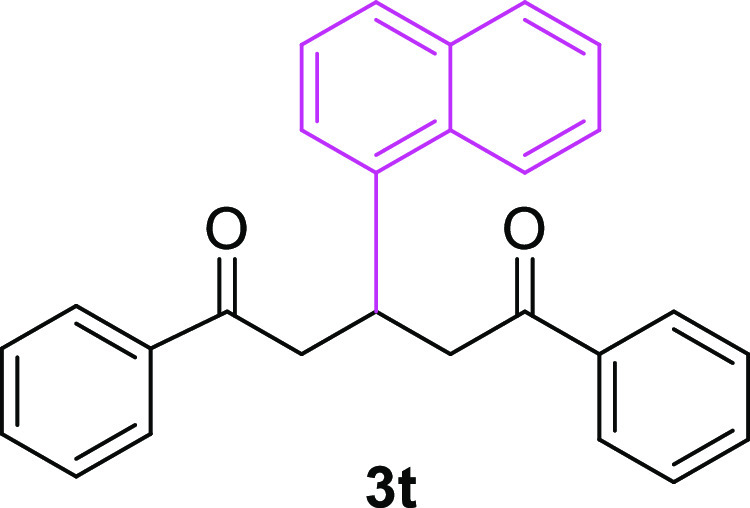
3-(Naphthalen-1-yl)-1,5-diphenylpentane-1,5-dione (3t)67
 White
solid (206.8 mg, 85% yield); mp 124–126 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.20 (d, J = 8.4 Hz, 1H, ArH), 7.92 (dd, J = 1.2,
8.3 Hz, 4H, ArH), 7.83 (d, J = 7.8 Hz, 1H, ArH),
7.70 (d, J = 8.2 Hz, 1H, ArH), 7.53–7.36 (m,
10H, ArH), 5.01 (p, J = 6.7 Hz, 1H, CH), 3.62 (dd, J = 7.3, 16.9 Hz, 2H, CH2), 3.52 (dd, J = 6.3, 16.9 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 198.8 (CO), 140.2 (C),
137.1 (C), 134.3 (C), 133.2 (CH), 131.4 (C), 129.1 (CH), 128.7 (CH),
128.3 (CH), 127.3 (CH), 126.4 (CH), 125.7 (CH), 125.5 (CH), 123.3
(CH), 44.6 (CH2), 31.3 (CH); HRMS (ESI) m/z: calcd for C27H22O2Na [M + Na]+, 401.1512; found, 401.1510.
White
solid (206.8 mg, 85% yield); mp 124–126 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.20 (d, J = 8.4 Hz, 1H, ArH), 7.92 (dd, J = 1.2,
8.3 Hz, 4H, ArH), 7.83 (d, J = 7.8 Hz, 1H, ArH),
7.70 (d, J = 8.2 Hz, 1H, ArH), 7.53–7.36 (m,
10H, ArH), 5.01 (p, J = 6.7 Hz, 1H, CH), 3.62 (dd, J = 7.3, 16.9 Hz, 2H, CH2), 3.52 (dd, J = 6.3, 16.9 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 198.8 (CO), 140.2 (C),
137.1 (C), 134.3 (C), 133.2 (CH), 131.4 (C), 129.1 (CH), 128.7 (CH),
128.3 (CH), 127.3 (CH), 126.4 (CH), 125.7 (CH), 125.5 (CH), 123.3
(CH), 44.6 (CH2), 31.3 (CH); HRMS (ESI) m/z: calcd for C27H22O2Na [M + Na]+, 401.1512; found, 401.1510.
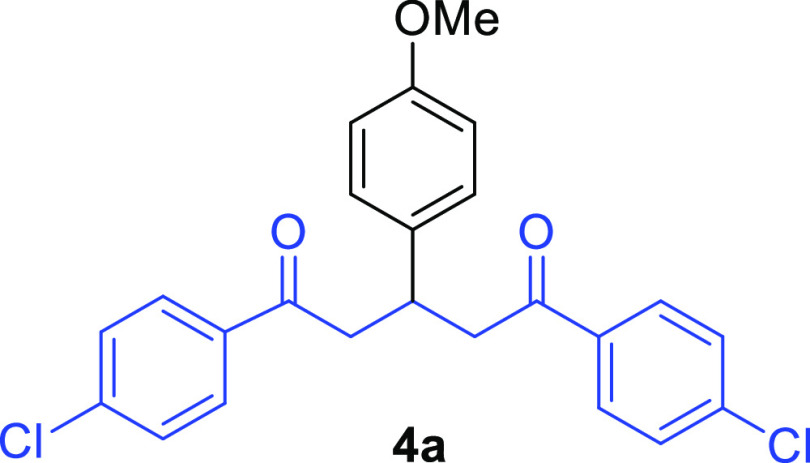
1,5-Bis(4-chlorophenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4a)15
 Colorless syrup (310.6 mg, 99% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.86 (d, J = 8.6 Hz, 4H, ArH), 7.39 (d, J = 8.7 Hz, 4H, ArH),
7.14 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.7 Hz, 2H, ArH), 3.94 (p, J = 7.0 Hz, 1H, CH),
3.73 (s, 3H, OCH3), 3.41 (dd, J = 7.0,
16.6 Hz, 2H, CH2), 3.24 (dd, J = 7.0,
16.5 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.6 (CO), 158.5 (C), 139.7 (C), 135.5 (C),
135.3 (C), 129.7 (CH), 129.1 (CH), 128.5 (CH), 114.2 (CH), 55.3 (OCH3), 45.2 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C24H20Cl2O3Na [M + Na]+, 449.0682; found, 449.0685.
Colorless syrup (310.6 mg, 99% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.86 (d, J = 8.6 Hz, 4H, ArH), 7.39 (d, J = 8.7 Hz, 4H, ArH),
7.14 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.7 Hz, 2H, ArH), 3.94 (p, J = 7.0 Hz, 1H, CH),
3.73 (s, 3H, OCH3), 3.41 (dd, J = 7.0,
16.6 Hz, 2H, CH2), 3.24 (dd, J = 7.0,
16.5 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.6 (CO), 158.5 (C), 139.7 (C), 135.5 (C),
135.3 (C), 129.7 (CH), 129.1 (CH), 128.5 (CH), 114.2 (CH), 55.3 (OCH3), 45.2 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C24H20Cl2O3Na [M + Na]+, 449.0682; found, 449.0685.
1,5-Bis(4-bromophenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4b)
 Colorless
syrup (316.8 mg, 84% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.78 (d, J = 8.5 Hz, 4H, ArH), 7.56 (d, J = 8.4 Hz, 4H, ArH),
7.14 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.7 Hz, 2H, ArH), 3.94 (p, J = 7.1 Hz, 1H, CH),
3.73 (s, 3H, OCH3), 3.40 (dd, J = 7.0,
16.5 Hz, 2H, CH2), 3.23 (dd, J = 7.1,
16.5 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.8 (CO), 158.5 (C), 135.7 (C), 135.4 (C),
132.1 (C), 129.8 (CH), 128.5 (CH), 114.2 (CH), 55.3 (OCH3), 45.2 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C24H20Br2O3Na [M + Na]+, 536.9671; found, 536.9670.
Colorless
syrup (316.8 mg, 84% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.78 (d, J = 8.5 Hz, 4H, ArH), 7.56 (d, J = 8.4 Hz, 4H, ArH),
7.14 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.7 Hz, 2H, ArH), 3.94 (p, J = 7.1 Hz, 1H, CH),
3.73 (s, 3H, OCH3), 3.40 (dd, J = 7.0,
16.5 Hz, 2H, CH2), 3.23 (dd, J = 7.1,
16.5 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.8 (CO), 158.5 (C), 135.7 (C), 135.4 (C),
132.1 (C), 129.8 (CH), 128.5 (CH), 114.2 (CH), 55.3 (OCH3), 45.2 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C24H20Br2O3Na [M + Na]+, 536.9671; found, 536.9670.
3-(4-Methoxyphenyl)-1,5-bis(4-methylphenyl)pentane-1,5-dione (4c)
 Colorless
syrup (257.8 mg, 91% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.83 (d, J = 8.2 Hz, 4H, ArH), 7.21 (d, J = 8.0 Hz, 4H, ArH),
7.16 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.7 Hz, 2H, ArH), 3.98 (p, J = 7.0 Hz, 1H, CH),
3.73 (s, 3H, OCH3), 3.40 (dd, J = 7.0,
16.4 Hz, 2H, CH2), 3.25 (dd, J = 7.2,
16.3 Hz, 2H, CH2), 2.38 (s, 6H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.6 (CO), 158.3
(C), 143.9 (C), 136.1 (C), 134.6 (C), 129.4 (CH), 128.5 (CH), 128.4
(CH), 114.1 (CH), 55.3 (OCH3), 45.2 (CH2), 36.8
(CH), 21.8 (CH3); HRMS (ESI) m/z: calcd for C26H26O3Na
[M + Na]+, 409.1774; found, 409.1769.
Colorless
syrup (257.8 mg, 91% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.83 (d, J = 8.2 Hz, 4H, ArH), 7.21 (d, J = 8.0 Hz, 4H, ArH),
7.16 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.7 Hz, 2H, ArH), 3.98 (p, J = 7.0 Hz, 1H, CH),
3.73 (s, 3H, OCH3), 3.40 (dd, J = 7.0,
16.4 Hz, 2H, CH2), 3.25 (dd, J = 7.2,
16.3 Hz, 2H, CH2), 2.38 (s, 6H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 198.6 (CO), 158.3
(C), 143.9 (C), 136.1 (C), 134.6 (C), 129.4 (CH), 128.5 (CH), 128.4
(CH), 114.1 (CH), 55.3 (OCH3), 45.2 (CH2), 36.8
(CH), 21.8 (CH3); HRMS (ESI) m/z: calcd for C26H26O3Na
[M + Na]+, 409.1774; found, 409.1769.
1,3,5-Tris(4-methoxyphenyl)pentane-1,5-dione (4d)31
 Colorless
syrup (307.6 mg, quantitative yield); 1H NMR (500 MHz,
CDCl3): δ (ppm) 7.93–7.90
(m, 4H, ArH), 7.17–7.15 (m, 2H, ArH), 6.91–6.88 (m,
4H, ArH), 6.79–6.76 (m, 2H, ArH), 3.96 (p, J = 7.0 Hz, 1H, CH), 3.84 (s, 6H, OCH3), 3.73 (s, 3H, OCH3), 3.38 (dd, J = 7.0, 16.2 Hz, 2H, CH2), 3.21 (dd, J = 7.3, 16.2 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 197.5 (CO), 163.6 (C), 158.3 (C), 136.1 (C), 130.6 (CH), 130.2
(C), 128.5 (CH), 114.1 (CH), 113.8 (CH), 55.6 (OCH3), 55.3
(OCH3), 45.1 (CH2), 37.1 (CH); HRMS (ESI) m/z: calcd for C26H26O5Na [M + Na]+, 441.1673; found, 441.1664.
Colorless
syrup (307.6 mg, quantitative yield); 1H NMR (500 MHz,
CDCl3): δ (ppm) 7.93–7.90
(m, 4H, ArH), 7.17–7.15 (m, 2H, ArH), 6.91–6.88 (m,
4H, ArH), 6.79–6.76 (m, 2H, ArH), 3.96 (p, J = 7.0 Hz, 1H, CH), 3.84 (s, 6H, OCH3), 3.73 (s, 3H, OCH3), 3.38 (dd, J = 7.0, 16.2 Hz, 2H, CH2), 3.21 (dd, J = 7.3, 16.2 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 197.5 (CO), 163.6 (C), 158.3 (C), 136.1 (C), 130.6 (CH), 130.2
(C), 128.5 (CH), 114.1 (CH), 113.8 (CH), 55.6 (OCH3), 55.3
(OCH3), 45.1 (CH2), 37.1 (CH); HRMS (ESI) m/z: calcd for C26H26O5Na [M + Na]+, 441.1673; found, 441.1664.
3-(4-Methoxyphenyl)-1,5-bis(4-phenylphenyl)pentane-1,5-dione (4e)
 White
solid (303.0 mg, 81% yield); mp 178–180 °C;
1H NMR (500 MHz, CDCl3): δ (ppm) 8.02 (d, J = 8.4 Hz, 4H, ArH), 7.66 (d, J = 8.4
Hz, 4H, ArH), 7.61 (dd, J = 1.2, 8.5 Hz, 4H, ArH),
7.45 (t, J = 7.3 Hz, 4H, ArH), 7.40–7.37 (m,
2H, ArH), 7.22 (d, J = 8.6 Hz, 2H, ArH), 6.82 (d, J = 8.6 Hz, 2H, ArH), 4.06 (p, J = 7.0
Hz, 1H, CH), 3.75 (s, 3H, OCH3), 3.50 (dd, J = 7.0, 16.4 Hz, 2H, CH2), 3.34 (dd, J = 7.1, 16.4 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 198.5 (CO), 158.4 (C), 145.9
(C), 140.0 (C), 135.9 (C), 135.8 (C), 129.1 (CH), 128.9 (CH), 128.6
(CH), 128.3 (CH), 127.4 (CH), 127.4 (CH), 114.2 (CH), 55.3 (OCH3), 45.4 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C36H30O3Na [M + Na]+, 533.2087; found, 533.2078.
White
solid (303.0 mg, 81% yield); mp 178–180 °C;
1H NMR (500 MHz, CDCl3): δ (ppm) 8.02 (d, J = 8.4 Hz, 4H, ArH), 7.66 (d, J = 8.4
Hz, 4H, ArH), 7.61 (dd, J = 1.2, 8.5 Hz, 4H, ArH),
7.45 (t, J = 7.3 Hz, 4H, ArH), 7.40–7.37 (m,
2H, ArH), 7.22 (d, J = 8.6 Hz, 2H, ArH), 6.82 (d, J = 8.6 Hz, 2H, ArH), 4.06 (p, J = 7.0
Hz, 1H, CH), 3.75 (s, 3H, OCH3), 3.50 (dd, J = 7.0, 16.4 Hz, 2H, CH2), 3.34 (dd, J = 7.1, 16.4 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 198.5 (CO), 158.4 (C), 145.9
(C), 140.0 (C), 135.9 (C), 135.8 (C), 129.1 (CH), 128.9 (CH), 128.6
(CH), 128.3 (CH), 127.4 (CH), 127.4 (CH), 114.2 (CH), 55.3 (OCH3), 45.4 (CH2), 36.9 (CH); HRMS (ESI) m/z: calcd for C36H30O3Na [M + Na]+, 533.2087; found, 533.2078.
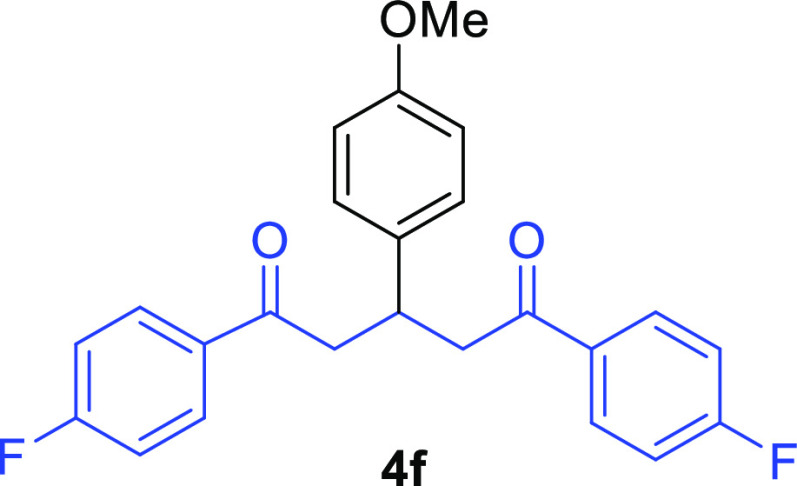
1,5-Bis(4-fluorophenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4f)32
 Colorless
syrup (195.9 mg, 68% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.97–7.94 (m, 4H,
ArH), 7.16–7.13 (m, 2H, ArH), 7.11–7.07 (m, 4H, ArH),
6.80–6.77 (m, 2H, ArH), 3.95 (p, J = 7.0 Hz,
1H, CH), 3.73 (s, 3H, OCH3), 3.42 (dd, J = 7.0, 16.5 Hz, 2H, CH2), 3.24 (dd, J = 7.1, 16.5 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 197.3 (CO), 166.9 (C), 164.9
(C), 158.5 (C), 135.6 (C), 133.5 (C), 133.4 (C), 131.0 (CH), 130.9
(CH), 128.5 (CH), 115.9 (CH), 115.7 (CH), 114.2 (CH), 55.3 (OCH3), 45.2 (CH2), 36.7 (CH); HRMS (ESI) m/z: calcd for C24H20F2O3Na [M + Na]+, 417.1273; found, 417.1266.
Colorless
syrup (195.9 mg, 68% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.97–7.94 (m, 4H,
ArH), 7.16–7.13 (m, 2H, ArH), 7.11–7.07 (m, 4H, ArH),
6.80–6.77 (m, 2H, ArH), 3.95 (p, J = 7.0 Hz,
1H, CH), 3.73 (s, 3H, OCH3), 3.42 (dd, J = 7.0, 16.5 Hz, 2H, CH2), 3.24 (dd, J = 7.1, 16.5 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 197.3 (CO), 166.9 (C), 164.9
(C), 158.5 (C), 135.6 (C), 133.5 (C), 133.4 (C), 131.0 (CH), 130.9
(CH), 128.5 (CH), 115.9 (CH), 115.7 (CH), 114.2 (CH), 55.3 (OCH3), 45.2 (CH2), 36.7 (CH); HRMS (ESI) m/z: calcd for C24H20F2O3Na [M + Na]+, 417.1273; found, 417.1266.
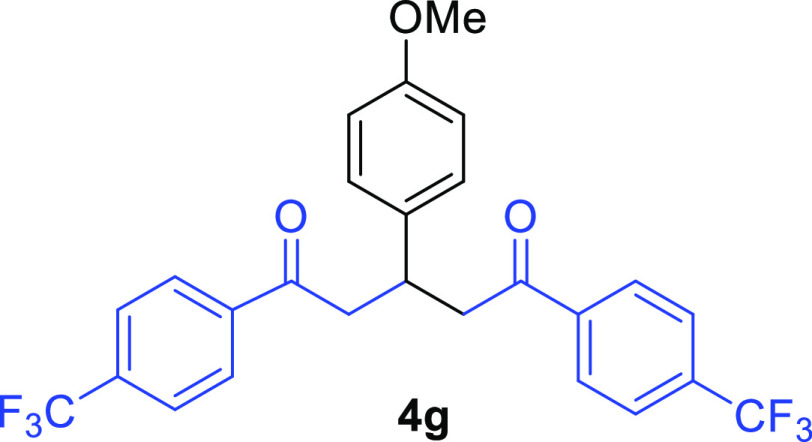
1,5-Bis(4-trifluoromethylphenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4g)
 Colorless syrup (118.0 mg, 33% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 8.02 (d, J = 8.1 Hz, 4H, ArH), 7.69 (d, J = 8.2 Hz, 4H, ArH),
7.15 (d, J = 8.3 Hz, 2H, ArH), 6.80 (d, J = 8.2 Hz, 2H, ArH), 3.98 (p, J = 6.9 Hz, 1H, CH),
3.74 (s, 3H, OCH3), 3.48 (dd, J = 7.0,
16.7 Hz, 2H, CH2), 3.31 (dd, J = 6.9,
16.7 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.9 (CO), 158.6 (C), 139.6 (C), 135.2 (C),
135.0 (C), 134.7 (C), 134.5 (C), 134.2 (C), 128.8 (CH), 128.6 (CH),
128.5 (CH), 127.0 (C), 125.9 (CH), 125.8 (CH), 125.8 (CH), 124.8 (C),
122.6 (C), 120.5 (C), 114.3 (CH), 55.4 (OCH3), 45.4 (CH2), 36.5 (CH); HRMS (ESI) m/z: calcd for C26H21F6O3 [M + H]+, 495.1394; found, 495.1395.
Colorless syrup (118.0 mg, 33% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 8.02 (d, J = 8.1 Hz, 4H, ArH), 7.69 (d, J = 8.2 Hz, 4H, ArH),
7.15 (d, J = 8.3 Hz, 2H, ArH), 6.80 (d, J = 8.2 Hz, 2H, ArH), 3.98 (p, J = 6.9 Hz, 1H, CH),
3.74 (s, 3H, OCH3), 3.48 (dd, J = 7.0,
16.7 Hz, 2H, CH2), 3.31 (dd, J = 6.9,
16.7 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.9 (CO), 158.6 (C), 139.6 (C), 135.2 (C),
135.0 (C), 134.7 (C), 134.5 (C), 134.2 (C), 128.8 (CH), 128.6 (CH),
128.5 (CH), 127.0 (C), 125.9 (CH), 125.8 (CH), 125.8 (CH), 124.8 (C),
122.6 (C), 120.5 (C), 114.3 (CH), 55.4 (OCH3), 45.4 (CH2), 36.5 (CH); HRMS (ESI) m/z: calcd for C26H21F6O3 [M + H]+, 495.1394; found, 495.1395.
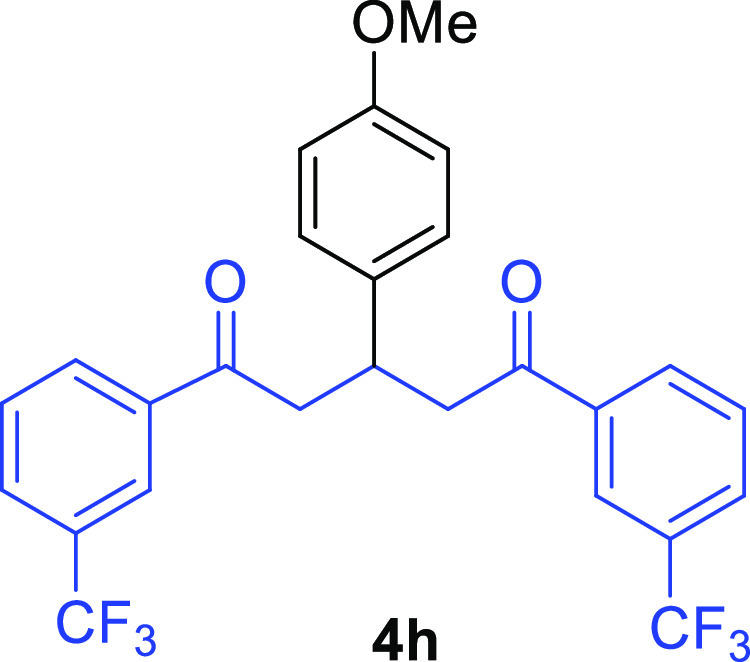
1,5-Bis(3-trifluoromethylphenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4h)
 Colorless syrup (116.2 mg, 32% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 8.15–8.11 (m, 4H,
ArH), 7.79 (d, J = 7.7 Hz, 2H, ArH), 7.56 (t, J = 7.9 Hz, 2H, ArH), 7.18 (d, J = 8.6
Hz, 2H, ArH), 6.80 (d, J = 8.6 Hz, 2H, ArH), 4.00
(p, J = 6.8 Hz, 1H, CH), 3.74 (s, 3H, OCH3), 3.48 (dd, J = 6.9, 16.8 Hz, 2H, CH2), 3.34 (dd, J = 7.0, 16.8 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
197.4 (CO), 158.6 (C), 137.5 (C), 135.3 (C), 131.5 (C), 131.4 (CH),
131.3 (C), 129.7 (CH), 129.7 (CH), 129.5 (CH), 128.5 (CH), 125.2 (CH),
125.1 (CH), 124.9 (CH), 122.7 (CH), 114.3 (CH), 55.4 (OCH3), 45.2 (CH2), 36.4 (CH); HRMS (ESI) m/z: calcd for C26H21F6O3 [M+], 494.1321; found, 494.1317.
Colorless syrup (116.2 mg, 32% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 8.15–8.11 (m, 4H,
ArH), 7.79 (d, J = 7.7 Hz, 2H, ArH), 7.56 (t, J = 7.9 Hz, 2H, ArH), 7.18 (d, J = 8.6
Hz, 2H, ArH), 6.80 (d, J = 8.6 Hz, 2H, ArH), 4.00
(p, J = 6.8 Hz, 1H, CH), 3.74 (s, 3H, OCH3), 3.48 (dd, J = 6.9, 16.8 Hz, 2H, CH2), 3.34 (dd, J = 7.0, 16.8 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
197.4 (CO), 158.6 (C), 137.5 (C), 135.3 (C), 131.5 (C), 131.4 (CH),
131.3 (C), 129.7 (CH), 129.7 (CH), 129.5 (CH), 128.5 (CH), 125.2 (CH),
125.1 (CH), 124.9 (CH), 122.7 (CH), 114.3 (CH), 55.4 (OCH3), 45.2 (CH2), 36.4 (CH); HRMS (ESI) m/z: calcd for C26H21F6O3 [M+], 494.1321; found, 494.1317.
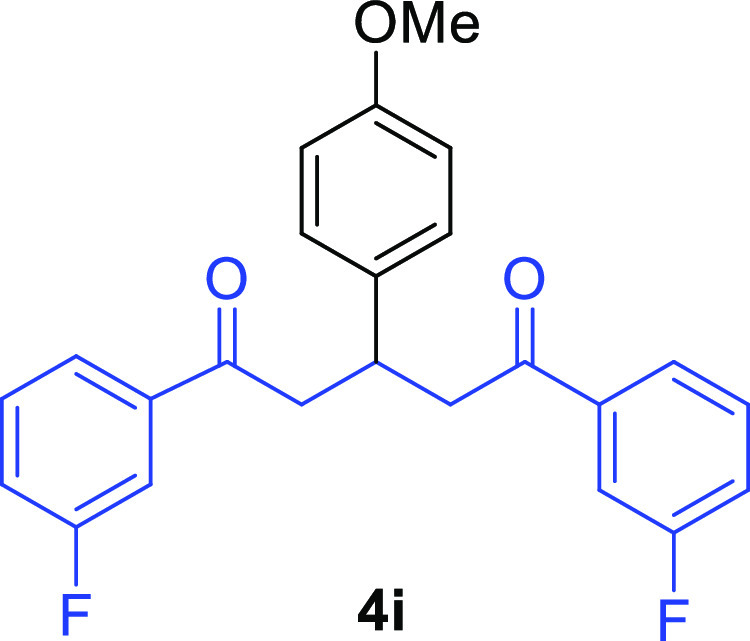
1,5-Bis(3-fluorophenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4i)
 White solid (136.9 mg, 47% yield); mp
111–114 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.71 (d, J = 8.0 Hz, 2H, ArH),
7.59 (d, J = 9.5
Hz, 2H, ArH), 7.43–7.39 (m, 2H, ArH), 7.25–7.21 (m,
2H, ArH), 7.16 (d, J = 8.6 Hz, 2H, ArH), 6.80 (d, J = 8.6 Hz, 2H, ArH), 3.97 (p, J = 7.0
Hz, 1H, CH), 3.74 (s, 3H, OCH3), 3.42 (dd, J = 6.9, 16.7 Hz, 2H, CH2), 3.27 (dd, J = 7.0, 16.6 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 197.5 (CO), 164.0 (C), 162.0
(C), 158.5 (C), 139.1 (C), 139.1 (C), 135.5 (C), 130.5 (CH), 130.4
(CH), 128.5 (CH), 124.1 (CH), 124.0 (CH), 120.4 (CH), 120.2 (CH),
115.1 (CH), 114.9 (CH), 114.2 (CH), 55.4 (OCH3), 45.3 (CH2), 36.5 (CH); HRMS (ESI) m/z: calcd for C24H20F2O3Na [M + Na]+, 417.1273; found, 417.1274.
White solid (136.9 mg, 47% yield); mp
111–114 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.71 (d, J = 8.0 Hz, 2H, ArH),
7.59 (d, J = 9.5
Hz, 2H, ArH), 7.43–7.39 (m, 2H, ArH), 7.25–7.21 (m,
2H, ArH), 7.16 (d, J = 8.6 Hz, 2H, ArH), 6.80 (d, J = 8.6 Hz, 2H, ArH), 3.97 (p, J = 7.0
Hz, 1H, CH), 3.74 (s, 3H, OCH3), 3.42 (dd, J = 6.9, 16.7 Hz, 2H, CH2), 3.27 (dd, J = 7.0, 16.6 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 197.5 (CO), 164.0 (C), 162.0
(C), 158.5 (C), 139.1 (C), 139.1 (C), 135.5 (C), 130.5 (CH), 130.4
(CH), 128.5 (CH), 124.1 (CH), 124.0 (CH), 120.4 (CH), 120.2 (CH),
115.1 (CH), 114.9 (CH), 114.2 (CH), 55.4 (OCH3), 45.3 (CH2), 36.5 (CH); HRMS (ESI) m/z: calcd for C24H20F2O3Na [M + Na]+, 417.1273; found, 417.1274.
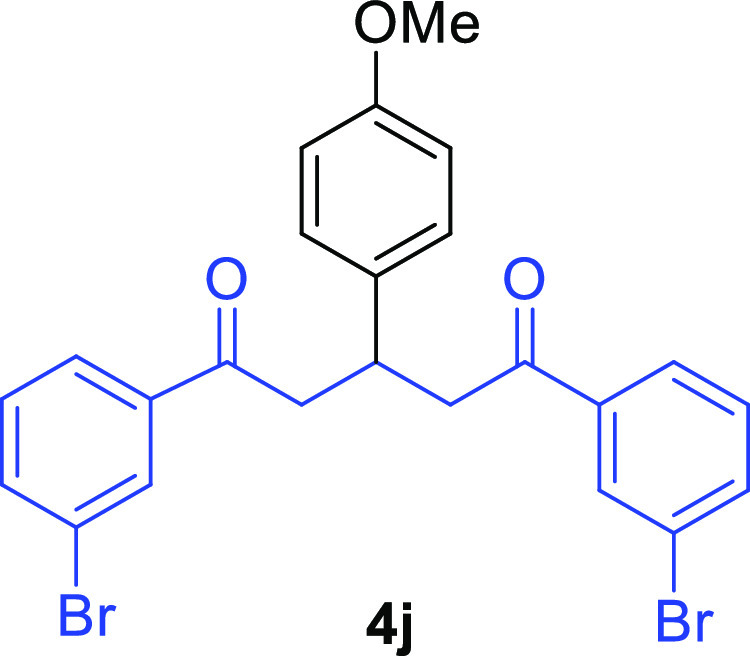
1,5-Bis(3-bromophenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4j)
 White solid (167.0 mg, 44% yield); mp
115–117 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.02 (t, J = 1.7 Hz, 2H, ArH),
7.84 (dt, J = 1.0,
7.9 Hz, 2H, ArH), 7.66–7.64 (m, 2H, ArH), 7.30 (t, J = 7.9 Hz, 2H, ArH), 7.17–7.14 (m, 2H, ArH), 6.80–6.79
(m, 2H, ArH), 3.96 (p, J = 6.9 Hz, 1H, CH), 3.74
(s, 3H, OCH3), 3.40 (dd, J = 6.9, 16.6
Hz, 2H, CH2), 3.26 (dd, J = 7.1, 16.7
Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.4 (CO), 158.5 (C), 138.7 (C), 136.1 (CH),
135.4 (C), 131.3 (CH), 130.3 (CH), 128.5 (CH), 126.8 (CH), 123.1 (C),
114.2 (CH), 55.4 (OCH3), 45.2 (CH2), 36.4 (CH);
HRMS (ESI) m/z: calcd for C24H20Br2O3Na [M + Na]+, 536.9671; found, 536.9667.
White solid (167.0 mg, 44% yield); mp
115–117 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.02 (t, J = 1.7 Hz, 2H, ArH),
7.84 (dt, J = 1.0,
7.9 Hz, 2H, ArH), 7.66–7.64 (m, 2H, ArH), 7.30 (t, J = 7.9 Hz, 2H, ArH), 7.17–7.14 (m, 2H, ArH), 6.80–6.79
(m, 2H, ArH), 3.96 (p, J = 6.9 Hz, 1H, CH), 3.74
(s, 3H, OCH3), 3.40 (dd, J = 6.9, 16.6
Hz, 2H, CH2), 3.26 (dd, J = 7.1, 16.7
Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 197.4 (CO), 158.5 (C), 138.7 (C), 136.1 (CH),
135.4 (C), 131.3 (CH), 130.3 (CH), 128.5 (CH), 126.8 (CH), 123.1 (C),
114.2 (CH), 55.4 (OCH3), 45.2 (CH2), 36.4 (CH);
HRMS (ESI) m/z: calcd for C24H20Br2O3Na [M + Na]+, 536.9671; found, 536.9667.
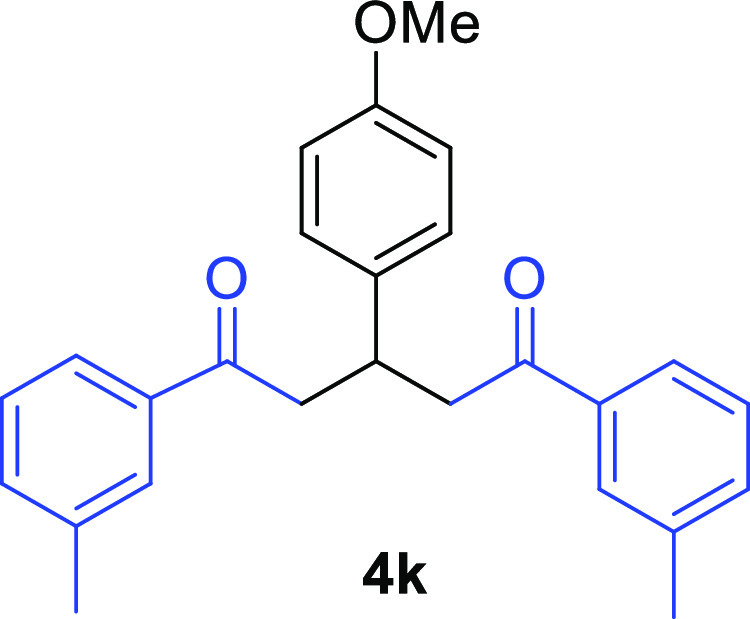
3-(4-Methoxyphenyl)-1,5-bis(3-methylphenyl)pentane-1,5-dione (4k)
 Colorless syrup (263.0 mg, 93% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.73–7.72 (m, 4H,
ArH), 7.34–7.29 (m, 4H, ArH), 7.19–7.17 (m, 2H, ArH),
6.81–6.78 (m, 2H, ArH), 3.99 (p, J = 7.1 Hz,
1H, CH), 3.73 (s, 3H, OCH3), 3.42 (dd, J = 6.9, 16.5 Hz, 2H, CH2), 3.28 (dd, J = 7.1, 16.4 Hz, 2H, CH2), 2.37 (s, 6H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
199.1 (CO), 158.3 (C), 138.5 (C), 137.1 (C), 136.1 (C), 133.9 (CH),
128.8 (CH), 128.6 (CH), 128.5 (CH), 125.5 (CH), 114.1 (CH), 55.3 (OCH3), 45.3 (CH2), 36.6 (CH), 21.5 (CH3);
HRMS (ESI) m/z: calcd for C26H26O3Na [M + Na]+, 409.1774;
found, 409.1773.
Colorless syrup (263.0 mg, 93% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.73–7.72 (m, 4H,
ArH), 7.34–7.29 (m, 4H, ArH), 7.19–7.17 (m, 2H, ArH),
6.81–6.78 (m, 2H, ArH), 3.99 (p, J = 7.1 Hz,
1H, CH), 3.73 (s, 3H, OCH3), 3.42 (dd, J = 6.9, 16.5 Hz, 2H, CH2), 3.28 (dd, J = 7.1, 16.4 Hz, 2H, CH2), 2.37 (s, 6H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
199.1 (CO), 158.3 (C), 138.5 (C), 137.1 (C), 136.1 (C), 133.9 (CH),
128.8 (CH), 128.6 (CH), 128.5 (CH), 125.5 (CH), 114.1 (CH), 55.3 (OCH3), 45.3 (CH2), 36.6 (CH), 21.5 (CH3);
HRMS (ESI) m/z: calcd for C26H26O3Na [M + Na]+, 409.1774;
found, 409.1773.
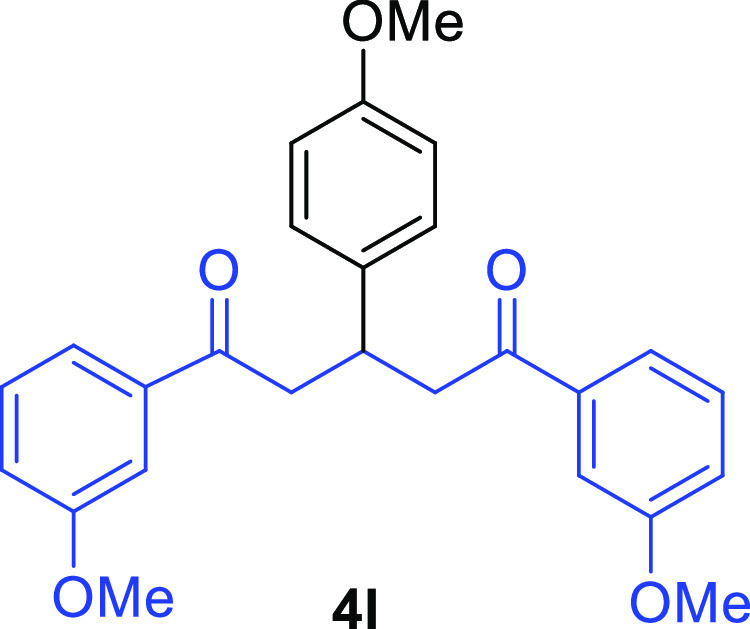
3-(4-Methoxyphenyl)-1,5-bis(3-methoxyphenyl)pentane-1,5-dione (4l)
 White solid (287.3 mg, 94% yield); mp
84–87 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.52 (d, J = 7.7 Hz, 2H, ArH),
7.45–7.44 (m, 2H, ArH), 7.33
(t, J = 8.1 Hz, 2H, ArH), 7.18–7.15 (m, 2H,
ArH), 7.08–7.06 (m, 2H, ArH), 6.80–6.78 (m, 2H, ArH),
3.99 (p, J = 7.1 Hz, 1H, CH), 3.82 (s, 6H, OCH3), 3.73 (s, 3H, OCH3), 3.42 (dd, J = 6.9, 16.6 Hz, 2H, CH2), 3.26 (dd, J = 7.2, 16.5 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 198.7 (CO), 160.0 (C), 158.4
(C), 138.4 (C), 135.9 (C), 129.7 (CH), 128.5 (CH), 121.0 (CH), 119.9
(CH), 114.1 (CH), 112.4 (CH), 55.6 (OCH3), 55.3 (OCH3), 45.4 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C26H26O5Na [M + Na]+, 441.1673; found, 441.1665.
White solid (287.3 mg, 94% yield); mp
84–87 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.52 (d, J = 7.7 Hz, 2H, ArH),
7.45–7.44 (m, 2H, ArH), 7.33
(t, J = 8.1 Hz, 2H, ArH), 7.18–7.15 (m, 2H,
ArH), 7.08–7.06 (m, 2H, ArH), 6.80–6.78 (m, 2H, ArH),
3.99 (p, J = 7.1 Hz, 1H, CH), 3.82 (s, 6H, OCH3), 3.73 (s, 3H, OCH3), 3.42 (dd, J = 6.9, 16.6 Hz, 2H, CH2), 3.26 (dd, J = 7.2, 16.5 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 198.7 (CO), 160.0 (C), 158.4
(C), 138.4 (C), 135.9 (C), 129.7 (CH), 128.5 (CH), 121.0 (CH), 119.9
(CH), 114.1 (CH), 112.4 (CH), 55.6 (OCH3), 55.3 (OCH3), 45.4 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C26H26O5Na [M + Na]+, 441.1673; found, 441.1665.
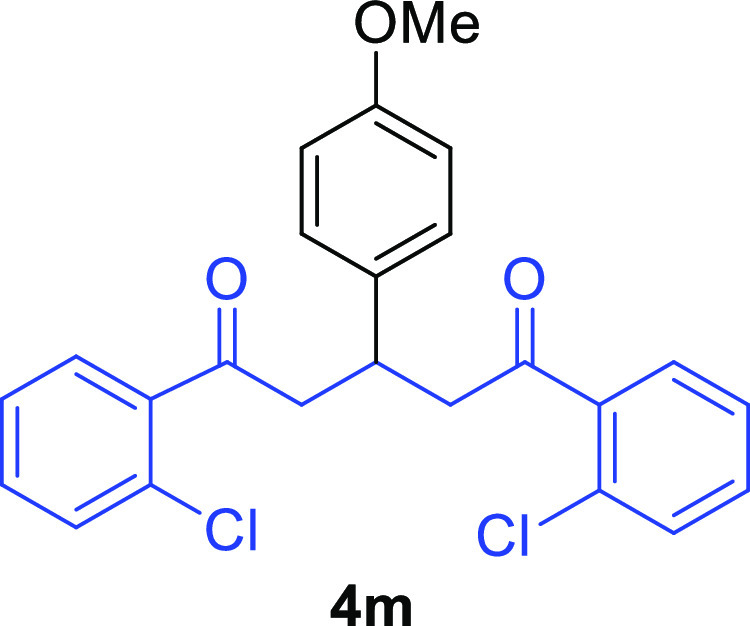
1,5-Bis(2-chlorophenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4m)
 Colorless syrup (236.1 mg, 75% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.35–7.30 (m, 4H,
ArH), 7.25–7.21 (m, 4H, ArH), 7.10–7.08 (m, 2H, ArH),
6.77–6.75 (m, 2H, ArH), 3.88 (p, J = 7.3 Hz,
1H, CH), 3.74 (s, 3H, OCH3), 3.39 (dd, J = 6.7, 16.9 Hz, 2H, CH2), 3.28 (dd, J = 7.9, 16.8 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 201.9 (CO), 158.4 (C), 139.5
(C), 134.9 (C), 131.7 (CH), 130.9 (C), 130.5 (CH), 129.0 (CH), 128.7
(CH), 127.0 (CH), 114.0 (CH), 55.4 (OCH3), 49.3 (CH2), 36.7 (CH); HRMS (ESI) m/z: calcd for C24H20Cl2O3Na [M + Na]+, 449.0682; found, 449.0686.
Colorless syrup (236.1 mg, 75% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.35–7.30 (m, 4H,
ArH), 7.25–7.21 (m, 4H, ArH), 7.10–7.08 (m, 2H, ArH),
6.77–6.75 (m, 2H, ArH), 3.88 (p, J = 7.3 Hz,
1H, CH), 3.74 (s, 3H, OCH3), 3.39 (dd, J = 6.7, 16.9 Hz, 2H, CH2), 3.28 (dd, J = 7.9, 16.8 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 201.9 (CO), 158.4 (C), 139.5
(C), 134.9 (C), 131.7 (CH), 130.9 (C), 130.5 (CH), 129.0 (CH), 128.7
(CH), 127.0 (CH), 114.0 (CH), 55.4 (OCH3), 49.3 (CH2), 36.7 (CH); HRMS (ESI) m/z: calcd for C24H20Cl2O3Na [M + Na]+, 449.0682; found, 449.0686.
3-(4-Methoxyphenyl)-1,5-bis(2-bromophenyl)pentane-1,5-dione (4n)
 Colorless syrup (324.2 mg, 86% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.53 (dd, J = 1.0, 7.8 Hz, 2H, ArH), 7.28–7.21 (m, 4H, ArH), 7.16 (dd, J = 1.9, 7.4 Hz, 2H, ArH), 7.11–7.09 (m, 2H, ArH),
6.78–6.75 (m, 2H, ArH), 3.88 (p, J = 7.3 Hz,
1H, CH), 3.74 (s, 3H, OCH3), 3.40 (dd, J = 6.7, 17.0 Hz, 2H, CH2), 3.25 (dd, J = 7.9, 17.0 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 202.6 (CO), 158.5 (C), 141.7
(C), 134.8 (C), 133.7 (CH), 131.6 (CH), 128.8 (CH), 128.6 (CH), 127.5
(CH), 118.7 (C), 114.1 (CH), 55.4 (OCH3), 48.9 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C24H20Br2O3Na [M + Na]+, 536.9671; found, 536.9674.
Colorless syrup (324.2 mg, 86% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.53 (dd, J = 1.0, 7.8 Hz, 2H, ArH), 7.28–7.21 (m, 4H, ArH), 7.16 (dd, J = 1.9, 7.4 Hz, 2H, ArH), 7.11–7.09 (m, 2H, ArH),
6.78–6.75 (m, 2H, ArH), 3.88 (p, J = 7.3 Hz,
1H, CH), 3.74 (s, 3H, OCH3), 3.40 (dd, J = 6.7, 17.0 Hz, 2H, CH2), 3.25 (dd, J = 7.9, 17.0 Hz, 2H, CH2); 13C{H} NMR (125
MHz, CDCl3): δ (ppm) 202.6 (CO), 158.5 (C), 141.7
(C), 134.8 (C), 133.7 (CH), 131.6 (CH), 128.8 (CH), 128.6 (CH), 127.5
(CH), 118.7 (C), 114.1 (CH), 55.4 (OCH3), 48.9 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C24H20Br2O3Na [M + Na]+, 536.9671; found, 536.9674.
3-(4-Methoxyphenyl)-1,5-bis(2-methoxyphenyl)pentane-1,5-dione (4o)
 White solid (245.8 mg, 80% yield); mp 102–104
°C; 1H NMR (500 MHz, CDCl3): δ (ppm)
7.45 (dd, J = 1.5, 7.6 Hz, 2H, ArH), 7.41–7.37
(m, 2H, ArH),
7.08 (d, J = 8.7 Hz, 2H, ArH), 6.92–6.89 (m,
4H, ArH), 6.74 (d, J = 8.7 Hz, 2H, ArH), 3.86 (p, J = 7.1 Hz, 1H, CH), 3.83 (s, 6H, OCH3), 3.73
(s, 3H, OCH3), 3.36–3.27 (m, 4H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 201.5
(CO), 158.3 (C), 158.0 (C), 136.7 (C), 133.3 (CH), 130.4 (CH), 128.8
(CH), 128.7 (CH), 120.7 (CH), 113.7 (CH), 111.5 (CH), 55.6 (OCH3), 55.3 (OCH3), 50.4 (CH2), 36.8 (CH);
HRMS (ESI) m/z: calcd for C26H26O5Na [M + Na]+, 441.1673;
found, 441.1672.
White solid (245.8 mg, 80% yield); mp 102–104
°C; 1H NMR (500 MHz, CDCl3): δ (ppm)
7.45 (dd, J = 1.5, 7.6 Hz, 2H, ArH), 7.41–7.37
(m, 2H, ArH),
7.08 (d, J = 8.7 Hz, 2H, ArH), 6.92–6.89 (m,
4H, ArH), 6.74 (d, J = 8.7 Hz, 2H, ArH), 3.86 (p, J = 7.1 Hz, 1H, CH), 3.83 (s, 6H, OCH3), 3.73
(s, 3H, OCH3), 3.36–3.27 (m, 4H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 201.5
(CO), 158.3 (C), 158.0 (C), 136.7 (C), 133.3 (CH), 130.4 (CH), 128.8
(CH), 128.7 (CH), 120.7 (CH), 113.7 (CH), 111.5 (CH), 55.6 (OCH3), 55.3 (OCH3), 50.4 (CH2), 36.8 (CH);
HRMS (ESI) m/z: calcd for C26H26O5Na [M + Na]+, 441.1673;
found, 441.1672.
3-(4-Methoxyphenyl)-1,5-bis-(2-methylphenyl)pentane-1,5-dione (4p)
 Colorless syrup (261.1 mg, 92% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.54 (dd, J = 1.1, 7.7 Hz, 2H, ArH), 7.32 (td, J = 1.3,15.0
Hz, 2H, ArH), 7.22–7.19 (m, 2H, ArH), 7.18–7.17 (m,
2H, ArH), 7.09–7.06 (m, 2H, ArH), 6.78–6.75 (m, 2H,
ArH), 3.87 (p, J = 7.3 Hz, 1H, CH), 3.74 (s, 3H,
OCH3), 3.34 (dd, J = 7.4, 16.4 Hz, 2H,
CH2), 3.18 (dd, J = 7.8, 16.4 Hz, 2H,
CH2), 2.23 (s, 6H, CH3); 13C{H} NMR
(125 MHz, CDCl3): δ (ppm) 203.2 (CO), 158.4 (C),
138.2 (C), 138.1 (C), 135.5 (C), 132.0 (CH), 131.3 (CH), 128.7 (CH),
128.4 (CH), 125.7 (CH), 114.1 (CH), 55.4 (OCH3), 48.3 (CH2), 37.1 (CH), 21.0 (CH3); HRMS (ESI) m/z: calcd for C26H26O3Na [M + Na]+, 409.1774; found, 409.1770.
Colorless syrup (261.1 mg, 92% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.54 (dd, J = 1.1, 7.7 Hz, 2H, ArH), 7.32 (td, J = 1.3,15.0
Hz, 2H, ArH), 7.22–7.19 (m, 2H, ArH), 7.18–7.17 (m,
2H, ArH), 7.09–7.06 (m, 2H, ArH), 6.78–6.75 (m, 2H,
ArH), 3.87 (p, J = 7.3 Hz, 1H, CH), 3.74 (s, 3H,
OCH3), 3.34 (dd, J = 7.4, 16.4 Hz, 2H,
CH2), 3.18 (dd, J = 7.8, 16.4 Hz, 2H,
CH2), 2.23 (s, 6H, CH3); 13C{H} NMR
(125 MHz, CDCl3): δ (ppm) 203.2 (CO), 158.4 (C),
138.2 (C), 138.1 (C), 135.5 (C), 132.0 (CH), 131.3 (CH), 128.7 (CH),
128.4 (CH), 125.7 (CH), 114.1 (CH), 55.4 (OCH3), 48.3 (CH2), 37.1 (CH), 21.0 (CH3); HRMS (ESI) m/z: calcd for C26H26O3Na [M + Na]+, 409.1774; found, 409.1770.
1,5-Bis-(3-chloro-4-methoxyphenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4q)
 Colorless syrup (336.6 mg, 94% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.95 (d, J = 2.1 Hz, 2H, ArH), 7.86 (dd, J = 2.1, 8.6 Hz,
2H, ArH), 7.15 (d, J = 8.7, 2H, ArH), 6.92 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.6,
2H, ArH), 3.96–3.90 (overlap, 7H, CH, OCH3), 3.73
(s, 6H, OCH3), 3.73 (s, 3H, OCH3), 3.37 (dd, J = 7.0, 16.4 Hz, 2H, CH2), 3.19 (dd, J = 7.1, 16.4 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 196.5 (CO), 158.8 (C),
158.3 (C), 135.6 (C), 130.6 (CH), 130.5 (C), 128.8 (CH), 128.4 (CH),
122.9 (C), 114.1 (CH), 111.3 (CH), 55.5 (OCH3), 55.3 (OCH3), 44.9 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C26H24O5Cl2Na [M + Na]+, 509.0893; found, 509.0899.
Colorless syrup (336.6 mg, 94% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.95 (d, J = 2.1 Hz, 2H, ArH), 7.86 (dd, J = 2.1, 8.6 Hz,
2H, ArH), 7.15 (d, J = 8.7, 2H, ArH), 6.92 (d, J = 8.6 Hz, 2H, ArH), 6.78 (d, J = 8.6,
2H, ArH), 3.96–3.90 (overlap, 7H, CH, OCH3), 3.73
(s, 6H, OCH3), 3.73 (s, 3H, OCH3), 3.37 (dd, J = 7.0, 16.4 Hz, 2H, CH2), 3.19 (dd, J = 7.1, 16.4 Hz, 2H, CH2); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 196.5 (CO), 158.8 (C),
158.3 (C), 135.6 (C), 130.6 (CH), 130.5 (C), 128.8 (CH), 128.4 (CH),
122.9 (C), 114.1 (CH), 111.3 (CH), 55.5 (OCH3), 55.3 (OCH3), 44.9 (CH2), 36.8 (CH); HRMS (ESI) m/z: calcd for C26H24O5Cl2Na [M + Na]+, 509.0893; found, 509.0899.
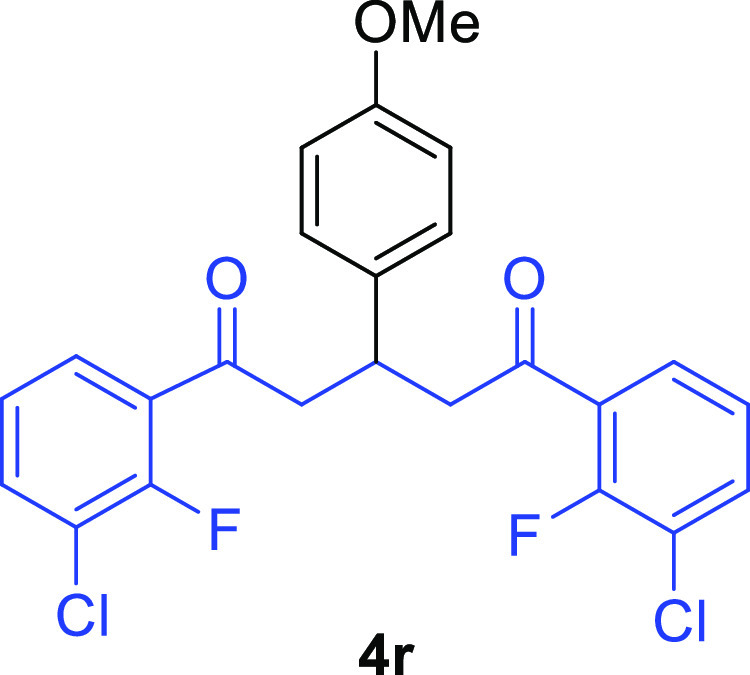
1,5-Bis-(3-chloro-2-fluorophenyl)-3-(4-methoxyphenyl)pentane-1,5-dione (4r)
 White solid (134.5 mg, 40% yield); mp
124–126 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.58 (t, J = 6.4 Hz, 2H, ArH),
7.53 (t, J = 6.8
Hz, 2H, ArH), 7.16 (d, J = 8.5 Hz, 2H, ArH), 7.11
(t, J = 8.0 Hz, 2H, ArH), 6.78 (d, J = 8.5 Hz, 2H, ArH), 3.98 (p, J = 6.9 Hz, 1H, CH),
3.74 (s, 3H, OCH3), 3.36 (dd, J = 2.5,
6.9 Hz, 4H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 196.1 (CO), 196.1 (CO), 158.4 (C), 158.3
(C), 156.3 (C), 135.6 (C), 134.8 (CH), 129.1 (CH), 128.6 (CH), 127.4
(C), 127.3 (C), 124.9 (CH), 124.9 (CH), 122.5 (C), 122.4 (C), 114.1
(CH), 55.3 (OCH3), 50.0 (CH2), 50.0 (CH2), 35.7 (CH); HRMS (ESI) m/z: calcd for C24H18Cl2O3F2Na [M + Na]+, 485.0493; found, 485.0487.
White solid (134.5 mg, 40% yield); mp
124–126 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.58 (t, J = 6.4 Hz, 2H, ArH),
7.53 (t, J = 6.8
Hz, 2H, ArH), 7.16 (d, J = 8.5 Hz, 2H, ArH), 7.11
(t, J = 8.0 Hz, 2H, ArH), 6.78 (d, J = 8.5 Hz, 2H, ArH), 3.98 (p, J = 6.9 Hz, 1H, CH),
3.74 (s, 3H, OCH3), 3.36 (dd, J = 2.5,
6.9 Hz, 4H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 196.1 (CO), 196.1 (CO), 158.4 (C), 158.3
(C), 156.3 (C), 135.6 (C), 134.8 (CH), 129.1 (CH), 128.6 (CH), 127.4
(C), 127.3 (C), 124.9 (CH), 124.9 (CH), 122.5 (C), 122.4 (C), 114.1
(CH), 55.3 (OCH3), 50.0 (CH2), 50.0 (CH2), 35.7 (CH); HRMS (ESI) m/z: calcd for C24H18Cl2O3F2Na [M + Na]+, 485.0493; found, 485.0487.
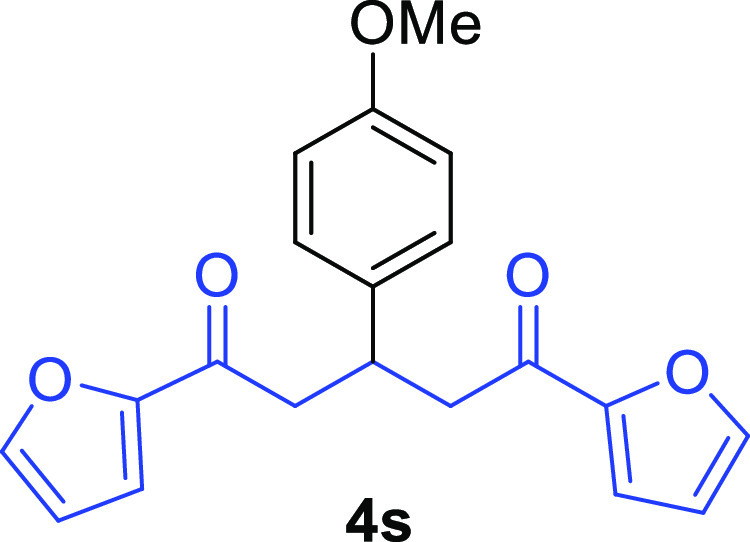
1,5-Di(furan-2-yl)-3-(4-methoxyphenyl)pentane-1,5-dione (4s)68
 White
solid (97.5 mg, 40% yield); mp 108–110 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.51 (s,
2H, ArH), 7.18–7.15 (m, 4H, ArH), 6.77 (d, J = 8.6 Hz, 2H, ArH), 6.47 (dd, J = 1.6, 3.5 Hz,
2H, ArH), 3.95 (p, J = 7.2 Hz, 1H, CH), 3.72 (s,
3H, OCH3), 3.26–3.13 (overlap, 4H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
187.7 (CO), 158.4 (CO), 152.9 (C), 146.5 (CH), 135.4 (C), 128.5 (CH),
117.4 (CH), 114.1 (CH), 112.3 (C), 55.3 (OCH3), 44.8 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C20H18O5Na [M + Na]+, 361.1046; found, 361.1039.
White
solid (97.5 mg, 40% yield); mp 108–110 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.51 (s,
2H, ArH), 7.18–7.15 (m, 4H, ArH), 6.77 (d, J = 8.6 Hz, 2H, ArH), 6.47 (dd, J = 1.6, 3.5 Hz,
2H, ArH), 3.95 (p, J = 7.2 Hz, 1H, CH), 3.72 (s,
3H, OCH3), 3.26–3.13 (overlap, 4H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
187.7 (CO), 158.4 (CO), 152.9 (C), 146.5 (CH), 135.4 (C), 128.5 (CH),
117.4 (CH), 114.1 (CH), 112.3 (C), 55.3 (OCH3), 44.8 (CH2), 36.6 (CH); HRMS (ESI) m/z: calcd for C20H18O5Na [M + Na]+, 361.1046; found, 361.1039.
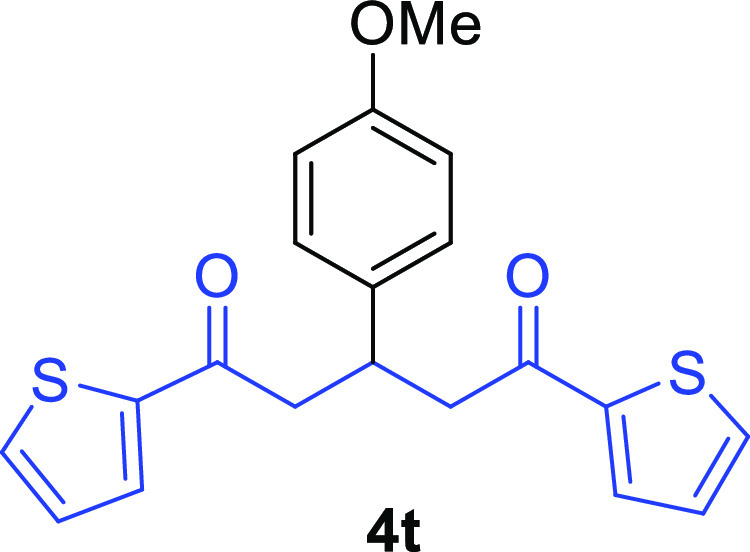
1,5-Di(thiophen-2-yl)-3-(4-methoxyphenyl)pentane-1,5-dione (4t)
 Colorless syrup (217.9 mg, 80% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.73 (dd, J = 1.0, 3.8 Hz, 2H, ArH), 7.58 (dd, J = 1.0, 4.9
Hz, 2H, ArH), 7.19–7.17 (m, 2H, ArH), 7.08 (dd, J = 3.9, 4.8 Hz, 2H, ArH), 6.80–6.77 (m, 2H, ArH), 4.00 (p, J = 7.1 Hz, 1H, CH), 3.73 (s, 3H, OCH3), 3.38
(dd, J = 7.0, 15.9 Hz, 2H, CH2), 3.22
(dd, J = 7.3, 15.9 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 191.6
(CO), 158.4 (CO), 144.4 (C), 135.3 (C), 133.8 (CH), 132.3 (CH), 128.5
(CH), 128.3 (CH), 114.2 (CH), 55.3 (OCH3), 45.7 (CH2), 37.4 (CH); HRMS (ESI) m/z: calcd for C20H18O3S2Na [M + Na]+, 393.0590; found, 393.0587.
Colorless syrup (217.9 mg, 80% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 7.73 (dd, J = 1.0, 3.8 Hz, 2H, ArH), 7.58 (dd, J = 1.0, 4.9
Hz, 2H, ArH), 7.19–7.17 (m, 2H, ArH), 7.08 (dd, J = 3.9, 4.8 Hz, 2H, ArH), 6.80–6.77 (m, 2H, ArH), 4.00 (p, J = 7.1 Hz, 1H, CH), 3.73 (s, 3H, OCH3), 3.38
(dd, J = 7.0, 15.9 Hz, 2H, CH2), 3.22
(dd, J = 7.3, 15.9 Hz, 2H, CH2); 13C{H} NMR (125 MHz, CDCl3): δ (ppm) 191.6
(CO), 158.4 (CO), 144.4 (C), 135.3 (C), 133.8 (CH), 132.3 (CH), 128.5
(CH), 128.3 (CH), 114.2 (CH), 55.3 (OCH3), 45.7 (CH2), 37.4 (CH); HRMS (ESI) m/z: calcd for C20H18O3S2Na [M + Na]+, 393.0590; found, 393.0587.
General Procedure for the Synthesis of 2,4,6-Triayl Pyridine Derivatives (5a–e)53,55,56,63
The representative 1,3,5-triayl-1,5-dione (3 and 4) (0.186 mmol, 1 equiv) was dissolved in EtOH (1.5 mL). To this, a stirred solution of ammonium acetate (1.93 mmol, 10.4 equiv) was added and the reaction mixture was refluxed at 80 °C for 4–10 h depending on the reactivity of the types of 1,5-diketones. Upon cooling to rt, the EtOH was removed by rotary vacuum, dissolved in ethyl acetate, and neutralized with water. The desired product was extracted from the aqueous phases with ethyl acetate and then washed three times with water. The combined organic phases were washed with saturated brine solution. Next, the organic layers were separated and dried over anhydrous MgSO4, filtered, and concentrated in rotary vacuum to give the crude products. Purification of the crude products by flash column chromatography using 1 to 5% ethyl acetate in hexane afforded the corresponding pure and known 2,4,6-triayl pyridine derivatives 5a,55,56,645b,535c,56,635d,56 and 5e.63
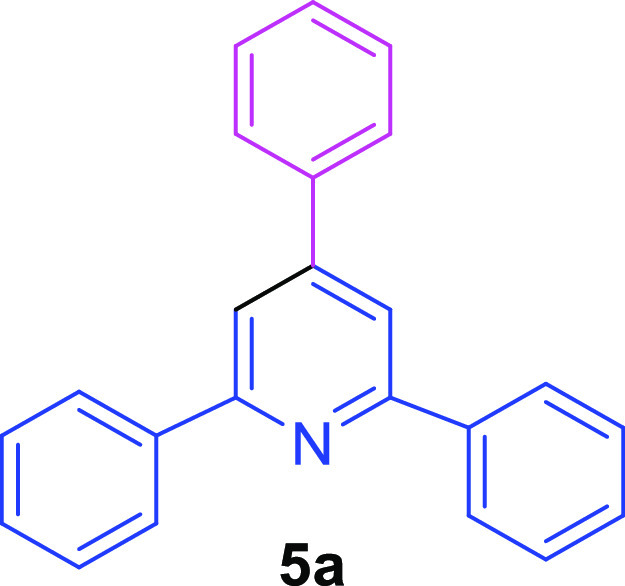
2,4,6-Triphenylpyridine (5a)55,56
 White solid (37.4 mg, 71% yield); mp 135–138
°C; 1H NMR (500 MHz, CDCl3): δ (ppm)
8.21 (d, J = 7.4 Hz, 4H, ArH), 7.89 (s, 2H, ArH),
7.75 (d, J = 7.3 Hz, 2H, ArH), 7.54–7.43 (m,
9H, ArH); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 157.7
(C), 150.3 (C), 139.7 (C), 139.2 (C), 129.3 (CH), 129.2 (CH), 129.1
(CH), 128.9 (CH), 127.3 (CH), 127.3 (CH), 117.3 (CH); HRMS (ESI) m/z: calcd for C23H16N [M + H]+, 308.1434; found, 308.1426.
White solid (37.4 mg, 71% yield); mp 135–138
°C; 1H NMR (500 MHz, CDCl3): δ (ppm)
8.21 (d, J = 7.4 Hz, 4H, ArH), 7.89 (s, 2H, ArH),
7.75 (d, J = 7.3 Hz, 2H, ArH), 7.54–7.43 (m,
9H, ArH); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 157.7
(C), 150.3 (C), 139.7 (C), 139.2 (C), 129.3 (CH), 129.2 (CH), 129.1
(CH), 128.9 (CH), 127.3 (CH), 127.3 (CH), 117.3 (CH); HRMS (ESI) m/z: calcd for C23H16N [M + H]+, 308.1434; found, 308.1426.
4-(2,4-Dichlorophenyl)-2,6-diphenylpyridine (5b)55
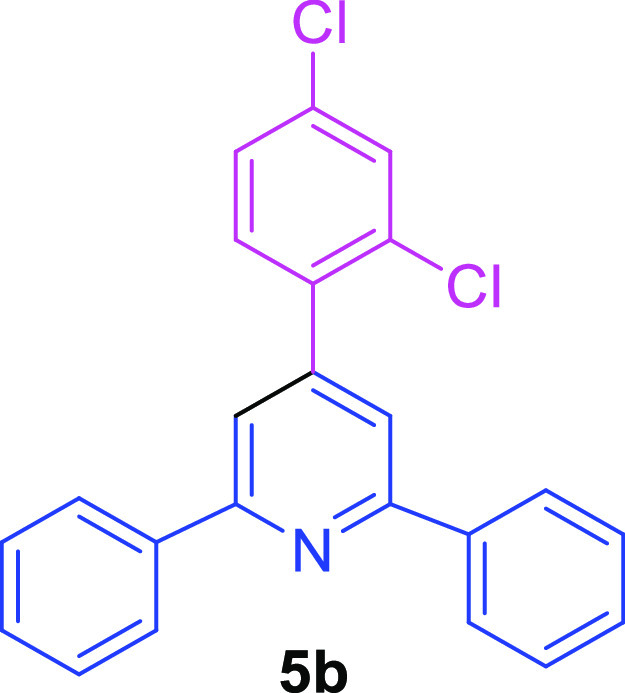 Colorless
paste (39.8 mg, 68% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 8.17–8.15 (m, 4H,
ArH), 7.72 (s, 2H, ArH), 7.56 (t, J = 1.2 Hz, 1H,
ArH), 7.51–7.48 (m, 4H, ArH), 7.45–7.41 (m, 2H, ArH),
7.37 (d, J = 1.2 Hz, 2H, ArH); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 157.2 (C), 147.7 (C),
139.4 (C), 137.2 (C), 135.2 (C), 133.3 (C), 131.8 (CH), 130.3 (CH),
129.4 (CH), 128.9 (CH), 127.7 (CH), 127.3 (CH), 119.4 (CH); HRMS (ESI) m/z: calcd for C23H16NCl2 [M + H]+, 376.0654; found, 376.0652.
Colorless
paste (39.8 mg, 68% yield); 1H NMR
(500 MHz, CDCl3): δ (ppm) 8.17–8.15 (m, 4H,
ArH), 7.72 (s, 2H, ArH), 7.56 (t, J = 1.2 Hz, 1H,
ArH), 7.51–7.48 (m, 4H, ArH), 7.45–7.41 (m, 2H, ArH),
7.37 (d, J = 1.2 Hz, 2H, ArH); 13C{H}
NMR (125 MHz, CDCl3): δ (ppm) 157.2 (C), 147.7 (C),
139.4 (C), 137.2 (C), 135.2 (C), 133.3 (C), 131.8 (CH), 130.3 (CH),
129.4 (CH), 128.9 (CH), 127.7 (CH), 127.3 (CH), 119.4 (CH); HRMS (ESI) m/z: calcd for C23H16NCl2 [M + H]+, 376.0654; found, 376.0652.
2,6-Bis(4-chlorophenyl)-4-(4-methoxyphenyl)pyridine (5c)56
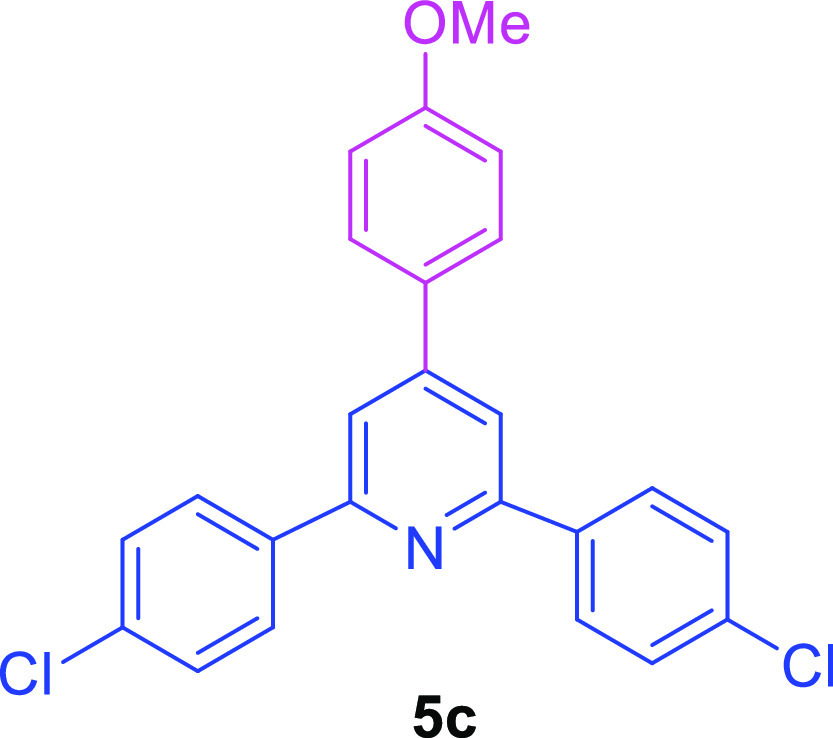 White solid (55.1 mg, 73% yield); mp 181–183 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.11–8.08
(m, 4H, ArH), 7.78 (s, 2H, ArH), 7.67–7.64 (m, 2H, ArH), 7.47–7.44
(m, 4H, ArH), 7.04–7.02 (m, 2H, ArH), 3.88 (s, 3H, OCH3); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 160.8 (C), 156.4 (C), 150.1 (C), 138.0 (C), 135.3 (C), 131.0
(CH), 129.1 (CH), 128.5 (CH), 128.4 (CH), 116.7 (CH), 114.7 (CH),
55.6 (OCH3); HRMS (ESI) m/z: calcd for C24H18NOCl2 [M + H]+, 406.0760; found, 406.0753.
White solid (55.1 mg, 73% yield); mp 181–183 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.11–8.08
(m, 4H, ArH), 7.78 (s, 2H, ArH), 7.67–7.64 (m, 2H, ArH), 7.47–7.44
(m, 4H, ArH), 7.04–7.02 (m, 2H, ArH), 3.88 (s, 3H, OCH3); 13C{H} NMR (125 MHz, CDCl3): δ
(ppm) 160.8 (C), 156.4 (C), 150.1 (C), 138.0 (C), 135.3 (C), 131.0
(CH), 129.1 (CH), 128.5 (CH), 128.4 (CH), 116.7 (CH), 114.7 (CH),
55.6 (OCH3); HRMS (ESI) m/z: calcd for C24H18NOCl2 [M + H]+, 406.0760; found, 406.0753.
2,6-Bis(4-methylphenyl)-4-(4-methoxyphenyl)pyridine (5d)56
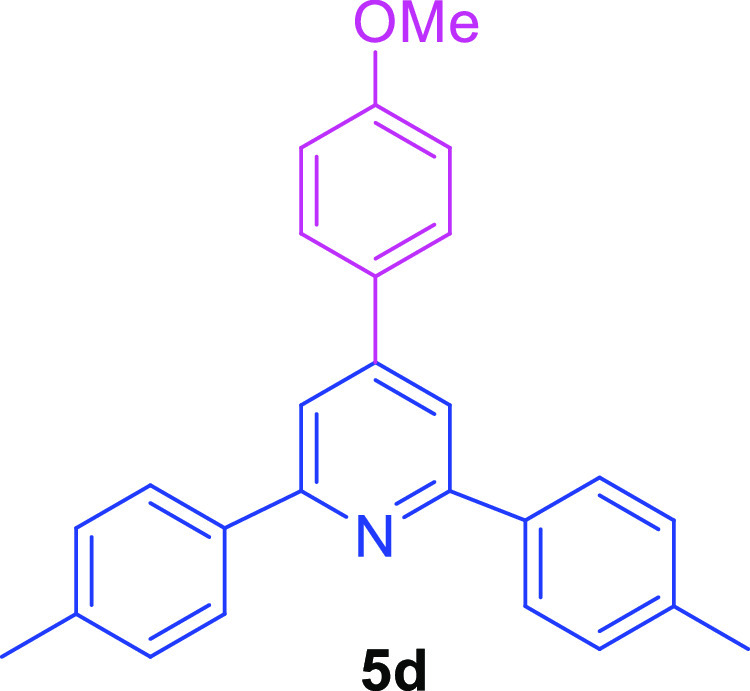 White solid (51.5 mg, 72% yield); mp157–158 °C; 1H NMR (500 MHz, CDCl3): (ppm) 8.10 (d, J = 8.1 Hz, 4H, ArH), 7.80 (s, 2H, ArH), 7.70–7.67
(m, 2H, ArH), 7.31 (d, J = 8.0 Hz, 4H, ArH), 7.05–7.02
(m, 2H, ArH), 3.87 (s, 3H, OCH3), 2.43 (s, 6H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
160.5 (C), 157.5 (C), 149.6 (C), 139.0 (C), 137.2 (C), 131.6 (C),
129.5 (CH), 128.4 (CH), 127.1 (CH), 116.1 (CH), 114.6 (CH), 55.5 (OCH3), 21.4 (CH3); HRMS (ESI) m/z: calcd for C26H24NO [M + H]+, 366.18524; found, 366.18492.
White solid (51.5 mg, 72% yield); mp157–158 °C; 1H NMR (500 MHz, CDCl3): (ppm) 8.10 (d, J = 8.1 Hz, 4H, ArH), 7.80 (s, 2H, ArH), 7.70–7.67
(m, 2H, ArH), 7.31 (d, J = 8.0 Hz, 4H, ArH), 7.05–7.02
(m, 2H, ArH), 3.87 (s, 3H, OCH3), 2.43 (s, 6H, CH3); 13C{H} NMR (125 MHz, CDCl3): δ (ppm)
160.5 (C), 157.5 (C), 149.6 (C), 139.0 (C), 137.2 (C), 131.6 (C),
129.5 (CH), 128.4 (CH), 127.1 (CH), 116.1 (CH), 114.6 (CH), 55.5 (OCH3), 21.4 (CH3); HRMS (ESI) m/z: calcd for C26H24NO [M + H]+, 366.18524; found, 366.18492.
2,6-Bis(4-methoxyphenyl)-4-(4-methoxyphenyl)pyridine (5e)55,56
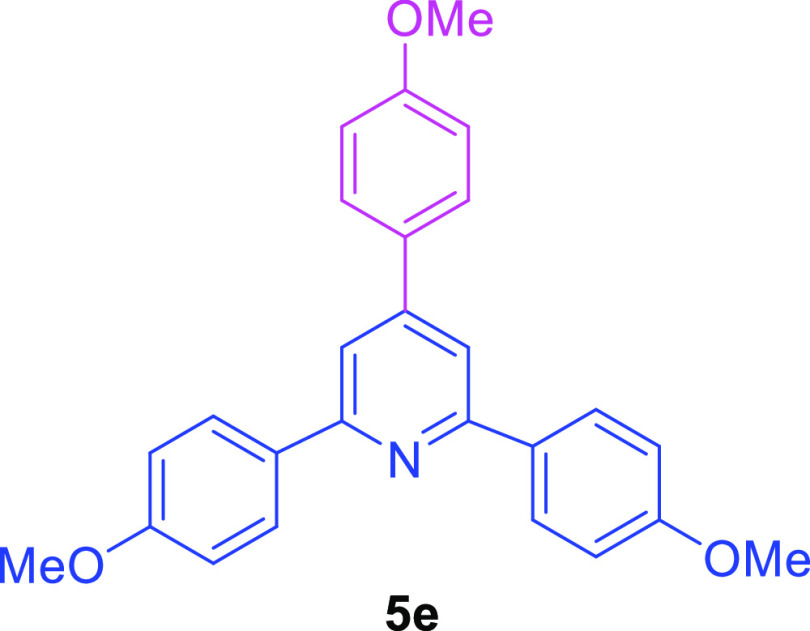 White
solid (43.0 mg, 61% yield); mp 135–137 °C; 1H NMR (500 MHz, CDCl3): (ppm) 8.15–8.12
(m, 4H, ArH), 7.72 (s, 2H, ArH), 7.69–7.66 (m, 2H, ArH), 7.03–7.00
(m, 6H, ArH), 3.87 (s, 9H, OCH3); 13C{H} NMR
(125 MHz, CDCl3): δ (ppm) 160.6 (C), 160.5 (C), 157.0
(C), 149.6 (C), 132.6 (C), 131.7 (C), 128.5 (CH), 128.4 (CH), 115.4
(CH), 114.6 (CH), 114.1 (CH), 55.6 (OCH3), 55.5 (OCH3); HRMS (ESI) m/z: calcd
for C26H24NO3 [M + H]+, 398.1751; found, 398.1750.
White
solid (43.0 mg, 61% yield); mp 135–137 °C; 1H NMR (500 MHz, CDCl3): (ppm) 8.15–8.12
(m, 4H, ArH), 7.72 (s, 2H, ArH), 7.69–7.66 (m, 2H, ArH), 7.03–7.00
(m, 6H, ArH), 3.87 (s, 9H, OCH3); 13C{H} NMR
(125 MHz, CDCl3): δ (ppm) 160.6 (C), 160.5 (C), 157.0
(C), 149.6 (C), 132.6 (C), 131.7 (C), 128.5 (CH), 128.4 (CH), 115.4
(CH), 114.6 (CH), 114.1 (CH), 55.6 (OCH3), 55.5 (OCH3); HRMS (ESI) m/z: calcd
for C26H24NO3 [M + H]+, 398.1751; found, 398.1750.
Acknowledgments
The authors thank Academia Sinica (AS-SUMMIT-109) and the Ministry of Science and Technology (MOST 108-3114-Y-001-002, 108-2113-M-001-019-) for the financial support. We also thank Dr. Su-Ching Lin, Ms. Yi-Ping Huang, and Ms. Mei-Man Chen for their help with NMR experiments, Dr. Yuh-Sheng Wen for the X-ray crystallography experiment, and Ms. Ping-Yu Lin for conducting mass spectrometry experiments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05328.
Detailed experimental procedures and spectroscopic data for all compounds (PDF)
The authors declare no competing financial interest.
Notes
CCDC 2031225, 2031234, 2031242, 2031245, and 2031246 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif or by emailing data_request@ccdc.cam.ac.uk, or by containing The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Supplementary Material
References
- Che C.; Qian Z.; Wu M.; Zhao Y.; Zhu G. Intermolecular Oxidative Radical Addition to Aromatic Aldehydes: Direct Access to 1,4- and 1,5-Diketones via Silver-Catalyzed Ring-Opening Acylation of Cyclopropanols and Cyclobutanols. J. Org. Chem. 2018, 83, 5665–5673. 10.1021/acs.joc.8b00666. [DOI] [PubMed] [Google Scholar]
- Kirsch S. F. Syntheses of Polysubstituted Furans: Recent Developments. Org. Biomol. Chem. 2006, 4, 2076–2080. 10.1039/b602596j. [DOI] [PubMed] [Google Scholar]
- Bellina F.; Rossi R. Synthesis and Biological Activity of Pyrrole, Pyrroline and Pyrrolidine Derivatives with Two Aryl Groups on Adjacent Positions. Tetrahedron 2006, 62, 7213–7256. 10.1016/j.tet.2006.05.024. [DOI] [Google Scholar]
- Davoodnia A.; Bakavoli M.; Moloudi R.; Tavakoli-Hoseini N.; Khashi M. Highly Efficient, One-Pot, Solvent-Free Synthesis of 2,4,6-Triarylpyridines Using a Brønsted-Acidic Ionic Liquid as Reusable Catalyst. Monatsh. Chem. 2010, 141, 867–870. 10.1007/s00706-010-0329-x. [DOI] [Google Scholar]
- Tabrizian E.; Amoozadeh A.; Rahmani S.; Imanifar E.; Azhari S.; Malmir M. One-Pot, Solvent-Free and Efficient Synthesis of 2,4,6-Triarylpyridines Catalyzed by Nano-Titania-Supported Sulfonic Acid as a Novel Heterogeneous Nanocatalyst. Chin. Chem. Lett. 2015, 26, 1278–1282. 10.1016/j.cclet.2015.06.013. [DOI] [Google Scholar]
- Boroujeni M. B.; Hashemzadeh A.; Faroughi M.-T.; Shaabani A.; Amini M. M. Magnetic MIL-101-SO3H: A Highly Efficient Bifunctional Nanocatalyst for the Synthesis of 1,3,5-Triarylbenzenes and 2,4,6-Triaryl Pyridines. RSC Adv. 2016, 6, 100195–100202. 10.1039/c6ra24574a. [DOI] [Google Scholar]
- Yang J.-X.; Tao X.-T.; Yuan C. X.; Yan Y. X.; Wang L.; Liu Z.; Ren Y.; Jiang M. H. A Facile Synthesis and Properties of Multicarbazole Molecules Containing Multiple Vinylene Bridges. J. Am. Chem. Soc. 2005, 127, 3278–3279. 10.1021/ja043510s. [DOI] [PubMed] [Google Scholar]
- Dong C.-p.; Kodama S.; Nomoto A.; Ueshima M.; Ogawa A. 4,6-Dihydroxysalicylic Acid-Catalyzed Oxidative Condensation of Benzylic Amines and Aromatic Ketones for the Preparation of 2,4,6-Trisubstituted Pyridines and Its Application to Metal-Free Synthesis of G-Quadruplex Binding Ligands. ACS Omega 2019, 4, 9029–9040. 10.1021/acsomega.9b00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y.-M.; Cai C. Three-Components Condensation Catalyzed by Molecular Iodine for the Synthesis of 2,4,6-Triarylpyridines and 5-Unsubstituted-3,4-Dihydropyrimidin- 2(1H)-Ones under Solvent-Free Conditions. Monatsh. Chem. 2009, 140, 49–52. 10.1007/s00706-008-0011-8. [DOI] [Google Scholar]
- Paul N.; Muthusubramanian S.; Bhuvanesh N. A Green Protocol for the Synthesis of Conformationally Rigid Sulfur Linked Bisquinolines by Double Friedlander Reaction in Water. New J. Chem. 2011, 35, 2607–2613. 10.1039/c1nj20539k. [DOI] [Google Scholar]
- Paul N.; Sathishkumar R.; Anuba C.; Muthusubramanian S. Reactions of Diphenacylaniline and Diphenacyl Sulfide under Gewald Conditions: Generation of Enamines and Thioamides. RSC Adv. 2013, 3, 7445–7451. 10.1039/c3ra21556c. [DOI] [Google Scholar]
- Müller C.; Wasserberg D.; Weemers J. J. M.; Pidko E. A.; Hoffmann S.; Lutz M.; Spek A. L.; Meskers S. C. J.; Janssen R. A. J.; van Santen R. A.; Vogt D. Donor-Functionalized Polydentate Pyrylium Salts and Phosphinines: Synthesis, Structural Characterization, and Photophysical Properties. Chem.—Eur. J. 2007, 13, 4548–4559. 10.1002/chem.200601650. [DOI] [PubMed] [Google Scholar]
- El-Idreesy T. T. Synthesis and Characterization of Pyrylogens Possessing Low Excitation Energies - Substituent Modification in Order to Improve Solar Energy Collection. Eur. J. Org Chem. 2012, 4515–4522. 10.1002/ejoc.201200514. [DOI] [Google Scholar]
- Clennan E. L.; Liao C.; Ayokosok E. Pyrylogens: Synthesis, Structural, Electrochemical, and Photophysical Characterization of a New Class of Electron Transfer Sensitizers. J. Am. Chem. Soc. 2008, 130, 7552–7553. 10.1021/ja802343v. [DOI] [PubMed] [Google Scholar]
- Chithiravel R.; Rajaguru K.; Muthusubramanian S.; Bhuvanesh N. A Direct Green Route towards the Synthesis of 2-Aroyl-3,5-Diarylthiophenes from 1,5-Diketones. RSC Adv. 2015, 5, 86414–86420. 10.1039/c5ra17829k. [DOI] [Google Scholar]
- Kanithi Y. Synthesis of 2, 4, 6-Triaryl Pyridines under Solvent Free Condition. Int. J. Appl. Agric. Sci. 2016, 2, 80–85. [Google Scholar]
- Liu L.; Feng S.; Li C. A Green Synthesis of Highly Substituted 1,5-Diketones. RSC Adv. 2015, 5, 56949–56953. 10.1039/c5ra08682e. [DOI] [Google Scholar]
- Takahashi H.; Arai T.; Yanagisawa A. 1,5-Diketone Synthesis Promoted by Barium Hydride or Barium Alkoxides. Synlett 2006, 2833–2835. 10.1055/s-2006-950269. [DOI] [Google Scholar]
- Yanagisawa A.; Takahashi H.; Arai T. One-Pot Synthesis of 1,5-Diketones Catalyzed by Barium Isopropoxide. Tetrahedron 2007, 63, 8581–8585. 10.1016/j.tet.2007.04.079. [DOI] [Google Scholar]
- Zhang Y.; Yang X.; Zhou H.-C. Direct Synthesis of Functionalized PCN-333 via Linker Design for Fe3+ Detection in Aqueous Media. Dalton Trans. 2018, 47, 11806–11811. 10.1039/c8dt01508b. [DOI] [PubMed] [Google Scholar]
- Ceylan M.; Gezegen H. Preparation of 1,5-Diketones by Addition of Cyclohexanone to Chalcones under Solvent-Free Phase Transfer Catalyst Condition. Turk. J. Chem. 2008, 32, 55–61. [Google Scholar]
- Kamble S. S.; Shankarling G. S. An Effect of H-Bonding in Synthesis of 1, 5-Diketones via Tandem Aldol-Michael Addition Reaction Using Room Temperature Ionic Liquid (RTIL). ChemistrySelect 2017, 2, 1917–1924. 10.1002/slct.201700034. [DOI] [Google Scholar]
- Yin Z.; Xiong C.; Guo J.; Hu X.; Shan Z.; Borovkov V. Highly Chemoselective Solvent-Free Synthesis of 1,3,5-Triaryl-1,5-Diketones: Crystallographic Investigation and Intramolecular Weak Bifurcated H Bonds Involving Aliphatic C-H Group. Synlett 2019, 30, 2143–2147. 10.1055/s-0039-1690224. [DOI] [Google Scholar]
- Rao Y. K.; Fang S.-H.; Tzeng Y.-M. Synthesis and Biological Evaluation of 3′,4′,5′-Trimethoxychalcone Analogues as Inhibitors of Nitric Oxide Production and Tumor Cell Proliferation. Bioorg. Med. Chem. 2009, 17, 7909–7914. 10.1016/j.bmc.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Wang H.-Y.; Chen L.-F.; Zhu X.-L.; Wang C.; Wan Y.; Wu H. Spectral Studies of Multi-Branched Fluorescence Dyes Based on Triphenylpyridine Core. Spectrochim. Acta, Part A 2014, 121, 355–362. 10.1016/j.saa.2013.10.087. [DOI] [PubMed] [Google Scholar]
- Zolfigol M. A.; Safaiee M.; Afsharnadery F.; Bahrami-Nejad N.; Baghery S.; Salehzadeh S.; Maleki F. Silica Vanadic Acid [SiO2-VO(OH)2] as an Efficient Heterogeneous Catalyst for the Synthesis of 1,2-Dihydro-1-Aryl-3H-Naphth[1,2-e][1,3]Oxazin-3-One and 2,4,6-Triarylpyridine Derivatives via Anomeric Based Oxidation. RSC Adv. 2015, 5, 100546–100559. 10.1039/c5ra21392d. [DOI] [Google Scholar]
- Koppolu S. R.; Balamurugan R. In Situ Formed Acetals Facilitated Direct Michael Addition of Unactivated Ketones. New J. Chem. 2017, 41, 1186–1192. 10.1039/c6nj02954j. [DOI] [Google Scholar]
- Marx A.; Yamamoto H. Aluminum Bis(Trifluoromethylsulfonyl)Amides: New Highly Efficient and Remarkably Versatile Catalysts for C-C Bond Formation Reactions. Angew. Chem. Int. Ed. 2000, 39, 178–181. . [DOI] [PubMed] [Google Scholar]
- Zhang F.-Y.; Corey E. J. Enantio- and Diastereoselective Michael Reactions of Silyl Enol Ethers and Chalcones by Catalysis Using a Chiral Quaternary Ammonium Salt. Org. Lett. 2001, 3, 639–641. 10.1021/ol015592k. [DOI] [PubMed] [Google Scholar]
- Paul N.; Shanmugam M. J.; Muthusubramanian S. Facile Microwave-Assisted Michael Addition of Diphenacyl Sulfides to Chalcones under Solvent-Free Conditions: Generation of Symmetrical and Unsymmetrical 1,5-Diketones. Synth. Commun. 2013, 43, 129–138. 10.1080/00397911.2011.593106. [DOI] [Google Scholar]
- Li Z.; Wen G.; He L.; Li J.; Jia X.; Yang J. Copper-Catalyzed Synthesis of 1,3,5-Triarylpentane-1,5-Diones from α,β-Unsaturated Ketones. RSC Adv. 2015, 5, 52121–52125. 10.1039/c5ra09155a. [DOI] [Google Scholar]
- Sodhi R. K.; Paul S.; Gupta V. K.; Kant R. Conversion of α,β-Unsaturated Ketones to 1,5-Diones via Tandem Retro-Aldol and Michael Addition Using Co(Acac)2 Covalently Anchored onto Amine Functionalized Silica. Tetrahedron Lett. 2015, 56, 1944–1948. 10.1016/j.tetlet.2015.02.057. [DOI] [Google Scholar]
- Charvieux A.; Giorgi J. B.; Duguet N.; Métay E. Solvent-Free Direct α-Alkylation of Ketones by Alcohols Catalyzed by Nickel Supported on Silica-Alumina. Green Chem. 2018, 20, 4210–4216. 10.1039/c8gc01958d. [DOI] [Google Scholar]
- Guo R.; Zhang G. Expedient Synthesis of 1,5-Diketones by Rhodium-Catalyzed Hydroacylation Enabled by C-C Bond Cleavage. J. Am. Chem. Soc. 2017, 139, 12891–12894. 10.1021/jacs.7b05427. [DOI] [PubMed] [Google Scholar]
- Waheed M.; Ahmed N.; Alsharif M. A.; Alahmdi M. I.; Mukhtar S. One-Pot Synthesis of 1,5-Diketones from 3-Acetyl-4-Hydroxycoumarin and Effective Cyclization to Unexpected 3,4-Dihydropyridines. Org. Biomol. Chem. 2018, 16, 3428–3437. 10.1039/c8ob00718g. [DOI] [PubMed] [Google Scholar]
- Min Z.-L.; Yin T.-Z.; Hu X.-M. A Facile One-Pot Synthesis of 2,4,6-Triarylpyridine in DMSO. Asian J. Chem. 2014, 26, 7977–7980. 10.14233/ajchem.2014.16841. [DOI] [Google Scholar]
- Huang B.; Zeng L.; Shen Y.; Cui S. One-Pot Multicomponent Synthesis of β-Amino Amides. Angew. Chem. Int. Ed. 2017, 56, 4565–4568. 10.1002/anie.201700840. [DOI] [PubMed] [Google Scholar]
- Chan C.-K.; Lai C.-Y.; Wang C.-C. TMSOTf-Catalyzed Synthesis of Substituted Quinazolines Using Hexamethyldisilazane as a Nitrogen Source under Neat and Microwave Irradiation Conditions. Org. Biomol. Chem. 2020, 18, 7201–7212. 10.1039/d0ob01507e. [DOI] [PubMed] [Google Scholar]
- Chan C.-K.; Lai C.-Y.; Lo W.-C.; Cheng Y.-T.; Chang M.-Y.; Wang C.-C. p-TsOH-Mediated Synthesis of Substituted 2,4-Diaryl-3-Sulfonylquinolines from Functionalized 2-Aminobenzophenones and Aromatic β-Ketosulfones under Microwave Irradiation. Org. Biomol. Chem. 2020, 18, 305–315. 10.1039/c9ob02445j. [DOI] [PubMed] [Google Scholar]
- Chan C.-K.; Lai C. Y.; Wang C. C. Environmentally Friendly Nafion-Mediated Friedländer Quinoline Synthesis under Microwave Irradiation: Application to One-Pot Synthesis of Substituted Quinolinyl Chalcones. Synth 2020, 52, 1779–1794. 10.1055/s-0039-1690088. [DOI] [Google Scholar]
- Ivanković A. Review of 12 Principles of Green Chemistry in Practice. Int. J. Sustainable Green Energy 2017, 6, 39. 10.11648/j.ijrse.20170603.12. [DOI] [Google Scholar]
- CCDC 2031225 (3o), 2031234 (3q), 2031246 (3t), 2031242 (4e), and 2031245 (4r), Contain the Supplementary Crystallographic Data for This Paper. This Data Can Be Obtained Free of Charge via Www.Ccdc.Cam.Ac.Uk/Conts/Retrieving.Html (or from the CCDC, 12 Unio).
- Doebelin C.; Wagner P.; Bihel F.; Humbert N.; Kenfack C. A.; Mely Y.; Bourguignon J.-J.; Schmitt M. Fully Regiocontrolled Polyarylation of Pyridine. J. Org. Chem. 2014, 79, 908–918. 10.1021/jo402200q. [DOI] [PubMed] [Google Scholar]
- Fang A. G.; Mello J. V.; Finney N. S. Structural Studies of Biarylpyridines Fluorophores Lead to the Identification of Promising Long Wavelength Emitters for Use in Fluorescent Chemosensors. Tetrahedron 2004, 60, 11075–11087. 10.1016/j.tet.2004.08.049. [DOI] [Google Scholar]
- Islam A.; Sugihara H.; Arakawa H. Molecular Design of Ruthenium(II) Polypyridyl Photosensitizers for Efficient Nanocrystalline TiO2 Solar Cells. J. Photochem. Photobiol., A 2003, 158, 131–138. 10.1016/s1010-6030(03)00027-3. [DOI] [Google Scholar]
- Roughley S. D.; Jordan A. M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- Guan A.-Y.; Liu C.-L.; Sun X.-F.; Xie Y.; Wang M.-A. Discovery of Pyridine-Based Agrochemicals by Using Intermediate Derivatization Methods. Bioorg. Med. Chem. 2016, 24, 342–353. 10.1016/j.bmc.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Rocco D.; Housecroft C. E.; Constable E. C. Synthesis of Terpyridines: Simple Reactions-What Could Possibly Go Wrong?. Molecules 2019, 24, 1799. 10.3390/molecules24091799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; He P.; Yu C. DPTA-Catalyzed One-Pot Regioselective Synthesis of Polysubstituted Pyridines and 1,4-Dihydropyridines. Tetrahedron 2012, 68, 4138–4144. 10.1016/j.tet.2012.03.104. [DOI] [Google Scholar]
- Yi Y.; Zhao M.-N.; Ren Z.-H.; Wang Y.-Y.; Guan Z.-H. Synthesis of Symmetrical Pyridines by Iron-Catalyzed Cyclization of Ketoxime Acetates and Aldehydes. Green Chem. 2017, 19, 1023–1027. 10.1039/c6gc03137d. [DOI] [Google Scholar]
- Saczewski J.; Raux E.; Barnes S.; Sullivan S.; Duszynska B.; Bojarski A. J.; Strekowski L. Synthesis of 4-Substituted 2- (4-Methylpiperazino) Pyrimidines and Quinazoline Analogs as Serotonin 5-HT 2A Receptor Ligands. J. Heterocycl. Chem. 2009, 46, 1259–1265. 10.1002/jhet.236. [DOI] [Google Scholar]
- Gadekar S. P.; Lande M. K. Solid Acid Catalyst TS-1 Zeolite-Assisted Solvent-Free One-Pot Synthesis of Poly-Substituted 2,4,6-Triaryl-Pyridines. Res. Chem. Intermed. 2018, 44, 3267–3278. 10.1007/s11164-018-3305-4. [DOI] [Google Scholar]
- Fu Y.; Wang P.; Guo X.; Wu P.; Meng X.; Chen B. Synthesis of Polyfunctional Pyridines via Copper-Catalyzed Oxidative Coupling Reactions. J. Org. Chem. 2016, 81, 11671–11677. 10.1021/acs.joc.6b02081. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Wang P.; Yuan X.; Qin M.; Chen B. Synthesis of Pyridine Derivatives from Acetophenone and Ammonium Acetate by Releasing CH4. Asian J. Org. Chem. 2019, 8, 1332–1335. 10.1002/ajoc.201900323. [DOI] [Google Scholar]
- Han J.; Guo X.; Liu Y.; Fu Y.; Yan R.; Chen B. One-Pot Synthesis of Benzene and Pyridine Derivatives via Copper-Catalyzed Coupling Reactions. Adv. Synth. Catal. 2017, 359, 2676–2681. 10.1002/adsc.201700053. [DOI] [Google Scholar]
- Rohokale R. S.; Koenig B.; Dhavale D. D. Synthesis of 2,4,6-Trisubstituted Pyridines by Oxidative Eosin Y Photoredox Catalysis. J. Org. Chem. 2016, 81, 7121–7126. 10.1021/acs.joc.6b00979. [DOI] [PubMed] [Google Scholar]
- Goh K. K. K.; Kim S.; Zard S. Z. Free-Radical Variant for the Synthesis of Functionalized 1,5-Diketones. Org. Lett. 2013, 15, 4818–4821. 10.1021/ol402213k. [DOI] [PubMed] [Google Scholar]
- Chang M.-Y.; Wu M.-H.; Tai H.-Y. NH4OAc Promoted Cyclocondensation of 3-(o-Allylphenyl)Pentane-1, 5-Diones: Synthesis of Tetracyclic Benzofused 1-Azahomoisotwistanes. Org. Lett. 2012, 14, 3936–3939. 10.1021/ol301693s. [DOI] [PubMed] [Google Scholar]
- Wang P.; Zhang X.; Liu Y.; Chen B. Synthesis of Pyrimidines with Ammonium Acetate as Nitrogen Source Under Solvent-Free Conditions. Asian J. Org. Chem. 2019, 8, 1122–1127. 10.1002/ajoc.201900248. [DOI] [Google Scholar]
- Ling F.; Shen L.; Pan Z.; Fang L.; Song D.; Xie Z.; Zhong W. B(C6F5)3-Catalyzed Oxidative Deamination/Cyclization Cascade Reaction of Benzylamines and Ketones for the Synthesis of 2,4,6-Triarylpyridines. Tetrahedron Lett. 2018, 59, 3678–3682. 10.1016/j.tetlet.2018.08.060. [DOI] [Google Scholar]
- Zhang X.; Wang Z.; Xu K.; Feng Y.; Zhao W.; Xu X.; Yan Y.; Yi W. HOTf-Catalyzed Sustainable One-Pot Synthesis of Benzene and Pyridine Derivatives under Solvent-Free Conditions. Green Chem. 2016, 18, 2313–2316. 10.1039/c5gc02747k. [DOI] [Google Scholar]
- Rekunge D. S.; Kale I. A.; Chaturbhuj G. U. An Efficient, Green Solvent-Free Protocol for the Synthesis of 2,4,6-Triarylpyridines Using Reusable Heterogeneous Activated Fuller’s Earth Catalyst. J. Iran. Chem. Soc. 2018, 15, 2455–2462. 10.1007/s13738-018-1434-8. [DOI] [Google Scholar]
- Safaiee M.; Ebrahimghasri B.; Zolfigol M. A.; Baghery S.; Khoshnood A.; Alonso D. A. Synthesis and Application of Chitosan Supported Vanadium Oxo in the Synthesis of 1,4-Dihydropyridines and 2,4,6-Triarylpyridines via Anomeric Based Oxidation. New J. Chem. 2018, 42, 12539–12548. 10.1039/c8nj02062k. [DOI] [Google Scholar]
- Le T. N. M.; Doan S. H.; Pham P. H.; Trinh K. H.; Huynh T. V.; Tran T. T. T.; Le M.-V.; Nguyen T. T.; Phan N. T. S. Synthesis of Triphenylpyridines via an Oxidative Cyclization Reaction Using Sr-Doped LaCoO3 Perovskite as a Recyclable Heterogeneous Catalyst. RSC Adv. 2019, 9, 23876–23887. 10.1039/c9ra04096j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F.; Lu Y.; Zhang Q.; Sun J.; Zeng X.; Li C. Homogeneous and Sensitive DNA Detection Based on Polyelectrolyte Complexes of Cationic Conjugated Poly(Pyridinium Salt)s and DNA. J. Mater. Chem. 2012, 22, 4106–4112. 10.1039/c2jm15491a. [DOI] [Google Scholar]
- Lantaño B.; Aguirre J. M.; Drago E. V.; Bollini M.; de la Faba D. J.; Mufato J. D. Synthesis of Benzylidenecycloalkan-1-Ones and 1,5-Diketones under Claisen–Schmidt Reaction: Influence of the Temperature and Electronic Nature of Arylaldehydes. Synth. Commun. 2017, 47, 2202–2214. 10.1080/00397911.2017.1367819. [DOI] [Google Scholar]
- Cabrera M.; Cerecetto H.; González M. New Hybrid Bromopyridine-Chalcones as in Vivo Phase II Enzyme Inducers: Potential Chemopreventive Agents. Medchemcomm 2016, 7, 2395–2409. 10.1039/c6md00456c. [DOI] [Google Scholar]
- Kopchuk D. S.; Chepchugov N. V.; Kim G. A.; Zyryanov G. V.; Kovalev I. S.; Rusinov V. L.; Chupakhin O. N. An Efficient Synthetic Approach to 4′,5,5″-Triaryl-2,2′:6′,2″-Terpyridines. Tetrahedron Lett. 2016, 57, 296–299. 10.1016/j.tetlet.2015.12.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.