Abstract
The global pandemic of novel coronavirus disease 2019 (COVID-19) has become an emergency of major international concern. We aim to assess the prevalence of clinical manifestations, pre-existing comorbidities, complications and treatment modalities in COVID-19 patients and compare incidence of these clinical data of severe patients with non-severe patients. An electronic search was performed in four databases to identify studies reporting clinical data of severe and non-severe COVID-19 patients. We calculated the odds ratio (OR) using fixed or random effect model. The analysis included 41 studies with 16,495 patients. The most prevalent clinical manifestations were fever 78.1%, cough 64.6%, fatigue 40.8%, and dyspnea 38.6%. Dyspnea (OR: 4.20, 95% CI: 3.09–5.72), cough (OR: 1.45, 95% CI: 1.18–1.78), and fatigue (OR: 1.40, 95% CI: 1.14–1.72) were found to be statistically significant higher in severe COVID-19 patients. We found that the most prevalent comorbidities were hypertension 32.2%, diabetes 17.1%, and cardiovascular disease 15.3%. Compared with non-severe group, proportion of hypertension (OR: 1.98, 95% CI: 1.62–2.42), diabetes (OR: 2.04, 95% CI: 1.67–2.50), cardiovascular disease (OR: 2.78, 95% CI: 2.00–3.86), and cancer (OR: 1.75, 95% CI: 1.40–2.18) were statistically significant higher in severe group. 24.7% patients presented with ARDS. The pooled effect of ARDS in severe and non-severe cases was 42.69 (OR: 42.69, 95% CI: 21.62–84.31). There was significant higher incidence of antiviral drugs, antibiotics, and glucocorticoids use in severe patients. Compared with non-severe patients, symptoms such as fever, cough, dyspnea, existing comorbidities, and complications are prevalent in severe COVID-19 patients.
Keywords: COVID-19, clinical features, comorbidities, complications, treatment
Introduction
Coronaviruses (CoVs) belonging to the family of Coronaviridae, were not considered as highly pathogenic for humans until the outbreak of severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012.1–4 SARS-CoV with fatality rate of around 11% appeared less lethal than MERS-CoV which has a fatality rate of 37%.1,3 Severe acute respiratory syndrome coronavirus (SARS-CoV-2), which causes coronavirus disease (COVID-19), has been believed to be originated in the Huanan animal market in Wuhan, China at the end of 2019 has widely and rapidly spread across the globe. 5 On 30 January 2020, a novel coronavirus was officially declared a public health emergency of international concern by the World Health Organization (WHO), and was subsequently declared as pandemic on March 11. 6 Despite continued international efforts to contain the spread, the number of COVID-19 cases are growing exponentially, as of 15 November, 53.7 million cases and 1.3 million deaths have been reported. 7 There is wide variability in the clinical features of COVID-19 ranging from mild to severe symptoms, in some cases patients may require specialized management at intensive care unit with poor long term outcomes.8–11 Alike in case of MERS-CoV, 12 comorbidities such as hypertension, diabetes, cardiovascular disease, and cancers have been identified as predisposing factors for adverse outcome in patients infected with SARS-CoV-2.8–11,13 To date, only limited number of meta-analysis have been published that compared clinical characteristics and complications of severe and non-severe patients with COVID-19. However, there were limited number of studies included in meta-analysis and the criteria used to categorize severe and non-severe cases were not uniform.13,14 Furthermore, previous meta-analyses included studies mainly from China only, so the conclusions of these cannot be generalized. Hence, prompt identification of clinical risk factors, comorbid conditions and complications which can predict progression toward to the severe form of disease is paramount for timely intervention to prevent fatal outcomes. This systemic review and meta-analysis of 41 studies from 11 countries aims to compare the clinical characteristics, comorbidities, complications, and treatment modalities among severe and non-severe COVID-19 patients, in order to obtain clear picture of risk factors of severe cases.
Methods
Ethical statement
Ethical approval and consent is not required as this is systematic review and meta-analysis with only a secondary analysis of data.
Search strategy and selection criteria
This systemic review and meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. We performed a literature search using PubMed, Scopus, Embase, and Web of Sciences databases from inception to 20 November 2020. Aiming to include all relevant articles, we used following search terms: “COVID-19,”“COVID 19,”“SARS-CoV-2,”“Novel coronavirus,”“Novel coronavirus 2019,”“Corona virus disease 2019,”“Clinical features,”“Clinical character,”“Comorbidities,” and “Complications.” Moreover, we searched the references of published articles to find additional studies. Our searches were restricted to English language.
The inclusion criteria of the meta-analysis were as follows: (a) Studies with patients diagnosed with COVID-19, (b) Studies involving the severe cases or ICU cases and non-severe or non-ICU cases, (c) at least one outcomes reported among clinical features, comorbidities, complications, and treatment options in severe and non-severe patients. Exclusion criteria were as follows: (a) studies with fewer than 20 cases, (b) studies with pediatric COVID-19 cases only, and (c) review articles, letters, comments, case reports, editorials, conference abstracts, viewpoints, and articles without abstracts.
Data extraction and quality assessment
The two researchers (MG and AP) independently extracted the following data from the included studies: first author’s name, year of publication, country of publication, type of publication, age, gender, sample size, and number of patients in severe and non-severe groups. Parameters regarding clinical features, comorbidities, complications and treatment modalities of participants in both severe and non-severe group were also recorded. Any discrepancy between the two researchers was resolved by consensus with third reviewer (TW). The primary outcome was to estimate the pooled prevalence of clinical feature, comorbidities, complications and treatment modalities in severe cases (ICU cases, and patients with elevated TnT level as the second choice if severe data was not given) and non-severe (non-ICU cases, and patients with normal TnT level as the second choice if non-severe data was not given). Study bias was assessed using the Methodological Index for Non-Randomized Studies (MINIORS) criteria. It consists of 12 items and the each item is scored as follows: 0 (content is not reported), 1 (reported but inadequate), and 2 (reported and adequate). The overall maximum ideal score is 24 for comparative studies. For comparative studies, the corresponding scores 0–6 indicates very low quality; 7–12 indicates low quality; 13–18 represents moderate quality; and 19–24 indicates high quality. 15
Statistical analysis
We used OpenMeta Analyst software with random-effects model to estimate the pooled prevalence with the corresponding 95% confidence interval (CI) of clinical data. We also used RevMan software version 5.3 to calculate pooled odds ratio (OR) and 95% CI for clinical features, comorbidities, complications, and treatment options in severe and non-severe COVID-19 patients. Heterogeneity among included studies was assessed using the Cochran’s Q test and I2 statistic. When I2 < 50%, a fixed effect model was used, otherwise a random effect model was selected. A p-value less than 0.05 was considered to be statistically significant.
Results
We identified 5225 potential articles by database searches, of which 75 full text articles were selected for full text review. A flow chart of studies selection process is presented in Figure 1. Of the 75 full text articles, 41 articles meet the inclusion criteria and were included in our systemic review and meta-analysis.
Figure 1.
Flowchart of the search and selection process.
Study characteristics
Most studies were from China5,9,11,16–38 (n = 26), followed by United States39–43 (n = 5), Switzerland44,45 (n = 2), Japan 8 (n = 1), Korea 46 (n = 1), Oman 47 (n = 1), Qatar 48 (n = 1), Iran 49 (n = 1), Denmark 50 (n = 1), Mexico 51 (n = 1), and Bulgaria 52 (n = 1). The sample size of included studies ranged from 41 to 5000 patients. The detailed characteristics of included studies are presented in Table 1. The MINORS scores were ≥18 for all the included studies (Table 2). The average MINORS score of the included studies was 19.4 (range 18–21). All studies were moderate to high methodological quality with a low risk of bias. The median or mean age of severe and non-severe patients is presented in detail (Table 1). The qualitative analysis showed that compared with non-severe group, the age of severe group was higher. Our study demonstrated that among all the confirmed patients with COVID-19 included in meta-analysis, 56% (CI: 49.4–62.7) were men. The overall proportion of severe patients in our study was 24%. There was significant difference between the severe and non-severe groups regarding the gender (p < 0.05).
Table 1.
Characteristics of the included studies in systemic review and meta-analysis.
| Study | Country | Totalpatients | Severe patients |
Non-severe patients |
||
|---|---|---|---|---|---|---|
| Age, years a | Male | Age, years a | Male | |||
| Argenziano MG | United States | 1000 | 62 (52–72) | 158 | 64 (51–77) | 353 |
| Cao J | China | 244 | 62.20 ± 13.43 | 63 | 59.79 ± 13.49 | 44 |
| Cao Z | China | 80 | 71 ± 15 | 16 | 44 ± 16 | 22 |
| Du RH | China | 109 | 68.4 ± 9.7 | 36 | 72.7 ± 11.6 | 38 |
| Ferguson J | United States | 72 | NA | NA | NA | NA |
| Giustino G | United States | 305 | 66 (56–74) | 132 | 58 (47–70) | 73 |
| Gregoriano C | Switzerland | 99 | 69 (57–75) | 28 | 63.5 (56–76) | 34 |
| Guan WJ | China | 1099 | 52 (40–65) | 100 | 45 (34–57) | 537 |
| Guo T | China | 187 | 71.4 (9.43) | 34 | 53.53 (13.22) | 57 |
| Hong KS | Korea | 98 | 63.2 ± 10.1 | 6 | 54.2 ± 17.7 | 32 |
| Huang C | China | 41 | 49 (41–61) | 11 | 49 (41–57.5) | 19 |
| Huang R | China | 202 | 49 (35–59) | 17 | 44 (33–53) | 99 |
| Israelsen SB | Denmark | 175 | 68 (60–72) | 16 | 73 (55–83) | 69 |
| Khamis F | Oman | 63 | 50 ± 17 | 21 | 47 ± 16 | 32 |
| Li C | China | 2068 | 69 (60–78) | 282 | 61 (49–68) | 723 |
| LI K | China | 83 | 53.7 ± 12.3 | 15 | 41.9 ± 10.6 | 29 |
| Li X | China | 548 | 65 (54–72) | 153 | 56 (44–66) | 126 |
| Lv Z | China | 354 | 62 (25–89) | 77 | 61 (23–79) | 58 |
| Omrani AS | Qatar | 5000 | 49.5 (39.5–60) | 100 | 38 (30–49) | 1067 |
| Ortiz-Brizuela E | Mexico | 309 | 53 (40–64) | 20 | 48 (29–60.5) | 65 |
| Pellaud C | Switzerland | 196 | 65 (56–71) | 30 | 74 (61–83) | 89 |
| Popov GT | Bulgaria | 138 | 63.0 ± 12.8 | 33 | 48.3 ± 15.7 | 54 |
| Shahriarirad R | Iran | 113 | NA | 7 | NA | 64 |
| Shi S | China | 416 | 74 (34–95) | 44 | 60 (21–90) | 161 |
| Suleyman G | United States | 463 | 63.8 ± 5.4 | 80 | 59.8 ± 15.2 | 85 |
| Tabata S | Japan | 104 | 73 (55–77) | 17 | 60 (40–71) | 22 |
| Tian S | China | 262 | 61.4 (1–94) | 26 | 44.5 (1–93) | 101 |
| Turcotte JJ | United States | 117 | 70.2 ± 12.1 | 26 | 62.6 ± 16.9 | 36 |
| Wan S | China | 135 | 56 (52–73) | 21 | 44 (33–49) | 52 |
| Wang D | China | 138 | 66 (57–78) | 22 | 51 (37–62) | 53 |
| Wang W | China | 421 | 56 (45–63) | 28 | 51 (38–60) | 186 |
| Wang Y | China | 222 | 70 (65.5–80) | 12 | 60.5 (48–67) | 96 |
| Wang Z | China | 69 | 70.5 (62–77) | 7 | 37 (32–51) | 25 |
| Wei Y | China | 276 | 65 (60–72.8) | 10 | 50 (39–57) | 145 |
| Wu J | China | 280 | 63.04 ± 10.20 | 45 | 37.55 ± 17.10 | 106 |
| Xiong F | China | 131 | 64.3 ± 12.4) | 17 | 63.1 ± 13.4 | 58 |
| Xiong S | China | 116 | 64 (53–76) | 38 | 56 (37–64) | 42 |
| Yang L | China | 200 | 71 ± 13.4 | 16 | 52 ± 16.2 | 82 |
| Zhang G | China | 221 | 62 (52–74) | 35 | 51 (36–64.3) | 73 |
| Zhang JJ | China | 140 | 64 (25–87) | 33 | 51.5 (26–78) | 38 |
| Zhou J | China | 201 | 57 (46–66) | 27 | 40 (31–53) | 75 |
Age data presented as median (IQR) or mean ± SD.
Table 2.
MINORS rating scale for quality assessment of included studies.
| Study | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | ⑩ | ⑪ | ⑫ | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Argenziano MG | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Cao J | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 19 |
| Cao Z | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Du RH | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Ferguson J | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Giustino G | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Gregoriano C | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 19 |
| Guan WJ | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Guo T | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Hong KS | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Huang C | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Huang R | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Israelsen SB | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Khamis F | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Li C | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| LI K | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 19 |
| Li X | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Lv Z | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Omrani AS | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Ortiz-Brizuela E | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Pellaud C | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Popov GT | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Shahriarirad R | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Shi S | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Suleyman G | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Tabata S | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Tian S | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Turcotte JJ | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Wan S | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Wang D | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Wang W | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Wang Y | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 19 |
| Wang Z | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
| Wei Y | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Wu J | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Xiong F | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Xiong S | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Yang L | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Zhang G | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 19 |
| Zhang JJ | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
| Zhou J | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 18 |
① A clearly stated aim; ② inclusion of consecutive patients; ③ prospective collection of data; ④ endpoints appropriate to the aim of the study; ⑤ unbiased assessment of the study endpoint; ⑥ follow-up period appropriate to the aim of the study; ⑦ loss to follow up less than 5%; ⑧ prospective calculation of the study size; ⑨ appropriate selection of control group; ⑩ synchronization of control group; ⑪ baseline comparable between groups; and ⑫ appropriately statistical analysis. The global ideal score being 24 for comparative studies.
Clinical manifestations
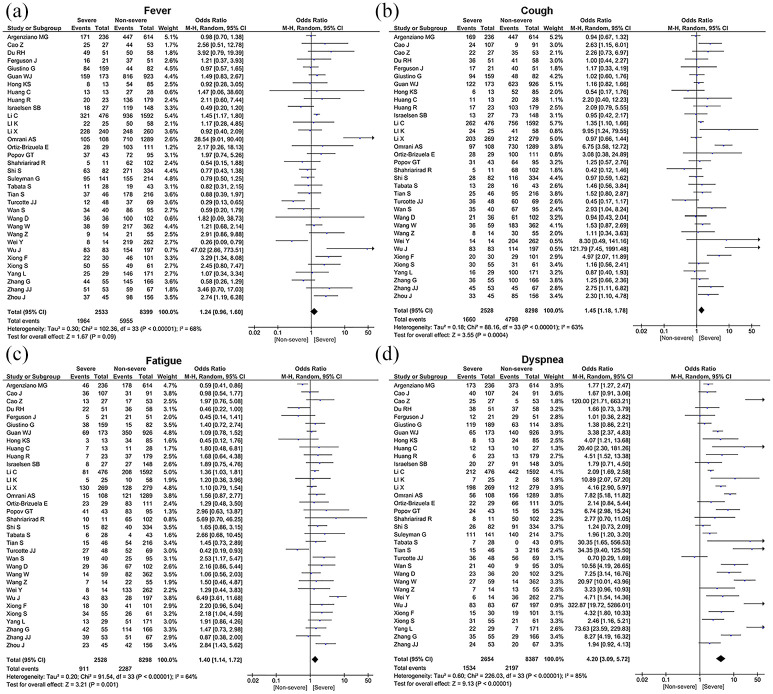
Results regarding the prevalence of clinical features of COVID-19 are summarized in the Table 3. The most prevalent symptom were fever (78.1%, 95% CI: 73.3%–82.9%), cough (64.6%, 95% CI: 60.0%–69.2%), fatigue (40.8%, 95% CI: 33.8%–47.8%), dyspnea (38.6%, 95% CI: 31.5%–45.8%), headache (16.9%, 95% CI: 11.9%–21.8%), sore throat (15.3%, 95% CI: 11.8%–18.8%), nausea or vomiting (13.8%, 95% CI: 10.4%–17.3%), and diarrhea (13.7%, 95% CI: 10.7%–16.7%) (Table 3). A significant heterogeneity was detected for clinical symptoms among the examined studies (p < 0.001) with an I2 index varying from 95% to 99%. Furthermore, we investigated the relationship between clinical characteristics of severe and non-severe cases with COVID-19. Among all clinical symptoms, dyspnea [OR = 4.20, 95% CI (3.09–5.72), Z = 9.13, p < 0.00001], cough [OR = 1.45, 95% CI (1.18–1.78), Z = 3.55, p = 0.0004], and fatigue [OR = 1.40, 95% CI (1.14–1.72), Z = 3.21, p = 0.001] were found to be statistically significant higher in severe COVID-19 (Table 4 and Figure 2). However, no statistically significant differences were found for the incidence of fever [OR = 1.24, 95% CI (0.96–1.60), Z = 1.67, p = 0.09], sore throat [OR = 1.16, 95% CI (0.81–1.67), Z = 0.81, p = 0.42], headache [OR = 0.95, 95% CI (0.69–1.31), Z = 0.31, p = 0.76], diarrhea [OR = 1.25, 95% CI (0.98–1.61), Z = 1.80, p = 0.07], and nausea or vomiting [OR = 1.28, 95% CI (0.85–1.92), Z = 1.19, p = 0.23] between severe and non-severe patients (Table 4 and Figure 2).
Table 3.
Meta-analysis outcomes of clinical data of COVID-19 patients.
| Variable | Number ofstudies | Prevalence(%) | 95% CI | N | Q ‡ | I 2§ | T 2† | p |
|---|---|---|---|---|---|---|---|---|
| Male | 40 | 56.0 | 49.4–62.7 | 10554 | 3151.95 | 99 | 0.045 | <0.001 |
| Female | 40 | 44.8 | 37.9–51.8 | 5967 | 3447.67 | 99 | 0.049 | <0.001 |
| Severe | 41 | 24.0 | 20.8–27.2 | 2376 | 2481.19 | 98 | 0.010 | <0.001 |
| Clinical features | ||||||||
| Fever | 33 | 78.1 | 73.3–82.9 | 7455 | 1430.19 | 98 | 0.019 | <0.001 |
| Cough | 34 | 64.6 | 60.0–69.2 | 6372 | 817.57 | 96 | 0.018 | <0.001 |
| Fatigue | 34 | 40.8 | 33.8–47.8 | 3430 | 2226.98 | 99 | 0.042 | <0.001 |
| Sore throat | 23 | 15.3 | 11.8–18.8 | 917 | 474.30 | 95 | 0.007 | <0.001 |
| Dyspnea | 33 | 38.6 | 31.5–45.8 | 3725 | 2297.79 | 99 | 0.043 | <0.001 |
| Headache | 28 | 16.9 | 11.9–21.8 | 1151 | 1430.78 | 98 | 0.017 | <0.001 |
| Diarrhea | 32 | 13.7 | 10.7–16.7 | 1457 | 879.13 | 96 | 0.007 | <0.001 |
| Nausea or vomiting | 23 | 13.8 | 10.4–17.3 | 800 | 662.07 | 97 | 0.007 | <0.001 |
| Co-morbidities | ||||||||
| Hypertension | 35 | 32.2 | 26.4–38.0 | 3537 | 1655.49 | 98 | 0.030 | <0.001 |
| Diabetes | 37 | 17.1 | 14.0–20.3 | 1869 | 865.71 | 96 | 0.009 | <0.001 |
| Cancer | 28 | 3.5 | 2.6–4.4 | 357 | 182.23 | 85 | 0.000 | <0.001 |
| COPD | 28 | 3.4 | 2.6–4.2 | 328 | 159.99 | 83 | 0.000 | <0.001 |
| Cardiovascular disease | 31 | 15.3 | 12.5–18.1 | 1094 | 786.10 | 96 | 0.006 | <0.001 |
| Chronic kidney disease | 19 | 7.6 | 5.6–9.7 | 42 | 555.38 | 97 | 0.002 | <0.001 |
| Complications | ||||||||
| ARDS | 17 | 24.7 | 16.0–33.4 | 1145 | 1768.78 | 99 | 0.032 | <0.001 |
| Shock | 11 | 6.8 | 4.3–9.4 | 201 | 185.0 | 95 | 0.001 | <0.001 |
| Acute kidney injury | 15 | 10.3 | 6.0–14.6 | 597 | 725.90 | 98 | 0.007 | <0.001 |
| Arrhythmia | 8 | 8.4 | 4.5–12.3 | 150 | 46.69 | 89 | 0.002 | <0.001 |
| Treatments | ||||||||
| Antiviral therapy | 25 | 68.7 | 53.6–83.8 | 3900 | 43,430.13 | 100 | 0.147 | <0.001 |
| Antibiotic therapy | 21 | 76.8 | 70.2–83.5 | 3615 | 2065.86 | 99 | 0.023 | <0.001 |
| Glucocorticoids | 28 | 35.4 | 27.4–43.5 | 2850 | 2254.03 | 99 | 0.046 | <0.001 |
| Oxygen support | 18 | 55.5 | 41.9–69.1 | 3740 | 2478.57 | 99 | 0.085 | <0.001 |
| CRRT | 15 | 3.3 | 2.2–4.5 | 190 | 143.35 | 90 | 0.000 | <0.001 |
| NIV | 16 | 23.2 | 16.1–30.2 | 987 | 1096.69 | 99 | 0.020 | <0.001 |
95% CI: 95% confidence interval; ARDS: acute respiratory distress syndrome; COPD: chronic obstructive pulmonary disease; CRRT: continuous renal replacement therapy; NIV: noninvasive ventilation.
Cochran’s Q statistic for heterogeneity.
I2 index to quantify the degree of heterogeneity.
Tau-squared as a measure of heterogeneity.
Table 4.
Analysis of severe and non-severe patients of COVID-19 by using Mantel–Haenszel test.
| Variable | Number ofstudies | OR | 95% CI | Severe | Non-severe | χ 2‡ | I 2§ | Z † | p |
|---|---|---|---|---|---|---|---|---|---|
| Male | 40 | 1.46 | 1.34–1.60 | 1859 | 5120 | 43.78 | 11 | 8.29 | <0.00001 |
| Female | 40 | 0.68 | 0.62–0.74 | 1159 | 4087 | 45.84 | 15 | 8.49 | <0.00001 |
| Clinical features | |||||||||
| Fever | 34 | 1.24 | 0.96–1.60 | 1964 | 5955 | 102.36 | 68 | 1.67 | 0.09 |
| Cough | 34 | 1.45 | 1.18–1.78 | 1660 | 4798 | 88.16 | 63 | 3.55 | 0.0004 |
| Fatigue | 34 | 1.40 | 1.14–1.72 | 911 | 2287 | 91.54 | 64 | 3.21 | 0.001 |
| Sore throat | 24 | 1.16 | 0.81–1.67 | 200 | 972 | 73.68 | 69 | 0.81 | 0.42 |
| Dyspnea | 34 | 4.20 | 3.09–5.72 | 1534 | 8387 | 226.03 | 85 | 9.13 | <0.00001 |
| Headache | 28 | 0.95 | 0.69–1.31 | 232 | 736 | 66.56 | 59 | 0.31 | 0.76 |
| Diarrhea | 33 | 1.25 | 0.98–1.61 | 434 | 979 | 69.95 | 54 | 1.80 | 0.07 |
| Nausea or vomiting | 22 | 1.28 | 0.85–1.92 | 169 | 516 | 67.31 | 69 | 1.19 | 0.23 |
| Co-morbidities | |||||||||
| Hypertension | 35 | 1.98 | 1.62–2.42 | 1315 | 2333 | 107.88 | 68 | 6.62 | <0.00001 |
| Diabetes | 37 | 2.04 | 1.67–2.50 | 741 | 1316 | 80.57 | 55 | 6.97 | <0.00001 |
| Cancer | 28 | 1.75 | 1.40–2.18 | 142 | 221 | 36.70 | 26 | 4.92 | <0.00001 |
| COPD | 28 | 1.90 | 1.49–2.41 | 131 | 178 | 46.70 | 42 | 5.26 | <0.00001 |
| Cardiovascular disease | 31 | 2.78 | 2.00–3.86 | 452 | 694 | 120.06 | 75 | 6.10 | <0.00001 |
| Chronic kidney disease | 20 | 2.74 | 1.68–4.48 | 260 | 289 | 72.08 | 74 | 4.03 | <0.00001 |
| Complications | |||||||||
| ARDS | 19 | 42.69 | 21.62–84.31 | 994 | 258 | 158.58 | 89 | 10.81 | <0.00001 |
| Shock | 11 | 23.95 | 9.50–60.35 | 180 | 21 | 23.84 | 58 | 6.74 | <0.00001 |
| Acute kidney injury | 17 | 11.12 | 6.07–20.38 | 560 | 263 | 102.42 | 84 | 7.80 | <0.00001 |
| Arrhythmia | 8 | 21.23 | 10.40–43.30 | 263 | 42 | 19.27 | 64 | 8.40 | <0.00001 |
| Treatments | |||||||||
| Antiviral | 25 | 2.18 | 1.43–3.34 | 992 | 3345 | 86.40 | 76 | 3.60 | 0.0003 |
| Antibiotics | 22 | 5.96 | 3.32–10.69 | 1296 | 3398 | 97.07 | 82 | 5.98 | <0.00001 |
| Glucocorticoids | 28 | 6.12 | 4.01–9.34 | 1347 | 1420 | 258.31 | 90 | 8.40 | <0.00001 |
| Oxygen support | 19 | 1.22 | 0.43–3.44 | 1104 | 2756 | 534.83 | 97 | 0.37 | 0.71 |
| CRRT | 16 | 18.92 | 8.20–43.66 | 183 | 32 | 34.53 | 57 | 6.89 | <0.00001 |
| NIV | 15 | 41.27 | 15.24–111.77 | 599 | 87 | 119.85 | 88 | 7.32 | <0.00001 |
OR: odds ratio; 95% CI: 95% confidence interval; ARDS: acute respiratory distress syndrome; COPD: chronic obstructive pulmonary disease; CRRT: continuous renal replacement therapy; NIV: noninvasive ventilation.
Chi-squared test for heterogeneity.
I2 index to quantify the degree of heterogeneity.
Z-statistics.
Figure 2.
Forest plot for clinical manifestations of severe patients compared with non-severe patients.
Pre-existing comorbidities
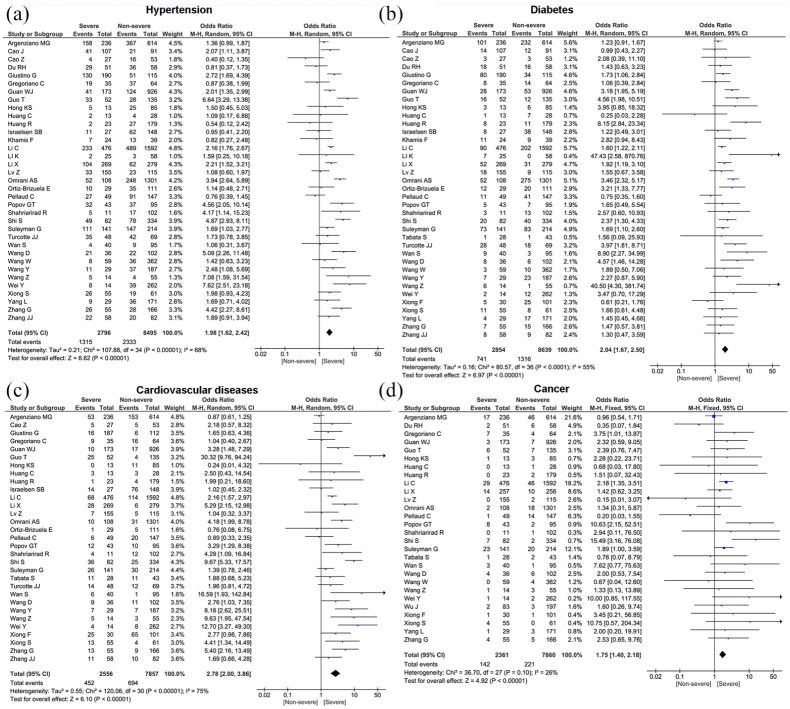
Meta-analysis showed that the most prevalent comorbidity was hypertension (32.2%, 95% CI: 26.4%–38.0%) followed by diabetes (17.1%, 95% CI: 14.0%–20.3%), cardiovascular disease (15.3%, 95% CI: 12.5%–18.1%), chronic kidney disease (7.6%, 95% CI: 5.6%–9.7%), cancer (3.5%, 95% CI: 2.6%–4.4%), and chronic obstructive pulmonary disease (COPD) (3.4%, 95% CI: 2.6%–4.2%). Significant heterogeneity (Cochran’s Q) with an I2 index ranging from 83% to 98% (p < 0.01) was observed among the included studies in the estimate of pre-existing comorbidities (Table 3). We also compared the difference in prevalence of comorbidities between severe and non-severe patients. The proportion of hypertension (OR = 1.98, 95% CI: 1.62–2.42, p < 0.00001), diabetes (OR = 2.04, 95% CI: 1.67–2.50, p < 0.00001), cancer (OR = 1.75, 95% CI: 1.40–2.18, p < 0.00001), COPD (OR = 1.90, 95% CI: 1.49–2.41, p < 0.00001), cardiovascular disease (OR = 2.78, 95% CI: 2.00–3.86, p < 0.00001), and chronic kidney disease (OR = 2.74, 95% CI: 1.68–4.48, p < 0.00001) were statistically significant higher in severe group compared to the non-severe group (Table 4 and Figure 3). For the pooled estimate of comorbidities, I2 varied from 26% to 75%.
Figure 3.
Forest plots depict the comparison of pre-existing comorbidities in severe and non-severe patients.
Complications
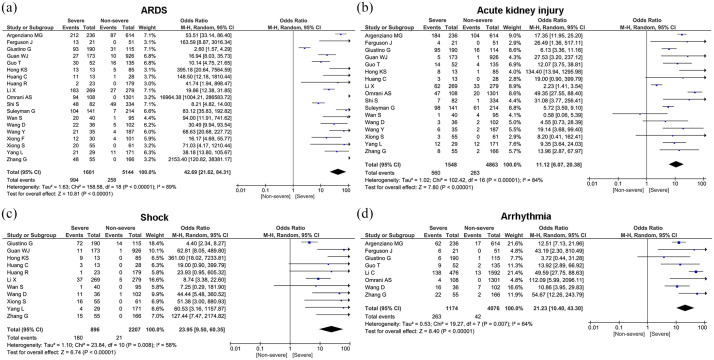
The most prevalent comorbidities in our study were ARDS (24.7%, 95% CI: 16.0%–33.4%), acute kidney injury (10.3%, 95% CI: 6.0%–14.6%), arrhythmia (8.4%, 95% CI: 4.5%–12.3%), and shock (6.8%, 95% CI: 4.3%–9.4%) (Table 3). Compared with non-severe patients, patients in severe group had higher risk on the incidence of ARDS (OR = 42.69, 95% CI: 21.62–84.31, p < 0.00001), shock (OR = 23.95, 95% CI: 9.50–60.35, p < 0.00001), acute kidney injury (OR = 11.12, 95% CI: 6.07–20.38, p < 0.00001), and arrhythmia (OR = 21.23, 95% CI: 10.40–43.30, p < 0.00001) (Figure 4).
Figure 4.
Forest plot for complications of severe patients compared with non-severe patients.
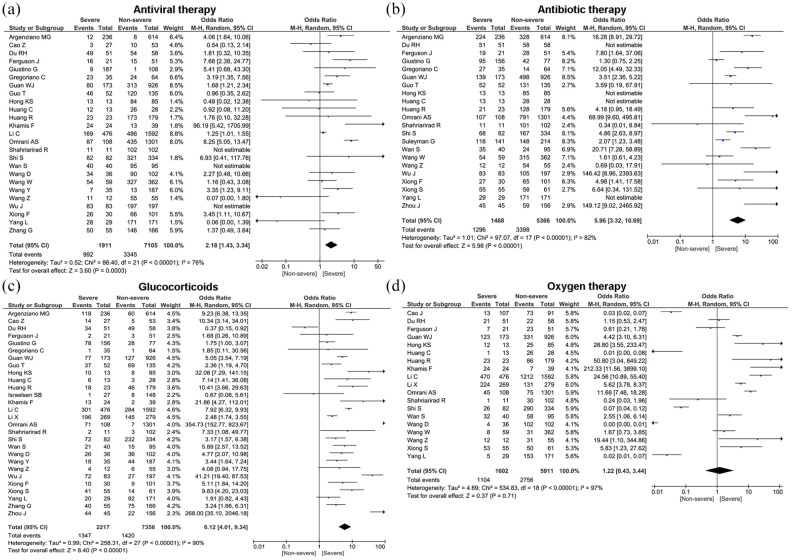
Treatment modalities
In terms of treatment, the majority of patients received antibiotic treatment (76.8%, 95% CI: 70.2%–83.5%), antiviral therapy (68.7%, 95% CI: 53.6%–83.8%), oxygen therapy (55.5%, 95% CI: 41.9%–69.1%), glucocorticoids (35.4%, 95% CI: 27.4%–43.5%), NIV (23.2%, 95% CI: 16.1%–30.2%), and CRRT (3.3%, 95% CI: 2.2%–4.5%) (Table 3). Compared to patients in the non-severe group, the patients in severe group were more likely to be treated with antibiotics therapy (OR = 5.96, 95% CI: 3.32–10.69, p < 0.00001), antiviral therapy (OR = 2.18, 95% CI: 1.43–3.34, p = 0.0003), oxygen therapy (OR = 1.22, 95% CI: 0.43–3.44, p = 0.71), glucocorticoids (OR = 6.12, 95% CI: 4.01–9.34, p < 0.00001), NIV (OR = 41.27, 95% CI: 15.24–111.77, p < 0.00001), and CRRT (OR = 18.92, 95% CI: 8.20–43.66, p < 0.00001) (Figure 5 and Table 4). However, no statistically significant difference was found for use of oxygen therapy in severe and non-severe cases (p = 0.71).
Figure 5.
Forest plots depict the comparison of the treatment modalities in severe and non-severe patients.
Discussion
Despite intense research and clinical investigations, much remains unknown about SARS-CoV-2. Until now, public health measures and control interventions are the only way to combat the COVID-19 pandemic. Proper and timely assessment of severe and non-severe coronavirus cases in the resource limited settings by healthcare professionals can save more lives and boost overwhelming health care system of low- and middle-income countries. This study systemically evaluated severe and non-severe patients with COVID-19 in terms of various clinical data. Our meta-analysis showed male predominance in the COVID-19 infections and incidence of severe courses. Recent studies have revealed that gender and age are major risk factors for SARS-CoV-2 infection. Alike in case of SARS and MERS infections, outcomes of COVID-19 were more severe in men compared to women.53,54 Possible factors causing different outcome of patients with COVID-19 between men and women can be related to differences in the immune system, sex hormones, physiological factors, and lifestyle.53–55
We also found that fever 78.1%, cough 64.6%, fatigue 40.8%, dyspnea 38.6%, headache 16.9%, sore throat 15.3%, nausea or vomiting 13.8%, and diarrhea 13.8% were common clinical manifestations of COVID-19. Our results are in line with previous pooled analysis showing similar trends in clinical features. 56 Mounting evidence suggests that clinical manifestations of COVID-19 are similar to SARS-CoV and MERS-CoV coronaviruses.56,57 Compared with the non-severe group, the severe group had higher pooled incidences of fever, cough, fatigue, dyspnea, headache, sore throat, nausea or vomiting, and diarrhea. These findings were in line with previous studies comparing clinical features in severe and non-severe COVID-19 cases.13,14
Studies have demonstrated that individuals with pre-existing comorbidities are more susceptible to infection and more likely to progress to severe COVID-19.31,45,58 Similar to the prior studies,58,59 the most prevalent comorbidities in our meta-analysis were hypertension, and diabetes, followed by cardiovascular disease, chronic kidney disease, cancer, and chronic obstructive pulmonary disease (COPD). Badawi and Ryoo 12 assessed the prevalence of comorbidities in the MERS-coronavirus patients and found that underlying disease such hypertension, diabetes, and cardiac disease were most prevalent comorbidities. The present meta-analysis showed that the incidence of hypertension, diabetes, cardiovascular disease, chronic kidney disease, cancer, and COPD is higher in severe than in non-severe COVID-19 patients. The incidence of hypertension, diabetes, cardiovascular disease, and chronic kidney disease was nearly two folds higher in severe cases than in non-severe counterparts. These findings are in agreement with findings for other respiratory diseases such as MERS, SARS, and influenza.60,61 Growing evidence suggests that proinflammatory state, and the attenuation of the innate immune response during comorbidities such as hypertension, diabetes, cardiovascular disease may be linked to the pathogenesis of COVID-19.62,63 In addition to this, mechanisms associated with increased COVID-19 severity in individuals with diabetes may be due to altered ACE2 receptor expression, dysregulated immune response, alveolar, and endothelial dysfunction. 64 Recent studies further confirmed that severe patients of COVID-19 had 2 to 100 times higher concentrations of IL-1, IL-10, and TNF-α than normal range, while IL-6 was markedly increased up to 1000 folds.5,65,66 This is in line with the concept of cytokine storm that may be responsible for critical illness in many conditions including viral infections.
Though COVID-19 mainly affects the respiratory system, it can spread to affect multiple organ systems with significant morbidity, mortality, and may lead to severe systemic complications. The present study revealed that ARDS with pooled prevalence of 24.7% was most prevalent complication, while acute kidney injury, arrhythmia, and shock were less prevalent. Additionally, we found that severe COVID-19 cases had almost 42 times the risk for ARDS than non-severe cases. The exact mechanism by which SARS-CoV-2 causes ARDS and the critical host immune factors that underlie the development of severe disease remains unclear. Recent study by Ruan et al. 67 demonstrated that severely ill patients tend to have a high concentration of pro-inflammatory cytokines such as IL-2, IL-7, IL-10, granulocyte-colony-stimulating factor, IP-10, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1A (MIP-1A) and tumor necrosis factor-α (TNF-α), compared to those who are moderately ill. Furthermore, study by Wu et al. 68 found that patients with COVID-19 who had developed ARDS had significantly higher neutrophil counts than non-ARDS patients which triggered a violent inflammatory immune response contributing to cytokine storm.
COVID-19 is a new entity and emerging infectious disease, currently there are no proven effective vaccines or therapeutic agents for this disease. In the absence of any clinically proven treatment strategy, the mainstay of treatment are supportive care, and prevention measures such as good hygiene, social distancing, and quarantine practices aimed at reducing transmission of the virus. Although currently there are no approved antiviral drugs for the treatment of COVID-19, however, broad spectrum antivirals that has been used for several RNA viruses, including SARS–CoV and MERS–CoV are widely used for the treatment of SARS–CoV-2.69,70 We found in our pooled analysis that antiviral therapy was used in 68.7% of the patients. Nevertheless, patients in severe group most commonly received antivirals than non-severe group.
While a variety of antibiotics have been used in COVID-19 patients, their role has yet to be established. Antibiotics such as cephalosporin, quinolones, carbapenems, tigecycline, and linezolid has been used as combination therapy in study by Chen et al. 71 (5) Similarly, in study by Wang et al., 9 severe patients received antibacterial therapy, such as moxifloxacin, ceftriaxone, and azithromycin. Consistent with results of previous studies,5,71 antibiotics therapy was most prevalent treatment modality used in our study. Furthermore, compared with non-severe patients, use of antibiotics was significantly higher in severe patients. Antibiotics such as azithromycin, doxycycline, and rapamycin inhibit protein synthesis and functionally reduce inflammation and viral replication. 72 However, clinicians should avoid the prolonged and inappropriate use of antimicrobials which may provoke the antimicrobial resistance and a decline in the effectiveness of these compounds.
The findings from previous studies provide evidence that treatment with corticosteroids improve the clinical condition of patients, reduce inflammation and prevent the development of ARDS in high risk patients.68,73 In contrast, other researchers revealed that corticosteroids did not improve symptoms in COVID-19 patients.74,75 Our pooled analysis showed that glucocorticoid was used in 35% of patients. Patients in the severe group had higher risk on the received glucocorticoid therapy, as well as oxygen support, CRRT and non-invasive ventilation than non-severe group. Debate regarding the use of corticosteroids in COVID-19 patients is far from conclusive, hence further studies are needed to delineate the use of corticosteroids for COVID-19.
The present study has some limitations. First, most of the data in this study are from retrospective studies. Second, high heterogeneity could be found in the analysis of some clinical data. This may be due to the different settings for study designs and large variation among studies in the sample size (9–5000 patients). Prospective multi-center randomized controlled trials are warranted to further confirm the conclusions of our study.
Conclusion
The most prevalent clinical symptoms of COVID-19 patients were fever, cough, fatigue, dyspnea, headache, sore throat, nausea or vomiting, and diarrhea. The incidences of these symptoms in severe patients were higher than non-severe group. Compared with non-severe patients, the comorbidities such hypertension, diabetes, cardiovascular disease, cancer, COPD, and chronic kidney disease were more common in severe patients. Severe patients are more prone to complications such as ARDS, shock, acute kidney injury, and arrhythmia. Although antiviral drugs, antibiotics, glucocorticoids are widely used in patients with COVID-19 but there is still no vaccine or definitive treatment against it. The COVID-19 pandemic is a public health emergency of international concern, there is a need for all countries to take joint actions to fight COVID-19. Our study results will help clinician to identify severe patients, which will contribute to early prediction, accurate diagnosis, and treatment of COVID-19 patients.
Author biographies
Mohan Giri is from Nepal and he is a clinical PhD student at the department of respiratory and critical care medicine, the First Affiliated Hospital of Chongqing Medical University. He has published more than 10 papers in reputed journals and his research field is interventional pulmonology and treatment of common respiratory disease.
Anju Puri is from Nepal and currently studying her Master degree in clinical nursing at the First Affiliated Hospital of Chongqing Medical University. Her research interests are critical care nursing and geriatric nursing.
Ting Wang is a master student at the department of respiratory and critical care medicine, the First Affiliated Hospital of Chongqing Medical University. Her research filed is lung cancer and pulmonary nodules.
Shuliang Guo is Director of the Department of Respiratory and Critical Care Medicine (National Clinical Key Specialty) of the First Affiliated Hospital of Chongqing Medical University. He is editor or special reviewers of more than 10 journals including the Chinese Journal of Tuberculosis and Respiratory Medicine. He has published more than 140 papers (including 30 in SCI). He is also the editor-in-chief, deputy editor-in-chief, and one of the textbooks compiled by national medical colleges and universities.
Footnotes
Author contributions: Conceptualization: M.G., G.S.L, A.P. Data curation: M.G., A.P., T.W. Formal analysis: M.G., A.P., T.W., G.S.L. Investigation: M.G., A.P. Project administration: M.G., A.P., T.W. Supervision: G.S.L., M.G., Validation: G.S.L., M.G., A.P. Visualization: M.G., A.P., T.W., G.S.L. Writing—M.G., A.P., T.W., G.S.L. Writing—review and editing: M.G., A.P., T.W., G.S.L.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from Chongqing Municipal Science and Technology Commission cstc2020jscx-fyzxX0040, cstc2020jscx-fyzxX0040; and Chongqing Municipal Education Commission KYYJ202006.
ORCID iD: Mohan Giri  https://orcid.org/0000-0001-8588-5482
https://orcid.org/0000-0001-8588-5482
References
- 1.de Wit E, van Doremalen N, Falzarano D, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016; 14: 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui DS, Azhar EI, Kim Y-J, et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis 2018; 18: e217–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song Z, Xu Y, Bao L, et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. Epub ahead of print January2019. DOI: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zumla A, Hui DS, Perlman S.Middle East respiratory syndrome. Lancet (London, England) 2015; 386: 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO announces COVID-19 outbreak a pandemic’ World Health Organzation Regional Office for Europe, http://www.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (2020, accessed 18 November 2020).
- 7.World Health Organization. COVID-19 weekly epidemiological update, https://www.who.int/publications/m/item/weekly-epidemiological-update---17-november-2020 (accessed 18 November 2020).
- 8.Tabata S, Imai K, Kawano S, et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis 2020; 20: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badawi A, Ryoo SG.Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis 2016; 49: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Sole F, Farcomeni A, Loffredo L, et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest 2020; 50: e13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D, Lian X, Song F, et al. Clinical features of severe patients infected with 2019 novel coronavirus: a systematic review and meta-analysis. Ann Transl Med 2020; 8: 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Sun J, Cao Z, et al. Epidemiological and clinical features of 201 COVID-19 patients in Changsha city, Hunan, China. Medicine (Baltimore) 2020; 99: e21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020; 75: 1730–1741. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 2020; 127: 104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Liu J, Zhang R, et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol 2020; 129: 104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong S, Liu L, Lin F, et al. Clinical characteristics of 116 hospitalized patients with COVID-19 in Wuhan, China: a single-centered, retrospective, observational study. BMC Infect Dis 2020; 20: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong F, Tang H, Liu L, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 2020; 31: 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Li W, Shi X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med 2020; 288: 128–138. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y, Zeng W, Huang X, et al. Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infect Dis 2020; 20: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Yang B, Li Q, et al. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020; 71: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zheng Y, Tong Q, et al. Cardiac injury and clinical course of patients with coronavirus disease 2019. Front Cardiovasc Med 2020; 7: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Xin C, Xiong Z, et al. Clinical characteristics and outcomes of 421 patients with coronavirus disease 2019 treated in a mobile cabin hospital. Chest 2020; 158: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 2020; 92: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect 2020; 80: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv Z, Cheng S, Le J, et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect 2020; 22: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020; 146: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020; 55: 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Jiang J, Wang F, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol 2020; 147: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang R, Zhu L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Negl Trop Dis 2020; 14: e0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du R-H, Liu L-M, Yin W, et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc 2020; 17: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Z, Li T, Liang L, et al. Clinical characteristics of coronavirus sisease 2019 patients in Beijing, China. PLoS One 2020; 15: e0234764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, Zheng Y, Luo Z, et al. Myocardial injury and COVID-19: serum hs-cTnI level in risk stratification and the prediction of 30-day fatality in COVID-19 patients with no prior cardiovascular disease. Theranostics 2020; 10: 9663–9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020; 369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson J, Rosser JI, Quintero O, et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March–April 2020. Emerg Infect Dis 2020; 26: 1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giustino G, Croft LB, Stefanini GG, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020; 76: 2043–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open 2020; 3: e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turcotte JJ, Meisenberg BR, MacDonald JH, et al. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PLoS One 2020; 15: e0237558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregoriano C, Koch D, Haubitz S, et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Med Wkly 2020; 150: w20316. [DOI] [PubMed] [Google Scholar]
- 45.Pellaud C, Grandmaison G, Pham Huu Thien HP, et al. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area – a retrospective cohort study. Swiss Med Wkly 2020; 150: w20314. [DOI] [PubMed] [Google Scholar]
- 46.Hong KS, Lee KH, Chung JH, et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J 2020; 61: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khamis F, Al-Zakwani I, Al Naamani H, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health 2020; 13: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omrani AS, Almaslamani MA, Daghfal J, et al. The first consecutive 5000 patients with coronavirus disease 2019 from Qatar; a nation-wide cohort study. BMC Infect Dis 2020; 20: 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shahriarirad R, Khodamoradi Z, Erfani A, et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis 2020; 20: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Israelsen SB, Kristiansen KT, Hindsberger B, et al. Characteristics of patients with COVID-19 pneumonia at Hvidovre Hospital, March–April 2020. Dan Med J 2020; 67: A05200313. [PubMed] [Google Scholar]
- 51.Ortiz-Brizuela E, Villanueva-Reza M, González-Lara MF, et al. Clinical and epidemiological characteristics of patients diagnosed with covid-19 in a tertiary care center in Mexico city: a prospective cohort study. Rev Investig Clin 2020; 72: 165–177. [DOI] [PubMed] [Google Scholar]
- 52.Popov GT, Baymakova M, Vaseva V, et al. Clinical characteristics of hospitalized patients with COVID-19 in Sofia, Bulgaria. Vector Borne Zoonotic Dis. Epub ahead of print October2020. DOI: 10.1089/vbz.2020.2679. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. Epub ahead of print August2020. DOI: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pradhan A, Olsson P-E.Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ 2020; 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scully EP, Haverfield J, Ursin RL, et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol 2020; 20: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Xie W, Wang Y, et al. A comparative overview of COVID-19, MERS and SARS: review article. Int J Surg 2020; 81: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health 2020; 13: 1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis 2020; 99: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alqahtani FY, Aleanizy FS, Ali El Hadi, Mohamed R, et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect 2018; 147: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 2013; 347: f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 2020; 8: 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renu K, Prasanna PL, Valsala Gopalakrishnan A.Coronaviruses pathogenesis, comorbidities and multi-organ damage – a review. Life Sci 2020; 255: 117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erener S.Diabetes, infection risk and COVID-19. Mol Metab 2020; 39: 101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Channappanavar R, Perlman S.Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan S, Yi Q, Fan S, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol 2020; 189: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos I, de A, Grosche VR, Bergamini FRG, et al. Antivirals against coronaviruses: candidate drugs for SARS-CoV-2 treatment? Front Microbiol 2020; 11: 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sargiacomo C, Sotgia F, Lisanti MP. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY) 2020; 12: 6511–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu F, Ji C, Luo J, et al. Clinical characteristics and corticosteroids application of different clinical types in patients with corona virus disease 2019. Sci Rep 2020; 10: 13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell CD, Millar JE, Baillie JK.Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet (London, England) 2020; 395: 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng W, Li Y, Cui L, et al. Efficacy and safety of corticosteroid treatment in patients with COVID-19: a systematic review and meta-analysis. Front Pharmacol 2020; 11: 571156. [DOI] [PMC free article] [PubMed] [Google Scholar]