Abstract
Acute myeloid leukemia (AML) is an aggressive hematological malignancy that is one of the more common pediatric malignancies in addition to occurring with high incidence in the aging population. Unfortunately, these patient groups are quite sensitive to toxicity from chemotherapy. Northern Labrador Tea, or Rhododendron tomentosum Harmaja (a.k.a. Ledum palustre subsp. decumbens) or “Tundra Tea”, is a noteworthy medicinal plant used by indigenous peoples in Alaska, Canada, and Greenland to treat a diversity of ailments. However, laboratory investigations of Northern Labrador Tea, and other Labrador Tea family members, as botanical sources for anticancer compounds have been limited. Utilizing an AML cell line in both in vitro and in vivo studies, as well as in vitro studies using primary human AML patient samples, this study demonstrated for the first time that Northern Labrador Tea extracts can exert anti-AML activity and that this may be attributed to ursolic acid as a constituent component. Therefore, this medicinal herb holds the potential to serve as a source for further drug discovery efforts to isolate novel anti-AML compounds.
Keywords: acute myeloid leukemia, Northern Labrador Tea, Rhododendron tomentosum, ursolic acid
Introduction
Northern Labrador Tea, or Rhododendron tomentosum Harmaja (a.k.a. Ledum palustre subsp. decumbens) or “Tundra Tea”, and Rhododendron groenlandicum (Oeder) Kron & Judd, or Bog Labrador Tea, are taxa in the genus Rhododendron as a section of Ledum. Importantly, they are some of the most prominent medical plants used by the Inuit, Cree, and other First Nations peoples of Northern Canada, Greenland, and Alaska (Black et al. 2011; Dampc & Luczkiewicz, 2013; Hébert & Thiffault, 2011; Popescu & Kopp, 2013). Indigenous people have used them to treat over 40 different heterogeneous ailments including toothaches, respiratory disease, diabetes, edema, infections, and rheumatism (Black et al. 2011; Dampc & Luczkiewicz, 2013; Hébert & Thiffault, 2011; Popescu & Kopp, 2013). Current populations still use R. tomentosum and R. groenlandicum for their inherent medicinal properties. It is commonly ingested as a tea but it is also used as a topical treatment for toothaches and skin sores. These procedures most commonly use the leaves of the plant but the shoots are used as well (Black et al. 2011; Dampc & Luczkiewicz, 2013; Hébert & Thiffault, 2011; Popescu & Kopp, 2013).
The Rhododendron genus is well known in current medicine for its anti-inflammatory, anti-nociceptive, anti-microbial, anti-oxidant, anti-diabetic, and cytotoxic activities (Black et al. 2011; Dampc & Luczkiewicz, 2013; Hébert & Thiffault, 2011; Popescu & Kopp, 2013). However, despite the widespread use of R. tomentosum and R. groenlandicum as ethnobotanicals there is a paucity of research identifying specific mechanisms of action. R. tomentosum has been reported to exert antioxidant and anti-inflammatory properties. In one report, R. tomentosum was shown to decrease LPS-stimulated production of the pro-inflammatory cytokine TNFα while antioxidant efficacy was demonstrated using the DPPH free radical scavenging assay (Black et al. 2011). These anti-inflammatory and antioxidant effects were attributed to constituent polyphenol components of R. tomentosum, including (+)-catechin and various quercetin derivatives (Black et al. 2011). Similarly, methanolic extracts of R. groenlandicum have demonstrated anti-inflammatory activities, including the inhibition of LPS-induced NO release in RAW 364.7 macrophages (Dufour et al. 2007). Additionally, extracts of R. groenlandicum leaves have been reported to be active against DLD-1 colon carcinoma and A-549 lung carcinoma cell lines (Dufour et al. 2007). These reports may be similar with those reporting anticancer efficacy for black teas, which has been attributed in part to theaflavin derivatives (Gao et al. 2016). In addition, R. groenlandicum has been shown to exert an anti adipogenic effect, which was attributed to its polyphenolic content (Eid et al. 2016), and has been described to attenuate insulin resistance in diet-induced obesity C57BL/6 mice (Ouchfoun et al. 2016). This was manifested through a reduction in blood glucose and a response to glucose tolerance while decreasing hepatic triglycerides, all likely driven by stimulation of insulin-dependent Akt pathway and increased GLUT-4 expression in skeletal muscle (Ouchfoun et al. 2016).
In the current study, we evaluated an anti-AML effect for Northern Labrador Tea (R. tomentosum) extracts in both cellular and animal models of acute myeloid leukemia (AML). This study is the first to evaluate in vitro and in vivo anti-AML activity for extracts of Northern Labrador Tea, and includes an evaluation of efficacy using primary patient AML samples. AML is an aggressive hematological malignancy impacting myeloid cells in the blood and bone marrow (Arber et al. 2016; Patel et al. 2012). Therapeutic advances for AML are limited and the disease generally has a poor clinical outcome (Arber et al. 2016; Patel et al. 2012). Therefore, there is high demand for new agents targeting AML and this study has identified Northern Labrador Tea as a potential source for the discovery of novel anti-AML compounds.
Materials and Methods
Cell Culture
Murine C1498 AML cells were maintained at 37°C, and 5% CO2, in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Patient AML samples were obtained using informed consent approved by the Penn State College of Medicine Institutional Review Board. Samples were prepared from peripheral blood or bone marrow using Ficoll-Paque separation of white blood cells (WBCs). For short term apoptosis assays primary human AML samples were maintained in RPMI-1640 supplemented with 10% FBS. For cellular viability assays primary human AML samples were maintained in Serum-Free Expansion Media (SFEM) (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 25 ng/ml each of granulocyte macrophage-colony stimulating factor (GM-CSF), stem cell factor (SCF), FLT3 ligand, and thrombopoietin (TPO) (each obtained from Reprokine, Rehovot, Israel).
In Vitro Assays
Cellular viability assays were performed as previously described using a Cell Titer 96 AQueous Non-Radioactive Cell Proliferation Assay according to the manufacturer’s instructions (Promega, Madison, WI) (Barth et al. 2014; Brown et al. 2013; McGill et al. 2014; McGill et al. 2018a). Briefly, C1498 cells were incubated for 48 hours, while primary human AML samples were incubated for 96 hours, with control or experimental treatments, prior to cellular viability assays. Apoptosis assays were performed as previously described using Annexin V and 7-aminoactinomycin D from BD Biosciences (San Jose, CA) (Brown et al. 2013; McGill et al. 2014; McGill et al 2018a), and a fluorophore-conjugated antibody targeting CD45 from Biolegend (San Diego, CA) to gate the blast population. Briefly, primary human AML samples were incubated for 48 hours, with control or experimental treatments, prior to apoptosis assays Flow cytometry was performed at the Penn State College of Medicine Flow Cytometry Core using a BD Biosciences LSR II flow cytometer and BD FACS Diva software.
Animal Trials
C57BL/6J mice were bred from founders obtained from the Jackson Laboratory (Bar Harbor, ME). As previously described (Barth et al. 2014; McGill et al. 2014; McGill et al. 2018a), male C57BL/6J mice were engrafted with C1498 cells (1×106 cells/mouse) by retro-orbital injection. After 12 days, mice were randomized and given ad libitum access to drinking water supplemented with the Northern Labrador Tea acetone extract (1 mg/ml), or to control water, and survival was monitored. Mice were euthanized once they reached moribund status. All procedures were approved by the Institutional Animal Care and Use Committees of the Penn State College of Medicine and the University of New Hampshire.
Northern Labrador Tea Extraction
Solvents and reagents were obtained from VWR (Radnor, PA) and Sigma (St. Louis, MO). Briefly, Northern Labrador Tea (R. tomentosum) was harvested at Murphy Dome 30 miles north of Fairbanks, Alaska in early August. Identification was performed by Dr. Steffi Ickert-Bond, curator of the herbarium at the University of Alaska Museum of the North located at the University of Alaska Fairbanks, by comparing with a voucher specimen harvested from the same location (UAM:Herb:16999, identifier ALAAC: V122899). Tea leaves were hand separated, dried, and stored at −80°C until extraction. Frozen, dried sample was finely crushed by mortar and pestle. 30 g sample was extracted in 100 mL solvent for 24 hours at 4°C with constant agitation. Extracts were prepared in water, 70% aqueous ethanol, isopropanol, acetone, or chloroform. Following extraction solids were removed by filtration, organic solvents were removed by rotary vaporization, and final volumes adjusted with water. Extracts were stored at −80°C until use. As these were whole extracts, the mass yield was slightly over 50% of the original starting material.
Ursolic Acid Quantification
Gas chromatography mass spectroscopy (GC/MS) was used to determine ursolic acid (UA) content of the acetone and aqueous extracts by adapting methods initially developed by Caligiani et al. (Caligiani et al. 2013). UA quantification was performed via the standard addition as described by Saxberg et al. (Saxberg & Kowalski, 1979). The instrument used was an Agilent 6890 GC with 5973N mass spectrometer operated in electron impact mode with a Restek Rxi-5ms column (30m × 0.25mm 0.25μm, Bellefonte, PA). Briefly, extracts (100 μL) were spiked with a range of UA masses (0, 10, 20, 50, and 100 μg) then evaporated (acetone) or lyophilized (aqueous), followed by derivatization in 1 mL 1:1 DMF:BSTFA (1% TMCS) at 90 °C for 1 hr. Extracts were injected (2 μL) in split mode (10:1) with helium carrier gas. Calibration was performed on extracted ion chromatograms (m/z = 320) at retention time = 57.1 min.
Statistical Analysis
Cellular viability and apoptosis assay comparisons of control treatments with Northern Laborador Tea extracts were made using a 1-way ANOVA followed by a Tukey’s multiple comparisons test. For in vivo studies, survival analysis was performed using the Mantel-Cox Logrank test. Graphical averages are depicted as +/− the standard deviation from the mean.
Results and Discussion
Extracts of Northern Labrador Tea were generated using a variety of different extraction solvents (water, chloroform, acetone, aqueous ethanol, and isopropanol). This can result in extracts that are enriched in varying degrees of different constituent compounds. Of note, the water-based extraction method would more closely resemble traditional indigenous preparations of the plant. In this study, the extracts of Northern Labrador Tea were first exposed to C1498 cells, which is a murine AML cell line, and cellular viability was assessed (Figure 1A). The water-based extract had no anti-AML effect. Variable anti-AML efficacy was observed for the chloroform, isopropanol, and aqueous ethanol extracts. However, the acetone extract demonstrated the most robust anti-AML efficacy against the C1498 cell line. Overall, these results showed that anti-AML compounds may not extract at all by methods analogous to traditional indigenous preparations of Northern Labrador Tea while organic extractions provide the best route to isolation of constituent anti-AML compounds. Next, the acetone extract was formulated into the drinking water of male C57BL/6J mice engrafted with C1498 cells. We have previously used this animal model of AML in experimental therapeutic studies (Barth et al. 2014; McGill et al. 2014; McGill et al. 2018a). This included an evaluation of the anti-AML efficacy of extracts from Devil’s Club (Oplopanax horridus), which is another Alaskan medicinal plant with profound indigenous use (McGill et al. 2014). This murine model is an aggressive and highly reproducible model of AML, which can be effectively used to evaluate anti-AML experimental therapeutics. Indeed, the in vitro anti-AML efficacy that was initially observed for the acetone extract of Northern Labrador Tea was predictive for an in vivo anti-AML efficacy that was observed as a significant extension in the lifespan of mice engrafted with C1498 cells from a median of 18 days to 25 days (Figure 1B). Altogether, these results demonstrate that Northern Labrador Tea contains constituent organic-soluble components with robust anti-AML activity. This rationalizes further effort to isolate and characterize these compounds as part of a natural products-driven drug discovery process.
Figure 1: In vitro and in vivo anti-AML therapeutic effect of Northern Labrador Tea extracts.
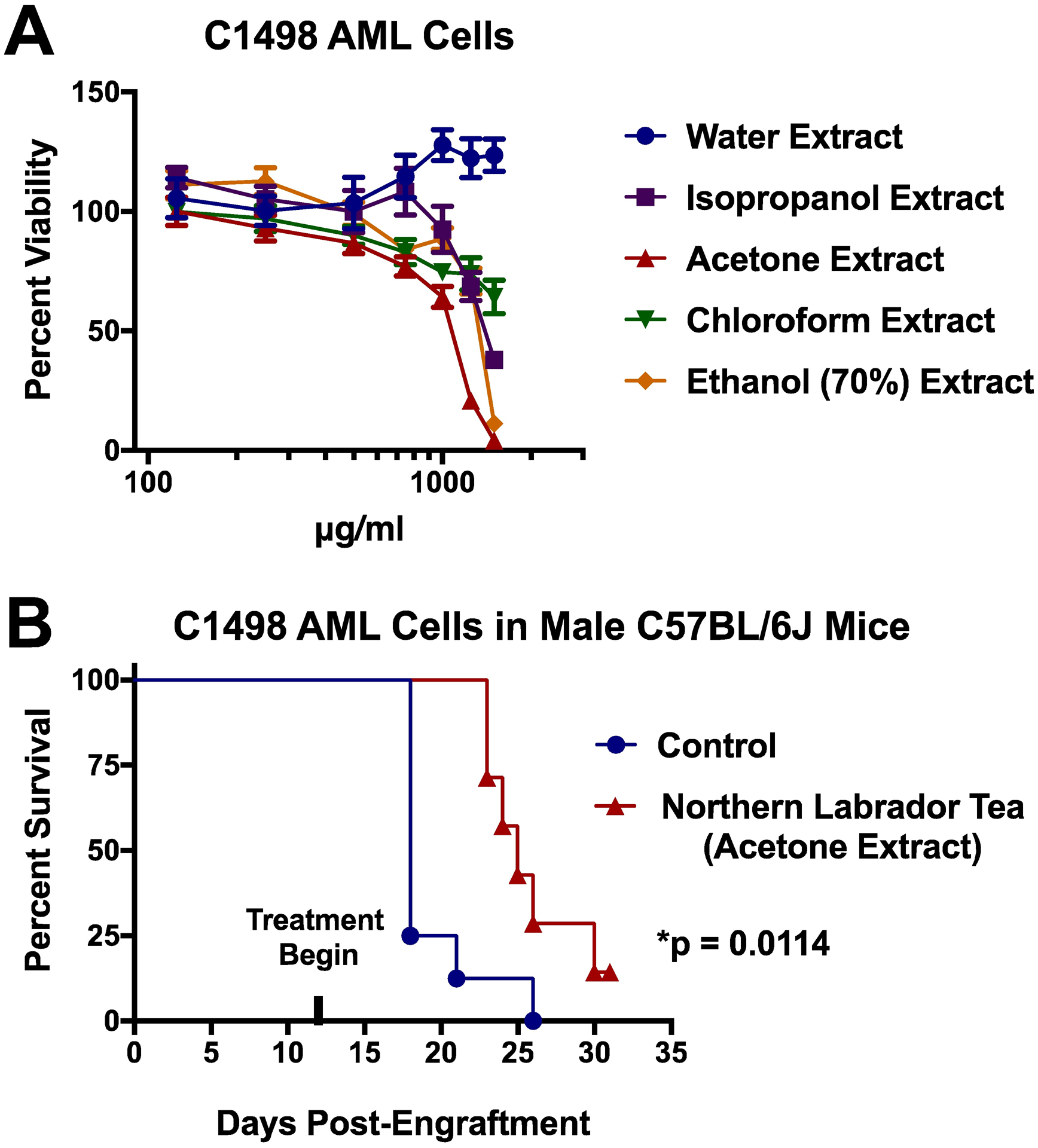
(A) Cellular viability was determined by MTS assay following 48-hour exposure of the C1498 AML cell line to various Northern Labrador Tea extracts. (B) Male C57BL/6J mice were engrafted with the murine AML cell line C1498 (1×106 cells/mouse by retro-orbital injection) and survival was monitored after mice were given ad libitum access to drinking water supplemented with the Northern Labrador Tea acetone extract (1 mg/ml), or to control water, starting one week following engraftment (Mantel-Cox Logrank test, p=0.0114, n≥7/group).
We further evaluated the acetone (most active) and water (least active) extracts by GC/MS and found that UA was a major component in the acetone extract (Figure 2A–B). Intriguingly, UA content in the acetone extract was determined to be 96 ppm, compared with 0 ppm in the water extract. Although we cannot assume that there was 100% extraction efficiency, we can approximate that Northern Labrador Tea contains a minimum of 0.19% UA by mass. To our knowledge, this is the first reported quantification of UA in any subspecies of Labrador Tea. Previously, quercetin and its derivatives were shown to be major components of Northern Labrador Tea (Black et al. 2011). Seasonal variation in the quantity of these and other polyphenols was associated with anti-inflammatory and antioxidant activities of Northern Labrador Tea (Black et al. 2011). We further evaluated the anti-AML activity for either quercetin or UA, or the combination of both, using the C1498 cell line. Interestingly, UA exerted a robust anti-AML effect while quercetin exerted a minimal anti-AML effect (Figure 2C). The anti-AML efficacy of the combination of UA and quercetin was similar with that of UA-alone. This demonstrates that UA might be responsible for the anti-AML effects of the Northern Labrador Tea, which are prevalent in the acetone extract but not the water extract. This also indicates that quercetin, while a prevalent component of Northern Labrador Tea associated with anti-inflammatory and antioxidant effects, may not be responsible for anti-AML effects of Northern Labrador Tea. This observation may be of further importance given that polyphenols such as quercetin have very poor oral bioavailability. Intriguingly, we have recently reported that quercetin can exert a combinatorial anti-AML efficacy in cell lines in combination with ceramide nanoliposomes and that this may be related to quercetin’s antioxidant and anti-inflammatory ability (McGill et al. 2018b). Perhaps quercetin, or extracts of plants rich in quercetin and its derivatives, may be better suited as adjuvant therapeutic strategies to minimize the toxic effects associated with other more powerful therapeutics. However, given poor bioavailability and other limitations of antioxidants as anticancer agents, in vitro effects attributed to polyphenols may not translate to in vivo. This may be a more profound limitation when the route of administration is dietary. In contrast, components of Northern Labrador Tea such as the pentacyclic tritepenoid UA may have better oral bioavailability and may readily accumulate to therapeutically-relevant doses in vivo. Furthermore, UA may act as a more potent anti-AML therapeutic because it may exert its effects through different pathways than quercetin. A more in-depth pharmacological and mechanistic study of UA as an anti-AML agent is needed to better assess its efficacy and optimal formulations and routes of administration.
Figure 2: Ursolic acid content of Northern Labrador Tea.
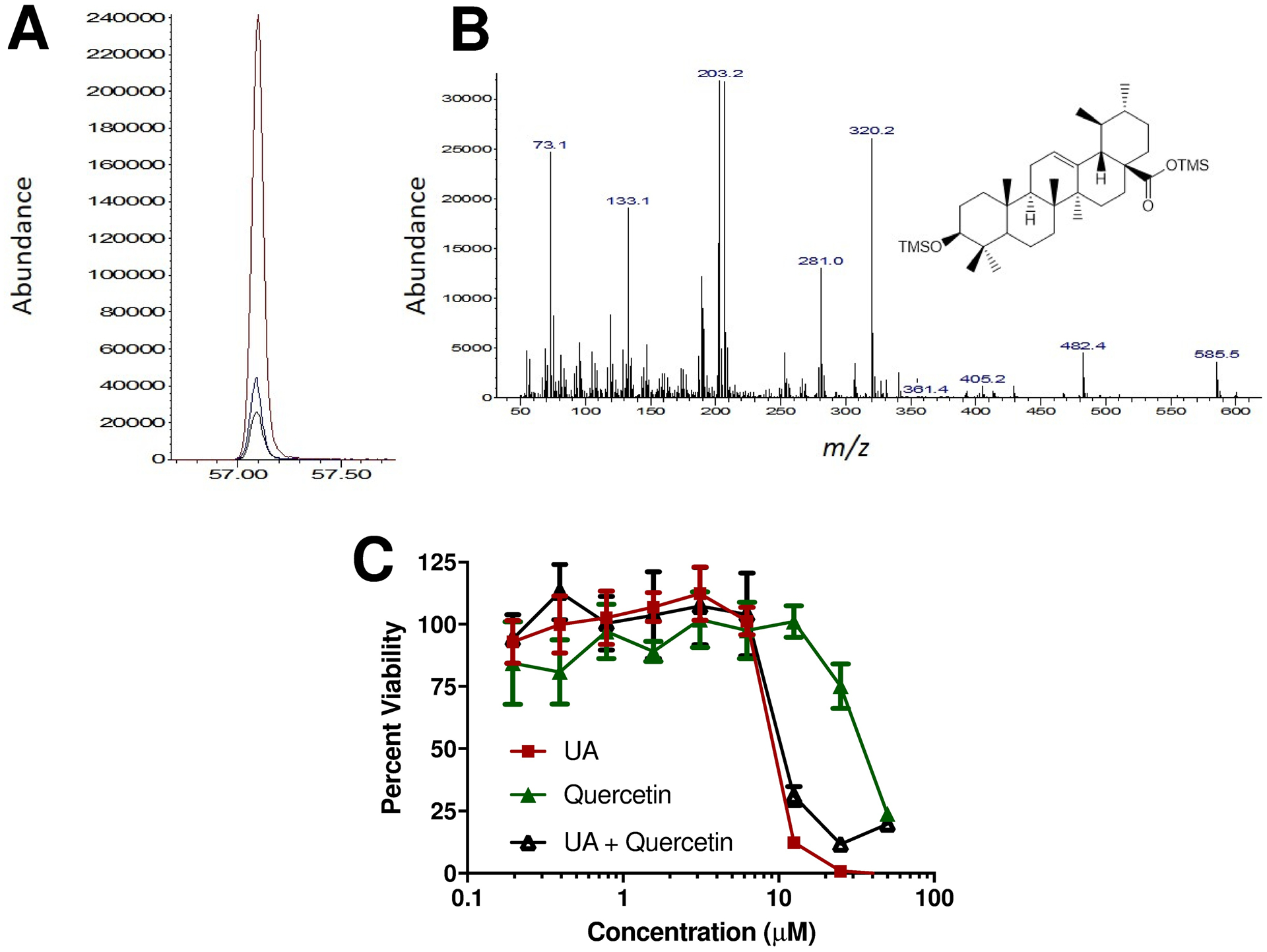
(A) Extracted ion chromatogram (m/z=320) corresponding to ursolic acid (UA) response in acetone extracts with 0, 10 and 100 μg UA standard added. (B) Full-scan mass spectrum of acetone extract at retention time = 57.1 min, consistent with Caligiani et al. (Caligiani et al. 2013). (C) Cellular viability was determined by MTS assay following 48-hour exposure of the C1498 AML cell line to UA or quercetin, a polyphenol prevalent in Northern Labrador Tea. UA exerted a more robust anti-AML effect than quercetin (n=6/group).
As a follow-up approach, primary human AML samples were exposed to the extracts of Northern Labrador Tea (Figure 3). Murine models such as the C57BL6/J mouse engrafted with C1498 cells are effective research tools, but translational studies utilizing human samples are needed to verify the potential of novel experimental therapeutics. Moreover, AML is a genetically heterogeneous malignancy (Arber et al. 2016; Patel et al. 2012). Different genetic variants of AML do not respond equally to the same therapeutics due to their inherent pathobiological differences (Patel et al. 2012). In this study, cellular viability was assessed in a growth factor-supplemented assay for five different human AML patient samples exposed to extracts of Northern Labrador Tea (Figure 3A). Not surprisingly, there were some substantial differences in the sensitivity of certain AML patient samples such as the sensitivity of patient sample #1000 to the water-based extract but not the isopropanol, acetone, or aqueous ethanol extracts. These observations highlight the existence of chemically-distinct compounds (aqueous-soluble versus organic-soluble) that can exert anti-AML activity towards different subtypes of AML. Finally, four of the human AML patient samples were exposed to extracts of Northern Labrador Tea in a short-term assay using basic cell culture media and apoptosis was evaluated (Figure 3B). This confirmed that the most prominent anti-AML activity resided in the organic extractions of Northern Labrador Tea.
Figure 3: Northern Labrador Tea extracts exert in vitro anti-AML efficacy towards primary human AML samples.
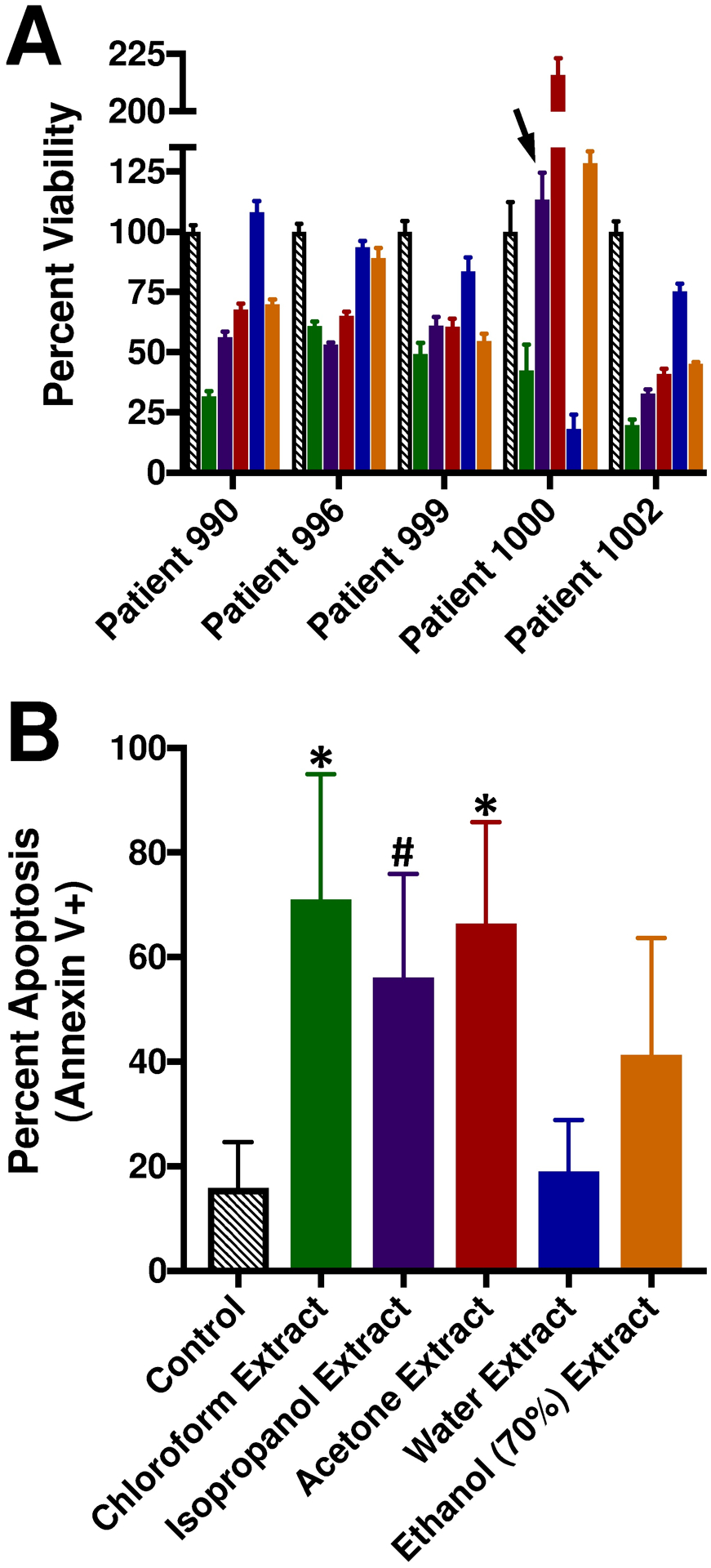
Ficol-prepared WBCs from human AML patients were exposed to 1 mg/ml Northern Labrador Tea extracts. (A) Cellular viability was determined following 96-hour exposure in SFEM supplemented with 25 ng/ml each of GM-CSF, SCF, FLT3 ligand, and TPO. For each patient AML sample, all experimental treatments are *p≤0.05 compared to control, except as indicated by an arrowhead (not significantly different), n=6 replicates per patient sample. (B) Apoptosis (Annexin V+ CD45-gated blast population) (Patient 990, 996, 1000, and 1002) was determined by flow cytometry following 48-hour exposure in RPMI-1640 supplemented with 10% FBS. *p≤0.012 compared to control, or #p=0.058 compared to control, n=4 samples.
Overall, this study is the first to show that extracts of Northern Labrador Tea contain constituent anti-AML compounds. While studies have suggested that Northern Labrador Tea may hold anticancer potential (Black et al. 2011; Dampc & Luczkiewicz, 2013; Hébert & Thiffault, 2011; Popescu & Kopp, 2013), this is the first to robustly demonstrate anti-AML efficacy using in vitro (cell line and primary patient sample) and in vivo models. Moreover, this study has attributed this anti-AML efficacy in part to the presence of UA in Northern Labrador Tea. It is worth noting that another study has shown that extracts of another Rhododendron family member (R. hainanense) can exert cytotoxicity towards the HL-60 human AML cell line (Zhao et al. 2012). Intriguingly though, we were not able to observe cytotoxicity to HL-60/vcr cells, a drug-resistant HL-60 variant, using our extracts of Northern Labrador Tea. Altogether, our present study demonstrates profound anti-AML activity for extracts of Northern Labrador Tea. This warrant further studies to isolate and characterize the constituent components of these extracts that are responsible for this anti-AML activity. Therefore, it is possible that Northern Labrador Tea holds promise as a source for the discovery of novel experimental compounds that may be used to treat AML.
Acknowledgements
We would like to thank Dr. Steffi Ickert-Bond, curator of the herbarium at the University of Alaska Museum of the North located at the University of Alaska Fairbanks, for identifying specimens of Northern Labrador Tea. This study was funded in part by the National Cancer Institute of the National Institutes for Health under award number K22-CA190674 (B.M.B.), the University of New Hampshire Collaborative Research Excellence Initiative, the Penn State University Kiesendahl Family Endowed Leukemia Research Fund, the Kenneth Noel Memorial Fund, and PA Tobacco Settlement funds. In addition, funding was provided by the University of Alaska Anchorage Office of Undergraduate Research and Scholarship-Alaska Heart Institute Fellowship. The authors have conflicts of interest to declare.
References
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, & Vardiman JW (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 127, 2391–2405. [DOI] [PubMed] [Google Scholar]
- Barth BM, Brown TJ, Adams MT, Garcia AM, Fisher LN, Fritz JL, Beck AJ, McGill CM, Kester M, Tran MA, & Claxton DF (2014). Combinatorial efficacy of 7,8-benzoflavone and liposomal ceramide in acute myeloid leukemia. Journal of Leukemia (Los Angeles), 2, 152. [Google Scholar]
- Black P, Saleem A, Dunford A, Guerrero-Analco J, Walshe-Roussel B, Haddad P, Cuerrier A, & Arnason JT (2011). Seasonal variation of phenolic constituents and medicinal activities of Northern Labrador tea, Rhododendron tomentosum ssp. subarcticum, an Inuit and cree First Nations traditional medicine. Planta Medica, 77, 1655–1662. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Garcia AM, Kissinger LN, Shanmugavelandy SS, Wang X, Cabot MC, Kester M, Claxton DF, & Barth BM (2013). Therapeutic combination of nanoliposomal safingol and nanoliposomal ceramide for acute myeloid leukemia. Journal of Leukemia (Los Angeles), 1, 110. [Google Scholar]
- Caligiani A, Malavasi G, Palla G, Marseglia A, Tognolini M, & Bruni R (2013). A simple GC–MS method for the screening of betulinic, corosolic, maslinic, oleanolic and ursolic acid contents in commercial botanicals used as food supplement ingredients. Food Chemistry, 136, 735–741. [DOI] [PubMed] [Google Scholar]
- Dampc A, & Luczkiewicz M (2013). Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia, 85, 130–143. [DOI] [PubMed] [Google Scholar]
- Dufour D, Pichette A, Mshvildadze V, Bradette-Hébert M, Lavoie S, Longtin A, Laprise C, & Legault J (2007). Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Ledum groenlandicum Retzius. Journal of Ethnopharmacology, 111, 22–28. [DOI] [PubMed] [Google Scholar]
- Eid HM, Ouchfoun M, Saleem A, Guerrero-Analco JA, Walshe-Roussel B, Musallam L, Rapinski M, Cuerrier A, Martineau LC, Arnason JT, & Haddad PS (2016). A combination of (+)-catechin and (−)-epicatechin underlies the in vitro adipogenic action of Labrador tea (Rhododendron groenlandicum), an antidiabetic medicinal plant of the Eastern James Bay Cree pharmacopeia. Journal of Ethnopharmacology, 178, 251–257. [DOI] [PubMed] [Google Scholar]
- Gao Y, Rankin GO, Tu Y, & Chen YC (2016). Inhibitory Effects of the Four Main Theaflavin Derivatives Found in Black Tea on Ovarian Cancer Cells. Anticancer Research, 36, 643–51. [PMC free article] [PubMed] [Google Scholar]
- Hébert F, & Thiffault N (2011). The biology of Canadian weeds. 146. Rhododendron groenlandicum (Oeder) Kron and Judd. Canadian Journal of Plant Science, 91, 725–738. [Google Scholar]
- McGill CM, Alba-Rodriguez EJ, Li S, Benson CJ, Ondrasik RM, Fisher LN, Claxton DF, & Barth BM (2014). Extract of Devil’s club (Oplopanax horridus) exert therapeutic efficacy in experimental models of acute myeloid leukemia. Phytotherapy Research, 28, 1308–1314. [DOI] [PubMed] [Google Scholar]
- McGill CM, Brown TJ, Cheng YY, Fisher LN, Shanmugavelandy SS, Gustafson SJ, Dunlap KL, Lila MA, Kester M, Toran PT, Claxton DF, & Barth BM (2018). Therapeutic Effect of Blueberry Extracts for Acute Myeloid Leukemia. International Journal of Biopharmaceutical Sciences, 1, 102. [PMC free article] [PubMed] [Google Scholar]
- McGill CM, Brown TJ, Fisher LN, Gustafson SJ, Dunlap KL, Beck AJ, Claxton DF, & Barth BM (2018). Combinatorial Efficacy of Quercitin and Nanoliposomal Ceramide for Acute Myeloid Leukemia. International Journal of Biopharmaceutical Sciences, 1, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchfoun M, Eid HM, Musallam L, Brault A, Li S, Vallerand D, Arnason JT, & Haddad PS (2016). Labrador tea (Rhododendron groenlandicum) attenuates insulin resistance in a diet-induced obesity model. European Journal of Nutrition, 55, 941–954. [DOI] [PubMed] [Google Scholar]
- Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, Huberman K, Cheng J, Viale A, Socci ND, Heguy A, Cherry A, Vance G, Higgins RR, Ketterling RP, Gallagher RE, Litzow M, van den Brink MR, Lazarus HM, Rowe JM, Luger S, Ferrando A, Paietta E, Tallman MS, Melnick A, Abdel-Wahab O, & Levine RL (2012). Prognostic relevance of integrated molecular profiling in acute myeloid leukemia. New England Journal of Medicine, 366, 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu R, & Kopp B (2013). The genus Rhododendron: an ethnopharmacological and toxicological review. Journal of Ethnopharmacology, 147, 42–62. [DOI] [PubMed] [Google Scholar]
- Saxberg BE, & Kowalski BR (1979). Generalized standard addition method. Analytical Chemistry, 51, 1031–1038. [Google Scholar]
- Zhao J, Ding HX, Zhao DG, Wang CM, & Gao K (2012). Isolation, modification and cytotoxic evaluation of flavonoids from Rhododendron hainanense. Journal of Pharmacy and Pharmacology, 64, 1785–1792. [DOI] [PubMed] [Google Scholar]


