Abstract
Purpose
There is a gap in knowledge regarding the impact of micrometastases (MIC) and isolated tumor cells (ITCs) found in the sentinel lymph nodes of patients with endometrial cancer. Here, we present a meta-analysis of the published literature on the rate of MIC and ITCs after lymphatic mapping and determine trends in postoperative management.
Methods
Literature search of Medline and PubMed was done using the terms: micrometastases, isolated tumor cells, endometrial cancer, and sentinel lymph node. Inclusion criteria were: English-language manuscripts, retrospectives, or prospective studies published between January 1999 and June 2019. We removed manuscripts on sentinel node mapping that did not specify information on micrometastases or isolated tumor cells, non-English-language articles, no data about oncologic outcomes, and articles limited to ten cases or less.
Results
A total of 45 manuscripts were reviewed, and 8 studies met inclusion criteria. We found that the total number of patients with MIC/ITCs was 286 (187 and 99, respectively). The 72% of patients detected with MIC/ITCs in sentinel nodes received adjuvant therapies. The MIC/ITCs group has a higher relative risk of recurrence of 1.34 (1.07, 1.67) than the negative group, even if the adjuvant therapy was given.
Conclusion
We noted that there is an increased relative risk of recurrence in patients with low-volume metastases, even after receiving adjuvant therapy. Whether adjuvant therapy is indicated remains a topic of debate because there are other uterine factors implicated in the prognosis. Multi-institutional tumor registries may help shed light on this important question.
Keywords: Micrometastases, Isolated tumor cells, Endometrial cancer, Sentinel lymph node
Introduction
Endometrial carcinoma is the most common gynecologic cancer in developed countries. In 2019, an estimated 61,880 new cases and 12,160 deaths from uterine cancer were diagnosed in the USA [1]. The standard management of patients diagnosed with endometrial cancer has changed in the last few years, the current recommendation is total hysterectomy and bilateral salpingo-oophorectomy along with sentinel lymph node mapping alone, to avoid full lymphadenectomy.
Sentinel nodes are considered positive for disease if they contain macrometastases (MAC > 2 mm), micrometastases (MIC 0.2–2 mm), or isolated tumor cells (ITC ≤ 0.2 mm) [2, 3]. The relationship between MIC or ITCs and increased risk of recurrence, as well as prognosis, has been demonstrated in a number of cancers such as breast cancer [4, 5], vulvar cancer [6–8], gastric cancer [9], esophageal cancer [10], colon cancer [11, 12], prostate cancer [13], and cervical cancer [14, 15].
In endometrial carcinoma, the clinical impact of low-volume metastasis remains unknown. Cibula et al. [16] published a study on the impact of MIC and ITCs in the sentinel lymph nodes (SLNs) and non-SLNs of cervical cancer patients. The patients selected for that study (17 patients in total) had cervical cancer and were at high risk of lymph node (LN) positivity (stage IB–IIA, biggest diameter ≥ 3 cm). A total of 573 pelvic LNs were examined through ultrastaging protocol (5762 slides). Metastatic involvement was detected in SLNs of eight patients (1 × MAC; 4 × MIC; 3 × ITCs) and in non-SLNs in two patients (2 × MIC). The authors found that using pathologic ultrastaging, there were no false-negative cases of positive non-SLN (MAC or MIC) and negative SLN. The presence of MAC and MIC was associated with a decrease in overall survival, but no difference in survival was found between patients with negative LN and ITCs.
It is hypothesized that MIC represents a truly small metastatic involvement, while ITC can be a different entity with a limited potential for the development of distant disease spread. Furthermore, there is a gap in knowledge regarding the prognosis impact and the ideal management of patients with endometrial cancer who have MIC or ITCs in the sentinel lymph nodes. The aim of this review is to explore the clinical significance of MIC or ITC in endometrial cancer and summarize the reported literature on the impact on postoperative management in patients with such findings.
Methods
Search strategy and selection criteria
Keywords including “micrometastases”, “isolated tumor cells”, “endometrial cancer”, and “sentinel lymph node” were used for literature searches in MEDLINE and PubMed. The search spanned from January 1999 to June 2019 and included all articles that contained information regarding “endometrial cancer” and “micrometastases and isolated tumor cells” in the titles and abstracts.
Articles had to meet the following inclusion criteria: English-language manuscripts limited to endometrial cancer, patients who had micrometastases and/or isolated tumor cells in the sentinel lymph nodes, studies that report oncologic outcomes, articles including ≥ 10 patients, patients who underwent open, laparoscopic or robotic surgery, and studies that did not present duplicated data. We included all retrospective and prospective studies. Two authors (NRGH and BN) reviewed the titles and abstracts of publications and excluded all unrelated articles (Fig. 1).
Fig. 1.
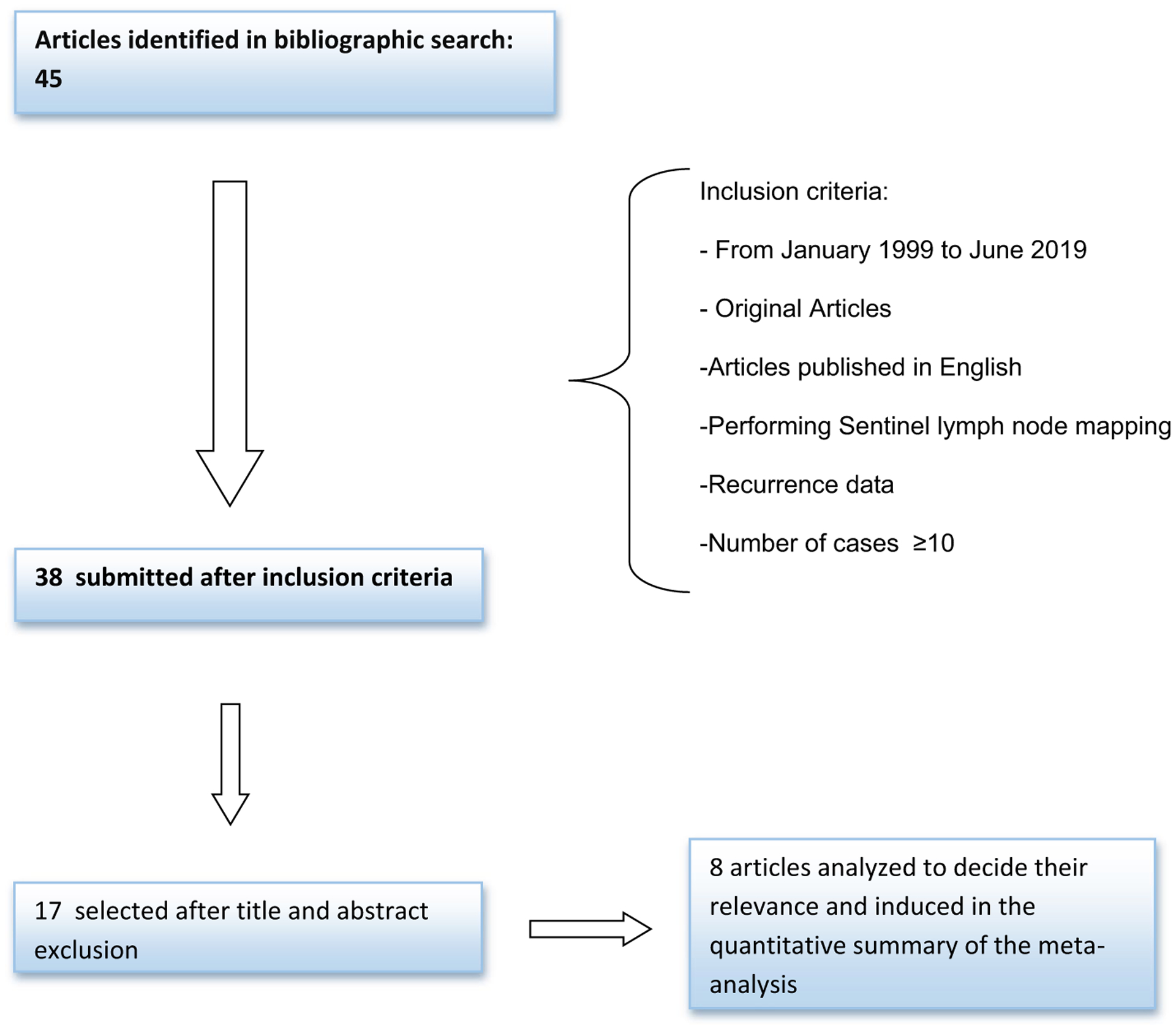
Flowchart of studies retrieved and finally included in the meta-analysis
We collected information on study design, year of publication, time period of study accrual, number of patients included, median age of patients, histological type, myometrial invasion (MI), lymphovascular invasion (LVI), grade, MIC/ITCs detection rate, and technique of detection (Table 1). We report the articles that compared the recurrences among patients with micrometastases, isolated tumor cells, and negative patients and studies that provided information on adjuvant therapy (Table 2).
Table 1.
Baseline characteristics of the included studies in the meta-analysis
| Authors | Design | Year | Study period | Number of patients | Median age | Endometrioid histology | Non endometrioid | MI | LVI | Grade 1 | Grade 2 | Grade 3 | Total MIC/ITCs patients | MIC/ITC (%) | Technique |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. [19] | Prospective cohort | 2013 | 2005–2011 | 425 | 58 | 415 | 10 | None: 241; < 50%: 184 | Yes: 58; no: 367 | 302 | 108 | 15 | 12 (9 ITCs) (3 MIC) | 3 | Ultrastaging (H&E + IHC: Anticytokeratin AE1/AE3) |
| Kim et al. [20] | Retrospective cohort | 2013 | 2005–2011 | 508 | 61 | 413 | Serous: 62; clear cell: 12; carcinosarcoma: 21 | None: 242; < 50%: 198; ≥ 50%:68 | Yes: 132; no: 376 | 261 | 116 | 131 | 23 (19 ITCs) (4 MIC) | 5 | Ultrastaging (H&E + IHC: Anticytokeratin AE1/AE3) |
| Erlanki et al. [21] | Retrospective cohort | 2010 | 2003–2006 | 47 | 60 | 37 | Adenosquamous: 7; serous papillary: 2; clear cell: 1 | None: 9; < 50%: 19; > 50%: 19 | Yes: 4; no: 40; no data: 3 | 15 | 21 | 8 | 7(MIC) | 15 | Ultrastaging (H&E + IHC: Standard ABC + Anticytokeratin AE1/AE3) |
| Clair et al. [22] | Retrospective cohort | 2015 | 2005–2013 | 844 | 61 | 724 | Serous: 104; clear cell: 16 | None: 422; < 50%: 310; ≥ 50%:112 | Yes: 201; no: 618; no data: 25 | 479 | 177 | 188 | 44 (23 ITCs) (21 MIC) | 5 | Ultrastaging (H&E + IHC: Anticytokeratin AE1/AE3) |
| Todo et al. [23] | Retrospective cohort | 2016 | 1997–2014 | 61 | 57 | 52 | 9 | < 50%: 29; ≥ 50%:32 | Yes: 15; no: 46 | 28 | 7 | 17 | 9 (6 ITCs) (3 MIC) | 15 | Ultrastaging (H&E + IHC: AE1/AE3 monoclonal antibody staining using an automated immunostainer) |
| Plante et al. [24] | Prospective cohort | 2017 | 2010–2015 | 519 | 64 | 448 | Serous: 36; carcinosarcoma: 25; clear cell: 7; other: 3 | None: 125; < 50%: 234; ≥ 50%:160 | Yes: 142; no: 362; no data: 15 | 260 | 150 | 109 | 42 (31 ITCs) (11 MIC) | 8 | Ultrastaging (H&E + IHC: Cytokeratin AE1/AE3) |
| Piedimonte et al. [27] | Retrospective case–control | 2018 | 2012–2018 | 41 | 64 | 41 | 0 | > 50%:16 | 17 | No data | No data | No data | 23 (11 ITCs) (12 MIC) | 56 | Ultrastaging (H&E + IHC: Cytokeratin-based) |
| Ignatov et al. [26] | Prospective cohort | 2019 | 2000–2017 | 428 | 69 | 399 | 26 | > 50%: 152; < 50%: 240 | No data | 145 | 174 | 100 | 126 (MIC) | 29 | Ultrastaging |
| Summary | Meta-analysis | 2873 | 62 | 2529 | 1490 | 753 | 568 | 17 |
SLN sentinel lymph node, MM micrometastases, ITCs isolated tumor cells, RT-PCR reverse transcription polymerase chain reaction, H&E hematoxylin and eosin; IHC immunohistochemistry, MI myometrial invasion, LVI lymphovascular space invasion, ABC avidin–biotin–peroxidase complex
Table 2.
Number of patients with adjuvant therapy and recurrences
| Total patients | Total negative patients | Total MIC/ITCs patients | |||||
|---|---|---|---|---|---|---|---|
| Adjuvant therapy | No adjuvant therapy | Adjuvant therapy | No adjuvant therapy | ||||
| Total patients | |||||||
| Kim et al. [19] | 425 | 400 | 94 | 306 | 12 (9 ITCs) (3 MIC) | 9 | 3 |
| Kim et al. [20] | 413 | 355 | No data | No data | 23 (19 ITCs) (4 MIC) | No data | No data |
| Erlanki et al. [21] | 47 | 40 | 12 | 28 | 7 (MIC) | 5 | 2 |
| Clair et al. [22] | 844 | 753 | No data | No data | 44 (23 ITCs) (21 MIC) | 40 | 4 |
| Todo et al. [23] | 61 | 52 | 34 | 18 | 9 (6 ITCs) (3 MIC) | 2 | 1 |
| Plante et al. [24] | 519 | 434 | No data | No data | 42 (31 ITCs) (11 MIC) | 39 | 3 |
| Piedimonte et al. [27] | 41 | 18 | 3 | 15 | 23 (11 ITCs) (12 MIC) | 16 | 2 |
| Ignatov et al. [26] | 428 | 302 | 0 | 302 | 126 (MIC) | 95 | 31 |
| Summary | 2778 | 2354 | 143 | 669 | 286 | 206 | 46 |
| Recurrences | |||||||
| Kim et al. [19] | 11 | 8 | 3 | 5 | 3 (2 ITCs). (1 MIC) | 2(ITCs) | 1(MIC) |
| Kim et al. [20] | 2 | No data | No data | 0 | 2 (ITCs) | 2 (ITCs) | 0 |
| Erlanki et al. [21] | 2 | 0 | 0 | 0 | 2 (MIC) | 1(MIC) | 1(MIC) |
| Clair et al. [22] | 51 | 47 | No data | No data | 4 (2 ITCs) (2 MIC) | 4 (2 ITCs) (2 MIC) | 0 |
| Todo et al. [23] | 12 | 8 | No data | 0 | 4 (ITCs/MIC)* | 2(ITCs/MIC)* | 2(ITCs/MIC)* |
| Plante et al. [24] | 1 | 0 | No data | No data | 1 (ITCs) | 1 (ITCs) | 0 |
| Piedimonte et al. [27] | 2 | 0 | 0 | 0 | 2 | 2 (MIC) | 0 |
| Ignatov et al. [26] | 231 | 221 | No data | No data | 10 (MIC) | No data | No data |
| Summary | 312 | 284 | 3 | 5 | 28 | 15 | 4 |
There is no difference between MIC and ITCS reported in the study
Statistical analysis
From each study, a number of cases and recurrences for each group of patients were extracted to calculate recurrence incidence. Relative risk and 95% confidence interval were calculated for each group number of cases. A random-effects meta-analysis was carried out for each comparison. Using the data, we created tables and forest plot was drawn. For each comparison, combined relative risk, given more weight for those studies with more cases, was calculated using DerSimonian–Laird random-effects mode, which accounts for both intra- and inter-study variability. All analyses were carried out with Stata 15.1
Results
We collected a total of 45 manuscripts, and 8 studies met our inclusion criteria (Fig. 1). Study characteristics are shown in Table 1. Studies totaled 2873 patients (range 41–508) among patients with MAC, negative lymph nodes, and MIC or ITCs. The median age was 62 years (range 54–69). Most of the patients (88%) reported an endometrioid histology on the final pathology, but 61% of total patients had more than 50% of myometrial invasion, 19% presented positive lymphovascular invasion, and Grade 3 was reported in the 20% of total patients. The median detection rate for MIC/ITCs was 17% (range 3–56). The ultrastaging technique was used in all the included studies.
Among all the studies which report data about oncologic outcomes, the total number of negative patients for MIC and ITCs was 2415, and the total number of patients with MIC/ITCs was 286 (187 and 99, respectively) (Table 2).
A total of 284 negative patients and 28 patients with either MIC or ITCs recurred. Table 3 shows the relative risk of recurrence between negative and MIC/ITCs patients.
Table 3.
Comparative recurrences between negative patients and MIC/ITC patients
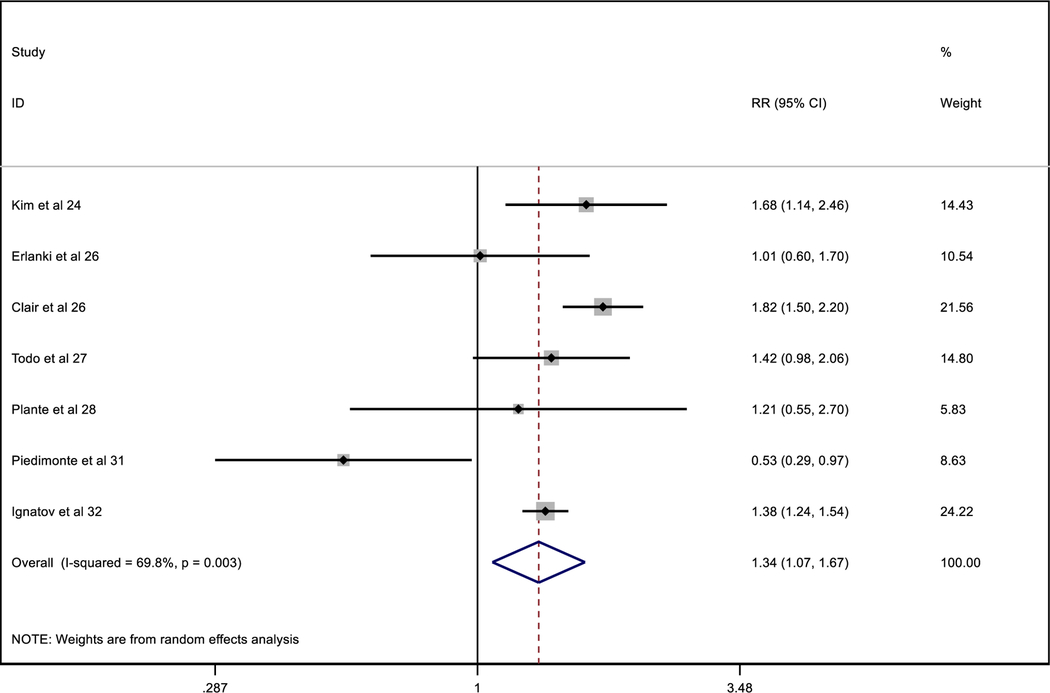
|
Considering only studies with clear data about the administration of adjuvant therapy (Tables 4 and 5), in the MIC/ITCs patients who did not receive adjuvant therapy, compared to negative patients and to MIC/ITCs patients who did receive adjuvant therapy, the relative risk of recurrence was similar in both groups not depending on adjuvant therapy.
Table 4.
Comparative recurrences between non-adjuvant negative patients and MIC/ITC non-adjuvant patients
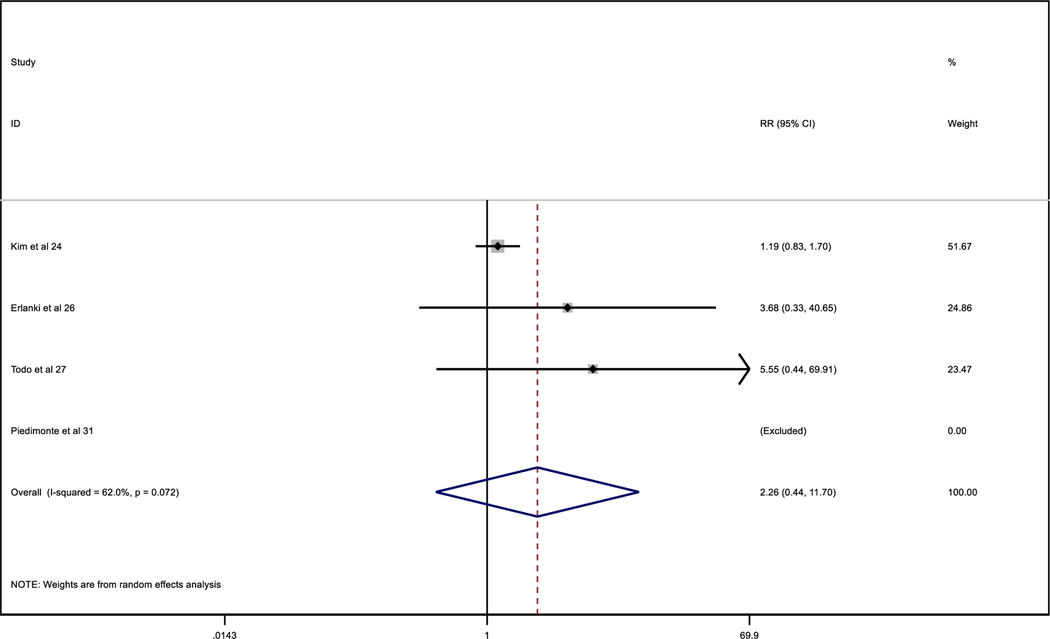
|
Table 5.
Comparative recurrences between MIC/ITC patients with adjuvant therapy and MIC/ITC non-adjuvant patients
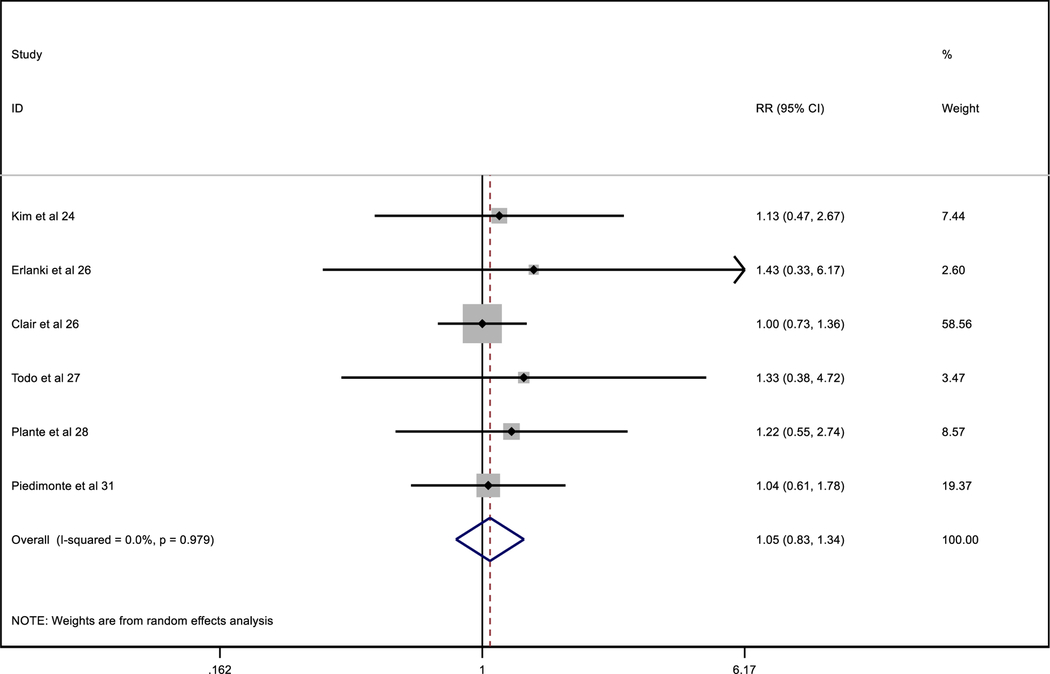
|
Discussion
Our findings suggest that there is a higher relative risk of recurrence in patients with low-volume metastases, even after receiving adjuvant therapy.
As previously noted, the incidence of MIC can differ according to the histological and biological technique used. Several studies proved that CK 20 is more sensitive than traditional histopathologic method with H&E (sensitivity was 94.5 and 91%, respectively) [17, 18]. Table 1 shows different ultrastaging techniques used in all the studies. Moreover, the SLN mapping with pathologic ultrastaging identified MIC or ITCs in 4.5% patients with endometrial cancer in whom no metastatic disease would have otherwise been detected by conventional pathologic processing [19, 20].
In terms of oncologic outcomes, the findings of low-volume metastases might have a negative impact on prognosis. Erlanki et al. [21] found that 2/7 (28%) of patients with micrometastases recurred and died of disease: both were of high risk—one had no adjuvant therapy, and the other one had both chemotherapy and radiotherapy. They reported a 36-month recurrence-free survival of 100% in patients who did not have micrometastases. Furthermore, Clair et al. [22] described a recurrence-free survival (RFS) of 86% for both MIC and ITCs patients. They observed that adjuvant therapy improves the survival rates in patients with low-volume metastasis compared to patients with macrometastasis. On the other hand, Todo et al. [23] reported that 28.6% of patients with ITC or MIC who received adjuvant therapy recurred (p = 0.17). Moreover, they found a higher rate of deep myometrial invasion in the ITCs or MIC patients than in node-negative patients (p = 0.028). However, this study presents some limitations: the majority of the patients had an early-stage carcinoma, received adjuvant therapy, or were patients with high-risk factors. In fact, histological grade, stage, and high-risk status are all important prognostic factors predicting disease recurrence. In addition, although we found that the 88% of the patients had an endometrioid histology on the final pathology, 61% of patients had more than 50% of myometrial invasion and 19% presented positive lymphovascular invasion.
Interestingly, Plante et al. [24] published a study on ITCs in patients with endometrial cancer, including 519 patients with a median follow-up of 29 months (range 0–67), and the progression-free survival (PFS) at 3 years for the ITC patients was 95.5%, similar to node-negative (87.6%) and micrometastasis patients (85.5%), but statistically better than patients with macrometastasis (58.5%) (p = 0.0012). Moreover, the latest prospective study to assess the association between treatment and recurrence-free survival in stage I–II endometrioid endometrial cancer patients with ITCs was published by Backes et al. [25]. They found that in a total of 175 patients with ITCs, 49% had stage IA, 39% stage IB, and 12% stage II disease (all with ITCs). Fifty-one percent underwent SLN assessment only, and the remainder underwent SLN and lymphadenectomy. A total of 76 (43%) received either no adjuvant therapy or vaginal brachytherapy only; 21 (12%) had external beam radiation; and 78 (45%) received chemotherapy + / − radiation. Patients who received chemotherapy more often had tumors with deep myometrial invasion, LVI, and higher grade. Nine (5.1%) patients recurred: 5 distant, 3 retroperitoneal, and 1 vaginal. After controlling for stage, LVI, and grade, chemotherapy was not associated with recurrence (HR = 0.63, 95% CI 0.11–3.52, P = 0.39). They concluded that the risk of retroperitoneal and/or distant recurrence is low (4.6%) for patients with stage I–II endometrioid EC and ITCs in SLNs regardless of adjuvant treatment or observation. The preliminary data suggest that adjuvant therapy does not appear to affect RFS.
The most recent publication is a multicenter, retrospective registry-based study of 2392 patients with endometrial cancer with and without MIC [26]. Without adjuvant therapy, the disease-free survival in the cohort of patients with MIC was reduced as compared with disease-free survival in the node-negative cohort, even after adjustment for age at diagnosis, myometrial invasion, histological grade and type, and performance status.
Although most of the studies recommended that the presence of isolated tumor cells should not drive the need for adjuvant treatments, the 72% of MIC/ITCs patients received some kind of adjuvant therapies. We could conclude that the benefit by giving additional treatments to ITCs patients depends on the presence of other high-risk uterine factors.
However, we recognize several important limitations. First, the number of the studies is small, given to the analysis a small power to make any conclusion. Second, in some studies, there were ITCs patients who received adjuvant therapy (chemotherapy or radiation) because of high-risk uterine factors or more advanced disease, and probably the prognosis could change. Lastly, given the favorable prognosis of endometrial cancer, our study is underpowered to detect small differences in survival.
In summary, when considering the association of MIC and ITCs with recurrence, we noted that patients with low-volume metastases had an increase relative risk of recurrence compared to negative patients, even if the adjuvant therapy was given. Further studies are needed in order to determine whether adjuvant therapy is indicated for both MIC and ITCs or only for those patients with MIC and to elucidate the specifics uterine factors that could change the indication of adjuvant therapy.
Conclusion
The current data show a higher sensibility and specificity of ultrastaging technique to detect MIC and ITCs; however, when we find these low-volume metastases, the clinical implications on adjuvant therapy remain a controversy. Currently, whether adjuvant therapy (chemotherapy or radiation) should be recommended in patients, at least, with MIC in regional LNs remains a topic of debate. In the near future, with the growing incorporation of SLN mapping and the initiatives of multi-institutional tumor registries, more data will elucidate the true clinical impact of MIC and ITCs on prognosis.
Funding
None.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
Ethical approval The present study was approved by the local ethical committee.
Informed consent Informed consent was not applicable.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Frumovitz M, Slomovitz BM, Singh DK, Broaddus RR, Abrams J, et al. Frozen section analyses as predictors of lymphatic spread in patients with early-stage uterine cancer. J Am Coll Surg. 2004;199(3):388–93. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Rustum NR. Update on sentinel node mapping in uterine cancer: 10-year experience at Memorial Sloan-Kettering Cancer Center. J Obstet Gynaecol Res. 2014;40(2):327–34. [DOI] [PubMed] [Google Scholar]
- 4.de Mascarel I, Bonichon F, Coindre JM, Trojani M. Prognostic significance of breast cancer axillary lymph node micrometastases assessed by two special techniques: reevaluation with longer follow-up. Br J Cancer. 1992;66(3):523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuckin MA, Cummings MC, Walsh MD, Hohn BG, Bennett IC, et al. Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer. 1996;73(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayansingh GV, Miller ID, Sharma M, Welch CJ, Sharp L, et al. The prognostic significance of micrometastases in node negative squamous cell carcinoma of the vulva. Br J Cancer. 2005;92(2):222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakim AA, Terada KY. Sentinel node dissection in vulvar cancer. Curr Treat Options Oncol. 2006;7(2):85–91. [DOI] [PubMed] [Google Scholar]
- 8.Knopp S, Holm R, Trope C, Nesland JM. Occult lymph node metastases in early stage vulvar carcinoma patients. Gynecol Oncol. 2005;99:383–7. [DOI] [PubMed] [Google Scholar]
- 9.Maehara Y, Oshiro T, Endo K, Baba H, Oda S, et al. Clinical significance of occult micrometastases lymph nodes from patients with early gastric cancer who died of recurrence. Surgery. 1996;119(4):397–402. [DOI] [PubMed] [Google Scholar]
- 10.Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337(17):1188–94. [DOI] [PubMed] [Google Scholar]
- 11.Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, et al. Identification of occult micrometastases in pericolic lymph nodes of Duke’s B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49 Correlation with long-term survival. Cancer. 1994;73(3):563–9. [DOI] [PubMed] [Google Scholar]
- 12.Liefers GJ, Cleton-Jansen AM, Van de Velde CJ, Hermans J, van Krieken JH, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339(4):223–8. [DOI] [PubMed] [Google Scholar]
- 13.Edelstein RA, Zietman AL, de las Morenas A, Krane RJ, Babayan RK, et al. Implications of prostate micrometastases in pelvic lymph nodes: an archival tissue study. Urology 1996;47(3):370–5. [DOI] [PubMed] [Google Scholar]
- 14.Juretzka MM, Jensen KC, Longacre TA, Teng NN, Husain A. Detection of pelvic lymph node micrometastasis in stage IA2–IB2 cervical cancer by immunohistochemical analysis. Gynecol Oncol. 2004;93:107–11. [DOI] [PubMed] [Google Scholar]
- 15.Barranger E, Cortez A, Commo F, Marpeau O, Uzan S, et al. Histopathological validation of the sentinel node concept in cervical cancer. Ann Oncol. 2004;15(6):870–4. [DOI] [PubMed] [Google Scholar]
- 16.Cibula D, Zikan M, Slama J, Fischerova D, Kocian R, et al. Risk of micrometastases in non-sentinel pelvic lymph nodes in cervical cancer. Gynecol Oncol. 2016;143:7–10. [DOI] [PubMed] [Google Scholar]
- 17.Fishman A, Klein A, Zemer R, Sc M, Zimlichman S, Bernheim J, et al. Detection of micrometastasis by cytokeratin-20 (reverse transcription polymerase chain reaction) in lymph nodes of patients with endometrial cancer. Gynecol Oncol. 2000;404:399–404. [DOI] [PubMed] [Google Scholar]
- 18.Bosquet JG, Keeney GL, Mariani A, Webb MJ, Cliby WA. Cytokeratin staining of resected lymph nodes may improve the sensitivity of surgical staging for endometrial cancer. Gynecol Oncol. 2003;91(3):518–25. [DOI] [PubMed] [Google Scholar]
- 19.Kim CH, Khoury-Collado F, Barber EL, Soslow RA, Makker V, et al. Sentinel lymph node mapping with pathologic ultrastaging: a valuable tool for assessing nodal metastasis in low grade endometrial cancer with superficial myoinvasion. Gynecol Oncol. 2013;131(3):714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23(5):964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlanki S, Bolat F, Seydaoglu G. Detection and importance of micrometastases in histologically negative lymph nodes in endometrial carcinoma. Eur J Gynaecol Oncol. 2011;32(6):619–25. [PubMed] [Google Scholar]
- 22.St Clair CM, Eriksson AG, Ducie JA, Jewell EL, Alektiar KM, et al. Low-volume lymph node metastasis discovered during sentinel lymph node mapping for endometrial carcinoma. Ann Surg Oncol. 2016;23(5):1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todo Y, Kato H, Okamoto K, Minobe S, Yamashiro K, et al. Isolated tumor cells and micrometastases in regional lymph nodes in FIGO stage I to II endometrial cancer. J Gynecol Oncol. 2016;27(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plante M, Stanleigh J, Renaud M-C, Sebastianelli A, Grondin K, et al. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: does adjuvant treatment matter? Gynecol Oncol. 2017;146:240–6. [DOI] [PubMed] [Google Scholar]
- 25.Backes FJ, Felix AS, Grégoire J, Plante M, Sullivane SA, et al. Sentinel lymph node (SLN) isolated tumor cells (ITCs) in otherwise stage I/II endometrioid endometrial cancer: To treat or not to treat? Abstracts presented for the 43rd annual meeting of the Society of Gynecologic Oncology. SGO 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ignatov A, Lebius C, Ignatov T, Ivros S, Knueppel R, et al. Lymph node micrometastases and outcome of endometrial cancer. Gynecol Oncol. 2019;154(3):475–9. [DOI] [PubMed] [Google Scholar]
- 27.Piedimonte S, Richer L, Souhami L, Arseneau J, Fu L, et al. Clinical significance of isolated tumor cells and micrometastasis in low-grade, stage I endometrial cancer. J Surg Oncol. 2018;118(7):1194–8. [DOI] [PubMed] [Google Scholar]
- 28.Clinton LK, Kondo J, Carney ME, Tauchi-Nishi P, Terada K, et al. Low-volume lymph node metastases in endometrial carcinoma. Int J Gynecol Cancer. 2017;27(6):1165–70. [DOI] [PubMed] [Google Scholar]


