Abstract
Background:
Growing evidence suggests that chronic pain and certain chronic pain conditions may increase risk for cognitive decline and dementia.
Objective:
In this systematic review, we critically evaluate available evidence regarding the association of chronic pain and specific common chronic pain conditions to subsequent decline in cognitive function, new onset cognitive impairment (CI), and incident Alzheimer’s disease and related dementias (ADRD); outline major gaps in the literature; and provide a preliminary conceptual model illustrating potential pathways linking pain to cognitive change.
Methods:
To identify qualifying studies, we searched seven scientific databases and scanned bibliographies of identified articles and relevant review papers. Sixteen studies met our inclusion criteria (2 matched case-control, 10 retrospective cohort, 2 prospective cohort), including 11 regarding the association of osteoarthritis (N = 4), fibromyalgia (N = 1), or headache/migraine (N = 6) to incident ADRD (N = 10) and/or its subtypes (N = 6), and 5 investigating the relation of chronic pain symptoms to subsequent cognitive decline (N = 2), CI (N = 1), and/or ADRD (N = 3).
Results:
Studies yielded consistent evidence for a positive association of osteoarthritis and migraines/headaches to incident ADRD; however, findings regarding dementia subtypes were mixed. Emerging evidence also suggests chronic pain symptoms may accelerate cognitive decline and increase risk for memory impairment and ADRD, although findings and measures varied considerably across studies.
Conclusion:
While existing studies support a link between chronic pain and ADRD risk, conclusions are limited by substantial study heterogeneity, limited investigation of certain pain conditions, and methodological and other concerns characterizing most investigations to date. Additional rigorous, long-term prospective studies are needed to elucidate the effects of chronic pain and specific chronic pain conditions on cognitive decline and conversion to ADRD, and to clarify the influence of potential confounding and mediating factors.
Keywords: Alzheimer’s disease, chronic pain, cognitive decline, cognitive impairment, dementia, fibromyalgia, headache, osteoarthritis, rheumatic
INTRODUCTION
Burden and epidemiology of Alzheimer’s disease and related dementias
Prevalence of Alzheimer’s disease and related dementias (ADRD), which encompass a range of progressive and devastating neurodegenerative disorders, continues to increase steeply in both developing and developed countries, paralleling the rapid aging of populations worldwide and constituting a serious and growing global public health crisis. A leading cause of disability, institutionalization, and death globally, ADRD imposes an enormous clinical, societal, and economic burden [1–3]. Even in high-income countries, the economic costs and associated societal burden of dementia threaten to overwhelm existing resources as the population ages and longevity increases [4].
The development of ADRD is generally insidious, with onset of clinical signs preceded years earlier by perceived and/or objective cognitive decline [5]. With effective disease-modifying treatments for cognitive impairment (CI) or ADRD still lacking after decades of disappointing clinical trials, research is shifting increasingly to prevention and early intervention [6, 7], with large-scale risk reduction trials now underway in multiple countries [7–11], including the U.S. [7, 12]. Although still incompletely understood, the development of late onset ADRD, which account for the vast majority of ADRD cases, is thought to reflect a complex interplay among multiple interacting factors, including those that are non-modifiable as well as those that are modifiable [3]. Most important among the former is age, with ADRD risk increasing exponentially with rising age [13]. For example, in the U.S., the percentage of adults with AD increases from 3% in those aged 65–74 to 17% in elders 75–84 and 32% in people 85 and older [3]. ADRD risk is also elevated in those with a family history of dementia and rises in a dose-dependent fashion with the presence of one or more APOE ε4 alleles [3, 14]. In addition, many studies have documented increased likelihood of ADRD in women and in African American and Hispanic adults, although some investigations suggest these disparities may reflect differences in socioeconomic, health-related, and other modifiable factors [3, 15].
In addition to these predisposing factors, a number of modifiable risk factors have also been linked to the development of CI and ADRD, providing potential opportunities for intervention. These include socioeconomic factors (e.g., low educational attainment [3, 16, 17], unemployment [18, 19], and poverty [3]); lifestyle behaviors (e.g., smoking, physical inactivity, and mid-life obesity [3, 20–29]); and psychosocial factors, including depression [30–34], anxiety [31, 35, 36], post-traumatic stress disorder [37–39], and other distressful states [40, 41], as well as sleep impairment [26, 42] and lack of social engagement [3, 25]. Several physical health conditions have also been linked to subsequent cognitive decline, CI, and ADRD, including: traumatic brain injury (TBI) and other head trauma [3, 43–45]; and history of specific chronic disorders, including hypertension in mid-life [3], diabetes [3, 46, 47], heart disease [48, 49], chronic obstructive pulmonary disease (COPD) [50], and stroke [20, 23, 49], as well as multimorbidity, the presence of multiple chronic conditions [51, 52]. In addition, certain medications have been linked to both increased and reduced risk for ADRD. For example, statin use has been consistently associated with reduced risk for dementia [53, 54]; conversely, benzodiazepines have been associated with elevated dementia risk in some studies [55, 56], although evidence for this association remains more equivocal [56–58]. Recent studies also suggest that polypharmacy may also increase risk for accelerated cognitive decline [59] and the development of dementia [60, 61].
However, while there has been considerable progress in identifying modifiable risk factors for ADRD, major knowledge gaps remain, hampering the development of effective prevention and treatment strategies. Important among these is our still limited understanding regarding the contribution of chronic pain and common chronic pain conditions to the development of CI and ADRD. Like ADRD, chronic pain continues to increase rapidly in prevalence worldwide, and itself poses a looming global public health crisis [62–65].
Chronic pain: Prevalence, burden, and relation to neurocognitive dysfunction
Chronic pain is a common and distressing condition associated with substantial clinical, economic, and societal burden in both developing and developed countries [66–68]. In the U.S., chronic pain affects up to 41% or more of the general population [67, 69–71] and up to 60% or more of older adults [72, 73], with prevalence continuing to increase [71]. Economic and social costs attributable to chronic pain are also high; for example, estimated excess annual healthcare costs in the U.S. range from $261–300 billion [74] and total costs, from $560 to $635 billion in 2010 dollars [75], far exceeding those for diabetes, hypertension, heart disease, cancer, and other common chronic disorders [73, 74]. Chronic pain can also lead to significant declines in productivity, physical function, quality of life, social engagement, and overall health, mood, and well-being [70, 72, 76–78], and is a leading cause of disability both in the U.S. and globally [65, 73, 79]. Moreover, as addressed in more detail in the discussion, many of these relationships are bidirectional, leading to a vicious cycle of increasing pain and social isolation, declining physical and mental health, disrupted sleep, and deterioration in mobility and function.
In addition, chronic pain can have significant effects on neurocognitive function. Because the neural systems involved in memory and cognition are closely linked to those involved in pain processing, these systems may affect one another reciprocally [76, 80] disrupting cognitive processing and contributing to a downward spiral of continuing pain, adverse neurostructural changes, and deteriorating cognitive function. Patients with chronic pain show changes in brain morphology paralleling those implicated in cognitive decline and CI, including grey matter volume reduction in brain regions involved not only in pain processing and emotional regulation, but in attention, memory consolidation, and cognitive processing [76, 80–84]. Chronic pain has also been shown to disrupt the functioning of the default mode network and other brain networks essential to normal cognitive function [82]. These alterations are thought to help explain the deterioration in memory and cognitive performance documented in many populations with chronic pain [76, 85], deterioration which may ultimately presage cognitive decline and the onset of CI and ADRD.
A growing body of evidence from epidemiologic studies suggests that chronic pain may be linked to subsequent cognitive decline, CI, and the development of ADRD. In this systematic review, we critically evaluate available evidence from published case-control and longitudinal studies regarding the association of chronic pain and specific common chronic pain conditions to incident dementia, as well as to adverse changes in cognitive function and new onset CI. We also outline major limitations and gaps in the current literature, provide a preliminary conceptual model illustrating potential pathways by which pain may influence cognitive change, and suggest directions for future research.
METHODS
To identify qualifying studies, we searched seven scientific databases, including Academic Search Complete, Scopus, MEDLINE, Pubmed, PsycARTICLES, and PsycINFO, as well as Google Scholar from their inceptions through February 2020 for original, peer-reviewed articles investigating the association of chronic pain or chronic pain conditions as a predictor of subsequent adverse changes in cognitive status. Search terms included (chronic pain or pain or arthritis or fibromyalgia or headache or migraine or rheumatic) AND (neurocogni* or neuropsych* or cogniti* or CI or memory or dementia or Alzheimer’s Disease). Titles and abstracts of the citations were scanned to identify potentially eligible articles for inclusion in this review. Potentially eligible papers were retrieved for more detailed review. In addition, we manually searched our own files, the citation sections of all identified articles, and the reference sections of recent review articles (2009–2020) regarding the relation of pain to cognition and/or dementia. Both authors reviewed the abstracts identified from the searches.
Original observational studies were included if they used longitudinal data to evaluate the association of chronic pain or common chronic pain conditions to subsequent changes in cognitive performance, new onset CI, and/or incident dementia. We excluded cross-sectional studies, studies that did not adjust at a minimum, for age and sex, studies for which the follow-up period was less than one year, as well as studies that were published only in dissertation or abstract form. We also excluded articles that were not published in English, compared only two clinical populations, included, as the major outcome variable, fewer than 50 cases, or did not report quantitative outcome data. In addition, studies assessing the association of rheumatological disorders to cognitive outcomes that did not distinguish types of arthritis were excluded from consideration. We also excluded studies regarding the relation of autoimmune and/or inflammatory rheumatic disorders (e.g., rheumatoid arthritis, systemic lupus erythematosus (SLE), ankylosing spondylitis, gout) to cognitive decline or dementia, as these relatively rare conditions are thought to be distinct in etiology and progression, and may thus differ in their effects on ADRD risk. We categorized into specific domains, both explanatory variables (specific individual chronic pain conditions, non-specific chronic pain symptoms) and cognitive outcomes (ADRD and specific ADRD subtypes, CI, and cognitive decline). For each domain, we summarized findings from relevant studies (with 95% confidence intervals), as well as indicators of external and internal validity. The latter included study design, sample size, data source, follow-up duration, study population selection procedures and characteristics, measures/assessment of pain/pain conditions and of cognitive outcomes, factors adjusted for in measures of association, attrition, and other information pertinent to study quality.
RESULTS
The initial literature search yielded 8,204 records. After removal of duplicates, a total of 7,176 potentially relevant records were scanned and 97 potentially eligible papers retrieved for full text review (Fig. 1). After excluding ineligible studies, a total of 16 papers reporting findings from 16 original studies, including two nested matched case-control studies [86, 87], 10 retrospective cohort investigations [87–94], and 4 prospective cohort studies [95–99] met our eligibility criteria and were included in the systematic review (Fig. 1). Table 1 provides a brief synopsis, and Table 2, a more comprehensive summary of the longitudinal studies identified for inclusion, detailing the characteristics, outcomes, and (Table 2) key findings of each study, stratified by chronic pain measure. Identified studies were conducted in a total of five countries, including Taiwan (N = 7, all using the Taiwan National Health Insurance Research Database (NHIRD) [86–88, 90, 91, 93, 100]), Japan (N = 1 [96]), the U.S. (N = 4 [94, 97–99]), Canada (N = 1 [89]), Norway (N = 2) [92, 95]), and England (N = 1 [101]). Study outcomes examined varied from all cause dementia/ADRD (N = 13 studies), Alzheimer’s disease (N = 8 studies), other dementias (N = 5 studies), and CI (N = 1 study) to measures of decline in cognitive function (N = 3), with 6 studies examining multiple outcomes (Table 1). Overall, 11 studies investigated the association of specific chronic pain conditions to dementia, including osteoarthritis (N = 4), fibromyalgia (N = 1), and headaches (N = 6) in a total of 701,593 adults (N = 26,327 AD/ADRD cases). Five investigations examined the relation of non-specific chronic pain symptoms to subsequent cognitive outcomes, including cognitive decline (N = 2), CI (N = 1), and dementia/dementia probability (N = 3).
Fig. 1.
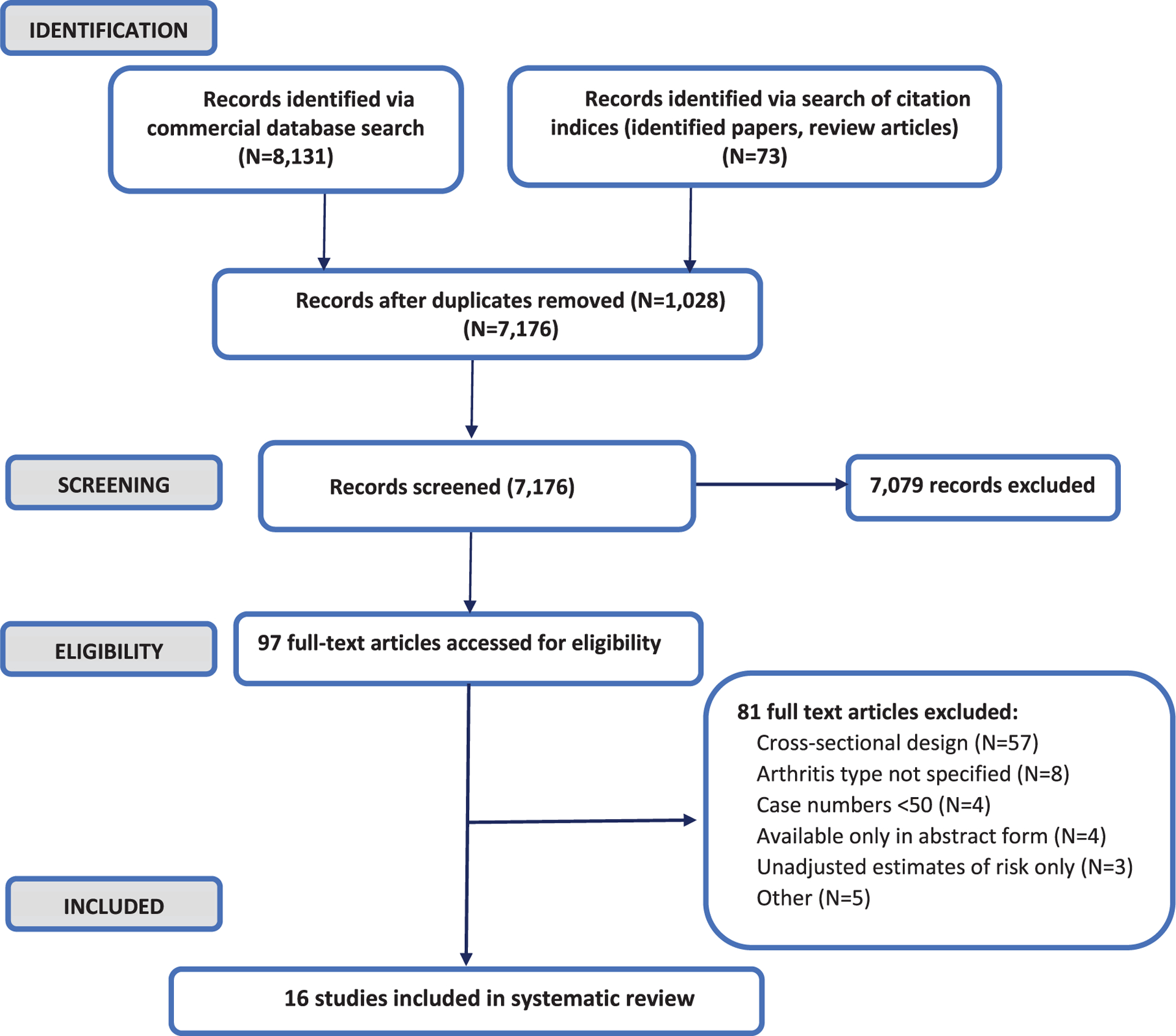
Study identification, screening, and selection for inclusion in systematic review.
Table 1.
Summary table of observational studies using longitudinal data to assess the relation of chronic pain and chronic pain conditions to subsequent cognitive decline and incident cognitive impairment and dementia
| First Author, y (Country) | Study Design | Max | Study Population | Outcome | Chronic pain condition | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f/u (y) | Age | N | N | ADRD | AD | Other Dem | CI | CD | OA | FMS | Headache | Chronic pain Sx | ||||||
| Any | Tension | Migraine | Y/N | Sev | PI | |||||||||||||
| Innes 2020 (USA) [94] | Retrospective cohort | 2 | Avg 75 y | 16,934 | 1,149 | X | X | X | ||||||||||
| Wang 2018 (Taiwan) [86] | Nested matched case control | 10 | Avg 76 y | 7854 | 2618 | X | X | |||||||||||
| Chen 2018 (Taiwan) [100] | Matched case control | 10 | ≥40 y | 71260 | 10180 | X | X | |||||||||||
| Huang 2015 (Taiwan) [87] | Retrospective matched cohort | 4 | ≥18 y | 105447 | 1071 | X | X | |||||||||||
| Tzeng 2018 (Taiwan) [88] | Retrospective matched cohort | 10 | ≥50 y | 166448 | 6123 | X | X | X | X | |||||||||
| Morton 2019 (Canada) [89] | Retrospective cohort | 5 | ≥65 y | 679 | 51 | X | X | X | X | |||||||||
| Tzeng 2017 (Taiwan) [90] | Retrospective matched cohort | 10 | ≥20 y | 14480 | 603 | X | X | X | X | X | X | |||||||
| Yang 2016 (Taiwan) [91] | Retrospective matched cohort | 10 | ≥20 y | 69,540 | 2,237 | X | X | X | X | |||||||||
| Røttereng 2015 (Norway) [92] | Retrospective matched cohort | 15 | 55–89 y | 16,443 | 746 | X | X | |||||||||||
| Hagen 2014 (Norway) [95] | Prospective cohort | 15 | ≥20 y | 51,383 | 378 | X | X | X | X | |||||||||
| Chuang 2013 (Taiwan) [93] | Retrospective matched cohort | 12 | Avg 42 y | 167,340 | 689 | X | X | |||||||||||
| Yamada 2019 (Japan) [96] | Prospective cohort | 3 | ≥65 y | 14,627 | 482 | X | X** | X** | ||||||||||
| Veronese 2018 (England) [101] | Longitudinal cohort | 4 | ≥50 y | 6,515 | n/a | X | X | X | ||||||||||
| van der Leeuw 2018 (USA) [97] | Prospective cohort | 6.2 | ≥65 y | 441 | 56 | X | X | X | ||||||||||
| Ezzati 2018 (USA) [98] | Retrospective cohort | 16.5 | ≥70 y | 1,114 | 114 | X | X | X | X | |||||||||
| Whitlock 2017 (USA) [99] | Retrospective cohort | 12 | ≥62 y≠≠ | 10,065 | n/a | X* | X | X | ||||||||||
Non-specific arthritis or ‘rheumatism’ (excluded from consideration)
Probability of incident dementia
Defined as self-reported presence and interference with daily activities
in 2000 (end of baseline period). AD, Alzheimer’s disease; ADRD, Alzheimer’s disease and related dementias; CD, cognitive decline; CI, cognitive impairment; Dem, dementia; f/u, follow-up; PI, pain interference; Sev, severity/intensity; sx, symptoms; y, year; Y/N, Yes/No (presence).
Table 2.
Observational studies using longitudinal data to assess the relation of chronic pain and chronic pain conditions to subsequent cognitive decline and incident cognitive impairment and dementia: characteristics and key findings
| First Author, y, Country | Design and Follow-up Period (y) | Data source | Study sample size | Population characteristics | Chronic pain measure | Outcome | Main Results AOR/ARR/AHR (95% CI) | Factors adjusted for | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Osteoarthritis (OA) | ||||||||||
| Innes 2020, USA [94] | Retrospective cohort (elders enrolled in fee-for-service (FFS) Medicare, 11 pooled cohorts). f/u: 2 y | Linked Medicare claims and Medicare Current Beneficiary Survey files (2001–2015) | 16,934 beneficiaries (1,149 ADRD, 4,545 OA) | Non-institutionalized adults 65 + y, ADRD-free at baseline, continuously enrolled in FFS Medicare | OA diagnosed at baseline (ICD-9-CM claims:≥2 out-patient claims≥ 90 days apart or≥ 1 inpatient claim); diagnosed joint, neck/ back, or neuropathic pain (ICD-9-CM) | Incident ADRD; Diagnosed y 2–3 (ICD-9-CM 290.0–290.3, 331.0–331.2, 331.7,331.8 or affirmative response to Health Status question re AD¥. | AORs for ADRD: OA: 1.23 (1.06,1.42) versus No OA or pain: OA+ pain: 1.31 (1.08,1.58) OA, no pain: 1.23 (0.94,1.66) No OA+pain: 1.13 (0.96,1.33) Incl depression, anxiety OA: 1.14 (0.98,1.32) versus No OA or pain: OA+pain: 1.20 (0.99, 1.45) Competing risk of death models: Similar AORs |
Age, sex, race/ethnicity, education; marital status, insurance, region, smoking, BMI, medications, diabetes, hypertension, heart failure, IHD, hypertension, kidney disease, cancer, diabetes, Parkinson’s disease, headache, migraine COPD, RA, SLE, hx of stroke, TBI. Also incl depression, anxiety, sleep disorders | ||
| Wang 2018, Taiwan [86] | Nested case control (random sample of 2 million pp; controls matched on age, sex). f/u: up to 10 y | National Health Insurance Research Database (NHIRD) (2001–11) | 7,854:2,618 AD, 5,236 age and sex-matched controls (2,678 OA) | Mean age 76.1 y; 59% F | Pain conditions (dx ≥ 1 y before AD): OA (ICD-9-CM 714, 715, 720, 721), OA/osteoporosis (OS) cluster (ICD-9-CM) | Incident AD (ICD-9-CM 331.0, 290.0, 290.2, 290.3, on AD meds approved for reimbursement) | AORs for AD: OA: 1.09 (0.95,1.26) OA/OS: 1.23 (1.07,1.41) | Age, sex | ||
| Chen 2018, Taiwan [100] | Matched case control (Matched age and sex). f/u: up to 10 y | Longitudinal Health Insurance Database (LHID; data drawn from NHIRD) (2000–10) | 71,260 adults (10,180 dementia, 61,080 controls matched on age, sex, index date): 37,051 OA | ≥40 y | Rheumatic Disorders, including OA: ICD-9-CM | All-cause dementia (2001–10) (ICD-9-CM codes 290.0–290.4, 294.1, 331.0) | AORs for dementia: OA: 1.47 (1.40–1.54) | Age, sex, comorbidities (not specified) | ||
| Huang 2015, Taiwan [87] | Retrospective matched cohort; controls matched 2:1 on age, sex. f/u: up to 4 y | National NHIRD-LHID (2004–2011) | Total: 105,447 OA (2004–7): 35,149 Controls: 70,298; 1,070 incident dementia | ≥ 18 mean age not reported | Osteoarthritis (5 +OA diagnoses (ICD-9) or hospitalization with OA primary dx) | All-cause dementia after OA dx (≥2 dx and 1 primary dx in hospital) | AHR (incident dementia): OA: 1.25 (1.10–1.43) | Baseline age, sex, urbanization; diabetes, dyslipidemia, RA, SLE, COPD, stroke (CVA), hypertension, IHD, Parkinson’s disease. | ||
| Fibromyalgia (FMS) | ||||||||||
| Tzeng 2018, Taiwan [88] | Retrospective matched cohort (controls matched on age, sex). f/u: Up to 10 y | NHIRD-LHID (2000–2010) | Total: 166,448 (41,614 Fibromyalgia (FMS); 124,836 controls). Exclude pts with ADRD before FMS dx; or with SLE, RA, SS. 6,123 with incident dementia. | ≥50 y (mean age not reported) 87.6% F | Newly diagnosed Fibromyalgia (ICD-9-CM ICD-9-CM 411.1, 413, 414.0, 414.8–414.9 + 3+outpt visits) in 2000 | All-cause dementia (2000–10); AD; VaD; non-VaD (ICD-9-CM 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.41, 290.42, 290.43, 290.8, 290.9, 331.0) | AHRs: Dementia: 2.77 (2.61–2.95); AD: 3.35 (2.57,4.32); VaD: 3.14 (2.60–3.78) non-VaD: 2.72 (2.55,2.90) | Age, sex, urbanization, income, region of residence insurance premium; diabetes, dyslipidemia, COPD, CKD, hypertension, IHD, MI, PVD, AF, HF, stroke, depression, anxiety & other MH disorders; head injury; Parkinson’s disease; cancer, inflammatory, connective tissue, rheumatologic, liver, kidney, GI, thyroid disease; Anti-DM drugs, antihypertensive drugs, statins | ||
| Headaches | ||||||||||
| Morton 2019, Canada[89] | Retrospective cohort: Of 1,790 pts (60.5% of those contacted), 1,355 w/o CI = >961 (71%) w/complete data = >245 died/LtF, 37 CI (non-ADRD) = >679 final sample. f/u: 1–5 y | Manitoba Study of Health and Aging | 679 adults cognitively intact at baseline with complete data on covariates, migraine | adults 65 + y at baseline | Migraine (Self-reported history of at baseline) (N= 72) | Dementia (DSM = IV) (N= 51) AD (NINCDS-ADRDA) (N= 34) VaD (NINDS-AIREN) (N= 12) | AORs for: Dementia: 2.97 (1.25, 6.61); AD: 4.22 (1.59,10.42); VaD: 1.52 (0.20,7.23) Adjusted for age, education (AD), & stroke (dementia), depression (VaD) | (See Results) Self-reported data on: age, sex, education, depression (‘confounding’); hypertension, diabetes, stroke, myocardial infarction and other heart conditions (‘intervening’) | ||
| Tzeng 2017, Taiwan [90] | Retrospective matched cohort (controls matched on age, sex). f/u: Up to 10 y | National level NHIRD-LHID (2000–2010) | Total: 14,480 (3,620 with newly diagnosed headaches (2000); 10,860 controls; 603 with incident dementia | ≥ 20 y; mean age not reported | Primary headache including Migraines (M) and Tension-type headache (TTH) | All-cause incident dementia; AD; VaD; non-VaD | Competing risk model: AHRs for: Dementia Any = 2.05 (1.71,2.46); M: 2.00 (1.57,2.53) TTH: 1.77 (1.41,2.22) Non-VaD: Any: 2.11 (1.75,2.55) M: 2.06 (1.53,2.51) TTH: 1.88 (1.49,2.37) VaD (N= 46 tot): NS | Age, sex, urbanization, location, DM, dyslipidemia, COPD, hypertension, IHD, AF, HF, MI, PAD, stroke, depression and other MH disorders, head injury, Parkinson’s disease, cancer, rheumatic, kidney, liver, and specific inflammatory/ connective tissue disease, epilepsy, pain, gout. | ||
| Yang 2016, Taiwan [91] | Retrospective matched cohort (controls frequency-matched 4:1 on age, sex, TTH dx date). f/u: Up to 10 years (until ADRD dx, death, or EOS), avg 8.1 y | National level NHIRD-LHID (2000–2010) | Total: 69,540 (13,908 with newly diagnosed TTH (2000–6); 55,632 TTH-free controls; 2,237 incident dementia | ≥ 20 y; mean age not reported; | Tension-type headaches (TTH) (ICD-9-CM 307.81 and 339.1) diagnosed 2000–6 (index date) | All-cause incident dementia (ICD-9-CM 290, 294.1, and 331.0); AD; VaD; non-VaD | Competing risk model: AHRs for: ADRD: 1.15 (1.05,1.27) Women: 1.25 (1.11,1.42)>65 y: 1.13 (1.01,1.27); non-VaD: 1.21 (1.09.1.34) AD, VaD: NS | Age, sex, diabetes, dyslipidemia, COPD, hypertension, IHD, AF, HF, stroke, depression, head injury, Parkinson’s disease and migraine | ||
| Straete Rottereng 2015 Norway [92] | Retrospective cohort. Of 64787 HUNT 2 pts (70% of those invited), 52,230 (56%) complete head-ache Q.¥¥ = >9617 (18%) died/LtF = >26197/52541 (62%) complete HUNT 3 Qs = >15697 55–89 y. f/u: up to 15 y | Nord-Trondelag Health Surveys: 1995–7 (HUNT2) and 2006–8 (HUNT3) and Dementia Regist (1998–2011); all residents≥20 y of NordTrøndelag invited | Reference group: 15,601 adults 55–89 y with headache info in HUNT2 and participate in HUNT3; 746 HUNT 2 pts in Dementia Regis; 96 confirmed healthy, no ADRD (HUNT3) | Age 55–89 y | At baseline (HUNT2): Any headache; migraine; non-migraine headache | Dementia (Dementia registry); confirmed non-demented (HUNT3) | AORs for any headache: Dementia: 1.24 (1.04–1.49) No Dementia: 0.62 (0.39,0.98) | Age, sex, education, HADS score, smoking, severe comorbid condition (Final analyses); also consider BMI, alcohol use, BP, physical activity. | ||
| Hagen 2014, Norway [95] | Prospective cohort. Of 64787 HUNT 2 pts (70% of those invited), 52,222 (56%) complete head-ache Q¥¥ =>51383 w/o CI or ADRD. f/u: up to 15 y (avg to dementia dx = 8.6 y) | Nord-Trondelag Health Surveys: 1995–7 (HUNT2) and Dementia Registry (1997–2010); all residents≥20 y of NordTrøndelag invited | 51,383 adults responding to headache questionnaire; 378 with incident dementia (63 VaD, 52 mixed dementia (D), 180 AD). Identified in Dementia Regis | Age≥20 y | At baseline (HUNT2): Any reported headache; migraine; non-migraine headache within past 12 months | Dementia-all cause, VaD, AD, dementia with Lewy bodies; FT D. Conservative dx (MRI, clin expert; ICD-10; NINCDS-ADRDA criteria required for AD, NINDS-AIREN for VaD) | AHRs for headache at baseline: All dementia: 1.3 (1.1–1.7) VaD: Any: 2.3 (1.4–3.8); M: 2.9 (1.3–6.6); Non-M:2.1 (1.2–3.7) Mixed D:2.0 (1.1,3.5) AD, other dementia subtypes: No association | Final models incl: All dementia: Age, sex Subtypes: age, sex, education, smoking, total HADS score Also info on: marital status, physical activity, alcohol use, anxiety, depressive sx; BMI, BP, lipids, glucose; HT meds; self-reported MI, DM, angina, stroke; daily med use. | ||
| Chuang 2013, Taiwan [93] | Retrospective matched cohort (adults with incident migraine frequency-matched to those without migraine on age, sex). f/u: up to 12 y until EOS, death, loss to f/u, or (controls) new migraine dx | NHIRD (1998–2010) | 167,340 adults: 33,468 with incident migraine; 133,872 with- out migraine. 689 with incident dementia. | Avg age = 42.4 (Migraine) and 42,1 (non-migraine); 71.3% F. All dementia-free at baseline | Newly diagnosed migraine (ICD-9 346) 1998–2010. | All cause dementia (ICD-9 290, 294.1 and 331.0) | AHRs for migraine: ADRD: 1.33 (1.22,1.46) | Age, sex; baseline diabetes, hypertension, coronary artery disease, head injury and depression | ||
| Chronic pain, unspecified/site-specific | ||||||||||
| Yamada 2019, Japan [96] | Prospective cohort (2013 baseline). f/u: 3 y to EOS (2017), leave area where registered, dementia dx, or death | Survey (mailed self-administered questionnaires) study of Japanese residents≥65 from 30 local govts (71% RR) in 2013 (baseline), linked to long- term care insurance registry (JAGE) | 14,627 dementia-free Japanese residents≥65 who applied to LT care but did not receive benefits (482 with incident dementia). | 65 + y at baseline, excl those with hx of stroke, cancer, injuries, depression, PD, dementia; who need daily living support or lack ADL info | Self-reported knee pain, low back pain, defined as presence of knee pain/low back pain in last year that interfered with daily activities (2 questions). | Incident dementia: data from LT care insurance; dx based on standardized home assessment (ADL, instrumental ADL; cognitive function, mental/ behavioral disorders** | Dementia: AHRs for Knee pain: 1.32 (1.06–1.64); appear strongest in those 65–79 (AHR 1.73 (1.11,2.68) and those who do not walk regularly (although interactions for latter NS) Back Pain: 0.79 (0.63–0.99) appear strongest in > 80 (AHR = 0.5 (0.3–0.8)) | Self-reported age group, sex, education, marital status, income, employment, loss events, social interaction, BMI, alcohol use (Y/N/ex-drinker), smoking ((Y/N/ex), DM, hyper-tension; mood/anxiety disorder (Kessler Psych Distress Scale (K6) 13+). Regular walking (modifier) | ||
| Veronese 2018, England [101] | Longitudinal cohort. 9,432 (85% of original cohort) complete WAVE 2, 8,960 with complete data = >2,429 died/LtF, 16 ADRD≠≠ = >6615 (60% original cohort) in WAVE 4. f/u: 4 y in 2 data waves (Wave 2:2002–3; Wave 4:2008–9) | English Longitudinal Study of Ageing (ELSA)-nationally representative ongoing cohort study; nurse visit+in person interviews | Total: 6,515 community dwelling adults | ≥50 y, avg 65 y; 57.3% women | Resp to question: “often troubled by pain?” If Y, what intensity (mild, mod, sev) | Change in cognitive function (verbal fluency, memory (immediate/ delayed recall; processing speed (letter cancellation test) | Pain (Y/N) and cognitive decline: No association overall Severe pain and memory decline: −0.36 (−0.68; −0.04), p = 0.04 | Baseline: Age, sex, marital status, education, household wealth, race, smoking, physical activity, alcohol, disability, BMI, diabetes, lung disease, asthma, hypertension, IHD, AF, HF, stroke, depression (CES-D), Parkinson’s disease, cancer, arthritis, osteoporosis (self-report except BMI)- Diffs in all but 2 vars (PD, cancer) | ||
| van der Leeuw 2018, USA [97] | Prospective cohort; of 590 CCMA pts enrolled 2011–17, 521 with baseline data on pain, cognition = >441 w/o MCI, MCR, or ADRD.≠≠≠ f/u: 1–6.2 y, mean = 2.75 y | Central Control of Mobility in Aging (CCMA) (lower Westchester Co): Community-dwelling adults | Total: 441; 285 with pain at baseline; 56 with incident major cognitive impairment (CI) | ≥65; avg 76 y; free of ADRD, MCI, or MCR at baseline. | MOS SF-36 pain severity scale, analyzed as continuous var and in tertiles/ quartiles (low, mild, moderate, severe) | Incident major cognitive impairment (CI) (Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); TMT Delta) | Major CI: No association with presence of pain (Y/N); In those with pain: Severe Pain: AHR= 3.47 (1.42–8.46) (versus low pain) for major memory impairment. | Age, sex, race, medications (incl analgesics, neuropathic/psych medications, sleep meds, antidepressants), General Health Score (summed conditions: DM, dyslipidemia, COPD, hypertension, IHD, AF, HF, stroke, PD, cancer, RA, etc.), depressive sx (GDS) | ||
| Ezzati 2018, USA [98] | Retrospective cohort Include EAS pts enrolled 1994–2015 with complete info on pain intensity/ interference and≥1 annual f/u. (cohort RR, LtF/deaths, missing data rates NR). f/u: 1–16.5 y; mean = 4.4 y | Einstein Aging Study (EAS), cohort of community-dwelling adults≥70 y in Bronx County, NY; dementia-free at baseline | Total: 1,114; 114 with incident dementia; 98 with probable AD | ≥70 y, avg 78 y | Pain intensity score (1–6); Pain interference (1–5) in past 4 weeks (2 q from SF-36): 91.4% with arthritis versus 60% in pain versus comparison group. | All-cause dementia; AD Assessed using DSM-IV criteria; also require meeting NINCDS-ADRDA criteria | Pain intensity: No association with AD/ADRD Pain interference: AHRs ADRD: 1.36 (1.07–1.73); Also incl depression, NSAIDs: 1.32 (1.03,1.69); <3 y versus≥3 y: 1.28 (0.69–1.83) versus 1.55 (1.11–2.2) AD: 1.26 (1.04–1.54) | Age, sex, race/ethnicity, education; medical comorbidity index (0–9): hypertension, diabetes, stroke, MI, angina, congestive heart failure, Parkinson’s disease, RA, COPD) | ||
| Whitlock 2017, USA [99] | Retrospective cohort: Of 12,058≥62 y (2000 wave), 10,065 with (non-proxy) data on pain, cognition (1998,2000 waves). f/u: up to 12 y until death/drop-out/EOS (2000–2012) | Health Retirement Survey (HRS, 2000–12): Population-based sample of community-dwelling older U.S. residents. In person/ telephone interviews ca. every 2 y | Total: 10,065; 1,120 with persistent pain (10.9 weighted %) | ≥62 y (2000 Wave); avg 73 y (interquartile range 67–78 y); 60% F | Persistent pain: troubled by mod/sev pain in 1998 and 2000 interviews (from items: “Are you often troubled with pain?” and “How bad is the pain most of the time: mild, moderate or severe?”) | Composite memory score and Dementia probability scores: developed using cognitive tests & interviews, i.e., using core HRS questions and models derived from ADAMS cohort. | Persistent pain: Associated with mean: 9.2% (CI 2.8–15%) faster cognitive decline; 7.7% (0.55–14.2%) faster increase in dementia probability after 10 y, corresponding to absolute 2.2% higher risk. | Baseline age, sex, race/ethnicity, education, marital status; financial assets; smoking, alcohol; med comorbidities (self- reported physician dx of DM, hypertension, COPD, heart disease, stroke, cancer [excl minor skin cancers]); depressive sx (CES-D), limitations in ADL. | ||
AD, Alzheimer’s disease; ADL, Activities of daily living; ADAMS, Aging, Demographics, and Memory Study; ADRD, Alzheimer’s disease and related dementia; ADRDA, National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association; AF, atrial fibrillation; AHR, adjusted hazards ratio; AOR, adjusted odds ratio; ARR, adjusted relative risk; avg, average; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes; dx, diagnosis; EOS, end of study; excl, excluding; F, female; f/u, follow-up; HF, heart failure; hx, history; HT, hypertensive; IHD, ischemic heart disease; incl, including; LtF, lost to follow-up; MCR, motoric cognitive risk syndrome; mod/sev, moderate/severe; MI, myocardial infarction; NINCDS-NINDS-AIREN, Neurological Disorders and Stroke (formerly NINCDS) and Association Internationale pour la Recherché et l’Enseignement en Neurosciences; NR, not reported; OA, osteoarthritis; RA, rheumatoid arthritis; RR, response rate; SLE, systemic lupus erythematosus; SS, Sjogren’s syndrome; sx, symptoms; VaD, vascular dementia; w/o, without; y, years;
Virtually universal;
Reported to correlate well with independent. physician panel assessment.
’Has a doctor ever told you that you have Alzheimer’s?’.
Those responding to headache questionnaire were younger, more likely to be women, and had higher socioeconomic status versus non-responders.
Those excluded at wave 4 due to missing data or death were significantly older, were more likely to report pain, and scored significantly worse in all cognitive tests at wave 2.
Eligible participants were significantly younger and more educated versus those excluded.
Association of chronic pain conditions to incident dementias
Osteoarthritis (OA) (N = 4 studies)
A total of four studies, two matched case-control studies [86, 100] and two retrospective cohort investigations [87, 94], assessed the relation of OA to incident ADRD [87, 100] or AD [86] using longitudinal data. Studies included one investigation of U.S. Medicare beneficiaries enrolled in fee-for-service plans [94] and three large, population-based studies of Taiwanese nationals (total N = 201,495 adults, 15,018 AD/ADRD cases). All of the latter studies used the Taiwanese National Health Insurance Research Database (NHIRD) or the Longitudinal Health Insurance Database (LHID, a randomly selected sample of 1 million adults from the NHIRD (1996–2011)) as the primary study data source (2000/2001/2004 to 2010/2011). The maximum follow-up period ranged from 2 [94] to 10 years [86, 100] (Tables 1 and 2).
In their retrospective matched cohort study of 105,447 Taiwanese adults ≥18 years (N = 1,070 with incident dementia), Huang and colleagues reported significantly increased risk for ADRD in those with a prior OA diagnosis after adjustment for age, sex, urbanization, and multiple comorbidities (adjusted hazards ratio (AHR)=1.25, 95% confidence interval (CI) 1.10,1.43) [87]. Consistent with these findings, in our recent retrospective cohort study of 16,934 Medicare beneficiaries ≥65 years (N = 1,149 with incident dementia), those with OA at baseline were significantly more likely to be diagnosed with incident ADRD at follow up after adjustment for sociodemographics, lifestyle characteristics, medications, multiple health conditions, and other factors linked to ADRD (Adjusted odds ratio (AOR) = 1.23, CI 1.06–1.42); this association was particularly pronounced in those with OA and accompanying pain (AOR versus no OA or pain = 1.31, CI 1.08–1.58), and appeared to be in part mediated by diagnosed anxiety and depression [94]. Likewise, findings of a large case-control study matched on age and sex (N = 71,260 ≥ 40 y, 10,180 with incident dementia) indicated significantly elevated odds of developing ADRD in those with diagnosed OA after adjustment for (unspecified) comorbidities (AOR = 1.47, CI 1.40–1.54) [100]. In contrast, in a nested case control of 7,854 older adults matched on age and sex showed little evidence of an association between OA alone and Alzheimer’s disease (AD), but reported elevated risk among those with both OA and osteoporosis (OR = 1.23, CI 1.07–1.41) [86].
Collectively, findings of these investigations suggest that older adults with OA may be at elevated risk for incident ADRD, although the association of OA to incident AD alone is less clear, especially given published data to date have been restricted to a single investigation, and findings were adjusted only for age and sex. Moreover, while findings support a potential link between OA and the development of dementia, interpretation is hampered by certain important limitations. These include short follow-up period [87, 94]; lack of information on cognitive trajectory, incident CI or specific dementia types [87, 94, 100]; and failure to assess the influence of OA-associated pain [87, 100], evaluate the potential role of psychosocial factors [87, 100] or survival bias [87, 100], and/or control for several potential confounders known to affect cognition, including certain: lifestyle and demographic factors, chronic health conditions, and medications. In addition, all but one study to date have been conducted in Taiwanese nationals using a similar data source, potentially limiting generalizability to other populations.
Fibromyalgia (N = 1 study)
Our search identified only one published longitudinal study to date evaluating the association of fibromyalgia (FMS) to cognitive outcomes, a large retrospective cohort study of over 165,000 Taiwanese nationals [88]. In this retrospective cohort study, the authors examined the association of newly diagnosed FMS to incident dementia in a nationally representative sample of adults ≥50 years of age drawn from the LHID (N = 41,614 with, 124,836 without FMS, of whom 6,213 were subsequently diagnosed with incident dementia) (Tables 1 and 2); the follow-up period ranged from 1 to a maximum of 10 years. In this cohort of older adults, FMS was associated with significantly increased risk for all cause dementia (AHR = 2.77, CI 2.61–2.95) after adjustment for a broad array of factors, including demographics, diagnosed chronic physical and mental health conditions, including arthropathies and connective tissue disorders, history of stroke and head injury, and certain medications. Those with newly diagnosed FMS were also at substantially increased risk for incident AD (AHR = 3.35, CI 2.57–4.32), vascular dementia (3.14, CI 2.60–3.78), and non-vascular dementia 2.72, CI 2.55–2.90). Findings of this single study suggest that FMS may contribute significantly to the development of dementia and its common subtypes. However, while this large cohort study has a number of strengths, information is lacking on certain demographic characteristics, lifestyle factors, and medications associated with cognitive risk, as well as on cognitive trajectory and pain symptoms. In addition to generalizability concerns, additional limitations include short follow-up times for a subset of participants, as well as the possibility of misclassification of incident dementia in those diagnosed shortly after identification of FMS.
Headache (migraine and non-migrainous) (N = 6 studies)
A total of six published longitudinal studies assessing the association of headache to incident dementia met our inclusion criteria (total N = 319,023 adults, 4,704 with incident ADRD) (Table 1). These included three large, population-based retrospective matched cohort studies of Taiwanese nationals, all using data from the NHIRD-LHID [90, 91, 93], two large cohort studies (one retrospective matched cohort and one prospective cohort) of Norwegian adults [92, 95], and a small retrospective cohort study of Canadian elders [89]. Of these, three investigations examined the relationship of any headache (including tension-type and migraine) to incident dementia [90, 92] and/or dementia subtypes [90, 95]; all suggested increased risk. In their retrospective matched cohort study of 14,480 Taiwanese nationals (603 incident ADRD), Tzeng and colleagues found adults with any newly diagnosed primary headache disorder to be at over two-fold increased risk for both incident dementia (AHR = 2.05, CI 1.71–2.46) and new onset non-vascular dementia (AHR = 2.11, CI 1.75–2.55) in competing risk analyses adjusted for a wide range of potential confounders (Table 2). Likewise, in a prospective study of 51,383 Norwegian adults (378 incident dementia cases) using linked Nord-Trondelag Health Survey (HUNT2, 1995–7) and Dementia Registry data (1997–2010), Hagen et al. reported a similarly increased risk for vascular and mixed dementia (HRs adjusted for age, sex education, and depressive symptoms = 2.3 (1.4–3.8) and 2.0 (CI 1.1–3.5), respectively) in those with self-reported headache at baseline [95]. In contrast, the authors indicated only a modest elevation in risk for all cause dementia (OR adjusted for age and sex = 1.3, CI 1.1–1.7). A subsequent study restricted to survey participants who also completed the next wave (HUNT3) (N = 15,601 adults, 746 with incident dementia) yielded comparable findings after adjustment for age, sex, education, depressive symptoms, and severe comorbid conditions (AOR for dementia in those who reported any headache at baseline = 1.24, CI 1.94–1.49) [92]. While reasons for the differing risk estimates for dementia is unknown, discrepancies may reflect differences in sample size, study population and design, follow-up period, ascertainment of headache and dementia, analysis and included covariates, and other factors.
Five studies assessed the relation of specific headache subtype, including tension-type [90, 91]/non-migrainous [95] and migraine headache [89, 90, 93] to risk for ADRD [89, 90, 93] and/or specific ADRD subtypes [89–91] [95] (Tables 1 and 2). In a large retrospective cohort study of Taiwanese adults, newly diagnosed tension-type headache was associated with significantly increased risk for both incident dementia (AHR = 1.77, CI 1.41–2.22) and non-vascular dementia (AHR = 1.88, CI 1.49–2.37) after adjustment for multiple potential confounders [90]. A second, larger Taiwanese study using the same data source likewise indicated significantly elevated risk for all-cause and non-vascular dementia in those with tension-type headache, although reported risk estimates were more modest (AHR = 1.15 (1.05–1.27) and 1.21 (1.09–1.34) [91]. In their prospective study of Norwegian adults, Hagen et al. also noted increased risk for vascular dementia in adults reporting non-migrainous headache at baseline (AHR = 2.1 (1.2–3.7) [95].
Similarly, all three studies assessing the relation of migraine to overall dementia risk indicated significant positive associations, although risk estimates again varied among studies (Table 2). For example, reported AHRs for dementia in those with newly diagnosed migraine varied from 1.33 (1.22–1.46) to 2.00 (1.57–2.53) in two large retrospective cohort studies of Taiwanese nationals [93], whereas a small retrospective cohort study of Manitoba residents indicated a 3-fold increased risk in those with self-reported migraine at baseline (AOR adjusted for age, education, and stroke = 2.97, CI 1.25–6.61) [89]. Three studies also provided support for a potential link between migraine and the development of other forms of dementia, including non-vascular dementia [89, 90] and vascular dementia [95], although findings varied across studies (Table 2). Notably, of the four studies [89–91, 95] that examined the relation of any headache to incident dementia subtypes, only one reported a significant association of headache to vascular dementia [95], and only one indicated an increased risk for AD [89].
Collectively, these findings support a link between headache and subsequent risk for ADRD, with two studies suggesting an increased risk for incident vascular dementia in those diagnosed with headache at baseline.
Association of chronic pain symptoms to cognitive decline, cognitive impairment, and dementia
We identified five studies using longitudinal data to investigate the relation of chronic pain symptoms to subsequent cognitive decline [99, 101], new onset CI [97], or incident dementia [96, 98]/dementia probability [99]; three were conducted in U.S. populations [97–99], with the remaining two studies based on data from Japanese [96] and British [101] cohorts. While providing support overall for a link between chronic pain and risk for adverse cognitive outcomes, findings differed across studies, likely due in part to discrepancies in study design, population, measures, length of follow-up, attrition, and other factors. For example, a survey study of 10,065 older U.S. adults followed for up to 12 years, indicated a modest association between persistent pain and acceleration in both overall cognitive decline and probability of dementia. Broadly consistent with these findings, pain interference, although not pain intensity, was associated with significantly increased risk for dementia in a retrospective cohort study of 1,114 adults (114 new dementia cases) followed for an average of 4.4 years [98]. Conversely, studies in a representative sample of British adults (N = 6,515) [101] and a small U.S. cohort (N = 441) [97] followed an average of 2.75 [97] to 4 years [101] suggested a link only between high pain severity and deterioration in memory but not in other cognitive domains. A recent investigation of 14,627 elderly Japanese applicants to a national long term care program followed up to 3 years (482 with incident dementia), self-reported knee pain, but not back pain at baseline were associated with significantly increased risk for incident dementia after adjustment for demographics, lifestyle factors, social interaction, and certain physical and mental health conditions (AHR = 1.32, CI 1.06–1.64) [96].
While collectively, these studies suggest that chronic pain may accelerate cognitive decline and increase risk for CI and dementia, findings remain somewhat mixed and several factors limit conclusions. The latter include small sample sizes [97, 98] as well as the lack of adjustment for certain lifestyle characteristics and comorbidities associated with chronic pain and ADRD, potential self-report and/or survival bias, and the relatively short follow-up periods characterizing most studies. Pain was ascertained based on a single question in 3 of the 5 studies identified; in the one study reporting largely negative findings, associations may have been attenuated by adjustment for chronic pain conditions—notably arthritis, the major contributor to pain in this population, as well as by attrition bias and other factors.
DISCUSSION
Together, findings of studies to date suggest that chronic pain and certain chronic pain conditions may increase risk for the development of ADRD. Of the 10 studies assessing the relation of OA [87, 94, 100], FMS [88], or headache [89–91, 95] [91–93] to risk for incident ADRD, all have yielded significant positive findings despite variation in study design, population, length of follow-up, and methodology. Specifically, as detailed above, recent large retrospective matched cohort studies in Taiwanese nationals [87, 88, 90, 91, 93, 100], a retrospective cohort study of U.S. Medicare beneficiaries [94], prospective cohort studies in Norwegian adults [92, 95], and a small retrospective cohort investigation of Manitoba residents [89] reported significantly increased risk for incident all cause dementia in those diagnosed with OA [87, 94, 100], FMS [88], and headache [89–93, 95] after adjustment for demographics, comorbidities, and other factors. While longitudinal investigations examining the relation of chronic pain symptoms to adverse cognitive outcomes remain few, and studies have varied widely in design, study population, measures, length of follow-up and other factors, overall findings likewise suggest that persistent pain [99], severe chronic pain [97, 101], and/or reported pain interference [98] may predict subsequent deterioration in memory [97, 101], accelerated cognitive decline [99], and dementia [98, 99].
In contrast, findings regarding the association of chronic pain conditions to specific forms of dementia have been mixed. For example, while a large retrospective cohort study of Taiwanese nationals reported significantly elevated risk for incident AD in older adults with FMS [88], the single study of OA and AD risk suggested no relation [86], nor did three of the four studies of headache that included AD as an outcome [90, 91, 95]. Likewise, of the five studies examining the association of FMS [88] or headache [89–91, 95] to incident vascular dementia, two studies reported significantly increased risk for vascular dementia in association with newly diagnosed FMS [88] or self-reported headache [95] at baseline, whereas the remaining three studies, including two in large samples of Taiwanese nationals [90, 91], found no evidence for an association of any headache type to vascular dementia risk [89–91].
In brief, as detailed above, findings of existing published studies of longitudinal data suggest that chronic pain and specific chronic pain conditions may contribute significantly to the subsequent deterioration in cognitive function and conversion to ADRD. However, conclusions are limited by a number of concerns. These include heterogeneity in study design, population, and methodology, as well as the short duration of follow-up, small sample sizes, potential self-report, detection, survival or other attrition bias, and/or incomplete adjustment for potentially confounding factors characterizing most studies. While external validity can generally be considered high in those studies based on large, nationally, or regionally representative samples, relatively low response rates, high attrition, and/or high missing data rates may have led to selection bias in some cohort studies, potentially influencing both internal and external validity of findings.
Future directions for research and preliminary conceptual model
Research regarding the relation of chronic pain and chronic pain conditions to subsequent cognitive decline and conversion to CI and dementia remains relatively sparse, and that regarding the potential relation of certain chronic pain conditions (i.e., FMS) or specific chronic pain manifestations (e.g., chronic knee or back pain) to any cognitive outcome is currently restricted to single studies. To our knowledge, no investigations have yet examined the potential link between certain common chronic pain manifestations, including neck pain and neuropathic pain, to any adverse cognitive outcome or examined the relation of any pain condition to the entire cognitive trajectory, from cognitive decline to dementia. Moreover, of the 11 studies examining the association of common chronic pain conditions to incident dementia, 7 were conducted in Taiwan using the same data source. Additional rigorous prospective studies in other regions and populations are thus clearly needed to confirm and extend the findings detailed above. These include studies designed to: investigate the independent influence of specific pain conditions and associated chronic pain symptoms on subsequent cognitive decline, new onset CI, and conversion to dementia; assess the effects of pain chronicity, intensity/severity, and overall burden (including sites affected) on these associations; evaluate the potential mediating role of mood impairment, sleep deficits, and other psychosocial factors; assess the possible modifying effects of these and other ADRD risk factors on the relation of chronic pain and chronic pain conditions to adverse cognitive outcomes; identify underlying mechanisms and, and ultimately, explore the potential role of effective chronic pain management approaches in buffering the putative adverse long term effects of chronic pain on cognitive function.
Moreover, given that the development of ADRD is insidious and that both chronic pain and ADRD are influenced by a constellation of interacting factors, large, long term prospective studies that take into account the role of the multiple potentially confounding and mediating factors are clearly needed. Figure 2 provides a preliminary conceptual model to help guide future epidemiologic research, illustrating specific key interacting pathways by which chronic pain and chronic pain conditions may contribute to cognitive decline and the development of CI and dementia and the factors linked to both chronic pain and ADRD risk that warrant consideration in causal models. While other psychosocial factors, including pain catastrophizing, self-efficacy, and coping strategies may also play a mediating role, emphasis in this conceptual model is on those factors for which information is generally available in large clinical, government, insurance, and other existing databases.
Fig. 2.

Conceptual Framework: Possible pathways by which chronic pain and chronic pain conditions may contribute to the development of cognitive decline, cognitive impairment, and ADRD. Hx, history; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; ADRD, Alzheimer’s disease and related dementias.
Link between chronic pain and adverse cognitive outcomes: Potential explanatory factors and underlying mechanisms
As illustrated in Fig. 2, chronic pain and chronic pain conditions may influence cognitive outcomes via multiple pathways. As summarized below, these pathways include both non-modifiable and modifiable factors linked to chronic pain and ADRD risk and are complex, interacting, and multidimensional.
Non-modifiable factors include increasing age and female gender, factors strongly and positively linked to risk for both chronic pain [15, 102] and dementia [3], as well as to certain risk factors for these conditions [3, 103–107]. In addition, black adults have been reported to be at elevated risk for ADRD [3] and to experience more frequent, severe, and disabling pain [108] relative to Caucasians, although other studies have found little or no evidence for this association after adjustment for socioeconomic status [15, 102].
Modifiable factors include multiple demographic/socioeconomic, lifestyle, and health-related risk factors. As illustrated in Fig. 2, most of the relationships of these factors to chronic pain and ADRD risk are likely reciprocal. Moreover, many of these risk factors may interact in a synergistic fashion to exacerbate risk for both pain and ADRD, contributing to a vicious cycle of increasing pain and pain-related dysfunction, deteriorating mood and sleep, reduced social engagement and physical activity, declining socioeconomic status and physical health, and increased use of medications, including those with documented adverse effects on cognitive health. Key demographic/socioeconomic factors include low educational level, poverty, unemployment, and social deprivation, which have been associated with significantly increased risk both for ADRD [3] and for chronic pain and specific chronic pain conditions [15, 102].
Lifestyle factors linked to increased risk for chronic pain and/or chronic pain disability include obesity [15, 109], physical inactivity [15, 109], poor diet [109], and social isolation/loneliness [110–112], as well as smoking [109], alcohol misuse [15], and substance abuse [113–115], factors which can contribute directly or indirectly to elevated risk for cognitive decline and the development of dementia [3, 116]. Moreover, most of the relationships between lifestyle factors and chronic pain/chronic pain disability are likely bidirectional [117, 118].
A number of physical and mental health conditions have also been linked to chronic pain and pain-related disability. These include diabetes [119–121], hypertension [122, 123], cardiovascular disease [15], COPD [15], and history of TBI [85] and stroke [124, 125], as well as multimorbidity [15]. As detailed above, these conditions have, in turn, been associated with increased ADRD risk [3, 20, 23, 46–52]. In addition, chronic stress [109], depression [15, 126–128], anxiety [15, 126, 128], and sleep impairment [129, 130] can both result from and exacerbate chronic pain. These factors have also been significantly associated with cognitive decline [33, 35, 131, 132] and incident dementia [35, 42, 94, 132–135], and may in part mediate the observed relationships between chronic pain and the subsequent accelerated deterioration in cognitive function and development of ADRD [83, 94]. Finally, chronic pain often leads to the use of certain medications, including analgesics, benzodiazepines, and specific anti-depressants, factors which have been linked to ADRD risk in some [55, 56], although not all studies [56–58]. Chronic pain has also been associated with polypharmacy [136–138], an emerging risk factor for dementia [61].
Link between chronic pain and adverse cognitive outcomes: Possible underlying mechanisms
As noted above, chronic pain can also have profound effects on neurocognitive function, with potentially significant implications for the development of CI and conversion to ADRD. In a recent review of 53 studies of cognitive performance in chronic pain populations, the authors concluded that chronic pain was related to deficits in several key neurocognitive domains, including memory, attention, and processing speed, and, albeit less consistently, executive functioning [85]. The neural systems involved in memory and cognition are closely linked to those involved in pain processing and likely affect one another reciprocally [76, 80], disrupting cognitive processing and contributing to a vicious cycle of continuing pain, adverse neurostructural changes, and deteriorating cognitive function [83]. Patients with chronic pain show changes in brain morphology paralleling those implicated in cognitive decline and CI, including grey matter volume reduction in the hippocampus, insular cortex, anterior cingulate cortex, thalamus, prefrontal cortex, and other brain regions involved in attention, memory consolidation, and cognitive processing [76, 80–84, 139]. Chronic pain has also been shown to disrupt specific brain networks critical to normal cognitive performance [82]. As noted above, these alterations are thought to help explain the deterioration in cognitive function documented in many chronic pain populations [76, 83, 85], deterioration which may ultimately contribute to accelerated cognitive decline and the development of CI and ADRD.
Chronic pain and chronic pain conditions may contribute to ADRD pathogenesis via other pathways as well. For example, OA and other chronic pain conditions have been associated with activation of central pain processing pathways [140, 141] and central nervous system hypersensitivity [141, 142], factors linked to disruption of cognitive functioning [76, 143]. Both systemic and peripheral inflammation may also play a role. For example, systemic increases in high-sensitivity C-reactive protein, interleukin-6, and other proinflammatory mediators have been implicated in the development and progression of both specific chronic pain conditions (e.g., OA, FMS) and CI [141, 144]. Likewise, local proinflammatory changes associated with chronic pain conditions have been linked to neuroinflammation [141, 144], cerebrovascular dysfunction [144], and adverse nociceptive and structural changes in the brain [141, 144, 145], alterations that may impair cognitive processing and promote cognitive dysfunction [141, 144, 146]. Chronic pain may also contribute to ADRD risk via adverse effects on cellular aging. For example, OA [147] and other chronic pain conditions [148] have been associated with shortened telomere length, a marker of accelerated cellular aging that has been linked to increased risk for ADRD [149–151].
Strengths and limitations
Our review included only studies that used longitudinal data, reported a minimum of 50 outcome cases, and that adjusted, at a minimum, for age and gender, helping to increase confidence in findings. In the tables provided, we include information on multiple domains of study quality, and take into account design and methodological limitations in our discussion of findings. However, this systematic review also has several potential limitations that warrant consideration. While studies were identified using a comprehensive, multi-pronged search strategy, our review was restricted to investigations published in English, potentially introducing bias. However, we did not identify any research published in non-English languages that met our inclusion criteria, reducing this likelihood. Publication bias may have resulted in disproportionate capture of studies reporting positive findings. However, pain is not yet an established risk factor for cognitive decline or ADRD, rendering this possibility less likely. Studies have, to date, been conducted in only a handful of countries, and the majority (7 of 11) of studies investigating the association of included chronic pain conditions were conducted in Taiwanese nationals using the same primary data source. It thus remains unclear if the findings discussed in the current review are generalizable to other populations, cultures, and/or regions. While overall, the investigations published to date have yielded results supportive of a link between chronic pain and the development of cognitive decline and dementia, the considerable heterogeneity in study populations (notably, those assessing the association of chronic pain symptoms to cognitive outcomes), design, follow-up periods, measures and ascertainment of outcome and explanatory variables, analysis, and other factors render direct comparisons across studies challenging. As detailed above, conclusions are limited by a number of design and/or methodological concerns characterizing the majority of studies, including small sample sizes; relatively short follow-up periods; lack of adjustment for the potential confounding influence of specific demographic, lifestyle, and health-related factors associated with both pain and dementia risk; potential selection, self-report, detection, and/or survival bias; and/or lack of information on pain chronicity, duration, intensity or other indicators of chronic pain burden.
Conclusions
Published case-control and cohort investigations to date suggest chronic pain and certain chronic pain conditions may increase risk for accelerated cognitive decline, new onset CI, and incident ADRD. However, research remains sparse, and interpretation of existing findings is hampered by the methodological and other limitations characterizing most studies. The relation of chronic pain and chronic pain conditions to specific forms of dementia also remains unclear. Additional large prospective investigations that address these concerns are needed to confirm and extend the findings of current studies, to elucidate the contributions of chronic pain and chronic pain conditions on the entire cognitive trajectory, to clarify the relation of chronic pain to specific dementia types, and to explore potential mediating factors and identify underlying mechanisms.
ACKNOWLEDGMENTS
This work was performed at West Virginia University, and was supported in part by the Alzheimer’s Research and Prevention Foundation and the National Institute of General Medical Sciences of the National Institutes of Health (Award Number 2U54GM104942). The contents are solely the responsibility of the authors and do not represent the official views of the Alzheimer’s Research and Prevention Foundation or the National Institutes of Health.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0960r1).
REFERENCES
- [1].Abubakar I, Tillmann T, Banerjee A (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, Abyu GY, Ahmed MB, Aichour AN, Aichour I (2017) Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16, 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alzheimer’s Association (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15 321–387. [Google Scholar]
- [4].Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H, Fratiglioni L, Frisoni GB, Gauthier S, Georges J, Graff C, Iqbal K, Jessen F, Johansson G, Jonsson L, Kivipelto M, Knapp M, Mangialasche F, Melis R, Nordberg A, Rikkert MO, Qiu C, Sakmar TP, Scheltens P, Schneider LS, Sperling R, Tjernberg LO, Waldemar G, Wimo A, Zetterberg H (2016) Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol 15, 455–532. [DOI] [PubMed] [Google Scholar]
- [5].Verlinden VJ, van der Geest JN, de Bruijn RF, Hofman A, Koudstaal PJ, Ikram MA (2016) Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement 12, 144–153. [DOI] [PubMed] [Google Scholar]
- [6].Pickett J, Bird C, Ballard C, Banerjee S, Brayne C, Cowan K, Clare L, Comas-Herrera A, Corner L, Daley S, Knapp M, Lafortune L, Livingston G, Manthorpe J, Marchant N, Moriarty J, Robinson L, van Lynden C, Windle G, Woods B, Gray K, Walton C (2018) A roadmap to advance dementia research in prevention, diagnosis, intervention, and care by 2025. Int J Geriatr Psychiatry 33, 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kulmala J, Ngandu T, Kivipelto M (2018) Prevention matters: Time for global action and effective implementation. J Alzheimers Dis 64, S191–S198. [DOI] [PubMed] [Google Scholar]
- [8].Imtiaz B, Tolppanen AM, Kivipelto M, Soininen H (2014) Future directions in Alzheimer’s disease from risk factors to prevention. Biochem Pharmacol 88, 661–670. [DOI] [PubMed] [Google Scholar]
- [9].Kivipelto M, Mangialasche F, Ngandu T (2018) Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 14, 653–666. [DOI] [PubMed] [Google Scholar]
- [10].Brodaty H, Heffernan M, Fiatarone Singh MA, Valenzuela M, Andrews G, Lautenschlager NT, Anstey KJ, Maeder A, Jorm LR, McNeil J (2017) Maintain your brain: A randomised controlled trial of an internet-based multi-component lifestyle intervention to prevent cognitive decline and dementia. Alzheimers Dement 13, P1216–P1216. [Google Scholar]
- [11].Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S, European Prevention of Alzheimer’s Dementia Consortium (2016) Development of interventions for the secondary prevention of Alzheimer’s dementia: The European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry 3, 179–186. [DOI] [PubMed] [Google Scholar]
- [12].Bott N, Kumar S, Krebs C, Glenn JM, Madero EN, Juusola JL (2018) A remote intervention to prevent or delay cognitive impairment in older adults: Design, recruitment, and baseline characteristics of the Virtual Cognitive Health (VC Health) Study. JMIR Res Protoc 7, e11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L (2012) Dementia prevention: Current epidemiological evidence and future perspective. Alzheimers Res Ther 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rasmussen KL, Tybjarg-Hansen A, Nordestgaard BG, Frikke-Schmidt R (2018) Absolute 10-year risk of dementia by age, sex and APOE genotype: A population-based cohort study. CMAJ 190, E1033–E1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mills SEE, Nicolson KP, Smith BH (2019) Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br J Anaesth 123, e273–e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee Y, Back JH, Kim J, Byeon H (2010) Multiple socioeconomic risks and cognitive impairment in older adults. Dement Geriatr Cogn Disord 29, 523–529. [DOI] [PubMed] [Google Scholar]
- [17].Meng X, D’Arcy C (2012) Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One 7, e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leist AK, Glymour MM, Mackenbach JP, van Lenthe FJ, Avendano M (2013) Time away from work predicts later cognitive function: Differences by activity during leave. Ann Epidemiol 23, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li G, Shen YC, Chen CH, Zhau YW, Li SR, Lu M (1991) A three-year follow-up study of age-related dementia in an urban area of Beijing. Acta Psychiatr Scand 83, 99–104. [DOI] [PubMed] [Google Scholar]
- [20].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11, 718–726. [DOI] [PubMed] [Google Scholar]
- [21].Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y (2015) Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 10, e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cao L, Tan L, Wang H-F, Jiang T, Zhu X-C, Lu H, Tan M-S, Yu J-T (2016) Dietary patterns and risk of dementia: A systematic review and meta-analysis of cohort studies. Mol Neurobiol 53, 6144–6154. [DOI] [PubMed] [Google Scholar]
- [23].Mayer F, Di Pucchio A, Lacorte E, Bacigalupo I, Marzolini F, Ferrante G, Minardi V, Masocco M, Canevelli M, Di Fiandra T, Vanacore N (2018) An estimate of attributable cases of Alzheimer disease and vascular dementia due to modifiable risk factors: The impact of primary prevention in Europe and in Italy. Dement Geriatr Cogn Dis Extra 8, 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014) Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol 13, 788–794. [DOI] [PubMed] [Google Scholar]
- [25].Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, Smidt N (2015) Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 22, 39–57. [DOI] [PubMed] [Google Scholar]
- [26].Zhao C, Noble JM, Marder K, Hartman JS, Gu Y, Scarmeas N (2018) Dietary patterns, physical activity, sleep, and risk for dementia and cognitive decline. Curr Nutr Rep 7, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mortimer JA, Stern Y (2019) Physical exercise and activity may be important in reducing dementia risk at any age. Neurology 92, 362–363. [DOI] [PubMed] [Google Scholar]
- [28].Nguyen JCD, Killcross AS, Jenkins TA (2014) Obesity and cognitive decline: Role of inflammation and vascular changes. Front Neurosci 8, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sellbom KS, Gunstad J (2012) Cognitive function and decline in obesity. J Alzheimers Dis 30(Suppl 2), S89–95. [DOI] [PubMed] [Google Scholar]
- [30].Mortamais M, Abdennour M, Bergua V, Tzourio C, Berr C, Gabelle A, Akbaraly TN (2018) Anxiety and 10-year risk of incident dementia-an association shaped by depressive symptoms: Results of the Prospective Three-City Study. Front Neurosci 12, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ford E, Greenslade N, Paudyal P, Bremner S, Smith HE, Banerjee S, Sadhwani S, Rooney P, Oliver S, Cassell J (2018) Predicting dementia from primary care records: A systematic review and meta-analysis. PLoS One 13, e0194735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cherbuin N, Kim S, Anstey KJ (2015) Dementia risk estimates associated with measures of depression: A systematic review and meta-analysis. BMJ Open 5, e008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mourao RJ, Mansur G, Malloy-Diniz LF, Castro Costa E, Diniz BS (2016) Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: Systematic review and meta-analysis. Int J Geriatr Psychiatry 31, 905–911. [DOI] [PubMed] [Google Scholar]
- [34].Gallagher D, Kiss A, Lanctot K, Herrmann N (2016) Depressive symptoms and cognitive decline: A longitudinal analysis of potentially modifiable risk factors in community dwelling older adults. J Affect Disord 190, 235–240. [DOI] [PubMed] [Google Scholar]
- [35].Gulpers B, Ramakers I, Hamel R, Kohler S, Oude Voshaar R, Verhey F (2016) Anxiety as a predictor for cognitive decline and dementia: A systematic review and meta-analysis. Am J Geriatr Psychiatry 24, 823–842. [DOI] [PubMed] [Google Scholar]
- [36].Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Pedersen NL, Gatz M (2016) Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimers Dement 12, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, Kluse M, Marmar C (2010) Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry 67, 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mawanda F, Wallace RB, McCoy K, Abrams TE (2017) PTSD, psychotropic medication use, and the risk of dementia among US veterans: A retrospective cohort study. J Am Geriatr Soc 65, 1043–1050. [DOI] [PubMed] [Google Scholar]
- [39].Flatt JD, Gilsanz P, Quesenberry CP Jr, Albers KB, Whitmer RA (2018) Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimers Dement 14, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Johansson L, Guo XX, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I (2010) Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain 133, 2217–2224. [DOI] [PubMed] [Google Scholar]
- [41].Kessing LV, Nilsson FM (2003) Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J Affect Disord 73, 261–269. [DOI] [PubMed] [Google Scholar]
- [42].Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, Shi J, Vitiello MV, Lu L (2018) Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 40, 4–16. [DOI] [PubMed] [Google Scholar]
- [43].Fann JR, Ribe AR, Pedersen HS, Fenger-Gron M, Christensen J, Benros ME, Vestergaard M (2018) Long-term risk of dementia among people with traumatic brain injury in Denmark: A population-based observational cohort study. Lancet Psychiatry 5, 424–431. [DOI] [PubMed] [Google Scholar]
- [44].Nordstrom A, Nordstrom P (2018) Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Med 15, e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, Tian G (2017) Head injury as a risk factor for dementia and Alzheimer’s disease: A systematic review and meta-analysis of 32 observational studies. PLoS One 12, e0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pal K, Mukadam N, Petersen I, Cooper C (2018) Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 53, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Biessels GJ, Despa F (2018) Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat Rev Endocrinol 14, 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Qiu C, Fratiglioni L (2015) A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 12, 267–277. [DOI] [PubMed] [Google Scholar]
- [49].Adelborg K, Horvath-Puho E, Ording A, Pedersen L, Toft Sorensen H, Henderson VW (2017) Heart failure and risk of dementia: A Danish nationwide population-based cohort study. Eur J Heart Fail 19, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kakkera K, Padala KP, Kodali M, Padala PR (2018) Association of chronic obstructive pulmonary disease with mild cognitive impairment and dementia. Curr Opin Pulm Med 24, 173–178. [DOI] [PubMed] [Google Scholar]
- [51].Vassilaki M, Aakre JA, Cha RH, Kremers WK, St Sauver JL, Mielke MM, Geda YE, Machulda MM, Knopman DS, Petersen RC, Roberts RO (2015) Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 63, 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fabbri E, An Y, Zoli M, Tanaka T, Simonsick EM, Kitner-Triolo MH, Studenski SA, Resnick SM, Ferrucci L (2016) Association of accelerated multi-morbidity and age-related cognitive decline in older, non-demented participants from the Baltimore longitudinal study of aging. J Am Geriatr Soc 64, 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Poly TN, Islam MM, Walther BA, Yang H-C, Wu C-C, Lin M-C, Li Y-C (2020) Association between use of statin and risk of dementia: A meta-analysis of observational studies. Neuroepidemiology 54, 214–226. [DOI] [PubMed] [Google Scholar]
- [54].Chu C-S, Tseng P-T, Stubbs B, Chen T-Y, Tang C-H, Li D-J, Yang W-C, Chen Y-W, Wu C-K, Veronese N (2018) Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci Rep 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E (2017) Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement 13, 406–418. [DOI] [PubMed] [Google Scholar]
- [56].Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, Poly TN, Masud JHB, Li YC, Shabbir SA (2016) Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis. Neuroepidemiology 47, 181–191. [DOI] [PubMed] [Google Scholar]
- [57].Baek YH, Lee H, Kim WJ, Chung JE, Pratt N, Ellett LK, Shin JY (2020) Uncertain association between benzodiazepine use and the risk of dementia: A cohort study. J Am Med Dir Assoc 21, 201–211.e202. [DOI] [PubMed] [Google Scholar]
- [58].Richardson K, Mattishent K, Loke YK, Steel N, Fox C, Grossi CM, Bennett K, Maidment I, Boustani M, Matthews FE, Myint PK, Campbell NL, Brayne C, Robinson L, Savva GM (2019) History of benzodiazepine prescriptions and risk of dementia: Possible bias due to prevalent users and covariate measurement timing in a nested case-control study. Am J Epidemiol 188, 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vetrano DL, Villani ER, Grande G, Giovannini S, Cipriani MC, Manes-Gravina E, Bernabei R, Onder G (2018) Association of polypharmacy with 1-year trajectories of cognitive and physical function in nursing home residents: Results from a multicenter European study. J Am Med Dir Assoc 19, 710–713. [DOI] [PubMed] [Google Scholar]
- [60].Park H-Y, Park J-W, Song HJ, Sohn HS, Kwon J-W (2017) The association between polypharmacy and dementia: A nested case-control study based on a 12-year longitudinal cohort database in South Korea. PLoS One 12, e0169463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Leelakanok N, D’Cunha RR (2019) Association between polypharmacy and dementia–A systematic review and metaanalysis. Aging Ment Health 23, 932–941. [DOI] [PubMed] [Google Scholar]
- [62].Foundation Arthritis (2019) Arthritis by the numbers-book of trusted facts & figures. Arthritis Foundation 3, 1–100. [Google Scholar]
- [63].Maiese K (2016) Picking a bone with WISP1 (CCN4): New strategies against degenerative joint disease. J Transl Sci 1, 83–85. [PMC free article] [PubMed] [Google Scholar]
- [64].Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, Bruyere O, Guillemin F, Hochberg MC, Hunter DJ, Kanis JA, Kvien TK, Laslop A, Pelletier J-P, Pinto D, Reiter-Niesert S, Rizzoli R, Rovati LC, Severens JLH, Silverman S, Tsouderos Y, Tugwell P, Reginster J-Y (2013) Health economics in the field of osteoarthritis: An expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 43, 303–313. [DOI] [PubMed] [Google Scholar]
- [65].Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L (2014) The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis 73, 1323–1330. [DOI] [PubMed] [Google Scholar]
- [66].Domenichiello AF, Ramsden CE (2019) The silent epidemic of chronic pain in older adults. Prog Neuropsychopharmacol Biol Psychiatry 93, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C (2018) Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR 67, 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dorner TE (2018) Pain and chronic pain epidemiology: Implications for clinical and public health fields. Wien Klin Wochenschr 130, 1–3. [DOI] [PubMed] [Google Scholar]
- [69].Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH (2010) The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain 11, 1230–1239. [DOI] [PubMed] [Google Scholar]
- [70].Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Anger-meyer MC, Borges GLG, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine J-P, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M (2008) Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain 9, 883–891. [DOI] [PubMed] [Google Scholar]
- [71].Nahin RL, Sayer B, Stussman BJ, Feinberg TM (2019) Eighteen-year trends in the prevalence of, and health care use for, noncancer pain in the United States: Data from the Medical Expenditure Panel Survey. J Pain 20, 796–809. [DOI] [PubMed] [Google Scholar]
- [72].Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT (2016) Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 6, e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Henschke N, Kamper SJ, Maher CG (2015) The epidemiology and economic consequences of pain. Mayo Clinic Proc 90, 139–147. [DOI] [PubMed] [Google Scholar]
- [74].Gaskin DJ, Richard P (2012) The economic costs of pain in the United States. J Pain 13, 715–724. [DOI] [PubMed] [Google Scholar]
- [75].Chinn S, Chuang E, Gritsenko K (2017) Epidemiology. In Pain Medicine: An Essential Review, Yong RJ, Nguyen M, Nelson E, Urman RD, eds. Springer, Switzerland, pp. 27–29. [Google Scholar]
- [76].Moriarty O, McGuire BE, Finn DP (2011) The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol 93, 385–404. [DOI] [PubMed] [Google Scholar]
- [77].Breivik H, Eisenberg E, O’Brien T, Openminds, Allegri M, Jaksch W, Kress H, Simpson K, Zylicz Z (2013) The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 13, 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Patel AS, Farquharson R, Carroll D, Moore A, Phillips CJ, Taylor RS, Barden J (2012) The impact and burden of chronic pain in the workplace: A qualitative systematic review. Pain Pract 12, 578–589. [DOI] [PubMed] [Google Scholar]
- [79].GBD 2013 DALYs and HALE Collaborators, Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah, Abera SF, Aboyans V, Abraham JP, Abubakar I, et al. (2015) Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 386, 2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Seminowicz DA, Ceko M (2015) Can we exploit cognitive brain networks to treat chronic pain? Pain Manag 5, 399–402. [DOI] [PubMed] [Google Scholar]
- [81].Apkarian AV, Hashmi JA, Baliki MN (2011) Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 152, S49–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DM (2014) Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. Neuroimage Clin 4, 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Innes KE, Sambamoorthi U (2018) The association of perceived memory loss with osteoarthritis and related joint pain in a large Appalachian population. Pain Med 9, 1340–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vachon-Presseau E, Roy M, Martel M-O, Caron E, Marin M-F, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P (2013) The stress model of chronic pain: Evidence from basal cortisol and hippocampal structure and function in humans. Brain 136, 815–827. [DOI] [PubMed] [Google Scholar]
- [85].Higgins DM, Martin AM, Baker DG, Vasterling JJ, Risbrough V (2018) The relationship between chronic pain and neurocognitive function: A systematic review. Clin J Pain 34, 262–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang J-H, Wu Y-J, Tee BL, Lo RY (2018) Medical comorbidity in Alzheimer’s disease: A nested case-control study. J Alzheimers Dis 63, 773–781. [DOI] [PubMed] [Google Scholar]
- [87].Huang S-W, Wang W-T, Chou L-C, Liao C-D, Liou T-H, Lin H-W (2015) Osteoarthritis increases the risk of dementia: A nationwide cohort study in Taiwan. Sci Rep 5, 10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tzeng NS, Chung CH, Liu FC, Chiu YH, Chang HA, Yeh CB, Huang SY, Lu RB, Yeh HW, Kao YC, Chiang WS, Tsao CH, Wu YF, Chou YC, Lin FH, Chien WC (2018) Fibromyalgia and risk of dementia-a nation-wide, population-based, cohort study. Am J Med Sci 355, 153–161. [DOI] [PubMed] [Google Scholar]
- [89].Morton RE, St John PD, Tyas SL (2019) Migraine and the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia: A prospective cohort study in community-dwelling older adults. Int J Geriatr Psychiatry 34, 1667–1676. [DOI] [PubMed] [Google Scholar]
- [90].Tzeng N-S, Chung C-H, Lin F-H, Yeh C-B, Huang S-Y, Lu R-B, Chang H-A, Kao Y-C, Chiang W-S, Chou Y-C, Tsao C-H, Wu Y-F, Chien W-C (2017) Headaches and risk of dementia. Am J Med Sci 353, 197–206. [DOI] [PubMed] [Google Scholar]
- [91].Yang FC, Lin TY, Chen HJ, Lee JT, Lin CC, Kao CH (2016) Increased risk of dementia in patients with tension-type headache: A nationwide retrospective population-based cohort study. Plos One 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Røttereng AKS, Bosnes O, Stordal E, Zwart J-A, Linde M, Stovner LJ, Hagen K (2015) Headache as a predictor for dementia: The HUNT Study. J Headache Pain 16, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chuang C-S, Lin C-L, Lin M-C, Sung F-C, Kao C-H (2013) Migraine and risk of dementia: A nationwide retrospective cohort study. Neuroepidemiology 41, 139–145. [DOI] [PubMed] [Google Scholar]
- [94].Innes KE, Sambamoorthi U (2020) The association of osteoarthritis and related pain burden to incident Alzheimer’s disease and related dementias: A retrospective cohort study of US Medicare beneficiaries. J Alzheimers Dis 75, 789–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hagen K, Stordal E, Linde M, Steiner TJ, Zwart J-A, Stovner LJ (2014) Headache as a risk factor for dementia: A prospective population-based study. Cephalalgia 34, 327–335. [DOI] [PubMed] [Google Scholar]
- [96].Yamada K, Kubota Y, Tabuchi T, Shirai K, Iso H, Kondo N, Kondo K (2019) A prospective study of knee pain, low back pain, and risk of dementia: The JAGES project. Sci Rep 9, 10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].van der Leeuw G, Ayers E, Leveille SG, Blankenstein AH, van der Horst HE, Verghese J (2018) The effect of pain on major cognitive impairment in older adults. J Pain 19, 1435–1444. [DOI] [PubMed] [Google Scholar]
- [98].Ezzati A, Wang C, Katz MJ, Derby CA, Zammit AR, Zimmerman ME, Pavlovic JM, Sliwinski MJ, Lipton RB (2019) The temporal relationship between pain intensity and pain interference and incident dementia. Curr Alzheimer Res 16, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, Smith AK (2017) Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med 177, 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen KT, Chen YC, Fan YH, Lin WX, Lin WC, Wang YH, Lin L, Chiou JY, Wei JC (2018) Rheumatic diseases are associated with a higher risk of dementia: A nation-wide, population-based, case-control study. Int J Rheum Dis 21, 373–380. [DOI] [PubMed] [Google Scholar]
- [101].Veronese N, Koyanagi A, Solmi M, Thompson T, Maggi S, Schofield P, Mueller C, Gale CR, Cooper C, Stubbs B (2018) Pain is not associated with cognitive decline in older adults: A four-year longitudinal study. Maturitas 115, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Grol-Prokopczyk H (2017) Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain 158, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kuehner C (2017) Why is depression more common among women than among men? Lancet Psychiatry 4, 146–158. [DOI] [PubMed] [Google Scholar]
- [104].Florindo AA, Guimaraes VV, Cesar CL, Barros MB, Alves MC, Goldbaum M (2009) Epidemiology of leisure, transportation, occupational, and household physical activity: Prevalence and associated factors. J Phys Act Health 6, 625–632. [DOI] [PubMed] [Google Scholar]
- [105].Nusselder WJ, Cambois EM, Wapperom D, Mesle F, Looman CWN, Yokota RTC, Van Oyen H, Jagger C, Robine JM (2019) Women’s excess unhealthy life years: Disentangling the unhealthy life years gap. Eur J Public Health 29, 914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nusselder WJ, Wapperom D, Looman CW, et al. (2019) Contribution of chronic diseases to gender differences in activity limitations. Eur J Public Health 29, 99–104. [DOI] [PubMed] [Google Scholar]
- [107].Nygård M, Härtull C, Wentjärvi A, Jungerstam S (2017) Poverty and old age in Scandinavia: A problem of gendered injustice? Evidence from the 2010 GERDA Survey in Finland and Sweden. Soc Indic Res 132, 681–698. [Google Scholar]
- [108].Aroke EN, Joseph PV, Roy A, Overstreet DS, Tollefsbol TO, Vance DE, Goodin BR (2019) Could epigenetics help explain racial disparities in chronic pain? J Pain Res 12, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nijs J, D’Hondt E, Clarys P, Deliens T, Polli A, Malfliet A, Coppieters I, Willaert W, Tumkaya Yilmaz S, Elma O, Ickmans K (2020) Lifestyle and chronic pain across the lifespan: An inconvenient truth? PM&R 12, 410–419. [DOI] [PubMed] [Google Scholar]
- [110].Karayannis NV, Baumann I, Sturgeon JA, Melloh M, Mackey SC (2019) The impact of social isolation on pain interference: A longitudinal study. Ann Behav Med 53, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Smith T (2017) “On their own”: Social isolation, loneliness and chronic musculoskeletal pain in older adults. Qual Ageing 18, 87–92. [Google Scholar]
- [112].Smith TO, Dainty JR, Williamson E, Martin KR (2019) Association between musculoskeletal pain with social isolation and loneliness: Analysis of the English longitudinal study of ageing. Br J Pain 13, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN (2015) Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain 156, 569–576. [DOI] [PubMed] [Google Scholar]
- [114].Witkiewitz K, Vowles KE (2018) Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: A critical review. Alcohol Clin Exp Res 42, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fishbain DA, Cole B, Lewis JE, Gao J, Rosomoff RS (2009) Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med 10, 829–839. [DOI] [PubMed] [Google Scholar]
- [116].Yarnell S, Li L, MacGrory B, Trevisan L, Kirwin P (2020) Substance use disorders in later life: A review and synthesis of the literature of an emerging public health concern. Am J Ger Psychiatry 28, 226–236. [DOI] [PubMed] [Google Scholar]
- [117].Boakye PA, Olechowski C, Rashiq S, Verrier MJ, Kerr B, Witmans M, Baker G, Joyce A, Dick BD (2016) A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. Clin J Pain 32, 327–336. [DOI] [PubMed] [Google Scholar]
- [118].Shpaner M, Tulipani LJ, Bishop JH, Naylor MR (2017) The vicious cycle of chronic pain in aging requires multidisciplinary non-pharmacological approach to treatment. Curr Behav Neurosci Rep 4, 176–187. [Google Scholar]
- [119].Mantyselka P, Miettola J, Niskanen L, Kumpusalo E (2008) Chronic pain, impaired glucose tolerance and diabetes: A community-based study. Pain 137, 34–40. [DOI] [PubMed] [Google Scholar]
- [120].Bair MJ, Brizendine EJ, Ackermann RT, Shen C, Kroenke K, Marrero DG (2010) Prevalence of pain and association with quality of life, depression and glycaemic control in patients with diabetes. Diabet Med 27, 578–584. [DOI] [PubMed] [Google Scholar]
- [121].Abaraogu UO, Ochi C, Umahi E, Ogbonnaya C, Onah I (2017) Individuals with type 2 diabetes are at higher risk of chronic musculoskeletal pain: A study with diabetes cohort. Int J Diabetes Dev Ctries 37, 267–271. [Google Scholar]
- [122].Bruehl S, Olsen RB, Tronstad C, Sevre K, Burns JW, Schirmer H, Nielsen CS, Stubhaug A, Rosseland LA (2018) Chronic pain-related changes in cardiovascular regulation and impact on comorbid hypertension in a general population: The Tromso study. Pain 159, 119–127. [DOI] [PubMed] [Google Scholar]
- [123].Lin KT, Lai SW, Hsu SD, Fan CY, Wang HJ, Chang PK, Liu W-H, Chen Y-L, Yen I-C, Lee S-Y (2019) Shoulder pain and risk of developing hypertension and cardiovascular disease: A nationwide population-based cohort study in Taiwan. J Med Sci 39, 127. [Google Scholar]
- [124].Delpont B, Blanc C, Osseby GV, Hervieu-Begue M, Giroud M, Bejot Y (2018) Pain after stroke: A review. Rev Neurol 174, 671–674. [DOI] [PubMed] [Google Scholar]
- [125].Tseng CH, Chen JH, Wang YC, Lin MC, Kao CH (2016) Increased risk of stroke in patients with fibromyalgia: A population-based cohort study. Medicine 95, e2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Velly AM, Mohit S (2018) Epidemiology of pain and relation to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 87, 159–167. [DOI] [PubMed] [Google Scholar]
- [127].Sheng JY, Liu S, Wang YC, Cui RJ, Zhang XW (2017) The link between depression and chronic pain: Neural mechanisms in the brain. Neural Plast 2017, 9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Harris ML (2016) Psychological factors in arthritis: Cause or consequence? In Psychosocial Factors in Arthritis: Perspectives on Adjustment and Management, Nicassio PM, ed. Springer International Publishing, pp. 53–77. [Google Scholar]
- [129].Koffel E, McCurry SM, Smith MT, Vitiello MV (2019) Improving pain and sleep in middle-aged and older adults: The promise of behavioral sleep interventions. Pain 160, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Nijs J, Mairesse O, Neu D, Leysen L, Danneels L, Cagnie B, Meeus M, Moens M, Ickmans K, Goubert D (2018) Sleep disturbances in chronic pain: Neurobiology, assessment, and treatment in physical therapist practice. Phys Ther 98, 325–335. [DOI] [PubMed] [Google Scholar]
- [131].Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, Fiocco AJ, Lupien SJ (2011) Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem 96, 583–595. [DOI] [PubMed] [Google Scholar]
- [132].Wu L, Sun D, Tan Y (2018) A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders. Sleep Breath 22, 805–814. [DOI] [PubMed] [Google Scholar]
- [133].de Almondes KM, Costa MV, Malloy-Diniz LF, Diniz BS (2016) Insomnia and risk of dementia in older adults: Systematic review and meta-analysis. J Psychiatr Res 77, 109–115. [DOI] [PubMed] [Google Scholar]
- [134].Yaffe K (2018) Modifiable risk factors and prevention of dementia what is the latest evidence? JAMA Intern Med 178, 281–282. [DOI] [PubMed] [Google Scholar]
- [135].Carroll S, Turkheimer E (2018) Midlife risk factors for late-life cognitive decline. Dev Rev 48, 201–222. [Google Scholar]
- [136].Halli-Tierney AD, Scarbrough C, Carroll D (2019) Polypharmacy: Evaluating risks and deprescribing. Am Fam Physician 100, 32–38. [PubMed] [Google Scholar]
- [137].Menditto E, Gimeno Miguel A, Moreno Juste A, Poblador Plou B, Aza Pascual-Salcedo M, Orlando V, Gonzalez Rubio F, Prados Torres A (2019) Patterns of multimorbidity and polypharmacy in young and adult population: Systematic associations among chronic diseases and drugs using factor analysis. PLoS One 14, e0210701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Giummarra MJ, Gibson SJ, Allen AR, Pichler AS, Arnold CA (2015) Polypharmacy and chronic pain: Harm exposure is not all about the opioids. Pain Med 16, 472–479. [DOI] [PubMed] [Google Scholar]
- [139].Ivo R, Nicklas A, Dargel J, Sobottke R, Delank K-S, Eysel P, Weber B (2013) Brain structural and psychometric alterations in chronic low back pain. Eur Spine J 22, 1958–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Sofat N, Ejindu V, Kiely P (2011) What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology 50, 2157–2165. [DOI] [PubMed] [Google Scholar]
- [141].Ji RR, Nackley A, Huh Y, Terrando N, Maixner W (2018) Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129, 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lluch E, Torres R, Nijs J, Van Oosterwijck J (2014) Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur J Pain 18, 1367–1375. [DOI] [PubMed] [Google Scholar]
- [143].Baker KS, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ (2017) Optimizing cognitive function in persons with chronic pain. Clin J Pain 33, 462–472. [DOI] [PubMed] [Google Scholar]
- [144].Al-Khazraji BK, Appleton CT, Beier F, Birmingham TB, Shoemaker JK (2018) Osteoarthritis, cerebrovascular dysfunction and the common denominator of inflammation: A narrative review. Osteoarthritis Cartilage 26, 462–470. [DOI] [PubMed] [Google Scholar]
- [145].Schaible H-G (2012) Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep 14, 549–556. [DOI] [PubMed] [Google Scholar]
- [146].Ikram M, Innes K, Sambamoorthi U (2019) Association of osteoarthritis and pain with Alzheimer’s diseases and related dementias among older adults in the United States. Osteoarthritis Cartilage 27, 1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Sibille KT, Chen H, Bartley EJ, Riley J 3rd, Glover, King CD, Zhang H, Cruz-Almeida Y, Goodin BR, Soto-longo A, Petrov ME, Herbert M, Bulls HW, Edberg JC, Staud R, Redden D, Bradley LA, Fillingim RB (2017) Accelerated aging in adults with knee osteoarthritis pain: Consideration for frequency, intensity, time, and total pain sites. Pain Rep 2, e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hassett AL, Epel E, Clauw DJ, Harris RE, Harte SE, Kairys A, Buyske S, Williams DA (2012) Pain is associated with short leukocyte telomere length in women with fibromyalgia. J Pain 13, 959–969. [DOI] [PubMed] [Google Scholar]
- [149].Fani L, Hila Saima, Sedaghat S, Broer L, Licher S, Arp PP, van Meurs JB, Ikram MK, Ikram MA (2020) Telomere length and the risk of Alzheimer’s disease: The Rotterdam Study. J Alzheimers Dis 73, 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhan Y, Song C, Karlsson R, Tillander A, Reynolds CA, Pedersen NL, Hagg S (2015) Telomere length shortening and alzheimer disease–a Mendelian randomization study. JAMA Neurol 72, 1202–1203. [DOI] [PubMed] [Google Scholar]
- [151].Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R (2012) Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol 69, 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]


