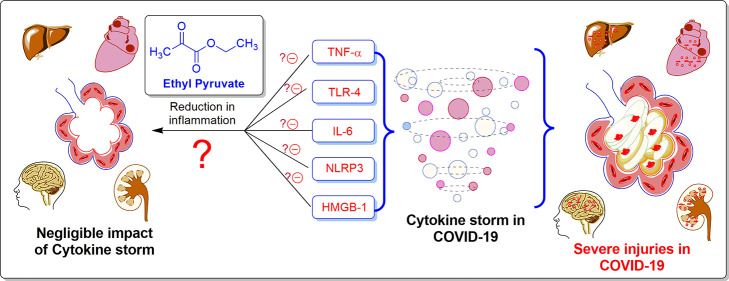
Abstract
COVID-19 is a deadly pandemic and has resulted in a huge loss of money and life in the past few months. It is well known that the SARS-CoV-2 gene mutates relatively slowly as compared to other viruses but still may create hurdles in developing vaccines. Therefore, there is a need to develop alternative routes for its management and treatment of COVID-19. Based on the severity of viral infection in COVID-19 patients, critically ill patients (∼5%, with old age, and comorbidities) are at high risk of morbidities. The reason for this severity in such patients is attributed to “misleading cytokine storm”, which produces ARDS and results in the deaths of critically ill patients. In this connection, ethyl pyruvate (EP) controls these cytokines/chemokines, is an anti-inflammatory agent, and possesses a protective effect on the lungs, brain, heart, and mitochondria against various injuries. Considering these facts, we propose that the site-selective EP formulations (especially aerosols) could be the ultimate adjuvant therapy for the regulation of misleading cytokine storm in severely affected COVID-19 patients and could reduce the mortalities.
Introduction
Coronavirus disease 2019 (COVID-19) is a highly infectious viral disease caused by severe acute respiratory syndrome coronavirus type-2 (SARS-CoV-2). Originated from China in December 2019, COVID-19 soon spread all around the world, and finally, on 11 March 2020, the World Health Organization (WHO) declared it a pandemic.1 Till 01 February 2021, ∼102,399,513 cases have been reported worldwide including ∼2,217,005 deaths.2 The battle is still on as the path of vaccine development is quite challenging due to the mutating nature of the virus.3,4 In SARS-COV-2 infection, most patients (∼80%) show mild symptoms or remain asymptomatic and thus need no hospitalization, while in some patients (∼15%), it becomes severe (need hospitalization) but can be recovered with proper care. However, a significant number of patients (∼5%), especially with older age and comorbidities like diabetes, hypertension, asthma, COPD, etc. are likely to develop interstitial pneumonia/acute respiratory distress syndrome (ARDS) and may eventually lead to death; therefore, such patients may need intensive care.5,6
Early pathogenesis of SARS-CoV-2 infection activates various immune cells (dendritic cell macrophages and neutrophils) leading to the further expression of several proinflammatory chemokines and cytokines such as IL-6, TNF-α, HMGB1, and so forth. Among them, being an imperative member of the cytokine network, interleukin-6 (IL-6) plays an important role as a proinflammatory mediator. The main activators of IL-6 production are tumor necrosis factor (TNF-α) and IL-1β. At the beginning of infectious inflammation, IL-6 plays a very crucial role in stimulating various host-cell defense mechanisms.27 Unfortunately, the overproduction of different chemokines and cytokines could develop an uncontrolled pathological condition, famously termed as “the Cytokine storm”.7 Incongruous development and misleading cytokine storm (majorly due to the increased level of TNF-α, IL-6, HMGB1, etc.) result in a plethora of immunopathological events after severe SARS-COV-2 infection. These uncontrolled immunopathological events develop ARDS and are responsible for deaths in COVID-19 patients.8−10 A huge amount of effort and money is being invested in the development of vaccines and drugs for the complete treatment of COVID-19; however, scientists are still struggling to get an ideal vaccine and/or drug for the same. Fortunately, corticosteroids (dexamethasone/hydrocortisone) have been found effective in patients with severe and critical COVID-19, and WHO has also recommended their use in special cases.11 Nonetheless, the research efforts should be diverted toward alternative treatment strategies such as management of misleading cytokine storm wherein the human body will get sufficient time to regulate its immunity to fight against this deadly infection.
To manage the misleading cytokine storm in COVID-19, we propose that the site-specific formulation (specifically an aerosol) of ethyl pyruvate (EP) could be a game-changer therapeutic intervention for the management of infection caused by SARS-COV-2 and other viruses.
Immunopathological Events in COVID-19
The spike proteins of SARS-COV or SARS-COV-2 attach to the angiotensin-converting enzyme 2 (ACE2) receptor for entering into the host cells. The ACE2 receptors are expressed on the alveolar epithelial type II cell, intestinal, cardiac, renal, and endothelial cells. The organs associated with these cells may be the targets for the development of new drugs for the treatment of COVID-19.5 The binding of the virus with the host cell receptor is a significant determinant of the pathogenesis of infection. As the virus enters into the host cell, its antigens activate antigen-presenting cells (APCs), and this phenomenon is the central part of the body’s immune system.5,8,12 The APC cells then activates the immune system and causes the production of a large amount of cytokines. In some patients, the cytokine activation gets misled (activates uninhibited “cytokine storm”), resulting in multiorgan failure and finally causing death in some critical cases.5,13−15
Relationship between TNF-α and COVID-19
TNF-α is a type of cell-signaling protein (a proinflammatory mediator) and an important member of the cytokine family, which is involved in the regulation of systemic inflammation. Typically, the infection caused by coronaviruses results in the upregulation of TNF-α and other cytokines (Figure 1);10,16 it has been observed that TNF-α levels get elevated in critical COVID-19 patients.17 Indeed, chloroquine and hydroxychloroquine have been proposed for the treatment of COVID-19 and found to inhibit the overproduction of these cytokines,18 and recent studies have also proven this hypothesis.19
Figure 1.
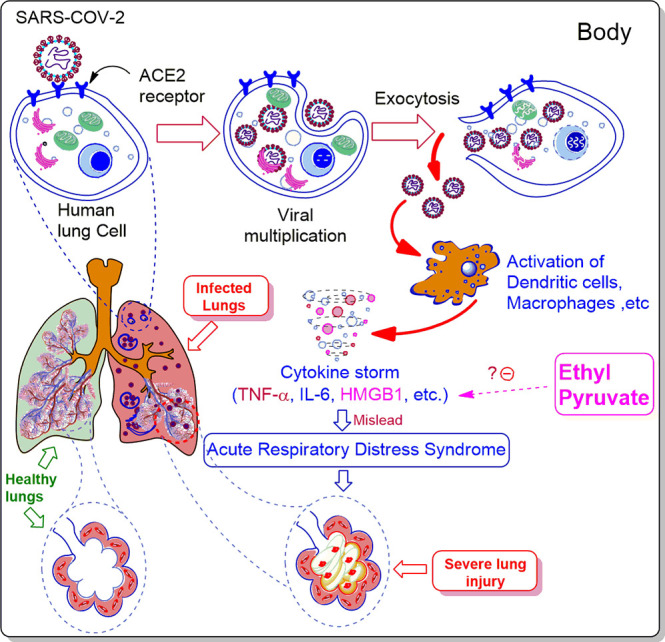
Immunopathological events (“cytokine storm”) of SARS-COV-2 infection and probable role of EP in ARDS management.
Role of IL-6 in COVID-19
Studies reveal that the uncontrolled production of interleukins, especially IL-6, plays a major role in the development of misleading cytokine storm (Figure 1).9 Therefore, it has been proposed that the early prediction of the elevated levels of IL-6 can be the hallmark for severely infected COVID-19 patients, which may require intensive care in the future.20 Moreover, such patients are being clinically tested for the effectiveness of tocilizumab and sarilumab in the treatment of COVID-19. These clinical trials typically cite the importance of these cytokines as the best strategies for the management of COVID-19.21,22 Thus, if we can reduce the production of these proinflammatory mediators (especially IL-6), then the uncontrolled hyperimmune response can be conquered, which in turn will provide a big relief to the severely affected patients of COVID-19 and could reduce the resulting deaths.23
Role of High-Mobility Group Box 1 Protein in COVID-19 (a Potential Therapeutic Target)
Cells infected with viruses or traumatic cells release many endogenous damage-associated molecular-pattern molecules (DAMPs) as emergency signals for intracellular homeostatic imbalance. High-mobility group box 1 protein (HMGB1) regulates the pathogenesis of many inflammatory diseases by acting as DAMPs and activates innate immunity as a result of viral infection or other cell damages such as oxidative damage, hypoxia, starvation, and pathogen infection.24,25 Moreover, excessive amounts of extracellular HMGB1 are associated with an increased release of IL-6, TNF, and IL-126 making it an another important mediator to be regulated in COVID-19 (Figure 1). It has been found that chloroquine inhibits HMGB1 and exerts its action in the treatment of COVID-19.25,27,28 However, the use of chloroquine and hydroxychloroquine is associated with some toxicities.29 Moreover, HMGB1 also has a major role in neuroinflammation and has been implicated in brain injury.30 This also correlates with the after-effects of SARS-COV-2 infection as some brain injuries have also been reported in COVID-19 patients who got successfully recovered from this deadly disease.31,32 Very recently, an experiment performed in Vero-E6 cells using genome-wide CRISPR screens proved that HMGB1 regulates ACE2 expression and results in SARS-CoV-2 infection.33 Therefore, drugs that control HMGB1 levels could provide potential benefits from mortality and the after-effects of COVID-19.24,34−37
EP: A Human Metabolite and a Novel Anti-inflammatory Agent
EP is also known as pyruvic acid ethyl ester or 2-oxo-propionate ethyl ester or as an investigation drug known as CTI-01 (Figure 2).38 EP is one of the compounds in the human metabolome database (HMDB).39,40 It belongs to the family of α-keto esters and derivatives.38,41 Different properties of EP are given in Table 1. EP is a derivative of the pyruvic acid/pyruvate which is the end product in the glycolysis cycle. It is well known that in Krebs’s cycle, pyruvate produces more energy in the form of ATP, while under anaerobic conditions, fewer ATPs are produced. Therefore, EP belongs to the indigenous compound family of our body and may produce less undesired effects if used as a drug. EP is also being used as a flavoring ingredient in food materials and can be consumed in decent quantities.42
Figure 2.
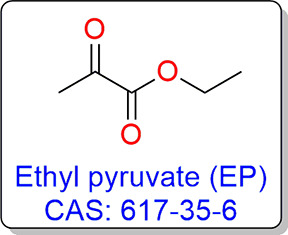
Structure and the CAS registry number of EP.
Table 1. Molecular Properties of EP.
| S. No | property name | property value |
|---|---|---|
| 1. | IUPAC name | ethyl 2-oxopropanoate |
| 2. | CAS number | 617-35-6 |
| 3. | SMILES | CCOC(=O)C(C)=O |
| 4. | molecular weight | 116.11 g/mol |
| 5. | number of hydrogen bond donors | 0 |
| 6. | number of hydrogen bond acceptors | 3 |
| 7. | number of rotatable bonds | 3 |
| 8. | logP value | 0.24 |
| 9. | pKa (strongest acidic) | 16.920 |
| 10. | pKa (strongest basic) | –7.720 |
| 11. | exact mass | 116.047344 g/mol |
| 12. | topological polar surface area | 43.4 Å2 |
| 13. | physical properties | colorless liquid, with a sweet, floral-fruity, warm odor |
| 14. | boiling point | 155.0 °C |
| 15. | melting point | 155.0 °C |
| 16. | solubility | slightly solubility (31.3 mg/mL);38 soluble in organic solvents, oils |
| 17. | density | 1.044–1.065 |
| 18. | rat acute toxicity | 1.0990 LD50, mol/kg38 |
EP is a novel anti-inflammatory agent and has been found useful in improving organ dysfunction in many preclinical models of critical illnesses, for example, ARDS, acute pancreatitis, severe sepsis, and stroke. It has been found that EP treatment in humans is safe when used at clinically relevant doses43 and also decreases the levels of various cytokines (e.g., IL-6, TNF-α, HMGB1, and so forth, Figures 3 and 4) in such critical illnesses, which promote the body’s inflammatory response.40
Figure 3.
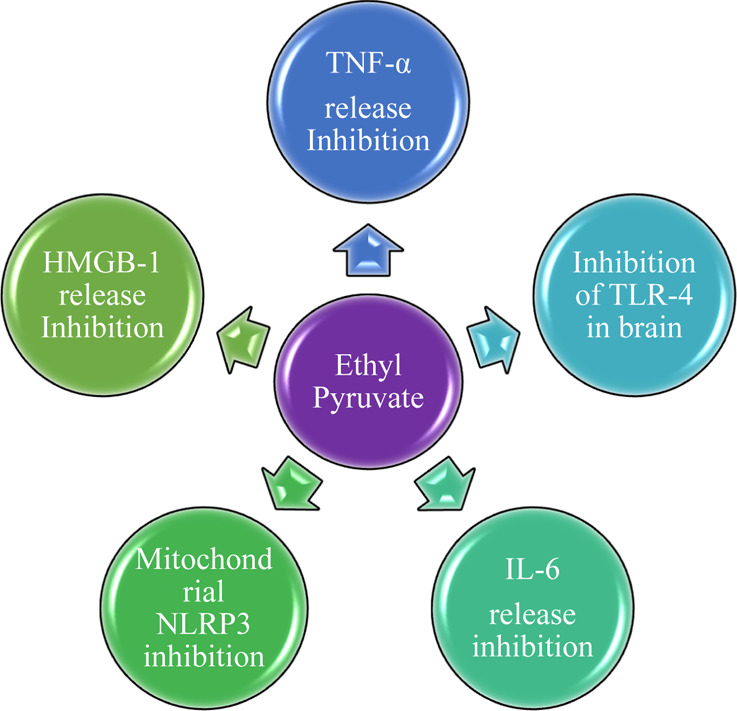
Different mechanisms of EP.
Figure 4.
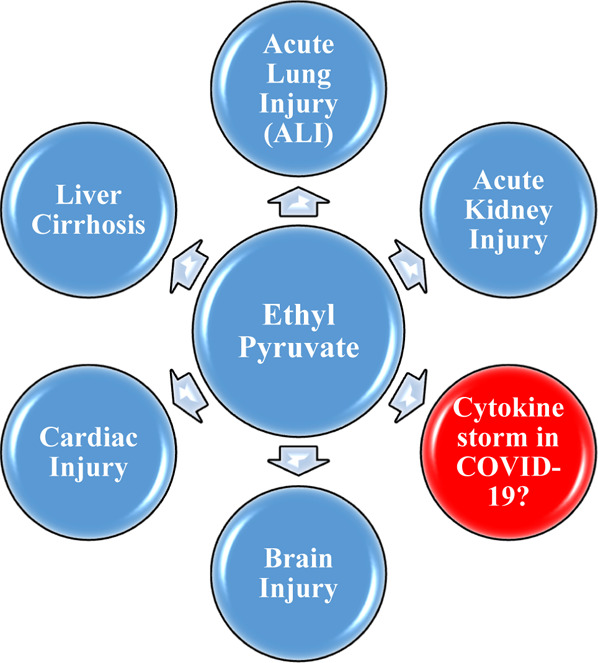
Various uses of EP.
Various Therapeutic Applications of EP
EP is found to be useful in treating many types of injuries of different organs including lungs, heart, brain, spinal cord, and so forth., as reported in the various literature studies (Figures 3 and 4).
Effect of EP in the Lung Function
Lipopolysaccharide (LPS)-induced acute lung injury (ALI) is a deadly disease in humans with high mortality rates and has symptoms that are similar to ARDS; it occurs due to diffuse alveolar damage and microvascular injury. EP is an anti-inflammatory agent which increases survival by reducing the level of IL-6 and by inhibiting the activation of nuclear factor-κB (NF-κB: a protein complex that controls the transcription of DNA, cytokine production, and cell survival). It also blocks the secretion of HMGB1 in LPS-induced ALI in mice and inhibits the circulation of HMGB1.44 By inhibiting autophagy in neutrophils, EP has the potential to dampen the granule release in ALI, and thus, it can be used in the treatment of LPS-induced ALI.45 Therefore, we propose that EP may inhibit the systemic release of both early (TNF-α, IL-1β, and IL-6) and late (HMGB1) cytokines in COVID-19 critical patients.
It has been observed that when the airway epithelium is persistently exposed to organic dust (OD), it results in several respiratory symptoms.46 EP has been found to decrease the expression levels of HMGB1 and receptors for advanced glycation end products (RAGEs) in cytoplasm.46
It has been found that radiation therapy overdose (especially in the case of lung cancer) induces the profibrotic cytokines (TGF-β1 and HMGB1) and proinflammatory mediators which lead to lung damage. EP treatment (at 4 weeks after irradiation) in radiation-induced lung injury (RILI) significantly reduces pulmonary inflammation infiltration and decreases the production of IL-6, HMGB1, IL-1β, and granulocyte-macrophage colony-stimulating factor (GM–CSF). It was also observed that EP has an anti-inflammatory action in RILI (by reducing the collagen deposition induced by radiation) and could ameliorate lung destruction resulted from harmful radiations.47
The efficacy of EP for bleomycin-induced lung fibrosis was investigated by the hyperpolarized 129Xe magnetic resonance imaging (MRI) protocol. EP treatment has been found to improve ventilation and gas-exchange impairment caused due to lung fibrosis and tissue damage. In the experiments, it was observed that HMGB1 is involved in chronic obstructive pulmonary disease (COPD) progression (histological analysis showed significant alveolar tissue destruction) and inhibition of HMGB1 resulted in a relatively normal alveolar structure.48,49 These studies suggest that the utility of EP can be one of the potential supportive therapies for the management of COVID-19 critical patients.
EP is a potent antioxidant and scavenges free radicals and has also been found to downregulate inflammatory gene expression in acute injuries. It has been found to attenuate pulmonary artery cytokine storm by inhibiting hypoxic pulmonary vasoconstriction.50 Similarly, hypoxia is also observed in COVID-19 patients and may be useful for improving the health of such patients.
Effect of EP on the Brain Function
EP has a neuroprotective effect in the postischemic brain51 and protects peripheral nerve degeneration via the inhibition of neuronal nitric oxide synthase.52 Moreover, by inhibiting HMGB1, EP has demonstrated neuroprotective effects against acute brain injury.53 Considering the neuroprotective action of EP, post-COVID-19 brain abnormalities can be treated with EP-based therapy.32
Neuroinflammation and reactive astrogliosis are responsible for neuronal degeneration and may develop spinal cord injury (SCI), which in turn may hinder neural regeneration and functional recovery. EP has shown its potential effects by inhibition of astrogliosis and neuroinflammation which promotes neuron survival and neural regeneration, with an improvement in the functional recovery of the spinal cord, indicating a potential neuroprotective effect of EP against SCI.54,55
Effect of EP on the Cardiac Function
EP plays its role in myocardial protection due to its antioxidant property, leading to the reduction of reactive oxygen species (ROS).56 It has been observed that EP improves cardiac functions by reducing apoptosis resulting from myocardial ischemia and reperfusion.57
Effect of EP on Inflammatory and Autoimmune Disorders
The mitochondria are known to control cellular oxidative homeostasis and are essential for the survival of human cells. It has been found that SARS-CoV-2 hijacks the host’s mitochondria and uses its machinery for replication.58,59 EP can be very useful in the protection of mitochondria in COVID-19 patients, as the recent studies show that EP acts as a novel NLR family pyrin domain containing 3 (NLRP3) inflammasome inhibitor that preserves the integrity of mitochondria during inflammation and significantly attenuates mitochondrial damage and cytoplasmic translocation of mitochondrial DNA.60
It is well known that immune response is mediated by specialized antigen-presenting cells specifically tolerogenic dendritic cells (tolDC). These cells have immunoregulatory properties and are implicated in multiple sclerosis as well as other autoimmune diseases. The in vitro experiments by Djedovic et al. established the relationship for the use of EP in the induction of tolDC cells.61 Similar observations were reported by Chakhtoura et al., wherein EP was found to modulate murine dendritic cell activation and survival through their immunometabolism.62 Another very important study was reported by Koprivica et al., wherein reduced inflammatory responses and resulting diminished destruction of insulin-producing pancreatic β-cells were observed in EP-treated mice.63
Effect of EP on Other Organ Systems
Apart from the above-mentioned positive effects, EP has many other positive effects on other organs such as (1) ameliorates hepatic injury,64 (2) attenuates sugar cataract,65,66 (3) provides durable protection against inflammation-induced gastrointestinal tract epithelial barrier dysfunction,67 (4) inhibits bacterial translocation, and decreases the production of malondialdehyde/myeloperoxidase in the intestine occurred due to thermal injury,68 (5) ameliorates intestinal epithelial barrier dysfunction,69 and (6) prevents renal damage induced by advanced glycation end products (AGEs).70 Therefore, administration of EP may be beneficial to COVID-19 patients when given before and/or after the treatment plan.
Conclusions
The present study is novel in the context of COVID-19 management as (1) EP could never be identified through molecular modeling studies as it is a fragment-like molecule. (2) The article proposes a pulmonary delivery system of EP, which could be a potential adjuvant therapy for the management of pre- as well as post-SARS-COV-2 infection in COVID-19 patients. Specifically, an EP–aerosol would be the ideal choice as it will have a minimal systemic side effect on other organs. (3) Such formulations are unavailable for the complete management of misleading cytokine storm in COVID-19 patients. (4) It may be one of the unique adjuvant therapies that can be utilized in the present scenario as there is no specific medication for the complete treatment of COVID-19 and handling mutating strains. Overall, the use of EP could be a boon for the management of misleading cytokine and reducing the deaths due to pneumonia-like/viral infections or diseases where patients succumb to death due to severe ARDS.
Based on various literature results mentioned above, it is clear that EP possesses various beneficial and protective effects against various organ injuries. Similar injuries are being observed in COVID-19 patients, which lead to the development of incongruous cytokine storm and finally death in many patients. The administration of EP locally (lungs) or systemically can be advantageous for COVID-19 patients and may reduce the mortalities of such patients. EP formulation can be a game-changer adjuvant therapy in controlling and managing the COVID-19 and related diseases. Thus, we propose to use EP formulations for controlling and managing the misleading cytokine storm in COVID-19 patients. Although this needs more clinical data as proof and may go through a long process, it is worth trying to get some benefits till ideal antiviral drugs reach the market.
Acknowledgments
We acknowledge the support of Director, Shri G.S. Institute of Technology and Science and Technology, Indore, Madhya Pradesh, India, for providing necessary facilities to compile this manuscript.
The authors declare no competing financial interest.
References
- WHO Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 30 August 2020).
- WHO WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ (accessed 01 February 2021).
- Baric R. S. Emergence of a Highly Fit SARS-CoV-2 Variant. N. Engl. J. Med. 2020, 383, 2684–2686. 10.1056/nejmcibr2032888. [DOI] [PubMed] [Google Scholar]
- Callaway E.The coronavirus is mutating — does it matter? https://www.nature.com/articles/d41586-020-02544-6 (accessed 02 February 2021). [DOI] [PubMed]
- Soy M.; Keser G.; Atagündüz P.; Tabak F.; Atagündüz I.; Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Front. Immunol. 2020, 39, 2085–2094. 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Clinical management of COVID-19. who.int/publications/i/item/clinical-management-of-covid-19 (accessed 08 September 2020).
- Samaiya P. K.; Kumar M. SARS-CoV-2 Therapy: Old Drugs as New Interventions. Coronaviruses 2020, 1, 1–9. 10.2174/2666796701999200721003212. [DOI] [Google Scholar]
- Li X.; Geng M.; Peng Y.; Meng L.; Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Liu J.; Zhang D.; Xu Z.; Ji J.; Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussman J. P. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front. Pharmacol. 2020, 11, 1169. 10.3389/fphar.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Corticosteroids for COVID-19. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed 01 February 2021).
- Sarzi-Puttini P.; Giorgi V.; Sirotti S.; Marotto D.; Ardizzone S.; Rizzardini G.; Antinori S.; Galli M. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome?. Clin. Exp. Rheumatol. 2020, 38, 337–342. [PubMed] [Google Scholar]
- Huang C.; Wang Y.; Li X.; Ren L.; Zhao J.; Hu Y.; Zhang L.; Fan G.; Xu J.; Gu X.; Cheng Z.; Yu T.; Xia J.; Wei Y.; Wu W.; Xie X.; Yin W.; Li H.; Liu M.; Xiao Y.; Gao H.; Guo L.; Xie J.; Wang G.; Jiang R.; Gao Z.; Jin Q.; Wang J.; Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020, 395, 497–506. 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.; Huang S.; Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2020, 93, 250–256. 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P.; Li W.; Xie J.; Hou Y.; You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Ye L.; Ye L.; Li B.; Gao B.; Zeng Y.; Kong L.; Fang X.; Zheng H.; Wu Z.; She Y. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007, 128, 1–8. 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadpoor P.; Rostaing L. Why the immune system fails to mount an adaptive immune response to a COVID-19 infection. Transpl. Int. 2020, 33, 824–825. 10.1111/tri.13611. [DOI] [PubMed] [Google Scholar]
- Jang C.-H.; Choi J.-H.; Byun M.-S.; Jue D.-M. Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology 2006, 45, 703–710. 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- Grassin-Delyle S.; Salvator H.; Brollo M.; Catherinot E.; Sage E.; Couderc L.-J.; Naline E.; Devillier P. Chloroquine Inhibits the Release of Inflammatory Cytokines by Human Lung Explants. Clin. Infect. Dis. 2020, 71, 2265–2268. 10.1093/cid/ciaa546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernatorova E. O.; Gorshkova E. A.; Polinova A. I.; Drutskaya M. S. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020, 53, 13–24. 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocilizumab vs CRRT in Management of Cytokine Release Syndrome (CRS) in COVID-19 (TACOS). https://clinicaltrials.gov/ct2/show/NCT04306705 (accessed 28 August 2020).
- Anti-il6 Treatment of Serious COVID-19 Disease With Threatening Respiratory Failure (TOCIVID). https://www.clinicaltrials.gov/ct2/show/NCT04322773 (accessed 28 August 2020).
- Mayor S. Intensive immunosuppression reduces deaths in covid-19-associated cytokine storm syndrome, study finds. Br. Med. J. 2020, 370, m2935. 10.1136/bmj.m2935. [DOI] [PubMed] [Google Scholar]
- Andersson U.; Ottestad W.; Tracey K. J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19?. Mol. Med. 2020, 26, 42. 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D.; Comish P.; Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020, 16, e1008536 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U.; Wang H.; Palmblad K.; Aveberger A.-C.; Bloom O.; Erlandsson-Harris H.; Janson A.; Kokkola R.; Zhang M.; Yang H.; Tracey K. J. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. H.; He L.; Long W.; Zhou Q.; Zhu S.; Wang P.; Fan S.; Wang H. Novel Mechanisms of Herbal Therapies for Inhibiting HMGB1 Secretion or Action. J. Evidence-Based Complementary Altern. Med. 2015, 2015, 456305. 10.1155/2015/456305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Cao L.; Xie M.; Yu Y.; Kang R.; Yang L.; Zhao M.; Tang D. Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem. Pharmacol. 2013, 86, 410–418. 10.1016/j.bcp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Porta A.; Bornstein K.; Coye A.; Montrief T.; Long B.; Parris M. A. Acute chloroquine and hydroxychloroquine toxicity: A review for emergency clinicians. Am. J. Emerg. Med. 2020, 38, 2209–2217. 10.1016/j.ajem.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel Y. N.; Angelopoulou E.; Piperi C.; Othman I.; Shaikh M. F. HMGB1-Mediated Neuroinflammatory Responses in Brain Injuries: Potential Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 4609. 10.3390/ijms21134609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov V.; Manina G.; Mikusova K.; Möllmann U.; Ryabova O.; Saint-Joanis B.; Dhar N.; Pasca M. R.; Buroni S.; Lucarelli A. P.; Milano A.; De Rossi E.; Belanova M.; Bobovska A.; Dianiskova P.; Kordulakova J.; Sala C.; Fullam E.; Schneider P.; McKinney J. D.; Brodin P.; Christophe T.; Waddell S.; Butcher P.; Albrethsen J.; Rosenkrands I.; Brosch R.; Nandi V.; Bharath S.; Gaonkar S.; Shandil R. K.; Balasubramanian V.; Balganesh T.; Tyagi S.; Grosset J.; Riccardi G.; Cole S. T. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science (New York, N.Y.) 2009, 324, 801–804. 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. W.; Brown R. L.; Benjamin L.; Nortley R.; Wiethoff S.; Bharucha T.; Jayaseelan D. L.; Kumar G.; Raftopoulos R. E.; Zambreanu L.; Vivekanandam V.; Khoo A.; Geraldes R.; Chinthapalli K.; Boyd E.; Tuzlali H.; Price G.; Christofi G.; Morrow J.; McNamara P.; McLoughlin B.; Lim S. T.; Mehta P. R.; Levee V.; Keddie S.; Yong W.; Trip S. A.; Foulkes A. J. M.; Hotton G.; Miller T. D.; Everitt A. D.; Carswell C.; Davies N. W. S.; Yoong M.; Attwell D.; Sreedharan J.; Silber E.; Schott J. M.; Chandratheva A.; Perry R. J.; Simister R.; Checkley A.; Longley N.; Farmer S. F.; Carletti F.; Houlihan C.; Thom M.; Lunn M. P.; Spillane J.; Howard R.; Vincent A.; Werring D. J.; Hoskote C.; Jäger H. R.; Manji H.; Zandi M. S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J.; Alfajaro M. M.; DeWeirdt P. C.; Hanna R. E.; Lu-Culligan W. J.; Cai W. L.; Strine M. S.; Zhang S.-M.; Graziano V. R.; Schmitz C. O.; Chen J. S.; Mankowski M. C.; Filler R. B.; Ravindra N. G.; Gasque V.; de Miguel F. J.; Patil A.; Chen H.; Oguntuyo K. Y.; Abriola L.; Surovtseva Y. V.; Orchard R. C.; Lee B.; Lindenbach B. D.; Politi K.; van Dijk D.; Kadoch C.; Simon M. D.; Yan Q.; Doench J. G.; Wilen C. B. Genome-wide CRISPR Screens Reveal Host Factors Critical for SARS-CoV-2 Infection. Cell 2021, 184, 76–91. 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.; Chen D.-Z.; Li J.; Czura C. J.; Tracey K. J.; Sama A. E.; Wang H. Pathogenic role of HMGB1 in SARS?. Med. Hypotheses 2004, 63, 691–695. 10.1016/j.mehy.2004.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.; Huang Y.; Quan J.; Liu J.; Wang H.; Billiar T. R.; Lotze M. T.; Zeh H. J.; Kang R.; Tang D. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon 2020, 6, e05672 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicco S.; Cicco G.; Racanelli V.; Vacca A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediat. Inflamm. 2020, 2020, 7527953. 10.1155/2020/7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelebier M.; Haznedaroğlu İ C. Could Targeting HMGB1 be useful for the Clinical Management of COVID-19 Infection?. Comb. Chem. High Throughput Screening 2020, 24, 587–590. 10.2174/1386207323999200728114927. [DOI] [PubMed] [Google Scholar]
- Drug Bank: Ethyl pyruvate. https://www.drugbank.ca/drugs/DB05869 (accessed 28 August 2020).
- Metabocard for Ethyl pyruvate (HMDB0031643). https://hmdb.ca/metabolites/HMDB0031643 (accessed 11 September 2020).
- Metabocard for Ethyl pyruvate (HMDB0031643). https://hmdb.ca/metabolites/HMDB0031643 (accessed 28 August 2020).
- Ethyl pyruvate. https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl-pyruvate (accessed 28 August 2020).
- Dictionary of food compounds with CD-ROM: additives, flavors, and ingredients; Shmuel Y., ed.; Chapman & Hall/CRC: Boca Raton, Fla, 2004. [Google Scholar]
- Fink M. P. Ethyl pyruvate: a novel anti-inflammatory agent. J. Intern. Med. 2007, 261, 349–362. 10.1111/j.1365-2796.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- Shang G.-H.; Lin D.-J.; Xiao W.; Jia C.-Q.; Li Y.; Wang A.-H.; Dong L. Ethyl pyruvate reduces mortality in an endotoxin-induced severe acute lung injury mouse model. Respir. Res. 2009, 10, 91. 10.1186/1465-9921-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.; Wang H.; Wang H.; Luo Y.; Yu Y.; Du Q.; Fei A.; Pan S. Protective effects of ethyl pyruvate on lipopolysaccharide-induced acute lung injury through inhibition of autophagy in neutrophils. Mol. Med. Rep. 2017, 15, 1272–1278. 10.3892/mmr.2017.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S. M.; Massey N.; Karriker L. A.; Singh B.; Charavaryamath C. Ethyl pyruvate reduces organic dust-induced airway inflammation by targeting HMGB1-RAGE signaling. Respir. Res. 2019, 20, 27. 10.1186/s12931-019-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.; Na F.; Yang H.; Li R.; Li M.; Sun X.; Hu B.; Huang G.; Lan J.; Xu H.; Tong R.; Mo X.; Xue J.; Lu Y. Ethyl pyruvate alleviates radiation-induced lung injury in mice. Biomed. Pharmacother. 2017, 92, 468–478. 10.1016/j.biopha.2017.05.111. [DOI] [PubMed] [Google Scholar]
- Hodono S.; Shimokawa A.; Stewart N. J.; Yamauchi Y.; Nishimori R.; Yamane M.; Imai H.; Fujiwara H.; Kimura A. Ethyl Pyruvate Improves Pulmonary Function in Mice with Bleomycin-induced Lung Injury as Monitored with Hyperpolarized (129)Xe MR Imaging. Magn. Reson. Med. Sci. 2018, 17, 331–337. 10.2463/mrms.mp.2017-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A.; Yamauchi Y.; Hodono S.; Stewart N. J.; Hosokawa O.; Hagiwara Y.; Imai H.; Fujiwara H. Treatment response of ethyl pyruvate in a mouse model of chronic obstructive pulmonary disease studied by hyperpolarized (129) Xe MRI. Magn Reson Med 2017, 78, 721–729. 10.1002/mrm.26458. [DOI] [PubMed] [Google Scholar]
- Tsai B. M.; Lahm T.; Morrell E. D.; Crisostomo P. R.; Poynter J.; Wang M.; Meldrum D. R. Ethyl pyruvate inhibits hypoxic pulmonary vasoconstriction and attenuates pulmonary artery cytokine expression. J. Surg. Res. 2008, 145, 130–134. 10.1016/j.jss.2007.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-W.; Jeong J.-Y.; Kim H. J.; Seo J.-S.; Han P.-L.; Yoon S.-H.; Lee J.-K. Combination Treatment with Ethyl Pyruvate and Aspirin Enhances Neuroprotection in the Postischemic Brain. Neurotoxic. Res. 2010, 17, 39–49. 10.1007/s12640-009-9075-4. [DOI] [PubMed] [Google Scholar]
- Chung H.-J.; Kim M.; Jung J.; Jeong N. Y. Inhibition of Neuronal Nitric Oxide Synthase by Ethyl Pyruvate in Schwann Cells Protects Against Peripheral Nerve Degeneration. Neurochem. Res. 2019, 44, 1964–1976. 10.1007/s11064-019-02830-4. [DOI] [PubMed] [Google Scholar]
- Su X.; Wang H.; Zhao J.; Pan H.; Mao L. Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-κB pathway after traumatic brain injury in the rat. Mediat. Inflamm. 2011, 2011, 807142. 10.1155/2011/807142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Su Z.; Pu Y.; Liu X.; Chen J.; Zhu F.; Zhu Y.; Zhang H.; He C. Ethyl pyruvate promotes spinal cord repair by ameliorating the glial microenvironment. Br. J. Pharmacol. 2012, 166, 749–763. 10.1111/j.1476-5381.2011.01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T.; Esposito E.; Mazzon E.; Di Paola R.; Meli R.; Caminiti R.; Bramanti P.; Fink M. P.; Cuzzocrea S. Beneficial effects of ethyl pyruvate in a mouse model of spinal cord injury. Shock 2009, 32, 217–227. 10.1097/shk.0b013e31818d4073. [DOI] [PubMed] [Google Scholar]
- Jun J. H.; Shim J.-K.; Oh J. E.; Shin E.-J.; Shin E.; Kwak Y.-L. Protective Effect of Ethyl Pyruvate against Myocardial Ischemia Reperfusion Injury through Regulations of ROS-Related NLRP3 Inflammasome Activation. Oxid. Med. Cell. Longevity 2019, 2019, 4264580. 10.1155/2019/4264580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.; Zhang J.; Luo X.; Luo W.; Lin C.; Zhang K.; Ji Y. Effects of ethyl pyruvate on cardiac function recovery and apoptosis reduction after global cold ischemia and reperfusion. Exp. Ther. Med. 2014, 7, 1197–1202. 10.3892/etm.2014.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. K.; Chaubey G.; Chen J. Y.; Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol.: Cell Physiol. 2020, 319, C258–C267. 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh J.; Peyssonnaux C.; Singh K. K.; Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020, 54, 1–7. 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Liang F.; Kwan K.; Tang Y.; Wang X.; Tang Y.; Li J.; Yang H.; Chavan S. S.; Wang H.; Andersson U.; Lu B.; Tracey K. J. Identification of ethyl pyruvate as a NLRP3 inflammasome inhibitor that preserves mitochondrial integrity. Mol. Med. 2018, 24, 8. 10.1186/s10020-018-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djedovic N.; Mansilla M. J.; Jevtić B.; Navarro-Barriuso J.; Saksida T.; Martínez-Cáceres E. M.; Miljković Đ. Ethyl Pyruvate Induces Tolerogenic Dendritic Cells. Front. Immunol. 2019, 10, 157. 10.3389/fimmu.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakhtoura M.; Chain R. W.; Sato P. Y.; Qiu C. C.; Lee M. H.; Meissler J. J.; Eisenstein T. K.; Koch W. J.; Caricchio R.; Gallucci S. Ethyl Pyruvate Modulates Murine Dendritic Cell Activation and Survival Through Their Immunometabolism. Front. Immunol. 2019, 10, 30. 10.3389/fimmu.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivica I.; Vujičić M.; Gajić D.; Saksida T.; Stojanović I. Ethyl Pyruvate Stimulates Regulatory T Cells and Ameliorates Type 1 Diabetes Development in Mice. Front. Immunol. 2019, 9, 3130. 10.3389/fimmu.2018.03130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N.; Dieteren S.; Franz N.; Köhler K.; Mörs K.; Nicin L.; Schmidt J.; Perl M.; Marzi I.; Relja B. Ethyl pyruvate ameliorates hepatic injury following blunt chest trauma and hemorrhagic shock by reducing local inflammation, NF-kappaB activation and HMGB1 release. PloS One 2018, 13, e0192171 10.1371/journal.pone.0192171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devamanoharan P. S.; Henein M.; Ali A. H.; Varma S. D. Attenuation of sugar cataract by ethyl pyruvate. Mol. Cell. Biochem. 1999, 200, 103–109. 10.1023/a:1007055503748. [DOI] [PubMed] [Google Scholar]
- Varma S. D.; Hegde K. R.; Kovtun S. Oxidative damage to lens in culture: reversibility by pyruvate and ethyl pyruvate. Ophthalmologica 2006, 220, 52–57. 10.1159/000089275. [DOI] [PubMed] [Google Scholar]
- Sappington P. L.; Fink M. E.; Yang R.; Delude R. L.; Fink M. P. Ethyl pyruvate provides durable protection against inflammation-induced intestinal epithelial barrier dysfunction. Shock (Augusta, Ga.) 2003, 20, 521–528. 10.1097/01.shk.0000092697.10326.8b. [DOI] [PubMed] [Google Scholar]
- Karabeyoğlu M.; Unal B.; Bozkurt B.; Dolapçi I.; Bilgihan A.; Karabeyoğlu I.; Cengiz O. The effect of ethyl pyruvate on oxidative stress in intestine and bacterial translocation after thermal injury. J. Surg. Res. 2008, 144, 59–63. 10.1016/j.jss.2007.02.050. [DOI] [PubMed] [Google Scholar]
- Sappington P. L.; Han X.; Yang R.; Delude R. L.; Fink M. P. Ethyl Pyruvate Ameliorates Intestinal Epithelial Barrier Dysfunction in Endotoxemic Mice and Immunostimulated Caco-2 Enterocytic Monolayers. J. Pharmacol. Exp. Ther. 2003, 304, 464–476. 10.1124/jpet.102.043182. [DOI] [PubMed] [Google Scholar]
- Jung E.; Kang W. S.; Jo K.; Kim J. Ethyl Pyruvate Prevents Renal Damage Induced by Methylglyoxal-Derived Advanced Glycation End Products. J. Diabetes Res. 2019, 2019, 4058280. 10.1155/2019/4058280. [DOI] [PMC free article] [PubMed] [Google Scholar]